Abstract
Radiologists play a central role in the diagnostic and prognostic evaluation of patients with acute mesenteric ischaemia (AMI). Unfortunately, more than half of AMI patients undergo imaging with no prior suspicion of AMI, making identifying this disease even more difficult. A confirmed diagnosis of AMI is ideally made with dynamic contrast-enhanced CT but the diagnosis may be made on portal-venous phase images in appropriate clinical settings. AMI is diagnosed on CT based on the identification of vascular impairment and bowel ischaemic injury with no other cause. Moreover, radiologists must evaluate the probability of bowel necrosis, which will influence the treatment options.
AMI is usually separated into different entities: arterial, venous, non-occlusive and ischaemic colitis. Arterial AMI can be occlusive or stenotic, the dominant causes being atherothrombosis, embolism and isolated superior mesenteric artery (SMA) dissection. The main finding in the bowel is decreased wall enhancement, and necrosis can be suspected when dilatation >25 mm is identified. Venous AMI is related to superior mesenteric vein (SMV) thrombosis as a result of a thrombophilic state (acquired or inherited), local injury (cancer, inflammation or trauma) or underlying SMV insufficiency. The dominant features in the bowel are hypoattenuating wall thickening with submucosal oedema. Decreased enhancement of the involved bowel suggests necrosis. Non-occlusive mesenteric ischaemia (NOMI) is related to impaired SMA flow following global hypoperfusion associated with low-flow states. There are numerous findings in the bowel characterised by diffuse extension. An absence of bowel enhancement and a thin bowel wall suggest necrosis in NOMI. Finally, ischaemic colitis is a sub-entity of arterial AMI and reflects localised colon ischaemia-reperfusion injury. The main CT finding is a thickened colon wall with fat stranding, which seems to be unrelated to SMA or inferior mesenteric artery lesions. A precise identification and description of vascular lesions, bowel involvement and features associated with transmural necrosis is needed to determine patient treatment and outcome.
Introduction
The bowel is a vital end organ like the brain or the heart. Significant impairment of the splanchnic vascularisation may lead to ischaemia (i.e., hypoxic-induced tissue damage characterised by complete cell death), organ necrosis (irreversible terminal ischaemic injury),and death. More than 90% of patients with mesenteric ischaemia died without appropriate treatment in the late 1970s 1 and the mortality rate remains as high as 80% in one of the most recent registry studies. 2 Paradoxically, AMI has been “the elephant in the room” for decades. Although the prognosis is known to be poor, there has been very little research on this life-threatening disease. Unlike the brain or myocardial infarction, where hundreds of clinical trials have been performed and solid recommendations exist, research in AMI has been limited, mainly due to its the lower incidence. 2 In 1926, A. J. Cokkinis wrote that « [the] occlusion of the mesenteric vessels is apt to be regarded as one of those conditions of which … the diagnosis is impossible, the prognosis hopeless, and the treatment almost useless ». 3 Unfortunately, this pessimism, expressed nearly a century ago, is still shared by many physicians today. This attitude plays a role in, and is also a result of, the poor outcome of most of these patients. However, AMI is curable when rapid revascularisation and/or bowel resection is performed with a mortality rate of only 10–20% in some cohorts 4,5 because the reversibility of ischaemic damage depends on the extent of tissue injury over time. Both survival and preventing extensive bowel resection and short bowel syndrome (resulting in high morbidity) should be the aim of treatment. 6 This shows the importance of raising awareness and collective education.
The clinical suspicion of AMI is difficult because the main clinical symptom is severe abdominal pain, which is fairly non-specific. AMI was not suspected before computed tomography (CT) in nearly 60% of confirmed cases and the diagnostic accuracy was not found to be different between patients with and without suspected AMI. 7 Since CT is usually performed in patients with severe abdominal pain, the diagnosis of AMI is often based on the radiologist’s experience. Our group showed that imaging without contrast administration was independently associated with a delayed diagnosis, thus emphasising the need for contrast-enhanced imaging in patients with severe abdominal pain. 8 Moreover, although arterial phase images should be acquired in case of suspected AMI to improve the detection and description of vascular lesions, the diagnosis can still be made with portal venous phase images, which are the only available images in most cases. 9
Unlike myocardial ischaemia, there are no reliable, accessible blood biomarkers of AMI. 10 Normal serum lactate values are often misleading and mistakenly reassuring. Indeed, normal values are found in up to 70% of patients with AMI 4 and have also been shown to be an independent predictor of a delayed diagnosis. 8 Thus, serum lactate should only be used as a prognostic marker associated with the onset and extent of transmural necrosis.
Moreover, academic research and reviews of AMI have mixed the different entities of this disease (venous, arterial and non-occlusive) 4,11–14 with confusing results for physicians. Venous and arterial AMI are based on different pathophysiological processes with different prognoses, 1 thus, evaluating them together results in a significant bias. 4,14–16 Arterial and venous origins are clearly separated in other organs such as the brain.
Survival depends on the severity of the ischaemic lesions and the survival rate with or without transmural necrosis is 65 to 98%, respectively. 4 Complete transmural necrosis of the entire intestinal wall defined histologically as diffuse cellular coagulative necrosis extending to the three intestinal tunics (mucosa, submucosa and muscularis propria) is always irreversible. Unfortunately, grading of the ischaemic injury is rarely reported in clinical studies. Therefore, it is often difficult to determine the extent and severity of disease in the included populations. 16–18 Furthermore, CT features that are directly linked to transmural necrosis are rarely reported even in reviews or textbooks. For example in a frequently cited review published by Clair et al. in the New England Journal of Medicine in 2016, the word “necrosis” only appears twice. 19
Another source of confusion about AMI is that occlusion of a vessel does not necessarily mean ischaemia, especially if none of the features of ischaemic bowel injury are observed. There has been the same confusion for decades about acute SMV thrombosis (SMVT) and acute venous mesenteric ischaemia. 20–23 Thus, it remains virtually impossible to precisely predict which patients with acute SMVT will progress to AMI, although transmural necrosis is identified in nearly 30% of patients with SMVT 24 and 23 to 43% of bowel resections. 25
In arterial occlusive AMI (AAOMI), revascularisation is the most effective bowel-saving treatment and bowel resection can be markedly limited to use for damage control. However, in the past, AMI has usually been managed by digestive surgeons, rather than gastroenterologists, radiologists or vascular surgeons. The rate of revascularisation in AAOMI patients is increasing, especially by endovascular means but remains low (approximately 20% in a recent regional cohort study in Finland and 9% in a national registry study from Estonia). 2,26,27 Descriptions of the lesions of the superior mesenteric artery (SMA) in the recent literature are insufficient, with only a general description of the location of lesions (“proximal” or “distal” SMA without clear anatomical landmarks) or their extent. 9,16,28 Therefore, the description of the SMA must be updated.
Thus, there is a clear need to improve the earlier detection as well as the description, reporting, stratification and prognosis of AMI, to optimise therapeutic decision-making and benefit patients. For this, a more systematic, precise, pragmatic and evidence-based approach is needed for this group of diseases, like ischaemia in other organs. This review presents an up-to-date, evidence-based description with precise insights from a specialised mesenteric stroke centre. Based on the evidence and a decade of clinical experience, we then illustrate our approach to suspected or confirmed cases of AMI.
Technical consideration
The use of a specific CT protocol is recommended and has been extensively described and reported. A triphasic protocol (unenhanced, early arterial, and portal venous phase) is recommended in patients with clinically suspected AMI or for monitoring after treatment. However, since most cases of AMI are initially diagnosed without a clear clinical suspicion, the complete protocol cannot be considered mandatory to reach the diagnosis. 7,29 Table 1 presents our detailed CT protocol.
Table 1.
