Abstract
The purpose of the present study was to characterize the pharmacokinetics profiles and identify important bioavailability barriers and pharmacological pathways of the key active components (KACs) of Antitumor B (ATB), a promising chemopreventive agent against oral cancer. KACs (matrine, dictamine, fraxinellone, and maackiain) were confirmed using an antiproliferative assay in oral squamous cancer cells (SCC-9, Cal-27) and an anti-inflammatory (anti-COX2) assay using LPS-induced SCC-9 cells, consistent with those predicted using an in silico network pharmacology approach. Pharmacokinetics and bioavailabilities of KACs were determined after i.v. (500 mg/kg ATB), i.p (500 mg/kg ATB), and oral administration (100, 500, 4000 mg/kg ATB and 5 mg/kg individual KACs) in mice (n = 5). The oral bioavailability values of KACs (500 mg/Kg ATB dose) were 9.0 ± 3.3%, 4.6 ± 2.8%, 3.9 ± 1.9%, and 0.2 ± 0.1% for matrine, maackiain, dictamine, and fraxinellone, respectively. The low bioavailability of orally administered KACs was mostly due to first-pass metabolism in the liver (for all KACs) and intestine (for only matrine and fraxinellone) because KACs have good solubilities and permeabilities. Multiple-dose PK studies following oral administration showed 23.2 and 8.5 fold accumulation of dictamine and maackiain in blood, respectively, after seven doses, whereas other KACs did not display measurable accumulation. Also, saliva levels of matrine were higher than their plasma levels, suggesting their preferential distribution to the targeted site of action. In conclusion, the systemic bioavailabilities of confirmed KACs of ATB were low but high levels of dictamine and matrine were found in saliva after repeated drug administration, which suggests that it may be fruitful to explore the use of local drug delivery system to achieve better efficacy in oral cancer chemoprevention. Moreover, significant concentrations of dictamine and matrine in saliva may be used as a drug-monitoring tool to track patient compliance during chemoprevention trials.
Keywords: Antitumor B, Zeng-sheng-ping, pharmacokinetics, oral squamous cell cancer, matrine, maackiain, dictamine, fraxinellone, entero-saliva recycling
1. Introduction
Oral cavity carcinomas account for 30% of head and neck cancer - a group of cancer originating from the upper aerodigestive tract - and 90% of oral cavity cancer is classified as squamous cell carcinomas (SCC).1,2 As the sixth most common cancer globally, approximately 600,000 new cases are diagnosed every year with a 5-year mortality rate of 40–50%.3 More than 53,000 men and women in the US are estimated to be diagnosed with these types of cancer.4 Conventional treatment therapies of oral cancer including chemotherapy, radiation and surgical resection, are often associated with considerable toxicity, drug resistance, impairment of speech and swallowing function.5–8 Moreover, the 5-year survival rate of these patients has not changed markedly in the past three decades.9 Therefore, the development of effective chemopreventive interventions against oral carcinoma is promising and significantly needed.
Antitumor B (ATB) - also known as Zeng Sheng Ping, ACAPHA - is a Chinese herbal mixture composed of six plants: Sophora tonkinensis Gagnep., Polygonum bistorta L., Prunella vulgaris L., Sonchus brachyotus DC., Dictamnus dasycarpus Turcz., and Dioscorea bulbifera L. (Supplemental Table S1). Previously, matrine, dictamine, maackiain, and fraxinellone were determined as the key active components (KAC) of ATB with significant in vitro activities against oral cancer cells. ATB is available as 300 mg tablets, which is manufactured according to Chinese Pharmacopeia’s GMP standards. It is currently used in China for treating upper digestive tract (e.g., esophagus) dysplasia to prevent cancer. A recent study by Sun Z et al. examined the chemo preventive effects of ATB in a short-term clinical trial of oral leukoplakia. No significant effect on body weight was reported and ATB intervention reduced the size of oral leukoplakia in 40 out of 59 patients whereas the placebo was effective in 9 out of 53 patients.10
Several studies in rodents have been published demonstrating the chemopreventive activity of ATB against various forms of cancer (e.g. lung, esophageal and oral).11–14 ATB has been shown to be very effective in the chemoprevention of upper aerodigestive tract tumors in humans and has a reasonable safety profile, based to clinical studies of several thousand subjects over more than two decades.10,15–17 However, there has been very few studies that can support and confirm which compounds are the KACs in this promising natural product, and questions about the absorption, metabolism, distribution and excretion of KACs persist.
Earlier studies focusing on the rat and human pharmacokinetics properties of matrine – the quality control chemical marker of ATB (based on Chinese Pharmacopeia).18,19 PK properties of other KACs are unknown, and barriers to their systemic bioavailabilities remain undefined. This information is critical for the quality control and dosing regimen of ATB for oral cancer chemoprevention. Our own studies using SCC2095 cells showed some promising active compounds and their tissue distribution in mice.14,20 However, absolute bioavailabilities were not reported and the reasons for low tissue levels of KACs were unknown. Moreover, the analytical method was not validated.21 Therefore, in order to further advance the ATB research in preparation for a clinical trial against oral cancer, we aim to 1) confirm the key active compounds of Antitumor B (ATB-KACs) against two more oral cancer cell lines; 2) conduct additional mechanism of action studies in vitro and in silico; and 3) characterize the pharmacokinetic profiles (including the bioavailability studies) of ATB-KACs in C57/BL6 mice using a validated UPLC-MS/MS analyzing method.
2. Materials and Methods
2.1. Materials
ATB powder was obtained from Shanghai Fanxin Pharmaceutical Co., Shanghai, China. Baohuoside I (Bao) was purchased from Chengdu Must Biotechnology Co. Ltd (Chengdu, China). Ammonium acetate (LC-MS grade) was purchased from Sigma (St. Louis, MO, USA). Dictamine (Dict) was purchased from Ambeed (Arlington Heights, IL, USA). Fraxinellone (Frax) and matrine (Matr) were purchased from TCI (Portland, OR, USA). PGE2 was obtained from Cayman Chemical (Ann Arbor, MI). All structures were confirmed using LC/MS. All other materials (typically analytical grade or better) were used as received.
2.2. Methods and conditions
2.2.1. Anti-proliferation Assay.
The anticancer activities (reported as IC50 values) of ATB, individual KACs were determined in SCC-9 and Cal-27 (oral squamous cell carcinoma cell lines) using a MTT assay. The cells were cultured in DMEM medium (Hyclone, USA), supplemented with 10% of fetal bovine serum in 5% CO2 at 37 °C. The assays were performed followed MTT method in 96-well plate that was reported earlier.22 Briefly, 6,000 cells were seeded into each well and allowed to adhere for 24 hours before treatment. The growth medium in each well was replaced by the medium containing test compound(s)/fractions at a suitable concentration. After 72 hours, cell viability was measured using colorimetric determination, and cell growth curve was plotted using the cell viability data.
2.2.2. PGE2 Assay.
SCC-9 cells (1 × 106) were seeded in 6-wells plates in DMEM-F12 medium and were allowed to attach to the surface overnight. Cells were treated with the indicated concentrations of ATB or KACs for 30 minutes. After that, the cells were stimulated with LPS (100 ng/mL). After 12 hours, cells were collected to measure the PGE2 levels. Briefly, cell suspensions were washed with 2 mL PBS, resuspended in 0.5 mL PBS and sonicated in 30 mins. The protein was precipitated by ACN (1:1, v/v) before analysis with an validated and published LC-MS/MS method [ref].
2.2.3. Network pharmacology analysis of KACs
2.2.3.1. Target prediction of KACs and oral cancer
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://ibts.hkbu.edu.hk/LSP/tcmsp.php) and Swiss Targets Prediction (http://www.swisstargetprediction.ch/) were employed as tools to obtain targets of the KACs by searching the compound names or uploading .mol format structures downloaded from TCMSP. The known therapeutic targets related to oral cancer were collected from the following sources. (1) Therapeutic Target Database (TTD, https://db.idrblab.org/ttd/); (2) DrugBank database (https://www.drugbank.ca/); (3) NCBI Gene Database (https://www.ncbi.nlm.nih.gov/gene/). “Oral cancer” was used as the keyword and “homo sapiens” was selected as the organism when searching targets. All the targets (protein) names were standardized into official gene symbols (Homo sapiens) via UniProt Knowledgebase (http://www.uniprot.org/).
