Abstract
Selected monoterpenes inhibited methane oxidation by methanotrophs (Methylosinus trichosporium OB3b, Methylobacter luteus), denitrification by environmental isolates, and aerobic metabolism by several heterotrophic pure cultures. Inhibition occurred to various extents and was transient. Complete inhibition of methane oxidation by Methylosinus trichosporium OB3b with 1.1 mM (−)-α-pinene lasted for more than 2 days with a culture of optical density of 0.05 before activity resumed. Inhibition was greater under conditions under which particulate methane monooxygenase was expressed. No apparent consumption or conversion of monoterpenes by methanotrophs was detected by gas chromatography, and the reason that transient inhibition occurs is not clear. Aerobic metabolism by several heterotrophs was much less sensitive than methanotrophy was; Escherichia coli (optical density, 0.01), for example, was not affected by up to 7.3 mM (−)-α-pinene. The degree of inhibition was monoterpene and species dependent. Denitrification by isolates from a polluted sediment was not inhibited by 3.7 mM (−)-α-pinene, γ-terpinene, or β-myrcene, whereas 50 to 100% inhibition was observed for isolates from a temperate swamp soil. The inhibitory effect of monoterpenes on methane oxidation was greatest with unsaturated, cyclic hydrocarbon forms [e.g., (−)-α-pinene, (S)-(−)-limonene, (R)-(+)-limonene, and γ-terpinene]. Lower levels of inhibition occurred with oxide and alcohol derivatives [(R)-(+)-limonene oxide, α-pinene oxide, linalool, α-terpineol] and a noncyclic hydrocarbon (β-myrcene). Isomers of pinene inhibited activity to different extents. Given their natural sources, monoterpenes may be significant factors affecting bacterial activities in nature.
Monoterpenes are naturally occurring compounds produced by plants and animals. The majority of these compounds are unsaturated hydrocarbons (C10), but there are also oxygenated derivatives, such as alcohols, ketones, and carboxylic acids, and collectively these compounds are known as monoterpenoids (12). These compounds, the main components in volatile essential oils of plants, are widely distributed throughout vegetation types but are found in especially high concentrations in plants such as the conifers (12, 23). The monoterpenes are a significant natural source of atmospheric nonmethane hydrocarbons. They are involved in a variety of atmospheric reactions (16, 23, 36) and can contribute to production of tropospheric ozone (16).
Monoterpenoids have long been used in the food, perfume, and pharmaceutical industries because of their flavoring and antimicrobial properties. They are currently of interest industrially as replacements for chlorofluorocarbons and halogenated solvents (21, 24). Recently, it has been suggested that monoterpenes play an important role in altering nitrogen (N) and carbon (C) cycling in forest soils (36).
The inhibition of activity and growth of some microorganisms by monoterpenes is well-known (22, 27). However, other microbes may be stimulated. Volatile oil from aromatic plants has increased CO2 production in soil samples sixfold (31). Microbial degradation of monoterpenes under both aerobic (24) and anaerobic (18) conditions has been described (reviewed in reference 28). The ability to inhibit some microorganisms but not others makes monoterpenes potential factors in the control of microbial processes in environments where they are abundant, such as forest soils (38).
It has been suggested that inhibition of nitrification by monoterpenes in forest soils has a major influence on N cycling in these environments (33–36). It has been proposed that monoterpenes have a direct effect on the primary enzyme of this process, ammonia monooxygenase (AMO) (34, 35). Methane (CH4) monooxygenase (MMO), the primary enzyme in the CH4 oxidation process, and AMO are susceptible to many of the same inhibitors (8). Thus, it might be expected that monoterpenes that inhibit nitrification should also inhibit CH4 oxidation. We recently found that a variety of monoterpenes inhibit methane consumption by forest soils under field-moist and slurry conditions (3). These compounds, which are typically most concentrated in the forest litter, along with other soluble, inhibitory soil components (5) may explain the lack of methane consumption in the top layers of many forest soils (1, 6). Preliminary studies showed that pure cultures of methanotrophs were also inhibited (3, 36).
In this study, we examined the effect of several monoterpenes on methane oxidation by pure cultures of the methanotrophs Methylosinus trichosporium OB3b and Methylobacter luteus, denitrification by six environmental isolates, and aerobic metabolism by several heterotrophic laboratory cultures.
MATERIALS AND METHODS
Bacterial cultures.
The methane oxidizers, Methylosinus trichosporium OB3b and Methylobacter luteus, were gifts from R. S. Hanson (University of Minnesota). Cells were grown in a nitrate mineral salts medium (NMS) (37) supplemented with 10 μM copper (Cu) under an atmosphere containing 20% CH4 in air. Methylosinus trichosporium OB3b was also grown in NMS containing no Cu in order to stimulate production of soluble MMO (sMMO) (17) under the same atmosphere. Cultures (volume, 0.5 liter) were grown in 2-liter Erlenmeyer flasks with a side arm at 25°C, with agitation provided by a magnetic stir bar.
Denitrifying isolates were obtained from a temperate swamp (isolates D1 and D3; obtained from Mt. St. Hilaire, Québec, Canada [4]) and a polluted sediment (isolates HH1, HH3, HH4, and HH6; obtained from Hamilton Harbour, Ontario, Canada [26]). These isolates were grown on a rotary shaker (250 rpm) in nutrient broth (NB) (BBL) supplemented with 10 mM KNO3 at 25°C under a helium atmosphere.
