Abstract
Natural ingredients have been used for centuries for skin treatment and care. Interest in the health effects of plants has recently increased due to their safety and applicability in the formulation of pharmaceuticals and cosmetics. Long-known plant materials as well as newly discovered ones are increasingly being used in natural products of plant origin. This review highlights the beneficial effects of plants and plant constituents on the skin, including moisturizing (e.g., Cannabis sativa, Hydrangea serrata, Pradosia mutisii and Carthamus tinctorius), anti-aging (e.g., Aegopodium podagraria, Euphorbia characias, Premna odorata and Warburgia salutaris), antimicrobial (e.g., Betula pendula and Epilobium angustifolium), antioxidant (e.g., Kadsura coccinea, Rosmarinus officinalis, Rubus idaeus and Spatholobus suberectus), anti-inflammatory (e.g., Antidesma thwaitesianum, Helianthus annuus, Oenanthe javanica, Penthorum chinense, Ranunculus bulumei and Zanthoxylum bungeanum), regenerative (e.g., Aloe vera, Angelica polymorpha, Digitaria ciliaris, Glycyrrihza glabra and Marantodes pumilum), wound healing (e.g., Agrimonia eupatoria, Astragalus floccosus, Bursera morelensis, Jatropha neopauciflora and Sapindus mukorossi), photoprotective (e.g., Astragalus gombiformis, Calea fruticose, Euphorbia characias and Posoqueria latifolia) and anti-tyrosinase activity (e.g., Aerva lanata, Bruguiera gymnorhiza, Dodonaea viscosa, Lonicera japonica and Schisandra chinensis), as well as their role as excipients in cosmetics (coloring (e.g., Beta vulgaris, Centaurea cyanus, Hibiscus sabdariffa and Rubia tinctiorum), protective and aromatic agents (e.g., Hyssopus officinalis, Melaleuca alternifolia, Pelargonium graveolens and Verbena officinalis)).
Keywords: plants, skin, photoprotection, wound healing, anti-aging, anti-tyrosinase, essential oils, colorants, cosmetics, pharmaceutics
1. Introduction
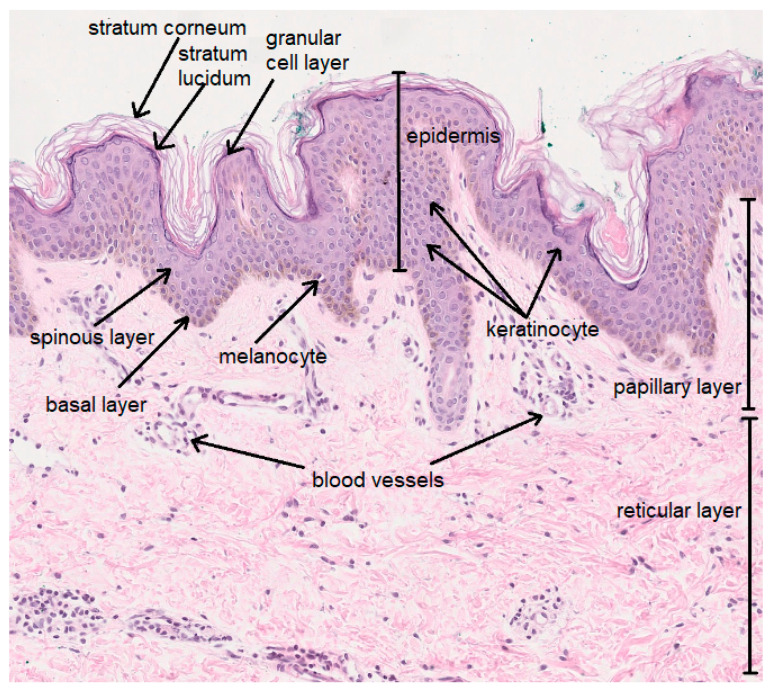
The skin consists of the epidermis and the dermis, below which lies subcutaneous tissue. The five-layer epidermis consists of keratinocytes—cells taking part in keratinization, melanocytes—pigment cells, Langerhans cells, mastocytes and Merkel cells. The dermis is composed of connective tissue and consists of a papillary layer and a reticular layer. It contains fibroblasts, which are responsible for the production of collagen, elastin and glycosaminoglycans (GAG), as well as numerous blood vessels, nerve endings and appendages, including hair follicles and sweat and sebaceous glands (Figure 1). The skin performs multiple complex functions; it takes part in metabolic and homeostatic processes and is responsible for the excretion, selective absorption and storage of substances. In addition, it protects against biological (e.g., microbes), physical (e.g., UV radiation) and chemical factors [1,2].
Figure 1.
Structure of the skin (own work; photo: Department of Clinical and Experimental Pathology, Medical College, Jan Kochanowski University).
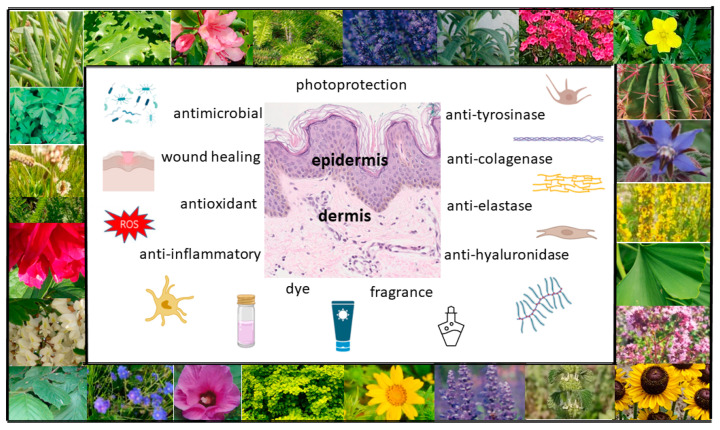
Botanical ingredients are one of the main sources of materials that are used in the cosmetics and pharmaceutical industries. Recent years have seen increasing interest in dermocosmetics and cosmeceuticals produced from plant materials, and thus, there has been greater interest in plant-based products with skin care properties. Plant materials can be applied topically for skin care purposes, as well as for the treatment of numerous skin diseases [2] (Figure 2). Their advantage is that they are gentle but effective, safe and non-toxic, without side effects. Cosmetics fortified with bioactive compounds are ideally suited to the needs of the skin and are more environmentally friendly than conventional cosmetics. A group of natural ingredients widely used in cosmetics is plant extracts, which are a rich source of biologically active substances significantly affecting human skin. They may exhibit a wide range of properties, both medicinal (in certain skin disorders, including inflammatory disorders such as acne, psoriasis or atopic dermatitis) and for use in skin care (e.g., antioxidant, antibacterial, astringent, moisturizing, regenerating, cleansing, smoothing or lightening) [3,4]. Plant extracts are obtained via extraction from various parts of raw plants, e.g., using an appropriately chosen solvent, such as water, ethyl alcohol, glycerine, glycols or vegetable oil. Plant extracts are obtained from whole plants or parts of plants (fruits, leaves, roots, bark, stems, branches, seeds or flowers). The composition and properties of plant extracts, which can be found in the formulas of natural cosmetics, depend on a variety of factors, including cultivation and harvest conditions, how and to what extent the material is broken up, or drying and extraction methods. Extracts from whole plants as well as individual chemical substances contained in them are used in cosmetics. Active plant substances are divided into primary and secondary metabolites. The former are basic substances that are essential to the plant for life, constituting building materials and energy sources. They include sugars, fats, proteins, amino acids and enzymes. Secondary metabolites include terpenes, steroids, saponins, tannins, alkaloids, volatile oils, resins, vitamins and phenolics [1,4].
Figure 2.
Possible uses of plants in skin care and treatment (own work; photos: M. Michalak).
The aim of the present paper is to describe plants as bioactive cosmetic and therapeutic substances. This review focuses on recent studies on the potential uses of plants and their constituents as photoprotective, anti-inflammatory, regenerative, wound-healing, anti-aging, depigmenting, aromatic and coloring agents.
2. Plants as Photoprotective Agents against Ultraviolet-Radiation-Induced Inflammation and Skin Damage
Ultraviolet (UV) radiation is a physical inflammatory, mutagenic and carcinogenic reagent, as well as a strong enhancer of reactive oxygen species (ROS) production. The biological effects of UV radiation on the skin may be the result of early reactions (erythema or sunburn) or long-term reactions (changes related to skin damage at the molecular and biochemical level). The first response of the skin to UV radiation is the activation of inflammation. UVB irradiation of keratinocytes leads to increased synthesis of pro-inflammatory cytokines in the epidermis, e.g., TNF-α (tumour necrosis factor α) and interleukins IL-1, IL-6, IL-8 and IL-10, which then influence immune cell activity. Another important mediator of inflammation induced by UV radiation is cyclooxygenase-2 (COX-2). COX-2 is an enzyme that is responsible for the synthesis of prostaglandins (PG) from arachidic acid; these play an important role in the regulation of the inflammatory reaction of skin exposed to UVB radiation [5,6,7,8]. Moreover, skin cells exposed to UV radiation respond by activating a cascade of signaling pathways. Disruptions in the activation of these pathways induced by UV radiation lead to disturbances of the homeostasis of the skin, changes in gene expression or the regulation of cytokine secretion, or a loss of control over the cell cycle, which in turn can lead to carcinogenesis [9]. Key signaling pathways activated by UV radiation include transcription factor NFκB (nuclear factor of kappa in B cells) and MAPKs (mitogen-activated protein kinases), including p38 kinases (p38 mitogen-activated protein kinases), JNK (Jun N-terminal kinase) and ERK 1/2 (extracellular signal-regulated kinase 1/2). The p38 kinase is activated by a number of pro-inflammatory cytokines or stress factors. Studies suggest that the p38 kinase is involved in the activation of inflammation induced by UVB radiation through the regulation of COX-2 activity, the production of IL-6, IL-8 and TNFα and the synthesis of nitric oxide (iNOS). Studies have shown that the JNK serine-threonine kinase pathway is more strongly activated by UVA radiation than by UVB radiation in human keratinocytes. The type and dosage of UV radiation have also been shown to determine the activation of ERK1/2 [10,11,12,13]. The exposure of human keratinocytes to UV radiation results in ROS accumulation. Oxidative stress may modulate various signaling cascades in human skin cells and mediate MAPK activity, and it may also be associated with elevated levels of activator protein 1 (AP-1) and NFκB in keratinocytes. Prolonged and intense exposure to UV radiation contributes not only to premature skin aging but also to melanoma and nonmelanoma skin cancers (cutaneous malignant melanoma, basal cell carcinoma or squamous cell carcinoma) [2,14].
Selected plant extracts and single compounds with antioxidant, anti-inflammatory and immunomodulatory effects play an important role in the photoprotection of the skin. Phytochemicals have shown the ability to act as free radical scavengers, radical chain reaction inhibitors, metal chelators, oxidative enzyme inhibitors and antioxidant enzyme cofactors. Some studies have reported that plant extracts promote endogenous antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX), which protect the skin against increasing ROS levels under oxidative stress. Moreover, plant materials can modulate the expression and activation of a wide variety of cytokines, such as TNF-α IL-1β, IL-6 and IL-8. Botanicals have also shown the ability to regulate the expression of various pro-inflammatory genes and inhibit the activity of pro-inflammatory enzymes such as inducible nitric oxide synthase (iNOS), COX-2 and lipoxygenase (LOX) [15,16,17].
Plant extracts and natural compounds from plants have been reported in the earlier literature to possess photoprotective properties. These include phytochemicals such as ferulic, caffeic, cinnamic, rosmarinic acid, quercetin, apigenin, rutin, luteolin, chrysin, hesperidin, dihydromyricetin, chrysanthemin, curcumin, genistein, resveratrol, carnosic, ursolic, ellagic, asiatic acid, zerumbone, astaxanthin, β-carotene, lycopene, zeaxantin, lutein and L-ergothioneine, as well as extracts from plants such as Opuntia humifusa [18], Camellia sinensis [19], Punica granatum [20], Hibiscus furcatus, Atalantia ceylanica, Mollugo cerviana, Leucas zeylanica, Ophiorrhiza mungos, Olax zeylanica [21] Silybum marianum [22], Polypodium leucotomos [23], Vaccinium myrtillus [24], Lonicera caerulea [25], Thymus vulgaris [26], Opuntia ficus-indica [27], Morinda citrifolia [28], Galinsoga parviflora, Galinsoga quadriradiata [29], Coffea arabica [30], Amaranthus cruentus, Moringa oleifera, Malaxis acuminata, Schinus terebinthifolius [31], Schinopsis brasiliensis [32], Crataegus pentagyna [33], Sambucus nigra, Helichrysum arenarium, Crataegus monogyna [34], Capnophyllum peregrinum [35], Dalbergia monetaria [36], Baccharis antioquensis [37], Juglans regia [38], Dimorphandra gardneriana and Lippia microphylla [39].
Some plants that are effective UV filters may be potential sunscreen ingredients [40]. These include plant extracts such as Astragalus gombiformis with an SPF value of 38 [41], Sloanea calva with an SPF value of 35.4 [42], Hylocereus polyrhizus with an SPF value of 35.02 [43] or Rosa centifolia with SPF values of 32 [44]. Moreover, plant extracts, through their synergistic effects with some physical or chemical UV filters (e.g., benzophenone-3 (BP-3), octyl methoxycinnamate (OMC) or titanium dioxide (TiO2)), may also play a role as cosmetic components that enhance the SPF of sunscreen formulations [40]. This effect has been shown for extracts from Sanionia uncinata [45,46], Vitis vinifera [47], Nephelium lappaceum [48], Psidium guajava [49], Campomanesia adamantium and Campomanesia xanthocarpa [50], as well as moss extracts from Leucobryum spp. and Holomitriopsis laevifolia [51].
Table 1 presents the results of research from the last five years on the protective effects of plant-derived products on UVB-mediated damage, with potential applications in photoprotective products.
Table 1.
Selected plant extracts from various species and their photoprotective properties.
| Species (Family) | Plant Material | Method | Effect | Ref |
|---|---|---|---|---|
|
Adenocaulon himalaicum (Asteraceae) |
leaf, EE | in vitro, HaCaT exposed to UVB | ↑ filaggrin, involucrin, loricrin expression ↓ MMP-1; MAPK, AP-1 activation |
[52] |
|
Alpinia officinarum (Zingiberaceae) |
rhizome, WE | in vivo, UVB-irradiated hairless mouse; in vitro, NIH-3T3 exposed to UVB |
↓ of MMP-1 expression and recovered the reduction in collagen content in mouse skin; ↓ IL-6, IL-8, MCP-3 expression and ↓ phospho-Akt and phospho-ERK in NIH-3T3 |
[53] |
|
Antidesma thwaitesianum (Euphorbiceae) |
fruit extract | in vitro, UVB-irradiated HaCaT | protects cells from UVB-induced cytotoxicity; anti-inflammatory effect through ↓ NO and ROS generation; ↓ phospho-p38 and phospho-JNK |
[54] |
|
Astragalus gombiformis (Fabaceae) |
aerial part, BE | in vitro, SPF via UV spectroscopy | SPF 37.78 | [41] |
|
Calea fruticosa (Asteraceae) |
aerial part, EE | in vitro, SPF via UV spectroscopy | SPF 9.66 | [55] |
|
Camellia sinensis (Theaceae) |
leaf extract | in vitro, NHEK exposed to UVB | efficacy in recovering TIMP-3 expression downregulated by UVB treatment | [56] |
|
Chrysophyllum lucentifolium (Sapotaceae) |
ME | in vitro, UVB and H2O2-treated HaCaT and HDF | ↓ expression of COX-2, MMP-1, and -9, HYAL-1, and -4 by downregulating the NF-κB and MAPK (ERK, JNK and p38) pathways; ↑ Col1a1 expression |
[57] |
|
Cistus incanus Cistus ladanifer (Cistaceae) |
aerial part extract |
in vitro, SPF via UV spectroscopy | SPF 3.33—4.37 | [58] |
|
Ceratonia siliqua (Fabaceae) |
pod and seed extract, WME | in vitro, SPF via UV spectroscopy | SPF 1.07–18.19 | [59] |
|
Corylus avellana (Betulaceae) |
hazelnut skin extract | in vitro, SPF via UV spectroscopy | extract ↑ SPF value of benzophenone 4.66–4.94 | [60] |
|
Cyclopia spp. (Fabaceae) |
leaf and branch, WAE | in vitro, SPF via UV spectroscopy | SPF 27.8 | [61] |
|
Diospyros kaki (Ebenaceae) |
fruit (pulp, skin and seed) extract | in vitro, HaCaT exposed to UVA and UVB | ↓ intracellular ROS production in cells; exerts a photoprotective and regenerative effect on UV-irradiated cells | [62] |
|
Elaeagnus angustifolia (Elaeagnaceae) |
leaf extract | in vitro, SPF via UV spectroscopy | SPF values of sunscreen formulation (with 2%, 4%, 6%, 8% extracts): 6.37–21.05 |
[63] |
|
Euphorbia characias (Euphorbiaceae) |
leaf, EE | in vitro, SPF via UV spectroscopy | SPF 9.10 | [64] |
|
Helianthus annuus (Asteraceae) |
flower, EE | in vitro, UVB-irradiated HDF | ↓ MMP-1, 3 and ROS production; ↓ procollagen type I reduction; anti-photoaging action via the activation of Nrf2, upregulation of TGF-β, downregulation of AP-1 and MAPK phosphorylation; ↓ UVB-induced VEGF and IL-6, COX-2, iNOS and TNF-α secretion |
[65] |
|
Hylocereus polyrhizus (Cactaceae) |
fruit peel, EE | in vitro, SPF via UV spectroscopy | SPF 35.02 | [43] |
|
Juglans regia (Juglandaceae) |
male flower, ME | in vitro, UVB-irradiated HaCaT | prevents the overexpression of MAPKs, AP-1, MMPs, Smad7; ↓ expression of TIMP-1/2, TGF-β1, Smad3 and procollagen type-1 in cells |
[66] |
|
Kadsura coccinea (Schisandraceae) |
root, stem, leaf and fruit, EE | in vitro, UVA and UVB-irradiated HaCaT | alleviates anti-proliferative and cytotoxic effects of UVA/UVB irradiation on cells; ↓ intracellular ROS level and keratinocyte damage | [67] |
|
Melaleuca leucadendron (Myrtaceae) |
flower, EE |
in vitro, UVB-induced HaCaT | ↓ COX-2 expression, ensures protection of DNA damage, prevents the increase in ROS; ↑ levels of the antioxidant enzymes SOD, GPx and CAT |
[68] |
| Moringa concanensis (Moringaceae) | stem bark extract | in vitro, SPF via UV spectroscopy, UVA/UVB absorption spectra | SPF 10.46 and broad absorption spectrum (UVA and UVB) ranges | [69] |
|
Oenanthe javanica (Apiaceae) |
EE | in vivo, UVB-exposed mouse | ↑ collagen types I and III productions; ↓ MMP-1 and MMP-3, TNF-α and COX-2 expression | [70] |
| Penthorum chinense (Penthoraceae) | EE | in vitro, HaCaT under UVB or H2O2 treatment | ↑ the promoter activity of the type 1 procollagen gene Col1A1; ↓ MMPs, COX-2, IL-6 expression and HYAL induced by UVB irradiation or H2O2-induced oxidative stress; ↓ phospho-p38 and phospho-JNK | [71] |
|
Pradosia mutisii (Sapotaceae) |
ME | in vitro, HaCaT, HDF under UVB or H2O2 treatment | ↓ MMP-1 and MMP-9; ↑ Sirt-1 |
[72] |
|
Posoqueria latifolia (Rubiaceae) |
flower, EE | in vitro, SPF via UV spectroscopy | SPF 35, broad-spectrum (UVA-UVB) protection efficacy | [44] |
|
Ranunculus bulumei (Ranunculaceae) |
aerial part, ME |
in vitro, UVB-irradiated HaCaT | ↓ mRNA levels of MMP-9, COX-2; ↑mRNA levels of Sirt-1, type-1 procollagen; ↓ phospho-p38; inactivates AP-1 |
[73] |
|
Rosa centifolia (Rosacea) |
flower, EE | in vitro, SPF via UV spectroscopy | SPF value of 32, broad-spectrum (UVA-UVB) protection efficacy |
[44] |
|
Rosmarinus officinalis (Lamiaceae) |
leaf, HE | in vivo, UV-irradiated rat | ↓ level of GSH, SOD, CAT; ↓ IL-1β, IL-6, and NF-kB; ↓ MMP-1, GM-CSF, NEP |
[74] |
|
Rubus idaeus (Rosaceae) |
EE | in vitro, HaCaT; in vivo, mouse exposed to UVB |
alleviate UVB-caused erythema in the skin; ↓ formation of 8-OHdG; recover the expression of Nrf2 and antioxidant enzyme proteins SOD and CAT; ↓ phospho-p38 and NF-κB expression |
[75] |
|
Sideritis raeseri (Lamiaceae) |
aerial part, WEE | in vitro, SPF via UV spectroscopy | SPF 4.54–18.01 | [76] |
|
Sloanea medusula Sloanea calva (Elaeocarpaceae) |
leaf, EE | in vitro, SPF via UV spectroscopy |
S. medusula, SPF 32.5 S. calva, SPF 35.4 |
[42] |
|
Spatholobus suberectus (Leguminosa) |
stem, WE, EE |
in vitro, HaCaT exposed to UVB | ↓ ROS production; block MAPK, NF-κB and c-Jun; ↑ Col1a1, ELN, HAS2 expression |
[77] |
| Syzygium formosum (Myrtaceae) | leaf, EE | in vitro, HaCaT exposed to UVB | ↓ IL-1 β, IL-6, IL-8 and COX-2 expression | [78] |
|
Silybum marianum (Asteraceae) |
sylimarin and flavonolignans | in vitro, SPF via UV spectroscopy | absorbs UVB and UVA; SPF 2.01–6.07 |
[79] |
|
Washingtonia filifera (Arecaceae) |
seed, EE, WE, ME | in vitro, H2O2-induced HaCaT; in vitro SPF via UV spectroscopy |
↓ ROS generation; SPF 1.52–3.35. |
[80] |
|
Zanthoxylum bungeanum (Rutaceae) |
sanshool, a major component | in vitro, UVB-irradiated HDF; in vivo, mouse |
↓ activation of JAK2-STAT3 signaling; ↓ MMP-1 and MMP-3 secretion |
[81] |
↓, inhibit/suppress/decrease; ↑, enhance/induce/increase; 8-OHdG, 8-hydroxydeoxyguanosine; AP-1, activator protein 1; BE, buthanol extract; Col1a1, collagen type I alpha 1; EE, ethanol extract; ELN, elastin; ERK, extracellular signal-regulated kinase; GM-CSF, granulocyte-macrophage colony-stimulating factor; HaCaT, human keratinocyte cell line; HAS2, hyaluronan synthase 2; HDF, human dermal fibroblast cell line; HE, hexane extract; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinases; MCP-3, monocyte chemotactic protein-3; ME, methanol extract; MMP, matrix metalloproteinases; NEP, neprilysin; NF-κB, nuclear factor-kappa B; NIH-3T3, skin fibroblast cells; NHEK, neonatal normal human epidermal keratinocytes; Nrf2, nuclear factor erythroid 2-related factor 2; Sirt-1, sirtuin 1; STAT3, signal transducer and activator of transcription; TIMP, tissue inhibitor of metalloproteinases; WAE, water/aceton extract; WE, water extract; WEE, water/ethanol extract; WME, water/methanol extract.
