Abstract
Background:
This systematic review and meta-analysis aimed to determine the efficacy of macitentan in patients with pulmonary hypertension (PH).
Methods:
A systematic search was made of PubMed, Embase, Cochrane Library, and clinicaltrials.gov, without language restrictions. Randomized controlled trials (RCTs) on treatment of PH with macitentan, compared with placebo or blank, were reviewed. Studies were pooled to weighted mean differences (WMDs) and risk ratios (RRs), with 95% confidence intervals (CIs).
Results:
Six RCTs (enrolling 1,003 participants) met the inclusion criteria. Macitentan showed significant effects on 6-min walk distance (6MWD) (WMD 12.06 m, 95% CI 2.12 to 21.99 m), pulmonary vascular resistance (PVR) (WMD –186.51 dyn·s/cm–5, 95% CI –232.72 to –140.29 dyn·s/cm–5), mean pulmonary artery pressure (mPAP) (WMD –3.20 mmHg, 95% CI –5.93 to –0.47 mmHg), N-terminal pro-brain natriuretic peptide (NT-proBNP) (WMD –232.47 ng/L, 95% wCI –318.22 to –146.72 ng/L), and cardiac index (WMD 0.39 L/min/m2, 95% CI 0.20 to 0.58 L/min/m2).
Conclusion:
Macitentan significantly improved 6MWD, PVR, mPAP, NT-proBNP, and cardiac index in patients with PH. Macitentan should be further validated in patients with PH.
Keywords: macitentan, pulmonary hypertension, efficacy, meta-analysis
Introduction
Pulmonary hypertension (PH) is a life-threatening disease defined as mean pulmonary arterial pressure (mPAP) ≥20 mmHg at rest, as measured by right heart catheterization [1,2]. According to the mechanism or underlying etiology, PH is divided into five clinical groups by the World Health Organization (WHO): Pulmonary arterial hypertension (PAH), PH secondary to left heart disease (PH-LHD), PH associated with hypoxemia, PH due to chronic thrombotic disease, embolic disease, or both, and miscellaneous [3,4]. Despite the availability of treatments for all subgroups of PH, the prognosis for PH remains poor [3,5]. The dual endothelin receptor antagonist (ERA) macitentan is characterized by sustained receptor binding [6], which is achieved by modifying the structure of bosentan to improve efficacy and safety [7]. Only a few randomized controlled trials (RCTs) have evaluated the efficacy of macitentan in patients with PH, and their results differed.
Therefore, the aim of this study was to perform a systematic review and meta-analysis of RCTs to determine the efficacy of macitentan in patients with PH.
Methods
Data sources and search strategy
This systematic review and meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [8]. The protocol was previously registered in November 2022 in the PROSPERO database (Review register: CRD42022371410). The PubMed, Embase, Cochrane Library, and clinicaltrials.gov were searched for studies up to November 2022.
Study selection
To be eligible for inclusion in the meta-analysis studies had to meet the following criteria: (a) inclusion of patients with PH aged ≥12 years, mPAP ≥25 mmHg, pulmonary vascular resistance (PVR) ≥240 dyn·s/cm–5, and 6-min walk distance (6MWD) ≥50 m, according to definition of WHO [1]; (b) use of a randomized controlled design to make a comparison of macitentan (10 mg once daily) with placebo or blank; and (c) follow-up for 12 weeks or longer to observe the efficacy. Studies were excluded if they included patients with the following: (a) severe co-existing diseases with poor functional status (eg, advanced renal failure, severe hepatic impairment); (b) current ERAs treatment; (c) previous pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty (BPA); (d) pregnant or lactating women; and (e) drug or alcohol abuse. The search strings used for the databases were (‘macitentan’ OR ‘Actelion-1’ OR ‘ACT-064992’) AND (‘pulmonary arterial hypertension’ OR ‘pulmonary hypertension’ OR ‘pulmonary artery pressure’ OR ‘PH’). The reference lists of any relevant review articles were also screened to identify studies that might have been missed in this search. No language restrictions were applied to our study selection process.