The effect of monoterpenes on aerobic activity was tested by using laboratory cultures of Pseudomonas aeruginosa, Escherichia coli, Serratia marcescens, Bacillus subtilis, and Staphylococcus aureus. Cells were grown in the presence of the ambient atmosphere in 50 ml of NB in 125-ml Erlenmeyer flasks at 25°C on a rotary shaker (250 rpm).
Incubations.
The effect of monoterpenes on CH4 oxidation by methanotrophs was tested by using 58-ml serum bottles capped with grey butyl stoppers and aluminum crimps. The bottles were acid washed (0.12 N HCl) and rinsed four times with deionized water to minimize contamination in experiments in which no Cu was added. Medium (10 ml) was added to the bottles and sterilized before monoterpenes were added. Pure monoterpenoids (approximately 0.6 to 6 μl) were added with a Hamilton glass microsyringe directly to the bottles to give final concentrations of 0.37 to 3.7 mM (0.5 to 5 ppm [wt/vol] for hydrocarbon monoterpenoids). These values are well below the aqueous solubilities of the compounds (32), but due to volatilization and adsorption they should be considered the upper limits of the dissolved concentrations. The bottles were then inoculated with late-log-phase methanotroph cells to give final optical densities at 600 nm (OD600) ranging from 0.04 to 0.095. Some bottles were preincubated without cells for 3 days to allow the monoterpene levels in the headspace to equilibrate. Incubations were carried out in the dark at 25°C on a rotary shaker (230 rpm) under atmospheres containing 4 to 20% CH4 in air. Consumption of CH4 and production of CO2 were monitored for 2 to 5 days, as described below.
Experiments with denitrifying isolates were carried out as described above except that NB containing KNO3 and a helium atmosphere were used. The bottles also received acetylene (10%, vol/vol), a monoterpene (3.1 mM), or acetylene plus a monoterpene to determine if N2O production (and hence denitrification) was affected by the monoterpene addition. The initial culture OD600 varied from 0.03 to 0.12.
The remaining laboratory cultures were grown aerobically in NB at 25°C on a rotary shaker (250 rpm) in 50-ml Erlenmeyer flasks capped with Suba-Seal stoppers (William Freeman Co., Barnsley, United Kingdom) in the presence of various concentrations of monoterpenes. Microbial activity was measured by measuring the production of CO2 over an incubation period of 38 h. The initial cell concentrations were equivalent to an OD600 of 0.01.
The data given below are the means ± standard errors of the means from duplicate or triplicate incubations.
Analyses.
Samples (0.3 to 0.5 ml) of the gases in the headspaces of incubation vessels were obtained with a syringe, and the gases were quantified by gas chromatography. The CH4 and CO2 levels were measured by thermal conductivity detection, while the N2O level was measured by electron capture detection (2, 4, 26). Standard gas mixtures were used to calibrate each measurement. The volatile monoterpenes in the headspaces were measured by using a Varian model 1700 gas chromatograph equipped with a flame ionization detector and a 3-m packed column containing 20% Carbowax 4000 (39) coated onto Chromsorb W, HP (80/100 mesh; Chromatographic Specialties Inc., Brockville, Ontario, Canada). The injector and detector temperatures used were 175 and 250°C, respectively. The column temperature was programmed to increase from 100 to 150°C at a rate of 4°C min−1. The measurements were not calibrated with a known standard and thus were measurements of the relative amounts of the volatile monoterpenes present in each flask. Changes in machine sensitivity were determined each day by injecting a freshly prepared volatile sample of (−)-α-pinene. No significant difference in sensitivity was observed during the experiment (data not shown). The minimum detection limits were estimated to be about 0.1 mM.
Cell densities were measured with a Spectronic 21 spectrophotometer at 600 nm.
Monoterpenoids.
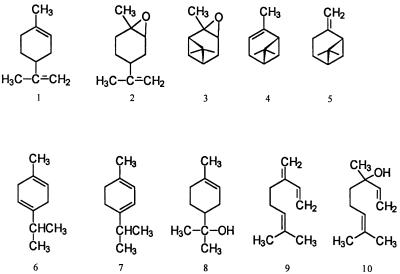
The following compounds were tested for their effects on microbial activities: (−)-α-pinene, α-terpinene, and β-myrcene from ICN Biochemicals, Aurora, Ohio; and (+)-α-pinene, (−)-β-pinene, γ-terpinene, (R)-(+)-limonene, (S)-(−)-limonene, (+)-limonene oxide, α-pinene oxide, α-terpineol, and (±)-linalool from Aldrich Chemical Co., Milwaukee, Wis. The monoterpenoids used are commonly found in nature, and their chemical structures are given in Fig. 1. The sterility of the monoterpenoids was confirmed by aseptically introducing 1 μl of each compound into 10 ml of NB. No growth was observed over a 15-day incubation period (data not shown).
FIG. 1.
Representative structural formulae of the monoterpenoids used. 1, (R)-(+)-limonene; 2, (+)-limonene oxide; 3, (+)-α-pinene oxide; 4, α-pinene; 5, (−)-β-pinene; 6, γ-terpinene; 7, α-terpinene; 8, α-terpineol; 9, β-myrcene; 10, linalool.