3. Plants as Regenerative and Wound-Healing Agents
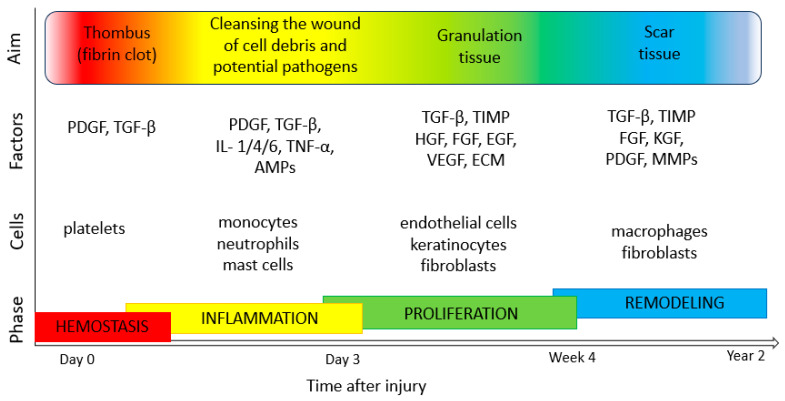
The process of the regeneration and healing of the skin involves interactions between many types of cells, including endothelial cells, inflammatory cells, keratinocytes and fibroblasts. It consists of stages such as coagulation (haemostasis, fibrin clot formation and activation of the clotting cascade by platelets), inflammation (neutrophil and monocyte migration, phagocytosis of bacteria and the release of proteolytic enzymes to debride the wound), proliferation (angiogenesis by endothelial cells, granulation tissue formation by fibroblasts and reepithelialization by keratinocytes) and tissue maturation (collagen/ECM remodeling by fibroblasts) [82,83,84] (Figure 3). An important step in tissue formation, repair and the maintenance of good skin conditions is proper cell proliferation and migration processes. These depend on many factors, such as biochemical communication, adhesion strength and mechanical flexibility, as well as organization of the cellular cytoskeleton [85,86,87]. Numerous regulators take part in keratinocyte migration and proliferation, including epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), fibroblast growth factor (FGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), angiopoietin-related growth factor (AGF), vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β), connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF) and platelet derived-endothelial cell growth factor (PD-ECGF). In addition, cytokines (e.g., IL-1, IL-6 and TNF-α), neuropeptides (G protein-coupled receptor (GCRP), vasoactive intestinal peptide (VIP) and substance P (SP)), MMPs and extracellular macromolecules also play various roles in the regulation of skin cell motility and proliferation [88,89].
Figure 3.
Phases of wound healing (own work based on [82,89,90,91]). TGF-β, transforming growth factor β; PDGF, platelet-derived growth factor; IL-1, 4, 6, interleukin-1, -4, -6); TNF-α, tumor necrosis factor α; AMPs, antimicrobial peptides; TIMP, tissue inhibitors of metalloproteinase; HGF, hepatocyte growth factor; FGF, fibroblast growth factor; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; KGF, keratinocyte growth factor; ECM, extracellular matrix; MMPs, matrix metalloproteinases.
A wound is an injury involving a breach of the integrity of the skin. A chronic wound may lead to complications, such as bacterial infections. Bacterial infections also delay the wound-healing process, prolonging inflammation. The surface of human skin is colonized by commensal bacteria with low virulence, such as coagulase-negative staphylococci and non-pathogenic corynebacteria and cutibacteria, but also by opportunistic pathogenic microbes (such as Candida spp., Malassezia spp. or Staphylococcus aureus) and bacteria with high pathogenic potential (e.g., Streptococcus pyogenes). The skin of hospitalized patients who have undergone antibiotic treatment may be colonized by Gram-negative non-fermenting bacteria (Pseudomonas aeruginosa or Acinetobacter baumannii) or yeasts, including the opportunistic pathogen Candida auris. The choice of treatment for skin and wound infections depends on various factors (e.g., the severity of the disease or host factors), but plants and drugs of natural origin can undoubtedly have broad applications alongside topical synthetic antibiotics and antiseptic agents [92,93,94].
Botanicals have been used topically for decades for skin regeneration and the treatment of dermatological problems, such as chronic diabetic wounds, ulcers, bedsores, burns and non-healing wounds. Numerous plants and drugs of natural origin support the normal repair systems of the skin and therefore show great therapeutic potential in skin regeneration and wound treatment by various mechanisms. These include effects on keratinocyte migration and proliferation rates, modulation of the release of various growth factors, cytokines, chemokines or neuropeptides by skin cells, increasing the formation of capillary vessels and increasing fibroblast activity. Another important group of raw materials comprises plants with astringent and antimicrobial properties, which contribute to wound contraction and increase the rate of epithelialization [83,84,95]. The scientific literature points to the important effects of plants (e.g., Achiella millefolium [96], Aloe vera [97], Althaea officinalis [98], Calendula officinalis [99], Curcuma longa [100], Eucalyptus globulus [101], Simmondsia chinensis [102], Pinus sylvestris [103] and Camellia sinensis [104]) and phytochemicals (e.g., triterpenes, alkaloids and flavonoids) on tissues and their potential to amplify skin regeneration and accelerate the process of wound repair and healing [84,95].
Table 2 cites some original research carried out in the last five years on selected plants and their constituents, as well as formulations based on raw materials of plant origin exhibiting wound-healing activity and potential applications in regeneration and skin treatment.
Table 2.
The impact of selected plant extracts and natural products of plant origin on skin regeneration and wound healing.
| Plant (Family) | Plant Material | Cell/Animal | Effect | Ref |
|---|---|---|---|---|
|
Agrimonia eupatoria (Rosaceae) |
WE | in vitro, NIH 3T3, HDF and HaCaT; in vivo, rat |
↑ ECM deposition, ↑ keratinocyte proliferation/differentiation; ↑ wound TS and contraction rates | [105] |
|
Angelica polymorpha (Umbelliferae) |
flower absolute | in vitro, HaCaT | ↑ cell migration, proliferation and collagen IV synthesis; ↑ phosphorylation of ERK1/2, JNK, MAPK p38 and Akt | [106] |
|
Annona reticulata (Annonaceae) |
leaf, EE | in vitro, HaCaT | ↑ VEGF and Akt; ↑ cell migration and proliferation | [107] |
|
Astragalus floccosus (Leguminosae) |
root, ME | in vitro, NHDF; in vivo, rat |
↑ scratch wound healing, cell proliferation, fibrosis and epithelization | [108] |
|
Betula pendula (Betulaceae) |
bark, WE | in vitro, HaCaT | strong activities against S. aureus, C. acnes and S. epidermidis; ↑ wound closure |
[109] |
| Boesenbergia rotunda (Zingiberaceae) | rhizome, EE | in vitro, HaCaT | ↑ ERK1/2 and Akt; ↑ cell migration and proliferation |
[110] |
|
Bursera morelensis (Burseraceae) |
terpenes α-pinene and α-phellandrene | in vivo, mouse | ↑ wound contraction due to collagen deposition from the early stages; provided better structure in scar tissue | [111] |
|
Centella asiatica (Apiaceae) |
WGE | in vitro, HaCaT | positively affected wound healing and cell migration | [112] |
|
Cumin carvi (Apiaceae) |
seed, WEE | in vivo, rat | healing effects: ↑ total protein content and biomechanical factors; ↑ re-epithelialization, granular tissue, connective tissue, collagen and angiogenesis index; ↓ inflammatory factors |
[113] |
|
Cyclopia spp. (Fabaceae) |
leaf and branch, WE, WEE | in vitro, HaCaT | ↑ cell migration | [61] |
|
Derris scandens (Fabaceae) |
stem, WE, EE | in vitro, HSF | ↑ cell migration and wound closure in a scratch assay | [114] |
|
Digitaria ciliaris (Poaceae) |
flower, EE | in vitro, CCD986sk HaCaT | ↑ cell proliferation and migration; ↑ collagen I and IV syntheses; ↑ phosphorylation of ERK1/2 and p38 MAPK | [115] |
|
Fagus sylvatica (Fagaceae) |
bark, WE | in vitro, HaCaT | strong activities against S. aureus, C. acnes and S. epidermidis; ↑ wound closure | [109] |
|
Glycyrrihza glabra (Fabaceae) |
root, EE | in vivo, rat | ↑ collagen synthesis, ↑ α-SMA, PDGFR-α, FGFR1 and Cytokeratin 14 expression; ↑ angiogenesis and collagen deposition through up-regulation of bFGF, VEGF and TGF-β gene expression levels | [116] |
|
Garcinia mangostana (Clusiaceae) |
pericarp, EE | in vitro, 3T3-CCL92 | ↑ fibroblast proliferation and wound recovery | [117] |
|
Greyia radlkoferi (Melianthaceae) |
leaf, EE | in vitro, HaCaT | antibacterial activity against wound-associated bacteria (S. aureus) | [118] |
|
Hydrangea serrata (Hydrangeaceae) |
leaf, WE | in vitro, HaCaT | improved transcription levels of keratin Ker5, Ker6 and Ker16 | [119] |
|
Jatropha neopauciflora (Euphorbiaceae) |
latex | in vivo, normal and diabetic mouse | accelerated and improved the wound-healing process | [120] |
|
Nigella sativa (Ranunculaceae) |
seed, EE | in vitro, 3T3-CCL92 | ↑ cell proliferation and wound recovery | [117] |
| Rosmarinus officinalis (Lamiaceae) | leaf, HE | in vitro, HaCaT | ↑ migration and repopulation of keratinocytes at the scratched area and considerably narrowed the scratched gap | [74] |
|
Salix koreensis (Salicaceae) |
flower absolute | in vitro, HaCaT | ↑ cell proliferation, migration and collagen I and IV production; ↑ phosphorylation of Akt, JNK, ERK1/2 and p38 MAPK | [121] |
|
Sapindus mukorossi (Sapindaceae) |
kernel oil | in vitro, CCD-966SK | ↑ cell proliferation and migration; anti-inflammatory and anti-microbial activities; ↑ wound healing, ↓ size of the wound | [122] |
|
Sorocea guilleminina (Moraceae) |
leaf, WE | in vitro, N3T3; in vivo rat |
↑ cell proliferation/migration rate, ↑ wound contraction | [123] |
|
Ulmus parvifolia (Ulmaceae) |
root bark, ME | in vitro, HaCaT; in vivo, mouse |
↑ cell migration; upregulated the expression of the MMP-2 and -9 protein, ↑ TGF-β |
[124] |
| Plant material | Formulation | Cell/animal | Effect | Ref |
| Aloe vera | gel with EE | in vitro, HaCaT, HFF1; in vivo, rat |
↑ cell proliferation; promoted wound healing; accelerated re-epithelialization and wound contraction | [125] |
| Avicennia schaueriana | cream with leaf WE | in vivo, mouse | ↑ re-epithelialization and the number of fibroblasts, exhibiting a healing activity on skin injuries | [126] |
| Caralluma europaea | ointment with aerial part WEE | in vivo, rat | ↑ wound healing | [127] |
| Cassia obtusifolia | gel with aerial part EE | in vivo, rat and mouse | ↑ wound healing | [128] |
| Clematis simensis | ointment with leaf WEE | in vivo, mouse and rat | ↑ wound contraction and epithelialization; extract reduced inflammation and demonstrated antioxidant activity | [129] |
| Cnestis ferruginea | creams with root bark ME | in vivo, rat | ↓ wound size; affected the formation of well-regenerated tissue | [130] |
| Convolvulus arvensis | ointment with stem ME | in vivo, rat | ↑ wound closure; improved skin architecture; healing potential comparable to that of gentamycin | [131] |
| Centella asiatica | hydrogel with asiaticoside-rich fraction | in vivo, rabbit | ↑ wound healing | [132] |
| Cynara humilis | ointment with root WE and EE | in vivo, rat | ↑ wound contraction, epithelialization, ↑ collagen production; ↓ the number of inflammatory cells during wound healing | [133] |
| Epilobium angustifolium | hydrogel with EE, IE and WE | in vitro, HDF | ↑ wound healing; activity against S. pneumoniae, E. coli, E. faecalis, E.
faecium, S. lutea and B. pseudomycoides |
[94] |
| Ginkgo biloba | O/W cream with leaf WE | in vivo, diabetic rats | ↑ wound closure associated with increased collagen synthesis | [134] |
| Loranthus acaci | gel with aerial part EE | in vivo, rat and mouse | ↑ wound healing | [128] |
| Marantodes pumilum | ointment with leaf and root WE | in vivo, rat | ↑ wound healing; re-epithelialization, collagen deposition, fibronectin content and fibroblast cells, and fiber transformation from collagen III to I | [135] |
| Phlomis russeliana | gel with aerial part extract | in vivo, mouse | ↑ dermal and epidermal regeneration, collagen formation, ↑ TGF-β, VEGF and FGF levels | [136] |
|
Punica granatum, Matricaria chamomilla |
ointment with methanol fraction of pomegranate and chamomile flowers | in vivo, rat | ↑ wound healing; activity against S. aureus, S. epidermidis and P. aeruginosa of plant extracts |
[137] |
| Roylea elegans | cream with leaf WE | in vivo, rat | ↑ wound contraction formation of collagen, and tissue re-epithelialization; ↑ protein, GSH, SOD and CAT levels, ↓ MPO levels; ↑ IL-10, ↓ TNF-α and IL-6 | [138] |
| Tamarix aphylla | nanoemulsion W/O with leaf ME | in vivo, rabbit | ↑ acid-burn wound-healing process (improved cell attachment at the edge of the wound, collagen content), ↓ healing duration | [139] |
| Urtica simensis | ointment with leaf WME | in vivo, mouse | ↑ wound contraction, ↓ periods of epithelialization | [140] |
| Virola oleifera | cream with resin | in vivo, rat | ↑ wound contraction; ↓ LPO and protein oxidation |
[141] |
| Plant essential oils |
polysaccharide-based hydrogel with eucalyptus, ginger and cumin EO | in vitro, L929 cells; in vivo, mouse |
antibacterial activity against S. aureus and E. coli; ↑ cell migration and improved burn wound healing | [142] |
| Cinnamaldehyde | nanoemulsion | in vivo, rat | ↓ wound size; ↑ CAT and SOD, ↓ NAP3; activity against S. aureus and S. typhimurium | [143] |
↓, inhibit/suppress/decrease; ↑, enhance/induce/increase; ECM, extracellular matrix; EE, ethanol extract; ERK, extracellular signal-regulated kinase; FGF, fibroblast growth factor; HE, hexane extract; IE, isopropanol extract; JNK, c-Jun N-terminal kinase; LPO, lipid peroxidation; MAPK, mitogen-activated protein kinases; ME, methanol extract; MMP, matrix metalloproteinases; MPO, myeloperoxidase; NAP3, cytokine neutrophil-activating protein 3; PDGFR-α, platelet-derived growth factor receptor-α; SMA, smooth muscle actin; TGF-β, transforming growth factor β; TS, tensile strength; VEGF, vascular endothelial growth factor; WE, water extract; WEE, water/ethanol extract; WGE, water/glycerin extract; WME, water/methanol extract.
4. Plants as Anti-Aging Agents
Preventing and combating signs of skin aging (dry skin, loss of firmness and elasticity or wrinkles) is an age-old challenge. The skin is the organ on which these processes are most noticeable, hence the great interest in age-related changes at the level of the epidermis, dermis and subcutaneous tissue. At the level of the epidermis, changes observed with age include (1) thinning of all layers of the epidermis and flattening of the dermo-epidermal junction; (2) disturbances of the production of natural moisturizing factor (NMF), leading to dryness and increased peeling of the epidermis; (3) a reduction in the level of epidermal lipids (mainly ceramides); and (4) oxidation of lipids of intercellular cement, leading to increased transepidermal water loss (TEWL) [144,145]. Disturbed production of lipids binding the corneocytes of the stratum corneum not only causes skin dryness but also disrupts the process of the exfoliation of keratinized epidermal cells. This is linked to the malfunction of enzymes, enabling exfoliation when the water content in the epidermis is low. For example, a deficiency of linoleic acid, a component of ceramide 1, with an important role in the cohesiveness of cement, is associated with dry skin symptoms [146,147]. Major age-related changes in the dermis include (1) a reduced number and activity of fibroblasts, which are cells that are responsible for the synthesis of collagen fibers, elastin fibers and hyaluronic acid; (2) degradation of collagen fibers, progressive collagen cross-linking and a reduction in skin resilience and resistance to stretching; (3) changes in the structure of elastin fibers, which clump together in an amorphous mass (elastosis), loss of elasticity and wrinkle formation; and (4) a reduction in hyaluronic acid, with insufficiently moisturized and resilient skin [144,145].
Over the centuries, the search for new substances to slow down the aging process and restore the skin’s young appearance has not diminished. Bioactive substances with anti-aging properties include moisturizers, which influence the hydrolipid barrier and minimize destructive lesions occurring in the stratum corneum. The skin may be hydrated through the external supply of water from moisturizing agents or via the application of agents forming an occlusive lipid film to slow down water loss from the skin. An important group of anti-aging agents comprises bioactive substances, which take part in the synthesis and metabolism of skin components (e.g., proteins and essential unsaturated fatty acids) and also exhibit collagenase, elastase and hyaluronidase inhibitory activity [1,144]. Collagenase is an enzyme belonging to the family of matrix metalloproteinases (MMP), which can degrade collagen, the fibrous component of the extracellular matrix (ECM) and the major structural protein in human skin. Elastase is a proteolytic enzyme involved in the degradation of elastin, a protein responsible for skin elasticity. Hyaluronidase is an enzyme (an endoglycosidase) responsible for the hydrolysis of hyaluronic acid, a skin glycosaminoglycan, which is a major component of ECM [148,149].
Botanicals that support the health, texture and integrity of the skin are widely used in cosmetic formulations for dry and mature skin. Plant extracts and natural products are recommended because they increase skin hydration, reduce TEWL, display skin-barrier-reinforcing properties, inhibit the degradation of skin components and help to maintain the integrity of the skin’s structure. These are promising approaches to preventing skin aging using products derived from plants. Plants can be a very interesting source of ingredients with potential anti-aging properties, as confirmed by the results of in vitro studies. However, further research is needed to confirm the efficacy of plant-derived materials in vivo, as the most important factor determining the effectiveness of active ingredients of natural origin is their bioavailability. In some studies, plants have been shown to exert notable in vivo anti-aging properties. According to the literature, skin parameters associated with skin aging, such as skin hydration (measured with a corneometer and tewameter), skin elasticity (measured with a cutometer and elastometer) or facial wrinkles (measured with a skin visiometer and camera for skin analysis) have been evaluated following the application of cosmetic formulations based on various plant extracts, alone or in combination [119,150,151,152]. Table 3 cites research from the last five years on selected plant species and their extracts with potential uses as agents preventing and slowing down skin aging.
Table 3.
