Abstract
Mushroom dietary fiber is a type of bioactive macromolecule derived from the mycelia, fruiting bodies, or sclerotia of edible or medicinal fungi. The use of mushroom dietary fiber as a prebiotic has recently gained significant attention for providing health benefits to the host by promoting the growth of beneficial microorganisms; therefore, mushroom dietary fiber has promising prospects for application in the functional food industry and in drug development. This review summarizes methods for the preparation and modification of mushroom dietary fiber, its degradation and metabolism in the intestine, its impact on the gut microbiota community, and the generation of short-chain fatty acids (SCFAs); this review also systematically summarizes the beneficial effects of mushroom dietary fiber on host health. Overall, this review aims to provide theoretical guidance and a fresh perspective for the prebiotic application of mushroom dietary fiber in the development of new functional foods and drugs.
Keywords: dietary fiber, mushroom, gut microbiota, beneficial effects, short-chain fatty acids
1. Introduction
Microorganisms are extensively distributed in all parts of the human body. Some microorganisms are synergistically associated with the human body and are thus called symbiotic microorganisms [1]. These microorganisms are mainly distributed on the skin surface, oral cavity, digestive system, respiratory system, and urogenital system [2], with 95% of them inhabiting the human intestinal tract [3]. The gut microbiota comprises up to trillions of microorganisms (10 times the number of human cells), with as many as 1000 species included. On average, each human has approximately 160 microbial species in the intestinal tract [4,5], with 90% of species belonging to the phyla Firmicutes and Bacteroidetes, followed by Proteobacteria, Actinobacteria, and Fusobacteria [6]. The gut microbiota encodes more than three million genes, 150 times more than those encoded by the human genome [7]. Therefore, the gut microbiota is also known as the “second human genome” [8]. Numerous studies have recently reported that the gut microbiota is closely involved in energy homeostasis [9], immune system regulation [10], metabolism [11], and other physiological processes in the host. Thus, the gut microbiota is also termed the “hidden metabolic organ” [12]. The human gut microbiota is a complex, interactive, and dynamically balanced ecosystem. Dietary changes, diseases, drugs, and other factors cause disturbances and changes in the composition of the gut microbiota, sometimes resulting in dysbiosis [13,14]. Numerous studies have shown that dysbiosis of the gut microbiota is correlated with the occurrence of several chronic diseases, such as obesity [15], diabetes [16], liver disease [17], inflammatory bowel disease [18], and cancer [19].
Mushrooms are valuable and healthy and they have a long history of consumption and have increased in popularity in recent years worldwide [20]. They are mainly composed of basidiomycete or ascomycete fungi and are prized for their nutritional and medicinal properties [21]. Bioactive compounds such as proteins, vitamins, minerals, dietary fibers, and trace elements from different mushroom varieties have been demonstrated to have high nutritional value [22] and enhance human health by promoting antioxidant, antimicrobial, anti-inflammatory, anticancer, antitumor, and immunostimulatory effects [23]. Mushrooms are popular among consumers as both a medicine and food. Peptides, lectins, ergosterol, terpenoids, phenols, and other biologically active compounds have been isolated and identified from various mushrooms [24]. However, mushrooms have relatively low levels of these active ingredients. Dietary fiber (DF), known as the “seventh nutrient” [25], positively affects blood sugar, blood pressure, lipid metabolism, and inflammation. The total DF content in the sclerotia of some mushrooms can exceed 80%. For example, the total DFs extracted from the sclerotia of Pleurotus tuber regium, Polyporus rhinocerus, and Wolfifiporia cocos were 81.7–96.3% of the total content [26,27]. A high fiber content raises the new possibility of using mushrooms as functional foods. Several studies have reported that DFs from mushrooms such as Lentinula edodes and Hericium erinaceus can change the gut microbiota, and therefore, DFs from mushrooms have attracted increasing attention [28,29,30]. DFs from mushrooms act as prebiotics. The selective growth of particular microorganisms in the intestine can stimulate the growth of beneficial microorganisms and inhibit the proliferation of pathogens, thus altering the gut microbiota to improve health [31]. This paper reviews the methods of preparing and modifying mushroom-derived DFs and their regulatory effects on the gut microbiota. In addition, we discuss how this modulation of the gut microbiota benefits the host. Our findings will provide theoretical guidance and insight for researchers seeking to develop new functional foods or drugs using mushroom-derived DFs.
A comprehensive literature search was conducted for studies published by 2023 by using the keywords “dietary fiber, mushroom, gut microbiota” on PubMed, Web of Science, cross ref, Elsevier, Springer Link, Google Scholar, and Scopus. The retrieved articles were characterized based on the methods of preparation and modification, degradation and metabolism in the intestine, and the beneficial effects of mushroom dietary fiber. The content of the review has been arranged and presented in specific sections.
2. Composition of DF from Mushrooms
Structurally, DF is a carbohydrate polymer with a polymerization degree of at least 10 and is typically associated with health benefits. DF cannot be digested or absorbed in the small intestine. DF can be naturally obtained from raw food materials or synthesized through physical, enzymatic, or chemical methods [32]. Based on its dissolution characteristics, DF is classified as soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) [33]. DFs from different sources exhibit different structures, chemical compositions, and physicochemical properties. Moreover, DF has various nutritional and physiological benefits. Compared with DFs from traditional sources, such as grains, vegetables, and fruits, the potential of DFs from mushrooms has not been fully realized [34,35]. In fact, mushrooms are rich in new types of DFs that are suitable for various members of the population, including children and those with diabetes. Thus, mushroom DFs have varied beneficial effects on human health [36]. DFs in mushrooms mainly include chitin (a straight-chain (1→4)-β-linked polymer of N-acetyl-glucosamine), β-glucan, and hemicellulose [37]. Among them, β-glucan is recognized as one of the most important components and is primarily linked by the mixed linkage of the β-1,4 and β-1,6 glycosidic bond, as well as the single linkage of β-1,3; β-1,4; and β-1,6 [38]. β-glucan is present in both SDF and IDF in mushrooms. However, its proportion in SDF and IDF considerably varies based on mushroom genera. In general, the proportion of β-glucan is higher in IDF [39].
3. Methods of the Preparation and Modification of DFs from Mushrooms
3.1. The Preparation of DFs from Mushrooms
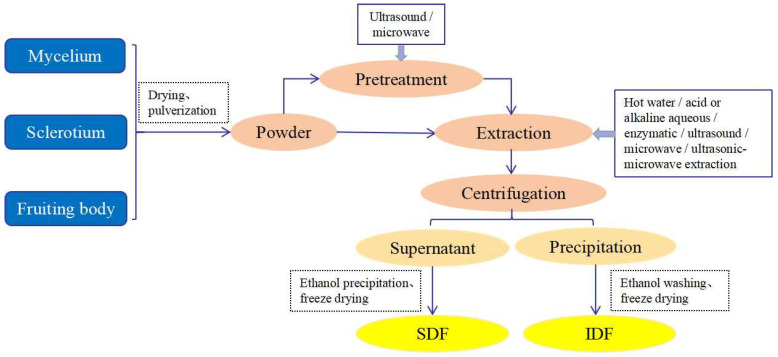
Using the extraction process shown in Figure 1, DFs from mushrooms are separated from the fruiting bodies, mycelia, or sclerotia [26,31]. As the first step of extraction, the dried mushroom fruiting bodies, mycelia, or sclerotia are ground into powder. The extraction is performed using ultrasonic or microwave treatment, or the powder is directly extracted with hot water, acid or alkaline aqueous solution, enzyme, ultrasonic waves, and other methods. Then, DFs are further isolated through centrifugation, ethanol precipitation, and freeze drying [40,41,42]. The type of extraction method affects the physicochemical properties and potential bioactivity of DFs; thus, the extraction method and extraction parameters, including solvent type, extraction temperature, extraction time, liquid–solid ratio, and equipment power, should be considered before DF extraction [43]. The methods for extracting DF from mushrooms include chemical, physical, enzyme, and microbial methods or a combination of these. Among them, alkali and enzymatic extraction methods are the most frequently used. The alkaline aqueous method is more widely used than the enzymatic method because it involves a simple protocol, has a low cost, and can be easily controlled [44,45]. The comparison of different methods and technical strategies used to extract DFs from mushrooms is shown in Table 1.
Figure 1.
Schematic showing an overview of mushroom DF extraction.
Table 1.
Different extraction methods for DF used on different mushroom varieties.
| Extraction Methods |
Materials | Extraction Conditions |
Extraction Features | Reference | |
|---|---|---|---|---|---|
| Physical method | Pressurized hot water | Pleurotus sajor-caju | 140 °C, 0.92 MPa, and 40 min | Water as a solvent, low cost, but poor impurity removal | [46] |
| Ultrasound-assisted | Agaricus bisporus | 15 min, 100 mm amplitude, and 1 h of precipitation in 80% ethanol |
Less time-consuming and highly efficient, but high cost and little capacity | [47] | |
| Microwave |
Cordyceps gunnii mycelia |
1:20 (w/v), 70 °C, 280 W, 5 min | High extraction efficiency, short time and low energy input, but the microwave power and microwave time should be strictly controlled | [48] | |
| Biological method | Enzymatic | Schizophyllum commune | α-amylase, 100 °C, 30 min; protease 60 °C, 30 min | High specificity of enzyme is needed, and the extraction conditions must be strictly controlled | [49] |
| Chemical method | Alkaline | Coprinus comatus | 2% NaOH in a ratio of 1:15, 85 °C, 2 h | High yield, but may degrade some compounds | [50] |
| Acid | Lentinula edodes stipe | 100 °C, 2 h; 0.8 M trichloroacetic acid, 4 °C, 3 h |
High yield, but may produce some byproducts | [51] | |
| Combined method | Hot water and alkaline | Cookeina tricholoma | 98 °C, 4 h; 2% KOH (w/v 1:4), 98 °C, 4 h | High yield and purity, but time-consuming | [52] |
| Acid–alkaline combined | Pleurotus eryngii | 0.1 M H2SO4 (1:10 w/v), 60 °C, 2 h; 0.25 M NaOH (1:8 w/v), 60 °C, 2 h | Higher purity, low cost, but may cause excessive degradation | [53] | |
3.2. Methods of Modification of DF from Mushrooms
There is a considerable difference in the DF content of different mushrooms. According to a paper by Cheung (2008) [54], the SDF content of some mushrooms is 0.50–4.42%, while the IDF content ranges from 23.6 to 43.1%. SDF has many crucial physiological functions because of its good gelling, water absorption, swelling, and fermentability properties [55,56,57]. The SDF content in high-quality DF should be more than 10% [58]. Therefore, increasing the content of SDF in mushrooms is a goal of modification. The currently used DF modification methods are divided into the following four main types: physical methods, chemical methods, biological methods, and combination methods. Treatment with different modification methods causes corresponding changes in the composition and structural characteristics of DFs, thereby affecting the physicochemical properties of DFs, including their oil holding capacity (OHC) and adsorption capacities [59]. The conditions, properties, and yield changes in mushroom DF after the application of different modification methods are shown in Table 2.
