Abstract
Background: Peripheral nerve pathology is frequently encountered in clinical practice among peripheral nerve and extremity surgeons. One major factor limiting nerve regeneration and possibly leading to revision surgeries is the development of traumatic or postoperative adhesions and scarring around nerves. In experimental models, different materials have been studied to limit scar tissue formation when wrapped around nerves. Methods: A systematic review of studies describing nerve-wrapping materials in a non-transectional rat sciatic nerve model was performed following the PRISMA guidelines. Literature describing nerve-wrapping methods for the prevention of peripheral nerve scarring in rat sciatic nerve models was identified using PubMed and Web of Science, scanned for relevance and analyzed. Results: A total of 15 original articles describing 23 different materials or material combinations for nerve wrapping were included. The heterogeneity of the methods used did not allow a meta-analysis, thus, a systematic review was performed. Out of 28 intervention groups, 21 demonstrated a preventive effect on scar tissue formation in at least one qualitative or quantitative assessment method. Conclusions: The analyzed literature describes a variety of materials from different origins to limit peripheral nerve scarring and adhesions. Thus, a scar-preventive effect by wrapping peripheral nerves as adhesion prophylaxis seems likely. However, a quantitative comparison of the studies to identify the optimal material or technique is not possible with the diversity of used models and study designs. Therefore, further research needs to be performed to identify the optimal nerve wraps to be used routinely in clinical practice.
Keywords: peripheral nerve injuries, biomaterials, microsurgery, nerve regeneration, nerve scarring, nerve adhesions
1. Introduction
Peripheral nerve pathology is frequent and poses both a clinical and economic challenge [1]. Postoperative or traumatic scarring and adhesions around peripheral nerves can cause debilitating symptoms in affected patients, hindering regeneration and sometimes leading to further surgical treatment [2]. Scar development is a normal and essential part of regeneration after peripheral nerve injuries, stabilizing the wound and giving the necessary tissue structure for axonal sprouting [3]. However, extensive scarring and adhesions may compress the nerve, impair its essential gliding ability, and can result in fibrosis within the neural and perineural tissue [4]. This, in turn, leads to decreased nerve perfusion and impaired regeneration after nerve injury [5,6]. Nerve scarring and adhesions occur in the connective tissue around nerve fiber bundles, known as perineurium and epineurium, and in the connective tissue between the nerve and its surrounding tissues, labeled as paraneurium [7].
Besides neurolysis and occasionally the provision of additional soft tissue coverage, effective clinical options for the treatment of primary or secondary nerve scarring are limited [2,8]. However, since the first clinical reports of positive effects and improved outcomes after vein wrapping in the surgical treatment of extensive scarring around nerves, additional research has been performed in this field and further treatment options have been explored [9].
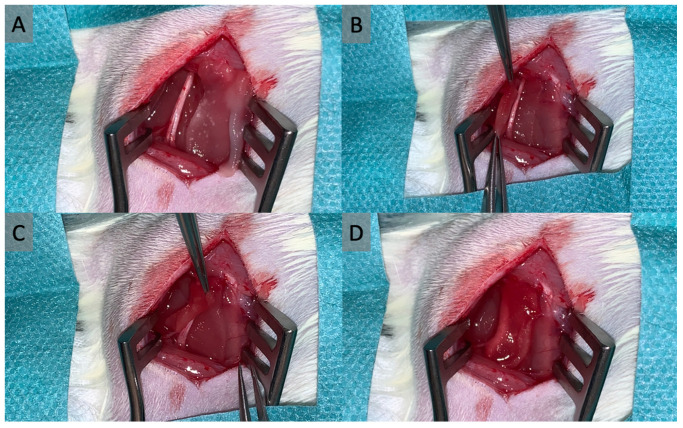
Numerous methods of wrapping with autologous or bioartificial materials have been described to improve peripheral nerve regeneration and prevent scar tissue formation. The underlying idea of this concept is to create a barrier around the nerve which prevents the formation of extensive scarring and adhesions between the nerve and its surrounding tissue and preserves the gliding ability of the nerve while allowing the diffusion of nutrients to the nerve [10]. However, assessing the effects of the material used is challenging when distinguishing between direct positive influences on nerve regeneration and secondary positive influences due to limited or prevented scarring. Especially the wrapping of peripheral nerves with a spacer material placed between the nerve and the surrounding tissue seems to provide favorable results in limiting peripheral nerve scarring [10]. One example of this technique is shown in Figure 1, where a synthetic collagen matrix is being wrapped around a part of a rat’s sciatic nerve.
Figure 1.
In this example for nerve wrapping, a collagen matrix is being wrapped around the sciatic nerve of a rat to limit scar tissue formation. The matrix is initially placed next to the nerve (A), then carefully pulled through underneath the nerve (B), and finally wrapped around it (C,D).
While the rat sciatic nerve model is the most popular animal model for peripheral nerve injury and scarring, various methods are described to induce peripheral nerve scarring and evaluate outcomes in an experimental setting [11]. A number of studies use transection and consecutive epineural sutures for scar induction, while some authors use less traumatic methods avoiding neurotmesis [11]. However, transection injuries are significantly more traumatic than other methods of scar induction, comparing materials tested with different injury types is hardly possible.
This study describes and reviews previously explored methods for nerve wrapping to prevent scarring around peripheral nerves in the rat sciatic nerve model after peripheral nerve scarring induction without neurotmesis.
2. Methods
This review was performed in accordance with the “Preferred Reporting Items for Systematic reviews and Meta-Analyses” (PRISMA) guidelines to ensure transparency and reproducibility [12].
2.1. Search Strategy
The scientific databases MEDLINE using the PubMed® interface and Web of ScienceTM were used to identify publications matching the search query “(rat) AND ((peripheral nerve) OR (sciatic nerve)) AND ((wrap) OR (wrapping) OR (cover) OR (covering) OR (coat) OR (coating) OR (barrier) OR (space) OR (spacer)) AND ((injury) OR (scar) OR (adhesion) NOT (transection) NOT (cut))”. The search was completed on 30 September 2022. The reference lists of studies included in the full-text screening were also searched and 10 additional publications were identified (citation search).
2.2. Inclusion Criteria
For this systematic review, the inclusion criteria were (1) a rat sciatic model inducing peripheral nerve scarring and perineural adhesions, (2) nerve coating/wrapping or any method to install a spacer material between the nerve and the surrounding tissue at the time of injury, (3) assessment of nerve adhesions/scar formation, and (4) available full text in English or German.
2.3. Exclusion Criteria
The review excluded studies matching the following criteria (1) nerve cut or transection injury, (2) other animal species than the rat, (3) non-sciatic nerve models, (4) in-vitro models, (5) other treatment/prevention methods than spacer material application, (6) no negative control group (defined as animals with injury but without spacer application), (7) study type of review/meta-analysis, (8) language other than English or German, and (9) publication date after September 2022.
2.4. Study Selection
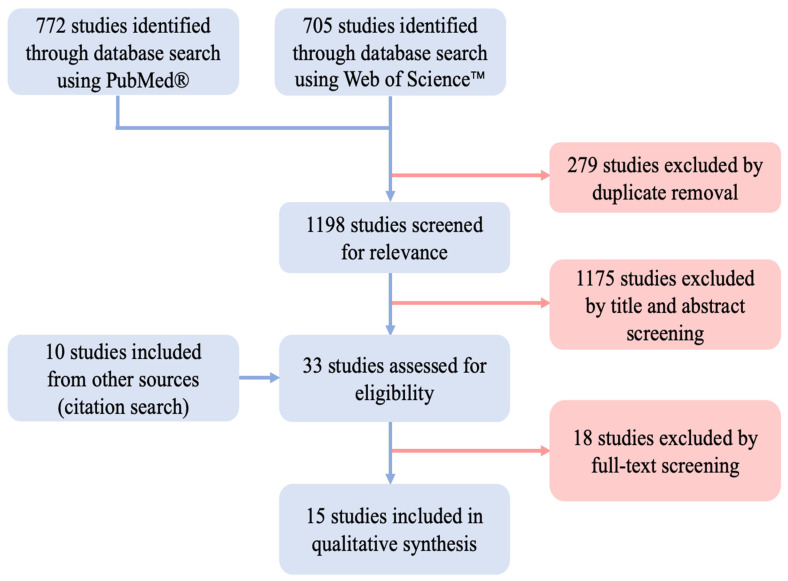
Two independent reviewers scanned the identified studies. First, titles and abstracts were screened for each included study. Next, full texts were screened for all studies not excluded in the abstract screening. In case of disagreement, a consensus decision was made with the support of a senior reviewer. The process is depicted as a flow diagram in Figure 2.
Figure 2.
Flow diagram illustrating the study screening and selection process performed according to the PRISMA guidelines [12].