RESULTS
Effects of pinenes on CH4 oxidation.
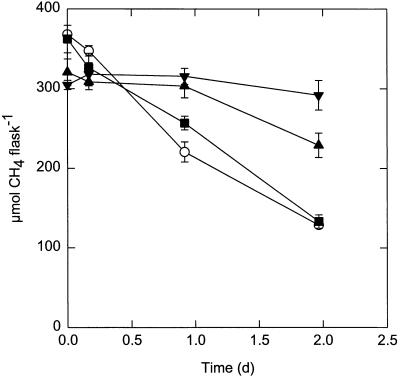
(−)-α-Pinene inhibited CH4 oxidation by Methylosinus trichosporium OB3b, as observed in preliminary studies (3, 36). After 2 days of incubation, significant inhibition (>50%) occurred with ≥0.73 mM (−)-α-pinene (0.1 mg ml−1) (Fig. 2). One-half this amount had no effect on CH4 oxidation, while a pinene concentration of 1.1 mM inhibited essentially all activity over a 2-day period. There was no lag phase in CH4 oxidation with 0.37 mM pinene, but 0.73 mM pinene resulted in a lag of about 1 day before oxidation began (Fig. 2). After an additional 2 to 3 days of incubation the culture to which 1.1 mM pinene was added also showed CH4 oxidation (data not shown). Thus, the inhibitory effect of this compound is transient under the experimental conditions described above. Furthermore, the more dilute the culture, the longer the lag period during which no oxidation occurred (data not shown). Because (−)-α-pinene was a strong inhibitor of CH4 oxidation, this compound was used extensively in further experiments.
FIG. 2.
Effects of different levels of (−)-α-pinene on CH4 oxidation by Methylosinus trichosporium OB3b. α-Pinene at final concentrations of 0 mM (○), 0.37 mM (▪), 0.73 mM (▴), and 1.10 mM (▾) was added to cultures having an initial OD600 of 0.05. The values are means ± standard errors of the means determined from triplicate experiments. d, day.
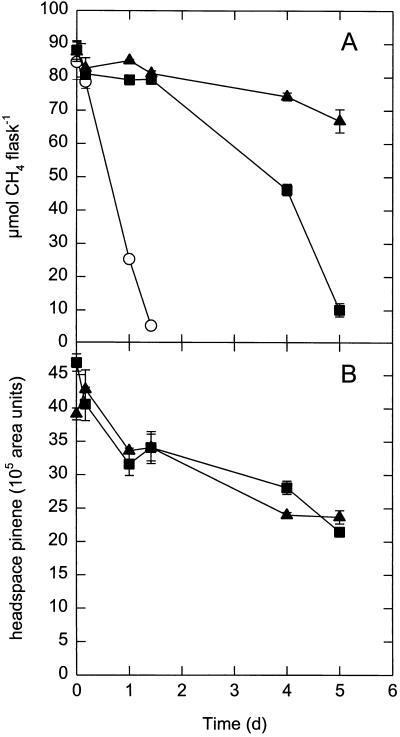
To determine if loss or degradation of the monoterpene was responsible for the observed transient inhibition, we monitored the headspace (−)-α-pinene content over time in inoculated and uninoculated serum bottles (Fig. 3). CH4 oxidation again occurred after a 2-day lag period and was nearly complete after 5 days when 1.1 mM (−)-α-pinene was added. However, the rate of oxidation was lower than the rate of oxidation in the inoculated control without (−)-α-pinene. The uninoculated control showed little change in CH4 concentration. The headspace pinene levels (and hence dissolved levels) decreased by one-half and at the same rate in both inoculated and uninoculated flasks (Fig. 3), indicating that microbial degradation or conversion of pinene was not significant. Furthermore, pinene addition did not stimulate CO2 production by the methanotrophs, and no conversion products were detected by gas chromatography (data not shown). Thus, it is likely that the decrease in headspace (−)-α-pinene content was due to adsorption to the walls or rubber stopper of the incubation vessel.
FIG. 3.
Changes in the levels of total CH4 (A) and headspace (−)-α-pinene (B) during CH4 oxidation by Methylosinus trichosporium OB3b. Control, inoculated flasks were incubated without (−)-α-pinene (○). (−)-α-Pinene (1.10 mM) was added to both inoculated (▪) and uninoculated (▴) flasks. An initial OD600 of 0.045 was used. The values are means ± standard errors of the means determined from duplicate experiments. d, day.
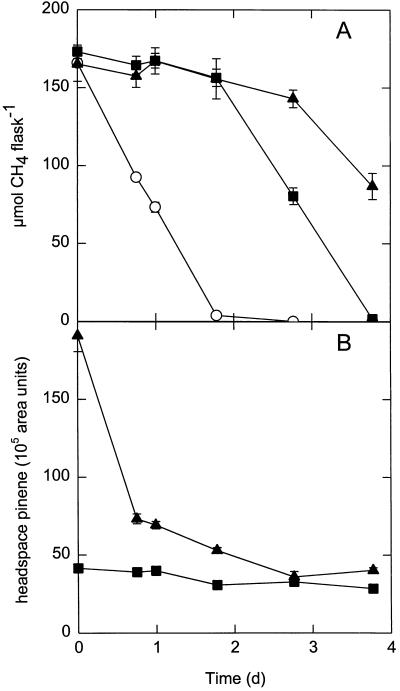
However, this decrease did not explain the transient nature of pinene inhibition. The level of volatile (−)-α-pinene in the headspace became stable after about 3 days of preincubation with shaking (Fig. 4). In preincubated flasks, in which the headspace (−)-α-pinene levels remained the same over the entire incubation period, the same pattern of inhibition was observed. Omitting the preincubation step resulted in a slightly longer lag phase in CH4 oxidation (Fig. 4).
