Abstract
Background and Objectives. Recent guidelines have downgraded the routine use of the intra-aortic balloon pump (IABP) in patients with cardiogenic shock (CS) due to ST-elevation myocardial infarction (STEMI). Despite this, its use in clinical practice remains high. The aim of this study was to evaluate the prognostic impact of the IABP in patients with STEMI complicated by CS undergoing primary PCI (pPCI), focusing on patients with anterior MI in whom a major benefit has been previously hypothesized. Materials and Methods. We enrolled 2958 consecutive patients undergoing pPCI for STEMI in our department from 2005 to 2018. Propensity score matching and mortality analysis were performed. Results. CS occurred in 246 patients (8.3%); among these patients, 145 (60%) had anterior AMI. In the propensity-matched analysis, the use of the IABP was associated with a lower 30-day mortality (39.3% vs. 60.9%, p = 0.032) in the subgroup of patients with anterior STEMI. Conversely, in the whole group of CS patients and in the subgroup of patients with non-anterior STEMI, IABP use did not have a significant impact on mortality. Conclusions. The use of the IABP in cases of STEMI complicated by CS was found to improve survival in patients with anterior infarction. Prospective studies are needed before abandoning or markedly limiting the use of the IABP in this clinical setting.
Keywords: acute myocardial infarction, cardiogenic shock, mechanical circulatory support, IABP
1. Introduction
During the last decades, the survival of patients affected by ST-segment elevation myocardial infarction (STEMI) showed a dramatic increase, mainly due to improvements in evidence-based therapies including early revascularization with primary percutaneous coronary intervention (pPCI) [1,2]. Nevertheless, the occurrence of cardiogenic shock (CS) related to STEMI remains one of the major causes of death, with a growing incidence in recent years and with mortality rates approaching 40–50% [3,4,5,6,7]. In this challenging scenario, mechanical circulatory support (MCS) devices are one of the available therapeutic options to improve hemodynamics and prognosis, limiting the toxicity of catecholamines [1,6,8,9]. The intra-aortic balloon pump (IABP) has been used for more than 50 years for its documented beneficial hemodynamic effects. Specifically, the IABP increases diastolic blood pressure and coronary perfusion, while it decreases the afterload and myocardial oxygen consumption [9,10]. Despite these theoretical benefits, in the last decade, growing evidence has challenged the beneficial role of the IAP [11,12,13,14] leading to its routine use in patients with myocardial infarction (MI) complicated by CS to be qualified as a Class III (level of evidence A) recommendation in the latest European guidelines [1]. Nevertheless, the IABP still represents the most widely used MCS device in clinical practice as it is perceived by many physicians as safe, affordable, easy to use and beneficial [8,9]. Moreover, recent studies have shown a favorable effect of IABP use in some high-risk subsets of patients, including those with anterior STEMI and persistent ischemia after pPCI [15,16,17,18,19].
The aim of the present study was to evaluate the potential benefit of IABP use in a large real-world cohort of consecutive patients with STEMI complicated by CS, focusing on patients with anterior localization of the infarction.
2. Materials and Methods
Study population and procedures. We retrospectively analyzed our registry in which all consecutive STEMI patients who underwent pPCI at Policlinico San Matteo in Pavia, Italy, between 1 January 2005 and 30 June 2018 were prospectively enrolled. STEMI was defined according to the current guidelines at the time of patient enrollment. The current STEMI definition recognizes the presence of typical symptoms of myocardial ischemia plus either ≥1 mV ST segment elevation for ≥20 min in ≥2 contiguous electrocardiogram leads or new left-bundle branch block in the presence of modified Sgarbossa criteria or hemodynamic instability [1]. We excluded patients undergoing PCI beyond 12 h from symptoms onset (24 h in case of cardiogenic shock), rescue PCI and urgent cardiac surgery. All revascularization procedures were performed by an experienced 24 h on-call team.
Clinical data collection. For each patient, informed written consent was obtained, and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee (date of Institutional Review Board approval: 1 January 2005). Demographic, clinical, procedural, electrocardiographic and laboratory data were prospectively collected into a dedicated database. Laboratory data were gathered at admittance (pre pPCI) and the following days in the Cardiac Intensive Care Unit (CICU). Detailed angiographic and procedural information of the pPCI were also collected. Follow-up data regarding the primary endpoint of this investigation (30-day all-cause mortality) were collected through an outpatient clinical visit at 30 days or via telephone contact by trained medical staff. PCI technique and all peri-procedural therapies were given according to institutional protocols and current guideline recommendations by the interventional and/or CICU cardiologists.
Definitions. The presence of a persistent (>30 min) systolic blood pressure (SBP) < 90 mmHg (or the need for pharmacological support to maintain SBP > 90 mmHg) due to cardiac dysfunction and signs of pulmonary congestion or impaired end-organ perfusion qualified for CS, in accordance with the previous literature [12]. Contrast-induced acute kidney injury (CI-AKI 0.5) was defined as a rise >0.5 mg/mL in serum creatinine occurring in the 96 h following pPCI [20]. Bleeding was defined according to TIMI criteria [21]; for the purpose of the current analysis, we considered all TIMI bleeding events, including both major or minor bleeding. According to the previous literature, ST resolution was defined as a ≥70% resolution of initial ST shift measured 20 milliseconds after the end of the QRS complex in the lead with maximal ST deviation twelve-lead ECGs performed at baseline (before coronary angiography) and at 60 min after reperfusion (elevation or depression) [22,23,24].