2.5. Data Extraction
The publications included in the final review were analyzed and their data were extracted and compiled for qualitative synthesis using Microsoft Excel Version 16.77.1 (Microsoft Corporation, Redmond, WA, USA). Tables for comparison of injury, intervention, assessment, follow-up time, and effect were used to estimate the trend of the results from the included studies. The used biomaterials were summarized regarding their regenerating qualities as a second objective. In studies with multiple intervention groups, including groups with transection injuries, only the groups matching our inclusion criteria were used for qualitative synthesis.
3. Results
3.1. Study Selection
Using PubMed®, 772 studies were identified and 705 studies were found using Web of ScienceTM. After excluding duplicates, 1198 studies remained. By applying the inclusion and exclusion criteria, 1175 studies were excluded during the title and abstract screening. By identifying studies from the references of the retrieved papers (citation search), 10 further studies were included for full-text assessment. Full-text assessment was performed on 33 studies in total. Of these, 15 studies met the final inclusion criteria and were used for qualitative synthesis [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27].
3.2. Study Characteristics
In total, eight different methods of scarring induction were used in all included studies [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27], and four studies used a combination of multiple methods [16,21,25,26]. Two studies compared the effects of their investigated prevention method on multiple scarring techniques [14,18]. Twenty-three different spacer materials and material combinations were investigated [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. The follow-up time until the final scarring assessment ranged from 4 weeks to 5 months, with most studies evaluating at 6 weeks after the initial surgery [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. In total, 475 sciatic nerves were assessed in all intervention groups, ranging from 7 to 42 per study, with a median of 15 assessed nerves per study group [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Basic study characteristics are shown in Table 1.
Table 1.
Basic study characteristics of all included studies and their intervention groups. Multiple intervention groups within the same study are labelled with letters A–D. For commercially produced wrappers, the production company is depicted in brackets behind the material. Abbr.: PBS = phosphate-buffered saline, AA = ascorbic acid, HA = hyaluronic acid, PLA = polylactide, PCL = poly(ε-caprolactone), CMC = carboxymethylcellulose (CMC), PE = phosphatidylethanolamine, n = number of animals in group, FU = follow-up time in weeks.
| Author | Year | Group | Scarring Induction | Wrapping Material | n | FU |
|---|---|---|---|---|---|---|
| Baltu et al. [27] | 2017 | A | Epineurectomy | Buccal mucosa graft | 24 | 8 |
| Dumanian et al. [13] | 1999 | A | Epineurectomy | Free fat grafts | 14 | 8 |
| Finsterbush et al. [26] | 1982 | A | Crush injury, muscle cauterization | Semirigid 15 mm silicone tube, cut longitudinally | 30 | 8 |
| Görgülü et al. [14] | 1998 | A | External neurolysis | Collagen fibers soaked with aprotinin | 22 | 6 |
| B | Abrasive injury | Collagen fibers soaked with aprotinin | 22 | 6 | ||
| C | External neurolysis | Collagen fibers soaked with PBS | 22 | 6 | ||
| D | Abrasive injury | Collagen fibers soaked with PBS | 22 | 6 | ||
| Hernandez-Cortes et al. [25] | 2010 | A | Perineurectomy + muscle cauterization | Oxidized regenerated cellulose wrap | 40 | 6 |
| Kikuchi et al. [24] | 2020 | A | Muscle cauterization | E8002 wrapping (Kawasumi Laboratories Inc., Tokyo, Japan) | 7 | 6 |
| B | Muscle cauterization | E8002 AA- wrapping (Kawasumi Laboratories Inc., Tokyo, Japan) | 7 | 6 | ||
| Li et al. [23] | 2018 | A | Crush injury | Chitosan conduit | 15 | 12 |
| B | Crush injury | HA gel | 15 | 12 | ||
| C | Crush injury | Chitosan conduit + HA gel | 15 | 12 | ||
| Murakami et al. [22] | 2014 | A | Chronic constriction injury by nerve ligation | Allogenic vein wrap | 30 | 20 |
| Ohsumi et al. [21] | 2005 | A | External/internal neurolysis + muscle cauterization | Viscous alginate sol | 8 | 6 |
| Okui et al. et al. [16] | 2010 | A | Internal neurolysis + muscle cauterization | Honeycomb poly-lactide film | 42 | 6 |
| B | Internal neurolysis + muscle cauterization | Cast poly-lactide film | 12 | 6 | ||
| Özgenel et al. [20] | 2004 | A | Epineurectomy | Human amniotic membrane | 12 | 12 |
| B | Epineurectomy | Human amniotic membrane + HA injection | 12 | 12 | ||
| Petersen et al. [15] | 1996 | A | Internal neurolysis | ADCON-T/N gel (Gliatech, Inc., Cleveland, OH, USA) | 9 | 4 |
| B | Internal neurolysis | Control gel | 9 | 4 | ||
| Shintani et al. [19] | 2018 | A | Muscle cauterization | PLA/PCL tube | 12 | 6 |
| B | Muscle cauterization | 1% HA | 8 | 6 | ||
| Smit et al. [18] | 2004 | A | External neurolysis | 1% HA | 5 | 6 |
| B | Crush injury | 1% HA | 7 | 6 | ||
| Yamamoto et al. [17] | 2010 | A | Internal neurolysis | 1% HA | 18 | 6 |
| B | Internal neurolysis | CMC-PE hydrogel, low viscosity | 18 | 6 | ||
| C | Internal neurolysis | CMC-PE hydrogel, high viscosity | 18 | 6 |
3.3. Scarring Assessment
Assessing the perineural scar formation with either qualitative or quantitative methods, 14 studies reported improved outcomes in at least one intervention group [13,14,15,16,17,18,19,20,21,22,23,24,26,27], and 1 study reported no significant difference in its only intervention group [25].
For scarring assessment, different approaches were used, including macroscopic evaluation (qualitative and quantitative), histological evaluation (qualitative and quantitative), and biomechanical testing (all quantitative). Eight studies reported quantified macroscopic scar assessment [14,15,17,19,20,23,24,27], in which twelve of nineteen intervention groups were reported to have significantly improved results after spacer application and the remaining seven not demonstrating a significant effect in this assessment method. All of these studies used the grading system for adhesions established by Petersen et al. [15], except for one study with three groups, where a modified system was used [17]. Using histological or microscopic methods, six studies with eleven groups combined reported quantified outcomes [15,20,23,24,25,27], of which eight intervention groups were significantly improved compared to their control groups. Biomechanical testing was performed in five studies with nine groups combined [13,17,18,19,21], in eight of which a significant improvement following spacer application was observed. Three studies used only descriptive methods for scarring assessment, all of which reported improved outcomes [16,22,26]. Assessment methods and overall outcome tendencies are shown in Table 2.
Table 2.
Scarring induction and assessment methods of all included studies and their intervention groups. Multiple intervention groups within the same study are labelled with letters A–D. For commercially produced wrappers, the production company is depicted in brackets behind the material. The right two columns show the outcomes as assessed in the study with quantitative or descriptive assessment methods. Abbr.: PBS = phosphate-buffered saline, AA = ascorbic acid, HA = hyaluronic acid, PLA = polylactide, PCL = poly(ε-caprolactone), CMC = carboxymethylcellulose (CMC), PE = phosphatidylethanolamine.