FIG. 4.
Changes in the levels of total CH4 (A) and headspace (−)-α-pinene (B) in preincubated (▪) and nonpreincubated (▴) flasks inoculated with Methylosinus trichosporium OB3b. Preincubated flasks were supplemented with (−)-α-pinene and shaken for 3 days before inoculation. Nonpreincubated flasks were inoculated immediately after (−)-α-pinene was added. Control flasks were incubated without (−)-α-pinene (○). An initial cell OD600 of 0.075 and 1.80 mM (−)-α-pinene were used. The values are means ± standard errors of the means determined from triplicate experiments. d, day.
The mechanism of this inhibition is not known. One possibility is that the (−)-α-pinene has a direct effect on the MMO enzyme, as has been suggested for the AMO enzyme (34, 36). Group II methanotrophs (e.g., Methylosinus trichosporium) express a sMMO enzyme under Cu-deficient conditions and a membrane-bound, particulate MMO (pMMO) enzyme under Cu-sufficient conditions (14, 17). We tested the effect of different forms of pinene on cultures expressing one or the other of these enzyme forms. CH4 consumption by Methylosinus trichosporium OB3b was the same in control cultures (cultures containing no pinene) grown with and without Cu (Table 1). However, when cultures were supplemented with different pinenes, CH4 consumption by Cu-sufficient cultures was inhibited to a greater extent than CH4 consumption by Cu-deficient cultures. This trend was especially evident when (+)-α-pinene and (−)-β-pinene were added; in these cases inhibition of Cu-sufficient cultures was approximately 40% greater. Methylobacter luteus, a Group I methanotroph which produces only the Cu-dependent pMMO (14, 17), also exhibited little CH4 consumption with (+)-α-pinene compared to Methylosinus trichosporium incubated without Cu (Table 1). Interestingly, (−)-β-pinene was less inhibitory than the α isomers in each case, despite the very similar molecular structures of these compounds (Fig. 1).
TABLE 1.
Effects of α- and β-pinenes on CH4 oxidation by methanotrophs incubated with and without Cu
| Supplement | Amt of CH4 oxidized (μmol flask−1)a
|
||
|---|---|---|---|
|
Methylosinus trichosporium OB3bb
|
Methylobacter luteus with Cuc | ||
| Without Cu | With Cu | ||
| None (control) | 300 ± 6 | 278 ± 20 | 133 ± 11 |
| (−)-α-Pinene | 73 ± 7 (76) | 45 ± 21 (84) | 11 ± 15 (92) |
| (+)-α-Pinene | 187 ± 7 (38) | 26 ± 8 (91) | 21 ± 11 (84) |
| (−)-β-Pinene | 287 ± 13 (4) | 158 ± 15 (43) | 56 ± 4 (58) |
The values are means ± standard errors of the means determined from triplicate experiments. The values in parentheses are percentages of inhibition compared with the control.
Measured after 2 days of incubation with an initial CH4 content of 20% (vol/vol) and a cell OD600 of 0.095.
Measured after 1 day of incubation with an initial CH4 content of 10% (vol/vol) and a cell OD600 of 0.095.
Effects of different monoterpenoids on CH4 oxidation.
Twelve monoterpenoids, including oxide and alcohol forms, were tested to determine their effects on CH4 oxidation (Table 2). Activity was measured by measuring the CO2 produced, since this procedure detected low rates of CH4 oxidation more sensitively than measuring CH4 consumption in a headspace containing 10% CH4 initially. Our experiments showed that no CO2 production occurred unless CH4 was present (data not shown), which confirmed the reliability of this method. All of the hydrocarbon monoterpenes except β-myrcene showed strong inhibition. β-Myrcene was the only noncyclic unsaturated hydrocarbon monoterpene used. (−)-α-Pinene, the limonenes, and γ-terpinene showed the greatest inhibition (>80%). Both α-pinene oxide and (R)-(+)-limonene oxide were much less inhibitory than the corresponding hydrocarbon forms. (±)-Linalool was significantly more inhibitory than β-myrcene, despite the fact that these compounds are structurally very similar (Fig. 1). This greater inhibition may have been related to the much higher aqueous solubility of the alcohol monoterpenoids (32). As observed previously (Table 1), (−)-β-pinene was less inhibitory than (−)-α-pinene. The difference between these two forms of pinene is the position of the unsaturated C-C bond (subterminal in the α isomer and terminal in the β isomer). The results suggest that in general, the lack of a subterminal double bond decreases the potential of monoterpenes to inhibit CH4 oxidation, as shown by the low levels of inhibition observed with α-pinene oxide, (−)-β-pinene, and (R)-(+)-limonene oxide, which lack the C-C double bond of the ring (Fig. 1). The presence of a C ring may also be important, as suggested by the low level of inhibition observed with β-myrcene (Table 2).
TABLE 2.