Statistical analysis. Categorical data were reported as absolute values and percentages. To evaluate the association between categorical data and IABP, Pearson’s χ2 test or Fisher exact test were used, as appropriate. Continuous variables were presented as median (with Q1–Q3 percentiles) and compared using Mann–Whitney U test. To evaluate the prognostic impact of IABP use, we performed propensity-matched analysis aiming to limit the influence of measured confounders. A propensity score was calculated for each patient using a logistic regression model in a dedicated analysis; the propensity score was an estimate of the probability that each patient received IABP and was created as follows. First, univariable associations were calculated for all variables known before IABP insertion that could have influenced the choice of inserting IABP. Second, all variables with p ≤ 0.05 (anterior STEMI, age > 75 years, three-vessel disease, persistent blood pressure ≤ 90 mmHg at admission in the cath-lab, hyperglycemia at admission, anemia at admission, TIMI final < 3, out of hospital cardiac arrest, diabetes, and female sex) were included in the propensity model. Based on the propensity score, each patient in whom IABP was used was matched to a unique control patient in whom IABP was not used. One-to-one matching was performed with nearest neighbor matching algorithm; the caliper width was equal to 0.1 of the standard deviation of the logit of the propensity score. Mortality analyses in the propensity-matched populations were performed using Kaplan–Meyer curves and log-rank test. The software used for the analysis was SPSS version 22 (SPSS Inc., Armonk, NY, USA), and the cut-off adopted for statistical significance was two-sided p value < 0.05.
3. Results
Primary PCI for STEMI was performed in 2958 patients between 1 January 2005 and 30 June 2018. Demographic, clinical, procedural and laboratory baseline variables of the overall STEMI population and the subset of patients with STEMI complicated by CS are shown in Table 1.
Table 1.
Characteristics of the overall population of the study and of the subset of patients with STEMI complicated by cardiogenic shock.
| Demographic Variables | STEMI (n = 2959) |
STEMI + Cardiogenic Shock (n = 246) |
p Value |
|---|---|---|---|
| Age (years; median (Q1–Q3)) | 63 (54–73) | 69.5 (60–77) | <0.001 |
| Age > 75 years (n, %) | 613 (20.4%) | 83 (33.7%) | <0.001 |
| Female sex (n, %) | 663 (22%) | 75 (30.5%) | <0.001 |
| Body mass index (kg/m2; median (Q1–Q3)) | 25.8 (23.4–28.7) | 24.2 (22.8–26.2) | <0.001 |
| CV risk factors | |||
| Smoke (n, %) | 1861 (63.1%) | 94 (38.5%) | <0.001 |
| Hypertension (n, %) | 1631 (55.3%) | 46 (60.1%) | <0.001 |
| Type II diabetes mellitus (n, %) | 496 (16.8%) | 46 (18.9%) | <0.001 |
| Dyslipidemia (n, %) | 1162 (39%) | 55 (22.6%) | <0.001 |
| Family history of cardiovascular disease (n, %) | 966 (32.8%) | 39 (16%) | <0.001 |
| Medical history | |||
| Peripheral arterial disease (n, %) | 312 (10.4%) | 47 (19.4%) | <0.001 |
| Previous myocardial infarction (n, %) | 392 (13.3%) | 40 (16.4%) | 0.056 |
| Previous percutaneous coronary intervention (n, %) | 357 (12.1%) | 33 (13.5%) | 0.199 |
| Previous coronary artery bypass grafting (n, %) | 53 (1.8%) | 8 (3.3%) | 0.712 |
| Chronic kidney disease (n, %) | 642 (21.4%) | 105 (52.8%) | <0.001 |
| Clinical and laboratory variables | |||
| Out of hospital cardiac arrest (n, %) | 284 (9.8%) | 92 (37.9%) | <0.001 |
| Anemia (n, %) | 528 (8.1%) | 106 (45.5%) | <0.001 |
| Heart rate (bpm; median (Q1–Q3)) | 75 (65–87) | 80 (67–99) | 0.001 |
| Left ventricular ejection fraction (median(Q1–Q3)) | 45 (38–50) | 35 (25–40) | <0.001 |
| Systolic arterial pressure (mmHg; median (Q1–Q3)) | 135 (120–150) | 90 (75–103) | <0.001 |
| Baseline blood sugar (mg/dL; median (Q1–Q3)) | 141 (120–176) | 175 (136–219) | <0.001 |
| Hyperglycemia (n,%) | 469 (16.5%) | 95 (43%) | <0.001 |