| Author | Year | Group | Scarring Induction | Wrapping Material | Scar Assessment Method | Scar Prevention Quantitative Ass. |
Scar Prevention Descriptive Ass. |
|---|---|---|---|---|---|---|---|
| Baltu et al. [27] | 2017 | A | Epineurectomy | Buccal mucosa graft | Adhesion score (Petersen et al. 1996 [15]), epineural scar density score | yes | yes |
| Dumanian et al. [13] | 1999 | A | Epineurectomy | Free fat grafts | Nerve stiffness | yes | yes |
| Finsterbush et al. [26] | 1982 | A | Crush injury, muscle cauterization | Semirigid 15 mm silicone tube, cut longitudinally | Desriptive histology | not described | yes |
| Görgülü et al. [14] | 1998 | A | External neurolysis | Collagen fibers soaked with aprotinin | Adhesion score (Petersen et al. 1996 [15]) | yes | yes |
| B | Abrasive injury | Collagen fibers soaked with aprotinin | Adhesion score (Petersen et al. 1996 [15]) | yes | yes | ||
| C | External neurolysis | Collagen fibers soaked with PBS | Adhesion score (Petersen et al. 1996 [15]) | no | no | ||
| D | Abrasive injury | Collagen fibers soaked with PBS | Adhesion score (Petersen et al. 1996 [15]) | no | no | ||
| Hernandez-Cortes et al. [25] | 2010 | A | Perineurectomy + muscle cauterization | Oxidized regenerated cellulose wrap | Connective tissue measurement | no | no |
| Kikuchi et al. [24] | 2020 | A | Muscle cauterization | E8002 wrapping (Kawasumi Laboratories Inc.) | Adhesion score (Petersen et al. 1996 [15]), optical scar density | yes | yes |
| B | Muscle cauterization | E8002 AA- wrapping (Kawasumi Laboratories Inc.) | Adhesion score (Petersen et al. 1996 [15]), optical scar density | no | no | ||
| Li et al. [23] | 2018 | A | Crush injury | Chitosan conduit | Adhesion score (Petersen et al. 1996 [15]), epineurium collagen density | yes | yes |
| B | Crush injury | HA gel | Adhesion score (Petersen et al. 1996 [15]), epineurium collagen density | yes | yes | ||
| C | Crush injury | Chitosan conduit + HA gel | Adhesion score (Petersen et al. 1996 [15]), epineurium collagen density | yes | yes | ||
| Murakami et al. [22] | 2014 | A | Chronic constriction injury by nerve ligation | Allogenic vein wrap | Descriptive histology | not described | yes |
| Ohsumi et al. [21] | 2005 | A | External/internal neurolysis + muscle cauterization | Viscous alginate sol | Biomechanical breaking strength, descriptive histology | yes | yes |
| Okui et al. et al. [16] | 2010 | A | Internal neurolysis + muscle cauterization | Honeycomb poly-lactide film | Descriptive histology, descriptive macroscopic adhesion strength | not described | yes |
| B | Internal neurolysis + muscle cauterization | Cast poly-lactide film | Descriptive macroscopic adhesion strength | not described | no | ||
| Özgenel et al. [20] | 2004 | A | Epineurectomy | Human amniotic membrane | Adhesion score (Petersen et al. 1996 [15]), scar thickness measurement | yes | yes |
| B | Epineurectomy | Human amniotic membrane + HA injection | Adhesion score (Petersen et al. 1996 [15]), scar thickness measurement | yes | yes | ||
| Petersen et al. [15] | 1996 | A | Internal neurolysis | ADCON-T/N gel (Gliatech, Inc., Cleveland, OH, USA) | Adhesion score (Petersen et al. 1996 [15]), scar area measurement | yes | yes |
| B | Internal neurolysis | Control gel | Adhesion score (Petersen et al. 1996 [15]), scar area measurement | no | no | ||
| Shintani et al. [19] | 2018 | A | Muscle cauterization | PLA/PCL tube | Adhesion score (Petersen et al. 1996 [15]), biomechanical breaking strength, descriptive histology | yes | yes |
| B | Muscle cauterization | 1% HA | Adhesion score (Petersen et al. 1996 [15]), biomechanical breaking strength, descriptive histology | no | no | ||
| Smit et al. [18] | 2004 | A | External neurolysis | 1% HA | Biomechanical breaking strength | yes | yes |
| B | Crush injury | 1% HA | Biomechanical breaking strength | yes | yes | ||
| Yamamoto et al. [17] | 2010 | A | Internal neurolysis | 1% HA | Adhesion score, biomechanical breaking strength, descriptive scar area measurement | no | yes |
| B | Internal neurolysis | CMC-PE hydrogel, low viscosity | Adhesion score, biomechanical breaking strength, descriptive scar area measurement | yes | yes | ||
| C | Internal neurolysis | CMC-PE hydrogel, high viscosity | Adhesion score, biomechanical breaking strength, descriptive scar area measurement | yes | yes |
3.4. Used Spacer Materials
With 23 different spacer materials or material combinations investigated, this collective of studies documents various approaches for the spacer technique [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Classifying biomaterials in a systematic way is important to identify trends and potentials within groups of materials. In the literature examining biomaterials used as nerve conduits, these materials are frequently divided into two large groups: materials of natural and materials of synthetic origin. These are further divided into subgroups regarding the main components [29]. Since tissue transplants are frequently used to wrap nerves for scarring prevention, but not as nerve conduits, they will be listed in a separate subgroup in this review. Looking at the underlying material origin, the spacer materials described in the examined literature can be divided into four different (sub-)groups, as shown in Table 3. These include tissue-derived materials, protein-based materials and polysaccharide materials, which are all counted as natural materials, and materials based on synthetic polymers.
Table 3.
Used biomaterials and their according groups. Abbr.: PBS = phosphate-buffered saline, PLA = polylactide, PCL = poly(ε-caprolactone), CMC = carboxymethylcellulose (CMC), PE = phosphatidylethanolamine.
| Biomaterial | Material Type | Origin |
|---|---|---|
| Buccal mucosa graft | Tissue-based | Natural |
| Fat graft | ||
| Vein graft | ||
| Human amniotic membrane | ||
| Collagen fibers + aprotinin | Protein-based | |
| Collagen fibers + PBS | ||
| ADCON-T/N gel (gel composed of gelatin and a carbohydrate polymer in PBS) | ||
| ADCON-T/N control gel | ||
| Oxidized regenerated cellulose wrap | Polysaccharide-based | |
| CMC-PE hydrogel | ||
| Hyaluronic acid (gel) | ||
| Alginate sol | ||
| Chitosan | ||
| Silicone | Synthetic Polymer | Synthetic |
| PLA | ||
| PLA-PCL | ||
| E8002 (PLA-based membrane with L-ascorbic acid) |
Tissue-derived materials: Baltu et al. described an improved epineural scar density score after autologous buccal mucosa graft application [27]. After applying free fat grafts from the rat’s groin region, Dumanian et al. showed a decreased nerve stiffness following epineurectomy [13].
Furthermore, allogenic vein transplants have been thoroughly investigated as a nerve wrap beforehand, and Murakami et al. showed reduced epineural scarring in descriptive histology [22]. Özgenel et al. investigated the use of a human amniotic membrane xenograft and found improved macroscopic adhesion scores after epineurectomy [20]. In another group, Özgenel et al. combined the human amniotic membrane with 1% hyaluronic acid (H.A.) and observed improved results in the adhesion scores and the scar thickness measurement [20].
Protein-based materials: Using collagen fibers to wrap the nerve and compare the effects of the proteinase inhibitor aprotinin to phosphate-buffered saline (PBS), Görgülü et al. found improved adhesion scores in the aprotinin group, but not in the PBS group [14]. ADCON-T/N is a gel composed of porcine gelatin and a polyglycan ester in PBS. The group treated with the ADCON-T/N gel was observed to have less macroscopic scar formation than both, the untreated control group as well as another group treated with a control gel, described by the authors as lacking a specific carbohydrate component [15,30]. While this, as many others, represents a hybrid between different categories, it was listed in this category because of gelatin’s main component, collagen.
Polysaccharide-based materials: Hyaluronic acid gel is part of eight investigated groups of the included studies, representing the most-used material in this cohort [17,18,19,20,23,25]. While it was investigated in combination with other materials in three groups, it was also tested on its own in five groups, showing a positive effect in four of these [17,18,19]. As mentioned above, it is described to have positive results in combination with a human amniotic membrane graft [20]. Another combination is hyaluronic acid with a chitosan conduit, showing a lower scar collagen density than the control group as described by Li et al. [23]. In this study, the chitosan conduit is also investigated on its own. While both the chitosan conduit and the HA show positive influences when applied individually, the best results are achieved after combining both [23]. As the only included study describing no improved results in any intervention group after spacer application, Hernández-Cortés et al. tested the effects of oxidized regenerated cellulose and evaluated scar formation using quantitative connective tissue measurement [25]. Ohsumi et al. examined a viscous alginate sol as a spacer material, which by resulting in a lower biomechanical breaking strength demonstrated scarring prevention, further supported by descriptive histology [21]. Two gels of different viscosities made by combining Carboxymethylcellulose (CMC) with the phosphoglyceride phosphatidylethanolamine (PE) were investigated by Yamamoto et al. and demonstrated to have positive effects in both versions. However, the group treated with the lower viscosity gel showed less scarring in the macroscopic evaluation and in breaking strength testing [17].
Materials based on synthetic polymers: Finsterbush et al. investigated the use of a longitudinally cut silicone tube, showing lower scar tissue formation than the control group in descriptive histology [26]. Three additional studies examined the effects of polylactide (PLA) in different forms [16,19,24]. Kikuchi et al. presented superior effects of E8002, a PLA-based membrane with ascorbic acid, over the control group in adhesion scores and optical scar density, but the same membrane without ascorbic acid did not show improved outcomes [24]. A PLA film with a honeycomb texture demonstrated better results in descriptive histological and adhesion strength analysis, which was not the case for the same film in a cast texture as examined by Okui et al. [16]. Shintani et al. found improved adhesion scores, biomechanical breaking strength, and descriptive histological results after applying a conduit made of PLA and poly(ε-caprolactone) [19].