KACs protein-protein interaction (PPI) network and oral cancer PPI network were performed by Cytoscape StringApp (http://apps.cytoscape.org/apps/stringappe). The targets related to KACs and oral cancer were imported into Cytoscape StringApp. The minimum required interaction score was set at medium.
2.2.3.2. Network construction and pathway enrichment analysis
KACs-oral cancer network was constructed using Cytoscape (Version 3.7.2). For the topological properties, “degree” was calculated to demonstrate the topological importance of nodes. Nodes with degree values more than the median were identified as candidate targets.
Database for Annotation, Visualization and Integrated Discovery (DAVID 6.8, https://david.ncifcrf.gov/home.jsp) webserver were employed to perform pathway enrichment analysis for the candidate targets, which are based on the pathway data obtained from the Kyoto Encyclopedia of Genes and Genomes database (KEGG, http://www.genome.jp/kegg/).
2.2.4. Stability studies.
Three batches of ATB (ATB_2009, ATB_2019_Shanghai and ATB_2019_Shanghai_different source) were tested according to ICH recommended conditions: long-term (12 months at 25 ± 2oC/60 ± 5% RH) , intermediate (6 months at 30 ± 2oC/65 ± 5% RH), and accelerated term (40 ± 2oC/75 ± 5% RH).23
2.2.5. UPLC-MS/MS
The LCMS/MS was developed with the similar approaching method that has been published before and validated according to FDA guidance.22,24 UPLC-MS/MS conditions for analyzing 4 ATB-KACs were: system, Waters Acquity™; column, BEH C18 column (50 × 2.1 mm I.D., 1.7 μm, Waters, Milford, MA, USA); mobile phase A (MPA), 2 mM ammonium acetate in water; mobile phase B (MPB), 100 % acetonitrile; gradient, 0 – 0.5 min, 5 % MPB, 0.5 –1.5 min, 5 – 30 % MPB, 1.5 – 4.0 min, 30 – 60 % MPB, 4.0 – 5.0 min, 60 – 80 % MPB, 5.0 – 5.5 min, 80 – 95 % MPB, 5.5 – 6.0 min, 95 % MPB, 6.0 – 6.1 min, 95 – 5 % MPB, 6.1 – 6.5 min, 95 % MPB; flow rate, 0.45 mL/min; column temperature, 45 °C; injection volume, 10 μL.
The UPLC-MS/MS analysis was performed on an AB Sciex 5500 mass spectrometer (Applied Biosystem/ MDS SCIEX, Foster City, CA, USA) equipped with an APCI TurboIonSpray™ source. Concentrations of ATB KACs in biological matrices and in vitro reaction mixtures were determined by using MRM (Multiple Reaction Monitoring) method in negative and positive modes simultaneously. The instrument dependent parameters for mass spectrum were set as follows: ion spray voltage, −4.5 kV (negative mode), 5.5 kV (positive mode); ion source temperature, 600 °C; nebulizer gas (gas 1), nitrogen, 50 psi; turbo gas (gas 2), nitrogen 50 psi; curtain gas, nitrogen 20 psi. Unit mass resolution was set in both mass-resolving quadruples Q1 and Q3. Compound-dependent parameters were listed in supplemental Table S2. The details of method development and validation were included in the Supplement material S1.
2.3. Pharmacokinetics study
2.3.1. Animals
C57BL/6 mice (20–25 g, 8–10 weeks old) were purchased from Taconic Biosciences, Inc. (New York, USA) and acclimatized in an environmentally controlled room (temperature: 25 ± 2 °C, humidity: 50 ± 5%, 12 hours dark-light cycle) for at least one week before experiments.
ATB extract for tail vein and intraperitoneal injection was prepared by sonicating ATB powder in saline for 15 minutes followed by centrifugation at 15,000 rpm for 15 minutes to collect the supernatant. The clear drug solution was aseptically filtered through a 0.2 μM membrane filter. ATB powder, a pure KAC, or a mixture of several KACs was dispersed in Ora Plus® suspending vehicle prior to oral administration.
2.3.2. Animal Dosing and Sample Collection
Pharmacokinetic studies were performed as per the animal protocol approved by the University of Houston’s Institutional Animal Care and Uses Committee (IACUC). For IV pharmacokinetic studies, drug solution was administered via I.V. bolus at a dose of 500 mg/kg. For i.p. PK studies, rats were administered once daily 50 mg/kg of ATB for seven days. For oral pharmacokinetic studies, ATB was administered by oral gavage with doses of 100, 500, or 4000 mg/kg. Blood samples (about 20 μL) were collected in heparinized tubes at 5, 15, 30, 60, 120, 240, 360, 480, and 1440 min, respectively using the mouse tail sectioning method. Saliva samples were collected by swabbing and weighing Salimetric™ foam in the animal oral cavity. The samples were stored at −80 °C until analysis.
2.3.3. Samples Analysis
The blood sample (10 μL) was spiked with 10 μL of 50% MeOH and 200 μL of 100 nM internal standard (IS - Baohuoside I) in ethyl acetate. The mixture was vortexed for 1 min. After centrifugation at 15,000 rpm for 15 min, the supernatant was transferred to a new tube and evaporated to dryness under a stream of airflow. The residue was reconstituted in 140 μL of 50% methanol and centrifuged at 15,000 rpm for 15 min. The supernatant (10 μL) was injected into the UPLC-MS/MS system for quantitative analysis. The saliva samples were processed similarly with 500 μL of extract solvent.
2.3.4. Pharmacokinetic parameter analysis
Pharmacokinetic parameters were obtained using the non-compartmental method by WinNonlin 8.1 (Pharsight Corporation, Mountain View, California).
2.4. Statistical Analysis
All the data were presented as means ± RSD, if not specified otherwise. Significance differences were assessed by using unpaired Student’s t-test. P-value of <0.05 was considered as statistically significant.
3. Results
3.1. Antiproliferative activity of ATB Key Active Components
In the previous publication, we reported that there were at least nine possible key active compounds in ATB: dictamine, matrine, maackiain, fraxinellone, obacunone, limonin, trifolirhizin, rutaevine and evodol.20 Based on the cell antiproliferation study, three compounds showed the best activities in a SCC-9 cell MTT assay were fraxinellone (Frax), dictamine (Dic) and maackiain (Maac) and therefore, they were selected as the marker compounds of ATB. However, the conclusion was made based on the IC50 value of ATB compounds on only one cell line SCC2095. In this study, we repeated the MTT assay to confirm the anticancer activity of those key active components in two cell lines SCC-9 and Cal-27. Matrine was also added to the experiments because it was previously the quality marker compound of ATB according to the Chinese pharmacopeia. In addition, matrine was reported to inhibit the adhesion and invasion properties of salivary gland adenoid cystic carcinoma cells (ACC-M) in vitro.25
The anticancer activities or IC50 values from the MTT assay (Table 1) showed that three compounds with the highest activities were Frax, Dict and Maac, with Maac being the best in Cal-27 cells (IC50: 11.5 ± 0.1 ug/mL) and Dict being the most active in SCC-9 cells (IC50: 27.8 ± 2.7 ug/mL) (p < 0.05). In this study, the quality control marker compound Matr also showed moderate inhibition effect against Cal-27 cells.
Table 1.