| Baseline hemoglobin (g/dL; median (Q1–Q3)) | 14.3 (13.5–15.3) | 13.6 (12–14.7) | <0.001 |
| Baseline white blood cells (n × 103/mcl; median (Q1–Q3)) | 11.2 (8.8–13.7) | 13.3 (9.5–20) | <0.001 |
| Troponin I peak (ng/dL; median (Q1–Q3)) | 80 (32–166) | 196 (81–355) | 0.001 |
| Creatine kinase peak (MU/L; median (Q1–Q3)) | 1.3 (0.6–2.4) | 2.8 (1.3–5.6) | <0.001 |
| Baseline creatinine (mg/dL; median (Q1–Q3)) | 0.9 (0.8–1.1) | 1 (0.8–1.3) | <0.001 |
| ECG | |||
| Anterior STEMI (n, %) | 1240 (46.5%) | 145 (59.7%) | <0.001 |
| ST resolution (n, %) | 1908 (69.9%) | 98 (49%) | <0.001 |
| Procedural data | |||
| Three-vessel disease (n, %) | 772 (26.4%) | 86 (36.3%) | 0.001 |
| Left main involvement (n, %) | 32 (1.1%) | 18 (8.1%) | <0.001 |
| Blood pressure ≤ 90 mmHg in the cath-lab (n,%) | 90 (3.1%) | 46 (20.3%) | <0.001 |
| Post-procedural TIMI flow <3 (n, %) | 278 (9.3%) | 56 (23.5%) | <0.001 |
| Length of stay | |||
| Coronary care unit (days; median (Q1–Q3)) | 4 (3–5) | 6 (2–10) | <0.001 |
| Hospital (days; median (Q1–Q3)) | 7 (6–9) | 9 (4–14) | <0.001 |
3.1. Characteristics of Patients with Cardiogenic Shock
CS occurred in 246 patients (8.3%) out of the whole population, 54.7% (n = 133) of whom received the IABP; 30-day mortality in CS patients was 45.5%. Among patients with STEMI complicated by CS, anterior localization of the infarction occurred in 59.8% of cases, with a higher 30-day mortality compared to patients with CS and non-anterior STEMI (49.6% vs. 37.5%, p < 0.001). Main significant differences in characteristics of patients with CS stratified for the localization of the infarction are shown in Table 2. Overall, patients with anterior STEMI presented a higher profile risk compared to their counterparts. The IABP was used, respectively, in 60.8% (n = 87) and 47.4% (n = 46) of patients with anterior vs. non-anterior STEMI complicated by CS (p = 0.04). Characteristics of patients with anterior STEMI complicated by CS stratified for IABP use are summarized in Table 3. IABP use in CS patients was neither associated with major complications nor with a significant increased rate of bleeding during hospital stay. Nevertheless, numerically greater, albeit not statistically significant, increases in the need for vascular surgery (8.5% vs. 5.5%, p = 0.366) and in the rate of bleeding (38.7% vs. 26.7%, p = 0.071) were found.
Table 2.
Characteristics of patients with STEMI complicated by CS in patients with anterior vs. non anterior myocardial infarction.
| Variables | Anterior STEMI | p-Value | |
|---|---|---|---|
| NO (n = 101) |
YES (n = 145) |
||
| Age > 75 years (n, %) | 39 (39.8%) | 44 (30.1%) | 0.128 |
| Female sex (n, %) | 34 (34.7%) | 39 (26.9%) | 0.193 |
| Type II diabetes mellitus (n, %) | 21 (21.9%) | 25 (17.4%) | 0.348 |
| Heart rate (bpm; median (Q1–Q3)) | 73 (62–82) | 80 (68–88) | <0.001 |
| Anemia (n, %) | 43 (46.7%) | 61 (44.2%) | 0.075 |
| Out of hospital cardiac arrest (n, %) | 29 (30.2%) | 61 (42.4%) | 0.057 |
| Hyperglycemia (n, %) | 34 (39.5%) | 59 (44.7%) | 0.451 |
| Creatine kinase peak (Mu/L; median (Q1–Q3)) | 1.2 (0.6–2.1) | 1.7 (0.8–3) | <0.001 |
| Baseline white blood cells (n × 103/mcl; median (Q1–Q3)) | 10.8 (8.7–13.1) | 11.5 (9–14.2) | <0.001 |
| Left ventricular ejection fraction (%; median(Q1–Q3)) | 40 (30–47) | 27 (20–37.5) | <0.001 |
| Contrast-induced acute kidney injury (n, %) | 5 (5.4%) | 13 (9.3%) | <0.001 |
| Bleeding (n, %) | 9 (9.6%) | 22 (15.6%) | <0.001 |
| Three-vessel disease (n, %) | 33 (34.7%) | 53 (38.1%) | 0.597 |
| Left main involvement (n, %) | 1(1.2%) | 16 (11.9%) | 0.004 |
| Systolic blood pressure < 90 mmHg in the cath-lab (n, %) | 23 (25%) | 22 (16.7%) | 0.126 |
| Post-procedural TIMI flow <3 (n, %) | 23 (23.7%) | 32 (23.2%) | 0.926 |
| ST resolution (n, %) | 78 (78%) | 88 (61%) | <0.001 |
Table 3.
Characteristics of patients with anterior AMI complicated by CS stratified for IABP use.