3.2.1. Physical Modification
The methods of physical modification involve modification through the destruction of the glycosidic bonds of DFs by applying external high temperature, high pressure, instantaneous decompression, explosion, high-speed impact, or shearing. Some examples of physical modification methods are steam treatment (SP) [60], high-pressure homogenization (HPH) [61], dynamic high-pressure microfluidization (DHPM) [62], ultrasonic comminution (UC) [63], high hydrostatic pressure (HHP) [63], extrusion [64], ultrasound [65], microwave [66], and cavitation jet processing [67]. The physical modification methods produce good results, have high production efficiency, and generate no chemical reagent residue; therefore, these methods are widely used but require a large investment in equipment.
3.2.2. Chemical Modification
In the methods for chemical modification, the structure and functional properties of DFs are modified through chemical reactions. Some examples of chemical modification methods include treatments with alkaline hydrogen peroxide [68], acid carboxymethylation [69], and hydroxypropylation [70]. DF modification using chemical methods is associated with a low cost but can alter the structural and functional properties of DFs. Furthermore, these methods may be associated with problems related to reagent residue.
3.2.3. Biological Modification
In the methods of biological modification, specific enzymes or microorganisms are utilized for the enzymolysis or fermentation of raw materials to modify DFs. Biological modification methods can be enzymatic [71] or based on microbial fermentation methods [72] and require mild treatment conditions that reduce DF loss; however, the production efficiency of these techniques is low. The high cost of the enzymatic method and the development of highly active strains involved in fermentation currently prevent the wider adoption of these techniques.
3.2.4. Combination Modification
The combination method refers to DF modification with two or more of the aforementioned methods [73]. The combination modification method can effectively compensate for the shortcomings of a single method and is likely to become the focus of future research.
Table 2.
Modification methods, properties, and yield changes of mushroom DFs.
| Modification Methods | Material | DFs | Modification Conditions | Property Changes | Reference | |
|---|---|---|---|---|---|---|
| Physical modification method | High-pressure homogenization |
Flammulina
velutiper |
IDF | 0, 10, 30, and 50 cycles at 700 bar | WHC ↑, interfacial properties ↑, particle size ↓, emulsification Performance ↑ |
[61] |
| Extrusion | Lentinula edodes residues | DF | 130 °C, moisture content 40%, 125 r/min | SDF ↑, OHC ↑, GAC ↑, glucose retardation and bile acid retardation index ↑ |
[64] | |
| High-temperature cooking |
Flammulina
velutiper |
DF | Liquid-to-material ratio 30:1, 125 °C, 50 min | SDF ↑, improves the physiological indices in obese mice | [74] | |
| High-pressure processing | Agrocybe chaxingu | DF | 400 MPa, 25 °C, 15 min | SDF ↑, polysaccharide solubility ↑, lower viscosity and greater fluidity |
[75] | |
| Chemical modification method | Alkaline | Lentinus edodes stem | DF | 13% NaOH, 80% ethanol, alkalization 120 min; 10% C2H2ClNaO2, 50 °C, etherification 3.5 h | WHC ↑, SC ↓, OHC ↑ | [76] |
| Biological modification method | Enzymatic | Lentinus edodes | DF | 1.5% cellulase, solid–liquid ratio 1:25, 50 °C, pH 5.5, 120 min | SC ↑, WHC ↑, OHC ↑, cation exchange capacity ↑, GAC ↑ | [71] |
| Fermentation | Lentinus edodes stem | IDFSDF | Material-liquid 1:10 g/mL, 6% Aspergillus niger, 28 °C, 2 d | IDF: WHC ↑, OHC ↑, SC ↑; SDF: WHC ↑, OHC ↑, SC ↑ |
[72] | |
| Combined modification method | Enzymatic-chemical | Auricularia polytricha | DF | 0.4% α-amylase 1.0% protamex, 66 °C, liquid material ratio 41 mL/g | SC ↑, WHC ↑, FAC ↑, GAC ↑, high constipation-relieving activity | [77] |
| Ultrasound-microwave-assisted enzymatic method |
Hericium Erinaceus residue |
DF | 3% celluloses, ultrasound (1.5 W/mL), 50 °C, 75 min, boiled to stop the enzyme | SDF ↑, particle size ↓, adsorption capacity ↑, better blood lipid-lowering effect in vitro | [78] | |
Note: WHC: water holding capacity; OHC: oil holding capacity; GAC: glucose adsorption capacity; SC: swelling capacity; FAC: fat adsorption capacity; ↑: increase; ↓: decrease.
4. Interaction between DFs and the Gut Microbiota
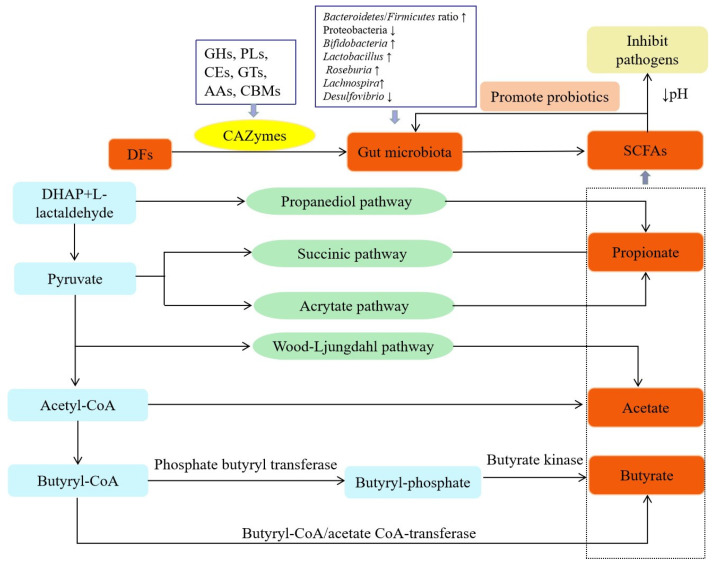
DFs can be utilized by the gut microbiota. DFs exert beneficial effects on the host mainly by fermentation and the production of metabolites. The effect of DFs on the gut microbiota is summarized in Figure 2.
Figure 2.
The effects of DFs on gut microbiota metabolism. Note: ↑: increase; ↓: decrease.
4.1. The Role of the Gut Microbiota in DF Metabolism
The gut microbiota affects the digestion, immunity, and nervous systems of human hosts by metabolizing carbohydrates, protein, fat, and other substances in the body [79]. The human genome cannot encode a sufficient amount of carbohydrate-active enzymes (CAZymes) for different glycosidic bonds [80]. Therefore, only some simple carbohydrates are digested in humans, and the remaining complex carbohydrates, including DFs, are transported to the large intestine for use by the gut microbiota [81]. Numerous CAZymes are produced by the gut microbiota and are involved in regulating the metabolism and utilization of carbohydrates such as DFs by the gut microbiota, thereby aiding the human digestive system in carbohydrate degradation and producing absorbable short-chain fatty acids (SCFAs) and other metabolites [82]. The gut microbiota can produce various CAZymes needed for DF degradation. According to differences in the similarity of amino acid sequences, protein structure, and catalytic function, CAZymes are categorized into five types of catalytic enzymes and the noncatalytic carbohydrate-binding module (CBM) [83]. The catalytic CAZymes include glycoside hydrolases (GHs), polysaccharide lyases (PLs), carbohydrate esterases (CEs), glycosyltransferases (GTs), and auxiliary activities (AAs). GHs degrade glycosidic bonds between two or more carbohydrates and those between carbohydrates and noncarbohydrates [84]. PLs degrade the long uronic-acid-containing polysaccharide chains through the β-elimination mechanism [85]. CEs remove ester groups in carbohydrates and participate in reactions involving side-chain degradation [86]. GTs catalyze the transfer of glycosyl groups from activated donor molecules to specific receptor molecules to form glycosidic bonds [87].
During DF degradation, collaboration between various CAZymes is necessary. For example, Ndeh et al. confirmed that the synergism of GHs, PLs, CEs, and other enzymes is needed for rhamnonic acid II degradation by Bacteroides polymorphus [88]. Some intestinal microorganisms can use numerous carbohydrates with different structures, whereas others can use only a small amount of carbohydrates [89]. According to Zhang et al., on average, Bacteroidetes encode four times more CAZyme genes than Firmicutes [90]. Approximately 81% of GHs and PLs in Bacteroidetes have signal sequences, whereas only 19% of GHs and PLs in Firmicutes have signal sequences [91]. Therefore, Bacteroides are better able to metabolize carbohydrates. In addition, intestinal microorganisms can degrade complex carbohydrates through cooperation. Degrading all carbohydrates in the intestinal tract is difficult for only one type of intestinal microorganism [92]. Thus, the short-chain primary products produced by some microorganisms that degrade complex carbohydrates can be transferred to other microorganisms for further degradation [93]. For example, Eubacterium rectale only decomposes the gum aldose side-chain of arabinoxylan, while Bifidobacterium longum further metabolizes these primary products to form monosaccharides, which are later consumed by E. rectale [94].
4.2. Effect of DFs on the Composition of the Gut Microbiota
Many factors affect the composition and function of the gut microbiota, including the host’s age and sex, genetic background, physiological status, living environment, diet habits, and drug treatment [95,96]. Of them, diet is considered among the most important factors because it significantly affects the composition, diversity, and abundance of the gut microbiota [97]. DFs are considered a nutritional source for the gut microbiota and play a major role in host health. Decreases in DF intake are associated with decreasing gut microbiota abundance, and vice versa [89,98,99]. Many recent in vivo and in vitro experimental studies have shown that DFs in mushrooms have a regulatory effect on the gut microbiota (Table 3). Mitsou et al. [100] suggested that mushrooms rich in β-glucans may exert beneficial in vitro effects on gut microbiota and/or SCFA production in elderly subjects. Zhang et al. [50] found that Coprinus comatus DFs regulated the gut microbiota composition by increasing the abundance of Bacteroides and Bifidobacterium and reducing the Firmicutes/Bacteroides ratio during an in vitro fermentation test; Zhao et al. [101] also confirmed that Flammulina velutipes DFs reduce the Firmicutes/Bacteroidetes ratio; and through in vitro experiments, Han et al. [53] demonstrated that Pleurotus eryngii DFs regulate the gut microbiota composition in mice fed a high-fat diet (HFD) by increasing the abundance of beneficial microorganisms such as Metallobacterium and Lactobacillus and reducing the abundance of harmful microorganisms such as unidentified_Lachnospiraceae and Helicobacter. All these results indicate that DFs from varying sources have a range of effects on the gut microbiota composition and abundance in vivo and in vitro, which may be related to differences in structure and glycosidic bond types [102,103].