Data extraction and quality assessment
Two reviewers independently screened articles according to the inclusion criteria. The reviewers compared selected studies and differences were resolved by consensus. A third reviewer acted as arbitrator in case of discrepancy between reviewers. Data tables were used to collect all relevant data from texts, tables and figures of each included trial, including author, year of publication or last update posted, patient number and age, duration of follow-up, treatment category, baseline 6MWD, WHO functional class (FC), and outcomes such as 6MWD, PVR, mPAP, N-terminal pro-brain natriuretic peptide (NT-proBNP), cardiac index, improvement in WHO FC, hospitalization for worsening of PH, death due to PH, and all-cause death. Study quality was assessed using the Detsky Quality Assessment Scale [9,10,11,12,13]. This is a 20-point scale for studies with statistically significant results and a 21-point scale for studies without statistically significant results.
Risk of bias of included trials
Two reviewers independently assessed the risk of bias using the Cochrane collaboration risk of bias tool for RCTs [14].
Data synthesis and statistical analysis
Meta-analyses were conducted where applicable; otherwise, outcomes were presented in narrative form. Data were analyzed using the RevMan Version 5.4.1 (The Cochrane Collaboration). Next, risk ratios (RRs) for discontinuous outcomes, and weighted mean differences (WMDs) for continuous outcomes, with corresponding 95% confidence intervals (CIs) were computed for individual trials. Chi-squared and Higgins I2 tests were used to assess heterogeneity among included trials. If significant heterogeneity (p ≤ 0.10 for Chi-squared test results or I2 ≥ 50%) was obtained, we used a random-effects model, otherwise a fixed-effects model was used. And a P value <0.05 was taken to indicate statistical significance. The P value of Egger’s linear regression test (STATA version 12.0) was used to assess the presence of publication bias in included studies for each outcome.
Results
Study selection and characteristics
Of 1,825 studies recognized by the initial search, 57 were retrieved for more detailed assessment, and 6 trials [15,16,17,18,19,20,28] were included in this meta-analysis (Figure 1). Baseline characteristics of trials included in this meta-analysis are shown in Table 1. A total of 1,003 patients were included: 498 assigned to the macitentan treatment groups and 505 to the control groups. The risk of bias results are summarized in Figure S1.
Figure 1.

Flow chart for selection of studies.
Table 1.
Baseline characteristics of trials included in meta-analysis.
|
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STUDY(REF. #) | YEAR | QUALITY SCORE | FOLLOW-UP WEEKS | PH TYPE | REGIMEN | n | AGE, YEARS (SD) | MALE, % | 6MWD, M (SD) | WHO FC, % | |||
|
| |||||||||||||
| I | II | III | IV | ||||||||||
|
| |||||||||||||
| Gatzoulis (15) | 2019 | 19 | 16 | PAH | Macitentan | 114 | 33 * | 28.1 | 368.7 (74.5) | 0 | 60.5 | 30.5 | 0 |
|
| |||||||||||||
| Placebo | 112 | 31 * | 39.3 | 380.3 (76.3) | 0 | 58.9 | 41.1 | 0 | |||||
|
| |||||||||||||
| Ghofrani (16) | 2017 | 20 | 24 | CTEPH | Macitentan | 40 | 58.2 (14.0) | 35 | 353.0 (87.9) | 0 | 30 | 70 | 0 |
|
| |||||||||||||
| Placebo | 40 | 56.9 (13.9) | 38 | 351.2 (73.8) | 0 | 15 | 82.5 | 2.5 | |||||
|
| |||||||||||||
| Pulido (17) | 2013 | 18 | 104 | PAH | Macitentan | 242 | 44.5 (16.3) | 19.8 | 363 (93.2) | 0.4 | 49.6 | 47.9 | 2.1 |
|
| |||||||||||||
| Placebo | 250 | 46.7 (17.0) | 26.1 | 352 (110.6) | 0 | 51.8 | 46.6 | 1.6 | |||||
|
| |||||||||||||
| Sitbon (19) | 2019 | 21 | 12 | PAH | Macitentan | 43 | 58.0 (8.7) | 51 | 385.8 (100.0) | 2 | 63 | 35 | 0 |
|
| |||||||||||||
| Placebo | 42 | 59.0 (9.5) | 52 | 383.2 (108.9) | 2 | 55 | 43 | 0 | |||||
|
| |||||||||||||
| Vachiéry (20) | 2018 | 17 | 12 | PH-LHD | Macitentan | 31 | 70.0 * | 19.4 | 300 * | 0 | 16.1 | 83.9 | 0 |
|
| |||||||||||||
| Placebo | 32 | 72.0 * | 50.0 | 305 * | 0 | 31.3 | 68.8 | 0 | |||||
|
| |||||||||||||
| SOPRANO (28) | 2021 | 16 | 12 | PH-LHD | Macitentan | 28 | 56.5 (8.2) | 78.6 | NR | NR | NR | NR | NR |
|
| |||||||||||||
| Placebo | 29 | 58.2 (7.0) | 79.3 | NR | NR | NR | NR | NR | |||||
|
| |||||||||||||
Abbreviations: CTEPH, chronic thromboembolic pulmonary hypertension; 6MWD, 6-min walk distance; NR, not reported; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PH-LHD, PH secondary to left heart disease; SD, standard deviation; WHO FC, World Health Organization functional class. * Median.