| Variables | IABP | p-Value | |
|---|---|---|---|
| NO (n = 58) |
YES (n = 87) |
||
| Age (years; median (Q1–Q3)) | 74 (68–82) | 67 (58–74) | <0.001 |
| Age > 75 years (n, %) | 22 (39.3%) | 21 (24.1%) | 0.054 |
| Body mass index (kg/m2, median (Q1–Q3)) | 24.2 (22.8–26.9) | 24.5 (22.8–28.7) | 0.535 |
| Female sex (%) | 16 (28.6%) | 21 (24.1%) | 0.555 |
| Hypertension (%) | 35 (62.5%) | 44 (51.2%) | 0.184 |
| Type II diabetes mellitus (%) | 7 (12.5%) | 18 (20.9%) | 0.197 |
| Anemia (n, %) | 29 (55.8%) | 32 (37.6%) | 0.038 |
| Previous myocardial infarction (%) | 12 (21.4%) | 13 (14.9%) | 0.319 |
| Peripheral arterial disease (%) | 16 (27.8%) | 12 (14%) | 0.044 |
| Out of hospital cardiac arrest (%) | 19 (34.5%) | 40 (46.5%) | 0.178 |
| Heart rate (bpm; median (Q1–Q3)) | 80 (72–102) | 91 (69–109) | 0.706 |
| Baseline blood sugar (mg/dL; median (Q1–Q3)) | 141 (133–181) | 214 (176–263) | 0.005 |
| Left ventricular ejection fraction (median(Q1–Q3)) | 30 (25–40) | 25 (20–35) | 0.041 |
| Baseline hemoglobin (g/dL; median (Q1–Q3)) | 12.3 (11.4–15.1) | 13.9 (12.4–15.1) | 0.058 |
| Baseline platelets (Mu/L, median (Q1–Q3)) | 232 (206–266) | 246 (203–304) | 0.276 |
| Platelets nadir (Mu/L, median (Q1–Q3)) | 188 (159–228) | 137 (101–200) | <0.001 |
| Baseline white blood cells (n × 103/mcl; median (Q1–Q3)) | 12.49 (9.8–16.4) | 14.6 (11.4–21.3) | 0.013 |
| Hyperglycemia (n, %) | 16 (31.4%) | 42 (52.5%) | 0.018 |
| Creatine kinase peak (mg/dMU/L; median (Q1–Q3)) | 2.83 (1.04–4.34) | 4.06 (2.7–7.1) | 0.001 |
| Baseline eGFR * (mg/mL, median (Q1–Q3)) | 44 (36–74) | 65 (43–88) | 0.087 |
| Systolic blood pressure < 90 mmHg in the cath-lab (n, %) | 3 (5.4%) | 19 (25%) | 0.003 |
| Three-vessel disease (%) | 22 (40%) | 31 (37.3%) | 0.754 |
| Left main involvement (n, %) | 2 (3.7%) | 12 (15.2%) | 0.034 |
| ST resolution (%) | 17 (34.7%) | 32 (50%) | 0.104 |
| CI-AKI 0.5 (%) | 14 (26.4%) | 21 (25%) | 0.853 |
| Post-procedural TIMI flow <3 (%) | 13 (23.6%) | 18 (22%) | 0.817 |
| GP IIbIIIa—inhibitors use (%) | 19 (37.3%) | 43 (52.4%) | 0.088 |
| Days in Coronary Care Unit (days; median (Q1–Q3)) | 7 (4–10) | 6 (4–10) | 0.219 |
| Days in hospital (days; median (Q1–Q3)) | 8 (6–15) | 12 (8–19) | 0.003 |
Legend: eGFR = estimated glomerular filtration rate; CI-AKI = contrast-induced acute kidney injury. * eGFR was estimated using Cockroft–Gault formula.
3.2. Mortality Analysis
In the univariate analysis for the whole CS group, IABP use was associated with a lower 30-day mortality (38.7% vs. 53%, OR 0.56, 95%CI 0.32–0.96, p = 0.002). In the subset of patients with anterior STEMI, IABP use was associated with a lower 30-day mortality (41% vs. 60.8%, OR 0.45, 95%CI 0.21–0.92, p = 0.013) whereas in the subgroup of patients with non-anterior STEMI, IABP use was not significantly associated with a lower 30-day mortality (34.1% vs. 41.3%, OR 0.74, 95%CI 0.39–1.76, p = 0.432).
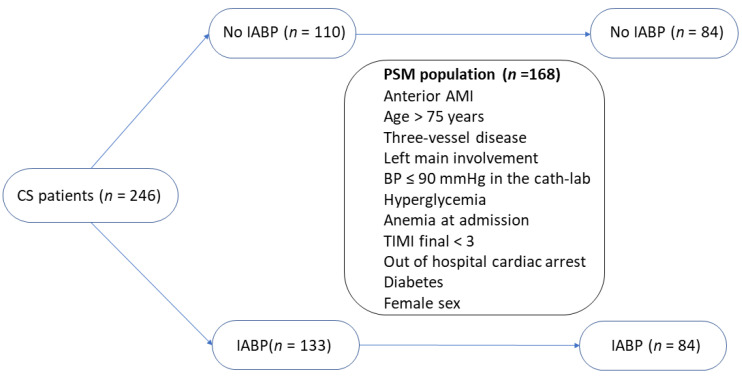
Furthermore, among 246 patients with CS, we successfully matched 84 patients who received the IABP with 84 patients who did not receive the IABP, but who showed a similar propension to receive the device according to the variables available before the decision. The matching flow diagram is presented in Figure 1. Characteristics of the two groups are summarized in Table 4.
Figure 1.
Matching flow diagram. Legend: CS = cardiogenic shock; IABP = intra-aortic balloon pump; PSM = propensity score matching; AMI = acute myocardial infarction; and TIMI = thrombolysis in myocardial infarction.
Table 4.