The function of DFs in regulating gut microbial diversity and composition has become a research hot spot. According to previous studies, DFs can be used as a substrate for intestinal microorganism CAZymes, and SCFAs produced through fermentation decrease the intestinal pH, thereby promoting the growth of beneficial microorganisms and inhibiting the growth of pathogenic microorganisms; this further affects the gut microbiota composition and the balance of microbial metabolites [104]. For example, during in vitro fermentation, DFs from Agaricus bisporus reduced the pH from 6.93 to 4.48 [105]. Similarly, DFs from Lentinus edodes and Ganoderma atram significantly reduced the pH when fermented [106,107]. On the other hand, pH significantly affects the abundance and diversity of the gut microbiota and enzyme activity [108,109]. pH may further affect the metabolism of the gut microbiota. For example, Bacteroides have a stronger adaptability at pH 6.7 than at pH 5.5, whereas Firmicutes have a stronger adaptability at pH 5.5 [110]. Moreover, the gut microbiota forms an interdependent community, wherein some intestinal microorganisms induce the growth of other microorganisms through cross-feeding behavior, thereby enriching the diversity and maintaining the stability of the gut microbiota [111,112]. For instance, Eubacterium hallii utilizes the products of 1,2-propanediol from the fermentation of rhamnose by Blautia spp. [113]. Bifidobacterium sp. can degrade starch or fructooligosaccharides, which can stimulate the growth of species in coculture that cannot degrade these complex substrates [114,115].
Table 3.
Effects of mushroom DF on the gut microbiota and SCFAs.
| DF Source | Model | Gut Microbiota Regulation | SCFA Generation | Effect on Host | Reference |
|---|---|---|---|---|---|
| Pleurotus eryngii | HFD-induced obese rat | The relative abundances of Roseburia and Lactobacillus ↓, the relative abundances of Anaerostipes, Clostridium and Lactococcus ↑. |
Increased the concentrations of total SCFAs. |
Reduced BW gain, adipose tissue weight, FBG level; the expression of FASN and ACC. |
[116] |
| Pleurotus eryngii | HFD-fed mice | The relative abundances of Methylobacterium and Lactobacillus ↑, the relative abundances of unidentified_Lachnospiraceae and Helicobacter ↓. | Increased the content of SCFAs, including acetic acid, propionic acid, and butyric acid. |
Decreased the weight, promoted the proliferation of beneficial bacteria, reduced the risks of many chronic diseases. | [53] |
| Agaricus blazei Murrill | Hyperlipidemia rats | The ratio of Firmicutes/Bacteroidetes ↓; the abundance of Peptostreptococcaceae, Erysipelaceae, and Clostridium ↑. |
Nm | Regulated dyslipidemia in rats with hyperlipidemia possibly by regulating imbalance in the intestinal microflora. | [117] |
| Hericium caput-medusae | One-day-old Arbor Acres male broilers | The count of Lactobacilli and Bifidobacteria ↑, the count of acecum Escherichia coli ↓. | Increased the concentration of propionic acid. | Decreased cholesterol content in broiler chickens. | [118] |
| Flammulina velutipes | Male C57BL/6 J mice | The relative abundance of some beneficial bacteria ↑, such as Akkermansia and Prevotellaceae UCG-001; the relative abundance of some harmful bacteria ↓, such as Lachnospiraceae NK4A136 group and Desulfovibrio. |
Nm | Reduced the weight gain, triglycerides and total cholesterol, low-density lipoprotein cholesterol; increased the activity of enzymes related to scavenging ability of oxygen free radicals. |
[119] |
| Flammulina velutipes | Mice | The relative abundance of Firmicutes ↓, the relative abundance of Bacteroidetes ↑; the ratio of Firmicutes/Bacteroidetes ↓. |
Increased the concentrations of total SCFAs, acetic acid, propionic acid, and n-butyric acid. |
Suppressed obesity and immune regulation. | [101] |
| Ganoderma lucidum | C57BL/6NCrlBltw genetic lineage mice | The ratio of Firmicutes/Bacteroidetes, Proteobacteria ↓. |
Nm | Reduced body weight gain, chronic inflammation, and insulin resistance in obese individuals. | [120] |
| Poria cocos | C57BL/6J mice | The relative abundance of Lachnospiracea, Clostridium ↑. | Increased butyrate levels. | Activated the intestinal PPAR-γ pathway, modulated gut microbiota to improve hyperglycemia and hyperlipidemia. | [121] |
| Agaricus bisporus | Human | The relative abundance of Firmicutes ↑, the relative abundance of Bacteroidetes ↓. | Increased the concentrations of acetic acid and propionic acid. | Increased the relative abundance of beneficial bacteria, exhibited an effective prebiotic regulation function on human gut microbiota. | [105] |
| Cordyceps militaris | Liver and kidney injury induced by lead acetate in mice | The relative abundance of Clostridium and Bacteroidetes ↑, the relative abundance of Firmicutes ↓. |
Nm | Reduced the Pb2+ content and organ index of liver and kidney in mice, had a protective effect on organs against damage in mice. | [122] |
| Pleurotus eryngii | C57BL/6 male mice | The relative abundances of Firmicutes ↓, Bacteroidetes ↑ |
Increased the concentrations of Acetate and Propionate. |
Regulated the host immune function effectively. | [123] |
| Ganoderma lucidum | Chronic pancreatitis mice | The relative abundance of Bacteroidetes ↓ and that of Firmicutes ↑; at the genus level, the relative abundance of beneficial bacteria such as Lactobacillus, Roseburia, and Lachnospira ↑. |
Nm | Indicated beneficial effects on pancreas fibrosis, and impeded an inflammatory response. | [124] |
| Dictyophora indusiata | Antibiotic-induced intestinal microflora disorder in mice | Beneficial bacteria ↑, including Lactobacilli and Ruminococcaceae; harmful bacteria ↓, such as Enterococcus, Bacteroides, and Proteobacteria. |
Nm | Enhanced the restoration of gut microbiota and gut barrier integrity, reduce the inflammation and endotoxin levels in mice. | [125] |
| Coprinus comatus | Human | The relative abundances of Bacteroides and Bifidobacterium ↑, the ratio of Firmicutes/Bacteroidetes ↓. |
Increased the production of propionic acid and butyric acid. | Demonstrated potential prebiotic effects. | [50] |
| Ganoderma lucidum | C57BL/6J mice | The relative abundances of Actinobacteria at the family level, and Leuconostoc, Lactobacillus spp. ↑. | Nm | Improved low-grade chronic inflammation, ectopic lipid accumulation, and systemic insulin sensitivity. | [126] |
| Hericium erinaceus | Mice | The relative abundance of Lachnospiraceae and Akkermansiaceae ↑, the relative abundance of Rikenellaceae and Bacteroidaceae ↓. | Nm | Promoted the production of NO, IL-6, IL-10, INF-γ, and TNF-α. | [127] |
| Auricularia auricular | ICR mice | The ratio of Firmicutes/Bacteroidetes ↓, the relative abundance of Porphyromoadaceae and Bacteroidaceae ↑. | Increased the concentration of total SCFAs and propanoic acid. | Increased microbial community diversity, and increased the immunoglobulin levels in mouse serum. | [128] |
| Ganoderma lucidum | DSS-induced colitis male Wistar rats |
The relative abundance of Firmicutes, Paraprevotella, etc. ↑, the relative abundance of Proteobacteria, Escherichia, etc. ↓. |
Increased total SCFAs, acetic acid, propionic acid, and butyric acid. |
Enhanced the immunity and reduced inflammatory response and colonic cancer risk. | [129] |
| Ganoderma lucidum | BALB/C mice | The ratio of Firmicutes/Bacteroidetes ↓, the relative abundance of Alistipes ↑. | Nm | Demonstrated tumor-suppressing activity in mice. | [130] |
| Ganoderma lucidum | BALB/c mice | The relative abundance of Oscillospira and unknown genus of Desulfovibrionaceae ↓. | Nm | Prevented colon from shortening and reduced mortality by 30% of mortality in CRC mice. | [131] |
Note: HFD: high-fat diet; BW: body weight; FBG: fasting blood glucose; FASN: fatty acid synthase; ACC: acetyl-CoA carboxylase; CRC: colorectal cancer; ↑: increase; ↓: decrease.
4.3. Effect of DFs on SCFA Production
The fermentation of DF by the gut microbiota yields SCFAs such as acetate, propionate, and butyrate and gases such as CH4, H2, and CO2. As the energy source for colonic epithelial cells, SCFAs play a vital role in cell renewal and recovery. SCFAs can also affect the intestinal mucosal barrier and play a critical role in the health of the intestine [132]. Acetate is the most abundant intestinal SCFA. Intestinal anaerobic microorganisms produce acetate to metabolize pyruvate through acetyl coenzyme A or the Wood Ljungdahl pathway [133]. Propionate is formed by converting succinic acid into methylmalonyl coenzyme A through the succinic acid pathway [134]. Alternatively, propionic acid can be synthesized through the acrylate pathway using acrylic acid and lactic acid as precursors. Additionally, propionic acid can be synthesized through the propanediol pathway, with deoxyhexose (such as trehalose and rhamnose) used as the substrate [135]. Butyrate is reduced to butyryl coenzyme A after condensation with two acetyl coenzyme A molecules. Butyryl coenzyme A is then converted to butyric acid (classical way) through phosphate butyryl transferase and butyrate kinase [136] or through the butyryl coenzyme A/acetic acid coenzyme A transferase pathway [137,138].