4. Discussion
Of 28 included intervention groups, 21 (75%) demonstrated a preventive effect on scar development after spacer material application in at least one qualitative or quantitative described endpoint. With 8 different scar induction methods and various outcome measures used to assess the effect of 23 different spacer materials, the overall heterogeneity of the described sample is high. Even though inclusion criteria for this systematic review were set tightly to increase comparability, a quantitative analysis was not possible.
Overall, the enormous efforts in testing a variety of used materials suggest that the technique of spacer material application around peripheral nerves to prevent perineural adhesions is promising, and results might be attributable to the technique as well as to the used material. Nerve wrapping might be a feasible technique to prevent extensive adhesion and scar formation after peripheral nerve injury. However, the current literature does not allow a conclusion on the relative significance of the material selection compared to the independent impact of the surgical technique itself. Nevertheless, feasible wrapping materials need to have certain basic properties to be suited for this indication. Biocompatibility plays a key role, and in future clinical settings, different aspects of patient-personalized material selection must be considered. The goal is to provide an effective mechanical barrier against adhesion formation between the nerve and the paraneural tissue and at the same time create an optimal environment for nerve perfusion, mobility, and nutrition and, thus, nerve regeneration [29].
Biocompatibility may vary from the animal model to the human model. Some materials, like silicone, might show favorable outcomes in animal studies with a limited follow-up, but lead to complications in clinical application [26,31,32]. Adverse reactions, as observed in some materials, run contrary to the goals of placement [31,33,34]. Furthermore, the size and diameter of the wrapping material are essential to avoid iatrogenic constriction or overfitting, possibly reducing the desired effects [35,36].
Tissue-based spacer materials were used in four of the included intervention groups [13,20,22,27]. Nerve wraps based on tissue and nerve covering techniques using transplanted tissue have been researched for decades, with cases of clinical application reported for different grafts and flaps [9,37,38,39,40,41]. Additional soft tissue coverage or wrapping with compatible tissues, like veins, shows promising results and is current practice as a last resort in clinical practice [8]. Their use comes with advantages including high biocompatibility, favorable biodegradation and no additional purchasing costs in the case of autologous transplants. Especially the vein graft has been well-researched from different perspectives in pre-clinical and clinical models [9,22,42,43,44,45,46,47,48,49]. Human amniotic membrane wrapping is described as an option in treatment for recurrent compression neuropathies due to its high biocompatibility and previously reported success in scar prevention [50,51]. Fat grafts or flaps are described as a successful salvage option in recurrent compression neuropathies [37,38,41]. While buccal mucosa has not been researched in larger-scale clinical application studies, it generally fulfills the criteria of biocompatibility and glide apparatus preservation. In general, tissue-based materials are well-researched in peripheral nerve surgery and frequently achieve significantly improved scar prevention and treatment results; some are already in clinical use. On the other hand, depending on the exact material, several aspects, including origin, donor site morbidity, availability, cost, and potential adverse reactions, must be considered individually for each patient [33]. While veins are frequently used due to their easy grafting and limited donor site morbidity, it is unclear if one of these materials produces superior outcomes regarding scarring and adhesion prevention.
Four groups used protein-based spacer materials to prevent peripheral nerve scarring, including collagen, cellulose, and gelatin [14,15,25]. Collagen has previously been described in clinical applications for nerve wrapping after peripheral nerve surgery [52,53,54,55]. Next to promising results in clinical testing, animal models have confirmed several positive effects of collagen, including immunomodulation [56], neuroregeneration [47,57,58,59], and pain alleviation after peripheral nerve injury [53,60]. Several collagen-based nerve wraps and conduits are available on the market and approved for in-patient use, bringing this technique from bench to bedside. Petersen et al. tested the effect of ADCON-T/N gel and the effect of a control gel without one specific carbohydrate component [15]. The basis for this gel is gelatin, largely composed of collagen, combined with a polyglycan ester in PBS [30]. While the control gel did not show scar preventive effects, ADCON gel has been demonstrated to have preventive effects on adhesion formation in tendon injuries and spinal peridural fibrosis additionally to peripheral nerves [15,30,61]. However, case reports of patients experiencing cerebrospinal fluid leaks after the use of the ADCON gel led to the suspension of its use in spinal surgery [62]. This is one example of materials that produce favorable outcomes in an experimental setting but might not be suitable for frequent clinical use, as conditions in humans differ.
The group of polysaccharide-based wrapping materials includes five used materials in the investigated studies. Although Hernández-Cortés et al. did not show an effect in preventing perineural adhesions using a cellulose-based wrapping material [25], cellulose has been examined with positive results in other research, including use around nerves and tendons to prevent adhesions [63,64,65]. Improved oxygen and glucose diffusion through cellulose conduits to nerves has been demonstrated [66,67]. Supporting the promising literature, Yamamoto et al. showed perineural adhesion prevention after using a CMC-PE gel [17]. Similarly, Urano et al. demonstrated improved neuroregeneration after CMC-PE application in an animal model of chronic nerve compression [68]. Hyaluronic acid gel is used in four of the included studies and shows scar preventive effects in three of them [17,18,19,23]. It has been described as a scar-preventive agent on its own after peripheral nerve injuries in previous studies [69,70]. Hyaluronic acid gel seems to show the best results when combined with other methods, as Li et al., Özgenel et al., and others have shown [20,23,71,72,73]. Another representative of the polysaccharide group is alginate, which is already under promising investigation as both a hydrogel and a conduit material in peripheral nerve research [74]. Due to its biochemical and biomechanical properties, it works well as a mechanical barrier to adhesion formation [74]. Last, chitosan, a polysaccharide naturally found in arthropod shells, has been broadly researched in peripheral nerve surgery [75]. Li et al. described its scar-preventive effects on its own and combined with hyaluronic acid [23]. As a conduit and in crystalline form, chitosan prevents epineural scar formation [76]. Its immunomodulatory properties seem to create a favorable regenerative environment after peripheral nerve injury. Therefore, chitosan was investigated clinically in its use to protect coaptation sites of peripheral nerves [75,77].
While silicone was described in earlier studies to prevent perineural scar formation and improve neural regeneration, its use has increasingly become unpopular [26,78]. Although silicone is biocompatible, its non-biodegradability poses clinical use problems, potentially leading to increased fibrosis [31,34]. Today, silastic tube cuffing is even used as a scarring induction method in experimental models [11]. Contrarily, polylactide generally is well-biodegradable [79]. Okui et al. investigate it in a honeycomb and a cast morphology, reporting superior results in the honeycomb and frequent dislocation in the cast morphology [16]. It can be modified using different other materials, as described by investigated studies with poly(ε-caprolactone) or ascorbic acid [19,24]. Clinically, adverse reactions, including inflammatory responses and delayed biodegradation, have been mentioned in combination with PLA- poly(ε-caprolactone) [34]. Ascorbic acid, on the other hand, has been preclinically observed to accelerate Wallerian degeneration and improve neural regeneration [80,81].
As indicated by the positive effect of the described studies, nerve wrapping appears to have a preventive effect on scar and adhesion formation around peripheral nerves. However, it remains unclear which materials are optimal for this purpose. While the concept per se seems to have the desired effect, further studies are needed in order to elaborate on the strengths and weaknesses of different materials. While some materials are already approved for clinical use, future comparative research needs to be conducted on their effects in order to optimize their indication in the sense of personalized treatments based on each patient’s individual case [10].
As illustrated by some of the investigated materials, biocompatibility is essential for the safe application of nerve-wrapping materials, and long-term effects need to be explored before widespread clinical use [10,15,26,31,34,62]. Attention should also be brought to the intraoperative technique of applying nerve wraps. If they are wrapped around the nerve too tightly, this can lead to adverse effects creating increased scar formation and hindering nerve regeneration [82].
Besides nerve wrapping, various essentially different approaches for the prevention of perineural adhesion formation have been explored. These range from local and systemic pharmaceutic interventions, like intraperitoneal verapamil injection [83], over cellular applications to external radiation and frequently show promising results [84,85,86,87]. However, the sheer quantity of research on wrapping spacers and mostly positive results indicates that the effect of wrapping the nerve should not be underestimated. From a practical viewpoint, the success of external radiation and repetitive pharmaceutical interventions, both described as scar-prevention methods, are highly patient-dependent and not easy or economical to administer. Considering the enormous amount of tested spacer materials, refining the spacer technique will be an increasingly big part of the research process in the future.