Characteristics of propensity-matched populations stratified for IABP use.
| Variables Included in the PMS | IABP | p-Value | |
|---|---|---|---|
| NO (n = 84) |
YES (n = 84) |
||
| Age > 75 years (%) | 30 (37.5%) | 26 (29.5%) | 0.275 |
| Anterior myocardial infarction (%) | 46 (57.5%) | 56 (63.6%) | 0.416 |
| Three-vessel disease (%) | 26 (32.5%) | 32 (36.4%) | 0.599 |
| Left main involvement (%) | 2 (2.5%) | 6 (6.8%) | 0.189 |
| Systolic blood pressure < 90 mmHg in the cath-lab (n, %) | 60 (62.5%) | 66 (75%) | 0.080 |
| Hyperglycemia at admission (%) | 26 (32.5%) | 44 (50%) | 0.022 |
| Anemia at admission (%) | 41 (51.2%) | 34 (38.6%) | 0.100 |
| Post-procedural TIMI flow <3 (%) | 17 (21.3%) | 20 (22.7%) | 0.817 |
| Out of hospital cardiac arrest (%) | 23 (28.7%) | 18 (20.5%) | 0.211 |
| Type II diabetes mellitus (%) | 11 (13.8%) | 22 (25%) | 0.067 |
| Female sex (%) | 24 (30%) | 27 (30.7%) | 0.924 |
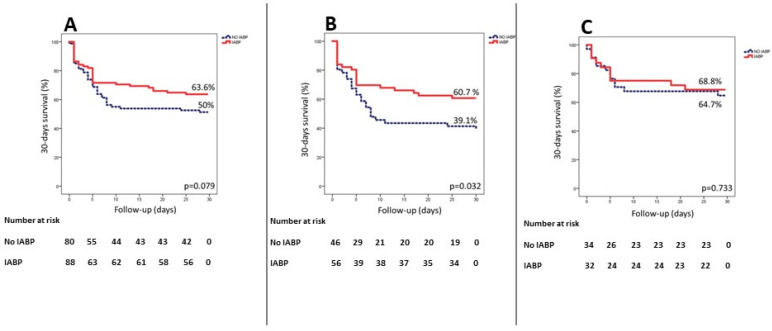
Figure 2 illustrates the Kaplan–Meier curves for 30-day mortality in the matched population for patients who received the IABP vs. those who did not receive the IABP in the whole group of CS patients (panel A) and in the subsets of patients with anterior (panel B) and non-anterior (panel C) STEMI. Indeed, in the first group, the overall 30-day mortality was 42.9% (n = 72), specifically 36.4% (n = 32) in patients in whom the IABP was used and 50% (n = 40) in the others (p = 0.079). In the subset of patients with anterior STEMI, the overall 30-day mortality was 49% (n = 50) to 39.3% (n = 22) in patients who received the IABP and 60.9% (n = 28) in those who did not (p = 0.032). Finally, in patients with non-anterior myocardial infarction, there was no significant difference in 30-day mortality according to IABP use (34.2% vs. 35.3%, p = 0.733).
Figure 2.
Panel (A): cumulative incidence of 30-day survival in the overall matched population of patients with STEMI complicated by cardiogenic shock; panel (B): cumulative incidence of 30-day survival in the subset of patients with anterior STEMI; and panel (C): cumulative incidence of 30-day survival in the subset of patients with non-anterior STEMI.
4. Discussion
The main finding of the present study is that treatment with IABP in patients with anterior STEMI complicated by cardiogenic shock is associated with decreased short-term mortality.
Cardiogenic shock due to STEMI still has an unacceptable mortality rate of 40–50% [3,4,5,6,7]. MCS devices represent an interesting therapeutic opportunity, as they offer the chance to assist the failing cardiac pump, limiting the use of intravenous inotropes, which may worsen myocardial ischemia. In our cohort of nearly three-thousands STEMI patients, 8.3% presented with CS with a short-term mortality of 45.5%, in accordance with the previous literature [3,4,5,6,7]. The IABP is still the most used device in patients with STEMI complicated by CS [8,9]. Despite this, its routine use has been downgraded to a class III recommendation [1]. The widespread use of the IABP can be explained considering several advantageous features: simplicity of use, an elevated safety profile, a low cost and a high perception of its usefulness by the operators [8,9,13,19]. Moreover, robust evidence in favor of more complex devices (i.e., axial pumps or percutaneous LVADs) in this clinical context is still lacking [25,26,27,28,29].
The downgrading of IABP routine use in the guidelines was mostly ascribable to the data provided by the IABP-SHOCK II trial. This was the first and only adequately powered randomized clinical trial evaluating the prognostic impact of the IABP in CS due to myocardial infarction, and it documented a neutral effect on short-term mortality, mainly due, according to the authors, to the modest effects of the device on cardiac output [12]. Long-term follow-up data at 12 months and 6 years were consistent with the initial findings of the study [30,31]. Despite its undoubted value as a landmark study in the field, a number of criticisms have been raised, in particular for the presence of a high rate of non-ST elevation MI (roughly 1/3 of the total population) and non-anterior STEMI patients (nearly 50%), who are less likely to receive a benefit from the IABP. Moreover, the lower than expected incidence of events in the control group configured the trial to be underpowered with regard to the primary hypothesis. Therefore, given the heterogeneous population enrolled in the trial, it is possible that a potential benefit of the IABP was lost, which could be present in peculiar high-risk subsets of patients such as those with an anterior STEMI. According to this hypothesis, some recent studies have found a potential benefit of non-routine IABP use in high-risk STEMI subgroups [16,17,18].