CT protocol for clinical suspicion of AMI. This protocol is also used as a baseline or for follow-up imaging before or after revascularisation or bowel resection
| Non-contrast phase | Arterial phase | Portal-venous phase | |
|---|---|---|---|
| Position | Supine | Supine | Supine |
| Coverage | Liver dome to pubic symphysis | Liver dome to pubic symphysis | Liver dome to pubic symphysis |
| Peak beam ernergy | 120 kv | 120 kv | 120kv |
| mA mode | Modulated | Modulated | Modulated |
| Pitch | 1.375 | 0.984 | 0.984 |
| Slice thickness | 2.5 mm | 1.25 mm | 1.25 mm |
| Image reconstruction overlap | 1.25 × 1 mm | 1.25 × 1 mm | 1.25 × 1 mm |
| Field of view | Large | Large | Large |
| Acquisition time | Bolus-triggered (beginning of the abdominal aorta) at 120 UH threshold | 50 s after arterial phase | |
| Iodine concentration | 350 mg ml−1 | ||
| Contrast media dose | 2 mL/kg | ||
| Contrast media Flow rate | 4 mL/s | ||
Arterial Occlusive Or Stenotic Acute Mesenteric Ischaemia
Vascular involvement
The dominant form of arterial involvement is the occlusion of the SMA with two main aetiologies: calcified atherosclerotic thrombosis (related to a subacute process of plaque remodelling which occurs more frequently in patients with multi-arterial disease) or a recent clot related to a cardiac or aortic embolus. However, it is difficult to precisely estimate the proportion of each aetiology because they differ among studies and are often not reported. Indeed, the relative proportion of atherosclerotic thrombosis AMI and embolic AMI ranges from 42 to 62% and 38 to 58%, respectively. 29,30
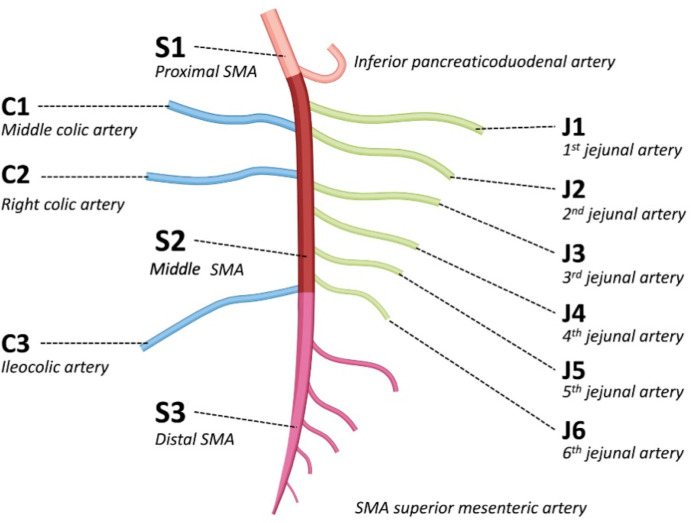
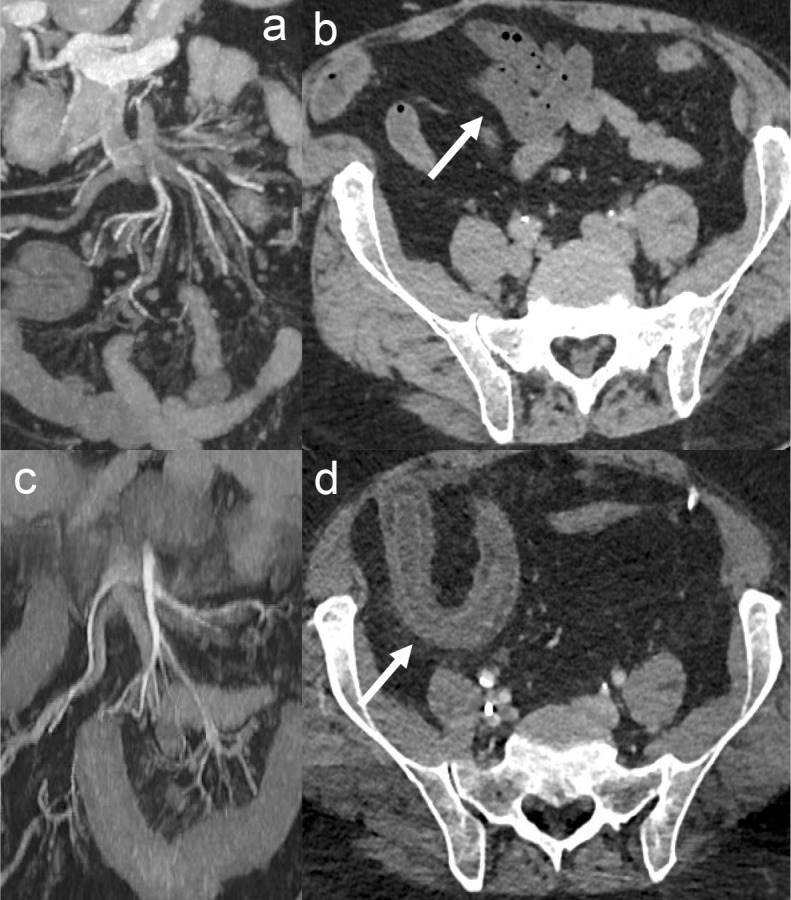
An accurate description of arterial occlusions is highly important to make therapeutic decisions, e.g., deciding the best revascularisation modality (endovascular or open) and technique (stenting, thrombus-aspiration, in situ thrombolysis, etc.). However, in a recent retrospective analysis of patients with arterial occlusive AMI, the location of the occlusion was not mentioned in the CT report in nearly 2/3 of the cases, was incorrect in 13%, incomplete in 8% and appropriate (with a correct number and localisation of the occlusions) in only 17%. 31 Therefore, we recently introduced a classification of the occlusive lesions of SMA (in AMI) similar to the existing anatomical descriptions of the brain and myocardial arterial anatomy. The goal of this classification was to improve the CT reporting and help standardise results in future studies. 31 The SMA was divided into three parts (Figure 1); proximal SMA (defined as the segment between the ostium and the origin of the inferior pancreaticoduodenal artery), middle SMA (defined as the segment between the origin of the inferior pancreaticoduodenal artery and the origin of the ileocolic artery) and distal SMA (defined as the vascular bed downstream from the ileocolic artery). A detailed description of the involvement of the jejunal and colic arteries was also included.
Figure 1.
Illustration of the superior mesenteric artery segmentation 31
Atherosclerotic
In atherosclerotic thrombosis, the SMA is infiltrated by diffuse calcified or non-calcified plaques that predominate around the ostium of the SMA. 30 Multiple associated stenoses of the SMA site are frequently observed below the occlusion. The downstream vascular bed is generally opacified as collaterals usually developed to compensate for the reduced blood flow. In particular, the celiac trunk is also often stenotic explaining why these patients may develop AMI, despite collaterals. 32 Although the etymological root of the word ischaemia is the ancient greek iskhaimos, which can be translated into “stopping of the blood,” blood flow does not need to stop completely to cause ischaemic damage. Like in other organ ischaemias, significant inflow impairment may be sufficient. Thus, although AMI can occur in patients with severe stenosis of the SMA without complete occlusion, this subentity has not been extensively studied so there is confusion about “occlusive” versus “non-occlusive” AMI. Indeed, haemodynamically significant yet stenotic AMI are part of so-called “occlusive AMI” and non-occlusive mesenteric ischaemia (NOMI) is a different entity (see below). Although there is no strict cut-off for the percentage of stenosis that increases the risk of AMI, it is believed that >90% stenosis of the SMA or >70% stenosis of both the celiac trunk and the SMA are risk factors. 32
Embolic
In embolic AAOMI, the site of occlusion can involve all parts of the SMA (segments S1, S2 or S3) 30 with a typical sharp end to contrast-enhancement associated with a filling defect in the distal SMA. Patients are usually younger than those with atherosclerotic AMI 1 and the process is more acute. Thus, patients have fewer arterial collaterals. Careful analysis of the distal mesenteric vasculature is recommended because small clots may be located in distal branches (jejunal arteries, colic arteries or the distal part of the SMA [segment S3]). 30 Synchronous emboli can be visualised in other organs in nearly 70% of patients. The most frequent sites are the lower limbs (27%), the kidney (18%), the brain (15%) and the spleen (10%). 30 In most patients, these emboli originate from the heart, especially from the auricle of the left atrium. 30
Isolated mesenteric artery dissection
Acute and spontaneous isolated mesenteric artery dissection rarely causes AAOMI (<5% of all causes). Isolated mesenteric dissection usually occurs in males between 45 and 55 years of age with no particular medical history. Most cases of AAOMI with isolated mesenteric artery dissection involve type III dissection according to the Yun classification 33 with the lesions leading to complete occlusion of the SMA. 34 Type III dissection is found in fewer than 20% of SMA 34,35 and can be confused with an embolus because complete thrombosis of the vessel can mask the true and false lumen. A wall hematoma or enlargement of the SMA suggests dissection. 36 It is important to consider that this aetiology in AMI in middle-aged patients, especially when no obvious cause of emboli is found. Since abdominal pain is related to both potential AAOMI and the dissection itself, it is not easy to clinically discriminate uncomplicated from complicated dissections. 34 In one retrospective study, our group reported that the total length of the dissection (approximatively>6 cm) and a ratio of true lumen/overall lumen>50% were associated with AMI. 36
Bowel involvement
Ischaemic injury – early form
Occlusive AMI is a dynamic process that worsens over time. There is ischaemic injury to the bowel in the early phase but no transmural necrosis. Thus, identifying the early features of ischaemic intestinal injury is highly important. The main feature is decreased bowel wall enhancement directly related to diminished perfusion in other organs (Figure 2). This decreased enhancement has been well illustrated in pre-clinical studies 37–39 and has been found to be highly specific for bowel ischaemia (specificity of 97% in the study by Kirkpatrick et al., 40 98% in Schieda et al. 9 and 99% according to Yikilmaz et al 41 ). These values do not seem to be influenced by the CT protocol. 9 However inter-reader agreement is still low 16 with significant variability across studies, mainly because of the purely qualitative and visual diagnosis. The reported prevalence of decreased bowel wall enhancement ranges from 18% to 92%. 4,14–16,40–47 This variability may be explained by the inclusion of AMI of various causes (arterial, venous and NOMI) in these studies. Calame et al. 46 specifically reported the prevalence of decreased enhancement due to an arterial cause in 81% of patients. One crucial point is that in most patients, the lesions are segmental and do not involve the whole bowel. Therefore, radiologists need to compare segments with suspected ischaemia with normal segments to improve the identification of decreased enhancement. This is especially true when portal venous phase images are available but no unenhanced or arterial phase images. To the best of our knowledge, no differential diagnosis exists in patients with decreased enhancement of the bowel wall.
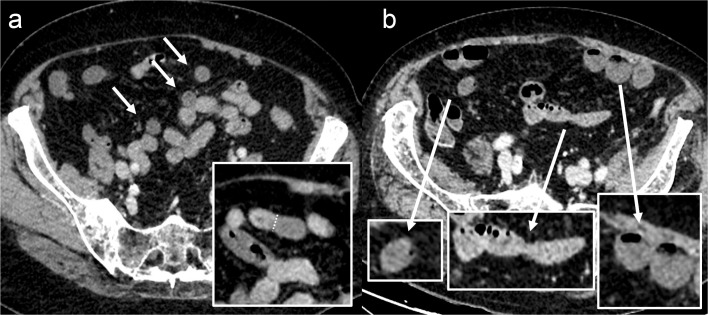
Figure 2.