MTT assay results of ATB and its KACs (IC50 values in µg/mL)
| Cell line | Frax | Dict | Maac | Matrine | ATB | 5-FU (control) |
|---|---|---|---|---|---|---|
| Cal-27 | 22.4 ± 0.6 | 19.7 ± 0.3 | 11.5 ± 0.1 | 21.0 ± 3.8 | > 1000 | 0.5 ± 0.1 |
| SCC-9 | 43.4 ± 10.2 | 27.8 ± 2.7 | 94.9 ± 43.1 | > 100 | > 1000 | 12.7 ± 1.8 |
3.2. ATB compounds suppress PGE2 overexpression in lipopolysaccharide-induced oral cancer cell
Prostaglandin E2 (PGE2) is a principal mediator of many inflammatory diseases and cancer.26,27 It also triggered promoting angiogenesis, which presents the undesirable effect of supporting the development and growth of cancer, as has been demonstrated in SCC.28 The results in Fig 3 showed that most ATB compounds suppressed the PGE2 level at the concentration of 10 μg/mL. Among all ATB-KACs, Frax was the best at inhibiting the PGE2 production in SCC-9 cells. Interestingly, in this anti-inflammation assay, Matr were more active than Dict and Maac. Consistently with these in vitro results, a ATB extract solution equivalent to a concentration of 5 mg/mL ATB also significantly inhibited the production of PGE2. Thus, ATB and ATB-KACs may be able to suppress inflammation in the oral cavity.
Figure 3.
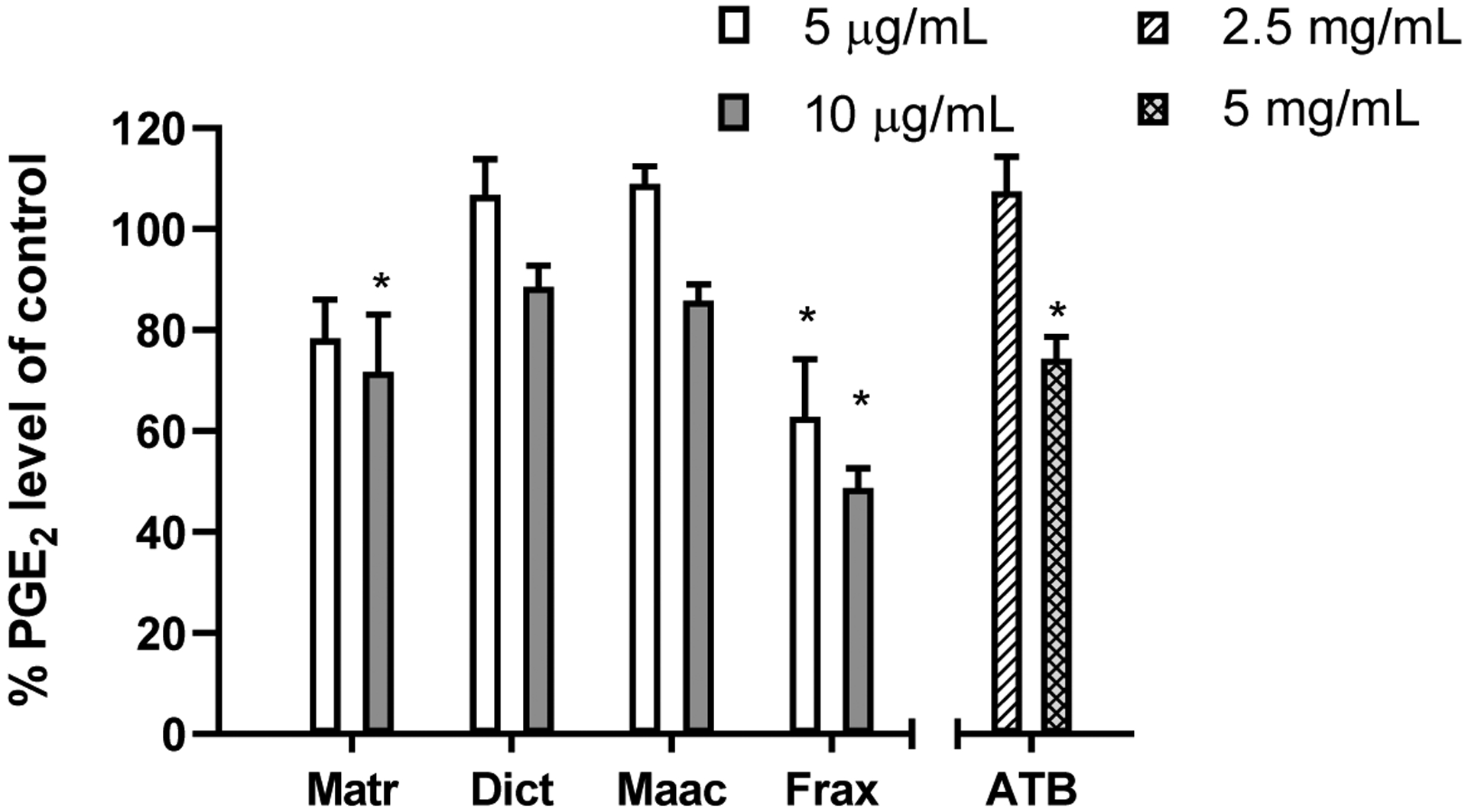
Percentages of PGE2 levels in SCC-9 cell lysates after treatment compared to control samples. (*) indicates the significant difference with the control samples
3.3. Network pharmacology analysis of KACs against oral cancer
3.3.1. Putative targets of KACs and oral cancer related targets
In total, 183 targets corresponding to KACs were predicted as putative targets of 4 KACs (Table S4). Three hundred and nineteen therapeutic targets related to oral cancer were collected after removing redundant genes (Table S4).
3.3.2. Identification of candidate targets for ATB compounds against oral cancer
KACs-oral cancer network was constructed by merging the KACs putative targets PPI network and oral cancer-related targets PPI networks. The topological properties of intersected targets were listed in Table S5. Thirteen candidate targets (VEGFA, CASP3, IL6, TNF, MYC, MAPK1, PTGS2, AR, MMP2, MTOR, RELA, CD44, CDK4) were identified, whose degree values larger than the median of all intersected targets, suggesting they may play important roles against oral cancer.
3.3.3. Pathway enrichment analysis
To investigate the biological functions of the KACs candidate targets, the pathway enrichment analysis was performed based on the KEGG database. Totally 57 pathways were enriched with P-value < 0.05. The pathways ranking according to the P-value and their involved genes were listed in Table S6, indicating that a major of the enriched signaling pathways were relevant with oral cancer-related pathology, namely, cell proliferation, apoptosis and inflammation. Among them, the tumorigenesis-related pathways potentially regulated by KACs were further summarized in Table 2.
Table 2.
The tumorigenesis-related pathways potentially regulated by ATB key active components
| No. | Term | Count | % | P Value | Genes |
|---|---|---|---|---|---|
| 1 | hsa04668: TNF signaling pathway | 6 | 46.15 | 6.02E-07 | MAPK1, CASP3, IL6, TNF, PTGS2, RELA |
| 2 | hsa04151: PI3K-Akt signaling pathway | 7 | 53.85 | 1.09E-05 | MAPK1, IL6, RELA, VEGFA, MTOR, CDK4, MYC |
| 3 | hsa04066: HIF-1 signaling pathway | 5 | 38.46 | 1.62E-05 | MAPK1, IL6, RELA, VEGFA, MTOR |
| 4 | hsa04010: MAPK signaling pathway | 5 | 38.46 | 7.00E-04 | MAPK1, CASP3, TNF, RELA, MYC |
| 5 | hsa04620: Toll-like receptor signaling pathway | 4 | 30.77 | 7.07E-04 | MAPK1, IL6, TNF, RELA |
| 6 | hsa04150: mTOR signaling pathway | 3 | 23.08 | 4.37E-03 | MAPK1, TNF, MTOR |
| 7 | hsa04370: VEGF signaling pathway | 3 | 23.08 | 4.82E-03 | MAPK1, PTGS2, VEGFA |
| 8 | hsa04210: Apoptosis | 3 | 23.08 | 4.98E-03 | CASP3, TNF, RELA |
| 9 | hsa04350: TGF-beta signaling pathway | 3 | 23.08 | 8.98E-03 | MAPK1, TNF, MYC |
| 10 | hsa04012: ErbB signaling pathway | 3 | 23.08 | 9.61E-03 | MAPK1, MTOR, MYC |
| 11 | hsa04064: NF-kappa B signaling pathway | 3 | 23.08 | 9.61E-03 | TNF, PTGS2, RELA |
Specifically, KACs were predicted to be involved in cell proliferation procession via regulating multiple targets (MTOR, MAPK, IL6, TNF) and mTOR signaling pathway, MAPK signaling pathway and NF-kappa B signaling pathway. Additionally, KACs are associated with cell apoptosis by hitting MYC, CAPS3, TNF and RELA, as well as involving in PI3K-Akt signaling pathway and TNF signaling pathway. Further, KACs also regulate inflammation related targets (PTGS2, IL6 and MMP2), and NF-kappa B signaling pathway and TNF signaling pathway. These potential actions related to inflammation and cell proliferation were in good agreement with what we observed experimentally here and those reported earlier.13,14,29
3.4. LC-MS/MS method development and validation of ATB KACs
The details of method development and validation were described in the Supplement Materials S1. The analyzing method was validated according to FDA guidance24 and the validation results revealed that the method is rapid, specific, sensitive, reproducible, and robust.