6-Min walk distance
Data on the change of 6MWD were extracted from five RCTs (929 patients). Compared with the control conditions, macitentan treatment significantly improved 6MWD (WMD 12.06 m, 95% CI 2.12 to 21.99 m; P = 0.02 [Figure 2A]). There was no significant heterogeneity (I2 = 43%; P = 0.14). Egger’s test (P = 0.742) did not show evidence of publication bias.
Figure 2.
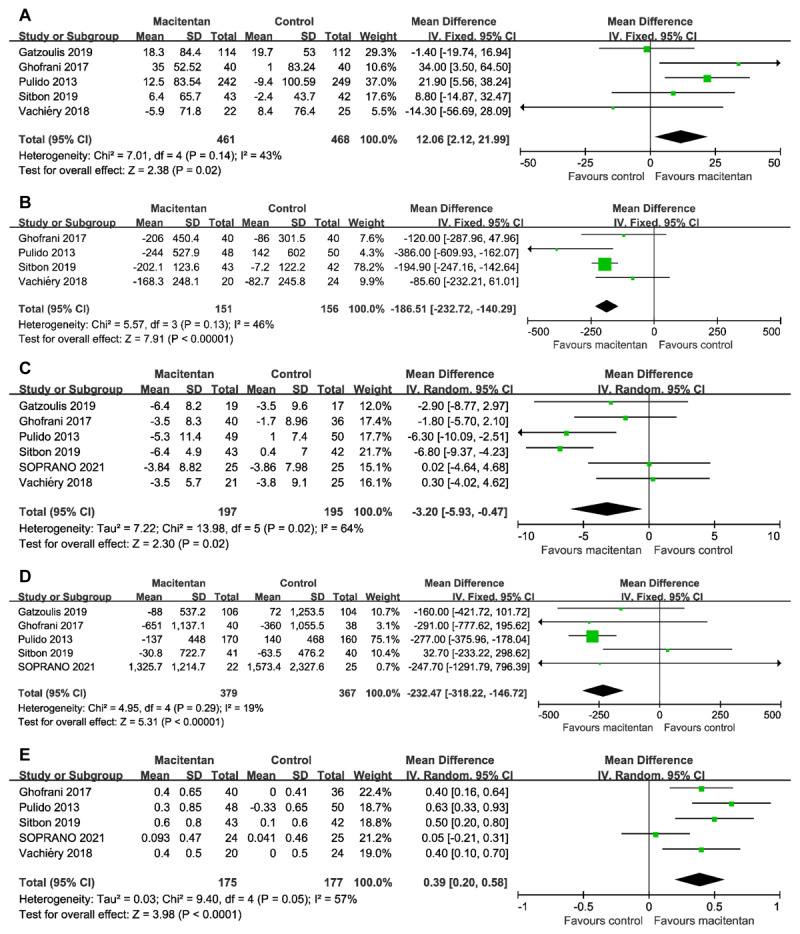
Forest plot assessing the efficacy of macitentan on (A) 6-min walk distance, (B) pulmonary vascular resistance, (C) mean pulmonary artery pressure, (D) NT-proBNP, and (E) cardiac index.
Pulmonary vascular resistance
The change of PVR was evaluated in four RCTs (307 patients). Compared with the control conditions, macitentan treatment significantly decreased PVR (WMD –186.51 dyn·s/cm–5, 95% CI –232.72 to –140.29 dyn·s/cm–5; P < 0.00001 [Figure 2B]). There was no significant heterogeneity (I2 = 46%; P = 0.13). Egger’s test (P = 0.980) did not show evidence of publication bias.