Selected plant extracts from various species with anti-aging activity.
| Species (Family) | Part/Extract | Method | Results | Ref |
|---|---|---|---|---|
|
Aegopodium podagraria (Apiaceae) |
WGE | enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA and COL activity | [112] |
|
Aerva lanata (Amaranthaceae) |
EE, WE | enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA, COL and HYAL activity | [153] |
|
Arachis hypogaea (Fabaceae) |
peanut shells, UAE | enzyme reaction assay, spectrophotometric method in vitro |
↓ COL activity | [154] |
|
Artemisia iwayomogi (Asteraceae) |
1% water fraction | in vivo study on 21 women volunteers | anti-wrinkle effect after using O/W cream for 8 weeks; ↓ depth of fine wrinkles on facial skin | [155] |
|
Asparagus officinalis (Asparagaceae) |
aerial parts, EE | enzyme reaction assay, spectrophotometric method in vitro |
↓ MMP-1, ELA and HYAL activity | [156] |
|
Borago officinalis (Boraginacea) |
aerial parts, ME, WME |
enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA, COL activity | [157] |
|
Bruguiera gymnorhiza (Rhizophoraceae) |
leaf, root, twig, fruit, EAE, ME | enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA activity | [158] |
|
Cannabis sativa (Cannabaceae) |
herb, WEE, MAE, UAE | enzyme reaction assay, spectrophotometric method in vitro; application analysis on 15 volunteers in vivo |
↓ COL and ELA activity; ↓ TEWL; ↑ skin moisture level |
[150] |
|
Cyclopia spp. (Fabaceae) |
leaf, branches, WE, WEE, WAE, BE | enzyme reaction assay, spectrophotometric method in vitro |
↓ COL and HYAL, weak influence on ELA activity | [61] |
|
Curculigo latifolia (Hypoxidaceae) |
root, steam, leaf, EAE, EE |
enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA activity | [159] |
|
Dimocarpus longan (Sapindaceae) |
seed extracts, PET, EAE, EE | enzyme reaction assay, spectrophotometric method in vitro |
↓ MMP-1 and HYAL activity | [160] |
|
Euphorbia characias (Euphorbiaceae) |
leaf, EE | enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA, COL and HYAL activity | [64] |
|
Hydrangea serrata (Hydrangeaceae) |
leaf, WE | in vitro, HaCaT and HDF; clinical study (22 subjects) | ↑ skin barrier components and HAS, ↓ mRNA levels of HYAL-1, -2, -3; ↑ mRNA expression of Col1a1; ↑ skin moisture level, ↓ skin wrinkles |
[119] |
|
Nelumbo nucifera (Nelumbonaceae) |
whole flower, stamen, EE | enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA, COL and HYAL activity | [161] |
| Olea europaea (Oleacea) |
leaf, WE, PPG, LA; MAE, UAE |
enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA and COL activity | [162] |
|
Plectranthus spp. (Lamiaceae) |
aerial part, WE, ME, AE, EAE | enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA and COL activity | [163] |
|
Pradosia mutisii (Sapotaceae) |
ME | in vitro, HaCaT and HDF | ↑ expression of moisturizing-related genes HAS-2, TGM-1 and Col1a1 gene | [72] |
|
Premna odorata (Verbenaceae) |
leaf, EO | enzyme reaction assay, spectrophotometric method in vitro | considerable anti-ELA and anti-HYAL and mild anti-COL potential | [164] |
|
Rosmarinus officinalis (Lamiaceae) |
leaf, HE | enzyme reaction assay, fluorometric and spectrophotometric methods in vitro |
↓ ELA, COL and HYAL activity | [74] |
|
Spatholobus suberectus (Fabaceae) |
stem, WE, EE | enzyme reaction assay, spectrophotometric method in vitro | ↓ ELA activity | [77] |
|
Thunbergia laurifolia (Acanthacea) |
leaf, EE, SE, RE | enzyme reaction assay, fluorometric and spectrophotometric methods in vitro; in vitro, 3T3 cells |
↓ MMP-1, MMP-2, -9 and HYAL activity |
[165] |
|
Vitis vinifera (Vitaceae) |
fruit, WEE | single-blind placebo-controlled in vivo study, 11 volunteers | improvement in skin moisture and elasticity after 12 weeks of applying W/O emulsion | [166] |
|
Washingtonia filifera (Arecaceae) |
pulp, seed, WE, EE, ME | in vitro, HaCaT | ↓ ELA and COL activity | [80] |
|
Warburgia salutaris (Canellacea) |
bark, WE | enzyme reaction assay, spectrophotometric method in vitro | activity against HYAL > ELA > COL | [167] |
|
Papaver rhoeas
Punica granatum Clitoria ternatea Carthamus tinctorius Gomphrena globasa |
flower, WEE | enzyme reaction assay, spectrophotometric method in vitro; application analysis on 15 volunteers in vivo |
↓ ELA and COL activity; SPF 20–31; ↓ TEWL; ↑ skin moisture level |
[168] |
|
Cannabis sativa
Foeniculum vulgare Punica granatum Vitis vinifera |
seed, EE, WEE, SFE UAE | enzyme reaction assay, spectrophotometric method in vitro |
↓ ELA and COL activity | [169] |
|
Phyllanthus emblica
Momordica cochinchinensis Centella asiatica |
leaf, fruit extract | randomized double-blind placebo-controlled in vivo study, 60 women | significant improvement in skin hydration, elasticity and wrinkles in eye and cheek areas after 60 days of emulsion application containing an extract combination | [151] |
↑, enhance/increase; ↓, inhibit/decrease; AE, aceton extract; BE, buthanol extract; COL, collagenase; Col1a1, collagen type I alpha 1; EAE, ethyl acetate extract; ELA, elastase; EE, ethanol extract; EO, essential oil; HAS, hyaluronic acid synthase; HE, hexane extract; HYAL, hyaluronidase; LA, lactic acid; MAE, magnetic-stirrer-assisted extraction; ME, methanol extract; MMP, metalloproteinases; PET, petroleum ether; PPG, polypropylene glycol; RE, reflux extraction; SE, Soxhlet extraction; SFE, supercritical fluid extraction; TGM-1, transglutaminase-1; UAE, ultrasound-assisted extraction; WE, water extract; WAE, water/aceton extract; WGE, water/glycerin extract; WEE, water/ethanol extract; WME, water/methanol extract.
5. Plants as Anti-Tyrosinase Agents
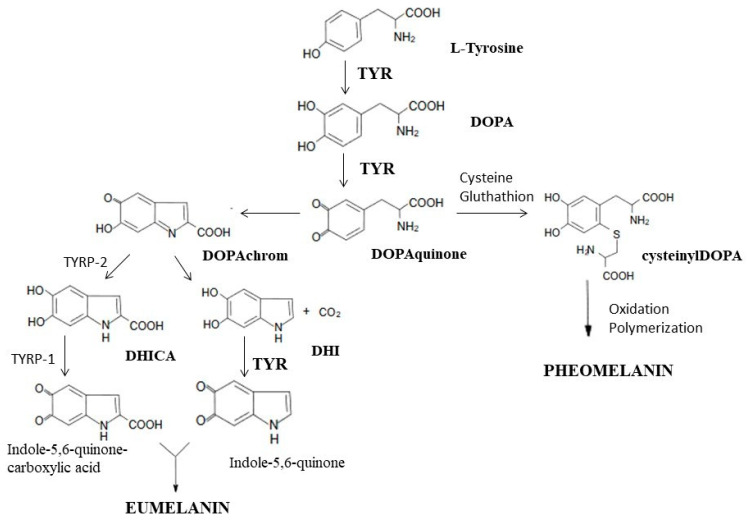
Tyrosinase is an enzyme that is widely distributed in the cells of animals, plants and microorganisms. It is a key enzyme in the biosynthesis of melanin, responsible for the catalysis of the first two synthesis reactions, i.e., the hydroxylation of tyrosine to DOPA and the oxidation of DOPA to dopaquinone. At the stage of dopaquinone formation, the eumelanin and pheomelanin pathways are separated. When thiol compounds (cysteine and glutathione) are present, they attach to dopaquinone, and the biosynthesis pathway is redirected toward pheomelanin. When the L-tyrosine concentration is low and that of cysteine is high, cysteine attaches to dopaquinone, and cysteinyldopa isomers are formed [170,171]. In the absence of thiol compounds, highly reactive dopaquinone easily undergoes intracellular cyclization, oxidation and transformation to dopachrome [170,171,172,173]. In the presence of TYRP2 (tyrosinase-related protein 2, also called dopachrome tautomerase—DCT) or metal cations (Cu2+, Zn2+, Fe2+, Co2+ or Ni2+), dopachrome may be converted to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) [170,173]. In the absence of DCT, dopachrome is converted to 5,6-dihydroxyindole (DHI) by nonenzymatic decarboxylation [170]. TYRP1 (tyrosinase-related protein 1) causes the oxidation of DHICA to indole-5,6-quinone-2-carboxylic acid, and TYR causes the oxidation of DHI to indole-5,6-quinone. The polymerization of the resulting monomers (indole and quinone) leads to the formation of eumelanin [171,173] (Figure 4).
Figure 4.
Participation of tyrosinase in the synthesis of melanins: eumelanin and pheomelanin. TYR, tyrosinase; DOPA, dihydroxyphenylalanine; TYRP2, tyrosinase-related protein 2; TYRP1, tyrosinase-related protein 1; DHICA, 5,6-dihydroxyindole-2-carboxylic acid; DHI, 5,6-dihydroksyindol (own work based on [170,172,174]).
As a metalloenzyme, tyrosinase has two copper atoms in its active site, determining its catalytic function. Substances belonging to the group of tyrosinase inhibitors inhibit melanin synthesis by interacting with copper ions in the active site of tyrosinase, thereby reducing the activity of the enzyme [175,176].
In recent years, anti-tyrosinase agents have attracted the attention of researchers searching for substances that can whiten the skin and also treat skin pigmentation disorders. Ongoing research indicates that many plant extracts and plant-derived chemicals are strong tyrosinase inhibitors and prevent the overproduction of melanin in the epidermal layers. At the same time, importantly, they inhibit melanogenesis without exerting cytotoxic or mutagenic effects on melanocytes [175,177,178,179]. Constituents of plant extracts with depigmenting properties resulting from the inhibition of tyrosinase activity include arbutin (found in, e.g., Pyrus pyrifolia peel (3.35 mg/g) [180], Origanum majorana herbs (51.3 mg/g) [181], Arctostaphylos uva-ursi leaves (6.4%) [182], Vaccinium vitis idaeae leaves (46.78 mg/g) [183] or Bergenia crassifolia leaves (22.59%) [184]), coumaric acid (present in, e.g., Artocapus altilis fruits (11.85 mg/100 g) [185,186]), ellagic acid (occurs in, e.g., Juglans regia leaves (16.25%), Castanea sativa stem bark (2.75%) or Eucalyptus camaldulensis leaves (0.28%) [187]), aloesin (isolated from the Aloe vera leaves (64 mg/L) [188]), baicalein (present in Scutellaria baicalensis roots (16.61 mg/g) [189,190]) and glabridin (found in Glycyrrhiza glabra roots (22.87 mg/g) [191]).
Table 4 presents research from the last five years on various plant species tested for anti-tyrosinase activity with potential uses in products for depigmenting or lightening the skin.
Table 4.
Selected plant species and their anti-tyrosinase properties.
| Plant Species | Family | Part of Plant | Ref |
|---|---|---|---|
| Acanthus mollis | Acanthaceae | leaves | [192] |
| Aerva lanata | Amaranthaceae | aerial parts | [153] |
| Allium galanthum | Amaryllidaceae | bulbus | [193] |
| Allium turkestanicum | |||
| Anacamptis pyramidalis | Orchidaceae | tubers | [194] |
| Anacardium occidentale | Anacardiaceae | leaves | [195] |
| Anacardium occidentale | Anacardiaceae | fruits | [196] |
| Andropogon virginicus | Poaceae | aerial parts | [197] |
| Angelica keiskei | Umbelliferae | leaves, roots | [198] |
| Arachis hypogaea | Fabaceae | peanut shell | [154] |
| Areca catechu | Palmaceae | fruits | [195] |
| Arctium minus | Asteraceae | flower heads, leaves, roots | [199] |
| Artemisia verlotiorum | Asteraceae | whole plant | [200] |
| Atractylodis macrocephalae | Asteraceae | rhizomes | [201] |
| Berberis thunbergii | Berberidaceae | leaves | [202] |
| Bergenia pacumbis | Saxifragaceae | plant and its rhizomes | [203] |
| Blepharis linariifolia | Acanthaceae | aerial parts | [204] |
| Bletilla striata | Orchidaceae | tubers, fibrous roots | [205] |
| Breynia retusa | Phyllanthaceae | leaves | [206] |
| Bridelia ferruginea | Phyllanthaceae | leaves, stem bark | [207] |
| Bruguiera gymnorhiza | Rhizophoraceae | leaves, roots, fruits | [158] |
| Cakile maritima | Brassicaceae | fruits, leaves, stems | [208] |
| Cannabis sativa | Cannabaceae | seeds | [169] |
| Carthamus tinctorius | Asteraceae | seeds | [209] |
| Celastrus hindsii | Celastracea | leaves | [210] |
| Cercis glabra | Fabaceae | leaves | [211] |
| Cladium mariscus | Cyperaceae | seeds | [212] |
| Clausena indica | Rutaceae | roots | [213] |
| Combretum micranthum | Combretaceae | leaves | [196] |
| Crotalaria burhia | Fabaceae | aerial parts, roots | [214] |
| Croton hirtus | Euphorbiaceae | aerial parts | [215] |
| Cudrania tricuspidata | Moraceae | fruits | [216] |
| Cytinus hypocistis | Cytinaceae | aerial parts | [217] |
| Dianella ensifolia | Liliaceae | roots | [218] |
| Dodonaea viscosa | Sapindaceae | stems | [219] |
| Elaeagnus angustifolia | Elaeagnaceae | fruits, leaves | [220] |
| Euphorbia hirta | Euphorbiaceae | whole plant | [196] |
| Feijoa sellowiana | Myrtaceae | leaves | [221] |
| Foeniculum vulgare | Apiaceae | seeds | [169] |
| Glochidion zeylanicum | Phyllanthaceae | leaves | [195] |
| Girardinia diversifolia | Urticaceae | shoot tips | [222] |
| Helichrysum rutilans | Asteraceae | aerial parts | [223] |
| Heliotropium procumbens | Boraginaceae | aerial parts | [224] |
| Heliotropium crispum | Boraginaceae | whole plant | [225] |
| Hibiscus tiliaceus | Malvaceae | leaves | [226] |
| Hypericum montbretii | Hypericaceae | aerial parts | [227] |
| Hypericum origanifolium | |||
| Iris pseudacorus | Iridaceae | aerial parts, rhizomes | [228] |
| Jatropha curcas | Euphorbiaceae | stems, bark, leaves | [229] |
| Jatropha gossipiifolia | |||
| Limonium effusum | Plumbaginaceae | aerial parts | [230] |
| Limonium sinuatum | |||
| Litchi chinensis | Sapindaceae | roots | [231] |
| Lonicera japonica | Caprifoliaceae | whole plant | [232] |
| Mangifera caloneura | Anacardiaceae | leaves | [195] |
| Manilkara kauki | Sapotaceae | fruits, leaves, seeds, stem bark, woods | [233] |
| Matthiola incana | Brassicacea | leaves, flower buds | [234] |
| Melastoma normale | Melastomacea | roots | [235] |
| Momordica cochinchinensis | Cucurbitacea | fruits (pulp, aril, seed) | [236] |
| Monotheca buxifolia | Sapotaceae | leaves, stems | [237] |
| Nelumbo nucifera | Nelumbonaceae | whole flower, stamen | [161] |
| Onosma bourgaei, | Boraginaceae | aerial parts | [238] |
| Onosma trachytricha | |||
| Paliurus spina-christi | Rhamnaceae | fruits, leaves, stems | [239] |
| Pistacia lentiscus | Anacardiaceae | leaves | [240] |
| Phaseolus vulgari | Fabaceae | seed coat | [241] |
| Phytolacca dioica | Phytolaccacea | fruits | [242] |
| Plectranthus ecklonii, P. namaensis, P. zuluensis | Lamiacea | aerial parts | [243] |
| Punica granatum | Punicaceae | seeds | [169] |
| Rheum palmatum | Polygonacea | roots, rhizomes | [244] |
| Rhizophora racemosa | Rhizophoraceae | leaves, stem bark | [245] |
| Rhizophora apiculata | Rhizophoraceae | leaves | [226] |
| Rhizophora mucronata | |||
| Rosa platyacantha | Rosaceae | flowers, leaves, buds | [246] |
| Rubus fraxinifolius | Rosaceae | leaves | [247] |
| Salvia chamelaeagnea, | Lamiacea | aerial parts | [243] |
| Salvia dolomitica | |||
| Sartoria hedysaroides | Fabaceae | aerial parts | [248] |
| Schisandra chinensis | Schisandraceae | fruits | [249] |
| Secamone afzelii | Asclepiadaceae | leaves | [250] |
| Streblus taxoides | Moraceae | wood | [251] |
| Strobilanthes glutinosus | Acanthaceae | whole plant | [252] |
| Tambourissa peltat | Monimiaceae | fruits, flowers, leaves | [200] |
| Vitis amurensis | Vitaceae | root | [253] |
| Vitis vinifera | Vitaceae | seeds | [169] |
| Warburgia salutaris | Canellacea | barks | [167] |
| Zingiber kerrii | Zingiberaceae | rhizomes | [254] |
| Ziziphora taurica | Lamiaceae | aerial parts | [255] |
The in vitro spectrophotometric enzyme tyrosinase inhibition assay was used to measure anti-tyrosinase activity, compared with kojic acid or β-arbutin as a reference tyrosinase inhibitor.
6. Plants as Aromatic Agents
Over the centuries, the aromatic applications of plant extracts have gained importance. Plant essential oils, considered to be those with an oil content above 0.01% of the fresh weight of the plant, are of particular importance. Some plant materials may contain even 20% essential oils (EOs) [256,257,258]. EOs are mainly obtained from plants of the Apiaceae, Asteraceae, Lamiaceae, Lauraceae, Myrtaceae, Rutaceae, Verbenaceae and Geraniaceae families [257,259] (Table 5). EOs can be found in all parts of the plant, i.e., the flowers (rose, lavender, jasmine or ylang-ylang), leaves (eucalyptus, peppermint, geranium, rosemary or tea tree), herbs (basil, hyssop and lemon balm), roots (ginger and vetiver), wood (cedarwood, camphor and sandalwood), bark (cinnamon and myrtle), seeds (anise, cumin, cardamom and fennel) and fruits (pepper, nutmeg and juniper). They are obtained from raw plant materials via distillation (water, steam or dry distillation), extraction (microwave, ultrasound, solvent extraction, maceration or enfleurage) or mechanical or cold pressing. EOs are mixtures of volatile substances, mostly colorless or light yellow, with an intense odor and an oily consistency, and they are soluble in liquid fats, alcohol, ether or chloroform. The biological activity and fragrance of EOs are determined according to their chemical composition. Their composition depends on numerous factors, including the origin of the plant materials or the conditions of plant growth. EOs are not chemically homogeneous. They may contain up to several hundred chemical compounds, including terpene hydrocarbons and their oxygen derivatives, alcohols, aldehydes, ketones, organic acids, esters and ethers [256,257,259,260]. Some compounds of EOs have a characteristic aroma, e.g., bisabolol, with a sweet floral odor; geraniol, with a fresh, sweet and rose-like odor; linalyl acetate, with a floral, sweet citrus odor; citronellol, with a strong floral, rose-like and sweet odor; limonene, with a strong orange odor; linalool with a floral, grassy, pleasant and citrus odor; myrcene, with a pleasant floral odor; terpineol, with a sweet, lilac odor; α-pinene, with a fresh, camphor, sweet and pine odor; or β-phellandrene, with a mint, turpentine odor [260].
Table 5.