3.4.1. Specificity, linearity and sensitivity
The method validation was conducted in blank mouse blood. The standard curves were linear in the concentration range of 0.96 – 500 nM for Matr and Maac; 2.4–1000 nM for Frax; and 0.1 – 100 nM for Dict. The lower limit of quantification (LLOQ) was 7.8 nM for Matr, Maac, and Frax, but was 0.8 nM for Dict.
3.4.2. Accuracy and precision
Accuracy, intra-day and inter-day precision were determined by measuring six replicates of QC samples at three concentration levels in mouse blood. The precision and accuracy were shown in Table 2. These results demonstrated that the precision and accuracy values were in the acceptance range (15%) based on FDA Guidance on Bioanalysis [ref].
3.4.3. Recovery, matrix effect, and stability
The mean extraction recoveries determined using three replicates of QC samples at three concentration levels (the same concentrations as QC sample) in mouse blood were shown in Table 3. Ethyl acetate was used to extract Matr, Dict, Maac and Frax from the biological samples. The result showed the recoveries were not less than 70% for these three analytes at low, medium, and high concentrations.
Table 3.
Precision and accuracy results on mouse blood
| Analyte | Linear range (nM) | Conc. (nM) |
Intra-day (n=6) | Inter-day (n=6, 3 days) | ||
|---|---|---|---|---|---|---|
| Accuracy (Bias, %) | Precision (CV, %) | Accuracy (Bias, %) | Precision (CV, %) | |||
| Matr | 2.0 – 500 | 7.8 | 116.7 | 9.4 | 111.3 | 12.9 |
| 125.0 | 104.2 | 10.6 | 93.8 | 6.9 | ||
| 500.0 | 93.0 | 8.4 | 90.6 | 1.1 | ||
| Dict | 0.8 – 100 | 0.8 | 112.9 | 10.0 | 113.8 | 7.9 |
| 12.5 | 112.3 | 9.00 | 106.5 | 9.1 | ||
| 50.0 | 94.1 | 6.6 | 101.5 | 5.6 | ||
| Maac | 2.0 – 1000 | 7.8 | 104.5 | 8.1 | 108.9 | 8.0 |
| 125.0 | 118.0 | 2.7 | 103.6 | 8.0 | ||
| 500.0 | 100.7 | 5.5 | 94.9 | 5.4 | ||
| Frax | 7.8 | 116.0 | 7.8 | 114.3 | 5.6 | |
| 7.8 – 1000 | 125.0 | 92.2 | 5.2 | 107.2 | 9.4 | |
| 500.0 | 86.9 | 3.8 | 91.3 | 3.1 | ||
As for testing the matrix effects of the samples, the relative peak areas of these three analytes after spiking evaporated blood samples at three concentration levels were comparable to similarly prepared aqueous standard solutions (ranged from 85 to 115%). It suggested that there was no measurable matrix effect that interfered with their determination in mouse blood.
The stabilities of ATB KACs in blood matrix were evaluated by analyzing three replicates of quality control samples at three different concentrations after short-term (25°C, 4 h), post-processing (20°C, 8 h), long-term cold storage (−80°C, 4 months), and after going through three freeze-thaw cycles (−80°C to 25°C). All the samples displayed 85–115% recoveries after various stability tests (Table S3).
3.5. Quality control of ATB products
The LC-MS/MS method was employed to determine the amounts of KACs in 3 different ATB batches: ATB_2009, ATB_2019_Shanghai and ATB_2019_another source. The results showed that the KACs concentration in ATB_2019_Shanghai and ATB_2019_different source were similar (p < 0.05). However, those ingredients in a year 2009 batch ATB_2009 were significantly lower than the new batches (p < 0.05). The difference may come from the source of materials and/or the long-term storage of the sample (more than 10 years of storage time for ATB_2009). It is noted that ATB, regardless of batch number, contains a much higher contents of Matr compared to the other KACs.
The stability of ATB was also tested in 3 storage conditions (accelerated, intermediate and long-term) according to ICH guidance.23 At the precision and accuracy level of 15%, we found that the active components were stable at different storage conditions. (Supplementary material, Table S11).
3.6. Pharmacokinetics of ATB in C57BL/6 mice
The objective of the PK studies is to characterize the pharmacokinetic behaviors and bioavailability of active components in ATB in order to determine the bioavailability barriers and monitor their exposure in vivo in mice. The pharmacokinetic studies of ATB were conducted in C57BL/6 mice by p.o., i.v. or i.p. administration of ATB. The pharmacokinetics of pure KACs (single or as a mixture) were also investigated at the dose of 5 mg/kg for each of the 4 KACs (Matr, Maac, Dict and Frax).
Intravenous Injection
ATB powder was extracted in saline as described in section 2.6. Pharmacokinetic profiles, which plots average blood concentration vs time curves of 4 ATB-KACs after i.v. dosing of 500 mg/kg (equal to 2.79 mg/kg Matr, 0.03 mg/kg Maac, 0.02 mg/kg Dict and 0.07 mg/kg Frax) were illustrated in Fig 4. The PK profiles showed significant higher levels of Matr in the blood samples (AUC0–24 = 5113.1 ± 3467.8 hr*ng/mL) compared to other 3 KACs, whose AUC0–24 values of Maac, Dict and Frax were 68.9 ± 42.4, 25.7 ± 14.4 and 95.2 ± 9.4 hr*μg/L, respectively. This corresponds to the much higher content of Matr in ATB (Table 5). The i.v. PK studies also showed that all four KACs of ATB were eliminated from systemic circulation. The t1/2 values of Matr, Maac, Dict and Frax were 7.5 ± 3.3, 4.9 ± 2.4, 0.4 ± 0.2 and 5.2 ± 2.0 hours, respectively (Table 6).
Figure 4.

Blood concentrations and bioavailability of ATB key active components after i.v., i.p and oral administration of ATB (n = 5)
Table 5.
Amount (μg/g herbal mixture) of ATB active compounds in different ATB product batches
| Sample batch | Matrine | Maackiain | Dictamine | Fraxinellone |
|---|---|---|---|---|
| ATB_Shanghai (*) | 5588.3 ± 75.8 | 56.9 ± 0.9 | 33.8 ± 1.2 | 145.6 ± 7.8 |
| ATB_Shanghai (different source) | 5882.6± 345.5 | 65.3 ± 3.5 | 31.2 ± 7.9 | 131.8 ± 15.3 |
| ATB 2009 | 1389.7± 185.0 | 8.9 ± 1.7 | 19.1 ± 2.2 | 165.2 ± 17.3 |
ATB Shanghai sample was used in this study
Table 6.