Mean pulmonary artery pressure
The change of mPAP was evaluated in six randomized studies (392 patients). Compared with the control conditions, macitentan treatment significantly decreased mPAP (WMD –3.20 mmHg, 95% CI –5.93 to –0.47 mmHg; P = 0.02 [Figure 2C]). There was significant heterogeneity (I2 = 64%; P = 0.02). Egger’s test (P = 0.271) did not show evidence of publication bias.
N-terminal pro-brain natriuretic peptide
Data on the change of NT-proBNP were extracted from five RCTs (746 patients). Compared with the control conditions, the use of macitentan significantly decreased the level of NT-proBNP (WMD –232.47 ng/L, 95% CI –318.22 to –146.72 ng/L; P < 0.00001 [Figure 2D]). There was no significant heterogeneity (I2 = 19%; P = 0.29). Egger’s test (P = 0.200) did not show evidence of publication bias.
Cardiac index
Data on the change of cardiac index were available from five trials (352 patients). Compared with the control groups, macitentan significantly improved cardiac index (WMD 0.39 L/min/m2, 95% CI 0.20 to 0.58 L/min/m2; P < 0.0001 [Figure 2E]). There was significant heterogeneity (I2 = 57%; P = 0.05). Egger’s test (P = 0.414) did not show evidence of publication bias.
Improvement in WHO functional class (FC)
Five RCTs reported data on improvement in WHO FC (933 patients). The percentage of patients with improvement in WHO FC did not differ significantly between the two groups (RR 1.32, 95% CI 0.99 to 1.74; P = 0.06 [Figure 3A]). There was no significant heterogeneity (I2 = 30%; P = 0.22). And the percentage in macitentan groups was 19.61% compared with 14.93% in control groups. Egger’s test (P = 0.251) did not show evidence of publication bias.
Figure 3.

Forest plot assessing the efficacy of macitentan on (A) improvement in WHO FC, (B) hospitalization for worsening of PH, (C) PH-related death, and (D) all-cause death.
Hospitalization for worsening of PH
Two trials reported data on hospitalization for worsening of PH (including 555 patients). The percentage of patients with hospitalization for worsening of PH did not differ significantly between the two groups (RR 1.00, 95% CI 0.25 to 4.05; P = 1.00 [Figure 3B]). There was significant heterogeneity among the studies (I2 = 70%; P = 0.07). And the percentage in macitentan groups was 18.32% compared with 28.72% in control groups.
P-Hrelated death
Five RCTs reported data on PH-related death (946 patients), and only two trials had patient deaths. There was no statistically significant difference in PH-related mortality between the two groups (RR 0.50, 95% CI 0.21 to 1.17; P = 0.11 [Figure 3C]). There was no significant heterogeneity (I2 = 0%; P = 0.79). The PH-related mortality in macitentan groups was 1.49% compared with 3.15% in control groups.
All-cause death
Six RCTs reported data on all-cause death (1,003 patients), and five trials had patient deaths. There was no statistically significant difference in all-cause mortality between the two groups (RR 0.82, 95% CI 0.46 to 1.91; P = 0.50 [Figure 3D]). There was no significant heterogeneity (I2 = 0%; P = 0.54). The all-cause mortality in macitentan groups was 3.61% compared with 4.55% in control groups. Egger’s test (P = 0.678) did not show evidence of publication bias.
Discussion
This systematic review and meta-analysis is designed specifically to evaluate RCTs that have explored the efficacy of macitentan in patients with PH. Based on the current results, we observed that macitentan significantly improved 6MWD, PVR, mPAP, NT-proBNP, and cardiac index. There was a trend for improvement in WHO FC and reduction in PH-related deaths with macitentan, although not statistically significant.