Inhibition of CH4 oxidation by Methylosinus trichosporium OB3b (OD600, 0.06) with selected monoterpenes (concentration, 1.8 mM)
| Expt | Compound addeda | % Inhibition of CH4 oxidationb |
|---|---|---|
| A | (−)-α-Pinene | 96 ± 5 |
| α-Pinene oxide | −3 ± 0.1 | |
| (−)-β-Pinene | 41 ± 4 | |
| (R)-(+)-Limonene | 84 ± 22 | |
| (S)-(−)-Limonene | 93 ± 16 | |
| (+)-Limonene oxide | 29 ± 7 | |
| α-Terpinene | 25 ± 6 | |
| γ-Terpinene | 82 ± 10 | |
| B | (−)-α-Pinene | 89 ± 5 |
| β-Myrcene | 7 ± 1 | |
| (±)-Linalool | 68 ± 3 | |
| α-Terpineol | 48 ± 3 |
The amounts used in experiments A and B were 81.1 and 44.6 μmol flask−1, respectively.
Based on CO2 production over 1.8 days and compared to a control to which no monoterpene was added. The values are means ± standard errors of the means determined from duplicate experiments.
Effects of monoterpenes on denitrifiers.
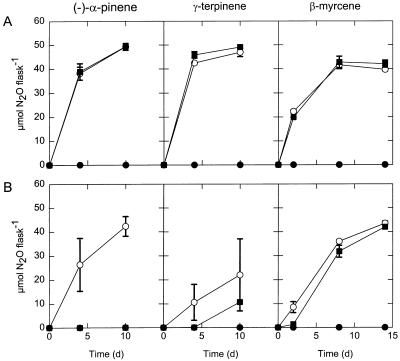
Three monoterpenes, (−)-α-pinene, γ-terpinene, and β-myrcene, were tested to determine their effects on denitrification by isolates from Hamilton Harbour and Mt. St. Hilaire. The accumulation of N2O by a Hamilton Harbour isolate (isolate HHI) (Fig. 5A) was the same in the presence of acetylene alone and in the presence of acetylene plus monoterpene, indicating that none of the monoterpenes tested inhibited denitrification of nitrate to N2O. In contrast, a swamp soil isolate (isolate D1) (Fig. 5B) showed N2O accumulation only with acetylene alone, and no denitrification occurred when (−)-α-pinene was present. Only partial N2O production (compared to flasks containing only acetylene) occurred with γ-terpinene, and β-myrcene had no effect. A similar pattern was obtained with four other isolates (HH3, HH4, HH6, and D3), in which case only swamp soil isolate D3 showed sensitivity to (−)-α-pinene (data not shown). Both isolate D1 and isolate D3 also failed to grow aerobically in the presence of (−)-α-pinene (data not shown), indicating that this compound exhibited general antimicrobial action against these organisms. We found no evidence of specific inhibition of N2O reduction by monoterpenes since no N2O accumulated with monoterpene alone. Our results illustrate the considerable differences in tolerance to monoterpenes of different microbes.
FIG. 5.
Effects of selected monoterpenes on denitrification (N2O accumulation) by environmental isolates from Hamilton Harbour (HH1)(A) and Mt. St. Hilaire (D1)(B). Flasks were incubated with acetylene (○), monoterpene (•), and acetylene plus monoterpene (▪). The initial culture densities (OD600) were 0.12 (for pinene and terpinene additions) and 0.03 (for myrcene additions). The monoterpene concentrations were 3.70 mM. The results for isolate HH1 (A) are representative of the results obtained with isolates HH3, HH4, HH6, and the results for isolate D1 (B) are similar to the results obtained with isolate D3 (see text). The values are means ± standard errors of the means determined from duplicate experiments. d, day.
Effects of monoterpenes on aerobic activity by several heterotrophs.
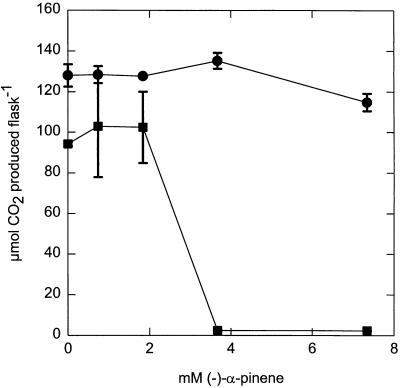
We compared the sensitivities of methanotrophs and denitrifiers used in this study with the sensitivities of some aerobically grown heterotrophic reference strains. (−)-α-Pinene (3.7 mM) had a negligible effect on the final CO2 concentrations in E. coli, P. aeruginosa, and Serratia marcescens cultures compared to control cultures, showing that these organisms were not inhibited (Table 3). This lack of effect occurred despite the high ratios of monoterpene content to cell density used compared to the experiments performed with methanotrophs (Fig. 1 through 3 and Table 2). However, Staphylococcus aureus produced 33% less CO2 and B. subtilis produced 98% less CO2 in the presence of (−)-α-pinene (Table 3). The levels of CO2 evolution during 38 h in E. coli and B. subtilis cultures (initial OD600, 0.01) were compared after different (−)-α-pinene concentrations were added (final concentrations, 0 to 7.3 mM) (Fig. 6). No significant effect was found for E. coli over the concentration range tested, but for B. subtilis there was a sharp decrease in CO2 evolution in the presence of (−)-α-pinene concentrations greater than 1.8 mM. Similar inhibition patterns for these bacteria have been observed with other monoterpenes (7, 27).