Pharmacokinetics parameters of ATB components after i.v. administration of 500 mg/kg ATB (n = 5)
| Parameters | Half-life (hr) |
Cmax (ng/mL) |
AUC0–24
(hr*ng/mL) |
AUC0–∞
(hr*ng/mL) |
V (L/kg) |
CL (L/hr/kg) |
MRT0–24 (hr) |
|---|---|---|---|---|---|---|---|
| Matrine | 7.5 ± 3.3 | 3929.1 ± 2331.9 | 5113.1 ± 3467.8 | 5170.7 ± 3462.8 | 9.1 ± 6.6 | 0.8 ± 0.5 | 1.8 ± 0.5 |
| Maackiain | 4.9 ± 2.4 | 67.5 ± 38.6 | 68.9 ± 42.4 | 71.5 ± 43.2 | 4.1 ± 3.1 | 0.6 ± 0.2 | 3.0 ± 1.8 |
| Dictamine | 0.4 ± 0.2 | 34.0 ± 27.1 | 24.5 ± 16.4 | 24.8 ± 16.5 | 0.6 ± 0.4 | 0.9 ± 0.7 | 0.5 ± 0.1 |
| Fraxinellone | 5. 2 ± 2.0 | 34.3 ± 13.5 | 95.2 ± 9.4 | 166.8 ± 58.4 | 2.9 ± 0.3 | 0.4 ± 0.1 | 3.2 ± 0.4 |
Oral administration
In order to fully understand the PK behaviors of 4 KACs, we determine the absolute oral bioavailability, blood exposure and PK profiles of KACs in mice, at doses of 100, 500 and 4000 mg/kg, respectively. A single dose of a mixture of 4 pure KACs at 5mg/kg each was also given orally for PK studies. In general, matrine always showed the highest concentration compared to other 3 compounds in all PK studies. Among the KACs, matrine and maackiain displayed relatively long half-life (t1/2 = 7.5 ± 2.8 hr, t1/2 Maac = 3.5 ± 1.0 hr) (Table 7, 8). Because matrine was detectable at all three doses, we were able to determine dose-normalized Cmax and AUC0–24, and the results showed dose-normalized AUC significantly increased (~400%) from 500 mg/kg (F=x%) to 4000 mg/kg (F=y%), suggesting that first-pass metabolism was probably saturated at the higher dose (Table 10). The dose-normalized Cmax value was also increased 50% at the higher dose but it was not significantly different compare to those values in the lower dose, due to large variabilities (Table 10). Interestingly, half-life of Dict at 4000 mg/kg p.o. dose increased nearly 6 times when compared to i.v. administered ATB at 500mg/kg.
Table 7.
Pharmacokinetic parameters of ATB components after oral administration of 500 mg/kg ATB (n = 5)
| Parameters | Tmax (hr) |
Cmax (ng/mL) |
AUC0–24
(hr*ng/mL) |
AUC0–∞
(hr*ng/mL) |
Half-life (hr) |
MRT0–24 (hr) |
|---|---|---|---|---|---|---|
| Matrine | 0.4 ± 0.1 | 53.8 ± 28.4 | 120.5 ± 59.4 | 123.7 ± 60.0 | 3.8 ± 2.4 | 3.6 ± 1.9 |
| Maackiain | 0.6 ± 0.4 | 0.5 ± 0.4 | 0.4 ± 0.3 | - | - | 0.6 ± 0.2 |
| Dictamine | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.1 | - | - | 0.4 ± 0.1 |
| Fraxinellone | - | - | - | - | - | - |
Table 8.
Pharmacokinetic parameters of ATB compounds after oral administration of 4000 mg/kg ATB (n = 5)
| Parameters | Tmax (hr) |
Cmax (ng/mL) |
AUC0–24 (hr*ng/mL) | AUC0–∞ (hr*ng/mL) | Half-life (hr) |
MRT0–24 (hr) |
|---|---|---|---|---|---|---|
| Matrine | 0.7 ± 0.6 | 709.1 ± 237.2 | 3667.7 ± 1340.4 | 5371.8 ± 1349.9 | 7.5 ± 2.8 | 4.5 ± 1.2 |
| Maackiain | 0.7 ± 0.6 | 16.4 ± 9.9 | 25.5 ± 15.3 | 27.5 ± 14.6 | 3.5 ± 1.0 | 3.6 ± 3.2 |
| Dictamine | 0.4 ± 0.3 | 4.2 ± 1.9 | 8.0 ± 3.8 | 9.4 ± 4.8 | 2.6 ± 2.0 | 3.6 ± 1.9 |
| Fraxinellone | 0.5 ± 0.3 | 4.7 ± 3.3 | 1.1 ± 1.5 | - | - | 0.4 ± 0.2 |
Table 10.
AUC/dose values of matrine in the herbal mixture and pure compound using different routes of administration
| Administration protocol | Matrine dosage (mg/kg) | Cmax/Dose (ng/mL)/(mg/kg) | AUC0-t /Dose (hr*ng/mL)/(mg/kg) |
|---|---|---|---|
| Blood concentration | |||
| 500 mg/kg ATB IV | 2.79 | 1408.3 ± 835.8 | 1830.0 ± 1241.2 |
| 100 mg/kg ATB Oral | 0.56 | 25.9 ± 20.5 | 58.5 ± 32.7 |
| 500 mg/kg ATB Oral | 2.79 | 19.3 ± 10.2 | 43.1 ± 21.3 |
| 4000 mg/kg ATB Oral | 22.32 | 31.8 ± 10.6 | 164.1 ± 60.0 |
| 5 mg/kg KAC Oral | 5.00 | 22.0 ± 7.4 | 46.9 ± 13.0 |
| 500 mg/kg ATB IP Day 1 | 2.79 | 292.4 ± 147.8 | 528.7 ± 200.9 |
| 500 mg/kg ATB IP Day 7 | 2.79 | 197.8 ± 144.1 | 592.0 ± 434.7 |
| Saliva concentration | |||
| 500 mg/kg ATB IP Day 7 | 2.79 | 1533.9 ± 823.5 | 952.8 ± 594.4 |
Intraperitoneal injection
In order to test the distribution of ATB compounds after absorption in the various organs including saliva, we dosed ATB via i.p. injection (500 mg/kg q.d., for a total of 7 consecutive days) to avoid contamination to the saliva samples, and then collected both the blood and saliva samples. In general, AUC and Cmax values of 4 KACs after i.p. dosing (500 mg/kg) were substantially higher (>10 fold) than those observed after orally administered ATB. Of the 4 KACs in ATB, only matrine and dictamine were detected in the mice saliva. On the 7th day, significant concentrations of matrine and dictamine were found in mouse saliva one hour post i.p. dosing, but there was no Frax or Maac detected. Significant salivary secretion of Matr (AUC0-t = 2680.4 ± 1658.4 hr*ng/mL) was quantified after i.p. dosing of ATB (Fig 5 and Table 10). The bioavailabilities of ATB-KACs following i.p. administration were 28.9 ± 11.0% (Matr), 4.8 ± 4.2% (Maac), 3.9 ± 1.9% (Dict), and 2.8 ± 2.1% (Frax) (Fig 4).
Figure 5.

Blood concentrations of ATB key active components after intraperitoneal administration of 500 mg/kg once a day in seven days and saliva concentration at day seventh (n = 5).
4. Discussion and Conclusion
This is the first-ever preclinical study reporting rapid and extensive secretion of certain active compounds (i.e., matrine and dictamine) of a multi-component botanical (Antitumor B, ATB) into the mouse saliva from the systemic circulation (Fig 5). The active salivary secretion has potential and significant utility not only as a marker of an active compound exposure at the target site but also as an indicator of patient compliance, which may be critical for a successful longitudinal clinical trial using botanicals. The mechanistic pathways (antiproliferative action and inhibition of PGE2 production) determined based on the in vitro activities of the selected key active components (KACs) of ATB were consistent with the results obtained from in silico network pharmacology pathway analysis. Simultaneous analysis of ATB-KACs using a sensitive and robust LC-MS/MS method allowed us to measure the systemic and target-site exposure and attribute extensive intestinal metabolism as the main reason for poor bioavailabilities of certain ATB-KACs in rodents.