Example of decreased bowel wall enhancement in early arterial occlusive acute mesenteric ischaemia. Panel A shows a non-dilated bowel, but some segments are hypoenhancing compared to others (arrows), with clear demarcation between ischaemic and non-ischaemic segments. Different bowel segments can be seen on panel B: mildly hypoenhancing non-dilated loop suggesting ischaemic injury (left side), a normal enhancing non-dilated loop free from ischaemic involvement (middle) and a hypoenhancing slightly dilated loop suggesting more advanced ischaemic, but still non-necrotic, lesions (right side).
Spontanenous bowel wall hyperattenuation on unenhanced CT has been suggested to be associated with ischaemia, especially in the setting of bowel obstruction. It corresponds to haemorrhagic congestion or intramural haematoma. In our experience, these are depicted in patients with reperfusion injury, venous mesenteric ischaemia or even in NOMI but not in pure forms of arterial occlusive mesenteric ischaemia.
Necrotic – late form
The diagnosis of late AMI (i.e., when irreversible transmural necrosis occurs) is of clinical importance as this significantly changes therapeutic management because patients are candidates for surgical bowel exploration (Figures 3 and 4). Although the time from ischaemia to infarction is unknown, like in cerebral or myocardial infarction, it probably depends on collateral vessels. 48,49 In one of the rare prospective studies of AMI, our group identified three features that independently predicted transmural necrosis: any type of organ failure, serum lactate≥2 mmol l−1 and small bowel dilatation (i.e., ≥ 25 mm). 4 The rate of transmural necrosis was shown to increase with the number of factors, reaching 100% when all three are present. Although a recent meta-analysis confirmed that small bowel dilatation is suggestive of necrosis, 50 this sign can only be interpreted as a sign of bowel necrosis if the diagnosis of AMI is made. Isolated small bowel dilatation does not suggest AMI.
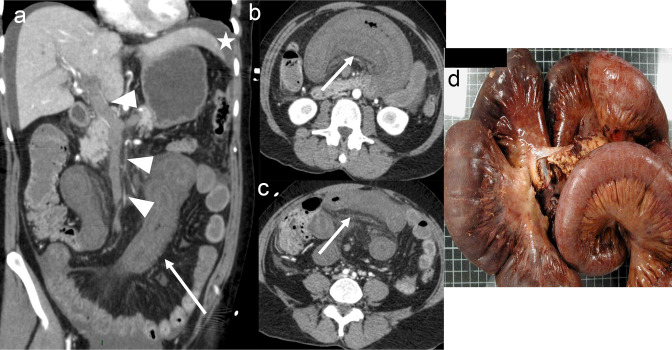
Figure 3.
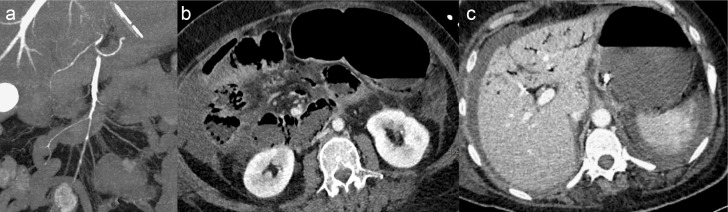
87-year-old female patient with early acute occlusive arterial mesenteric ischaemia (AMI). Image A shows occlusion of the distal segment of the superior mesenteric artery (SMA, segment S3) by a clot (arrow) with patent proximal and middle SMA (segment S1 and S2) and ileocolic artery (arrowheads). Images B and C show decreased enhancement of a non-dilated ileal wall (arrows) with normal enhancement of the adjacent small bowel (star). These features suggest early non-necrotic AMI. However, the patient underwent ileocecal resection in another institution. The pathologic examination showed a yellowish mucosa with a tiger stripe appearance but with a normal outer layer (macroscopic appearance D) and no transmural necrosis on pathology. This patient had a Clichy score of 0/3 (small bowel diameter <25 mm, lactate <2 mmol l−1 and absence of organ failure) and could have avoided resection if properly revascularised.
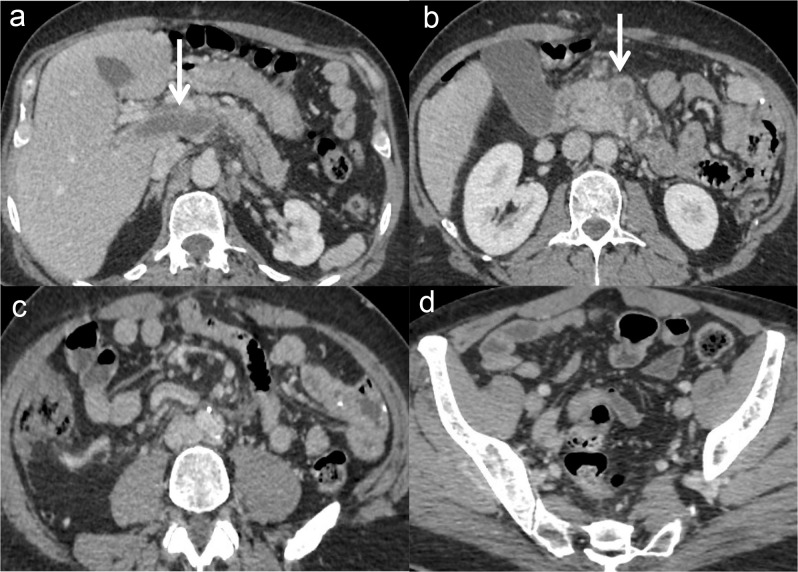
Figure 4.
Example of a delayed diagnosis resulting in transmural necrosis in a 56-year-old male patient. This patient presented in the emergency department with marked and intense abdominal pain. Because of acute kidney injury, he underwent non-contrast abdominal computed tomography (to rule out a perforated ulcer), showing isolated dilatation of the ileum (A). He was admitted for observation, but he presented with sepsis and lactic acidosis 12 hours later. He underwent a contrast-enhanced computed tomography (B, portal venous phase), showing a hypo-enhancing dilated ileum and occlusion of the superior mesenteric artery (not depicted here). Two and a half meters of small bowel were resected. The patient is alive but now has short bowel syndrome.
Bowel wall pneumatosis is normally described in late AMI. 44,50,51 However, it develops late and only 5% of patients with necrotic AMI were found to have pneumatosis in the study by Nuzzo et al.. 4 It is important to note that several studies failed to identify this feature as a marker of transmural necrosis because the bowel can still be viable in certain patients with bowel wall pneumatosis. 14,52–54 This is especially true when pneumatosis is isolated and not associated with portal venous gas. Thus, the impact of this entity is still a matter of controversy. 55
Interestingly, one study reported that higher thrombus attenuation values (> 36 HU) were associated with transmural necrosis in embolic cases of arterial occlusive AMI. 56 This was interpreted as a sign of a higher erythrocyte (higher HU values) over fibrin (lower HU values) ratio in clots and, therefore an indicator of prolonged ischaemia because the proportion of erythrocytes in thrombi gradually increases as they become trapped in a fibrin mesh. In addition, a few studies have reported that ascites may be associated with transmural necrosis 44,57 but the evidence is limited. Finally, our group recently showed that patients with colonic involvement in CT had a higher rate of transmural necrosis, mortality and morbidity rates than those without. 58,59 Thus, colonic lesions should be carefully evaluated on the initial CT scan.
Post-therapeutic aspects
Ischaemia-reperfusion injury (RI) following mesenteric ischaemia is characterised by massive acute neutrophilic inflammation caused by secondary blood reflow. It is the result of oxidative stress following reperfusion that increases reactive oxygen species and local inflammation leading to cell death. This has been studied in pre-clinical models in various organs, especially the heart, the brain, the liver and the bowel. Our group recently described the appearance of RI after revascularisation in arterial occlusive AMI on CT. 60 Reperfusion injury occurred in approximatively 40% of patients a median of 40 h after revascularisation. RI can mimic residual or recurrent AMI because patients present with abdominal pain, possible rectal bleeding, and elevated CRP. Lesions typically present as bowel wall thickening (mean 9 mm) on CT, hypoattenuating oedema of the submucosa, hyperenhancement of the mucosa, fat stranding, dilated draining veins and fluid adjacent to the involved bowel loop. 60 These features were present in nearly 90% of the patients with RI (Figure 5). Intra-luminal haemorrhage was inconsistent and found in nearly 20% of patients. It is important to note that RI is not associated with a poorer prognosis. 60
Figure 5.
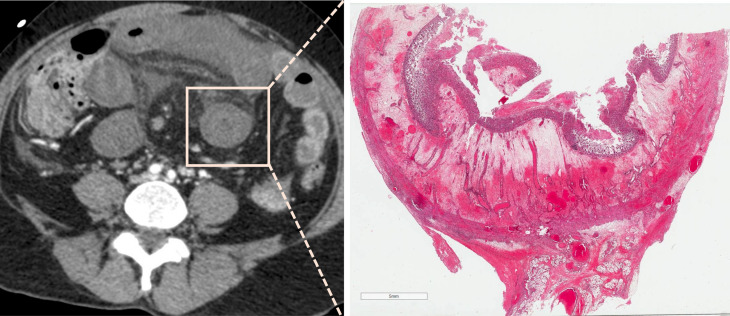
An 86-year-old female patient presented to the emergency department with marked abdominal pain requiring morphine and elevated serum lactate at 3.2 mmol l−1. Image A shows an embolus of the middle and distal segments of the superior mesenteric artery (segment S2 and S3, arrow). Unenhanced computed tomography (B) showed dilatation of the ileum (arrow) and portal-venous phase (C) showed decreased bowel enhancement. This patient underwent bowel resection because of extensive macroscopic signs of necrosis (D) with 1 m of small bowel left in place. Transmural necrosis was found in microscopic evaluation.