TABLE 3.
Aerobic metabolism by heterotrophic bacteria (CO2 evolution over a 38-h period; initial OD600, 0.01) in the presence and absence of (−)-α-pinene (concentration, 3.7 mM)
| Organism | Amt (μmol) of CO2 produced day−1
|
% Inhibition | |
|---|---|---|---|
| Control | Culture containing α-pinene | ||
| Escherichia coli | 151 ± 1a | 139 ± 0.1 | 8 |
| Pseudomonas aeruginosa | 255 ± 9 | 268 ± 10 | −5 |
| Serratia marcescens | 188 ± 1 | 203 ± 14 | −8 |
| Staphylococcus aureus | 167 ± 6 | 111 ± 15 | 33 |
| Bacillus subtilis | 125 ± 2 | 2.6 ± 0.1 | 98 |
The values are means ± standard errors of the means determined from duplicate experiments.
FIG. 6.
Effects of different levels of (−)-α-pinene on aerobic metabolism (CO2 production) of E. coli (•) and B. subtilis (▪) over a 38-h period. The initial cell OD600 was 0.01. The values are means ± standard errors of the means determined from duplicate experiments.
DISCUSSION
The effects of monoterpenoids on microbial growth and activity have been studied primarily to determine the potential use of these compounds in preventing growth of pathogens in the food industry (20, 27). Microbial biotransformation of monoterpenoids into commercially valuable compounds (10) and degradation of monoterpene-containing wastes from industrial sources (19, 24) have also received attention. However, relatively little is known about how monoterpenes affect bacterial processes in nature, such as the cycling of elements. Monoterpene inputs into forest soils stimulate microbial metabolism (31) and can enhance assimilation of ammonium (9). It has been suggested that monoterpenes are important inhibitors of nitrification in some forest soils (34, 36) and in cultures of Nitrosomonas europaea (11). Recently, we found that forest soil methanotrophs are similarly inhibited (3). In the current study, we found that cultures of methanotrophs and some denitrifying environmental isolates are more sensitive to monoterpene inhibition than are several other bacteria.
The magnitude of the inhibitory effect of (−)-α-pinene depended on the concentration of the compound. For example, a (−)-α-pinene concentration of 0.73 mM was required to cause a significant effect on Methylosinus trichosporium OB3b cultures having an initial OD600 of 0.05 (6.75 × 106 cells ml−1 [14]). Other workers have reported that inhibition depends on the ratio of monoterpene to cells for bacteria (27) and yeasts (30). This effect has been interpreted as indicating that cellular uptake of monoterpenes occurs, perhaps into the hydrophobic membrane (30). However, up to tenfold more (−)-α-pinene had little effect on E. coli cultures having much lower initial densities (OD600, 0.01). Large differences in susceptibility among different bacteria, especially differences among E. coli, Serratia marcescens, and B. subtilis (see above), have also been described by other workers (7, 27), and these differences may depend on the mechanism of action of the compounds (see below). The high sensitivity which we observed in methanotrophs, however, is important because it has an impact on one of the major global CH4 sinks, biological CH4 consumption (17).
The concentrations of monoterpenes used in this study (0.37 to 3.7 mM or 0.05 to 0.5 mg ml of culture−1) are ecologically relevant. For example, monoterpene levels in excess of 3 to 5 mg g−1 occur in forest litter layers and fresh foliage (36, 38). Thus, inhibition of methanotrophy in nature by these compounds is possible, as recently observed with aqueous extracts of other forest soil components (5). Such an effect is consistent with the lack of methane consumption seen in the top layers of many forest soils (1, 6).
The differences in susceptibilities to monoterpenes among denitrifying environmental isolates suggest that any control of denitrification by these compounds in different environments depends on the denitrifiers present. The lack of sensitivity of the Hamilton Harbour isolates (HH1, HH3, HH4, and HH6) is interesting in view of the fact that plant-derived terpenoids induce polychlorinated biphenyl degradation by bacteria (13) because of structural similarities between the molecules. Hamilton Harbour is a highly polluted site containing a variety of industrial wastes, including aromatic compounds (26). It is possible that bacteria surviving in this environment acquire a tolerance for cyclic organic compounds. Species-specific differences, as observed for E. coli and B. subtilis, may also explain the different levels of tolerance of the denitrifiers to monoterpenes. Although none of the isolates could use (−)-α-pinene, α-terpinene, or β-myrcene as a single carbon source (25), denitrifying monoterpene degraders have been isolated from Hamilton Harbour sediments (25), as well as from activated sludge and waterlogged forest soil (18). Although we cannot conclude that monoterpenes are specific inhibitors of denitrification, it is clear that these compounds inhibit a subset of denitrifiers, which makes them potential factors in controlling the process in some environments.