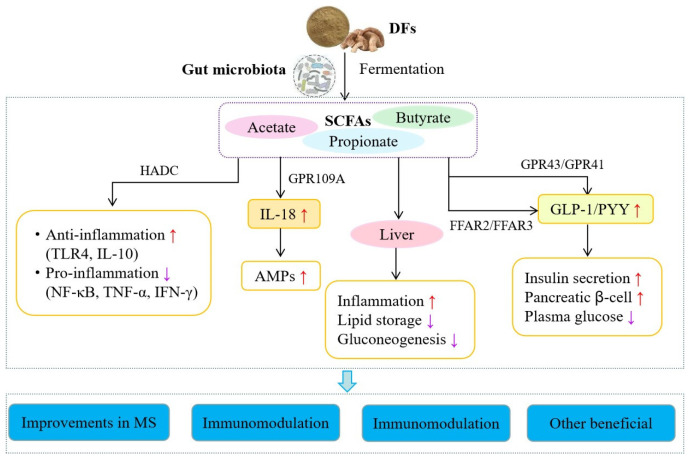
SCFAs produced during microbial fermentation participate in metabolism related to different human organs. SCFAs can be quickly absorbed and used by colon cells, transported to the liver through the portal vein system, or enter the circulatory system. Only 5–10% of SCFAs are excreted through feces [139]. SCFAs, especially butyrate, serve as histone deacetylase (HDAC) inhibitors and bind to SCFA receptors to regulate cell proliferation, apoptosis, and differentiation by inhibiting HDACs and altering the expression of functional genes, thereby affecting intestinal function [140]. SCFAs inhibit HDAC and promote the proliferation of macrophages and other cells, the expression of receptors such as Toll-like receptor 4 (TLR4), and the release of anti-inflammatory factors such as interleukin-10 (IL-10). They also inhibit the expression of nuclear factor-κB (NF-κB), tumor necrosis factor (TNF), IL-8, and other cytokines [141,142]. They also combine with different receptors to perform various functions. For instance, butyric acid can induce the arrest of the human colon cancer cell cycle by upregulating the expression of cell cycle regulators and binding to G-protein-coupled receptor 109A (GPR109A) [143]. SCFAs can activate GPR41 and GPR43 and stimulate the secretion of intestinal hormones such as glucagon-like peptide-1 (GLP-1) and peptide tyrosine tyrosine (PYY) in the colon. GLP-1 promotes the body’s secretion of insulin, reduces the secretion of glucagon, and enhances the body’s sensitivity to insulin. PYY can regulate intestinal motility, slow gastric emptying, induce a sense of fullness, and reduce food intake [144]. SCFAs have also been shown to help maintain the integrity of the intestinal mucosal barrier and regulate intestinal motility and the immune response [145,146].
DFs from different mushrooms may affect SCFA production (Table 3). For example, P. eryngii DFs increased acetic acid and propionic acid concentrations [123], while F. velutipes DFs increased the concentration of total SCFAs, acetic acid, propionic acid, and butyric acid [101]. Moreover, the species and abundance of gut microbiota, substrate source, substrate utilization rate, host genotype, and intestinal transport are factors that influence SCFA production [139].
5. Health Benefits of DFs
The gut microbiota, as the core microecological system in the human intestinal tract, helps maintain the normal physiological function of the human body by preventing the invasion of various viral antigens. DFs are fermented by intestinal microorganisms to yield SCFAs, which can improve host health and have many beneficial effects in the human body (Figure 3).
Figure 3.
DFs improve host health after oral administration. Note: HADC: histone deacetylase; TLR4: toll-like receptor-4; IL-10: interleukin-10; NF-κB: nuclear factor-κB; TNF-α: tumor necrosis factor-α; IFN-γ: interferon-γ; GPR109A: G-protein-coupled receptor 109A; AMPs: antimicrobial peptides; FFAR2: free fatty acid receptor 2; GLP-1: glucagon-like peptide-1; PYY: peptide YY; MS: metabolic syndrome; ↑: increase; ↓: decrease.
5.1. Improving Metabolic Syndromes
Metabolic syndrome (MS) is a dysbiosis of physiological metabolism caused by insulin resistance. MS manifests as a pathological state of metabolic disorders related to nutrition, including hyperglycemia, dyslipidemia, central obesity, and hypertension. An increasing amount of evidence indicates that the etiology of MS is associated with dysbiosis of the gut microbiota [147,148,149]. The HFD-induced dysbiosis of the gut microbiota may disrupt intestinal barrier function and increase endotoxin levels in the circulatory system. This leads to metabolic endotoxemia and induces MSs, such as insulin resistance, obesity, and even diabetes [150]. By generating enzymes such as CAZymes and proteases, the gut microbiota promotes the digestion of carbohydrates, such as DFs, and produces metabolites such as SCFAs that can be absorbed and used by the body. Studies have shown that changes in the gut microbiota and SCFAs are associated with the development of metabolic diseases [151]. According to experimental evidence, an increase in Firmicutes and a decrease in Bacteroides have been observed in obese individuals and mouse models [152]. Indeed, DFs from mushrooms can effectively improve diet-induced MSs in mice and rats (Table 3). For example, P. eryngii DFs reduced LDL cholesterol levels and body weight in HFD-fed mice by altering the abundance of SCFA-producing gut microbiota [116]. G. lucidum DFs reverse the HFD-induced dysbiosis of gut microbiota by reducing the proportion of Firmicutes/Bacteroides and the Proteobacteria level. They also maintain the integrity of the intestinal barrier and reduce metabolic endotoxemia [120]. F. velutipes DFs can alleviate lipid metabolism dysbiosis in obese mice by regulating the intestinal-flora-mediated AMPK signaling pathway [119]. In addition, other types of DFs from mushrooms can play a positive role in MSs by regulating the gut microbiota composition (Table 3). Thus, mushroom DFs appear to play a positive role in regulating dysbiosis in the intestine that is induced by metabolic disturbances and maintaining the integrity of the intestinal barrier, further highlighting the potential value of mushroom DFs as foods or drugs.
5.2. Immunomodulatory Effects
The intestinal tract is the largest immune organ of the human body, and it is involved in immune and inflammatory reactions [153]. Although DFs cannot be completely digested in the intestine, they are decomposed into various metabolites through enzymes produced by intestinal microorganisms. Some microbial metabolites, such as tryptophan metabolites and SCFAs, interact with host cells through the intestinal barrier, thereby affecting the immune response [154]. An increasing number of studies have shown that SCFAs can inhibit the expression of inflammatory factors or alleviate inflammation by promoting histone acetylation or activating GPRs [155], activating peroxisome-proliferator-activated receptors [156], inhibiting the NF-κB signaling pathway [157], facilitating T-cell apoptosis [158], increasing antimicrobial peptide production [159], and downregulating the expression of signal transduction and activating transcription factor-3 [160]. Relevant studies have also reported that DFs from mushrooms can promote SCFA production and the growth of intestinal microorganisms to stimulate the host immune response and regulate the differentiation, maturation, and function of immune cells (Table 3). Vlassopoulou et al. [161] found that supplementation with mushroom β-(1→3, 1→6)-d-glucan is well tolerated and promotes health through the potentiation of the immune system. In addition, F. velutipes DF may affect immune function regulation by mediating the gut microbiota [101]. H. erinaceus DFs can regulate the gut microbiota composition and immune activity through the NF-κB, MAPK, and PI3K/Akt pathways [127]. G. lucidum DFs change the diversity of the gut microbiota and significantly alleviate pancreatitis symptoms in mice by reducing the levels of lipase, interferon-γ (IFN-γ), and TNF-α and increasing SOD levels and total antioxidant activity [124]. A. auricular DFs may affect intestinal nutritional metabolism and immune regulation by changing the composition of the gut microbiota [128]. G. lucidum DFs not only regulate the gut microbiota composition and SCFA production but also participate in the regulation of gene expression in KEGG pathways related to different types of inflammation [129]. Thus, these studies indicate that DFs from different mushrooms may be associated with different immune regulatory pathways. This immune regulation can be attributed to the diversity of the gut microbiota and SCFA production, which may act as signaling molecules for mediating and maintaining the host’s immune system.
5.3. Antitumor Effects
The gut microbiota affects the metabolism and endocrine and immune systems of the host. The gut microbiota is associated with the occurrence of many diseases, including inflammatory bowel disease, nonalcoholic fatty liver disease, type 2 diabetes, and neurodegenerative diseases [162,163,164]. Importantly, increasing evidence shows that the gut microbiota can affect tumor occurrence, tumor progression, and the response to treatment [19]. For example, Helicobacter pylori induces gastritis and canceration by producing toxic factors such as cytotoxin-associated gene A and vacuolating cytotoxin A [165]. A study investigating the gut microbiota of patients with early lung cancer reported that Akkermansia muciniphila may cause lung cancer [166]. The decrease in the abundance of Lactobacillidae and Bifidobacteriaceae in colorectal cancer (CRC) patients is related to colon and rectal tumors, respectively [167]. In patients with multiple polypoid adenoma and intramucosal carcinoma, significant changes in the microbiome and metabolome have been observed. The relative abundance of Fusobacterium nucleatum spp. increased during the progression of intramucosal carcinoma to the more advanced stage, while the abundance of Atobobium parvulum and Actinomyces odontolyticus significantly increased in patients with multiple polypoid adenoma and/or intramucosal carcinoma [168]. Moreover, the gut microbiota is closely correlated with the effect of chemotherapy and immunotherapy [169,170]. For example, the gut microbiota can alleviate chemotherapy-induced adverse reactions in CRC patients [171]. Butyric acid, a metabolite of the gut microbiota, can directly improve the antitumor cytotoxic effect of CD8+ T cells in vitro and in vivo in an ID2-dependent manner by promoting the IL-12 signaling pathway. Butyric acid can also promote the antitumor efficacy of oxaliplatin [172]. L. rhamnosus GG-induced cGAS/STING-dependent type I interferon can enhance the response to immune checkpoint inhibitors [173].
DFs from mushrooms play an anticancer/antitumor role by regulating the gut microbiota composition and diversity, and this role has attracted increasing attention (Table 3). For example, G. lucidum polysaccharide can reverse the proportion of Firmicutes/Bacteroides and increase the levels of Alistipes, resulting in the production of SCFAs, and Helicobacter and Riskenella, which are related to immunosuppression and carcinogenesis [130]. Moreover, G. lucidum DFs can alleviate CRC by altering special intestinal microorganisms [131]. Thus, these studies indicate that mushroom DFs inhibit tumor growth or metastasis by regulating the gut microbiota composition and diversity; furthermore, immunomonitoring mediated by gut microbiota-produced metabolites such as SCFAs may be beneficial. However, the exact anticancer/antitumor mechanism of mushroom DFs remains unclear.