Anterior localization of myocardial infarction is a well-known marker of high risk. Indeed, among our STEMI patients, it was associated with a higher mortality (7.1% vs. 3.7%, p < 0.001), higher CK peak and inflammatory markers, a lower LVEF and higher rate of hyperglycemia, incomplete ST resolution, TIMI flow < 3 after revascularization, CI-AKI and bleeding complications. Due to all these features, patients with anterior MI appear to be a good target for interventions aiming to support systemic perfusion without increasing myocardial oxygen consumption. Accordingly, the CRISP-AMI trial [19] explored the potential benefit of the routine use of the IABP in anterior MI not complicated by CS. Despite the trial being neutral, a subsequent sub-study showed a prognostic benefit in patients with larger infarction (defined as total sum of ST elevation > 15 mm) or incomplete ST resolution after reperfusion [18]. In our population, the IABP was used more frequently in patients with CS and anterior STEMI. This group of patients presented the greatest risk profile features as illustrated in Table 3, showing lower LVEF, greater CK peak and white blood cell values at admission and a numerically higher incidence of out-of-hospital cardiac arrest. Interestingly, patients with advanced peripheral artery disease were less likely to receive the IABP, possibly due to the fear of technical difficulties in the insertion. Of note, the median age was higher in patients who did not receive the IABP; nevertheless, elderly age was included in the propensity score and thus its influence on the mortality analysis is unlikely. The IABP was associated with a survival benefit in the overall population in the univariate analysis. However, after stratification for infarct localization, the benefit was consistent only in patients with anterior MI. Nonetheless, these results could be the consequence of a selection bias (i.e., the device utilization preferably in those patients where is not perceived to be futile). To account for known confounding factors, we performed a propensity matching score including in the match only the variables known before the insertion of the IABP, as they could influence the choice of inserting the device but could not be influenced by the device itself. With this method, we obtained two propensity-matched cohorts of patients each made of 84 patients with a similar propension to receive the IABP. As this constitutes a retrospective analysis, we opted not to provide a formal sample size calculation. However, it is important to acknowledge that the matching process did result in a reduction in the population of patients with CS included in the analysis, potentially affecting the power of our analysis. In the overall CS population, there was a non-significant trend towards a better survival in patients in whom the IABP was used. In the subset of patients with anterior infarction and CS, the benefit of IABP use was shown to be significant (39.3% vs. 60.9%, p = 0.032), while this beneficial effect was not present in those with non-anterior STEMI.
The greater benefit of the IABP in patients with CS and anterior STEMI could be explained considering several factors. First, the larger infarct size (expressed by a significantly higher CK peak) in these patients suggests a greater portion of jeopardized but potentially salvageable myocardium. The balloon pump, offering both systolic unloading and diastolic coronary flow augmentation, could help to increase oxygen delivery and decrease its consumption. In fact, the ratio between these two factors is a key factor in the ischemic damage setting [32]. With regard to this, the possible benefit of pre-revascularization 30 min unloading of the left ventricle using Impella CP in patients with STEMI without cardiogenic shock was investigated in the Door-To-Unload in STEMI pilot trial [33]. The trial was neutral regarding major adverse cardiovascular and cerebrovascular events and the infarct size measured at 30 days, demonstrating the feasibility and safety of this strategy, which is currently under investigation in a larger and adequately powered study (NCT03947619).
Second, as demonstrated by De Silva et al. [34], the effect of the IABP in augmenting the diastolic coronary perfusion is counterbalanced by the vasoconstriction of coronary arteries due to the self-regulation of the coronary circle. Thus, only when these self-regulatory mechanisms are lost (i.e., in the context of persistent ischemia as suggested by the authors), was a beneficial increase in the coronary flow due to the IABP observed. This condition is more likely to happen in the context of CS due to anterior infarction, as suggested by a significantly lower rate of ST resolution (61% vs. 78%, p < 0.001).
Finally, a certain number of patients with CS in the context of inferior MI are more likely to experience hypotensive episodes due to marked vagal activation or acute right ventricular dysfunction rather than true states of CS. In such cases, a prompt revascularization on top of OMT can reverse the hypotension, whereas MCS devices would not be beneficial.
Limitations
The present analysis has some limitations. First, our study was a single-center study. Thus, our results refer to a specific population, so they could be potentially confounded by local practice and may not apply to different populations around the world. Second, we did not assess the effects of the IABP in a randomized study, but with a post hoc analysis of consecutive patients using propensity score matching to account for imbalances between groups. Therefore, it cannot be excluded that some confounders not considered in the matching process could be unevenly distributed between the two groups in this way, affecting the results of the study. Third, we did not systematically collect data regarding serum lactates, the length of IABP use and the timing of IABP insertion (i.e., before or after the procedure); nevertheless, this last variable did not have an impact on prognosis in several studies [35,36]. Fourth, at the time of data gathering we did not use other percutaneous MCS devices such as Impella in our center, thus we could not make comparisons between patients treated with different MCS devices.
5. Conclusions
The present analysis shows that the use of the IABP in patients with STEMI and CS is associated with a reduced short-term mortality in patients with anterior infarction, whereas it has no effect on the outcome in patients presenting with non-anterior infarction. Future prospective randomized trials aiming to evaluate the prognostic role of IABP use in patients with CS due to myocardial infarction should focus on this specific high-risk subset of patients.