Görgülü et al. described the combination of collagen fibers as a wrapping material in combination with aprotinin acting as a pharmaceutic anti-scarring agent [14]. The combination of wrapping materials with bioactive agents has been used to improve peripheral nerve regeneration and prevent nerve adhesions in research. This includes drugs limiting fibroblast activity and suppressing inflammatory responses [83,86,87,88,89]. Furthermore, different bioactive agents, including drugs and mesenchymal stem cells, can be used to improve nerve regeneration and create a favorable environment for neurite outgrowth [28,90,91,92]. Four studies included in this systematic review compared hybrid models, adding different agents to wrapping materials, with the use of only one of the components [14,20,23,24]. Favorable results in using these hybrid models suggest that they will be a future refinement enhancing the positive effects of spacer application for the prevention of peripheral nerve adhesions.
In this study, several limitations exist: (1) by excluding transection injuries to homogenize results, potential candidates were left out; (2) the heterogeneity of scarring induction and assessment methods makes direct comparisons barely possible; and (3) all investigated studies use the rat sciatic nerve model which is frequently used. However, it is not optimal in mimicking human peripheral nerve physiology and pathology [93,94]. Therefore, the direct translation of positive effects from the animal model to humans can be limited and requires thorough investigation before the clinical implementation of wrapping materials in patients. Mainly, the heterogeneity in injury types and, in some cases, the lack of distinction between primarily aiming for improved nerve regeneration or decreased scarring introduces difficulties in the comparison of results and thus slows a transition to systematic clinical testing.
5. Conclusions
Our systematic review of different methods for peripheral nerve wrapping demonstrates that most of the literature describes positive effects on preventing peripheral nerve adhesions and scarring by applying a biocompatible spacer material in the animal model. The existing wrapping materials have to be evaluated using standardized and comparative animal models to filter out the most promising candidates before a transition from bench to bedside can be made.
Author Contributions
Conceptualization, M.M.-S. and A.H.B.; methodology, M.M.-S. and A.H.B.; validation, U.K. and L.H.; formal analysis, M.M.-S. and T.T.K.; data curation, T.T.K. and M.A.; writing—original draft preparation, M.M.-S. and A.H.B.; writing—review and editing, L.H. and K.R.E.; visualization, T.T.K. and M.A.; supervision, A.H.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
K.R.E. is a consultant for AxoGen, Checkpoint, Integra, and Tissium. The remaining authors have no potential conflicts of interest with respect to the research, authorship, mentioned products or devices, and publication of this article.
Funding Statement
The authors received no financial support for this article’s research, authorship, and publication.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Aman M., Zimmermann K.S., Thielen M., Thomas B., Daeschler S., Boecker A.H., Stolle A., Bigdeli A.K., Kneser U., Harhaus L. An Epidemiological and Etiological Analysis of 5026 Peripheral Nerve Lesions from a European Level I Trauma Center. J. Pers. Med. 2022;12:1673. doi: 10.3390/jpm12101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tos P., Crosio A., Pugliese P., Adani R., Toia F., Artiaco S. Painful Scar Neuropathy: Principles of Diagnosis and Treatment. Plast. Aesthet. Res. 2015;2:156–164. doi: 10.4103/2347-9264.160878. [DOI] [Google Scholar]
- 3.Wang M.L., Rivlin M., Graham J.G., Beredjiklian P.K. Peripheral Nerve Injury, Scarring, and Recovery. Connect. Tissue Res. 2019;60:3–9. doi: 10.1080/03008207.2018.1489381. [DOI] [PubMed] [Google Scholar]
- 4.Millesi H., Zöch G., Rath T. The Gliding Apparatus of Peripheral Nerve and Its Clinical Significance. Ann. Hand Up. Limb Surg. 1990;9:87–97. doi: 10.1016/S0753-9053(05)80485-5. [DOI] [PubMed] [Google Scholar]
- 5.Atkins S., Smith K.G., Loescher A.R., Boissonade F.M., O’Kane S., Ferguson M.W.J., Robinson P.P. Scarring Impedes Regeneration at Sites of Peripheral Nerve Repair. Neuroreport. 2006;17:1245–1249. doi: 10.1097/01.wnr.0000230519.39456.ea. [DOI] [PubMed] [Google Scholar]
- 6.Clark W.L., Trumble T.E., Swiontkowski M.F., Tencer A.F. Nerve Tension and Blood Flow in a Rat Model of Immediate and Delayed Repairs. J. Hand Surg. Am. 1992;17:677–687. doi: 10.1016/0363-5023(92)90316-H. [DOI] [PubMed] [Google Scholar]
- 7.Millesi H., Zöch G., Reihsner R. Mechanical Properties of Peripheral Nerves. Clin. Orthop. Relat. Res. 1995;314:76–83. doi: 10.1097/00003086-199505000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Pripotnev S., Mackinnon S.E. Revision of Carpal Tunnel Surgery. J. Clin. Med. 2022;11:1386. doi: 10.3390/jcm11051386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masear V.R., Colgin S. The Treatment of Epineural Scarring with Allograft Vein Wrapping. Hand Clin. 1996;12:773–779. doi: 10.1016/S0749-0712(21)00365-6. [DOI] [PubMed] [Google Scholar]
- 10.Dy C.J., Aunins B., Brogan D.M. Barriers to Epineural Scarring: Role in Treatment of Traumatic Nerve Injury and Chronic Compressive Neuropathy. J. Hand Surg. 2018;43:360–367. doi: 10.1016/j.jhsa.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosio A., Ronchi G., Fornasari B.E., Odella S., Raimondo S., Tos P. Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring. J. Clin. Med. 2021;10:1613. doi: 10.3390/jcm10081613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumanian G.A., McClinton M.A., Brushart T.M. The Effects of Free Fat Grafts on the Stiffness of the Rat Sciatic Nerve and Perineural Scar. J. Hand Surg. 1999;24:30–36. doi: 10.1053/jhsu.1999.jhsu24a0030. [DOI] [PubMed] [Google Scholar]
- 14.Görgülü A., Imer M., Şimşek O., Sencer A., Kutlu K., Çobanoǧlu S. The Effect of Aprotinin on Extraneural Scarring in Peripheral Nerve Surgery: An Experimental Study. Acta Neurochir. 1998;140:1303–1307. doi: 10.1007/s007010050254. [DOI] [PubMed] [Google Scholar]
- 15.Petersen J., Russell L., Andrus K., MacKinnon M., Silver J., Kliot M. Reduction of Extraneural Scarring by ADCON-T/N after Surgical Intervention. Neurosurgery. 1996;38:976–984. doi: 10.1097/00006123-199605000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Okui N., Yamamoto M., Fukuhira Y., Kaneko H., Hirata H. Artificial Perineurium to Enhance Nerve Recovery from Damage after Neurolysis. Muscle Nerve. 2010;42:570–575. doi: 10.1002/mus.21727. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M., Endo N., Ito M., Okui N., Koh S., Kaneko H., Hirata H. Novel Polysaccharide-Derived Hydrogel Prevents Perineural Adhesions in a Rat Model of Sciatic Nerve Adhesion. J. Orthop. Res. 2010;28:284–288. doi: 10.1002/jor.21004. [DOI] [PubMed] [Google Scholar]
- 18.Smit X., Van Neck J.W., Afoke A., Hovius S.E.R. Reduction of Neural Adhesions by Biodegradable Autocrosslinked Hyaluronic Acid Gel after Injury of Peripheral Nerves: An Experimental Study. J. Neurosurg. 2004;101:648–652. doi: 10.3171/jns.2004.101.4.0648. [DOI] [PubMed] [Google Scholar]