5.4. Other Beneficial Effects
Because of their strong water absorption and swelling capacities, DFs from mushrooms, especially IDF, can promote intestinal peristalsis, increase stool volume, increase the frequency of bowel movements, and avert constipation, thereby preventing and treating gastrointestinal diseases. Feeding constipated rats with A. polytricha DFs increased the wet weight of their stool and intestinal propulsion rate, thereby indicating high constipation-relieving activity [73]. Furthermore, DFs from mushrooms have antioxidant capacity and can eliminate free radicals from the body. For example, DFs extracted from Boletus edulis have significant reducing power and chelating activity and strong antioxidant activity [174].
6. Conclusions and Prospects
DFs have various biological activities and are a crucial component of foods that can benefit health. Intestinal microorganisms selectively degrade DFs from mushrooms, thus conferring benefits to the host. The physiological activities of mushroom DFs are usually affected by the extraction method or modified extraction method used and their raw material, such as mycelia, fruiting bodies, and sclerotia. The intake of mushroom DFs induces changes in the host gut microbiota, thereby affecting the host’s immune system. The beneficial effect of DFs on the host may be mediated by gut microbiota-produced metabolites. In particular, SCFAs participate in the regulation of the host’s metabolic homeostasis and immune response. However, until now, the regulatory effect of mushroom DF on the gut microbiota has not been completely investigated. Therefore, future studies on mushroom DFs should aim to (1) investigate the full spectrum of metabolites produced through mushroom DF ingestion and their effect on host immunity by using a combination of multiomics analysis techniques (metagenomics, metabonomics, and other omics); (2) investigate the characteristics of the gut microbiota in different populations and the functional role and mechanisms by which DFs from mushrooms help maintain gut microbiota and host health; and (3) diagnose disease on the basis of specific microorganisms or metabolites and realize the goal of targeted prevention and treatment by using specific DFs. Importantly, additional animal trials and human preclinical and clinical trials are required to fully understand the multiple beneficial effects of DFs from mushrooms on human health using a combination of multiomics analysis techniques.
Author Contributions
Conceptualization, C.Y. and A.M.; methodology, C.Y. and L.Z.; writing—original draft, C.Y.; investigation, Q.D., R.Z., M.Z. and B.Z.; formal analysis, Q.D., R.Z., M.Z. and B.Z.; funding acquisition, M.C.; project administration, M.C. and Y.Z.; supervision, M.C. and A.M.; data curation, L.Z.; writing—review and editing, Y.Z. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by Shanghai Committee of Science and Technology (21N51900500); National Natural Science Foundation of China (32260797); Shanghai Academy of Agricultural Sciences (JCYJ231601).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jansma J., El Aidy S. Understanding the host-microbe interactions using metabolic modeling. Microbiome. 2021;9:16. doi: 10.1186/s40168-020-00955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malard F., Dore J., Gaugler B., Mohty M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2021;14:547–554. doi: 10.1038/s41385-020-00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X., Ning Z.B., Mayne J., Deeke S.A., Li J., Starr A.E., Chen R., Singleton R., Butcher J., Mack D.R., et al. In vitro metabolic labeling of intestinal microbiota for quantitative metaproteomics. Anal. Chem. 2016;88:6120–6125. doi: 10.1021/acs.analchem.6b01412. [DOI] [PubMed] [Google Scholar]
- 4.Rajilic-Stojanovic M., de Vos W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crow J.M. Microbiome: That healthy gut feeling. Nature. 2011;480:S88–S89. doi: 10.1038/480S88a. [DOI] [PubMed] [Google Scholar]
- 6.Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 7.Xu X.F., Xu P.P., Ma C.W., Tang J., Zhang X.W. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013;31:318–337. doi: 10.1016/j.biotechadv.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Almeida A., Mitchell A.L., Boland M., Forster S.C., Gloor G.B., Tarkowska A., Lawley T.D., Finn R.D. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Petterson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 10.Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., Costea P.I., Godneva A., Kalka I.N., Bar N., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H.S., Sparks J.B., Karyala S.V., Settlage R., Luo X.M. Host adaptive immunity alters gut microbiota. ISME J. 2015;9:770–781. doi: 10.1038/ismej.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caitriona M.G., Paul D.C. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D.T., Wang P., Wang P.P., Hu X.S., Chen F. Targeting the gut microbiota by dietary nutrients: A new avenue for human health. Crit. Rev. Food Sci. Nutr. 2019;59:181–195. doi: 10.1080/10408398.2017.1363708. [DOI] [PubMed] [Google Scholar]
- 14.Collins S.M., Denou E., Verdu E.F., Bercik P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig. Liver Dis. 2009;41:850–853. doi: 10.1016/j.dld.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Wang X.S., Chen D., Li Y.L., Zhao S.L., Chen C.Y., Ning D.L. Alleviating effects of walnut green husk extract on disorders of lipid levels and gut bacteria flora in high fat diet-induced obesity rats. J. Funct. Foods. 2019;52:576–586. doi: 10.1016/j.jff.2018.11.022. [DOI] [Google Scholar]
- 16.Wang P.P., Wang T., Zheng X.J., Cui W., Shang J., Zhao Z.Z. Gut microbiota, key to unlocking the door of diabetic kidney disease. Nephrology. 2021;26:641–649. doi: 10.1111/nep.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H., Yan Y.P., Yi X.Y., Duan Y.C., Wang J.F., Li S.S., Luo L.L., Huang T.Z., Lnglis B., Li X., et al. Histopathological features and composition of gut microbiota in rhesus monkey of alcoholic liver disease. Front. Microbiol. 2019;10:165. doi: 10.3389/fmicb.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananthakrishnan A.N., Issa M., Binion D.G. Clostridium difficile and inflammatory bowel disease. Med. Clin. N. Am. 2010;94:135–153. doi: 10.1016/j.mcna.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Zitvogel L., Galluzzi L., Viaud S., Vetizou M., Daillere R., Merad M., Kroemer G. Cancer and the gut microbiota: An unexpected link. Sci. Transl. Med. 2015;7:271ps1. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotowski M.A. History of mushroom consumption and its impact on traditional view on mycobiota-an example from Poland. Microb. Biosyst. 2019;4:1–13. [Google Scholar]
- 21.Balan V., Munafo Jr J.P., Pattathil S., Merritt B.B., Venketachalam S., Ng W. Protocols to evaluate the nutritional and potential health benefits of edible mushrooms. Curr. Biotechnol. 2018;7:34–58. doi: 10.2174/2211550105666160503170750. [DOI] [Google Scholar]
- 22.Wani B.A., Bodha R.H., Wani A.H. Nutritional and medicinal importance of mushrooms. J. Med. Plants Res. 2010;4:2598–2604. [Google Scholar]
- 23.Wasser S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014;37:6. doi: 10.4103/2319-4170.138318. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q., Wang F., Xu Z.H., Ding Z.Y. Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules. 2017;22:955. doi: 10.3390/molecules22060955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO/FAO Diet, Nutrition and the Preparation of Chronic Disease; Proceedings of the WHO Technical Report Series 916; Geneva, Switzerland. 28 January–1 February 2002. [Google Scholar]
- 26.Wong K.H., Cheung P.C.K. Dietary fibers from mushroom sclerotia: 1. preparation and physicochemical and functional properties. J. Agric. Food Chem. 2005;53:9395–9400. doi: 10.1021/jf0510788. [DOI] [PubMed] [Google Scholar]
- 27.Cheung P.C.K., Lee M.Y. Fractionation and characterization of mushroom dietary fiber (Nonstarch polysaccharides) as potential nutraceuticals frorm sclerotia of Pleurotus tuber-regium (Fries) singer. J. Agric. Food Chem. 2000;48:3148–3151. doi: 10.1021/jf000382s. [DOI] [PubMed] [Google Scholar]
- 28.Musco N., Vassalotti G., Mastellone V., Cortese L., Della Rocca G., Molinari M.L., Calabrò S., Tudisco R., Cutrignelli M.I., Lombardi P. Effects of a nutritional supplement in dogs affected by osteoarthritis. Vet. Med. Sci. 2019;5:325–335. doi: 10.1002/vms3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Y.L., Geng Y., Du Y., Li W., Lu Z.M., Xu H.Y., Xu G.H., Shi J.S., Xu Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018;57:67–76. doi: 10.1016/j.jnutbio.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Lu J.H., He R.J., Sun P.L., Zhang F.M., Linhardt R.J., Zhang A.Q. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020;150:765–774. doi: 10.1016/j.ijbiomac.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 31.Kumari K. Mushrooms as source of dietary fiber and its medicinal value: A review article. J. Pharmacogn. Phytochem. 2020;9:2075–2078. [Google Scholar]
- 32.Cummings J.H., Mann J.I., Nishida C., Vorster H.H. Dietary fiber: An agreed definition. Lancet. 2009;373:365–366. doi: 10.1016/S0140-6736(09)60117-3. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Ye R., Yin L., Zhang N. Novel blasting extrusion processing improved the physicochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J. Food Eng. 2014;120:1–8. doi: 10.1016/j.jfoodeng.2013.07.011. [DOI] [Google Scholar]
- 34.Shea N.O., Arendt E.K., Gallagher E. Dietary fiber and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. 2012;16:1–10. doi: 10.1016/j.ifset.2012.06.002. [DOI] [Google Scholar]
- 35.Elleuch M., Bedigian D., Roiseux O., Besbes S., Blecker C., Attia H. Dietary fiber and fiber-rich by-products of food processing: Characterisation, technological functionality and commerical application: A review. Food Chem. 2010;124:411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- 36.Zhao R.Q., Yang W.J., Pei F., Zhao L.Y., Hu Q.H. In vitro fermentation of six kinds of edible mushrooms and its effects on fecal microbiota composition. LWT—Food Sci. Technol. 2018;96:627–635. doi: 10.1016/j.lwt.2018.06.012. [DOI] [Google Scholar]