Selected plants with identified essential oil compounds and a description of their aroma.
| Family | Aromatic Plants | Extraction of EO | Single Constituent | Aroma Description | Ref. |
|---|---|---|---|---|---|
| Lamiaceae |
Lavandula
officinalis |
hydrodistillation of air-dried flowers | linalool, linalyl acetate, geraniol, β-caryophyllene, lavandulyl acetate | fresh, herbaceous, floral | [261] |
|
Origanum
vulgare |
hydrodistillation of air-dried aerial parts | carvacrol, γ-terpinene, p-cymene, trans-sabinene hydrate, thymol | warm, spicy, camphoraceous | [262] | |
|
Thymus
vulgaris |
hydrodistillation of shade-dried flowers and leaves | thymol, γ-terpinene, p-cymene, linalool, myrcene, α-pinene, α-thujene | strong, spicy, herbaceous | [263] | |
|
Mentha
piperita |
hydrodistillation of shade-dried aerial parts | camphane, menthone, menthol, β-pinene, pulegone, β-cubebene, α-pinene, γ-terpinene, γ-carane, piperiton | fresh, sweetish, menthol | [264] | |
|
Hyssopus
officinalis |
steam distillation, simple hydrodistillation and hydrodistillation in Dean–Stark apparatus of air-dried flowering aerial parts | elemol, spathulenol, α-eudesmol, γ-eudesmol, virdiflorol, hedycaryol, isopinocamphone, cis-jasmone. |
fresh, herbal, slightly sweet, camphorous | [265] | |
| Apiaceae |
Pimpinella
anisum |
hydrodistillationof mature fruits | trans-anethole, γ-himachalene, trans-pseudoisoeugenyl 2-methylbutyrate, cis-dihydrocarvone, methyl chavicol, α-himachalen, β-himachalene | fresh, warm, sweet, mildly pungent | [266] |
|
Carum
carvi |
hydrodistillation and microwave-assisted hydrodistillation of air-dried seeds |
carvone, limonene, apiole, andrographolide, aromadendrene, β-cadinene, friedelanol, barrigenol, 3-benzyloxyphenol |
pungent, anise-like, herbaceous | [267] | |
| Rutaceae |
Citrus
limon |
hydrodistillation of peels | limonene, α-citral, β-pinene, α-terpinene, β-elemene, neryl acetate |
sharp, lemon, sweet | [268] |
|
Citrus
paradisi |
molecular distillation from cold-pressed fruits | limonene, β-myrcene, α-pinene, sabinene (0.60%), carvone (0.41%), cis-limonene oxide (0.43%), and trans-limonene oxide (0.33%), caryophyllene (0.20%), β-cubebene (0.14%), α-copaene (0.13%), | fresh, sharp, citrus | [269] | |
| Verbenaceae |
Verbena
officinalis |
steam distillation of leaves | limonene, 1,8-cineole, ar-curcumeme, caryophyllene oxide, spathulenol | lemony scent with sweet, fruity undertones | [270] |
| Lauraceae | Cinnamomum verum | hydrodistillation of shade-dried leaves | eugenol, linalol, benzyl benzoate, | sweet, spicy, slightly woody, clove-like | [271] |
| Asteraceae |
Anthemis
nobilis |
hydrodistillation of shade-dried flowers | en-yn-dicycloether, β-caryophyllene, aristolene epoxide, germacrene D, widdrol, cis-caryophyllene |
crisp, sweet, herbal, floral, soft fruity (reminiscent of apples) | [272] |
| Myrtaceae |
Eucalyptus
globulus |
steam distillation of dried leaves | eucalyptol, α-pinene, p-cymene, β- myrcene, terpinen-4-ol, γ-terpinene |
fresh, camphoraceous, medicinal | [273] |
|
Melaleuca
alternifolia |
steam distillation of young branches and leaves | terpinen-4-ol,-terpinene, 1,8-cineole, p-cymene | fresh, camphoraceous | [274] | |
|
Syzygium
aromaticum |
supercritical fluid extraction assisted by cold pressing buds | eugenol, eugenyl acetate, β- caryophyllene, α-humulene |
clove, strong | [275] | |
| Geraniaceae |
Pelargonium
graveolens |
hydrodistillation of fully grown aerial parts | citronellol, geraniol, caryophyllene oxide, menthone, linalool, β-bourbonene, iso-menthone, geranyl formate | floral, sweet, rose-like with minty undertones | [276] |
Cosmetic aromatherapy utilizes EOs for skin, body, face and hair products. EOs are added to skincare and bath cosmetics or massage preparations as substances providing fragrance and as active ingredients. Smell is an important criterion in purchasing cosmetic products. A wide range of essential oils is available, and their marketing potential is enormous. Fragrance composition is an important element of the formulation of new cosmetic preparations. Fragrances also play an important role in masking unpleasant aromas from fatty acids, oils and surfactants used in cosmetic formulations [256,258,260].
EOs and their constituents, in addition to their aromatic effects, are also used in modern cosmetics and dermocosmetics as absorption promoters and preservatives [258]. The absorption of active substances by the skin can also be increased by EOs, such as eucalyptus, peppermint or terpentine oil, as well as by components of essential oils, such as menthol, limonene, carvacrol, linalool, α-pinene or terpineol [258,259]. Due to their antimicrobial action, EOs can act as natural preservatives to prolong the durability of cosmetics, e.g., essential oils from lavender (Lavandula angustifolia) [261], thyme (Thymus vulgaris) [263], peppermint (Mentha piperita) [264], cajuput (Melaleuca cajuputi), cinnamon (Cinnamomum zeylanicum) [271], clove (Syzygium aromaticum) [275], eucalyptus (Eucalyptus globulus) [273], sage (Salvia officinalis) [277] and tea tree (Melaleuca alternifolia) [274]. EO constituents performing this function include phenols, aldehydes, alcohols, ketones and esters [258,259].
The use of EOs may have side effects, such as allergic reactions, irritation or temporary sensitivity to UV radiation. An allergic reaction or skin irritation may occur following the use of cinnamon, clove or lemon grass oil, and oils with a photosensitizing effect include citrus oils (e.g., bergamot, lime, bitter orange, lemon or grapefruit), as well as EOs present in angelica root (Angelica archangelica), rue (Ruta graveolens), parsley leaf (Petroselinum crispum) and marigold (Tagetes minuta). Constituents of EOs that may trigger allergic reactions include benzyl alcohol, cinnamyl alcohol, eugenol, hydroxycitronellal, isoeugenol, benzyl salicylate, cinnamaldehyde, coumarin, geraniol, anisyl alcohol, benzyl cinnamate, farnesol, linalol, benzyl benzoate, citronellol or limonene [258,259,260]. EO safety in the cosmetic industry is monitored in a variety of ways, e.g., by the International Fragrance Association (IFRA) and the International Organization for Standardization (ISO) [260].
7. Plants as Colorants and Dye Agents
The history of the human use of pigments dates back to prehistoric times. Dye plants that are known to have been used in various periods include dyer’s madder (Rubia tinctorum), true indigo (Indigofera tinctoria), dyer’s woad (Isatis tinctoria), dyer’s weed (Reseda luteola) and logwood (Haematoxylum campechianum) [278]. Dyes that are currently used in cosmetics were once used in various branches of industry. It is believed that dyes were originally used for ornamental purposes. In ancient Egypt, mainly the skin and hair were dyed, e.g., using henna (a pigment obtained from the shrub Lawsonia inermis). In modern cosmetology, plant pigments are added to cosmetic products to give them an aesthetic appearance. Like aroma, color plays an important role in marketing cosmetics and pharmaceutical products [278,279,280]. In addition, colorants and dyes are used as beauty enhancers, masking imperfections or correcting minor skin defects. Apart from color cosmetics (e.g., fluids, lip pencils, lipstick, rouge or eyeshadow), plant pigments are also a component of skin care cosmetics with protective and antioxidant properties, with the ability to strengthen blood vessels and improve the condition of skin [281,282].
Plant dyes, which are varied in terms of chemical structure, are a group of compounds that are present in plant parts such as flowers, fruits and leaves. Plant pigments include quinones, polyphenols, chlorophylls, carotenoids and betalains [279,281,282,283,284] (Table 6).
Quinones are compounds whose color ranges from yellow to orange to red to brown. Quinones, which include benzoquinones, naphthoquinones and anthraquinones, are a large group of pigments. Anthraquinones are anthracene derivatives that are widespread in the plant world. They can be found among plants of the Polygonaceae, Rubiaceae, Rhamnaceae, Scrophulariaceae, Liliaceae, Hypericaceae and Fabaceae families. In traditional dyeing, hypericin, a red dye obtained from St John’s wort (Hypericum perforatum), was used as well. Natural fibers were also dyed using rhamnotoxin—a red pigment obtained from the bark of alder buckthorn—as well as with alkannin, from the rhizomes and roots of dyer’s alkanet (Alkanna tinctoria). This dye has been used since ancient times in color cosmetics, such as lipsticks. Another source of alkannin, which is a naphthoquinone derivative, is the root of common bugloss (Anchusa officinalis) [279,281,282].
A wealth of flavonoids can be found in plants of the Apiaceae, Asteraceae, Betulaceae, Polygonaceae, Brassicaceae, Ericaceae, Fabaceae, Hypericaceae, Primulaceae, Lamiaceae, Rosaceae, Rubiaceae, Rutaceae and Scrophulariaceae families. Apart from their role in skin care, flavonoids are used in cosmetics as natural plant dyes, including flavonols (intense yellow), flavones (light yellow and cream-colored), chalcones (light yellow) and aurones (intense yellow) [279,281,282].
Anthocyanins are widespread plant dyes, the most common of which include red pelargonidin (geranium and dahlia), blue-to-red peonidin (elderberry and peony) and cyanidin (cornflower, chokeberry, cranberry and cherry), purple malvidin (mallow and grapes), petunidin (petunia) and delphinidin (grape, elderberry and cranberry). Tannins are broadly distributed in the plant kingdom and are generally classified into two types: hydrolysable tannins (e.g., gallotannins and ellagitannins) and condensed tannins (catechins and leucoanthocyanidins). Plants supplying brown, gray or sometimes rust-colored tannin dyes include the species Uncaria gambir, Galla chinensis (Chinese gallnut), Acacia catechu, Schinopsis balansae, Pteropcarpus marspinum, Eucalyptus rostrata, Quercus infectoria, Quercus robur, Quercus sessilis, Potentilla erecta, Alchemilla vulgaris, Sanguisorba officinalis and Polygonum bistorta [279,281,282,285].
Chlorophylls are a pigment that is present in all green plants (in the stems, leaves, flowers, fruits or seeds), e.g., Urtica dioica, Medicago sativa, spinach, lettuce and broccoli. Among the known plant chlorophylls, two have significance as dyes: chlorophyll a (blue-green) and chlorophyll b (yellow-green). Chemically, chlorophyll is an ester (magnesium porphyrin composed of four pyrrole rings) with two alcohols (phytol and methanol) [280,282].
Carotenoids are polyene dyes, i.e., they have a conjugated double-bond system. Plant sources of carotenoids include Crocus sativus, from which the stigma, containing the yellow carotenoid pigment crocin, is used; Bixa orellana, whose fruits supply the yellow-orange carotenoid pigment bixin (annato, orlean); and Calendula officinalis, whose flowers contain α- and β-carotene, lutein, lycopene and violaxanthin [281,282,286].
Betalains are found in plants of the order Caryophyllales. Sources of betalain pigments include beet root (Beta vulgaris), the fruits of the prickly pear (Opuntia ficus-indica) or cacti of the Hylocereus genus and the flowers of numerous species of the Amaranthaceae family [281,282].
Table 6.
Classification of natural colorants according to chemical functional groups (structure) [279,282,287].
| Chemical Class | Example of Class | Source | Color Produced | |
|---|---|---|---|---|
| Quinones | benzoquinone | 1,4-benzoquinone | Pyrus lindleyi | brown |
| anthraquinones | alizarin | Rubia tinctiorum | red | |
| napthoqinones | lawsone (2-hydroxy-1,4-naphthoquinone) |
Lawsonia inermis | brown, purple grey and shades of orange |
|
| juglone (5-hydroxy-1,4-naphthoquinon) |
Juglans regia | |||
| Polyphenols | flavones | luteolin | Reseda luteola | yellow and brown |
| apigenin | Chamomilla recutita | |||
| chrysin | Passiflora incarnata | |||
| anthocyanins | protocyanins | Centaurea cyanus | red, violet or blue (depending on pH) | |
| malvidin, peonidin, delphinidin | Althaea rosea | |||
| 3-delphinidin sambubioside (hibiscin), 3-cyanidin sambubioside, 3-delphinidin glucoside | Hibiscus sabdariffa | |||
| 3-cyanidin glucoside (chrysanthemum), 3-cyanidin sambubioside |
Sambucus nigra
fructus |
|||
| Betalains | betacyanins | betanin |
Beta vulgaris
Amaranthus cruentus Opuntia ficus-indica |
red and purple |
| betaxanthins | vulgaxanthin I and II, indicaxanthin |
Hylocereus polyrhizus, Opuntia ficus-indica, Beta vulgaris |
yellow and orange | |
| Carotenoids | carotenes | α-, β-, γ-carotene, lycopene |
Daucus carota, Solanum lycopersicum, Sorbus aucuparia |
orange, red and yellow |
| xanthophylls | lutein, zeaxanthin, violaxanthin | Spinacia oleracea, Zea mays, Tagetes erecta |
Natural colorants and dyes of plant origin have the important advantages of being nontoxic, safe, without side effects, non-carcinogenic, environmentally friendly (biodegradable and compatible with the environment) and economical. For these reasons, they are becoming an object of consumer interest with broad applications in the cosmetic industry. Plant dyes can be an alternative to synthetic dyes, which involve the use of petrochemical-based materials, and due to their allergic, toxic, mutagenic, genotoxic and carcinogenic effects, they are responsible for various health and skin problems [280,283,287].
8. Future Perspectives and Challenges
In the European Union, before cosmetic products can be sold to customers, they must be evaluated for safety in accordance with Regulation (EC) No. 1223/2009 of the European Parliament and of the Council, and in the United States, the safety of cosmetics is regulated by the Food and Drug Administration (FDA), mainly through the Federal Food, Drug, and Cosmetics Act (FD&C Act) and the Fair Packaging and Labeling Act (FPLA). The global cosmetics industry (encompassing products for the face, eyes, hair, nails, mouth and body, which may be used externally for cleansing, beautifying or altering one’s appearance) is continually growing, together with consumer awareness regarding health care, including hygiene and skin care [288]. Among the entire range of cosmetics, plant-based products have seen tremendous growth of about 15–20 per cent over the past five years. This review presents a wide assortment of plants with various applications in cosmetic preparations that have been reported in the last five years. It is also important to consider certain aspects of the use of plants and bioactive compounds of plant origin in cosmetics and the associated challenges.
First, attention should be paid to the ability of active ingredients of natural origin to penetrate the first skin barrier, as the bioavailability of bioactive compounds is an important factor determining their effectiveness. One promising solution for the future is the development of delivery systems for bioactive ingredients that facilitate penetration, through improved encapsulation and targeted delivery. A related issue is the fact that the effects of these agents have not been conclusively demonstrated in all cases. For example, although some natural agents appear to have promising sun-protection effects, when they are added to sunscreens, this effect has been shown to be poor and to ensure only a modest or low increase in SPF (e.g., lycopene [289] and Cucumis sativus extract [290]). Therefore, in vitro research into the biological activity of plants must also be supported by in vivo studies. Even when preliminary studies show promising effects, confirmation in clinical trials is needed.
Second, it is important to consider the mechanism of action and the safety of plant-derived bioactive ingredients. A good example is bergamot oil. The use of methoxypsoralens from the Citrus bergamia essential oil following sun exposure has been shown to increase photosensitivity, causing further damage rather than providing photoprotection, despite its stimulating effect on tyrosinase activity [291]. Other adverse effects, such as acute toxicity, skin and eye irritation or skin sensitization, may occur following the topical application of materials of plant origin. This is why it is essential to conduct research not only on the effectiveness of these substances but on their safety as well, prior to including them in a cosmetic formulation.
In addition, discussions about ingredients of plant origin and their biological activity should take into account their chemical structure. One example is the role of flavonoids and their effect on melanogenesis in relation to the chemical structure of this complex group of compounds. For example, hesperetin [292] and genistein [293] have been shown to stimulate melanogenesis, whereas compounds such as epicatechin (EGCG) [294] or baicalein [189] act as inhibitors of melanin formation. It is interesting to compare the two structurally similar compounds apigenin and luteolin. One additional hydroxyl group in luteolin results in different cellular functions: apigenin stimulates melanin synthesis [295], whereas luteolin inhibits it [296]. This suggests that the characteristic chemical structure of individual bioactive compounds leads to differences in how they regulate melanogenesis. Conflicting reports in the scientific literature regarding quercetin may also be puzzling, as some data suggest that it stimulates melanogenesis [297], whereas other data indicate an inhibitory effect against melanogenesis [298]. This demonstrates that there is still a need for in-depth research leading to a better understanding of these plant-derived molecules.
Another important consideration is how the plant material to be used as a cosmetic component is obtained (e.g., the extraction/separation technique, temperature or type of solvent used). Some of the active compounds present in plants (e.g., polyphenols, essential oils or vitamins) have low stability, and their sensitivity to light and heat limits their use in cosmetics. Research in this area is aimed at the development of more stable derivatives or the encapsulation of active substances in liposomes, which protects them from degradation.
The implementation of new solutions for obtaining and preparing plant-derived materials and including them in a cosmetic product is associated with the issue of intellectual property. The mechanisms of the legal protection of innovations, such as patents, are also worthy of attention. Naturally, not all research results can be patented. In the context of plant-based cosmetic materials, no plant or substance extracted from it can be protected by the patent system; however, a complex or mixture of plant extracts or isolated molecules, if it meets the criteria of novelty, inventive activity and industrial application, is patentable [299,300]. Patents involving pharmaceutical and cosmetic applications may refer to the ingredients, formulation, product type, use of pharmaceutical carrier systems or cosmetic production/manufacturing methods [300]. In patents filed in the National Institute of Industrial Property (INPI), types of applications of plant extracts in cosmetics include multifunctional product innovation (e.g., the use of a plant extracts for the treatment of gynoid lipodystrophy and acne), extraction processes used to isolate active ingredients with potential applications in cosmetics and the use of extracts with anti-aging, skin/hair pigmentation and conditioning or photoprotection properties [299,301]. An analysis of patents related to cosmetics containing plant ingredients reveals a high proportion of innovations involving the use of species from the Fabaceae, Asteraceae, Rosaceae, Lamiaceae, Poaceae, Rutaceae, Lilliacae and Apiaceae families [301]. Examples of plants described in patents for cosmetic applications include Pothomorphe umbellata root extract for anti-aging activity and the treatment of cell damage caused by exposure to UV rays; Glycyrrhiza glabra and Shophora flavecens roots for the treatment of skin hyperpigmentation; the Artemisia plant species for whitening the skin and delaying aging; or the Pueraria plant species for rejuvenation, lightening the skin and treating skin inflammation [299,301].