Collections of saliva samples to measure endogenous biomarkers of drug efficacy or drug monitoring have been reported in about 1,314 clinical studies in the www.clinicaltrials.gov website, out of which 550 studies used therapeutic drugs, whereas about 25 used dietary supplements or botanical drugs. In most of the trials using natural products, saliva samples were either collected to measure the biomarker for product efficacy or count the number of oral bacteria. For example, the safety, tolerability, and behavioral effects of Souroubea-Platanu (for stress-reduction) in healthy volunteers were measured using salivary cortisol levels (NCT03904511). In the recently completed clinical studies (NCT03971981 and NCT01504932), the release of active compound(s) into saliva from the xylitol chewing gum and freeze-dried black raspberry lozenges were measured to determine active compounds’ exposure in the oral cavity. Currently, there are only two clinical studies from our collaborators at Medical College of Wisconsin (NCT03459729 and NCT04278989), which has not yet started recruiting subjects, where active salivary secretion of systemically-available active compounds from oral botanical products will be used for drug monitoring and pharmacokinetic profiling of Antitumor B in the oral cancer patients.
Our data strongly indicated an active secretion of matrine and dictamine into the mouse saliva based on their comparable levels in saliva and blood after the administration of ATB (Fig 5 and Table 10), which may contribute to the overall oral tissue exposure required for anticancer activity of ATB (AUCsaliva/AUCblood = 1.27 for Matr at day 7 after multiple-dose IP administration of 500 mg/Kg ATB) (Table 9–10). Once saliva containing Matr and Dict is swallowed, both compounds will probably enter “entero-saliva recycling” after reabsorption from the gastrointestinal tract into the blood, contributing to their prolonged half-life. More interesting, this represents an opportunity to develop Matr and Dict as a drug-monitoring tool to track patient compliance during the upcoming chemoprevention trials of ATB (NCT03459729 and NCT04278989).
Table 9.
Pharmacokinetic parameters of ATB compounds after intraperitoneal administration of 500 mg/kg ATB once a day in seven days (n = 5).
| Compound | Tmax (hr) |
Cmax (ng/mL) |
AUC0–24 (hr*ng/mL) | AUC0–∞ (hr*ng/mL) | Half-life (hr) |
MRT0–24 (hr) |
|
|---|---|---|---|---|---|---|---|
| Matrine | Day 1 | 0.2 ± 0.1 | 815.9 ± 412.4 | 1477.3 ± 561.3 | 1660.4 ± 508.8 | 7.3 ± 2.6 | 5.3 ± 0.8 |
| Day 7 | 0.5 ± 0.4 | 551.8 ± 402.0 | 1651.6 ± 1212.9 | 2254.0 ± 1862.3 | 8.2 ± 3.7 | 8.0 ± 3.2 | |
| Maackiain | Day 1 | 0.2 ± 0.1 | 15.6 ± 13.4 | 5.6 ± 5.6 | - | - | 0.3 ± 0.1 |
| Day 7 | 0.6 ± 0.3 | 17.2 ± 3.7 | 47.4 ± 7.5* | 51.8 ± 7.5 | 2.2 ± 0.7 | 8.4 ± 1.5 | |
| Dictamine | Day 1 | 0.2 ± 0.1 | 1.9 ± 0.6 | 1.0 ± 0.5 | - | - | 0.4 ± 0.2 |
| Day 7 | 0.5 ± 0.0* | 3.8 ± 1.8 | 23.2 ± 5.2* | 31.4 ± 9.2 | 5.2 ± 1.5 | 11.7 ± 1.6* | |
| Fraxinellone | Day 1 | 0.5 ± 0.4 | 4.1 ± 3.9 | 2.7 ± 2.0 | - | - | 0.6 ± 0.2 |
| Day 7 | - | - | - | - | - | - |
indicates the significant difference from Day 1 with Day 7 values, p < 0.05.
The comprehensive pharmacokinetic studies of ATB and its KACs suggested poor oral bioavailabilities for 4 ATB-KACs are due to extensive first-pass metabolism in the intestine and/or liver. The oral bioavailabilities of ATB-KACs were 9.0 ± 3.3% (matrine), 4.6 ± 2.8% (maackain), 3.9 ± 1.9% (dictamine), and 0.2 ± 0.1% (fraxinellone) (Fig 4, Table 10). Comparative pharmacokinetics of Matr and Dict after intraperitoneal (IP) and oral administration of ATB in mice provided evidence of major first-pass effect in the intestine (Fig 4, bioavailability panel). This was supported by the published reports on the fair to good aqueous solubilities of our active compounds (49 mg/mL, 0.51 mg/mL, 0.032 mg/mL and 0.014 mg/mL) and high permeabilities (P = 4.25 × 10−5 cm/s, 4.27 × 10−5 cm/s, 4.33 × 10−5 cm/s, and 2.58 × 10−5 cm/s) for Matr, Maac, Dict, and Frax, respectively.20,30–32 Single-dose IP versus oral administration of ATB suggested intestinal first-pass effect as one of the major bioavailability barriers for Frax (FIP /Foral = 14) and Matr (FIP /Foral = 3.2) in addition to their extensive liver metabolism. On the other hand, the liver was the dominant metabolic organ responsible for the poor oral bioavailabilities of Maac and Dict (Fig 4). Multiple-dose (7-day) IP administration showed a significantly high accumulation of Dict (AUC7-day/AUC1-day = 23.2) and Maac (AUC7-day/ AUC1-day = 8.5) in the blood (Fig 5), providing a possible mechanism to achieve higher therapeutic tissue exposure of ATB-KACs during long-term chemopreventive treatment. However, since extensive first-pass metabolism was the major bioavailability barrier to achieving the desired exposure of KACs, drug delivery technologies for local delivery of ATB in the oral cavity (trans-buccal delivery using the buccal patch, chewing gum, lozenges, etc.) needs to be explored.
Some of the pharmacokinetic findings for Dict were unexpected because of observed plasma accumulation (after 3 days of once-daily dose) and prolonged half-life upon i.p. multiple dosing. After single-dose (at a low dose of 5 mg/Kg) I.V. administration, Dict was cleared very rapidly (t1/2 = 0.4 ± 0.2 hr) from the circulation. However, multiple-dose (at 500 mg/Kg dose) IP administration showed accumulation of Dict. The two possible reasons for such contradictory elimination profiles could be 1) the accumulation of Dict in certain tissues at the beginning and 2) the auto-inhibition of the metabolic enzyme responsible for Dict metabolism when a higher concentration of Dict is achieved after repeated administration of ATB at a high dose. Moreover, the single-dose oral administration (at 4000 mg/kg dose) showed prolonged half-life (t1/2 = 2.6 ± 2.0 hr) as compared to IV and IP (at a lower dose), possibly because of the saturation of the metabolic enzyme and transporters involved in the elimination of Dict at such a high dose. Wang et al. reported the half-life of Dict after IV (1 mg/kg dose) (t1/2 = 0.92 ± 0.26 hr) and oral (t1/2 =1.17 ± 0.27 hr) (5 mg/kg dose) administration in rats,33 the moderate differences in half-lives could be explained as differences in metabolism between the two species.
The main pharmacological pathways for oral anticancer activities of the identified and confirmed ATB-KACs (Matr, Dict, Maac and Frax) were found to be antiproliferative and anti-inflammatory. The significant inhibition of PGE2 production (measured using LC-MS/MS) in SCC cells by Frax (25 μM), Matr (50 μM) and ATB (5 mg/ml) (Figure 3) supported this conclusion. Using the network pharmacology application on multi-component traditional Chinese medicine, PPI (protein-protein interaction) networks merging KACs putative targets (total 183) (Table S4) and oral cancer-related targets (total 319) (Table S5), identified 11 major candidate targets (Table 2) for KACs efficacy against oral cancer. The pathway enrichment analysis of these candidate targets based on the KEGG database predicted involvement of TNFα, PI3K-Akt, and NF-kappa B signaling pathways, which will impact inflammation, cell proliferation and apoptosis.