The general antimicrobial properties of monoterpenoids may be related to their interactions with microbial membranes, because of their hydrophobicity (30). At high levels (5 mM) they can disrupt electron transport and uncouple oxidative phosphorylation in bacteria (22). Andrews et al. (7) found that α-pinene (2 mM) disrupted the cytoplasmic membranes of Saccharomyces cerevisiae and the gram-positive organism Bacillus thuringiensis, but that gram-negative bacteria were more resistant to terpenes. β-Pinene inhibited respiration at the cytochrome b portion of the electron transport chain of yeast cells (30). White (34, 35) proposed a more specific mechanism for the inhibition of nitrification by monoterpenes, a mechanism involving direct binding to the AMO enzyme, based on the similarity of these compounds to many known nitrification inhibitors. Indeed, monoterpene-dependent inhibition of nitrification by pure cultures of N. europaea does occur (11), but the actual mechanism remains speculative. The similar properties of the AMO of nitrifiers and the MMO of methanotrophs (17), including sensitivity to the same inhibitors (8), suggest that monoterpenes might inhibit CH4 oxidation and nitrification in similar ways.
Green and Dalton (15) found that purified sMMO of Methylococcus capsulatus (Bath) converted β-pinene to β-pinene oxide and another product. This implies that β-pinene bound to the active site of the enzyme, a characteristic of a competitive inhibitor. We did not detect any volatile conversion products of β-pinene or other monoterpenes resulting from incubation with methanotrophic cultures. However, such conversions may have occurred but resulted in products that were below the level of sensitivity of the analytical method used. The higher level of inhibition seen under conditions that support expression of the pMMO than under those that support expression of the sMMO may indicate that there is an indirect effect involving membrane disruption by the monoterpenes. It is possible that a variety of specific and general effects work in concert to give the inhibition seen with the methanotrophs and other bacteria used in our study.
No matter what the actual mechanisms involved, inhibition showed specificity with regard to monoterpene structure. We found that in general, the presence of a C ring and a subterminal C-C double bond was important for inhibition. This differs slightly from White’s proposal (34). White postulated that a terminal C-C double bond, when associated with a six-carbon ring structure, should be highly inhibitory to nitrification, since this structure is similar to the structure of many nongaseous inhibitors of AMO. Of course, as discussed above, inhibition by monoterpenes may occur via a variety of actions that do not necessarily involve specific action on an enzyme. Indirect action on associated proteins and indirect action by chelation of required metals, such as Cu2+ (8), are examples of other mechanisms. It should also be noted that some inhibitors (for example, nitrapyrin) may function differently in nitrifiers and methanotrophs (8).
Understanding the mechanism of inhibition should help explain the transient nature of CH4 oxidation inhibition that we observed. One possible explanation is that, upon initial contact with the monoterpene, a significant fraction of the cells are killed, leading to a lag phase during which activity by the survivors is undetectable. A variety of physical and chemical factors may also influence the inhibitory properties of monoterpenes. For example, it has been proposed that molecular aggregation and droplet size affect the toxicity of monoterpenes (29).
In conclusion, monoterpenes appear to be potentially important inhibitors of processes such as CH4 oxidation and, in some cases, denitrification. The monoterpene concentrations that we used are environmentally relevant in view of the reported natural levels (36, 38). Their inhibitory effects of monoterpenes on methanotrophs, for example, may contribute to the stratification of methane consumption in forest soils (1, 5, 6). Thus, given their widespread distribution, monoterpenes may be regulators of microbial processes in nature. Because of the increased use of these compounds by industries (21, 24) and their large-scale release by wood pulping processes (19), these effects may be even more important.
ACKNOWLEDGMENTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and by NSERC-BOREAS (Boreal Ecosystem Atmosphere Study).
REFERENCES
- 1.Adamsen A P S, King G. Methane consumption in temperate and subarctic forest soils: rates, vertical zonation, and responses to water and nitrogen. Appl Environ Microbiol. 1993;59:485–490. doi: 10.1128/aem.59.2.485-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral J A, Archambault C, Richards S R, Knowles R. Denitrification associated with Groups I and II methanotrophs in a gradient enrichment system. FEMS Microbiol Ecol. 1995;18:289–298. [Google Scholar]
- 3.Amaral, J. A., and R. Knowles. Unpublished data.
- 4.Amaral J A, Knowles R. Methane metabolism in a temperate swamp. Appl Environ Microbiol. 1994;60:3945–3951. doi: 10.1128/aem.60.11.3945-3951.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaral, J. A., and R. Knowles. Inhibition of methane consumption in forest soils and pure cultures of methanotrophs by aqueous forest soil extracts. Soil Biol. Biochem., in press.