Author Contributions
Conceptualization, A.S., S.C., S.L., M.F., G.C., S.D.S. and G.M.D.F.; Methodology, A.S., S.C., A.D., A.M.-M., F.F., M.F., G.C. and R.C.; Software, A.S., S.C., A.D., A.M.-M., F.F., M.F., G.C. and R.C.; Validation, S.L., M.G., L.O.V., S.D.S. and G.M.D.F.; Formal Analysis, A.S., S.C., A.D., A.M.-M., F.F., M.F., G.C. and R.C.; Investigation, A.S., S.C., A.D., A.M.-M., F.F., M.F., G.C. and R.C.; Resources, S.L., M.G., L.O.V., S.D.S. and G.M.D.F.; Data Curation, A.S., S.C., A.D., A.M.-M., F.F., M.F., G.C. and R.C.; Writing—Original Draft Preparation, A.S. and S.C.; Writing—Review and Editing, S.L., M.G., L.O.V., S.D.S., G.M.D.F., A.D., A.M.-M., F.F., M.F., G.C. and R.C.; Visualization, A.S. and S.C.; Supervision, S.L., M.G., L.O.V., S.D.S. and G.M.D.F.; Project Administration, S.L., M.G., L.O.V., S.D.S. and G.M.D.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Boar of IRCCS Policlinico San Matteo.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 2.Hochman J.S., Sleeper L.A., Webb J.G., Sanborn T.A., White H.D., Talley J.D., Buller C.E., Jacobs A.K., Slater J.N., Col J., et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N. Engl. J. Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 3.Panteleev O.O., Vyshlov E.V., Kercheva M.A., Ryabov V.V. Analysis of results from intra-aortic balloon pump counterpulsation in patients with myocardial infarction and cardiogenic shock. Sib. J. Clin. Exp. Med. 2022;37:21–27. doi: 10.29001/2073-8552-2022-37-2-21-27. [DOI] [Google Scholar]
- 4.Kimman J.R., Van Mieghem N.M., Endeman H., Brugts J.J., Constantinescu A.A., Manintveld O.C., Dubois E.A., den Uil C.A. Mechanical support in early cardiogenic shock: What is the role of intra-aortic balloon counterpulsation? Curr. Heart Fail. Rep. 2020;17:247–260. doi: 10.1007/s11897-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babaev A., Frederick P.D., Pasta D.J., Every N., Sichrovsky T., Hochman J.S., NRMI Investigators Trends in Management and Outcomes of Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA. 2005;294:448. doi: 10.1001/jama.294.4.448. [DOI] [PubMed] [Google Scholar]
- 6.Mandawat A., Rao S.V. Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock. Circ. Cardiovasc. Interv. 2017;10:4337. doi: 10.1161/CIRCINTERVENTIONS.116.004337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosentino N., Resta M.L., Somaschini A., Campodonico J., D’aleo G., Di Stefano G., Lucci C., Moltrasio M., Bonomi A., Cornara S., et al. ST-Segment Elevation Acute Myocardial Infarction Complicated by Cardiogenic Shock: Early Predictors of Very Long-Term Mortality. J. Clin. Med. 2021;10:2237. doi: 10.3390/jcm10112237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossini R., Valente S., Colivicchi F., Baldi C., Caldarola P., Chiappetta D., Cipriani M., Ferlini M., Gasparetto N., Gilardi R., et al. ANMCO POSITION PAPER: Role of Intra-Aortic Balloon Pump in Patients with Acute Advanced Heart Failure and Cardiogenic Shock. Eur. Heart J. Suppl. 2021;23:C204–C220. doi: 10.1093/eurheartj/suab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rihal C.S., Naidu S.S., Givertz M.M., Szeto W.Y., Burke J.A., Kapur N.K., Kern M., Garratt K.N., Goldstein J.A., Dimas V., et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care. J. Am. Coll. Cardiol. 2015;65:e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Kantrowitz A., Tjønneland S., Freed P.S., Phillips S.J., Butner A.N., Sherman J.L. Initial Clinical Experience With Intraaortic Balloon Pumping in Cardiogenic Shock. JAMA J. Am. Med. Assoc. 1968;203:113–118. doi: 10.1001/jama.1968.03140020041011. [DOI] [PubMed] [Google Scholar]
- 11.Sjauw K.D., Engström A.E., Vis M.M., Van Der Schaaf R.J., Baan J., Koch K.T., De Winter R.J., Piek J.J., Tijssen J.G.P., Henriques J.P.S. A Systematic Review and Meta-Analysis of Intra-Aortic Balloon Pump Therapy in ST-Elevation Myocardial Infarction: Should We Change the Guidelines? Eur. Heart J. 2009;30:459–468. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 12.Thiele H., Zeymer U., Neumann F.J., Ferenc M., Olbrich H.G., Hausleiter J., Richardt G., Hennersdorf M., Empen K., Fuernau G., et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 13.Combes A., Price S., Slutsky A.S., Brodie D. Temporary Circulatory Support for Cardiogenic Shock. Lancet. 2020;396:199–212. doi: 10.1016/S0140-6736(20)31047-3. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad Y., Sen S., Shun-Shin M.J., Ouyang J., Finegold J.A., Al-Lamee R.K., Davies J.E.R., Cole G.D., Francis D.P. Intra-Aortic Balloon Pump Therapy for Acute Myocardial Infarction: A Meta-Analysis. JAMA Intern. Med. 2015;175:931–939. doi: 10.1001/jamainternmed.2015.0569. [DOI] [PubMed] [Google Scholar]