PH is a major global health problem. The prevalence of PH in the global population is about 1%, and even reaches 10% in people over 65 years old [21]. In recent years, research on macitentan has focused on PAH, PH-LHD, and chronic thromboembolic PH (CTEPH). Pulmonary arterial hypertension (group 1) is characterized by loss and obstructive remodeling of the pulmonary vascular bed. Pulmonary arterial hypertension is characterized by precapillary PH, defined as mPAP ≥20 mm Hg, pulmonary artery wedge pressure (PAWP) ≤15 mm Hg, and PVR ≥3 Wood units (WU) [1]. Chronic elevation of PVR may cause progressive right ventricular (RV) dysfunction and RV failure (RVF) [22]. Persistence of RVF may lead to increased right atrial pressure and decreased cardiac index [3]. Despite guidelines recommending combination therapy targeting multiple pathways of PAH, patient outcomes remain poor [21,23]. PH-LHD (group 2) is initially caused by a passive increase in left atrial pressure and develops further in response to endothelial dysfunction and vasoconstriction [24]. In patients with chronic heart failure and PH-LHD, elevated plasma endothelin-1 levels are associated with increased pulmonary pressure [25] and greater risk of death [26]. Chronic thromboembolic PH (group 4) is characterized by pulmonary artery obstruction and vascular remodeling caused by chronic organized thrombus. Although CTEPH has treatment options of PEA and BPA. Some patients who are ineligible for treatment or have residual or recurrent PH still lack effective treatments.
Limitations
This study met most of the methodological criteria recommended for systematic reviews and meta-analyses [27]. However, some limitations need to be considered when interpreting the results of this study. Firstly, some included studies had small sample sizes, which may have reduced the power of the results. Secondly, the number of included studies was small. Thirdly, some potential confounding between-study variables could have influenced outcomes and thus this may have also affected our meta-analysis results. Fourthly, this meta-analysis included three different clinical groups of PH that may respond differently to macitentan. Finally, because the inability to analyze individual patient data, this pooled data meta-analysis was not patient-level, and there may be within-study heterogeneity as well as between-study heterogeneity, so the results should be considered provisional.
Future RCTs should elucidate the efficacy of macitentan on long-term outcomes of PH, particularly mortality, and explore the possibilities of macitentan in different clinical groups with PH.
Conclusion
Macitentan significantly improved 6MWD, PVR, mPAP, NT-proBNP, and cardiac index in patients with PH. Macitentan should be further validated in patients with PH.
Data Accessibility Statement
Extracted data are available on request to the corresponding author.
Additional Files
The additional files for this article can be found as follows:
Figure s1.
PRISMA 2020 Checklist.
Acknowledgements
The authors would like to thank Stephanie Sun for her music, which always heals the heart.
Competing Interests
The authors have no competing interests to declare.
Authors Contributions
All authors, led by D.H., were involved in the concept and protocol design of the meta-analysis. J.Q. and G.W. screened the titles and abstracts and extracted data from the articles. J.Q. was primarily responsible for statistical analyses. D.H. was primarily involved in the interpretation of the quality data. All authors contributed to interpreting the results. G.W. and D.H accessed and verified the data. All authors contributed to the writing of the article and approved its submission. D.H. was responsible for the decision to submit the article.
References
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019; 53(1). DOI: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin J, Wang G, Han D. Selexipag in patients with pulmonary hypertension: a systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol. 2023; 48(2): 101466. DOI: 10.1016/j.cpcardiol.2022.101466 [DOI] [PubMed] [Google Scholar]
- 3.Mandras SA, Mehta HS, Vaidya A. Pulmonary hypertension: a brief guide for clinicians. Mayo Clin Proc. 2020; 95(9): 1978–1988. DOI: 10.1016/j.mayocp.2020.04.039 [DOI] [PubMed] [Google Scholar]