9. Conclusions
Plants and their constituents can be used to maintain the physiological balance of human skin. Ongoing research provides valuable information on the chemical composition and pharmacological properties of botanicals. Moreover, studies have confirmed their effectiveness and have demonstrated new potential applications of plant materials in products for topical use as skin care and therapeutic agents with multifaceted effects. Natural products of plant origin can be used as a safe and efficacious alternative to synthetic products. This is reflected in growing consumer interest in natural cosmetics and the market trend expressed by the development and increasing number of products based on plant-derived ingredients.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This project was financed under the program of the Minister of Education and Science ‘Regional Initiative of Excellence’ in the years 2019–2023, project no. 024/RID/2018/19, with an amount of funding of 11,999,000.00 PLN.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Michalak M., Pierzak M., Kręcisz B., Suliga E. Bioactive Compounds for Skin Health: A Review. Nutrients. 2021;13:203. doi: 10.3390/nu13010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalak M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022;23:585. doi: 10.3390/ijms23020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menaa F. Skin anti-aging benefits of phytoterapeutics-based emulsions. Pharm. Anal. Acta. 2014;5:168. doi: 10.4172/2153-2435.1000e168. [DOI] [Google Scholar]
- 4.Jadoon S., Karim S., Bin Asad M.H., Akram M.R., Khan A.K., Malik A., Chen C., Murtaza G. Anti-Aging Potential of Phytoextract Loaded-Pharmaceutical Creams for Human Skin Cell Longetivity. Oxid. Med. Cell Longev. 2015;2015:709628. doi: 10.1155/2015/709628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clydesdale G.J., Dandie G.W., Muller H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 6.Brink N., Szamel M., Young A.R., Wittern K.P., Bergemann J. Comparative quantification of IL-1beta, IL-10, IL-10r, TNF-alpha and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm. Res. 2000;49:290–296. doi: 10.1007/PL00000209. [DOI] [PubMed] [Google Scholar]
- 7.Rundhaug J.E., Fischer S.M. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem. Photobiol. 2008;84:322–329. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 8.Muller-Decker K. Cyclooxygenase-dependent signaling is causally linked to non-melanoma skin carcinogenesis: Pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011;30:343–361. doi: 10.1007/s10555-011-9306-z. [DOI] [PubMed] [Google Scholar]
- 9.de Gruijl F.R., van Kranen H.J., Mullenders L.H. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J. Photochem. Photobiol. B. 2001;63:19–27. doi: 10.1016/S1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 10.He Y.-Y., Huang J.-L., Chignell C.F. Delayed and Sustained Activation of Extracellular Signal-regulated Kinase in Human Keratinocytes by UVA: Implications in carcinogenesis. J. Biol. Chem. 2004;279:53867–53874. doi: 10.1074/jbc.M405781200. [DOI] [PubMed] [Google Scholar]
- 11.Muthusamy V., Piva T.J. The UV response of the skin: A review of the MAPK, NFκB and TNF signal transduction pathways. Arch. Dermatol. Res. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- 12.Meng X., Qiu L., Song H., Dang N. MAPK pathway involved in epidermal terminal differentiation of normal human epidermal keratinocytes. Open Med. 2018;13:189–195. doi: 10.1515/med-2018-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chouinard N., Valerie K., Rouabhia M., Huot J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem. J. 2002;365:133–145. doi: 10.1042/BJ20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C.H., Wu S.B., Hong C.H., Yu H.S., Wei Y.H. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: The implication in UV-based phototherapy. Int. J. Mol. Sci. 2013;14:6414–6643. doi: 10.3390/ijms14036414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solano F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules. 2020;25:1537. doi: 10.3390/molecules25071537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skarupova D., Vostalova J., Rajnochova Svobodova A. Ultraviolet A protective potential of plant extracts and phytochemicals. Biomed. Pap. Med. Fac. Univ. Palacký Olomouc Czechoslov. 2020;164:1–22. doi: 10.5507/bp.2020.010. [DOI] [PubMed] [Google Scholar]
- 17.Torres-Contreras A.M., Garcia-Baeza A., Vidal-Limon H.R., Balderas-Renteria I., Ramírez-Cabrera M.A., Ramirez-Estrada K. Plant Secondary Metabolites against Skin Photodamage: Mexican Plants, a Potential Source of UV-Radiation Protectant Molecules. Plants. 2022;11:220. doi: 10.3390/plants11020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park K., Choi H.S., Hong Y.H., Jung E.Y., Suh H.J. Cactus cladodes (Opuntia humifusa) extract minimizes the effects of UV irradiation on keratinocytes and hairless mice. Pharm. Biol. 2017;55:1032–1040. doi: 10.1080/13880209.2017.1286357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobi S.E., Gilbert M., Paul N., McMillan T.J. The green tea polyphenol, epi gallocatechin-3-gallate, protects against the oxidative cellular and genotoxic damage of UVA radiation. Int. J. Cancer. 2002;102:439–444. doi: 10.1002/ijc.10730. [DOI] [PubMed] [Google Scholar]
- 20.Afaq F., Zaid M.A., Khan N., Dreher M., Mukhtar H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009;18:553–561. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Napagoda M.T., Malkanthi B.M.A.S., Abayawardana S.A.K., Qader M.M., Jayasinghe L. Photoprotective potential in some medicinal plants used to treat skin diseases in Sri Lanka. BMC Complement. Altern. Med. 2016;16:479. doi: 10.1186/s12906-016-1455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svobodová A., Zdařilová A., Walterová D., Vostálová J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J. Dermatol. Sci. 2007;48:213–224. doi: 10.1016/j.jdermsci.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Alonso-Lebrero J.L., Domínguez-Jiménez C., Tejedor R., Brieva A., Pivel J.P. Photoprotective properties of a hydrophilic extract of the fern Polypodium leucotomos on human skin cells. J. Photochem. Photobiol. B. 2003;70:31–37. doi: 10.1016/S1011-1344(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 24.Calò R., Marabini L. Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a human keratinocyte cell line (HaCaT cells) J. Photochem. Photobiol. B. 2014;132:27–35. doi: 10.1016/j.jphotobiol.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Svobodová A., Zdařilová A., Vostálová J. Lonicera caerulea and Vaccinium myrtillus fruit polyphenols protect HaCaT keratinocytes against UVB-induced phototoxic stress and DNA damage. J. Dermatol. Sci. 2009;56:196–204. doi: 10.1016/j.jdermsci.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z., Park S.Y., Hwang E., Zhang M., Seo S.A., Lin P., Yi T.H. Thymus vul garis alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE antioxidant system. J. Cell Mol. Med. 2017;21:336–438. doi: 10.1111/jcmm.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petruk G., Di Lorenzo F., Imbimbo P., Silipo A., Bonina A., Rizza L., Piccoli R., Monti D.M., Lanzetta R. Protective effect of Opuntia ficus-indica L. cladodes against UVA-induced oxidative stress in normal human keratinocytes. Bioorg. Med. Chem. Lett. 2017;27:5485–5489. doi: 10.1016/j.bmcl.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 28.West B.J., Deng S., Palu A.K., Jensen C.J. Morinda citrifolia Linn. (Rubiaceae) leaf extracts mitigate UVB-induced erythema. J. Nat. Med. 2009;63:351–354. doi: 10.1007/s11418-009-0327-7. [DOI] [PubMed] [Google Scholar]
- 29.Bazylko A., Borzym J., Parzonko A. Determination of in vitro antioxidant and UV-protecting activity of aqueous and ethanolic extracts from Galinsoga parviflora and Galinsoga quadriradiata herb. J. Photochem. Photobiol. B. 2015;149:189–195. doi: 10.1016/j.jphotobiol.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Cho Y.-H., Bahuguna A., Kim H.-H., Kim D.-I., Kim H.-J., Yu J.-M., Jung H.-G., Jang J.-Y., Kwak J.-H., Park G.-H., et al. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells. J. Photochem. Photobiol. B. 2017;174:323–332. doi: 10.1016/j.jphotobiol.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Bulla M.K., Hernandes L., Baesso M.L., Nogueira A.C., Bento A.C., Bortoluzzi B.B., Serra L.Z., Cortez D.A.G. Evaluation of photoprotective potential and percutaneous penetration by photoacoustic spectroscopy of the Schinus terebinthifolius raddi extract. Photochem. Photobiol. 2015;91:558–566. doi: 10.1111/php.12419. [DOI] [PubMed] [Google Scholar]
- 32.de Lima-Saraiva S.R.G., da Silva Oliveira F.G., de Oliveira Junior R.G., de Souza Araújo C., de Oliveira A.P., Pacheco A.G.M., Rolim L.A., de Amorim E.L.C., César F.C.S., da Silva Almeida J.R.G. Chemical analysis and evaluation of antioxidant, antimicrobial, and photoprotective activities of Schinopsis brasiliensis Engl. (Anacardiaceae) Sci. World J. 2017;2017:1713921. doi: 10.1155/2017/1713921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebrahimzadeh M., Enayatifard R., Khalili M., Ghaffarloo M., Saeedi M., Charati J.Y. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran. J. Pharm. Res. 2014;13:1041–1047. [PMC free article] [PubMed] [Google Scholar]
- 34.Jarzycka A., Lewińska A., Gancarz R., Wilk K.A. Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J. Photochem. Photobiol. B. 2013;128:50–57. doi: 10.1016/j.jphotobiol.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Lefahal M., Zaabat N., Ayad R., Makhloufi E.H., Djarri L., Benahmed M., Laouer H., Nieto G., Akkal S. In vitro assessment of total phenolic and flavonoid contents, antioxidant and photoprotective activities of crude methanolic extract of aerial parts of Capnophyllum peregrinum (L.) Lange (Apiaceae) growing in Algeria. Medicines. 2018;5:26. doi: 10.3390/medicines5020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins F.J., Caneschi C.A., Vieira J.L.F., Barbosa W., Raposo N.R.B. Antioxidant activity and potential photoprotective from amazon native flora extracts. J. Photochem. Photobiol. B. 2016;161:34–39. doi: 10.1016/j.jphotobiol.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Mejía-Giraldo J.C., Winkler R., Gallardo C., Sánchez-Zapata A.M., Puertas-Mejía M.A. Photoprotective potential of Baccharis antioquensis (Asteraceae) as natural sunscreen. Photochem. Photobiol. 2016;92:742–752. doi: 10.1111/php.12619. [DOI] [PubMed] [Google Scholar]
- 38.Muzaffer U., Paul V.I., Prasad N.R., Karthikeyan R., Agilan B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine. 2018;42:100–111. doi: 10.1016/j.phymed.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Nunes A.R., Rodrigues A.L.M., de Queiróz D.B., Vieira I.G.P., Neto J.F.C., Junior J.T.C., Tintino S.R., de Morais S.M., Coutinho H.D.M. Photoprotective potential of medicinal plants from Cerrado biome (Brazil) in relation to phenolic content and antioxidant activity. J. Photochem. Photobiol. B. 2018;189:119–123. doi: 10.1016/j.jphotobiol.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 40.He H., Li A., Li S., Tang J., Li L., Xiong L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF) Biomed. Pharmacother. 2021;134:111161. doi: 10.1016/j.biopha.2020.111161. [DOI] [PubMed] [Google Scholar]
- 41.Lekmine S., Boussekine S., Akkal S., Martín-García A.I., Boumegoura A., Kadi K., Djeghim H., Mekersi N., Bendjedid S., Bensouici C., et al. Investigation of Photoprotective, Anti-Inflammatory, Antioxidant Capacities and LC–ESI–MS Phenolic Profile of Astragalus gombiformis Pomel. Foods. 2021;10:1937. doi: 10.3390/foods10081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quintero-Rincón P., Mesa-Arango A.C., Flórez-Acosta O.A., Zapata-Zapata C., Stashenko E.E., Pino-Benítez N. Exploring the Potential of Extracts from Sloanea medusula and S. calva: Formulating Two Skincare Gels with Antioxidant, Sun Protective Factor, and Anti-Candida albicans Activities. Pharmaceuticals. 2023;16:990. doi: 10.3390/ph16070990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vijayakumar R., Salwa Abd Gani S., Hasanah Zaidan U., Effendi Halmi I., Karunakaran T., Razak Hamdan M. Exploring the Potential Use of Hylocereus polyrhizus Peels as a Source of Cosmeceutical Sunscreen Agent for Its Antioxidant and Photoprotective Properties. Evid.-Based Complement. Altern. Med. 2020;2020:7520736. doi: 10.1155/2020/7520736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuentes J.L., Pedraza Barrera C.A., Villamizar Mantilla D.A., Flórez González S.J., Sierra L.J., Ocazionez R.E., Stashenko E.E. Flower Extracts from Ornamental Plants as Sources of Sunscreen Ingredients: Determination by In Vitro Methods of Photoprotective Efficacy, Antigenotoxicity and Safety. Molecules. 2022;27:5525. doi: 10.3390/molecules27175525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandes A.S., Mazzei J.L., Oliveira C.G., Evangelista H., Marques M.R.C., Ferraz E.R.A., Felzenszwalb I. Protection against UV-induced toxicity and lack of mutagenicity of Antarctic Sanionia uncinata. Toxicology. 2017;376:126–136. doi: 10.1016/j.tox.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Fernandes Ada S., Santos Alencar A., Evangelista H., Luiz Mazzei J., Felzenszwalb J. Photoprotective and toxicological activities of extract from the Antarctic moss Sanionia uncinata. Pharmacogn. Mag. 2015;11:38–43. doi: 10.4103/0973-1296.149701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hubner A., Sobreira F., Vetore Neto A., Pinto C.A.S.d.O., Dario M.F., Díaz I.E.C., Lourenço F.R., Rosado C., Baby A.R., Bacchi E.M. The synergistic behavior of antioxidant phenolic compounds obtained from Winemaking waste’s valorization, increased the efficacy of a sunscreen system. Antioxidants. 2019;8:530. doi: 10.3390/antiox8110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mota M.D., da Boa Morte A.N., Silva L.C.R.C., Chinalia F.A. Sunscreen protection factor enhancement through supplementation with Rambutan (Nephelium lappaceum L.) ethanolic extract. J. Photochem. Photobiol. B. 2020;205:111837. doi: 10.1016/j.jphotobiol.2020.111837. [DOI] [PubMed] [Google Scholar]
- 49.Mota M.D., Costa R.Y.S., Guedes A.A.S., Silva L.C.R.C., Chinalia F.A. Guava-fruit extract can improve the UV-protection efficiency of synthetic filters in sun cream formulations. J. Photochem. Photobiol. B. 2019;201:111639. doi: 10.1016/j.jphotobiol.2019.111639. [DOI] [PubMed] [Google Scholar]
- 50.Catelan T.B.S., Gaiola L., Ferreira Duarte B., Lima Cardoso C.A. Evaluation of the in vitro photoprotective potential of ethanolic extracts of four species of the genus Campomanesia. J. Photochem. Photobiol. B. 2019;197:111500. doi: 10.1016/j.jphotobiol.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Fernandes A.S., Mazzei J.L., Evangelista H., Marques M.R.C., Ferraz E.R.A., Felzenszwalb I. Protection against UV-induced oxidative stress and DNA damage by Amazon moss extracts. J. Photochem. Photobiol. B. 2018;183:331–341. doi: 10.1016/j.jphotobiol.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 52.Ahn H.S., Kim H.J., Na C., Jang D.S., Shin Y.K., Lee S.H. The protective effect of Adenocaulon himalaicum Edgew. and its bioactive compound neochlorogenic acid againstUVB-induced skin damage in human dermal fibroblasts and epidermal keratinocytes. Plants. 2021;10:1669. doi: 10.3390/plants10081669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung J.M., Kwon O.Y., Choi J.K., Lee S.H. Alpinia officinarum Rhizome ameliorates the UVB induced photoaging through attenuating the phosphorylation of AKT and ERK. BMC Complement. Med. Ther. 2022;22:232. doi: 10.1186/s12906-022-03707-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Natewong S., Niwaspragrit C., Ratanachamnong P., Samatiwat P., Namchaiw P., Jaisin Y. Photo-Protective and Anti-Inflammatory Effects of Antidesma thwaitesianum Müll. Arg. Fruit Extract against UVB-Induced Keratinocyte Cell Damage. Molecules. 2022;27:5034. doi: 10.3390/molecules27155034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seregheti T.M.Q., Pinto A.P.R., Gonçalves M.C., Antunes A.S., Almeida W.A.S., Machado R.S., Silva J.N., Ferreira P.M.P., Pessoa C., Dos Santos V.M.R., et al. Antiproliferative and photoprotective activities of the extracts and compounds from Calea fruticosa. Braz. J. Med. Biol. Res. 2020;53:e9375. doi: 10.1590/1414-431x20209375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park S., Eun-Soo Lee E.-S., Park N.-H., Hwang K., Cho E.-G. Circadian Expression of TIMP3 Is Disrupted by UVB Irradiation and Recovered by Green Tea Extracts. Int. J. Mol. Sci. 2019;20:862. doi: 10.3390/ijms20040862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song C., Lorz L.R., Lee J., Cho J.Y. In Vitro Photoprotective, Anti-Inflammatory, Moisturizing, and Antimelanogenic Effects of a Methanolic Extract of Chrysophyllum lucentifolium Cronquist. Plants. 2022;11:94. doi: 10.3390/plants11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaweł-Bęben K., Kukula-Koch W., Hoian U., Czop M., Strzępek-Gomółka M., Antosiewicz B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants. 2020;9:202. doi: 10.3390/antiox9030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kavvoura D.-A., Stefanakis M.K., Kletsas D., Katerinopoulos H.E., Pratsinis H. Biological Activities of Ceratonia siliqua Pod and Seed Extracts: A Comparative Analysis of Two Cretan Cultivars. Int. J. Mol. Sci. 2023;24:12104. doi: 10.3390/ijms241512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ivanović S., Avramović N., Dojćinović B., Trifunović S., Novaković M., Tešević V., Mandi B. Chemical Composition, Total Phenols and Flavonoids Contents and Antioxidant Activity as Nutritive Potential of Roasted Hazelnut Skins (Corylus avellana L.) Foods. 2020;9:430. doi: 10.3390/foods9040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hering A., Stefanowicz-Hajduk J., Gucwa M., Wielgomas B., Ochocka J.R. Photoprotection and Antiaging Activity of Extracts from Honeybush (Cyclopia sp.)—In Vitro Wound Healing and Inhibition of the Skin Extracellular Matrix Enzymes: Tyrosinase, Collagenase, Elastase and Hyaluronidase. Pharmaceutics. 2023;15:1542. doi: 10.3390/pharmaceutics15051542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gea-Botella S., Moreno-Chamba B., de la Casa L., Salazar-Bermeo J., Martí N., Martínez-Madrid M.C., Valero M., Saura D. Carotenoids from Persimmon (Diospyros kaki Thunb.) Byproducts Exert Photoprotective, Antioxidative and Microbial Anti-Adhesive Effects on HaCaT. Pharmaceutics. 2021;13:1898. doi: 10.3390/pharmaceutics13111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmady A., Amini M.H., Zhakfar A.M., Babak G., Sediqi M.N. Sun protective potential and physical stability of herbal sunscreen developed from afghan medicinal plants. Turk. J. Pharm. Sci. 2020;17:285–292. doi: 10.4274/tjps.galenos.2019.15428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pintus F., Floris S., Fais A., Era B., Porcedda C., Tuberoso C.I.G., Caddeo C. Euphorbia characias Extract: Inhibition of Skin Aging-Related Enzymes and Nanoformulation. Plants. 2022;11:1849. doi: 10.3390/plants11141849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang E., Gao W., Xiao Y.K., Ngo H.T.T., Yi H.Y. Helianthus annuus L. flower prevents UVB-induced photodamage in human dermal fibroblasts by regulating the MAPK/AP-1, NFAT, and Nrf2 signaling pathways. J. Cell. Biochem. 2019;120:601–612. doi: 10.1002/jcb.27417. [DOI] [PubMed] [Google Scholar]
- 66.Muzaffer U., Paul V.I., Agilan B., Prasad N.R. Protective effect of Juglans regia L., against ultraviolet-B induced photoaging in human epidermal keratinocytes. Biomed. Pharmacother. 2019;111:724–732. doi: 10.1016/j.biopha.2018.12.129. [DOI] [PubMed] [Google Scholar]