- 37.Manzi P., Gambelli L., Marconi S., Vivanti V., Pizzoferrato L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999;65:477–482. doi: 10.1016/S0308-8146(98)00212-X. [DOI] [Google Scholar]
- 38.Mullins J.T. Regulatory mechanism of β-glucan synthetases in bacteria, fungi and plants. Physiol. Plant. 1990;78:309–314. doi: 10.1111/j.1399-3054.1990.tb02096.x. [DOI] [Google Scholar]
- 39.Manzi P., Pizzoferrato L. Bata-glucans in edible mushrooms. Food Chem. 2000;68:315–318. doi: 10.1016/S0308-8146(99)00197-1. [DOI] [Google Scholar]
- 40.Guo X., Meng H., Zhu S., Tang Q., Pan R., Yu S. Stepwise ethanolic precipitation of sugar beet pectins from the acidic extract. Carbohyd. Polym. 2016;136:316–321. doi: 10.1016/j.carbpol.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Gong X., Wang S., Li Y., Qu H. Separation characteristics of ethanol precipitation for the purification of the water extract of medicinal plants. Sep. Purif. Technol. 2013;107:273–280. doi: 10.1016/j.seppur.2013.01.029. [DOI] [Google Scholar]
- 42.Sen D., Gosling A., Stevens G.W., Bhattacharya P.K., Barber A.R., Kentish S.E., Bhattacharjee C., Gras S.L. Galactosyl oligosaccharide purification by ethanol precipitation. Food Chem. 2011;128:773–777. doi: 10.1016/j.foodchem.2011.03.076. [DOI] [Google Scholar]
- 43.Praveen M.A., Parvathy K.R.K., Balasubramanian P., Jayabalan R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019;92:46–64. doi: 10.1016/j.tifs.2019.08.011. [DOI] [Google Scholar]
- 44.Wang S., Fang Y., Xu Y., Zhu B., Piao J., Zhu L., Yao L., Liu K., Wang S., Zhang Q., et al. The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from Rubus chingii Hu. Fruits. J. Funct. Foods. 2022;93:105081. doi: 10.1016/j.jff.2022.105081. [DOI] [Google Scholar]
- 45.Ma R., Chen J.N., Zhou X.J., Lin H., Gao Q., Peng X., Tanokura M., Xue Y.L. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.) LWT-Food Sci. Technol. 2021;145:111313. doi: 10.1016/j.lwt.2021.111313. [DOI] [Google Scholar]
- 46.Sakdasri W., Arnutpongchai P., Phonsavat S., Bumrungthaichaichan E., Sawangkeaw R. Pressurized hot water extraction of crude polysaccharides, β-glucan, and phenolic compounds from dried gray oyster mushroom. LWT-Food Sci. Technol. 2022;168:113895. doi: 10.1016/j.lwt.2022.113895. [DOI] [Google Scholar]
- 47.Aguilo-Aguayo I., Walton J., Vinas I., Tiwari B.K. Ultrasound assisted extraction of polysaccharides from mushroom by-products. LWT-Food Sci. Technol. 2017;77:92–99. doi: 10.1016/j.lwt.2016.11.043. [DOI] [Google Scholar]
- 48.Zhu Z.Y., Dong F.Y., Liu X.C., Lv Q., Yang Y., Liu F., Chen L., Wang T.T., Wang Z., Zhang Y.M. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohyd. Polym. 2016;140:461–471. doi: 10.1016/j.carbpol.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 49.Wunjuntuk K., Ahmad M., Techakriengkrai T., Chunhom R., Jaraspermsuk E., Chaisri A., Kiwwongngam R., Wuttimongkolkul S., Charoenkiatkul S. Proximate composition, dietary fibre, beta-glucan content, and inhibition of key enzymes linked to diabetes and obesity in cultivated and wild mushrooms. J. Food Compos. Anal. 2022;105:104226. doi: 10.1016/j.jfca.2021.104226. [DOI] [Google Scholar]
- 50.Zhang Z., Zhao L., Qu H., Zhou H., Yang H., Chen H. Physicochemical characterization, adsorption function and prebiotic effect of chitin-glucan complex from mushroom Coprinus comatus. Int. J. Biol. Macromol. 2022;206:255–263. doi: 10.1016/j.ijbiomac.2022.02.152. [DOI] [PubMed] [Google Scholar]
- 51.Chou W.T., Sheih I.C., Fang T.J. The applications of polysaccharides from various mushroom wastes as prebiotics in different systems. J. Food Sci. 2013;78:M1041–M1048. doi: 10.1111/1750-3841.12160. [DOI] [PubMed] [Google Scholar]
- 52.Moreno R.B., Ruthes A.C., Baggio C.H., Vilaplana F., Komura D.L., Iacomini M. Structure and antinociceptive effects of β-D-glucans from Cookeina tricholoma. Carbohyd. Polym. 2016;141:220–228. doi: 10.1016/j.carbpol.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Han X., Yang D., Zhang S., Liu X., Zhao Y., Song C., Sun Q. Characterization of insoluble dietary fiber from Pleurotus eryngii and evaluation of its effects on obesity-preventing or relieving effects via modulation of gut microbiota. J. Future Foods. 2023;3:55–66. doi: 10.1016/j.jfutfo.2022.09.009. [DOI] [Google Scholar]
- 54.Cheung P.C.K. Nutritional value and health benefifits of mushrooms. In: Cheung P.C.K., editor. Mushrooms as Functional Foods. Vol. 2. John Wiley & Sons; Hoboken, NJ, USA: 2008. pp. 71–109. [Google Scholar]
- 55.Liu C., Lin X.L., Wan Z., Zou Y., Cheng F.F., Yang X.Q. The physicochemical properties, in vitro binding capacities and in vivo hypocholesterolemic activity of soluble dietary fiber extracted from soy hulls. Food Funct. 2016;7:4830–4840. doi: 10.1039/C6FO01340F. [DOI] [PubMed] [Google Scholar]
- 56.Agnihotri M.A., Khan A. Effect of water-soluble gummy fiber, water-insoluble neutral detergent fiber isolated from Syzygium cumini seeds on biliary and fecal bile acids and sterols in rats fed a high cholesterol diet. Int. J. Med. Sci. Public Health. 2015;4:23–26. doi: 10.5455/ijmsph.2015.030120148. [DOI] [Google Scholar]
- 57.Barber T.M., Kabisch S., Pfeiffer A.E.H., Weickert M.O. The health benefits of dietary fiber. Nutrients. 2020;12:3029. doi: 10.3390/nu12103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Y., Liu W., Wu B., Wu P., Duan Y., Yang Q., Ma H. Modification of garlic skin dietary fiber with twin-screw extrusion process and in vivo evaluation of Pb binding. Food Chem. 2018;268:550–557. doi: 10.1016/j.foodchem.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 59.Wang K., Li M., Wang Y., Liu Z., Ni Y. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa) Food Hydrocoll. 2021;110:106162. doi: 10.1016/j.foodhyd.2020.106162. [DOI] [Google Scholar]
- 60.Wang L., Xu H., Yuan F., Fan R., Gao Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015;185:90–98. doi: 10.1016/j.foodchem.2015.03.112. [DOI] [PubMed] [Google Scholar]
- 61.He K., Zhang X., Li Y., Li B., Liu S. Water-insoluble dietary-fibers from Flammulina velutiper used as edible stabilizers for oil-in-water Pickering emulsions. Food Hydrocolloid. 2020;101:105519. doi: 10.1016/j.foodhyd.2019.105519. [DOI] [Google Scholar]
- 62.Wang H., Huang T., Tu Z.C., Ruan C.Y., Lin D. The adsorption of lead(II) ions by dynamic high pressure micro-fluidization treated insoluble soybean dietary fiber. J. Food Sci. Technol. 2016;53:2532–2539. doi: 10.1007/s13197-016-2203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu G., Bei J., Zhao J., Li Q., Cheng C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018;257:333–340. doi: 10.1016/j.foodchem.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 64.Xue Z., Ma Q., Guo Q., Santhanam R.K., Gao X., Chen Z., Wang C., Chen H. Physicochemical and functional properties of extruded dietary fiber from mushroom Lentinula edodes residues. Food Biosci. 2019;32:100452. doi: 10.1016/j.fbio.2019.100452. [DOI] [Google Scholar]
- 65.Huang L., Ding X., Zhao Y., Li Y., Ma H. Modification of insoluble dietary fiber from garlic straw with ultrasonic treatment. J. Food Process. Preserv. 2019;42:e13399. doi: 10.1111/jfpp.13399. [DOI] [Google Scholar]
- 66.Wei E., Yang R., Zhao H., Wang P., Zhao S., Zhai W., Zhang Y., Zhou H. Microwave-assisted extraction releases the antioxidant polysaccharides from seabuckthorn (Hippophae rhamnoides L.) berries. Int. J. Biol. Macromol. 2019;123:280–290. doi: 10.1016/j.ijbiomac.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 67.Wu C., Teng F., McClements D.J., Zhang S., Li Y., Wang Z. Effect of cavitation jet processing on the physicochemical properties and structural characteristics of okara dietary fiber. Food Res. Int. 2020;134:109251. doi: 10.1016/j.foodres.2020.109251. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida B.Y., Prudencio S.H. Alkaline hydrogen peroxide improves physical, chemical, and techno-functional properties of okara. Food Chem. 2020;323:126776. doi: 10.1016/j.foodchem.2020.126776. [DOI] [PubMed] [Google Scholar]
- 69.Zhang M.Y., Liao A.M., Thakur K., Huang J.H., Zhang J.G., Wei Z.J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019;297:124983. doi: 10.1016/j.foodchem.2019.124983. [DOI] [PubMed] [Google Scholar]
- 70.Zheng Y., Li Y., Tian H. Effects of carboxymethylation, acidic treatment, hydroxypropylation and heating combined with enzymatic hydrolysis on structural and physicochemical properties of palm kernel expeller dietary fiber. LWT-Food Sci. Technol. 2020;133:109909. doi: 10.1016/j.lwt.2020.109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z.D. Master’s Thesis. Liaoning University; Shenyang, China: 2018. Study on Extraction and Enzymatic Modification of Lentinus edodes Dietary Fiber. [Google Scholar]
- 72.Pei Z.W. Master’s Thesis. Liaoning University; Shenyang, China: 2021. Study on Properties and Application of Dietary Fiber from Lentinus edodes Stem Modified by Aspergillus niger. [Google Scholar]
- 73.Gan J., Xie L., Peng G., Xie J., Chen Y., Yu Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021;119:106872. doi: 10.1016/j.foodhyd.2021.106872. [DOI] [Google Scholar]
- 74.Liu X., Wang W., Gong Z., Wang Y., Cui W., Song S., Zhang J., Jia F. Modification, physicochemical properties and lipid-lowering and antioxidant activity of dietary fiber from Flammulina velutipes. Food Sci. 2021;42:60–98. (In Chinese with English abstract) [Google Scholar]
- 75.Lv G., Zhang Z., Pan H., Fan L. Effect of physical modifification of mushroom (A. chaxingu) powders on their physical and chemical properties. Food Sci. Technol. Res. 2014;20:731–738. doi: 10.3136/fstr.20.731. [DOI] [Google Scholar]
- 76.Wang C.C. Master’s Thesis. Liaoning University; Shenyang, China: 2020. Study on Technologies of Chemical Modification and Properties of Dietary Fiber from Lentinus edodes Stem. [Google Scholar]
- 77.Jia F., Yang S., Ma Y., Gong Z., Cui W., Wang Y., Wang W. Extraction optimization and constipation-relieving activity of dietary fiber from Auricularia polytricha. Food Biosci. 2020;33:100506. doi: 10.1016/j.fbio.2019.100506. [DOI] [Google Scholar]
- 78.Liu T., Wang N., Xu X., Wang D. Effect of high quality dietary fiber of Hericium erinaceus on lowering blood lipid in hyperlipidemia mice. J. Future Foods. 2022;2:61–68. doi: 10.1016/j.jfutfo.2022.03.018. [DOI] [Google Scholar]