Venous Acute Mesenteric Ischaemia
This section discusses bowel ischaemia complicating splanchnic vein thrombosis or occlusion. Ischaemia complicating mechanical bowel obstruction is not included because the primary cause is not vascular per se. Indeed, the ischaemic complication is caused by torsion of the vascular pedicle of the bowel. Nevertheless, the appearance of bowel lesions on imaging is comparable to that observed in venous AMI. 46,61
Data on venous AMI are scarce compared to AAOMI mainly because most studies evaluate portal vein or SMV thrombosis specifically or it is mixed with arterial occlusive AMI. However, there are still several important messages.
Vascular involvement
SMVT is part of the broader spectrum of portal vein thrombosis (PVT). Nearly 60% of patients with PVT have associated SMVT. 62 Chronic SMVT detected in the presence of non-enhancing and narrowed mesenteric veins will not be discussed because it is not a cause of AMI.
Acute SVMT has been found to be isolated in approximately 25% of cases. 63 One specificity of acute SMVT (rather than PVT) is that the prognosis is directly linked to the onset of bowel ischaemia which may evolve into necrosis. 63 There are numerous causes of acute SMVT that are usually classified as primary (idiopathic) or secondary, 23 but the clinical relevance of this simplification is limited because an increasing number of predisposing disorders have been discovered and diagnosed. The classification inspired by Acosta et al. 20 separates causes into three main groups (i.e., local inflammation, thrombophilia, and chronic venous insufficiency) and is preferable, although associated local and systemic factors often co-exist. 64
Thrombophilia
Thrombophilia is defined as a disorder that is associated with an increased tendency to develop thrombosis, either acquired or inherited. Discussing the numerous causes of thrombophilia is not clinically relevant for imaging because the features of SVMT are not believed to differ on CT. The JAK2-V617F mutations seem to be dominant in acquired thrombophilia. 65 On imaging, thrombosis can involve the entire mesenteric venous network. Involvement of the proximal vein in thrombophilia-related SMVT makes it more prone to bowel ischaemia, as illustrated in an old animal study. 66 Thrombosis is often seen as a luminal filling defect with well-defined peripheral rim enhancement of the venous wall (vasa vasorum) in thrombophilia-related SMVT (Figure 6).
Figure 6.
Acute embolic occlusive arterial mesenteric ischaemia in a 60-year-old male patient. Images A and B show an embolus of the superior mesenteric artery with decreased enhancement of the ileum (arrow). This patient underwent open surgical thrombectomy resulting in complete revascularisation. Images C and D show the follow-up CT scan with a patent SMA and bowel wall thickening (with associated hypoattenuating oedema of the submucosa and hyperenhancement of the mucosa) of the ileum (arrow) suggesting a reperfusion injury.
Underlying chronic mesenteric venous insufficiency
Underlying chronic mesenteric venous insufficiency corresponds to decreased venous drainage with decreased venous blood flow, favouring the onset of partial or complete thrombosis. The main aetiology is portal hypertension complicating advanced chronic liver disease. It can also be found with other causes of portal hypertension or segmental portal hypertension. PVT occurs in approximately 10% of patients with cirrhosis 67,68 and SMVT develops by direct extension. Amitrano et al found that SMVT occurs in 15% of patients with cirrhosis and PVT, and mesenteric ischaemia in 10% of the cases of cirrhosis-related SMVT. 67
Local inflammation of the superior mesenteric vein or its branches
A local risk factor for thrombosis is identified in 25% of patients with SMVT. 20 The three leading causes are cancer, inflammation or trauma (surgical or not). These cases of thrombosis are believed to be less prone to bowel infarction because they are generally localised segmental thromboses that are rapidly compensated by the development of collaterals. Tumours that encase or occlude the SMV, especially distal branches, rarely cause ischaemia because the vascular occlusion is not acute. It gradually worsens with tumour growth and collateral circulation usually develops. Tumours that invade smaller and more proximal branches (e.g., the ileal veins), such as midgut neuroendocrine tumours, are more likely to be complicated by bowel ischaemia. 69 Acute pancreatitis, especially necrotising, often causes splenic vein thrombosis but is rarely associated with an SMVT, with an estimated incidence ranging from 2 to 14%. 70–72 Bowel necrosis following acute pancreatitis is also highly uncommon. 71 SMVT complicating inflammatory bowel diseases is even rarer, with a reported prevalence of nearly 1%. 73 Finally, SMVT associated or not with bowel ischaemia is extremely rare after surgery or mesenteric trauma (estimated<1%).
Bowel involvement
Acute SVMT is symptomatic in nearly 90% of patients, but almost half of the patients with symptomatic SMVT do not experience bowel ischaemia. 17,63,65 Ischaemia is difficult to detect in patients with SMVT and ischaemia is difficult to differentiate from necrosis for several reasons:
The presence of isolated abdominal pain does not suggest ischaemia;
The CT features of benign bowel venous congestion overlaps with those of ischaemia; and
CT studies are rare in these cases and rarely differentiate between ischaemia and necrosis.
Ischaemic injury – early form
The presence of hypoattenuating bowel wall thickening with a multiple-layer appearance suggesting submucosal bowel oedema is the consistent finding on CT. In addition, ascites is also common, corresponding to mesenteric congestion or oedema 17 and intramural haematoma can also been found.
Necrotic – late form
The factors associated with necrosis in venous MI are not well known because studies usually focus on patients with SMVT and not venous AMI per se. For instance, Elkrief et al showed that patients with acute SMVT and underlying diabetes mellitus have an increased risk of requiring intestinal resection. 74 Neither local factors nor systemic prothrombotic conditions were associated with intestinal resection. When second-order radicles of the SMV were found to be preserved on CT scan the risk of severe resection was low. 74
In one recent study, Jiang M et al. included more than 200 patients with venous AMI and found that decreased bowel enhancement was an independent predictor of transmural necrosis 24 (Figures 7 and 8). This finding has already been suggested in smaller studies 25,75 and in a meta-analysis. 50 Bowel dilation is not a good predictor of transmural necrosis because it is one of the first features of venous AMI. In addition, there is still no predictive score for necrosis in venous AMI (Table 2).
Figure 7.
Example of acute superior mesenteric venous thrombosis in a 48-year-old female patient with abdominal pain. Images A and B show complete thrombosis of the superior mesenteric vein with parietal enhancement and adjacent fat stranding (arrows) corresponding to thrombosis. In images C and D, the bowel shows no features of ischaemic injury (i.e., normal enhancement, no wall thickening).
Figure 8.
Necrotic acute venous mesenteric ischaemia in a 59-year-old female patient who presented to the emergency department for marked abdominal pain requiring morphine. Images A, B and C show hypoattenuating bowel wall thickening with a multiple-layer appearance, suggesting submucosal bowel oedema (arrow) with complete superior mesenteric vein thrombosis (A, arrowhead) and ascites (A, star). In addition, the involved bowel loops showed decreased enhancement suggesting necrosis. Image D shows the macroscopic analysis with a dark brown small bowel and congestive mesentery with transmural necrosis plus haemorrhage on microscopy.
Table 2.
Short-term research priorities in the radiological evaluation of acute mesenteric ischaemia.
| Arterial |
|
| Venous |
|
| Non-occlusive |
|
| Ischaemic colitis |
|
AMI, acute mesenteric ischaemia; CT, computed tomography.
Non-occlusive mesenteric ischaemia
NOMI corresponds to mesenteric vascular insufficiency related to a low-flow state, hypotension or hypoxemia. It usually occurs in critically ill patients with prolonged shock or high doses of catecholamines. Although NOMI is usually regarded as a spasm of the splanchnic vessels, especially the SMA, this is an oversimplification of a more complex multifactorial process. Coronary and cerebral spasms are well known, but until recently there have been very few studies of NOMI.
Vascular involvement
As in all vasospasms, the SMA is patent in NOMI, but its calibre may be decreased (especially compared to prior CT). 77–79 Some authors have also reported a decrease in the diameter of the SMV. 78 At present, irregularity of the vessel with focal narrowing (as described in cerebral vasospasms 80 ) with a tendency to spare the bifurcations is considered to be highly suggestive of this condition, rather than a decrease in the calibre of the SMA, which may be difficult to identify. Thus, CT is an appropriate non-invasive method for diagnosing NOMI 81 and invasive angiography should not be performed for diagnostic purposes. It should be noted that the long-standing association between atherosclerosis and NOMI was challenged in a recent study suggesting that SMA atherosclerosis is not a risk factor for NOMI in critically ill patients. 82
Bowel involvement
Ischaemic injury – early form
NOMI is a dynamic, locally reversible and changing state of vascular impairment.