- 67.Jeon J.S., Kang H.M., Park J.H., Kang J.S., Lee Y.J., Park Y.H., Je B.I., Park S.Y., Choi Y.W. A Comparative Study on Photo-Protective and Anti-Melanogenic Properties of Different Kadsura coccinea Extracts. Plants. 2021;10:1633. doi: 10.3390/plants10081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bianchini Silva L.S., Perasoli F.B., Carvalho K.V., Vieira K.M., Paz Lopes M.T., Bianco de Souza G.H., Henrique Dos Santos O.D., Freitas K.M. Melaleuca leucadendron (L.) L. flower extract exhibits antioxidant and photoprotective activities in human keratinocytes exposed to ultraviolet B radiation. Free Radic. Biol. Med. 2020;159:54–65. doi: 10.1016/j.freeradbiomed.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 69.Santhanam R., Karunakaran T., Sowndhararajan K., Zulkifli M.F., Kothandaraman M.G., Aravindhan V., Ismail W.I.W. Photoprotective Potential, Cytotoxicity, and UPLC-QTOF/MS Analysis on Bioactive Solvent Fractions of Moringa concanensis Nimmo Bark. Evid.-Based Complement. Altern. Med. 2022;2022:3781189. doi: 10.1155/2022/3781189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Her Y., Shin B.-N., Lee Y.L., Park J.H., Kim D.W., Kim K.S., Kim H., Song M., Kim J.-D., Won M.-H., et al. Oenanthe javanica Extract Protects Mouse Skin from UVB Radiation via Attenuating Collagen Disruption and Inflammation. Int. J. Mol. Sci. 2019;20:1435. doi: 10.3390/ijms20061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeong D., Lee J., Park S.H., Kim Y.A., Park B.J., Oh J., Sung G.-H., Aravinthan A., Kim J.-H., Kang H., et al. Antiphotoaging and Antimelanogenic Effects of Penthorum chinense Pursh Ethanol Extract due to Antioxidant- and Autophagy-Inducing Properties. Oxid. Med. Cell. Longev. 2019;2019:9679731. doi: 10.1155/2019/9679731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lorz L.R., Yoo B.C., Kim M.-Y., Cho J.Y. Anti-Wrinkling and Anti-Melanogenic Effect of Pradosia mutisii Methanol Extract. Int. J. Mol. Sci. 2019;20:1043. doi: 10.3390/ijms20051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong Y.H., Kim J.H., Cho J.Y. Photoaging Protective Effects of Ranunculus bulumei Methanol Extract. Evid.-Based Complement. Altern. Med. 2020;2020:1761785. doi: 10.1155/2020/1761785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ibrahim N., Abbas H., El-Sayed N.S., Gad H.A. Rosmarinus officinalis L. hexane extract: Phytochemical analysis, nanoencapsulation, and in silico, in vitro, and in vivo anti-photoaging potential evaluation. Sci. Rep. 2022;12:13102. doi: 10.1038/s41598-022-16592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang P.-W., Cheng Y.-C., Hung Y.-C., Lee C.-H., Fang J.-Y., Li W.-T., Wu Y.-R., Pan T.-L. Red Raspberry Extract Protects the Skin against UVB-Induced Damage with Antioxidative and Anti-inflammatory Properties. Oxid. Med. Cell Longev. 2019;2019:9529676. doi: 10.1155/2019/9529676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krgović N., Jovanović M., Aradski A.A., Janković T., Stević T., Zdunić G., Laušević S.D., Šavikin K. Bioassay-Guided Skin-Beneficial Effects of Fractionated Sideritis raeseri subsp. raeseri Extract. Plants. 2022;11:2677. doi: 10.3390/plants11202677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwon K.-R., Alam B., Park J.-H., Kim T.-H., Lee S.-H. Attenuation of UVB-Induced Photo-Aging by Polyphenolic-Rich Spatholobus suberectus Stem Extract Via Modulation of MAPK/AP-1/MMPs Signaling in Human Keratinocytes. Nutrients. 2019;11:1341. doi: 10.3390/nu11061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park H.-A., Kim M.Y., Lee N.-Y., Lim J., Park K.-B., Lee C.-K., Nguyen V.D., Kim J., Park J.-T., Park J.-I. Variation of Triterpenic Acids in 12 Wild Syzygium formosum and Anti-Inflammation Activity on Human Keratinocyte HaCaT. Plants. 2021;10:2428. doi: 10.3390/plants10112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vostálová J., Tinková E., Biedermann D., Kosina P., Ulrichová J., Rajnochová Svobodová A. Skin Protective Activity of Silymarin and its Flavonolignans. Molecules. 2019;24:1022. doi: 10.3390/molecules24061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Era B., Floris S., Sogos V., Porcedda C., Piras A., Medda R., Fais A., Pintus F. Anti-Aging Potential of Extracts from Washingtonia filifera Seeds. Plants. 2021;10:151. doi: 10.3390/plants10010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hao D., Wen X., Liu L., Wang L., Zhou X., Li Y., Zeng X., He G., Jiang X. Sanshool improves UVB-induced skin photodamage by targeting JAK2/STAT3-dependent autophagy. Cell. Death. Dis. 2019;10:19–31. doi: 10.1038/s41419-018-1261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzalez A.C., Costa T.F., Andrade Z.A., Medrado A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016;91:614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shedoeva A., Leavesley D., Upton Z., Fan C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019;2019:2684108. doi: 10.1155/2019/2684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bass S., Chowdhury M., Raj S., Raj Chaudhary N. Effects of Phytomedicines on Wound Healing. Eur. J. Exper. Biol. 2021;11:133–138. [Google Scholar]
- 85.Bindschadler M., McGrath J.L. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J. Cell Sci. 2007;120:876–884. doi: 10.1242/jcs.03395. [DOI] [PubMed] [Google Scholar]
- 86.Parent C.A., Devreotes P.N. A cell’s sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- 87.Horwitz R., Webb D. Cell migration. Curr. Biol. 2003;13:756–759. doi: 10.1016/j.cub.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 88.Sivamani R.K., Garcia M.S., Rivkah Isseroff R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007;12:2849–2868. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- 89.Seeger M.A., Paller A.S. The Roles of Growth Factors in Keratinocyte Migration. Adv. Wound Care. 2015;4:213–224. doi: 10.1089/wound.2014.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zulkefli N., Che Zahari C.N.M., Sayuti N.H., Kamarudin A.A., Saad N., Hamezah H.S., Bunawan H., Baharum S.N., Mediani A., Ahmed Q.U., et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023;24:4607. doi: 10.3390/ijms24054607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang Y., He J., Guo B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano. 2021;15:12687–12722. doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 92.Chittasupho C., Manthaisong A., Okonogi S., Tadtong S., Samee W. Effects of Quercetin and Curcumin Combination on Antibacterial, Antioxidant, In Vitro Wound Healing and Migration of Human Dermal Fibroblast Cells. Int. J. Mol. Sci. 2022;23:142. doi: 10.3390/ijms23010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bittner Fialová S., Rendeková K., Mučaji P., Nagy M., Slobodníková L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, European Legislation and Folk Medicine-A Review. Int. J. Mol. Sci. 2021;22:10746. doi: 10.3390/ijms221910746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nowak A., Zagórska-Dziok M., Perużyńska M., Cybulska K., Kucharska E., Ossowicz-Rupniewska P., Piotrowska K., Duchnik W., Kucharski Ł., Sulikowski T., et al. Assessment of the Anti-Inflammatory, Antibacterial and Anti-Aging Properties and Possible Use on the Skin of Hydrogels Containing Epilobium angustifolium L. Extracts. Front. Pharmacol. 2022;13:896706. doi: 10.3389/fphar.2022.896706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Albahri G., Badran A., Hijazi A., Daou A., Baydoun E., Nasser M., Merah O. The Therapeutic Wound Healing Bioactivities of Various Medicinal Plants. Life. 2023;13:317. doi: 10.3390/life13020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pain S., Altobelli C., Boher A., Cittadini L., Favre-Mercuret M., Gaillard C., Sohm B., Vogelgesang B., André-Frei V. Surface rejuvenating effect of Achillea millefolium extract. Int. J. Cosmet. Sci. 2011;33:535–542. doi: 10.1111/j.1468-2494.2011.00667.x. [DOI] [PubMed] [Google Scholar]
- 97.Sajithlal G.B. Influence of aloe vera on collagen turnover in healing of dermal wounds in rats. Indian J. Exp. Biol. 2000;36:896–901. [PubMed] [Google Scholar]
- 98.Rezaei M., Dadgar Z., Noori-Zadeh A., Mesbah-Namin S.A., Pakzad I., Davodian E. Evaluation of the antibacterial activity of the Althaea officinalis L. leaf extract and its wound healing potency in the rat model of excision wound creation. Avicenna J. Phytomed. 2015;5:105–112. [PMC free article] [PubMed] [Google Scholar]
- 99.Preethi K.C., Kuttan R. Wound healing activity of flower extract of Calendula officinalis. J. Basic Clin. Physiol. Pharmacol. 2009;20:73–80. doi: 10.1515/JBCPP.2009.20.1.73. [DOI] [PubMed] [Google Scholar]
- 100.Yen Y.H. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-α, MMP-9,-SMA, and collagen. Int. Wound J. 2018;15:605–617. doi: 10.1111/iwj.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mumtaz R., Zubair M., Khan M.A., Muzammil S., Siddique M.H. Extracts of Eucalyptus alba Promote Diabetic Wound Healing by Inhibiting α-Glucosidase and Stimulating Cell Proliferation. Evid. Based Complement. Altern. Med. 2022;2022:4953105. doi: 10.1155/2022/4953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ranzato E., Martinotti S., Burlando B. Wound healing properties of jojoba liquid wax: An in vitro study. J. Ethnopharmacol. 2011;134:443–449. doi: 10.1016/j.jep.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 103.Wang C., Shang H., Cui W., Zhou F., Zhang S., Wang X., Gao P., Wei K., Zhu R. Pine pollen polysaccharides promote cel proliferation and accelerate wound healing by activating the JAK2-STAT3 signaling pathway. Int. J. Biol. Macromol. 2022;210:579–587. doi: 10.1016/j.ijbiomac.2022.04.210. [DOI] [PubMed] [Google Scholar]
- 104.Chen G., He L., Zhang P., Zhang J., Mei X., Wang D., Zhang Y., Ren X., Chen Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C. 2020;110:110686. doi: 10.1016/j.msec.2020.110686. [DOI] [PubMed] [Google Scholar]
- 105.Vasilenko T., Kováč I., Slezák M., Ďurkáč J., Peržel’ová N., Čoma M., Kaňuchová M., Urban L., Szabo P., Dvořánková B., et al. Agrimonia eupatoria L. Aqueous Extract Improves Skin Wound Healing: An In Vitro Study in Fibroblasts and Keratinocytes and In Vivo Study in Rats. In Vivo. 2022;36:1236–1244. doi: 10.21873/invivo.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee S.-Y., Won K.-J., Kim D.-Y., Kim M.-J., Won Y.-R., Kim N.-Y., Lee H.-M. Wound Healing-Promoting and Melanogenesis-Inhibiting Activities of Angelica polymorpha Maxim. Flower Absolute In Vitro and Its Chemical Composition. Molecules. 2021;26:6172. doi: 10.3390/molecules26206172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazumdar S., Ghosh A.K., Dinda M., Das A.K., Das S., Jana K., Karmakar P. Evaluation of wound healing activity of ethanol extract of Annona reticulata L. leaf both in vitro and in diabetic mice model. J. Tradit. Complement. Med. 2021;11:27–37. doi: 10.1016/j.jtcme.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akbari F., Azadbakht M., Bagheri A., Vahedi L. In Vitro and In Vivo Wound Healing Activity of Astragalus floccosus Boiss. (Fabaceae) Adv. Pharmacol. Pharm. Sci. 2022;2022:7865015. doi: 10.1155/2022/7865015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Emrich S., Schuster A., Schnabel T., Oostingh G.J. Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts. Molecules. 2022;27:2817. doi: 10.3390/molecules27092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruttanapattanakul J., Wikan N., Okonogi S., Na Takuathung M., Buacheen P., Pitchakarn P., Potikanond S., Nimlamool W. Boesenbergia rotunda extract accelerates human keratinocyte proliferation through activating ERK1/2 and PI3K/Akt kinases. Biomed. Pharmacother. 2021;133:111002. doi: 10.1016/j.biopha.2020.111002. [DOI] [PubMed] [Google Scholar]
- 111.Salas-Oropeza J., Jimenez-Estrada M., Perez-Torres A., Castell-Rodriguez A.E., Becerril-Millan R., Rodriguez-Monroy M.A., Jarquin-Yañez K., Canales-Martinez M.M. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules. 2021;26:2488. doi: 10.3390/molecules26092488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nizioł-Łukaszewska Z., Zagórska-Dziok M., Ziemlewska A., Bujak T. Comparison of the Antiaging and Protective Properties of Plants from the Apiaceae Family. Oxid. Med. Cell Longev. 2020;2020:5307614. doi: 10.1155/2020/5307614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rafsanjani S.M., Naeini A.T., Meimandi-Parizi A., Nowzari F., Wani M.M., Iraji A. Wound healing effect of Carum carvi L. on the incised skin wound in male rats: Histopathology, total protein and biomechanical evaluations. Vet. Med. Sci. 2022;8:2726–2737. doi: 10.1002/vms3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Somwong P., Kamkaen N. Wound-healing activity and quantification of bioactive compounds from Derris scandens extract. J. Adv. Pharm. Technol. Res. 2022;13:38–43. doi: 10.4103/japtr.japtr_208_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park S.M., Won K.J., Hwang D.I., Kim D.Y., Kim H.B., Li Y., Lee H.M. Potential Beneficial Effects of Digitaria ciliaris Flower Absolute on the Wound Healing-Linked Activities of Fibroblasts and Keratinocytes. Planta Med. 2020;86:348–355. doi: 10.1055/a-1101-9326. [DOI] [PubMed] [Google Scholar]
- 116.Assar D.H., Elhabashi N., Mokhbatly A.A.A., Ragab A.E., Elbialy Z.I., Rizk S.A., Albalawi A.E., Althobaiti N.A., Jaouni S.A., Atiba A. Wound healing potential of licorice extract in rat model: Antioxidants, histopathological, immunohistochemical and gene expression evidences. Biomed. Pharmacother. 2021;143:112151. doi: 10.1016/j.biopha.2021.112151. [DOI] [PubMed] [Google Scholar]
- 117.Siriwattanasatorn M., Itharat A., Thongdeeying P., Ooraikul B. In Vitro Wound Healing Activities of Three Most Commonly Used Thai Medicinal Plants and Their Three Markers. Evid. Based. Complement. Alternat. Med. 2020;2020:6795383. doi: 10.1155/2020/6795383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Loggenberg S.R., Twilley D., De Canha M.N., Meyer D., Mabena E.C., Lall N. Evaluation of Wound Healing and Antibacterial Potential of Greyia radlkoferi Szyszyl. Ethanolic Leaf Extract. Front. Pharmacol. 2022;13:806285. doi: 10.3389/fphar.2022.806285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoon J.H., Park S.H., Yoon S.E., Hong S.Y., Lee J.B., Lee J., Cho J.Y. Hydrangea serrata Hot Water Extract and Its Major Ingredient Hydrangenol Improve Skin Moisturization and Wrinkle Conditions via AP-1 and Akt/PI3K Pathway Upregulation. Nutrients. 2023;15:2436. doi: 10.3390/nu15112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hernandez-Hernandez A.B., Alarcon-Aguilar F.J., Garcia-Lorenzana M., Rodriguez-Monroy M.A., Canales-Martinez M.M. Jatropha neopauciflora Pax Latex Exhibits Wound-Healing Effect in Normal and Diabetic Mice. J. Evid. Based. Integr. Med. 2021;26:2515690X20986762. doi: 10.1177/2515690X20986762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim N.Y., Won K.J., Kim H.B., Kim D.Y., Kim M.J., Won Y.R., Lee H.M. Chemical Composition of Salix koreensis Anderss Flower Absolute and Its Skin Wound Healing Activities In Vitro. Plants. 2022;11:246. doi: 10.3390/plants11030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen C.-C., Nien C.-J., Chen L.-G., Huang K.-Y., Chang W.-J., Huang H.-M. Effects of Sapindus mukorossi Seed Oil on Skin Wound Healing: In Vivo and in Vitro Testing. Int. J. Mol. Sci. 2019;20:2579. doi: 10.3390/ijms20102579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Freitas Figueiredo F., Filho F.C., Damazo A.S., Arunachalam K., Colodel E.M., Ribeiro M., Venturini C.L., Oliveira D.M., Machado M.T., Pavan E., et al. Sorocea guilleminiana Gaudich.: Wound healing activity, action mechanisms, and chemical characterization of the leaf infusion. J. Ethnopharmacol. 2020;248:112307. doi: 10.1016/j.jep.2019.112307. [DOI] [PubMed] [Google Scholar]
- 124.Kang M.C., Yumnam S., Park W.S., So H.M., Kim K.H., Shin M.C., Ahn M.-J., Kim S.Y. Ulmus parvifolia Accelerates Skin Wound Healing by Regulating the Expression of MMPs and TGF-β. J. Clin. Med. 2020;9:59. doi: 10.3390/jcm9010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rahman S., Islam R., Rana M., Spitzhorn L.-S., Rahman M.S., Adjaye J., Asaduzzaman S.M. Characterization of burn wound healing gel prepared from human amniotic membrane and Aloe vera extract. BMC Complement. Altern. Med. 2019;19:115. doi: 10.1186/s12906-019-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lopes C.M.I., Baratella-Evêncio L., DE Souza I.A., DE Oliveira E.B., Sá J.G.A., Santana M.A.N., Neto P.P.M., DE Santana E.S., DA Silva L.A., Vieira J.R.C. Evaluation of cytotoxicity and wound healing activity of Avicennia schaueriana in cream. An. Acad. Bras. Cienc. 2019;91:e20180171. doi: 10.1590/0001-3765201920180171. [DOI] [PubMed] [Google Scholar]
- 127.Amrati F.E.-Z., Chebaibi M., Galvão de Azevedo R., Conte R., Slighoua M., Mssillou I., Kiokias S., de Freitas Gomes A., Soares Pontes G., Bousta D. Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma europaea Extracts. Molecules. 2023;28:1780. doi: 10.3390/molecules28041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moglad E.H., Hamad A.M., Fatima F., Devanathadesikan Seshadri V., Naz M. Antimicrobial and wound healing activities of certain Sudanese medicinal plants. Saudi. J. Biol. Sci. 2020;27:1766–1772. doi: 10.1016/j.sjbs.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Teshome N., Degu A., Ashenafi E., Ayele E., Abebe A. Evaluation of Wound Healing and Anti-Inflammatory Activity of Hydroalcoholic Leaf Extract of Clematis simensis Fresen (Ranunculaceae) Clin. Cosmet. Investig. Dermatol. 2022;15:1883–1897. doi: 10.2147/CCID.S384419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ankomah A.D., Boakye Y.D., Agana T.A., Boamah V.E., Sampene Ossei P.P., Adu F., Agyare C. Evaluation of Dermal Toxicity and Wound Healing Activity of Cnestis ferruginea Vahl ex DC. Adv. Pharmacol. Pharm. Sci. 2022;2022:5268613. doi: 10.1155/2022/5268613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saleem U., Khalid S., Zaib S., Anwar F., Akhtar M.F., Hussain L., Saleem A., Ahmad B. Wound Healing Potential and In Silico Appraisal of Convolvulus arvensis L. Methanolic Extract. Biomed. Res. Int. 2022;2022:1373160. doi: 10.1155/2022/1373160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ahmed A.S., Taher M., Mandal U.K., Jaffri J., Susanti D., Mahmood S., Zakaria Z.A. Pharmacological properties of Centella asiatica hydrogel in accelerating wound healing in rabbits. BMC Complement Altern. Med. 2019;19:213. doi: 10.1186/s12906-019-2625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salhi N., El Guourrami O., Rouas L., Moussaid S., Moutawalli A., Benkhouili F.Z., Ameggouz M., Alshahrani M.M., Al Awadh A.A., Bouyahya A., et al. Evaluation of the Wound Healing Potential of Cynara humilis Extracts in the Treatment of Skin Burns. Evid. Based. Complement. Alternat. Med. 2023;2023:5855948. doi: 10.1155/2023/5855948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bardaa S., Makni K., Boudaouara O., Bardaa T., Ktari N., Hachicha S., Ben Salah R., Kallel R., Sahnoun Z., Boufi S. Development and Evaluation of the Wound Healing Effect of a Novel Topical Cream Formula Based on Ginkgo biloba Extract on Wounds in Diabetic Rats. Biomed. Res. Int. 2021;2021:6474706. doi: 10.1155/2021/6474706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahmad S.U., Binti Aladdin N.-A., Jamal J.A., Shuid A.N., Mohamed I.N. Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model. Molecules. 2021;26:228. doi: 10.3390/molecules26010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Okur M.E., Karadağ A.E., Üstündağ Okur N., Özhan Y., Sipahi H., Ayla S., Daylan B., Demirci B., Demirci F. In Vivo Wound Healing and In Vitro Anti-Inflammatory Activity Evaluation of Phlomis russeliana Extract Gel Formulations. Molecules. 2020;25:2695. doi: 10.3390/molecules25112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Niknam S., Tofighi Z., Faramarzi M.A., Abdollahifar M.A., Sajadi E., Dinarvand R., Toliyat T. Polyherbal combination for wound healing: Matricaria chamomilla L. and Punica granatum L. DARU J. Pharm. Sci. 2021;29:133–145. doi: 10.1007/s40199-021-00392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Upadhyay G., Tiwari N., Maurya H., Upadhyay J., Joshi R., Ansari M.N. In Vivo wound-healing and antioxidant activity of aqueous extract of Roylea elegans leaves against physically induced burn model in Wistar albino rats. 3 Biotech. 2021;11:442. doi: 10.1007/s13205-021-02993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gul H., Naseer R.D., Abbas I., Khan E.A., Rehman H.U., Nawaz A., Azad A.K., Albadrani G.M., Altyar A.E., Albrakati A., et al. The Therapeutic Application of Tamarix aphylla Extract Loaded Nanoemulsion Cream for Acid-Burn Wound Healing and Skin Regeneration. Medicina. 2023;59:34. doi: 10.3390/medicina59010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Abeje B.A., Bekele T., Getahun K.A., Asrie A.B. Evaluation of Wound Healing Activity of 80% Hydromethanolic Crude Extract and Solvent Fractions of the Leaves of Urtica simensis in Mice. J. Exp. Pharmacol. 2022;14:221–241. doi: 10.2147/JEP.S363676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Carvalho G.R., Braz D.S., Gonçalves T.C.O., Aires R., Côco L.Z., Guidoni M., Fronza M., Endringer D.C., Júnior A.D.S., Campos-Toimil M., et al. Development and Evaluation of Virola oleifera Formulation for Cutaneous Wound Healing. Antioxidants. 2022;11:1647. doi: 10.3390/antiox11091647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang H., Liu Y., Cai K., Zhang B., Tang S., Zhang W., Liu W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn. Trauma. 2021;9:tkab041. doi: 10.1093/burnst/tkab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qureshi K.A., Mohammed S.A.A., Khan O., Ali H.M., El-Readi M.Z., Mohammed H.A. Cinnamaldehyde-Based Self-Nanoemulsion (CA-SNEDDS) Accelerates Wound Healing and Exerts Antimicrobial, Antioxidant, and Anti-Inflammatory Effects in Rats’ Skin Burn Model. Molecules. 2022;27:5225. doi: 10.3390/molecules27165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.George J., Sneed K., Pathak Y. The Skin Aging Process and Anti-Aging Strategies. Biomed. J. Sci. Tech. Res. 2022;42:33377–33386. doi: 10.26717/BJSTR.2022.42.006712. [DOI] [Google Scholar]