- 79.Illiano P., Brambilla R., Parolini C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020;287:833–855. doi: 10.1111/febs.15217. [DOI] [PubMed] [Google Scholar]
- 80.Grondin J.M., Tamura K., Dejean G., Abbott D.W., Brumer H. Polysaccharide utilization loci: Fueling microbial communities. J. Bacteriol. 2017;199:10–1128. doi: 10.1128/JB.00860-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cockburn D.W., Koropatkin N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016;428:3230–3252. doi: 10.1016/j.jmb.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 82.Luis A.S., Martens E.C. Interrogating gut bacterial genomes for discovery of novel carbohydrate degrading enzymes. Curr. Opin. Chem. Biol. 2018;47:126–133. doi: 10.1016/j.cbpa.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Lombard V., Golaconda R.H., Drula E., Coutinbo P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White B.A., Lamed R., Bayer E.A., Flint H.J. Biomass utilization by gut microbiomes. Annu. Rev. Microbiol. 2014;68:279–296. doi: 10.1146/annurev-micro-092412-155618. [DOI] [PubMed] [Google Scholar]
- 85.Lombard V., Bernard T., Rancurel C., Brumer H., Coutinbo P.M., Henrissat B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010;432:437–444. doi: 10.1042/BJ20101185. [DOI] [PubMed] [Google Scholar]
- 86.Biely P. Microbial carbohydrate esterases deacetylating plant polysaccharides. Biotechnol. Adv. 2012;30:1575–1588. doi: 10.1016/j.biotechadv.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 87.Zhu F., Zhang H., Wu H. Glycosyltransferase-mediated sweet modification in oral Streptococci. J. Dent. Res. 2015;94:659–665. doi: 10.1177/0022034515574865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ndeh D., Rogowski A., Cartmell A., Lois A.S., Basle A., Gray J., Venditto I., Briggs J., Zhang X., Labourel A., et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature. 2017;544:65–70. doi: 10.1038/nature21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koropatkin N.M., Cameron E.A., Martens E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang T., Yang Y., Liang Y., Jiao X., Zhao C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients. 2018;10:1055. doi: 10.3390/nu10081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaoutari A.E., Armougom F., Gordon J.I., Raoult D., Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 92.Pokusaeva K., Fitzgerald G.F., van Sinderen D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011;6:285–306. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mahowald M.A., Rey F.E., Seedorf H., Gordon J.I. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA. 2009;106:5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rivière A., Gagnon M., Weckx S., Roy D., De Vuyst L. Mutual cross-feeding interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 explain the bifidogenic and butyrogenic effects of arabinoxylan oligosaccharides. Appl. Environ. Microb. 2015;81:7767–7781. doi: 10.1128/AEM.02089-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nie Q., Chen H., Hu J., Fan S., Nie S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 2019;59:848–863. doi: 10.1080/10408398.2018.1536646. [DOI] [PubMed] [Google Scholar]
- 96.Shah B.R., Li B., Al Sabbah H., Xu W., Mraz J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020;102:178–192. doi: 10.1016/j.tifs.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Makki K., Deehan E.C., Walter J., Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 98.Chen J., Li Y., Tian Y., Huang C., Li D., Zhong Q., Ma X. Interaction between microbes and host intestinal health: Modulation by dietary nutrients and gut-brain-endocrine-immune axis. Curr. Protein Pept. Sci. 2015;16:592–603. doi: 10.2174/1389203716666150630135720. [DOI] [PubMed] [Google Scholar]
- 99.Zimmer J., Lange B., Frick J.S., Sauer H., Zimmermann K., Schwiertz A., Rusch K., Klosterhalfen S., Enck P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012;66:53–60. doi: 10.1038/ejcn.2011.141. [DOI] [PubMed] [Google Scholar]
- 100.Mitsou E., Savami G., Stamoulou E., Kerezoudi E., Terzi E., Koutrotsios G., Bekiaris G., Zervakis G., Mountzouris K., Pletsa V., et al. Effects of rich in β-glucans edible mushrooms on aging gut microbiota characteristics: An in vitro study. Molecules. 2020;25:2806. doi: 10.3390/molecules25122806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao R., Hu Q., Ma G., Su A., Xie M., Li X., Chen G., Zhao L. Effects of Flammulina velutipes polysaccharide on immune response and intestinal microbiota in mice. J. Funct. Foods. 2019;56:255–264. doi: 10.1016/j.jff.2019.03.031. [DOI] [Google Scholar]
- 102.Hamaker B.R., Tuncil Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014;426:3838–3850. doi: 10.1016/j.jmb.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 103.Yang J., Martinez I., Walter J., Keshavarzian A., Rose D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe. 2013;23:74–81. doi: 10.1016/j.anaerobe.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 104.Comino P., Williams B.A., Gidley M.J. In vitro fermentation gas kinetics and end-products of soluble and insoluble cereal flour dietary fibers are similar. Food Funct. 2018;9:898–905. doi: 10.1039/C7FO01724C. [DOI] [PubMed] [Google Scholar]
- 105.Xiang Q.R., Li W.Y., Feng T. Regulating effects of dietary fiber and powder of Agaricus bisporus based on in vitro fermentation on human gut microbiota. Sci. Technol. Food Ind. 2022;44:130–137. (In Chinese) [Google Scholar]
- 106.Xue Z., Ma Q., Chen Y., Lu Y., Wang Y., Jia Y., Zhang M., Chen H. Structure characterization of soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler and the effects on fermentation and human gut microbiota in vitro. Food Res. Int. 2020;129:108870. doi: 10.1016/j.foodres.2019.108870. [DOI] [PubMed] [Google Scholar]
- 107.Ding Q., Nie S., Hu J., Zong X., Li Q., Xie M. In vitro and in vivo gastrointestinal digestion and fermentation of the polysaccharide from Ganoderma atrum. Food Hydrocoll. 2017;63:646–655. doi: 10.1016/j.foodhyd.2016.10.018. [DOI] [Google Scholar]
- 108.Feng W., Ao H., Peng C., Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 109.Walker A.W., Duncan S.H., Leitch E.C., Child M.W., Flint H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microb. 2005;71:3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duncan S.H., Louis P., Thomson J.M., Flint H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009;11:2112–2122. doi: 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 111.Payling L., Fraser K., Loveday S.M., Sims I., Roy N., McNabb W. The effects of carbohydrate structure on the composition and functionality of the human gut microbiota. Trends Food Sci. Technol. 2020;97:233–248. doi: 10.1016/j.tifs.2020.01.009. [DOI] [Google Scholar]
- 112.Smith N.W., Shorten P.R., Altermann E., Roy N.C., McNabb W.C. The classification and evolution of bacterial cross-feeding. Front. Ecol. Evol. 2019;7:153. doi: 10.3389/fevo.2019.00153. [DOI] [Google Scholar]
- 113.Reichardt N., Vollmer M., Holtrop G., Farquharson F.M., Wefers D., Bunzel M., Duncan S.H., Drew J.E., Williams L.M., Milligan G., et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018;12:610–622. doi: 10.1038/ismej.2017.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Belenguer A., Duncan S.H., Calder A.G., Holtrop G., Louis P., Lobley G.E., Flint H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microb. 2006;72:3593–3599. doi: 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Falony G., Viachou A., Verbrugghe K., Vuyst L.D. Cross-feeding between Bifidobacterium longum BB536 and acetate converting, butyrate-producing colon bacteria during growth on oligofructose. Appl. Environ. Microb. 2006;72:7835–7841. doi: 10.1128/AEM.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakahara D., Nan C., Mori K., Hanayama M., Kikuchi H., Hirai S., Egashira Y. Effect of mushroom polysaccharides from Pleurotus eryngii on obesity and gut microbiota in mice fed a high-fat diet. Eur. J. Nutr. 2020;59:3231–3244. doi: 10.1007/s00394-019-02162-7. [DOI] [PubMed] [Google Scholar]
- 117.Li Y., Lu X., Li X., Guo X., Sheng Y., Li Y., Xu G., Han X., An L., Du P. Effects of Agaricus blazei Murrill polysaccharides on hyperlipidemic rats by regulation of intestinal microflora. Food Sci. Nutr. 2020;8:2758–2772. doi: 10.1002/fsn3.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shang H.M., Song H., Wang L.N., Wu B., Ding G.D., Jiang Y.Y., Yao X., Shen S.J. Effects of dietary polysaccharides from the submerged fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. On performance, gut microflora, and cholesterol metabolism in broiler chickens. Livest. Sci. 2014;167:276–285. doi: 10.1016/j.livsci.2014.07.004. [DOI] [Google Scholar]
- 119.Wang W., Yang S., Song S., Zhang J., Jia F. Flammulina velutipes mycorrhizae dietary fiber improves lipid metabolism disorders in obese mice through activating AMPK signaling pathway mediated by gut microbiota. Food Biosci. 2021;43:101246. doi: 10.1016/j.fbio.2021.101246. [DOI] [Google Scholar]
- 120.Chang C.J., Lin C.S., Lu C.C., Martel J., Ko Y.F., Ojcius D.M., Tseng S.F., Wu T.R., Chen Y.Y.M., Yong J.D., et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun S.S., Wang K., Ma K., Bao L., Liu H.W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019;17:3–14. doi: 10.1016/S1875-5364(19)30003-2. [DOI] [PubMed] [Google Scholar]
- 122.Song Q., Zhu Z. Using Cordyceps militaris extracellular polysaccharides to prevent Pb2+-induced liver and kidney toxicity by activating Nrf2 signals and modulating gut microbiota. Food Funct. 2020;11:9226–9239. doi: 10.1039/D0FO01608J. [DOI] [PubMed] [Google Scholar]
- 123.Ma G., Kimatu B.M., Zhao L., Yang W., Pei F., Hu Q. In vivo fermentation of a Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response. Food Funct. 2017;8:1810–1821. doi: 10.1039/C7FO00341B. [DOI] [PubMed] [Google Scholar]