Unlike AAOMI, it is not linearly time-dependent. Thus, a complex association of ischaemia, reperfusion and necrosis may coexist. The CT features of NOMI are therefore numerous, alternating from bowel wall thickening (hypothesised to be related to reperfusion injury 77 ) and thinning, abnormal bowel wall enhancement (increased or decreased), and normal segments with abnormal ones. In approximately 25% of patients, the intestinal injury is diffuse. 29,77 Bowel dilatation is found in 40% of patients, and pneumatosis (with or without portal venous gas) in 30 to 40% of patients on the first CT evaluation. 29,77 They should not be regarded as features of transmural necrosis. Indeed, NOMI is frequent in ICU or post-operative cases where a diffuse bowel ileus is nearly always present. Also, due to the non-linear time-dependent pathophysiology, pneumatosis (which can be interpreted as a mere disruption of the mucosa without transmural necrosis), favoured by bacterial translocation, is more frequent in NOMI than in AAOMI 46 (Figure 9). Non-specific signs such as ascites or fat stranding are usually present. 29,77
Figure 9.
Non-necrotic non-occlusive mesenteric ischaemia in a 69-year-old male patient after aortocoronary bypass. Biological laboratory tests showed a lactate concentration of 5 mmol l−1, a prothrombin rate of 79% and a bicarbonate concentration of 22 mmol l−1. Image A shows a diffuse spasm of the superior mesenteric artery. Image B shows a small bowel dilatation with persistent wall enhancement, and image C shows portal venous gas (secondary to bowel wall pneumatosis, not shown). Despite the presence of pneumatosis, this patient had a low risk of necrosis according to the NOMI-ITN score. 76 The patient underwent a laparotomy that did not reveal any bowel necrosis.
Necrotic – late form
Approximately 50 to 65% of patients with NOMI undergo bowel resection for suspected transmural necrosis. 29,83 In 2021, Calame et al showed that the Clichy score 4 (initially developed for patients with AAOMI) inaccurately predicted transmural necrosis in NOMI. The authors identified two CT factors (i.e., an absence of bowel wall enhancement and a thin bowel wall 76 ) as independent predictors of transmural necrosis in NOMI. These features, along with prothrombin rate (inferior to 40%) and plasma bicarbonate concentration (inferior to 15 mmol l−1), were combined into a NOMI-ITN score whose performance is excellent. 4 In addition, Verdot et al confirmed that the absence of wall enhancement was the most consistent feature of bowel wall necrosis regardless of the involved bowel or colonic segment, while a thin bowel wall was the most specific CT feature of small bowel transmural necrosis. 83
Implication for prognosis
Besides making the diagnosis of transmural necrosis to determine which patients can benefit from bowel resection, abdominal CT provides crucial features about the prognosis of patients with NOMI. Indeed, visualisation of kidney infarction on CT was found to be an independent predictor of 28-day mortality 84 while atherosclerosis (quantified by a mesenteric calcium score) was associated with death within 24 h. 85 The occurrence of RI (bowel wall thickening) seems to have a positive impact on survival 77 but the bowel enhancement pattern is not associated with survival. 86
Ischaemic Colitis
Although for some authors, ischaemic colitis (IC) is part of the spectrum of arterial AMI, it has certain specificities and should be described separately. 87 It is different from colonic ischemia complicating AMI, or from colonic ischemia complicating vascular or abdominal surgery. IC is a localised ischaemia-reperfusion injury mostly caused by transient haemodynamic variations (systemic hypotension, decreased cardiac output, aortic surgery, dialysis, and so on). However, the cause is rarely identified and colonic arteries (from the SMA or the inferior mesenteric artery) are usually patent. IC is usually segmental and rarely affects the whole colon. 88 The main CT finding is hypoattenuating bowel wall thickening with adjacent fat stranding 88,89 but this is highly non-specific since infectious or inflammatory colitis can have the same findings. Submucosal oedema is frequent and it is believed to correspond to the resorption of oedema that generates local inflammation. 90 The left colon is involved in 30–50% of the patients and the right colon in 25–30%. 88,91 Importantly, right-sided IC should be considered to be a form of AMI until proven otherwise.
The median length of the abnormal colon is approximately 20 cm, and mean wall thickening is approximately 8 mm. 88 Pneumatosis is reported in nearly 5% of cases, and the resection rate is approximately 20%. 88,91 Indications for resection are mostly based on the importance of the inflammatory process (local and systemic), but most cases resolve spontaneously. Revascularisation can be discussed in case of significant SMA stenosis, but should be delayed, and prompt resection of the injured colon should be performed when needed. 90 The mortality rate is approximately 10%, 88,91 higher when the right colon is involved. 91,92 Recurrence rarely occurs. 92 Clinical and biological features (rather than CT findings) are associated with outcomes and are valuable in assessing the severity of disease. 90
Conclusion
This review article proposes a diagnostic approach to AMI with particular focus on the classification of the different forms of disease. There are several specific messages for radiologists. First, CT is the cornerstone of the diagnosis and should be performed rapidly, 24/7 and be contrast-enhanced. While the appropriate triphasic CT protocol is highly recommended, portal venous phase images identify the main features: decreased bowel wall enhancement, bowel wall thickening or thinning, pneumatosis, submucosal oedema, dilatation and even occlusion of the proximal and middle part of the SMA. Second, besides the diagnosis, radiologists must also search for and report features of bowel necrosis to assess disease severity. This important semiology differs according to the type of AMI. In Table 3, we have synthetised the typical clinical presentation and recanalisation options depending on different forms of AMI. Radiologists should remember that serum lactate is not a diagnostic biomarker of AMI but merely a prognostic indicator. Normal serum lactate level does not rule out the diagnosis. Finally, acute vascular lesions can cause intense abdominal pain (directly related to the vessel wall injury) and may not be related to ischaemia. This is especially true for SMVT-related thrombophilia or spontaneously isolated dissection of the SMA. However, the visualisation of any type of vascular injury should lead to a careful analysis of the bowel to detect any ischaemic bowel injury.
Table 3.
Typical clinical presentation and recanalisation options depending on the type of acute mesenteric ischaemia.
| Typical clinical presentation | Recanalisation options (Laparoscopic bowel evaluation is driven by the prediction of transmural necrosis) |
|
|---|---|---|
| Atherosclerotic AMI | Possible history of chronic mesenteric ischaemia Sudden abdominal pain in a patient with multi-arterial disease |
Endovascular revascularisation:
Open revascularisation:
|
| Embolic AMI | Sudden marked pain in a patient with a history of atrial fibrillation | Endovascular revascularisation:
Open revascularisation:
|
| Isolated SMA dissection | Sudden marked abdominal pain (epigastric) in middle-aged males without known atherosclerosis | Revascularisation only if bowel ischaemia (low evidence):
|
| Venous AMI | Abdominal pain and inflammatory syndrome in females with history of venous thrombosis | Anticoagulation therapy. If worsening despite adequate anticoagulation:
|
| Non-occlusive mesenteric ischaemia | Intensive care unit patient with shock and abdominal pain/distension | Intensive resuscitation. Local intra-arterial vasodilator infusion (low evidence) |
| Ischaemic colitis | Abdominal guarding with inflammatory syndrome mimicking infectious colitis. | No revascularisation. |
AMI, acute mesenteric ischaemia.
Most pre-existing reviews have mixed features of ischaemic bowel injury with different aetiologies and degrees of severity, resulting in unclear messages. The lack of prospective studies and the disproportionate number of pre-clinical studies are also a major drawback in modern AMI research. This is why the current review is supported by recent references, purposely excluding review papers. The current approach should improve our understanding of the ischaemic-to-necrotic process in occlusive arterial, venous or non-occlusive forms of this disease. We call for international collaborations to bridge the gap between AMI and cardiovascular or neurovascular research. Table 3 provides a short list of the main research priorities in AMI.
Patient stratification and prognostic criteria are needed. We encourage the use of classifications and scores (Table 4) including anatomical segmentation of the SMA, and scores to assess the probability of necrosis in occlusive AMI and NOMI or to describe spontaneous isolated dissection of the SMA. Moreover, standardisation of terminology is highly important. For example, the features of ischaemia should be separated from those of necrosis, and this needs to be done for each AMI sub-entity separately. Similarly, the features of reperfusion should not be confused with ischaemia, although the two can coexist.
Table 4.
Summary of the main vascular and bowel features on CT according to the type of acute mesenteric ischaemia (AMI)
| Imaging features of vascular lesions | Imaging features of ischaemic injury (early AMI) | Imaging features of transmural necrosis (late AMI) | Predictive score of transmural necrosis | |
|---|---|---|---|---|
| Arterial occlusive AMI |
|
Decreased enhancement of the bowel wall |
|
|
| Venous AMI |
|
|
|
|
| Non-Occlusive Mesenteric Ischaemia | Low flow states: spasm of the SMA | Various, simultaneous, and diffuse features |
|
|
| Ischaemic colitis | Normal Possible stenosis or occlusions of the SMA/IMA are not related to the bowel lesions |
Non-specific signs Prognosis directly linked to the local and systemic inflammatory process | ||
IMA, inferior mesenteric artery; PV, portal vein; SMA/V, superior mesenteric artery/vein.
Besides the need to raise awareness and adopt a common rigorous language for AMI, its management should be based on a dynamic and coordinated network of dedicated physicians, surgeons and radiologists. The mesenteric stroke centre we represent is an example of this type of organisation.
Contributor Information
Lorenzo Garzelli, Email: lorenzo.garzelli@aphp.fr.
Iannis Ben Abdallah, Email: iannis.benabdallah@aphp.fr.
Alexandre Nuzzo, Email: alexandre.nuzzo@aphp.fr.
Marco Dioguardi Burgio, Email: marco.dioguardiburgio@aphp.fr.