- 19.Shintani K., Uemura T., Takamatsu K., Yokoi T., Onode E., Okada M., Nakamura H. Protective Effect of Biodegradable Nerve Conduit against Peripheral Nerve Adhesion after Neurolysis. J. Neurosurg. 2018;129:815–824. doi: 10.3171/2017.4.JNS162522. [DOI] [PubMed] [Google Scholar]
- 20.Özgenel G.Y., Fílíz G. Combined Application of Human Amniotic Membrane Wrapping and Hyaluronic Acid Injection in Epineurectomized Rat Sciatic Nerve. J. Reconstr. Microsurg. 2004;20:153–157. doi: 10.1055/S-2004-820772. [DOI] [PubMed] [Google Scholar]
- 21.Ohsumi H., Hirata H., Nagakura T., Tsujii M., Sugimoio T., Miyamoto K., Horiuchi T., Nagao M., Nakashima T., Uchida A. Enhancement of Perineurial Repair and Inhibition of Nerve Adhesion by Viscous Injectable Pure Alginate Sol. Plast. Reconstr. Surg. 2005;116:823–830. doi: 10.1097/01.prs.0000176893.44656.8e. [DOI] [PubMed] [Google Scholar]
- 22.Murakami K., Kuniyoshi K., Iwakura N., Matsuura Y., Suzuki T., Takahashi K., Ohtori S. Vein Wrapping for Chronic Nerve Constriction Injury in a Rat Model: Study Showing Increases in VEGF and HGF Production and Prevention of Pain-Associated Behaviors and Nerve Damage. J. Bone Jt. Surg. 2014;96:859–867. doi: 10.2106/JBJS.L.01790. [DOI] [PubMed] [Google Scholar]
- 23.Li R., Liu H., Huang H., Bi W., Yan R., Tan X., Wen W., Wang C., Song W., Zhang Y., et al. Chitosan Conduit Combined with Hyaluronic Acid Prevent Sciatic Nerve Scar in a Rat Model of Peripheral Nerve Crush Injury. Mol. Med. Rep. 2018;17:4360–4368. doi: 10.3892/mmr.2018.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kikuchi K., Setoyama K., Takada S., Otsuka S., Nakanishi K., Norimatsu K., Tani A., Sakakima H., Kawahara K.I., Hosokawa K., et al. E8002 Inhibits Peripheral Nerve Adhesion by Enhancing Fibrinolysis of L-Ascorbic Acid in a Rat Sciatic Nerve Model. Int. J. Mol. Sci. 2020;21:3972. doi: 10.3390/ijms21113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Cortés P., Peregrina M., Aneiros-Fernández J., Tassi M., Pajares-López M., Toledo M., O’Valle F. Oxidized Regenerated Cellulose Does Not Prevent the Formation of Experimental Postoperative Perineural Fibrosis Assessed by Digital Analysis. Histol. Histopathol. 2010;25:741–747. doi: 10.14670/HH-25.741. [DOI] [PubMed] [Google Scholar]
- 26.Finsterbush A., Porat S., Rousso M., Ashur H. Prevention of Peripheral Nerve Entrapment Following Extensive Soft Tissue Injury, Using Silicone Cuffing: An Experimental Study. Clin. Orthop. Relat. Res. 1982;162:276–281. doi: 10.1097/00003086-198201000-00043. [DOI] [PubMed] [Google Scholar]
- 27.Baltu Y., Uzun H., Özgenel Y.G. The Reduction of Extraneural Scarring with Buccal Mucosa Graft Wrapping around the Sciatic Nerve: An Experimental Study in a Rat Model. J. Plast. Surg. Hand Surg. 2017;51:259–263. doi: 10.1080/2000656X.2016.1241790. [DOI] [PubMed] [Google Scholar]
- 28.Gärtner A., Pereira T., Simões M.J., Armada-da-Silva P.A.S., França M.L., Sousa R., Bompasso S., Raimondo S., Shirosaki Y., Nakamura Y., et al. Use of Hybrid Chitosan Membranes and Human Mesenchymal Stem Cells from the Wharton Jelly of Umbilical Cord for Promoting Nerve Regeneration in an Axonotmesis Rat Model. Neural Regen. Res. 2012;7:2247. doi: 10.3969/J.ISSN.1673-5374.2012.29.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornasari B.E., Carta G., Gambarotta G., Raimondo S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020;8:554257. doi: 10.3389/fbioe.2020.554257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golash A., Kay A., Wagner J.G., Peck F., Watson J.S., Lees V.C. Efficacy of ADCON-T/N after Primary Flexor Tendon Repair in Zone II: A Controlled Clinical Trial. J. Hand Surg. 2003;28:113–115. doi: 10.1016/S0266-7681(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 31.Merle M., Lee Dellon A., Campbell J.N., Chang P.S. Complications from Silicon-Polymer Intubulation of Nerves. Microsurgery. 1989;10:130–133. doi: 10.1002/micr.1920100213. [DOI] [PubMed] [Google Scholar]
- 32.Derebaşınlıoğlu H., Demirkazık A., Çiçek Doğan İ., Eğilmez H.R., Çam S., Yeldir N. The Effect of a Silicone Sheet on Sciatic Nerve Healing in Rats. Eur. J. Plast. Surg. 2023;46:453–463. doi: 10.1007/s00238-023-02071-3. [DOI] [Google Scholar]
- 33.Koenig Z.A., Burns J.C., Hayes J.D. Necrotic Granulomatous Inflammation after Use of Small Intestine Submucosa Matrix for Recurrent Compression Neuropathy. Plast. Reconstr. Surg. Glob. Open. 2022;10:e4378. doi: 10.1097/GOX.0000000000004378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stang F., Keilhoff G., Fansa H. Biocompatibility of Different Nerve Tubes. Materials. 2009;2:1480–1507. doi: 10.3390/ma2041480. [DOI] [Google Scholar]
- 35.Giusti G., Shin R.H., Lee J.Y., Mattar T.G., Bishop A.T., Shin A.Y. The Influence of Nerve Conduits Diameter in Motor Nerve Recovery after Segmental Nerve Repair. Microsurgery. 2014;34:646–652. doi: 10.1002/micr.22312. [DOI] [PubMed] [Google Scholar]
- 36.Isaacs J., Mallu S., Yan W., Little B. Consequences of Oversizing: Nerve-to-Nerve Tube Diameter Mismatch. J. Bone Jt. Surg.-Am. Vol. 2014;96:1461–1467. doi: 10.2106/JBJS.M.01420. [DOI] [PubMed] [Google Scholar]
- 37.Mathoulin C., Bahm J., Roukoz S. Pedicled Hypothenar Fat Flap for Median Nerve Coverage in Recalcitrant Carpal Tunnel Syndrome. Hand Surg. 2000;5:33–40. doi: 10.1142/S0218810400000120. [DOI] [PubMed] [Google Scholar]
- 38.De Smet L., Vandeputte G. Pedicled fat flap coverage of the median nerve after failed carpal tunnel decompression. J. Hand Surg. Br. 2002;27:350–353. doi: 10.1054/jhsb.2002.0780. [DOI] [PubMed] [Google Scholar]
- 39.Dahlin L.B., Lekholm C., Kardum P., Holmberg J. Coverage of the Median Nerve with Free and Pedicled Flaps for the Treatment of Recurrent Severe Carpal Tunnel Syndrome. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2002;36:172–176. doi: 10.1080/028443102753718069. [DOI] [PubMed] [Google Scholar]
- 40.Krzesniak N.E., Sarnowska A., Figiel-Dabrowska A., Osiak K., Domanska-Janik K., Noszczyk B.H. Secondary Release of the Peripheral Nerve with Autologous Fat Derivates Benefits for Functional and Sensory Recovery. Neural Regen. Res. 2021;16:856–864. doi: 10.4103/1673-5374.297081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goitz R.J., Steichen J.B. Microvascular Omental Transfer for the Treatment of Severe Recurrent Median Neuritis of the Wrist: A Long-Term Follow-Up. Plast. Reconstr. Surg. 2005;115:163–171. doi: 10.1097/01.PRS.0000138808.77038.D7. [DOI] [PubMed] [Google Scholar]
- 42.Ruch D.S., Spinner R.M., Koman L.A., Challa V.R., O’Farrell D., Levin L.S. The Histological Effect of Barrier Vein Wrapping of Peripheral Nerves. J. Reconstr. Microsurg. 1996;12:291–296. doi: 10.1055/s-2007-1006488. [DOI] [PubMed] [Google Scholar]
- 43.Hallock G.G. Vein Donor Site Morbidity after Coronary Bypass Surgery: An Overlooked but Important Issue. Plast. Surg. (Chir. Plast.) 1999;7:117–121. doi: 10.1177/229255039900700304. [DOI] [Google Scholar]
- 44.Hirosawa N., Uchida K., Kuniyoshi K., Murakami K., Inoue G., Miyagi M., Matsuura Y., Orita S., Inage K., Suzuki T., et al. Vein Wrapping Promotes M2 Macrophage Polarization in a Rat Chronic Constriction Injury Model. J. Orthop. Res. 2018;36:2210–2217. doi: 10.1002/jor.23875. [DOI] [PubMed] [Google Scholar]