Previous study from collaborating group has also suggested ATB as an effective chemopreventive agent against lung tumorigenesis and the corresponding microarray analysis revealed that genes modulated by ATB belonged to several signaling pathways, such as MAPK (Table 2)13, NOTCH, FGF, MAPK3 (Table S5)13,29, CASP3 (Table 2, S5, S6)13, EGFR (Table S5)14. It was also reported that MAPK pathway (P-value = 7.00E-04, MAPK1, CASP3, TNF, RELA, MYC) and apoptosis pathway (P-value = 4.98E-03, CASP3, TNF, RELA) could be regulated by the KACs, which also plays an important role in the oral cancer due to their involvement in tumor cell proliferation, differentiation, apoptosis, angiogenesis, invasion, and metastasis.34 These biological procession predictions may help elucidate the mechanisms of action against oral cancer of ATB-KACs in future research.
A highly specific, sensitive, reproducible, and robust LC-MS/MS method was developed for the quantification of Matr, Maac, Dict and Frax in the biological samples generated from this study (Fig 2, Table 3, 4). The developed LC-MS/MS method had distinct advantages of using small sample volume (10 μl); rapid simultaneous analysis of KACs (run time = 6.5 min); simple sample preparation; minor matrix effect; high specificity and sensitivity; and good recovery of KACs etc. (Table 3–5). Moreover, the method can be run in dual mode (positive and negative modes simultaneously) to help reduce per sample analysis time. In this study, this method was used for the quality control and stability studies of KACs in the ATB mixture, as well as for analyzing the concentrations of KACs in the biological samples from the animal PK studies. The robustness and sensitivity of the method allows for its application in the planned clinical studies of ATB at the Medical College of Wisconsin.
Figure 2.

UPLC-MS/MS chromatogram of ATB key active components and the internal standard (Baohuoside I). (A) Total ion chromatogram, (B) chromatogram in positive mode, (C) chromatogram in negative mode different from the control value (p < 0.05).
Table 4.
Recovery and matrix effect results of matrine, dictamine, maackiain and fraxinellone at high, medium, and low concentrations in mouse blood and saliva
| Conc. | Matr | Dict | Maac | Frax | ||||
|---|---|---|---|---|---|---|---|---|
| Recovery (%) | Matrix effect (%) | Recovery (%) | Matrix effect (%) | Recovery (%) | Matrix effect (%) | Recovery (%) | Matrix effect (%) | |
| LOQ | 84.9 ± 1.5 | 88.3 ± 4.0 | 110.0 ± 3.3 | 114.7 ± 6.3 | 95.2 ± 9.3 | 102.7 ± 8.9 | 107.5 ± 12.8 | 111.4 ± 24.0 |
| MQC | 79.6 ± 3.3 | 94.6 ± 6.4 | 89.5 ± 6.1 | 87.6 ± 1.5 | 103.6±2.5 | 85.7 ± 1.0 | 82.6 ± 7.7 | 102.4 ± 4.8 |
| HQC | 76.0 ± 7.4 | 89.7 ± 2.8 | 90.4 ± 12.7 | 88.1 ± 6.1 | 101.4 ± 4.8 | 102.5 ± 6.6 | 87.1 ± 12.6 | 107.3 ± 12.8 |
There are few limitations in our present study. First, the drug concentrations in mouse saliva showed more interindividual variability than desired, possibly due to low amounts of saliva collected from the animal. Most published studies involving mouse saliva measurement used pilocarpine (IP) to stimulate saliva secretion to measure the effect of the disease or the intervention on the salivary gland function.35,36 However, we did not use any pilocarpine in our study to accurately measure the drug concentrations secreted in saliva. Therefore, in future studies, we need to develop better method of saliva collection from small animals to reduce this variability (e.g., cannulation of salivary glands).37 Second, the low blood levels of Maac and Frax at different time points made it difficult to accurately estimate the pharmacokinetic parameters for all the animals. In future studies, this can be overcome by using either the higher oral dose of individual compounds or ATB mixture for the pharmacokinetic studies.
Patient non-compliance to the prescribed medication is a challenge in everyday clinical practice, more so for botanical drugs because they are easily mistaken as supplements and missing a dose occasionally does not seem very harmful. However, the consequences of non-compliance to the prescribed medication schedule could be serious including treatment ineffectiveness and/or failure38–41, patient health status deterioration, additional hospitalizations or even deaths.39,42 Significant salivary secretion of Matr after administration of ATB (Fig 5, Table 10) indicated its potential for being used as a drug monitoring tool to follow patient compliance in clinical trials. In addition, the stability in saliva (6 months in −80oC, 7 days in −20oC, and 4 hrs in room temperature) (Table S3), easy quantification at lower concentrations (LLOQ = 2 nM) and no accumulation in the body after multiple-dose administration (Fig 5) add to its suitability to be used as compliance marker compound in long-term intervention studies, where patients can collect a daily saliva sample at home and store the samples in −20°C freezer before submitting them for analysis on their next visit to doctor. Though, the continuous intragastric administration of 5 g/kg Radix S. tonkinenis (equivalent to approximately 25 mg/kg Matr) for 14 days showed the inhibition of rat liver enzymes,43 a much lower proposed dose of 1.2 mg/kg ATB three times a day for 7–24 days would be safe for Matr to be used a compliance marker in the planned clinical trials (NCT03459729 and NCT04278989).
Although ATB has not been marketed in the USA yet, many ATB individual herb or their liquid extract such as Shan Dou Gen (S. tonkinensis Gagnep. - which contains Matr), Bai Xian Pi (D. dasycarpus Turcz. - which contains Dict), Huang Yao Zi, Xia Ku Cao, Quan Shen are available as natural supplements on many online stores (Amazon, Ebay, Walmart, Bonanza, Chineseherbsdirect, Lifeirl) for over-the-counter purchase. Therefore, the PK information obtained here may be broadly applicable to the matrine in these products, and a lack of accumulation and high sensitivity meant that matrine can be used in the clinical studies of these products in humans.
In conclusion, the comprehensive preclinical studies undertaken here showed that Matr is excreted into the mouse saliva by using a fully validated LC-MS/MS method. We also showed the importance of intestine versus liver in first-pass metabolism of 4 KACs in ATB, and the pattern suggests we should try to develop local delivery of these four active compounds since their oral bioavailability is low. The convenient detection of Matr should lead to its use as a tool for monitoring patient compliance and drug exposure in the planned and upcoming ATB clinical trials (NCT03459729 and NCT04278989).
Supplementary Material
Figure 1.
Chemical structure of ATB key active components
Significant statement.
This study contributed the in vitro and in vivo pharmacokinetic data in animals submitted to FDA for the Investigational New Drug (IND) application for testing Antitumor B as a botanical drug. Currently, there are two open clinical trials of ATB approved by FDA to investigate the pharmacokinetics (NCT03459729) and tumor inhibition (NCT04278989) of ATB in oral cancer patients. Matrine, dictamine, maackiain, and fraxinellone were determined as the key active components of ATB with significant in vitro activities against oral cancer. The oral bioavailability of ATB active compounds were low but significant levels of dictamine and matrine were found in saliva upon multiple administrations, providing for support that KACs are available for the pharmacological actions in the oral tissue. The PK profiles of ATB components suggested that modifications such as KAC-enriched ATB formula with targeted delivery in oral cavity may be needed to better leverage the chemopreventive efficacy of ATB against oral carcinoma.
Acknowledgments
This project is supported by the NIH Grant CA205633 and GM070737
Footnotes
Conflict of interest Disclosure
The authors declare no competing financial interest.