- 4.Pahal P, Sharma S. Idiopathic pulmonary artery hypertension. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- 5.Wang G, Shang W, Ren Y, Liu S, Ren X, Wei S, et al. Benefits of statins in chronic obstructive pulmonary disease patients with pulmonary hypertension: A meta-analysis. Eur J Intern Med. 2019; 70: 39–42. DOI: 10.1016/j.ejim.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 6.Gatfield J, Mueller Grandjean C, Sasse T, Clozel M, Nayler O. Slow receptor dissociation kinetics differentiate macitentan from other endothelin receptor antagonists in pulmonary arterial smooth muscle cells. PLoS One. 2012; 7(10): e47662. DOI: 10.1371/journal.pone.0047662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolli MH, Boss C, Binkert C, Buchmann S, Bur D, Hess P, et al. The discovery of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N’-propylsulfamide (Macitentan), an orally active, potent dual endothelin receptor antagonist. J Med Chem. 2012; 55(17): 7849–61. DOI: 10.1021/jm3009103 [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151(4): 264–9. DOI: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 9.Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’Abbé KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992; 45(3): 255–65. DOI: 10.1016/0895-4356(92)90085-2 [DOI] [PubMed] [Google Scholar]
- 10.Shang W, Zhang Y, Wang G, Han D. Anakinra was not associated with lower mortality in hospitalised COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials. Rev Med Virol. 2023; 33(2): e2418. DOI: 10.1002/rmv.2418 [DOI] [PubMed] [Google Scholar]
- 11.Shang W, Wang Y, Wang G, Han D. Benefits of ozone on mortality in patients with COVID-19: A systematic review and meta-analysis. Complement Ther Med. 2023; 72: 102907. DOI: 10.1016/j.ctim.2022.102907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren Y, Wang G, Han D. Statins in hospitalized COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials. J Med Virol. 2023; 95(6): e28823. DOI: 10.1002/jmv.28823 [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Qin J, Han D. Long-term safety of macitentan in patients with pulmonary hypertension: A meta-analysis of randomised controlled trials. Eur J Clin Invest. 2023: e14059. DOI: 10.1111/eci.14059 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343: d5928. DOI: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatzoulis MA, Landzberg M, Beghetti M, Berger RM, Efficace M, Gesang S, et al. Evaluation of Macitentan in Patients With Eisenmenger Syndrome. Circulation. 2019; 139(1): 51–63. DOI: 10.1161/CIRCULATIONAHA.118.033575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghofrani HA, Simonneau G, D’Armini AM, Fedullo P, Howard LS, Jaïs X, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT–1): Results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017; 5(10): 785–794. DOI: 10.1016/S2213-2600(17)30305-3 [DOI] [PubMed] [Google Scholar]
- 17.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013; 369(9): 809–18. DOI: 10.1056/NEJMoa1213917 [DOI] [PubMed] [Google Scholar]
- 18.Galiè N, Jansa P, Pulido T, Channick RN, Delcroix M, Ghofrani HA, et al. SERAPHIN haemodynamic substudy: The effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension. Eur Heart J. 2017; 38(15): 1147–1155. DOI: 10.1093/eurheartj/ehx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitbon O, Bosch J, Cottreel E, Csonka D, de Groote P, Hoeper MM, et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med. 2019; 7(7): 594–604. DOI: 10.1016/S2213-2600(19)30091-8 [DOI] [PubMed] [Google Scholar]
- 20.Vachiéry JL, Delcroix M, Al-Hiti H, Efficace M, Hutyra M, Lack G, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018; 51(2). DOI: 10.1183/13993003.01886-2017 [DOI] [PubMed] [Google Scholar]
- 21.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016; 4(4): 306–22. DOI: 10.1016/S2213-2600(15)00543-3 [DOI] [PubMed] [Google Scholar]
- 22.Rose-Jones LJ, McLaughlin VV. Pulmonary hypertension: Types and treatments. Curr Cardiol Rev. 2015; 11(1): 73–9. DOI: 10.2174/1573403X09666131117164122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019; 53(1). DOI: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiéry JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016; 37(12): 942–54. DOI: 10.1093/eurheartj/ehv512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cody RJ, Haas GJ, Binkley PF, Capers Q, Kelley R. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation. 1992; 85(2): 504–9. DOI: 10.1161/01.CIR.85.2.504 [DOI] [PubMed] [Google Scholar]
- 26.Pousset F, Isnard R, Lechat P, Kalotka H, Carayon A, Maistre G, et al. Prognostic value of plasma endothelin-1 in patients with chronic heart failure. Eur Heart J. 1997; 18(2): 254–8. DOI: 10.1093/oxfordjournals.eurheartj.a015228 [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151(4): W65–94. DOI: 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 28.Clinical Study to Assess the Efficacy and Safety of Macitentan in Patients With Pulmonary Hypertension After Left Ventricular Assist Device Implantation (SOPRANO). NCT02554903 trial. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT02554903. Accessed November 3, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure s1.
PRISMA 2020 Checklist.
Data Availability Statement
Extracted data are available on request to the corresponding author.