- 45.Mukai M., Uchida K., Hirosawa N., Murakami K., Inoue G., Miyagi M., Shiga Y., Sekiguchi H., Inage K., Orita S., et al. Frozen Vein Wrapping for Chronic Nerve Constriction Injury Reduces Sciatic Nerve Allodynia in a Rat Model. BMC Neurosci. 2022;23:37. doi: 10.1186/s12868-022-00719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J., Varitimidis S.E., Fisher K.J., Tomaino M.M., Sotereanos D.G. The Effect of Wrapping Scarred Nerves with Autogenous Vein Graft to Treat Recurrent Chronic Nerve Compression. J. Hand Surg. 2000;25:93–103. doi: 10.1053/jhsu.2000.jhsu025a0093. [DOI] [PubMed] [Google Scholar]
- 47.Mathieu L., Adam C., Legagneux J., Bruneval P., Masmejean E. Reduction of Neural Scarring after Peripheral Nerve Suture: An Experimental Study about Collagen Membrane and Autologous Vein Wrapping. Chir. Main. 2012;31:311–317. doi: 10.1016/j.main.2012.10.167. [DOI] [PubMed] [Google Scholar]
- 48.Hirosawa N., Uchida K., Kuniyoshi K., Murakami K., Inoue G., Miyagi M., Matsuura Y., Orita S., Inage K., Suzuki T., et al. Vein Wrapping Facilitates Basic Fibroblast Growth Factor-Induced Heme Oxygenase-1 Expression Following Chronic Nerve Constriction Injury. J. Orthop. Res. 2018;36:898–905. doi: 10.1002/jor.23674. [DOI] [PubMed] [Google Scholar]
- 49.Xu J., Sotereanos D.G., Moller A.R., Jacobsohn J., Tomaino M.M., Fischer K.J., Herndon J.H. Nerve Wrapping with Vein Grafts in a Rat Model: A Safe Technique for the Treatment of Recurrent Chronic Compressive Neuropathy. J. Reconstr. Microsurg. 1998;14:323–330. doi: 10.1055/s-2007-1000185. [DOI] [PubMed] [Google Scholar]
- 50.Mirzayan R., Russo F., Yang S.-J.T., Lowe N., Shean C.J., Harness N.G. Human Amniotic Membrane Wrapping of the Ulnar Nerve during Cubital Tunnel Surgery Reduces Recurrence of Symptoms. Arch. Bone Jt. Surg. 2022;10:969–975. doi: 10.22038/ABJS.2021.60743.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaspar M.P., Abdelfattah H.M., Welch I.W., Vosbikian M.M., Kane P.M., Rekant M.S. Recurrent Cubital Tunnel Syndrome Treated with Revision Neurolysis and Amniotic Membrane Nerve Wrapping. J. Shoulder Elbow Surg. 2016;25:2057–2065. doi: 10.1016/j.jse.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Gao Y.B., Liu Z.G., Lin G.D., Guo Y., Chen L., Huang B.T., Yin Y.B., Yang C., Sun L.Y., Rong Y.B., et al. Safety and Efficacy of a Nerve Matrix Membrane as a Collagen Nerve Wrapping: A Randomized, Single-Blind, Multicenter Clinical Trial. Neural Regen. Res. 2021;16:1652–1659. doi: 10.4103/1673-5374.303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Economides J.M., Defazio M.V., Attinger C.E., Barbour J.R. Prevention of Painful Neuroma and Phantom Limb Pain after Transfemoral Amputations through Concomitant Nerve Coaptation and Collagen Nerve Wrapping. Neurosurgery. 2016;79:508–512. doi: 10.1227/NEU.0000000000001313. [DOI] [PubMed] [Google Scholar]
- 54.Soltani A.M., Allan B.J., Best M.J., Mir H.S., Panthaki Z.J. Revision Decompression and Collagen Nerve Wrap for Recurrent and Persistent Compression Neuropathies of the Upper Extremity. Ann. Plast. Surg. 2014;72:572–578. doi: 10.1097/SAP.0b013e3182956475. [DOI] [PubMed] [Google Scholar]
- 55.Kokkalis Z.T., Mavrogenis A.F., Ballas E.G., Papagelopoulos P.J., Soucacos P.N. Collagen Nerve Wrap for Median Nerve Scarring. Orthopedics. 2015;38:117–121. doi: 10.3928/01477447-20150204-04. [DOI] [PubMed] [Google Scholar]
- 56.Smith M.J., Smith D.C., Bowlin G.L., White K.L. Modulation of Murine Innate and Acquired Immune Responses Following in Vitro Exposure to Electrospun Blends of Collagen and Polydioxanone. J. Biomed. Mater. Res. A. 2010;93:793–806. doi: 10.1002/JBM.A.32579. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J., Zhang Y., Jiang Y.K., Li J.A., Wei W.F., Shi M.P., Wang Y.B., Jia G.L. The Effect of Poly(Lactic-Co-Glycolic Acid) Conduit Loading Insulin-like Growth Factor 1 Modified by a Collagen-Binding Domain on Peripheral Nerve Injury in Rats. J. Biomed. Mater. Res. B Appl. Biomater. 2022;110:2100–2109. doi: 10.1002/jbm.b.35064. [DOI] [PubMed] [Google Scholar]
- 58.Kim P.D., Hayes A., Amin F., Akelina Y., Hays A.P., Rosenwasser M.P. Collagen Nerve Protector in Rat Sciatic Nerve Repair: A Morphometric and Histological Analysis. Microsurgery. 2010;30:392–396. doi: 10.1002/micr.20760. [DOI] [PubMed] [Google Scholar]
- 59.Bozkurt A., Boecker A., Tank J., Altinova H., Deumens R., Dabhi C., Tolba R., Weis J., Brook G.A., Pallua N., et al. Efficient Bridging of 20 Mm Rat Sciatic Nerve Lesions with a Longitudinally Micro-Structured Collagen Scaffold. Biomaterials. 2016;75:112–122. doi: 10.1016/j.biomaterials.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Mukai M., Uchida K., Hirosawa N., Murakami K., Kuniyoshi K., Inoue G., Miyagi M., Sekiguchi H., Shiga Y., Inage K., et al. Wrapping with Basic Fibroblast Growth Factor-Impregnated Collagen Sheet Reduces Rat Sciatic Nerve Allodynia. J. Orthop. Res. 2019;37:2258–2263. doi: 10.1002/jor.24349. [DOI] [PubMed] [Google Scholar]
- 61.Kazanci A., Gurcan O., Gurcay A.G., Onder E., Kazanci B., Yaman M.E., Bavbek M. Effects of Topical CovaTM, Tisseel® and Adcon® Gel Application on the Development of Spinal Peridural Fibrosis: An Experimental Study in Rats. Turk. Neurosurg. 2017;27:962–968. doi: 10.5137/1019-5149.JTN.16809-15.1. [DOI] [PubMed] [Google Scholar]
- 62.Rabb C.H. Failed Back Syndrome and Epidural Fibrosis. Spine J. 2010;10:454–455. doi: 10.1016/j.spinee.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 63.Temiz A., Ozturk C., Bakunov A., Kara K., Kaleli T. A New Material for Prevention of Peritendinous Fibrotic Adhesions after Tendon Repair: Oxidised Regenerated Cellulose (Interceed), an Absorbable Adhesion Barrier. Int. Orthop. 2008;32:389–394. doi: 10.1007/s00264-007-0335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biçer M., Bayram A.S., Gürbüz O., Şenkaya I., Yerci Ö., Tok M., Anǧ E., Moǧol E.B., Saba D. Assesssment of the Efficacy of the Bio-Absorbable Oxidized Regenerated Cellulose for Prevention of Post-Operative Pericardial Adhesion in the Rabbit Model. J. Int. Med. Res. 2008;36:1311–1318. doi: 10.1177/147323000803600619. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda K., Yamauchi D., Tomita K. Preliminary Study for Prevention of Neural Adhesion Using an Absorbable Oxidised Regenerated Cellulose Sheet. Hand Surg. 2002;7:11–14. doi: 10.1142/S0218810402000911. [DOI] [PubMed] [Google Scholar]
- 66.Towne J., Carter N., Neivandt D.J. COMSOL Multiphysics® Modelling of Oxygen Diffusion through a Cellulose Nanofibril Conduit Employed for Peripheral Nerve Repair. Biomed. Eng. Online. 2021;20:60. doi: 10.1186/s12938-021-00897-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter N., Towne J., Neivandt D.J. Finite Element Analysis of Glucose Diffusivity in Cellulose Nanofibril Peripheral Nerve Conduits. Cellulose. 2021;28:2791–2803. doi: 10.1007/s10570-021-03724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urano H., Iwatsuki K., Yamamoto M., Ohnisi T., Kurimoto S., Endo N., Hirata H. Novel Anti-Adhesive CMC-PE Hydrogel Significantly Enhanced Morphological and Physiological Recovery after Surgical Decompression in an Animal Model of Entrapment Neuropathy. PLoS ONE. 2016;11:e0164572. doi: 10.1371/journal.pone.0164572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikeda K., Yamauchi D., Osamura N., Hagiwara N., Tomita K. Hyaluronic Acid Prevents Peripheral Nerve Adhesion. Br. J. Plast. Surg. 2003;56:342–347. doi: 10.1016/S0007-1226(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 70.Özgenel G.Y. Effects of Hyaluronic Acid on Peripheral Nerve Scarring and Regeneration in Rats. Microsurgery. 2003;23:575–581. doi: 10.1002/micr.10209. [DOI] [PubMed] [Google Scholar]