Dominique Cazals-Hatem, Email: dominique.cazals-hatem@aphp.fr.
Pierre-Emmanuel Rautou, Email: pierre-emmanuel.rautou@aphp.fr.
Valérie Vilgrain, Email: valerie.vilgrain@aphp.fr.
Paul Calame, Email: calame.paul@gmail.com.
Maxime Ronot, Email: maxime.ronot@aphp.fr.
REFERENCES
- 1. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease Aetiology. Br J Surg 2004; 91: 17–27. doi: 10.1002/bjs.4459 [DOI] [PubMed] [Google Scholar]
- 2. Lemma A, Tolonen M, Vikatmaa P, Mentula P, Kantonen I, But A, et al. Editor’s choice - epidemiology, diagnostics, and outcomes of acute occlusive arterial mesenteric ischaemia: A population based study. Eur J Vasc Endovasc Surg 2022; 64: 646–53. doi: 10.1016/j.ejvs.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 3. Cokkinis AJ. Mesenteric vascular occlusion. Southern Medical Journal 1926; 19: 655. doi: 10.1097/00007611-192608000-00028 [DOI] [Google Scholar]
- 4. Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, et al. Predictive factors of intestinal necrosis in acute mesenteric ischemia: prospective study from an intestinal stroke center. Am J Gastroenterol 2017; 112: 597–605. doi: 10.1038/ajg.2017.38 [DOI] [PubMed] [Google Scholar]
- 5. Najdawi M, Garzelli L, Nuzzo A, Huguet A, Raynaud L, Paulatto L, et al. Endovascular Revascularization of acute arterial mesenteric ischemia: report of a 3-year experience from an intestinal stroke center unit. Eur Radiol 2022; 32: 5606–5615. doi: 10.1007/s00330-022-08660-3 [DOI] [PubMed] [Google Scholar]
- 6. Amiot A, Messing B, Corcos O, Panis Y, Joly F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin Nutr 2013; 32: 368–74. doi: 10.1016/j.clnu.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 7. Anglaret S, Dallongeville A, Beaussier H, Touloupas C, Boulay I, Tardivel AM, et al. Influence of clinical suspicion on CT accuracy of acute mesenteric ischemia: retrospective study of 362 patients. Eur J Radiol 2021; 138: 109652. doi: 10.1016/j.ejrad.2021.109652 [DOI] [PubMed] [Google Scholar]
- 8. Nuzzo A, Joly F, Ronot M, Castier Y, Huguet A, Paugam-Burtz C, et al. Normal lactate and Unenhanced CT-scan result in delayed diagnosis of acute mesenteric ischemia. Am J Gastroenterol 2020; 115: 1902–1905. doi: 10.14309/ajg.0000000000000836 [DOI] [PubMed] [Google Scholar]
- 9. Schieda N, Fasih N, Shabana W. Triphasic CT in the diagnosis of acute mesenteric ischaemia. Eur Radiol 2013; 23: 1891–1900. doi: 10.1007/s00330-013-2797-y [DOI] [PubMed] [Google Scholar]
- 10. Nuzzo A, Guedj K, Curac S, Hercend C, Bendavid C, Gault N, et al. Accuracy of Citrulline, I-FABP and D-lactate in the diagnosis of acute mesenteric ischemia. Sci Rep 2021; 11: 18929. doi: 10.1038/s41598-021-98012-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Copin P, Zins M, Nuzzo A, Purcell Y, Beranger-Gibert S, Maggiori L, et al. Acute mesenteric ischemia: A critical role for the Radiologist. Diagn Interv Imaging 2018; 99: 123–34. doi: 10.1016/j.diii.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 12. Garzelli L, Nuzzo A, Copin P, Calame P, Corcos O, Vilgrain V, et al. Contrast-enhanced CT for the diagnosis of acute mesenteric ischemia. AJR Am J Roentgenol 2020; 215: 29–38. doi: 10.2214/AJR.19.22625 [DOI] [PubMed] [Google Scholar]
- 13. Nuzzo A, Maggiori L, Paugam-Burtz C, Cazals-Hatem D, Ronot M, Huguet A, et al. Oral antibiotics reduce intestinal necrosis in acute mesenteric ischemia: A prospective cohort study. Am J Gastroenterol 2019; 114: 348–351. doi: 10.1038/s41395-018-0389-9 [DOI] [PubMed] [Google Scholar]
- 14. Canfora A, Ferronetti A, Marte G, Maio VD, Mauriello C, Maida P, et al. Predictive factors of intestinal necrosis in acute mesenteric ischemia. Open Med (Wars) 2019; 14: 883–889. doi: 10.1515/med-2019-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barrett T, Upponi S, Benaglia T, Tasker AD. Multidetector CT findings in patients with mesenteric ischaemia following cardiopulmonary bypass surgery. Br J Radiol 2013; 86(): 20130277. doi: 10.1259/bjr.20130277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Copin P, Ronot M, Nuzzo A, Maggiori L, Bouhnik Y, Corcos O, et al. Inter-reader agreement of CT features of acute mesenteric ischemia. Eur J Radiol 2018; 105: 87–95. doi: 10.1016/j.ejrad.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 17. Acosta S, Alhadad A, Ekberg O. Findings in multi-detector row CT with portal phase Enhancement in patients with mesenteric venous thrombosis. Emerg Radiol 2009; 16: 477–82. doi: 10.1007/s10140-009-0807-9 [DOI] [PubMed] [Google Scholar]
- 18. Gopee-Ramanan P, Patlas MN, Pindiprolu B, Katz DS. Utility of Biphasic multi-detector computed tomography in suspected acute mesenteric ischemia in the emergency Department. Emerg Radiol 2019; 26: 523–529. doi: 10.1007/s10140-019-01698-9 [DOI] [PubMed] [Google Scholar]
- 19. Clair DG, Beach JM. Mesenteric ischemia. N Engl J Med 2016; 374: 959–968. doi: 10.1056/NEJMra1503884 [DOI] [PubMed] [Google Scholar]
- 20. Acosta S, Alhadad A, Svensson P, Ekberg O. Epidemiology, risk and Prognostic factors in mesenteric venous thrombosis. Br J Surg 2008; 95: 1245–51. doi: 10.1002/bjs.6319 [DOI] [PubMed] [Google Scholar]
- 21. Morasch MD, Ebaugh JL, Chiou AC, Matsumura JS, Pearce WH, Yao JS. Mesenteric venous thrombosis: a changing clinical entity. J Vasc Surg 2001; 34: 680–84. doi: 10.1067/mva.2001.116965 [DOI] [PubMed] [Google Scholar]
- 22. Alvi AR, Khan S, Niazi SK, Ghulam M, Bibi S. Acute mesenteric venous thrombosis: improved outcome with early diagnosis and prompt anticoagulation therapy. Int J Surg 2009; 7: 210–213. doi: 10.1016/j.ijsu.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 23. Duran R, Denys AL, Letovanec I, Meuli RA, Schmidt S. Multidetector CT features of mesenteric vein thrombosis. Radiographics 2012; 32: 1503–22. doi: 10.1148/rg.325115100 [DOI] [PubMed] [Google Scholar]
- 24. Jiang M, Li CL, Pan CQ, Lv WZ, Ren YF, Cui XW. Nomogram for predicting Transmural bowel infarction in patients with acute superior mesenteric venous thrombosis. World J Gastroenterol 2020; 26: 3800–3813. doi: 10.3748/wjg.v26.i26.3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HK, Hwang D, Park S, Lee JM, Huh S. Treatment outcomes and risk factors for bowel infarction in patients with acute superior mesenteric venous thrombosis. J Vasc Surg Venous Lymphat Disord 2017; 5: 638–46. doi: 10.1016/j.jvsv.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 26. Beaulieu RJ, Arnaoutakis KD, Abularrage CJ, Efron DT, Schneider E, Black JH. Comparison of open and Endovascular treatment of acute mesenteric ischemia. J Vasc Surg 2014; 59: 159–64. doi: 10.1016/j.jvs.2013.06.084 [DOI] [PubMed] [Google Scholar]
- 27. Kase K, Reintam Blaser A, Tamme K, Mändul M, Forbes A, Talving P, et al. Epidemiology of acute mesenteric ischemia: A population-based investigation. World J Surg 2023; 47: 173–81. doi: 10.1007/s00268-022-06805-5 [DOI] [PubMed] [Google Scholar]
- 28. Blachar A, Barnes S, Adam SZ, Levy G, Weinstein I, Precel R, et al. Radiologists' performance in the diagnosis of acute intestinal ischemia, using MDCT and specific CT findings, using a variety of CT protocols. Emerg Radiol 2011; 18: 385–94. doi: 10.1007/s10140-011-0965-4 [DOI] [PubMed] [Google Scholar]
- 29. Lehtimäki TT, Kärkkäinen JM, Saari P, Manninen H, Paajanen H, Vanninen R. Detecting acute mesenteric ischemia in CT of the acute abdomen is dependent on clinical suspicion: review of 95 consecutive patients. Eur J Radiol 2015; 84: 2444–53. doi: 10.1016/j.ejrad.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 30. Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg 2005; 241: 516–22. doi: 10.1097/01.sla.0000154269.52294.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tual A, Garzelli L, Nuzzo A, Corcos O, Castier Y, Ben Abdallah I, et al. Strengthening the description of superior mesenteric artery Occlusions in acute mesenteric ischaemia: proposition for an anatomical classification. Eur J Vasc Endovasc Surg 2023; 65: 802–8. doi: 10.1016/j.ejvs.2023.01.041 [DOI] [PubMed] [Google Scholar]