- 124.Li K., Zhuo C., Teng C., Yu S., Wang X., Hu Y., Ren G., Yu M., Qu J. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int. J. Biol. Macromol. 2016;93:904–912. doi: 10.1016/j.ijbiomac.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 125.Kanwal S., Joseph T.P., Owusu L., Ren X., Li M., Xin Y. A polysaccharide isolated from Dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients. 2018;10:1003. doi: 10.3390/nu10081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu S., Dou Y., Ye B., Wu Q., Wang Y., Hu M., Ma F., Rong X., Guo J. Ganoderma lucidum polysaccharide insulin sensitivity by regulating inflammatory cytokines and gut microbiota composition in mice. J. Funct. Foods. 2017;38:545–552. doi: 10.1016/j.jff.2017.09.032. [DOI] [Google Scholar]
- 127.Yang Y., Ye H., Zhao C., Ren L., Wang C., Georgiev M.I., Xiao J., Zhang T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohyd. Polym. 2021;262:117668. doi: 10.1016/j.carbpol.2021.117668. [DOI] [PubMed] [Google Scholar]
- 128.Zhao R., Cheng N., Nakata P.A., Zhao L., Hu Q. Consumption of polysaccharides from Auricularia auricular modulates the intestinal microbiota in mice. Food Res. Int. 2019;123:383–392. doi: 10.1016/j.foodres.2019.04.070. [DOI] [PubMed] [Google Scholar]
- 129.Xie J., Liu Y., Chen B., Zhang G., Ou S., Luo J., Peng X. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 2019;63:1559. doi: 10.29219/fnr.v63.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li L.F., Liu H.B., Zhang Q.W., Li Z.P., Wong T.L., Fung H.Y., Zhang J.X., Bai S.P., Lu A.P., Han Q.B. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G. sinense: Chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci. Rep. 2018;8:6172. doi: 10.1038/s41598-018-22885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luo J., Zhang C., Liu R., Gao L., Ou S., Liu L., Peng X. Ganoderma lucidum polysaccharide alleviating colorectal cancer by alteration of special gut bacteria and regulation of gene expression of colonic epithelial cells. J. Funct. Foods. 2018;47:127–135. doi: 10.1016/j.jff.2018.05.041. [DOI] [Google Scholar]
- 132.Cotter P.D., Hill C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003;67:429–453. doi: 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ragsdale S.W., Pierce E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. BBA-Proteins Proteom. 2008;1784:1873–1898. doi: 10.1016/j.bbapap.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hetzel M., Brock M., Selmer T., Pierik A.J., Golding B.T., Buckel W. Acryloyl-CoA reductase from Clostridium propionicum: An enzyme complex of propionyl-CoA dehydrogenase and electron-transferring flavoprotein. Eur. J. Biochem. 2003;270:902–910. doi: 10.1046/j.1432-1033.2003.03450.x. [DOI] [PubMed] [Google Scholar]
- 135.Scott K.P., Martin J.C., Campbell G., Mayer C.D., Flint H.J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J. Bacteriol. 2006;188:4340–4449. doi: 10.1128/JB.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Louis P., Duncan S.H., McCrae S.I., Millar J., Jackson M.S., Flint H.J. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 2004;186:2099–2106. doi: 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H.J. Acetate utilization and butyryl coenzyme A (CoA): Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microb. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blaak E.E., Canfora E.E., Theis S., Frost G., Groen A.K., Mithieux G., Nauta A., Scott K., Stahl B., van Harsselaar J., et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes. 2020;11:411–455. doi: 10.3920/BM2020.0057. [DOI] [PubMed] [Google Scholar]
- 139.Wong J.M., De Souza R., Kendall C.W., Emam A., Jenkins D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 140.Martin-Gallausiaux C., Marinelli L., Blottiere H.M., Larraufie P., Lapaque N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021;80:37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 141.Park J., Kim M., Kang S.G., Jannasch A.H., Cooper B., Patterson J., Kim C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen Y., Cui W., Li X., Yang H. Interaction between commensal bacteria, immune response and the intestinal barrier in inflammatory bowel disease. Front. Immunol. 2021;12:761981. doi: 10.3389/fimmu.2021.761981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 144.Beglinger C., Degen L. Gastrointestinal satiety signals in humans—Physiologic roles for GLP-1 and PYY? Physiol. Behav. 2006;89:460–464. doi: 10.1016/j.physbeh.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 145.Beisner J., Filipe Rosa L., Kaden-Volynets V., Stolzer I., Gunther C., Bischoff S.C. Prebiotic lnulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front. Immunol. 2021;12:678360. doi: 10.3389/fimmu.2021.678360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mirzaei R., Afaghi A., Babakhani S., Sohrabi M.R., Hosseini-Fard S.R., Babolhavaeji K., Akbari S.K.A., Yousefimashouf R., Karampoor S. Role of microbiotaderived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021;139:111619. doi: 10.1016/j.biopha.2021.111619. [DOI] [PubMed] [Google Scholar]
- 147.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 148.Larsen N., Vogensen F.K., Van Den Berg F.W., Nielsen D.S., Andreasen A.S., Pedersen B.K., AI-Soud W.A., Sorensen S.J., Hansen L.H., Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxmia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 150.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 151.Backhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Nat. Acad. Sci. USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V.M., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 153.Hachimura S., Totsuka M., Hosono A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018;82:584–599. doi: 10.1080/09168451.2018.1433017. [DOI] [PubMed] [Google Scholar]
- 154.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From Dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 155.Luu M., Pautz S., Kohl V., Singh R., Romero R., Lucas S., Hofmann J., Raifer H., Vachharajani N., Carrascosa L.C., et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019;10:760. doi: 10.1038/s41467-019-08711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kinoshita M., Suzuki Y., Saito Y. Butyrate reduces colonic paracellular permeability by enhancing PPARγ activation. Biochem. Biophys. Res. Commun. 2002;293:827–831. doi: 10.1016/S0006-291X(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 157.Lee C., Kim B.G., Kim J.H., Chun J., Im J.P., Kim J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL- 10 independent manner. Int. Immunopharmacol. 2017;51:47–56. doi: 10.1016/j.intimp.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 158.Ogawa K., Yasumura S., Atarashi Y., Minemura M., Miyazaki T., Lwamoto M., Higuchi K., Watanabe A. Sodium butyrate enhances Fas-mediated apoptosis of human hepatoma cells. J. Hepatol. 2004;40:278–284. doi: 10.1016/j.jhep.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 159.Xiong H., Guo B., Gan Z., Song D., Lu Z., Yi H., Wu Y., Wang Y., Du H. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci. Rep. 2016;6:27070. doi: 10.1038/srep27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kanauchi O., Serizawa I., Araki Y., Suzuki A., Andoh A., Fujitama Y., Mitsuyama K., Takaki K., Toyonaga A., Sata M., et al. Germinated barley foodstuff, a prebiotic product, ameliorates inflammation of colitis through modulation of the enteric environment. J. Gastroenterol. 2003;38:134–141. doi: 10.1007/s005350300022. [DOI] [PubMed] [Google Scholar]
- 161.Vlassopoulou M., Yannakoulia M., Pletsa V., Zervakis G.I., Kyriacou A. Effects of fungal beta-glucans on health—A systematic review of randomized controlled trials. Food Funct. 2021;12:3366. doi: 10.1039/D1FO00122A. [DOI] [PubMed] [Google Scholar]
- 162.Castellarin M., Warren R.L., Freeman J.D., Dreolini L., Krzywinski M., Stauss J., Barnes R., Watson P., Allen-Vercoe E., Moore R.A., et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Song H., Yoo Y., Hwang J., Na Y., Kim H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immun. 2016;137:852–860. doi: 10.1016/j.jaci.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 164.Amar J., Chabo C., Waget A., Klopp P., Vachoux C., Bermudez-Humaran L.G., Smirnova N., Berge M., Sulpice T., Lahtinen S., et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang F., Meng W., Wang B., Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 166.Zheng Y., Fang Z., Xue Y., Zhang J., Zhu J., Gao R. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes. 2020;11:1030–1042. doi: 10.1080/19490976.2020.1737487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Youssef O., Lahti L., Kokkola A., Karla T., Tikkanen M., Ehsan H., Carpelan-Holmstrom M., Koskensalo S., Bohling T., Rautelin H., et al. Stool microbiota composition differs in patients with stomach, colon, and rectal neoplasms. Digest. Dis. Sci. 2018;63:2950–2958. doi: 10.1007/s10620-018-5190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yachida S., Mizutani S., Shiroma H., Shiba S., Nakajima T., Sakamoto T., Watanabe H., Masuda K., Nishimoto Y., Kubo M., et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019;25:968–976. doi: 10.1038/s41591-019-0458-7. [DOI] [PubMed] [Google Scholar]
- 169.Viaud S., Saccheri F., Mignot G., Yamazaki T., Daillere R., Hannani D., Enot D.P., Pfirschke C., Engblom C., Pittet M.J., et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mego M., Chovanec J., Vochyanova-Andrezalova I., Konkolovsky P., Mikulova M., Reckova M., Miskovska V., Bystricky B., Beniak J., Medvecova L., et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015;23:356–362. doi: 10.1016/j.ctim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 172.He Y., Fu L., Li Y., Wang W., Gong M., Zhang J., Fu Y.X., Chen Y., Guo X. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021;33:988–1000. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 173.Si W., Liang H., Bugno J., Xu Q., Ding X., Yang K., Fu Y., Weichselbaum R.R., Zhao X., Wang L. Lactobacillus rhamnosus GG induces cGAS/STING-dependent type I interferon and improves response to immune checkpoint blockade. Gut. 2022;71:521–533. doi: 10.1136/gutjnl-2020-323426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang A., Xiao N., He P., Sun P. Chemical analysis and antioxidant activity in vitro of dietary fibers extracted from Boletus edulis. Int. J. Biol. Macromol. 2011;49:1092–1095. doi: 10.1016/j.ijbiomac.2011.09.005. [DOI] [PubMed] [Google Scholar]