- 71.Adanali G., Verdi M., Tuncel A., Erdogan B., Kargi E. Effects of Hyaluronic Acid-Carboxymethylcellulose Membrane on Extraneural Adhesion Formation and Peripheral Nerve Regeneration. J. Reconstr. Microsurg. 2003;19:29–35. doi: 10.1055/s-2003-37188. [DOI] [PubMed] [Google Scholar]
- 72.Mekaj A.Y., Manxhuka-Kerliu S., Morina A.A., Duci S.B., Shahini L., Mekaj Y.H. Effects of Hyaluronic Acid and Tacrolimus on the Prevention of Perineural Scar Formation and on Nerve Regeneration after Sciatic Nerve Repair in a Rabbit Model. Eur. J. Trauma Emerg. Surg. 2017;43:497–504. doi: 10.1007/s00068-016-0683-4. [DOI] [PubMed] [Google Scholar]
- 73.Park J.S., Lee J.H., Han C.S., Chung D.W., Kim G.Y. Effect of Hyaluronic Acid-Carboxymethylcellulose Solution on Perineural Scar Formation after Sciatic Nerve Repair in Rats. Clin. Orthop. Surg. 2011;3:315–324. doi: 10.4055/cios.2011.3.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdelbasset W.K., Jasim S.A., Sharma S.K., Margiana R., Bokov D.O., Obaid M.A., Hussein B.A., Lafta H.A., Jasim S.F., Mustafa Y.F. Alginate-Based Hydrogels and Tubes, as Biological Macromolecule-Based Platforms for Peripheral Nerve Tissue Engineering: A Review. Ann. Biomed. Eng. 2022;50:628–653. doi: 10.1007/s10439-022-02955-8. [DOI] [PubMed] [Google Scholar]
- 75.Boecker A., Daeschler S.C., Kneser U., Harhaus L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019;13:104. doi: 10.3389/fncel.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcol W., Larysz-Brysz M., Kucharska M., Niekraszewicz A., Slusarczyk W., Kotulska K., Wlaszczuk P., Wlaszczuk A., Jedrzejowska-Szypulka H., Lewin-Kowalik J. Reduction of Post-Traumatic Neuroma and Epineural Scar Formation in Rat Sciatic Nerve by Application of Microcrystallic Chitosan. Microsurgery. 2011;31:642–649. doi: 10.1002/micr.20945. [DOI] [PubMed] [Google Scholar]
- 77.Neubrech F., Sauerbier M., Moll W., Seegmüller J., Heider S., Harhaus L., Bickert B., Kneser U., Kremer T. Enhancing the Outcome of Traumatic Sensory Nerve Lesions of the Hand by Additional Use of a Chitosan Nerve Tube in Primary Nerve Repair: A Randomized Controlled Bicentric Trial. Plast. Reconstr. Surg. 2018;142:415–424. doi: 10.1097/PRS.0000000000004574. [DOI] [PubMed] [Google Scholar]
- 78.Ducker T.B., Hayes G.J. Experimental Improvements in the Use of Silastic Cuff for Peripheral Nerve Repair. J. Neurosurg. 1968;28:582–587. doi: 10.3171/jns.1968.28.6.0582. [DOI] [PubMed] [Google Scholar]
- 79.Tokiwa Y., Calabia B.P. Biodegradability and Biodegradation of Poly(Lactide) Appl. Microbiol. Biotechnol. 2006;72:244–251. doi: 10.1007/s00253-006-0488-1. [DOI] [PubMed] [Google Scholar]
- 80.Li L., Li Y., Fan Z., Wang X., Li Z., Wen J., Deng J., Tan D., Pan M., Hu X., et al. Ascorbic Acid Facilitates Neural Regeneration After Sciatic Nerve Crush Injury. Front. Cell. Neurosci. 2019;13:108. doi: 10.3389/fncel.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L., Xu Y., Wang X., Liu J., Hu X., Tan D., Li Z., Guo J. Ascorbic Acid Accelerates Wallerian Degeneration after Peripheral Nerve Injury. Neural. Regen. Res. 2021;16:1078–1085. doi: 10.4103/1673-5374.300459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicolas C.F., Corvi J.J., Zheng Y., Park K.H., Akelina Y., Engemann A., Strauch R.J. Resorbable Nerve Wraps: Can They Be Overtightened? J. Reconstr. Microsurg. 2022;38:694–702. doi: 10.1055/s-0042-1744274. [DOI] [PubMed] [Google Scholar]
- 83.Xue J.W., Jiao J.B., Liu X.F., Jiang Y.T., Yang G., Li C.Y., Yin W.T., Ling L. Inhibition of Peripheral Nerve Scarring by Calcium Antagonists, Also Known as Calcium Channel Blockers. Artif. Organs. 2016;40:514–520. doi: 10.1111/aor.12584. [DOI] [PubMed] [Google Scholar]
- 84.Görgülü A., Uzal C., Doǧanay L., Imer M., Eliuz K., Çobanoǧlu S., Loeffler J.S., Gerszten P.C., Kondziolka D., Kline D.G. The Effect of Low-Dose External Beam Radiation on Extraneural Scarring after Peripheral Nerve Surgery in Rats. Neurosurgery. 2003;53:1389–1396. doi: 10.1227/01.NEU.0000093827.05319.E5. [DOI] [PubMed] [Google Scholar]
- 85.Soto P.A., Vence M., Piñero G.M., Coral D.F., Usach V., Muraca D., Cueto A., Roig A., van Raap M.B.F., Setton-Avruj C.P. Sciatic Nerve Regeneration after Traumatic Injury Using Magnetic Targeted Adipose-Derived Mesenchymal Stem Cells. Acta Biomater. 2021;130:234–247. doi: 10.1016/j.actbio.2021.05.050. [DOI] [PubMed] [Google Scholar]
- 86.Albayrak B.S., Ismailoglu O., Ilbay K., Yaka U., Tanriover G., Gorgulu A., Demir N. Doxorubicin for Prevention of Epineurial Fibrosis in a Rat Sciatic Nerve Model: Outcome Based on Gross Postsurgical, Histopathological, and Ultrastructural Findings. J. Neurosurg. Spine. 2010;12:327–333. doi: 10.3171/2009.9.SPINE09407. [DOI] [PubMed] [Google Scholar]
- 87.Vural E., Yilmaz M., Ilbay K., Ilbay G. Prevention of Epidural Fibrosis in Rats by Local Administration of Mitomycin C or Daunorubicin. Turk. Neurosurg. 2016;26:291–296. doi: 10.5137/1019-5149.JTN.7705-12.1. [DOI] [PubMed] [Google Scholar]
- 88.Ilbay K., Etus V., Yildiz K., Ilbay G., Ceylan S. Topical Application of Mitomycin C Prevents Epineural Scar Formation in Rats. Neurosurg. Rev. 2005;28:148–153. doi: 10.1007/s10143-004-0370-5. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald J.J. Suppression of Scarring in Peripheral Nerve Implants by Drug Elution. J. Neural Eng. 2016;13:026006. doi: 10.1088/1741-2560/13/2/026006. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J., Chen Y., Huang Y., Wu W., Deng X., Liu H., Li R., Tao J., Li X., Liu X., et al. A 3D-Printed Self-Adhesive Bandage with Drug Release for Peripheral Nerve Repair. Adv. Sci. 2020;7:2002601. doi: 10.1002/advs.202002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boecker A.H., van Neerven S.G.A., Scheffel J., Tank J., Altinova H., Seidensticker K., Deumens R., Tolba R., Weis J., Brook G.A., et al. Pre-Differentiation of Mesenchymal Stromal Cells in Combination with a Microstructured Nerve Guide Supports Peripheral Nerve Regeneration in the Rat Sciatic Nerve Model. Eur. J. Neurosci. 2016;43:404–416. doi: 10.1111/ejn.13052. [DOI] [PubMed] [Google Scholar]
- 92.Aman M., Schulte M., Li Y., Thomas B., Daeschler S., Mayrhofer-Schmid M., Kneser U., Harhaus L., Boecker A. Benefit of Adjuvant Mesenchymal Stem Cell Transplantation to Critical-Sized Peripheral Nerve Defect Repair: A Systematic Review and Meta-Analysis of Preclinical Studies. J. Clin. Med. 2023;12:1306. doi: 10.3390/jcm12041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Angius D., Wang H., Spinner R.J., Gutierrez-Cotto Y., Yaszemski M.J., Windebank A.J. A Systematic Review of Animal Models Used to Study Nerve Regeneration in Tissue-Engineered Scaffolds. Biomaterials. 2012;33:8034–8039. doi: 10.1016/j.biomaterials.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaplan H.M., Mishra P., Kohn J. The Overwhelming Use of Rat Models in Nerve Regeneration Research May Compromise Designs of Nerve Guidance Conduits for Humans. J. Mater. Sci. Mater. Med. 2015;26:226. doi: 10.1007/s10856-015-5558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.