- 32. Bordet M, Tresson P, Huvelle U, Long A, Passot G, Bergoin C, et al. Natural history of asymptomatic superior mesenteric arterial stenosis depends on coeliac and inferior mesenteric artery status. European Journal of Vascular and Endovascular Surgery 2021; 61: 810–18. doi: 10.1016/j.ejvs.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 33. Yun WS, Kim YW, Park KB, Cho SK, Do YS, Lee KB, et al. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg 2009; 37: 572–77. doi: 10.1016/j.ejvs.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 34. Ben Abdallah I, Huguet A, Nuzzo A, Mirault T, Roussel A, El Batti S, et al. Acute isolated mesenteric artery dissection: four year experience from a French intestinal stroke centre. European Journal of Vascular and Endovascular Surgery 2022; 64: 656–64. doi: 10.1016/j.ejvs.2022.08.032 [DOI] [PubMed] [Google Scholar]
- 35. Yuan Z, Hu G, Sheng S, You Y, Wang J. Management strategy and Radiologic outcomes of symptomatic spontaneous isolated superior mesenteric artery dissection based on angiographic classification: the follow-up experience in a single center. J Endovasc Ther 2022; 15266028221133700. doi: 10.1177/15266028221133700 [DOI] [PubMed] [Google Scholar]
- 36. Ben Abdallah I, Craiem D, Casciaro M, Deza D, Ronot M, Corcos O, et al. Case-control study of 3d morphology in isolated mesenteric artery dissection. Cardiovasc Eng Technol 2023; 14: 230–38. doi: 10.1007/s13239-022-00649-9 [DOI] [PubMed] [Google Scholar]
- 37. Khan S, Goh V, Tam E, Wellsted D, Halligan S. Perfusion CT assessment of the colon and Rectum: feasibility of Quantification of bowel wall perfusion and Vascularization. Eur J Radiol 2012; 81: 821–24. doi: 10.1016/j.ejrad.2011.02.033 [DOI] [PubMed] [Google Scholar]
- 38. Shi H, Li R, Qiang J, Li Y, Wang L, Sun R, et al. Computed tomography perfusion imaging detection of Microcirculatory dysfunction in small intestinal ischemia-reperfusion injury in a porcine model. PLOS ONE 2016; 11: e0160102. doi: 10.1371/journal.pone.0160102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosow DE, Sahani D, Strobel O, Kalva S, Mino-Kenudson M, Holalkere NS, et al. Imaging of acute mesenteric ischemia using Multidetector CT and CT angiography in a porcine model. J Gastrointest Surg 2005; 9: 1262–74. doi: 10.1016/j.gassur.2005.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kirkpatrick IDC, Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology 2003; 229: 91–98. doi: 10.1148/radiol.2291020991 [DOI] [PubMed] [Google Scholar]
- 41. Yikilmaz A, Karahan OI, Senol S, Tuna IS, Akyildiz HY. Value of Multislice computed tomography in the diagnosis of acute mesenteric ischemia. Eur J Radiol 2011; 80: 297–302. doi: 10.1016/j.ejrad.2010.07.016 [DOI] [PubMed] [Google Scholar]
- 42. Taourel PG, Deneuville M, Pradel JA, Régent D, Bruel JM. Acute mesenteric ischemia: diagnosis with contrast-enhanced CT. Radiology 1996; 199: 632–36. doi: 10.1148/radiology.199.3.8637978 [DOI] [PubMed] [Google Scholar]
- 43. Kärkkäinen JM, Saari P, Kettunen HP, Lehtimäki TT, Vanninen R, Paajanen H, et al. Interpretation of abdominal CT findings in patients who develop acute on chronic mesenteric ischemia. J Gastrointest Surg 2016; 20: 791–802. doi: 10.1007/s11605-015-3013-y [DOI] [PubMed] [Google Scholar]
- 44. Wang X, Chu C, Sun S, Xie T, Duan Z, Wang K, et al. Outcomes and clinical characteristics of Transmural intestinal necrosis in acute mesenteric ischemia. Scand J Gastroenterol 2019; 54: 953–59. doi: 10.1080/00365521.2019.1646800 [DOI] [PubMed] [Google Scholar]
- 45. Chen Y C, Huang T Y, Chen R C, Tsai S H, Chang W C, Fan H L, et al. Comparison of ischemic and nonischemic bowel segments in patients with mesenteric ischemia: Multidetector row computed tomography findings and measurement of bowel wall Attenuation changes. Mayo Clin Proc 2016; 91: 316–28. doi: 10.1016/j.mayocp.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 46. Calame P, Malakhia A, Turco C, Grillet F, Piton G, Delabrousse E. Transmural bowel necrosis from acute mesenteric ischemia and Strangulated small-bowel obstruction: distinctive CT features. AJR Am J Roentgenol 2020; 214: 90–95. doi: 10.2214/AJR.19.21693 [DOI] [PubMed] [Google Scholar]
- 47. Barmase M, Kang M, Wig J, Kochhar R, Gupta R, Khandelwal N. Role of Multidetector CT angiography in the evaluation of suspected mesenteric ischemia. Eur J Radiol 2011; 80: e582–e587. doi: 10.1016/j.ejrad.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 48. Liebeskind DS, Saber H, Xiang B, Jadhav AP, Jovin TG, Haussen DC, et al. Collateral circulation in Thrombectomy for stroke after 6 to 24 hours in the DAWN trial. Stroke 2022; 53: 742–48. doi: 10.1161/STROKEAHA.121.034471 [DOI] [PubMed] [Google Scholar]
- 49. Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation 1992; 86: 81–90. doi: 10.1161/01.cir.86.1.81 [DOI] [PubMed] [Google Scholar]
- 50. Zeng Y, Yang F, Hu X, Zhu F, Chen W, Lin W. Radiological predictive factors of Transmural intestinal necrosis in acute mesenteric ischemia: systematic review and meta-analysis. Eur Radiol 2023; 33: 2792–99. doi: 10.1007/s00330-022-09258-5 [DOI] [PubMed] [Google Scholar]
- 51. Atre ID, Eurboonyanun K, O’Shea A, Lahoud RM, Shih A, Kalva S, et al. Predictors of Transmural intestinal necrosis in patients presenting with acute mesenteric ischemia on computed tomography. Abdom Radiol 2022; 47: 1636–1643. doi: 10.1007/s00261-020-02558-8 [DOI] [PubMed] [Google Scholar]
- 52. Milone M, Di Minno MND, Musella M, Maietta P, Iaccarino V, Barone G, et al. Computed tomography findings of Pneumatosis and Portomesenteric venous gas in acute bowel ischemia. World J Gastroenterol 2013; 19: 6579–84. doi: 10.3748/wjg.v19.i39.6579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kernagis LY, Levine MS, Jacobs JE. Pneumatosis Intestinalis in patients with ischemia: correlation of CT findings with viability of the bowel. AJR Am J Roentgenol 2003; 180: 733–36. doi: 10.2214/ajr.180.3.1800733 [DOI] [PubMed] [Google Scholar]
- 54. Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros PR. Pneumatosis Intestinalis and Portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol 2001; 177: 1319–23. doi: 10.2214/ajr.177.6.1771319 [DOI] [PubMed] [Google Scholar]
- 55. Calame P, Delabrousse É, Ronot M. Letter to the editor: Pneumatosis in bowel ischemia: time to change the optics to improve patient care. Insights Imaging 2022; 13(): 25. doi: 10.1186/s13244-022-01165-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang W, Zhang J, Kuang LQ, Yi KM, Li CX, Wang Y. Relationship of superior mesenteric artery thrombus density with Transmural intestinal necrosis on Multidetector computed tomography in acute mesenteric ischemia. Quant Imaging Med Surg 2021; 11: 3120–32. doi: 10.21037/qims-20-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Emile SH. Predictive factors for intestinal Transmural necrosis in patients with acute mesenteric ischemia. World J Surg 2018; 42: 2364–72. doi: 10.1007/s00268-018-4503-3 [DOI] [PubMed] [Google Scholar]
- 58. Ksouri A, Copin P, Bonvalet F, Bozi L, Cazals-Hatem D, Garzelli L, et al. Colonic involvement in acute mesenteric ischemia: prevalence, risk factors, and outcomes. Eur Radiol 2022; 32: 2813–23. doi: 10.1007/s00330-021-08318-6 [DOI] [PubMed] [Google Scholar]
- 59. Sinz S, Schneider MA, Graber S, Alkadhi H, Rickenbacher A, Turina M. Prognostic factors in patients with acute mesenteric ischemia-novel tools for determining patient outcomes. Surg Endosc 2022; 36: 8607–18. doi: 10.1007/s00464-022-09673-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garzelli L, Nuzzo A, Hamon A, Ben Abdallah I, Gregory J, Raynaud L, et al. Reperfusion injury on computed tomography following Endovascular Revascularization of acute mesenteric ischemia: prevalence, risk factors, and patient outcome. Insights Imaging 2022; 13(): 194. doi: 10.1186/s13244-022-01339-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Inoue A, Nitta N, Ota S, Takaki K, Imai Y, Misaki S, et al. MR imaging-based evaluation of mesenteric ischemia caused by Strangulated small bowel obstruction and mesenteric venous occlusion: an experimental study using rabbits. Magn Reson Med Sci 2020; 19: 125–34. doi: 10.2463/mrms.mp.2019-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Plessier A, Darwish-Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010; 51: 210–18. doi: 10.1002/hep.23259 [DOI] [PubMed] [Google Scholar]