- 6.Amaral J A, Knowles R. Localization of methane consumption and nitrification activities in some boreal forest soils and the stability of methane consumption on storage and disturbance. J Geophys Res-Atmos. 1997;102:29,255–29,260. [Google Scholar]
- 7.Andrews R E, Parks L W, Spence K D. Some effects of Douglas fir terpenes on certain microorganisms. Appl Environ Microbiol. 1980;40:301–304. doi: 10.1128/aem.40.2.301-304.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bédard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bremner J M, McCarty G W. Effects of terpenoids on nitrification in soil. Soil Sci Soc Am J. 1988;52:1630–1633. [Google Scholar]
- 10.Chang H C, Oriel P. Bioproduction of perillyl alcohol and related monoterpenes by isolates of Bacillus stearothermophilus. J Food Sci. 1994;59:660–662. [Google Scholar]
- 11.Courtney K J, Ward B B, Langenheim J H. The effect of coastal redwood monoterpenes on Nitrosomonas europaea. Am J Bot Suppl. 1991;78:144–145. [Google Scholar]
- 12.Dev S. Handbook of terpenoids-monoterpenoids. 1 and 2. Boca Raton, Fla: CRC Press; 1982. [Google Scholar]
- 13.Gilbert E S, Crowley D E. Plant compounds that induce polychlorinated biphenyl biodegradation by Arthrobacter sp. strain B1B. Appl Environ Microbiol. 1997;63:1933–1938. doi: 10.1128/aem.63.5.1933-1938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham D W, Chaudhary J A, Hanson R S, Arnold R G. Factors affecting competition between type I and type II methanotrophs in two-organism, continuous-flow reactors. Microb Ecol. 1993;25:1–17. doi: 10.1007/BF00182126. [DOI] [PubMed] [Google Scholar]
- 15.Green J, Dalton H. Substrate specificity of soluble methane monooxygenase: mechanistic implications. J Biol Chem. 1989;264:17698–17703. [PubMed] [Google Scholar]
- 16.Hakola H, Shorees B, Arey J, Atkinson R. Product formation from the gas-phase reactions of OH radicals and O3 with β-phellandrene. Environ Sci Technol. 1993;27:278–283. [Google Scholar]
- 17.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harder J, Probian C. Microbial degradation of monoterpenes in the absence of molecular oxygen. Appl Environ Microbiol. 1995;61:3804–3808. doi: 10.1128/aem.61.11.3804-3808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keith L H. Identification of organic compounds in unbleached Kraft paper mill wastewaters. Environ Sci Technol. 1976;10:555–564. [Google Scholar]
- 20.Kim J, Marshall M R, Wei C. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem. 1995;43:2839–2845. [Google Scholar]
- 21.Kirchner E M. Environment, health concerns force shift in use of organic solvents. Chem Eng News. 1994;72:13–20. [Google Scholar]
- 22.Knobloch K, Weigand H, Weis N, Schwarm H-M, Vigenschow H. Action of terpenoids on energy metabolism. In: Brunke E-J, editor. Progress in essential oil research. Berlin, Germany: Walter de Gruyter and Co.; 1986. pp. 429–445. [Google Scholar]
- 23.Lerdau M, Guenther A, Monson R. Plant production and emission of volatile organic compounds. BioScience. 1997;47:373–383. [Google Scholar]
- 24.Misra G, Pavlostathis S G, Perdue E M, Araujo R. Aerobic biodegradation of selected monoterpenes. Appl Microbiol Biotechnol. 1996;45:831–838. doi: 10.1007/s002530050770. [DOI] [PubMed] [Google Scholar]
- 25.Richards, S. R., and R. Knowles. Unpublished data.
- 26.Richards S R, Knowles R. Inhibition of nitrous oxide reduction by a component of Hamilton Harbour sediment. FEMS Microbiol Ecol. 1995;17:39–46. [Google Scholar]
- 27.Subba M S, Soumithri T C, Suryanarayana Rao R. Antimicrobial action of citrus oils. J Food Sci. 1967;32:225–227. [Google Scholar]
- 28.Trudgill P W. Microbial metabolism of monoterpenes—recent developments. Biodegradation. 1990;1:93–105. doi: 10.1007/BF00058829. [DOI] [PubMed] [Google Scholar]
- 29.Uribe S, Peña A. Toxicity of allelopathic monoterpene suspensions on yeast. J Chem Ecol. 1990;16:1399–1408. doi: 10.1007/BF01021035. [DOI] [PubMed] [Google Scholar]
- 30.Uribe S, Ramirez J, Peña A. Effects of β-pinene on yeast membrane functions. J Bacteriol. 1985;161:1195–1200. doi: 10.1128/jb.161.3.1195-1200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vokou D, Margaris N S. Decomposition of terpenes by soil microorganisms. Pedobiologia. 1988;31:413–419. [Google Scholar]
- 32.Weidenhamer J D, Macias F A, Fischer N H, Williamson G B. Just how insoluble are monoterpenes? J Chem Ecol. 1993;19:1799–1807. doi: 10.1007/BF00982309. [DOI] [PubMed] [Google Scholar]
- 33.White C S. Volatile and water-soluble inhibitors of nitrogen mineralization and nitrification in forest ecosystems in New Mexico. Biol Fertil Soils. 1986;2:97–104. [Google Scholar]
- 34.White C S. Nitrification inhibition by monoterpenoids: theoretical mode of action based on molecular structures. Ecology. 1988;69:1631–1633. [Google Scholar]
- 35.White C S. Comments on “Effects of terpenoids on nitrification in soil.”. Soil Sci Soc Am J. 1990;54:296–297. [Google Scholar]
- 36.White C S. Monoterpenes: their effects on ecosystem nutrient cycling. J Chem Ecol. 1994;20:1381–1405. doi: 10.1007/BF02059813. [DOI] [PubMed] [Google Scholar]
- 37.Whittenbury R, Phillips K C, Wilkinson J F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 38.Wilt F M, Miller G C, Everett R L, Hackett M. Monoterpene concentrations in fresh, senescent, and decaying foliage of single-leaf pinyon (Pinus monophylla Torr. & Frem.:Pinaceae) from the western Great Basin. J Chem Ecol. 1993;19:185–194. doi: 10.1007/BF00993688. [DOI] [PubMed] [Google Scholar]
- 39.Zubyk W J, Conner A Z. Analysis of terpene hydrocarbons and related compounds by gas chromatography. Anal Chem. 1960;32:912–917. [Google Scholar]