- 145.Russell-Goldman E., Murphy G.F. The Pathobiology of Skin Aging New Insights into an Old Dilemma. Am. J. Pathol. 2020;190:1356–1369. doi: 10.1016/j.ajpath.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feingold K.R., Elias P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barier. Biochim. Biophys. Acta. 2014;1841:280–294. doi: 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 147.van Smeden J., Janssens M., Gooris G.S., Bouwstra J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta. 2014;1841:295–313. doi: 10.1016/j.bbalip.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 148.Gębalski J., Graczyk F., Załuski D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure–activity relationship. J. Enzyme Inhib. Med. Chem. 2022;37:1120–1195. doi: 10.1080/14756366.2022.2061966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thring T.S., Hili P., Naughton D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009;9:27. doi: 10.1186/1472-6882-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zagórska-Dziok M., Bujak T., Ziemlewska A., Nizioł-Łukaszewska Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules. 2021;26:802. doi: 10.3390/molecules26040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Poomanee W., Yaowiwat N., Pattarachaidaecharuch T., Leelapornpisid P. Optimized multiherbal combination and in vivo anti-skin aging potential: A randomized double blind placebo controlled study. Sci. Rep. 2023;13:5633. doi: 10.1038/s41598-023-32738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ghimeray A.K., Jung U.S., Lee H.Y., Ki Y.H., Ryu E.K., Chang M.S. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica and Morus alba fruits extract. Clin. Cosmet. Investig. Dermatol. 2015;8:389–396. doi: 10.2147/CCID.S80906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pieczykolan A., Pietrzak W., Dos Santos Szewczyk K., Gawlik-Dziki U., Wang R. LC-ESI-MS/MS Polyphenolic Profile and In Vitro Study of Cosmetic Potential of Aerva lanata (L.) Juss. Herb Extracts. Molecules. 2022;27:1259. doi: 10.3390/molecules27041259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gam D.H., Hong J.W., Kim J.H., Kim J.W. Skin-Whitening and Anti-Wrinkle Effects of Bioactive Compounds Isolated from Peanut Shell Using Ultrasound-Assisted Extraction. Molecules. 2021;26:1231. doi: 10.3390/molecules26051231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim K.Y., Lee E.J., Whang W.K., Park C.H. In vitro and in vivo anti-aging effects of compounds isolated from Artemisia iwayomogi. J. Anal. Sci. Technol. 2019;10:35. doi: 10.1186/s40543-019-0193-1. [DOI] [Google Scholar]
- 156.Sriyab S., Laosirisathian N., Punyoyai C., Anuchapreeda S., Tima S., Chiampanichayakul S., Chaiyana W. Nutricosmetic effects of Asparagus officinalis: A potent matrix metalloproteinase-1 inhibitor. Sci. Rep. 2021;11:8772. doi: 10.1038/s41598-021-88340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Michalak M., Zagórska-Dziok M., Klimek-Szczykutowicz M., Szopa A. Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago officinalis Extracts on Skin Cells. Molecules. 2023;28:868. doi: 10.3390/molecules28020868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sadeer N.B., Sinan K.I., Cziáky Z., Jek J., Zengin G., Jeewon R., Abdallah H.H., Rengasamy K.R.R., Mahomoodally M.F. Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules. 2020;10:731. doi: 10.3390/biom10050731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nur S., Setiawan H., Hanafi M., Elya B. Phytochemical composition, antioxidant, in vitro and in silico studies of active compounds of Curculigo latifolia extracts as promising elastase inhibitor. Saudi. J. Bio. Sci. 2023;30:103716. doi: 10.1016/j.sjbs.2023.103716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hong-in P., Chaiyana W. Potential cosmeceutical lamellar liquid crystals containing black longan (Dimocarpus longan Lour.) seed extract for MMP-1 and hyaluronidase inhibition. Sci. Rep. 2022;12:7683. doi: 10.1038/s41598-022-11554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tungmunnithum D., Drouet S., Hano C. Validation of a High-Performance Liquid Chromatography with Photodiode Array Detection Method for the Separation and Quantification of Antioxidant and Skin Anti-Aging Flavonoids from Nelumbo nucifera Gaertn. Stamen Extract. Molecules. 2022;27:1102. doi: 10.3390/molecules27031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marijan M., Mitar A., Jakupovic L., Prlic Kardum J., Zovko Končic M. Optimization of Bioactive Phenolics Extraction and Cosmeceutical Activity of Eco-Friendly Polypropylene-Glycol– Lactic-Acid-Based Extracts of Olive Leaf. Molecules. 2022;27:529. doi: 10.3390/molecules27020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Andradea J.M., Domínguez-Martín E.M., Nicolai M., Faustino C., Rodrigues L.M., Rijo P. Screening the dermatological potential of Plectranthus species components: Antioxidant and inhibitory capacities over elastase, collagenase and tyrosinase. J. Enzyme Inhib. Med. Chem. 2021;36:258–270. doi: 10.1080/14756366.2020.1862099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Altyar A.E., Ashour M.L., Youssef F.S. Premna odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling. Biomolecules. 2020;10:879. doi: 10.3390/biom10060879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chaiyana W., Chansakaow S., Intasai N., Kiattisin K., Lee K.-H., Lin W.-C., Lue S.-C., Leelapornpisid P. Chemical Constituents, Antioxidant, Anti-MMPs, and Anti-Hyaluronidase Activities of Thunbergia laurifolia Lindl. Leaf Extracts for Skin Aging and Skin Damage Prevention. Molecules. 2020;25:1923. doi: 10.3390/molecules25081923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Waqas M.K., Akhtar N., Rasul A., Sethi A., Abbas K., Hussain T. Non-invasive in vivo evaluations of cosmetic emulsion containing phytoextract of grape seeds in the treatment of skin aging by using noninvasive bio-engineering techniques. Acta Pol. Pharm. Drug Res. 2018;75:97–105. [Google Scholar]
- 167.Abdelfattah M.A.O., Dmirieh M., Bakrim W.B., Mouhtady O., Ghareeb M.A., Wink M., Sobeh M. Antioxidant and anti-aging effects of Warburgia salutaris bark aqueous extract: Evidences from in silico, in vitro and in vivo studies. J. Ethnopharmacol. 2022;292:115187. doi: 10.1016/j.jep.2022.115187. [DOI] [PubMed] [Google Scholar]
- 168.Bujak T., Zagórska-Dziok M., Ziemlewska A., Nizioł-Łukaszewska Z., Wasilewski T., Hordyjewicz-Baran Z. Antioxidant and Cytoprotective Properties of Plant Extract from Dry Flowers as Functional Dyes for Cosmetic Products. Molecules. 2021;26:2809. doi: 10.3390/molecules26092809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Michailidis D., Angelis A., Nikolaou P.E., Mitakou S., Skaltsounis A.L. Exploitation of Vitis vinifera, Foeniculum vulgare, Cannabis sativa and Punica granatum By-Product Seeds as Dermo-Cosmetic Agents. Molecules. 2021;26:731. doi: 10.3390/molecules26030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ito S. A chemist’s view of melanogenesis. Pigment. Cell Res. 2003;16:230–236. doi: 10.1034/j.1600-0749.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 171.Solano F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014;2014:498276. doi: 10.1155/2014/498276. [DOI] [Google Scholar]
- 172.Simon J.D., Peles D., Wakamatsu K., Ito S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment. Cell Melanoma Res. 2009;22:563–579. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 173.d’Ischia M., Wakamatsu K., Cicoira F., Di Mauro E., Garcia-Borron J.C., Commo S., Galván I., Ghanem G., Kenzo K., Meredith P., et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment. Cell Melanoma Res. 2015;28:520–544. doi: 10.1111/pcmr.12393. [DOI] [PubMed] [Google Scholar]
- 174.Hearing V.J. Determination of melanin synthetic pathway. J. Investig. Dermatol. 2011;131:E8–E11. doi: 10.1038/skinbio.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chang T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Solano F., Briganti S., Picardo M., Ghanem G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment. Cell Res. 2006;19:550–571. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 177.Couteau C., Coiffard L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics. 2016;3:27. doi: 10.3390/cosmetics3030027. [DOI] [Google Scholar]
- 178.Smit N., Vicanova J., Pavel S. The Hunt for Natural Skin Whitening Agents. Int. J. Mol. Sci. 2009;10:5326–5349. doi: 10.3390/ijms10125326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Agbai O.N., Taylor S.C. Melasma and Depigmentation Agents. Cosmeceuticals and Active Cosmetics. In: Sivamani R.K., Jagdeo J., Elsner P., Maibach H.I., editors. Cosmeceuticals and Active Cosmetics. CRC Press; Boca Raton, FL, USA: 2016. pp. 343–356. [Google Scholar]
- 180.Lee B.D., Eun J.B. Optimum extraction conditions for arbutin from Asian peal peel by supercritical fluid extraction (SFE) using Box-Behnken design. J. Med. Plant Res. 2012;6:2348–2364. [Google Scholar]
- 181.Lukas B., Schmiderer C., Mitteregger U., Novak J. Arbutin in marjoram and oregano. Food Chem. 2010;121:185–190. doi: 10.1016/j.foodchem.2009.12.028. [DOI] [Google Scholar]
- 182.Kenndler I.E., Schwer C., Fritsche B., Pöhm M. Determination of arbutin in Uvae-ursi folium (bearberry leaves) by capillary zone electrophoresis. J. Chromatogr. A. 1990;514:383–388. doi: 10.1016/S0021-9673(01)89414-0. [DOI] [Google Scholar]
- 183.Pyka A., Bober K., Stolarczyk A. Densitometric Determination of arbutin in cowberry leaves (Vaccinium vitis idaeae) Acta Pol. Pharmaceutic. Drug Res. 2007;63:395–400. [PubMed] [Google Scholar]
- 184.Pop C., Vlase L., Tamas M. Natural Resources Containing Arbutin. Determination of Arbutin in the Leaves of Bergenia crassifolia (L.) Fritsch. acclimated in Romania. Not. Bot. Hort. Agrobot. Cluj. 2009;37:129–132. [Google Scholar]
- 185.Nguyen M.H.K., Nguyen H.X., Nguyen M.T.T., Nguyen N.T. Phenolic Constituents from the Heartwood of Artocapus altilis and their Tyrosinase Inhibitory Activity. Nat. Prod. Commun. 2012;7:185–186. doi: 10.1177/1934578X1200700214. [DOI] [PubMed] [Google Scholar]
- 186.Soifoini T., Donno D., Jeannoda V., Rakoto D.D., Msahazi A., Farhat S.M.M., Oulam M.Z., Beccaro G.L. Phytochemical Composition, Antibacterial Activity, and Antioxidant Properties of the Artocarpus altilis Fruits to Promote Their Consumption in the Comoros Islands as Potential Health-Promoting Food or a Source of Bioactive Molecules for the Food Industry. Foods. 2021;10:2136. doi: 10.3390/foods10092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Özer Ö., Mutlu B., Kıvçak B. Antityrosinase Activity of Some Plant Extracts and Formulations Containing Ellagic Acid. Pharmaceut. Biol. 2007;45:519–524. doi: 10.1080/13880200701446746. [DOI] [Google Scholar]
- 188.Jones K., Hughes J., Hong M., Jia Q., Orndorff S. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigment. Cell Res. 2002;15:335–340. doi: 10.1034/j.1600-0749.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- 189.Li X., Guo L., Sun Y., Zhou J., Gu Y., Li Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int. J. Mol. Med. 2010;25:923–927. doi: 10.3892/ijmm_00000423. [DOI] [PubMed] [Google Scholar]
- 190.Zheng Y., Zhou S., Zhang H., Lu Z., Deng R., Feng Y., Li P. Comparative Study of the Flavonoid Content in Radix Scutellaria from Different Cultivation Areas in China. Int. J. Anal. Chem. 2023;2023:3754549. doi: 10.1155/2023/3754549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eghlima G., Kheiry A., Sanikhani M., Hadian J., Aelaei M., Ebrahimi S.N. Investigation of Phytochemical Variability, Antioxidant Activity and Ecological Conditions of Native Iranian Glycyrrhiza glabra L. Int. J. Hort. Sci. Technol. 2020;7:387–400. [Google Scholar]
- 192.Matos P., Paranhos A., Batista M.T., Figueirinha A. Synergistic Effect of DIBOA and Verbascoside from Acanthus mollis Leaf on Tyrosinase Inhibition. Int. J. Mol. Sci. 2022;23:13536. doi: 10.3390/ijms232113536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kadyrbayeva G., Zagórska J., Grzegorczyk A., Gaweł-Beben K., Strzepek-Gomółka M., Ludwiczuk A., Czech K., Kumar M., Koch W., Malm A., et al. The Phenolic Compounds Profile and Cosmeceutical Significance of Two Kazakh Species of Onions: Allium galanthum and A. turkestanicum. Molecules. 2021;26:5491. doi: 10.3390/molecules26185491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fawzi Mahomoodally M., Picot-Allain M.C.N., Zengin G., Llorent-Martínez E.J., Abdullah H.H., Ak G., Senkardes I., Chiavaroli A., Menghini L., Recinella L., et al. Phytochemical Analysis, Network Pharmacology and in Silico Investigations on Anacamptis pyramidalis Tuber Extracts. Molecules. 2020;25:2422. doi: 10.3390/molecules25102422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chaikhong K., Chumpolphant S., Rangsinth P., Sillapachaiyaporn C., Chuchawankul S., Tencomnao T., Prasansuklab A. Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction. Plants. 2023;12:65. doi: 10.3390/plants12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zeitoun H., Khan Z., Banerjee K., Salameh D., Lteif R. Antityrosinase Activity of Combretum micranthum, Euphorbia hirta and Anacardium occidentale Plants: Ultrasound Assisted Extraction Optimization and Profiling of Associated Predominant Metabolites. Molecules. 2020;25:2684. doi: 10.3390/molecules25112684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Anh L.H., Quan N.V., Lam V.Q., Iuchi Y., Takami A., Teschke R., Xuan T.D. Antioxidant, Anti-tyrosinase, Anti-α-amylase, and Cytotoxic Potentials of the Invasive Weed Andropogon virginicus. Plants. 2021;10:69. doi: 10.3390/plants10010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee J.-H., Mei H.-C., Kuo I.-C., Lee T.-H., Chen Y.-H., Lee C.-K. Characterizing Tyrosinase Modulators from the Roots of Angelica keiskei Using Tyrosinase Inhibition Assay and UPLC-MS/MS as the Combinatorial Novel Approach. Molecules. 2019;24:3297. doi: 10.3390/molecules24183297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ilgün S., Karatoprak G.S., Polat D.Ç., ¸Safak E.K., Yıldız G., Küpeli Akko E., Sobarzo-Sánchez E. Phytochemical Composition and Biological Activities of Arctium minus (Hill) Bernh.: A Potential Candidate as Antioxidant, Enzyme Inhibitor, and Cytotoxic Agent. Antioxidants. 2022;11:1852. doi: 10.3390/antiox11101852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Suroowan S., Llorent-Martínez E.J., Zengin G., Buskaran K., Fakurazi S., Abdalla A.N., Khalid A., Le Van B., Mahomoodally M.F. Unveiling the Antioxidant, Clinical Enzyme Inhibitory Properties and Cytotoxic Potential of Tambourissa peltata Baker—An Understudied Endemic Plant. Molecules. 2023;28:599. doi: 10.3390/molecules28020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu Y.-Q., Xu C.-Y., Liang F.-Y., Jin P.-C., Qian Z.-Y., Luo Z.-S., Qin R.-G. Selecting and Characterizing Tyrosinase InhibiTors from Atractylodis macrocephalae Rhizoma Based on Spectrum-Activity Relationship and Molecular Docking. J. Anal. Methods Chem. 2021;2021:5596463. doi: 10.1155/2021/5596463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.del Pilar Fernández-Poyatos M., Ruiz-Medina A., Zengin G., Llorent-Martínez E.J. Phenolic Characterization, Antioxidant Activity, and Enzyme Inhibitory Properties of Berberis thunbergii DC. Leaves: A Valuable Source of Phenolic Acids. Molecules. 2019;24:4171. doi: 10.3390/molecules24224171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pandey B.P., Pradhan S.P., Adhikari K., Nepal S. Bergenia pacumbis from Nepal, an astonishing enzymes inhibitor. BMC Complem. Med. Ther. 2020;20:198–209. doi: 10.1186/s12906-020-02989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dirar A.M., Wada M., Watanabe T., Devkota H.P. Phenolic Compounds from the Aerial Parts of Blepharis linariifolia Pers. and Their Free Radical Scavenging and Enzyme Inhibitory Activities. Medicines. 2019;6:113. doi: 10.3390/medicines6040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Luo Y., Wang J., Li S., Wu Y., Wang Z., Chen S., Chen H. Discovery and identification of potential anti-melanogenic active constituents of Bletilla striata by zebrafish model and molecular docking. BMC Complem. Med. Ther. 2022;22:9. doi: 10.1186/s12906-021-03492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dall’Acqua S., Sinan K.I., Ferrarese I., Sut S., Bene K., Mahomoodally M.F., Sadeer N.B., Ak G., Zengin G. Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts. Molecules. 2020;25:5537. doi: 10.3390/molecules25235537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mahomoodally M.F., Jugreet S., Sinan K.I., Zengin G., Ak G., Ceylan R., Jekő J., Cziáky Z., Angelini P., Angeles Flores G., et al. Pharmacological Potential and Chemical Characterization of Bridelia ferruginea Benth.—A Native Tropical African Medicinal Plant. Antibiotics. 2021;10:223. doi: 10.3390/antibiotics10020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Placines C., Castañeda-Loaiza V., Rodrigues M.J., Pereira C.G., Stefanucci A., Mollica A., Zengin G., Llorent-Martínez E.J., Castilho P.C., Custódio L. Phenolic Profile, Toxicity, Enzyme Inhibition, In Silico Studies, and Antioxidant Properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal. Plants. 2020;9:142. doi: 10.3390/plants9020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yeom S.-H., Gam D.-H., Kim J.-H., Kim J.-W. Development of Ultrasound-Assisted Extraction to Produce Skin-Whitening and anti-Wrinkle Substances from Safflower Seed. Molecules. 2022;27:1296. doi: 10.3390/molecules27041296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Viet T.D., Xuan T.D., Anh L.H. α-Amyrin and β-Amyrin Isolated from Celastrus hindsii Leaves and Their Antioxidant, Anti-Xanthine Oxidase, and Anti-Tyrosinase Potentials. Molecules. 2021;26:7248. doi: 10.3390/molecules26237248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lou Y., Xu T., Cao H., Zhao Q., Zhang P., Shu P. Natural Antioxidants, Tyrosinase and Acetylcholinesterase Inhibitors from Cercis glabra Leaves. Molecules. 2022;27:8667. doi: 10.3390/molecules27248667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rodrigues M.J., Custódio L., Mecha D., Zengin G., Cziáky Z., Sotkó G., Pereira C.G. Nutritional and Phyto-Therapeutic Value of the Halophyte Cladium mariscus L. (Pohl.): A Special Focus on Seeds. Plants. 2022;11:2910. doi: 10.3390/plants11212910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Van Quan N., Xuan T.D., Anh L.H., Tran H.-D. Bio-Guided Isolation of Prospective Bioactive Constituents from Roots of Clausena indica (Dalzell) Oliv. Molecules. 2019;24:4442. doi: 10.3390/molecules24244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Anwar S., Faisal Nadeem M., Pervaiz I., Khurshid U., Akmal N., Aamir K., Haseeb ur Rehman M., Almansour K., Alshammari F., Shaikh M.F., et al. A comprehensive phytochemical, biological, and toxicological studies of roots and aerial parts of Crotalaria burhia Buch.-Ham: An important medicinal plant. Front. Plant Sci. 2022;13:988352. doi: 10.3389/fpls.2022.988352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dall’Acqua S., Sinan K.I., Sut S., Ferrarese I., Etienne O.K., Mahomoodally M.F., Lobine D., Zengin G. Evaluation of Antioxidant and Enzyme Inhibition Properties of Croton hirtus L’Hér. Extracts Obtained with Different Solvents. Molecules. 2021;26:1902. doi: 10.3390/molecules26071902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Oh H.-N., Park D.-H., Park J.-Y., Song S.-Y., Lee S.-H., Yoon G., Moon H.-S., Oh D.-S., Rhee S.-H., Im E.-O., et al. Tyrosinase Inhibition Antioxidant Effect and Cytotoxicity Studies of the Extracts of Cudrania tricuspidata Fruit Standardized in Chlorogenic Acid. Molecules. 2019;24:3266. doi: 10.3390/molecules24183266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zengin G., Cádiz-Gurrea M.d.l.L., Fernández-Ochoa Á., Leyva-Jiménez F.J., Carretero A.S., Momotko M., Yildiztugay E., Karatas R., Jugreet S., Mahomoodally M.F., et al. Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles. Molecules. 2022;27:5788. doi: 10.3390/molecules27185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen Y.-C., Su S.-H., Huang J.-C., Chao C.-Y., Sung P.-J., Chen Y.-F., Ko H.-H., Kuo Y.-H. Tyrosinase Inhibitors Derived from Chemical Constituents of Dianella ensifolia. Plants. 2022;11:2142. doi: 10.3390/plants11162142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sagara T., Sugimoto S., Yamano Y., Nehira T., Masuda K., Otsuka H., Matsunam K. Isolation of Three New Diterpenes from Dodonaea viscosa. Chem. Pharm. Bull. 2021;69:40–47. doi: 10.1248/cpb.c20-00327. [DOI] [PubMed] [Google Scholar]