- 63. Amitrano L, Guardascione MA, Scaglione M, Pezzullo L, Sangiuliano N, Armellino MF, et al. Prognostic factors in Noncirrhotic patients with Splanchnic vein Thromboses. Am J Gastroenterol 2007; 102: 2464–70. doi: 10.1111/j.1572-0241.2007.01477.x [DOI] [PubMed] [Google Scholar]
- 64. Elkrief L, Payancé A, Plessier A, d’Alteroche L, Ronot M, Paradis V, et al. Management of Splanchnic vein thrombosis. JHEP Rep 2023; 5(): 100667. doi: 10.1016/j.jhepr.2022.100667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De Stefano V, Fiorini A, Rossi E, Za T, Farina G, Chiusolo P, et al. Incidence of the Jak2 V617F Mutation among patients with Splanchnic or cerebral venous thrombosis and without overt chronic Myeloproliferative disorders. J Thromb Haemost 2007; 5: 708–14. doi: 10.1111/j.1538-7836.2007.02424.x [DOI] [PubMed] [Google Scholar]
- 66. H L. Gradual occlusion of the mesenteric vessels. Surgery 1943; 13: 406–10. [Google Scholar]
- 67. Amitrano L, Guardascione MA, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol 2004; 40: 736–41. doi: 10.1016/j.jhep.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 68. Pan J, Wang L, Gao F, An Y, Yin Y, Guo X, et al. Epidemiology of portal vein thrombosis in liver cirrhosis: A systematic review and meta-analysis. Eur J Intern Med 2022; 104: 21–32. doi: 10.1016/j.ejim.2022.05.032 [DOI] [PubMed] [Google Scholar]
- 69. Landau M, Wisniewski S, Davison J. Jejunoileal Neuroendocrine tumors complicated by intestinal ischemic necrosis are associated with worse overall survival. Archives of Pathology & Laboratory Medicine 2016; 140: 461–66. doi: 10.5858/arpa.2015-0105-OA [DOI] [PubMed] [Google Scholar]
- 70. Gonzelez HJ, Sahay SJ, Samadi B, Davidson BR, Rahman SH. Splanchnic vein thrombosis in severe acute Pancreatitis: a 2-year, single-institution experience. HPB 2011; 13: 860–64. doi: 10.1111/j.1477-2574.2011.00392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Junare PR, Udgirkar S, Nair S, Debnath P, Jain S, Modi A, et al. Splanchnic venous thrombosis in acute Pancreatitis: does anticoagulation affect outcome. Gastroenterology Res 2020; 13: 25–31. doi: 10.14740/gr1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mortelé KJ, Mergo PJ, Taylor HM, Wiesner W, Cantisani V, Ernst MD, et al. Peripancreatic vascular abnormalities complicating acute Pancreatitis: contrast-enhanced Helical CT findings. Eur J Radiol 2004; 52: 67–72. doi: 10.1016/j.ejrad.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 73. Hatoum OA, Spinelli KS, Abu-Hajir M, Attila T, Franco J, Otterson MF, et al. Mesenteric venous thrombosis in inflammatory bowel disease. J Clin Gastroenterol 2005; 39: 27–31. [PubMed] [Google Scholar]
- 74. Elkrief L, Corcos O, Bruno O, Larroque B, Rautou P-E, Zekrini K, et al. Type 2 diabetes mellitus as a risk factor for intestinal resection in patients with superior mesenteric vein thrombosis. Liver Int 2014; 34: 1314–21. doi: 10.1111/liv.12386 [DOI] [PubMed] [Google Scholar]
- 75. Lee SS, Ha HK, Park SH, Choi EK, Kim AY, Kim JC, et al. Usefulness of computed tomography in differentiating Transmural infarction from Nontransmural ischemia of the small intestine in patients with acute mesenteric venous thrombosis. J Comput Assist Tomogr 2008; 32: 730–37. doi: 10.1097/RCT.0b013e318159f135 [DOI] [PubMed] [Google Scholar]
- 76. Calame P, Winiszewski H, Doussot A, Malakhia A, Grillet F, Verdot P, et al. Evaluating the risk of irreversible intestinal necrosis among critically ill patients with Nonocclusive mesenteric ischemia. Am J Gastroenterol 2021; 116: 1506–13. doi: 10.14309/ajg.0000000000001274 [DOI] [PubMed] [Google Scholar]
- 77. Mazzei MA, Guerrini S, Cioffi Squitieri N, Vindigni C, Imbriaco G, Gentili F, et al. Reperfusion in non-occlusive mesenteric ischaemia (NOMI): effectiveness of CT in an emergency setting. Br J Radiol 2016; 89: 20150956. doi: 10.1259/bjr.20150956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nakamura Y, Urashima M, Toyota N, Ono C, Iida M, Fukumoto W, et al. Non-occlusive mesenteric ischemia (NOMI): utility of measuring the diameters of the superior mesenteric artery and superior mesenteric vein at Multidetector CT. [Japanese journal of radiology]. Jpn J Radiol 2013; 31: 737–43. doi: 10.1007/s11604-013-0245-1 [DOI] [PubMed] [Google Scholar]
- 79. Pérez-García C, de Miguel Campos E, Fernández Gonzalo A, Malfaz C, Martín Pinacho JJ, Fernández Álvarez C, et al. Non-occlusive mesenteric ischaemia: CT findings, clinical outcomes and assessment of the diameter of the superior mesenteric artery. Br J Radiol 2018; 91: 20170492. doi: 10.1259/bjr.20170492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chaudhary SR, Ko N, Dillon WP, Yu MB, Liu S, Criqui GI, et al. Prospective evaluation of Multidetector-row CT angiography for the diagnosis of vasospasm following subarachnoid hemorrhage: a comparison with Digital subtraction angiography. Cerebrovasc Dis 2008; 25: 144–50. doi: 10.1159/000112325 [DOI] [PubMed] [Google Scholar]
- 81. Kammerer S, Schuelke C, Berkemeyer S, Velasco A, Heindel W, Koehler M, et al. The role of Multislice computed tomography (MSCT) angiography in the diagnosis and therapy of non-occlusive mesenteric ischemia (NOMI): could MSCT replace DSA in diagnosis PLOS One 2018; 13(): e0193698. doi: 10.1371/journal.pone.0193698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Konan A, Piton G, Ronot M, Hassoun Y, Winiszewski H, Besch G, et al. Abdominal Atherosclerosis is not a risk factor of Nonocclusive mesenteric ischemia among critically ill patients: a propensity matching study. Ann Intensive Care 2022; 12(): 117. doi: 10.1186/s13613-022-01096-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Verdot P, Calame P, Winiszewski H, Grillet F, Malakhia A, Lakkis Z, et al. Diagnostic performance of CT for the detection of Transmural bowel necrosis in non-occlusive mesenteric ischemia. Eur Radiol 2021; 31: 6835–45. doi: 10.1007/s00330-021-07728-w [DOI] [PubMed] [Google Scholar]
- 84. Calame P, Winiszewski H, Lakkis Z, Verdot P, Pili-Floury S, Heyd B, et al. Prognostic factors in non-occlusive mesenteric ischemia: A pragmatic pre-operative score for the prediction of 28-day mortality. The American Journal of Surgery 2022; 224: 617–23. doi: 10.1016/j.amjsurg.2022.03.048 [DOI] [PubMed] [Google Scholar]
- 85. Juif A, Calame P, Winiszewski H, Turco C, Verdot P, Pili-Floury S, et al. Atherosclerosis is associated with poorer outcome in non-occlusive mesenteric ischemia. Eur J Radiol 2021; 134: 109453. doi: 10.1016/j.ejrad.2020.109453 [DOI] [PubMed] [Google Scholar]
- 86. Miyazawa R, Kamo M. What affects the prognosis of NOMI patients? analysis of clinical data and CT findings. Surg Endosc 2020; 34: 5327–30. doi: 10.1007/s00464-019-07321-9 [DOI] [PubMed] [Google Scholar]
- 87. Carlson RM, Madoff RD. Is "ischemic" colitis ischemic Dis Colon Rectum 2011; 54: 370–73. doi: 10.1007/DCR.0b013e31820481a9 [DOI] [PubMed] [Google Scholar]
- 88. Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211: 381–88. doi: 10.1148/radiology.211.2.r99ma28381 [DOI] [PubMed] [Google Scholar]
- 89. Iacobellis F, Berritto D, Fleischmann D, Gagliardi G, Brillantino A, Mazzei MA, et al. CT findings in acute, subacute, and chronic ischemic colitis: suggestions for diagnosis. BioMed Research International 2014; 2014: 1–7. doi: 10.1155/2014/895248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ, American College of Gastroenterology . ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol 2015; 110: 18–44. doi: 10.1038/ajg.2014.395 [DOI] [PubMed] [Google Scholar]
- 91. Brandt LJ, Feuerstadt P, Blaszka MC. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: a study of 313 cases supported by histology. Am J Gastroenterol 2010; 105: 2245–52. doi: 10.1038/ajg.2010.217 [DOI] [PubMed] [Google Scholar]
- 92. Yadav S, Dave M, Edakkanambeth Varayil J, Harmsen WS, Tremaine WJ, Zinsmeister AR, et al. A population-based study of incidence, risk factors, clinical spectrum, and outcomes of ischemic colitis. Clin Gastroenterol Hepatol 2015; 13: 731–38. doi: 10.1016/j.cgh.2014.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]