Associated Content
Supporting information
References
- 1.Masthan KM, Babu NA, Sankari SL & Priyadharsini C Leukoplakia: A short review on malignant potential. J Pharm Bioallied Sci 7, S165–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dionne KR, Warnakulasuriya S, Zain RB & Cheong SC Potentially malignant disorders of the oral cavity: current practice and future directions in the clinic and laboratory. Int J Cancer 136, 503–515 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136, E359–86 (2015). [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Facts & Figures 2019. Am. Cancer Soc (2019).
- 5.Friedlander P et al. Functional status after primary surgical therapy for squamous cell carcinoma of the base of the tongue. Head Neck 24, 111–114 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Epstein JB, Robertson M, Emerton S, Phillips N & Stevenson-Moore P Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck 23, 389–398 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Gellrich NC et al. Pain, function, and psychologic outcome before, during, and after intraoral tumor resection. J. Oral Maxillofac. Surg 60, 772–777 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Perlmutter MA, Johnson JT, Snyderman CH, Cano ER & Myers EN Functional outcomes after treatment of squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg 128, 887–891 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Am. Cancer Society. Cancer Facts & Figures. Am. Cancer Soc (2017).
- 10.Sun Z, Guan X, Li N, Liu X & Chen X Chemoprevention of oral cancer in animal models, and effect on leukoplakias in human patients with ZengShengPing, a mixture of medicinal herbs. Oral Oncol. 46, 105–110 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Fan X Inhibitory effect of antitumor-B and retinamide on precancerous lesions of the bladder in rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. (1993). [PubMed] [Google Scholar]
- 12.Wang Y, Zhang Z, Kastens E, Lubet RA & You M Mice with alterations in both p53 and Ink4a/Arf display a striking increase in lung tumor multiplicity and progression: differential chemopreventive effect of budesonide in wild-type and mutant A/J mice. Cancer Res. 63, 4389–4395 (2003). [PubMed] [Google Scholar]
- 13.Zhang Z et al. Cancer chemopreventive activity of a mixture of Chinese herbs (antitumor B) in mouse lung tumor models. Oncogene (2004). doi: 10.1038/sj.onc.1207496 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y et al. Chemopreventive effect of a mixture of Chinese Herbs (antitumor B) on chemically induced oral carcinogenesis. Mol. Carcinog (2013). doi: 10.1002/mc.20877 [DOI] [PubMed] [Google Scholar]
- 15.Lin PZ et al. [Secondary prevention of esophageal cancer--intervention on precancerous lesions of the esophagus]. Zhonghua Zhong Liu Za Zhi 10, 161–166 (1988). [PubMed] [Google Scholar]
- 16.Lin P [Medicamentous inhibitory therapy of precancerous lesions of the esophagus--3 and 5 year inhibitory effect of antitumor B, retinamide and riboflavin]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 12, 235–245 (1990). [PubMed] [Google Scholar]
- 17.Lin P et al. Studies on medicamentous inhibitory therapy for esophageal precancerous lesions--3- and 5-year inhibitory effects of antitumor-B, retinamide and riboflavin. Proc. Chin. Acad. Med. Sci. Peking Union Med. Coll 5, 121–129 (1990). [PubMed] [Google Scholar]
- 18.Gao G & Law FCP Physiologically based pharmacokinetics of matrine in the rat after oral administration of pure chemical and ACAPHA. Drug Metab. Dispos 37, 884–891 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao G Commparative pharmacokinetics of matrine: pure matrine vs. crude chemical in ACAPHA. (Simon Fraser University, 2007). [Google Scholar]
- 20.Yin T et al. Developing an activity and absorption-based quality control platform for Chinese traditional medicine: Application to Zeng-Sheng-Ping(Antitumor B). J. Ethnopharmacol 172, 195–201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y et al. Chemopreventive effect of a mixture of Chinese Herbs (antitumor B) on chemically induced oral carcinogenesis. Mol. Carcinog 52, 49–56 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Bui D et al. Pharmacokinetic and Metabolic Profiling of Key Active Components of Dietary Supplement Magnolia officinalis Extract for Prevention against Oral Carcinoma. J. Agric. Food Chem (2020). doi: 10.1021/acs.jafc.0c01475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ICH Expert Working Group. ICH Guideline Q1A(R2) Stability Testing of New Drug Substances and Products. in International Conference on Harmonization (2003). doi: 10.1136/bmj.333.7574.873-a [DOI] [Google Scholar]
- 24.Cder FDA US Food and Drug Administration. Guidance for industry: bioanalytical method validation guidance for industry bioanalytical method validation. 1–22 (2018). [Google Scholar]
- 25.Zhao J et al. [Effect and mechanism of matrine on adhesion and invasion of salivary gland adenoid cystic carcinoma cells in vitro]. Shanghai Kou Qiang Yi Xue 18, 401–405 (2009). [PubMed] [Google Scholar]
- 26.Lee YJ et al. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther 130, 157–176 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Wang D & Dubois RN Eicosanoids and cancer. Nature Reviews Cancer (2010). doi: 10.1038/nrc2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasry WHS, Rodriguez-Lecompte JC & Martin CK Role of COX-2/PGE2 mediated inflammation in oral squamous cell carcinoma. Cancers (Basel). 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim KJ, Rajan K & Eberhart CG Effects of Zeng Sheng Ping/ACAPHA on malignant brain tumor growth and notch signaling. Anticancer Res. 32, 2689–2696 (2012). [PMC free article] [PubMed] [Google Scholar]
- 30.Gao S, Yang Z, Yin T, You M & Hu M Validated LC-MS/MS method for the determination of maackiain and its sulfate and glucuronide in blood: Application to pharmacokinetic and disposition studies. J. Pharm. Biomed. Anal 55, 288–293 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z et al. Biopharmaceutical and pharmacokinetic characterization of matrine as determined by a sensitive and robust UPLC-MS/MS method. J. Pharm. Biomed. Anal (2010). doi: 10.1016/j.jpba.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ran Q-Q, Ruan L-P, Zhu D-N & Yu B-Y [Improving the solubility of fraxinellone to increase its oral bioavailability and hepatoprotective action against acute liver injury in mice]. Yao Xue Xue Bao 42, 675–680 (2007). [PubMed] [Google Scholar]
- 33.Wang P et al. Pharmacokinetics, tissue distribution and excretion study of dictamnine, a major bioactive component from the root bark of Dictamnus dasycarpus Turcz. (Rutaceae). J. Chromatogr. B Anal. Technol. Biomed. Life Sci 942–943, 1–8 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Peng Q et al. Mitogen-activated protein kinase signaling pathway in oral cancer (Review). Oncol. Lett 15, 1379–1388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamy E et al. Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci. 8, 65 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagavant H et al. A method for the measurement of salivary gland function in mice. J. Vis. Exp 2018, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuriki Y et al. Cannulation of the mouse submandibular salivary gland via the wharton’s duct. J. Vis. Exp (2011). doi: 10.3791/3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochieng W et al. Implementation and Operational Research: Correlates of Adherence and Treatment Failure Among Kenyan Patients on Long-term Highly Active Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr 69, e49–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kardas P Patient non-compliance as a cause of treatment failure. Pol. Merkur. Lekarski 9, 732–735 (2000). [PubMed] [Google Scholar]
- 40.Lailulo Y, Kitenge M, Jaffer S, Aluko O & Nyasulu PS Factors associated with antiretroviral treatment failure among people living with HIV on antiretroviral therapy in resource-poor settings: a systematic review and metaanalysis. Syst. Rev 9, 292 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bezabhe WM, Chalmers L, Bereznicki LR & Peterson GM Adherence to Antiretroviral Therapy and Virologic Failure: A Meta-Analysis. Medicine (Baltimore). 95, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J, Sklar GE, Oh V. M. Sen & Li SC Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther. Clin. Risk Manag 4, 269–286 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai J et al. Effect of Radix Sophorae Tonkinensis on the activity of cytochrome P450 isoforms in rats. Int. J. Clin. Exp. Med 8, 9737–9743 (2015). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