- 220.Carradori S., Cairone F., Garzoli S., Fabrizi G., Iazzetti A., Giusti A.M., Menghini L., Uysal S., Ak G., Zengin G., et al. Phytocomplex Characterization and Biological Evaluation of Powdered Fruits and Leaves from Elaeagnus angustifolia. Molecules. 2020;25:2021. doi: 10.3390/molecules25092021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sabera F.R., Ashour R.M., El-Halawanya A.M., Mahomoodally M.F., Akd G., Zengind G., Mahrous E.A. Phytochemical profile, enzyme inhibition activity and molecular docking analysis of Feijoa sellowiana O. Berg. J. Enzym. Inhib. Med. Chem. 2021;36:618–626. doi: 10.1080/14756366.2021.1880397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shrestha S.S., Sut S., Ferrarese I., Di Marco S.B., Zengin G., De Franco M., Pant D.R., Mahomoodally M.F., Ferri N., Biancorosso N., et al. Himalayan Nettle Girardinia diversifolia as a Candidate Ingredient for Pharmaceutical and Nutraceutical Applications—Phytochemical Analysis and In Vitro Bioassays. Molecules. 2020;25:1563. doi: 10.3390/molecules25071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Popoola O.K., Marnewick J.L., Iwuoha E.I., Hussein A.A. Methoxylated Flavonols and ent-Kaurane Diterpenes from the South African Helichrysum rutilans and Their Cosmetic Potential. Plants. 2023;12:2870. doi: 10.3390/plants12152870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ozntamar-Pouloglou K.-M., Cheilari A., Zengin G., Graikou K., Ganos C., Karikas G.-A., Chinou I. Heliotropium procubens Mill: Taxonomic Significance and Characterization of Phenolic Compounds via UHPLC–HRMS-In Vitro Antioxidant and Enzyme Inhibitory Activities. Molecules. 2023;28:1008. doi: 10.3390/molecules28031008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Arshad A., Ahemad S., Saleem H., Saleem M., Zengin G., Abdallah H.H., Tousif M.I., Ahemad N., Fawzi Mahomoodally M. RP-UHPLC-MS Chemical Profiling, Biological and In Silico Docking Studies to Unravel the Therapeutic Potential of Heliotropium crispum Desf. as a Novel Source of Neuroprotective Bioactive Compounds. Biomolecules. 2021;11:53. doi: 10.3390/biom11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lim W.Y., Chan E.W.C., Phan C.W., Wong C.W. Tyrosinase Inhibiting Extracts from Coastal Plants as Potential Additives in Skin Whitening Formulations. Curr. Appl. Sci. Technol. 2021;21:481–494. [Google Scholar]
- 227.Sut S., Dall’Acqua S., Zengin G., Senkardes I., Uba A.I., Bouyahya A., Aktumsek A. Novel Signposts on the Road from Natural Sources to Pharmaceutical Applications: A Combinative Approach between LC-DAD-MS and Offline LC-NMR for the Biochemical Characterization of Two Hypericum Species (H. montbretii and H. origanifolium) Plants. 2023;12:648. doi: 10.3390/plants12030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yehia S.M., Ayoub I.M., Watanabe M., Devkota H.P., Singab A.N.B. Metabolic profiling, antioxidant, and enzyme inhibition potential of Iris pseudacorus L. from Egypt and Japan: A comparative study. Sci Rep. 2023;13:5233. doi: 10.1038/s41598-023-32224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zengin G., Mahomoodally M.F., Sinan K.I., Ak G., Etienne O.K., Sharmeen J.B., Brunetti L., Leone S., Di Simone S.C., Recinella L., et al. Chemical Composition and Biological Properties of Two Jatropha Species: Different Parts and Different Extraction Methods. Antioxidants. 2021;10:792. doi: 10.3390/antiox10050792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Baysal I., Ekizoglu M., Ertas A., Temiz B., Agalar H.G., Yabanoglu-Ciftci S., Temel H., Ucar G., Turkmenoglu F.P. Identification of Phenolic Compounds by LC-MS/MS and Evaluation of Bioactive Properties of Two Edible Halophytes: Limonium effusum and L. sinuatum. Molecules. 2021;26:4040. doi: 10.3390/molecules26134040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Saive M., Genva M., Istasse T., Frederich M., Maes C., Fauconnier M.-L. Identification of a Proanthocyanidin from Litchi chinensis Sonn. Root with Anti-Tyrosinase and Antioxidant Activity. Biomolecules. 2020;10:1347. doi: 10.3390/biom10091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fan Z., Li L., Bai X., Hhang H., Liu Q., Zhang H., Fu Y., Moyo R. Extraction optimization, antioxidant activity, and tyrosinase inhibitory capacity of polyphenols from Lonicera japonica. Food Sci. Nutr. 2019;7:1786–1794. doi: 10.1002/fsn3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Srisupap S., Chaicharoenpong C. In vitro antioxidant and antityrosinase activities of Manilkara kauki. Acta Pharm. 2021;71:153–162. doi: 10.2478/acph-2021-0009. [DOI] [PubMed] [Google Scholar]
- 234.Taviano M.F., Miceli N., Acquaviva R., Malfa G.A., Ragusa S., Giordano D., Cásedas G., Les F., López V. Cytotoxic, Antioxidant, and Enzyme Inhibitory Properties of the Traditional Medicinal Plant Matthiola incana (L.) R. Br. Biology. 2020;9:163. doi: 10.3390/biology9070163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.He R.-J., Li J., Huang Y.-L., Wang Y.-F., Yang B.-Y., Liu Z.-B., Ge L., Yang K.-D., Li D.-P. Structural Characterization and Assessment of Anti-Inflammatory and Anti-Tyrosinase Activities of Polyphenols from Melastoma normale. Molecules. 2021;26:3913. doi: 10.3390/molecules26133913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kim J., Hong S.-C., Lee E.H., Lee J.W., Yang S.-H., Kim J.-C. Preventive Effect of M. cochinchinensis on Melanogenesis via Tyrosinase Activity Inhibition and p-PKC Signaling in Melan-A Cell. Nutrients. 2021;13:3894. doi: 10.3390/nu13113894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ali J.S., Saleem H., Mannan A., Zengin G., Mahomoodally M.F., Locatelli M., Abidin S.A.Z., Ahemad N., Zia M. Metabolic fingerprinting, antioxidant characterization, and enzyme-inhibitory response of Monotheca buxifolia (Falc.) A. DC. extracts. BMC Complement. Med. Ther. 2020;20:313. doi: 10.1186/s12906-020-03093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Istifli E.S. Chemical Composition, Antioxidant and Enzyme Inhibitory Activities of Onosma bourgaei and Onosma trachytricha and in Silico Molecular Docking Analysis of Dominant Compounds. Molecules. 2021;26:2981. doi: 10.3390/molecules26102981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zengin G., Fernández-Ochoa Á., Cádiz-Gurrea M.d.l.L., Leyva-Jiménez F.J., Segura-Carretero A., Elbasan F., Yildiztugay E., Malik S., Khalid A., Abdalla A.N., et al. Phytochemical Profile and Biological Activities of Different Extracts of Three Parts of Paliurus spina-christi: A Linkage between Structure and Ability. Antioxidants. 2023;12:255. doi: 10.3390/antiox12020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Elloumi W., Maalej A., Ortiz S., Michel S., Chamkha M., Boutefnouchet S., Sayadi S. Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities. Molecules. 2022;27:855. doi: 10.3390/molecules27030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fonseca-Hernández D., Lugo-Cervantes E.D.C., Escobedo-Reyes A., Mojica L. Black Bean (Phaseolus vulgaris L.) Polyphenolic Extract Exerts Antioxidant and Antiaging Potential. Molecules. 2021;26:6716. doi: 10.3390/molecules26216716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Di Petrillo A., González-Paramás A.M., Rosa A., Ruggiero V., Boylan F., Kumar A., Pintus F., Santos-Buelga C., Fais A., Era B. Chemical composition and enzyme inhibition of Phytolacca dioica L. seeds extracts. J. Enzyme Inhib. Med. Chem. 2019;34:519–527. doi: 10.1080/14756366.2018.1563077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Etsassala N.G.E.R., Waryo T., Popoola O.K., Adeloye A.O., Iwuoha E.I., Hussein A.A. Electrochemical Screening and Evaluation of Lamiaceae Plant Species from South Africa with Potential Tyrosinase Activity. Sensors. 2019;19:1035. doi: 10.3390/s19051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xiong Y., Kim H.K., Özer Ö.Ç., van Duijn B., Korthout H.A.A.J., Zi L., Cai A. Synergistic Inhibiting Effect of Phytochemicals in Rheum palmatum on Tyrosinase Based on Metabolomics and Isobologram Analyses. Molecules. 2023;28:944. doi: 10.3390/molecules28030944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chiavaroli A., Sinan K.I., Zengin G., Mahomoodally M.F., Sadeer N.B., Etienne O.K., Cziáky Z., Jek J., Glamoclija J., SokoviĆ M., et al. Identification of Chemical Profiles and Biological Properties of Rhizophora racemosa G. Mey. Extracts Obtained by Different Methods and Solvents. Antioxidants. 2020;9:533. doi: 10.3390/antiox9060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sabitov A., Gaweł-Beben K., Sakipova Z., Strzepek-Gomółka M., Hoian U., Satbayeva E., Głowniak K., Ludwiczuk A. Rosa platyacantha Schrenk from Kazakhstan—Natural Source of Bioactive Compounds with Cosmetic Significance. Molecules. 2021;26:2578. doi: 10.3390/molecules26092578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Desmiaty Y., Hanafi M., Saputri F.C., Elya B., Rifai E.A., Syahdi R.R. Two triterpenoids from Rubus fraxinifolius leaves and their tyrosinase and elastase inhibitory activities. Sci. Rep. 2021;11:20452. doi: 10.1038/s41598-021-99970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dall’Acqua S., Sut S., Sinan K.I., Zengin G., Ferrarese I., Peron G., Yildiztugay E., Picot-Allain C., Mahomoodally M.F. An Integrated NMR, LC-DAD-MS, LC-QTOF Metabolomic Characterization of Sartoria hedysaroides: Correlation of Antioxidant and Enzyme Inhibitory Activity with Chemical Composition by Multivariate Data Analysis. Antioxidants. 2022;11:110. doi: 10.3390/antiox11010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zagórska-Dziok M., Wójciak M., Ziemlewska A., Nizioł-Łukaszewska Z., Hoian U., Klimczak K., Szczepanek D., Sowa I. Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra chinensis Extracts and Their Applicability in Skin Care Product. Molecules. 2022;27:8877. doi: 10.3390/molecules27248877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sinan K.I., Yagi S., Llorent-Martínez E.J., Ruiz-Medina A., Gordo-Moreno A.I., Stefanucci A., Mollica A., Bene K., Zengin G. Understanding the Chemical Composition and Biological Activities of Different Extracts of Secamone afzelii Leaves: A Potential Source of Bioactive Compounds for the Food Industry. Molecules. 2023;28:3678. doi: 10.3390/molecules28093678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Parndaeng K., Pitakbut T., Wattanapiromsakul C., Hwang J.S., Udomuksorn W., Dej-adisai S. Chemical Constituents from Streblus taxoides Wood with Their Antibacterial and Antityrosinase Activities Plus in Silico Study. Antibiotics. 2023;12:319. doi: 10.3390/antibiotics12020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Aziz M., Ahmad S., Khurshid U., Pervaiz I., Lodhi A.H., Jan N., Khurshid S., Arshad M.A., Ibrahim M.M., Mersal G.A.M., et al. Comprehensive Biological Potential, Phytochemical Profiling Using GC-MS and LC-ESI-MS, and In-Silico Assessment of Strobilanthes glutinosus Nees: An Important Medicinal Plant. Molecules. 2022;27:6885. doi: 10.3390/molecules27206885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Oh K.-E., Shin H., Lee M.K., Park B., Lee K.Y. Characterization and Optimization of the Tyrosinase Inhibitory Activity of Vitis amurensis Root Using LC-Q-TOF-MS Coupled with a Bioassay and Response Surface Methodology. Molecules. 2021;26:446. doi: 10.3390/molecules26020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pintatum A., Laphookhieo S., Logie E., Berghe W.V., Maneera W. Chemical Composition of Essential Oils from Different Parts of Zingiber kerrii Craib and Their Antibacterial, Antioxidant, and Tyrosinase Inhibitory Activities. Biomolecules. 2020;10:228. doi: 10.3390/biom10020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tomczyk M., Ceylan O., Locatelli M., Tartaglia A., Ferrone V., Sarikurkcu C. Ziziphora taurica subsp. taurica: Analytical Characterization and Biological Activities. Biomolecules. 2019;9:367. doi: 10.3390/biom9080367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ali B., Al-Wabel N.A., Shams S., Ahamad A., Khan S.A., Anwar F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015;5:601–611. doi: 10.1016/j.apjtb.2015.05.007. [DOI] [Google Scholar]
- 257.Elshafie H.S., Camele I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. Biomed. Res. Int. 2017;2017:9268468. doi: 10.1155/2017/9268468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Michalak M. Aromatherapy and methods of applying essential oils. Arch. Physiother. Glob. Res. 2018;22:25–31. [Google Scholar]
- 259.Guzmán E., Lucia A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics. 2021;8:114. doi: 10.3390/cosmetics8040114. [DOI] [Google Scholar]
- 260.Sharmeen J.B., Mahomoodally F.M., Zengin G., Maggi F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Śmigielski K., Raj A., Krosowiak K., Gruska R. Chemical Composition of the Essential Oil of Lavandula angustifolia Cultivated in Poland. J. Essent. Oil Bear. Plants. 2009;12:338–347. doi: 10.1080/0972060X.2009.10643729. [DOI] [Google Scholar]
- 262.Khan M., Khan S.T., Khan N.A., Mahmood A., Al-Kedhairy A.A., Alkhathlan H.Z. The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in Saudi Arabia and evaluation of their antibacterial activity. Arabian J. Chem. 2018;11:1189–1200. doi: 10.1016/j.arabjc.2018.02.008. [DOI] [Google Scholar]
- 263.Shabnum S., Wagay M.G. Essential Oil Composition of Thymus vulgaris L. and their Uses. J. Res. Develop. 2011;11:23617694. [Google Scholar]
- 264.Golparvar A.R., Hadipanah A. Chemical compositions of the essential oil from peppermint (Mentha piperita L.) cultivated in Isfahan conditions. J. Herb. Drugs. 2013;4:75–80. [Google Scholar]
- 265.Wesołowska A., Jadczak D., Grzeszczuk M. Essential oil composition of hyssop (Hyssopus officinalis L.) cultivated in north-western Poland. Herba Pol. 2020;56:57–65. [Google Scholar]
- 266.Acimovic M., Tesevic V., Todosijevic M., Djisalov J., Oljaca S. Compositional characteristics of the essential oil of Pimpinella anisum and Foeniculum vulgare grown in Serbia. Botanica Serbica. 2015;39:9–14. [Google Scholar]
- 267.Jiang Z.T., Sun M.L., Li R., Wang Y. Essential oil Composition of Chinese Caraway (Carum carvi L.) J. Essen. Oil Bear. Pl. 2011;14:379–382. doi: 10.1080/0972060X.2011.10643949. [DOI] [Google Scholar]
- 268.Louiza H., Salah M., Malika B. Chemical composition of Citrus limon (Eureka variety) essential oil and evaluation of its antioxidant and antibacterial activities. Afr. J. Biotechnol. 2018;17:356–361. doi: 10.5897/AJB2016.15693. [DOI] [Google Scholar]
- 269.Deng W., Liu K., Cao S., Sun J., Zhong B., Chun J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules. 2020;25:217. doi: 10.3390/molecules25010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chalchat J., Garry R. Chemical composition of the leaf oil of Verbena officinalis L. J. Essen. Oil Res. 1996;8:419–420. doi: 10.1080/10412905.1996.9700653. [DOI] [Google Scholar]
- 271.Chakraborty A., Sankaran V., Ramar M., Chellappan D.R. Chemical analysis of leaf essential oil of Cinnamomum verum from Palni hills, Tamil Nadu. J. Chem. Pharm. Sci. 2015;8:476–479. [Google Scholar]
- 272.Sadiki F.Z., El Idrissi M. Chemical composition of essential oil of Anthemis nobilis L. flowers from Morocco. Appl. J. Envir. Eng. Sci. 2019;5:342–348. [Google Scholar]
- 273.Almas I., Innocent E., Machumi F., Kisinza W. Chemical Composition of Essential Oils from Eucalyptus globulus and Eucalyptus maculata Grown in Tanzania. Sci. Afr. 2021;12:e00758. doi: 10.1016/j.sciaf.2021.e00758. [DOI] [Google Scholar]
- 274.Borotová P., Galovicová L., Vukovic N.L., Vukic M., Tvrdá E., Kacániová M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants. 2022;11:558. doi: 10.3390/plants11040558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hatami T., Johner J.C.F., Zabot G.L., Meireles M.A.A. Supercritical fluid extraction assisted by cold pressing from clove buds: Extraction performance, volatile oil composition, and economic evaluation. J. Supercrit. Fluids. 2019;144:39–47. doi: 10.1016/j.supflu.2018.10.003. [DOI] [Google Scholar]
- 276.Sharopov F.S., Zhang H., Setzer W.N. Composition of geranium (Pelargonium graveolens) essential oil from Tajikistan. Am. J. Essent. Oils Nat. Prod. 2014;2:13–16. [Google Scholar]
- 277.Khalil R., Li Z.G. Antimicrobial activity of essential oil of Salvia officinalis L. collected in Syria. Afr. J. Biotechnol. 2011:10. doi: 10.5897/AJB10.2615. [DOI] [Google Scholar]
- 278.Dweck A.C. Natural ingredients for colouring and styling. Int. J. Cosmet. Sci. 2002;24:287–302. doi: 10.1046/j.1467-2494.2002.00148.x. [DOI] [PubMed] [Google Scholar]
- 279.Yusuf M., Shabbir M., Mohammad F. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospect. 2017;7:123–145. doi: 10.1007/s13659-017-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mohana Priya M., Chidambara Rajan P., Lavanya M. Use of natural pigments as colorants in cosmetics—A review. J. Emerg. Technol. Innov. Res. 2020;7:907–917. [Google Scholar]
- 281.Delgado-Vargas F., Jiménez A.R., Paredes-López O. Natural pigments: Carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000;40:173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- 282.Michalak M., Glinka R. Sources of vegetable dyes and their use in cosmetology. Pol. J. Cosmetol. 2017;20:196–205. [Google Scholar]
- 283.Bhalekar O.S., Waghmare S.A., Kamble H.V., Dhamal K.S. Natural colourants and dyes from plant origin. Int. J. Sci. Res. Eng. Dev. 2022;5:537–549. [Google Scholar]
- 284.Brudzyńska P., Sionkowska A., Grisel M. Plant-Derived Colorants for Food, Cosmetic and Textile Industries: A Review. Materials. 2021;14:3484. doi: 10.3390/ma14133484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Cui H., Xie W., Hua Z., Cao L., Xiong Z., Tang Y., Yuan Z. Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review. Molecules. 2022;27:8062. doi: 10.3390/molecules27228062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chandrasekar R., Sivagami B., Swapna D. Herbal Cosmetics an Overview. Int. J. Pharm. Res. Rev. 2016;5:1–20. [Google Scholar]
- 287.Prabhu K.H., Bhute A.S. Plant based natural dyes and mordnats: A Review. J. Nat. Prod. Plant Resour. 2012;2:649–664. [Google Scholar]
- 288.Morel S., Sapino S., Peira E., Chirio D., Gallarate M. Regulatory Requirements for Exporting Cosmetic Products to Extra-EU Countries. Cosmetics. 2023;10:62. doi: 10.3390/cosmetics10020062. [DOI] [Google Scholar]
- 289.Sopyan I., Gozali D., Tiassetiana S. Formulation of tomato extracts (Solanum lycopersicum L.) as a sunscreen lotion. Natl. J. Physiol. Pharm. Pharmacol. 2017;8:453–458. doi: 10.5455/njppp.2017.7.1039921112017. [DOI] [Google Scholar]
- 290.Maheshwar G.H., Patil B.S., Prashant D. Comparative sun protection factor determination of fresh fruits extract of cucumber vs marketed cosmetic formulation. Res. J. Pharm. Biol. Chem. Sci. 2010;1:55–59. [Google Scholar]
- 291.Ortel B., Gange R.W. An Action Spectrum for the Elicitation of Erythema in Skin persistently Sensitized by Photobound 8-Methoxypsoralen. J. Investig. Dermatol. 1990;94:781–785. doi: 10.1111/1523-1747.ep12874639. [DOI] [PubMed] [Google Scholar]
- 292.Huang Y.C., Liu K.C., Chiou Y.L. Melanogenesis of murine melanoma cells induced by hesperetin, a Citrus hydrolysate-derived flavonoid. Food Chem. Toxicol. 2012;50:653–659. doi: 10.1016/j.fct.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 293.Wang H.Z., Zhang Y., Xie L.P., Yu X.Y., Zhang R.Q. Effects of genistein and daidzein on the cell growth, cell cycle, and differentiation of human and murine melanoma cells. J. Nutr. Biochem. 2002;13:421–426. doi: 10.1016/S0955-2863(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 294.Kim D.-S., Park S.-H., Kwon S.-B., Li K., Youn S.-W., Park K.-C. (-)-Epigallocatechin-3-gallate and hinokitiol reduce melanin synthesis via decreased MITF production. Arch. Pharm. Res. 2004;27:334–339. doi: 10.1007/BF02980069. [DOI] [PubMed] [Google Scholar]
- 295.Ye Y., Chou G.-X., Wang H., Chu J.-H., Yu Z.-L. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine. 2010;18:32–35. doi: 10.1016/j.phymed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 296.An S.M., Kim H.J., Kim J.-E., Boo Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother. Res. 2008;22:1200–1207. doi: 10.1002/ptr.2435. [DOI] [PubMed] [Google Scholar]
- 297.Takekoshi S., Matsuzaki K., Kitatani K. Quercetin stimulates melanogenesis in hair follicle melanocyte of the mouse. Tokai J. Exp. Clin. Med. 2013;38:129–134. [PubMed] [Google Scholar]
- 298.Fujii T., Saito M. Inhibitory effect of quercetin isolated from rose hip (Rosa canina L.) against melanogenesis by mouse melanoma cells. Biosci. Biotechnol. Biochem. 2009;73:1989–1993. doi: 10.1271/bbb.90181. [DOI] [PubMed] [Google Scholar]
- 299.Magalhães W.V., Baby A.R., Robles Velasco M.V., Mendes Pereira D.M., Kaneko T.M. Patenting in the cosmetic sector: Study of the use of herbal extracts. Braz. J. Pharm. Sci. 2011;47:693–700. doi: 10.1590/S1984-82502011000400005. [DOI] [Google Scholar]
- 300.Yapar E.A. Intellectual Property and Patent in Cosmetics. Marmara Pharm. J. 2017;21:419–424. doi: 10.12991/marupj.306786. [DOI] [Google Scholar]
- 301.Césara F.C.S., Carnevale Netob F., Portoc e Patrícia G.S., Camposa M.B.G.M. Patent analysis: A look at the innovative nature of plant-based cosmetics. Quim. Nova. 2017;40:840–847. doi: 10.21577/0100-4042.20170022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.