- 15.Gelsomino S., Johnson D.M., Lorusso R. Intra-Aortic Balloon Pump: Is the Tide Turning? Crit. Care. 2018;18:345. doi: 10.1186/s13054-018-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawranek M., Gierlotka M., Pres D., Zembala M., Ga M. Nonroutine Use of Intra-Aortic Balloon Pump in Cardiogenic Shock Complicating Myocardial Infarction With Successful and Unsuccessful Primary Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2018;11:1885–1893. doi: 10.1016/j.jcin.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Gul B., Bellumkonda L. Usefulness of Intra-Aortic Balloon Pump in Patients With Cardiogenic Shock. Am. J. Cardiol. 2019;123:750–756. doi: 10.1016/j.amjcard.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 18.van Nunen L.X., van ’t Veer M., Schampaert S., Rutten M.C.M., van de Vosse F.N., Patel M.R., Pijls N.H.J. Intra-Aortic Balloon Counterpulsation Reduces Mortality in Large Anterior Myocardial Infarction Complicated by Persistent Ischaemia: A CRISP-AMI Substudy. EuroIntervention. 2015;11:286–292. doi: 10.4244/EIJY14M09_10. [DOI] [PubMed] [Google Scholar]
- 19.Patel M.R., Smalling R.W., Thiele H., Barnhart H.X., Zhou Y., Chandra P., Chew D., Cohen M., French J., Perera D., et al. Intra-Aortic Balloon Counterpulsation and Infarct Size in Patients with Acute Anterior Myocardial Infarction without Shock: The CRISP AMI Randomized Trial. JAMA J. Am. Med. Assoc. 2011;306:1329–1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 20.Marenzi G., Cosentino N., Moltrasio M., Rubino M., Crimi G., Buratti S., Grazi M., Milazzo V., Somaschini A., Camporotondo R., et al. Acute Kidney Injury Definition and In-Hospital Mortality in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction. J. Am. Heart Assoc. 2016;5:e003522. doi: 10.1161/JAHA.116.003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesebro J.H., Knatterud G., Roberts R., Borer J., Cohen L.S., Dalen J., Dodge H.T., Francis C.K., Hillis D., Ludbrook P., et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A Comparison between Intravenous Tissue Plasminogen Activator and Intravenous Streptokinase* Clinical Findings through Hospital Discharge. Circulation. 1987;76:142–154. doi: 10.1161/01.CIR.76.1.142. [DOI] [PubMed] [Google Scholar]
- 22.van’t Hof A.W., ten Berg J., Heestermans T., Dill T., Funck R.C., van Werkum W., Dambrink J.H.E., Suryapranata H., van Houwelingen G., Ottervanger J.P., et al. Prehospital Initiation of Tirofiban in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Angioplasty (On-TIME 2): A Multicentre, Double-Blind, Randomised Controlled Trial. Lancet. 2008;372:537–546. doi: 10.1016/S0140-6736(08)61235-0. [DOI] [PubMed] [Google Scholar]
- 23.Montalescot G., Van’t Hof A.W., Lapostolle F., Silvain J., Flensted Lassen J., Bolognese L., Cantor W.J., Cequier Á., Chettibi M., Goodman S.G., et al. Prehospital Ticagrelor in ST-Segment Elevation Myocardial Infarction. N. Engl. J. Med. 2014;371:1016–1043. doi: 10.1056/NEJMoa1407024. [DOI] [PubMed] [Google Scholar]
- 24.Somaschini A., Cornara S., Ferlini M., Crimi G., Camporotondo R., Gnecchi M., Ferrario Ormezzano M., Oltrona Visconti L., De Ferrari G.M., De Servi S. Favorable Effect of Glycoprotein IIbIIIa Inhibitors among STEMI Patients Treated with Primary PCI and Incomplete ST Resolution. Platelets. 2020;31:48–54. doi: 10.1080/09537104.2018.1562171. [DOI] [PubMed] [Google Scholar]
- 25.Dhruva S.S., Ross J.S., Mortazavi B.J., Hurley N.C., Krumholz H.M., Curtis J.P., Berkowitz A., Masoudi F.A., Messenger J.C., Parzynski C.S., et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-Aortic Balloon Pump with In-Hospital Mortality and Major Bleeding among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA J. Am. Med. Assoc. 2020;323:734–745. doi: 10.1001/jama.2020.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seyfarth M., Sibbing D., Bauer I., Fröhlich G., Bott-Flügel L., Byrne R., Dirschinger J., Kastrati A., Schömig A. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. J. Am. Coll. Cardiol. 2008;4:1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 27.Schrage B., Ibrahim K., Loehn T., Werner N., Sinning J.M., Pappalardo F., Pieri M., Skurk C., Lauten A., Landmesser U., et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock: Matched-Pair Iabp-Shock II Trial 30-Day Mortality Analysis. Circulation. 2019;139:1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614. [DOI] [PubMed] [Google Scholar]
- 28.Ouweneel D.M., Eriksen E., Seyfarth M., Henriques J.P.S. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump for Treating Cardiogenic Shock: Meta-Analysis. J. Am. Coll. Cardiol. 2017;24:358–360. doi: 10.1016/j.jacc.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Kuno T., Takagi H., Ando T., Kodaira M., Numasawa Y., Fox J., Bangalore S. Safety and Efficacy of Mechanical Circulatory Support with Impella or intra-aortic Balloon Pump for high-risk Percutaneous Coronary Intervention and/or Cardiogenic Shock: Insights from a Network meta-analysis of Randomized Trials. Catheter. Cardiovasc. Interv. 2020;97:E636–E645. doi: 10.1002/ccd.29236. [DOI] [PubMed] [Google Scholar]
- 30.Thiele H., Zeymer U., Neumann F.-J., Ferenc M., Olbrich H.-G., Hausleiter J., de Waha A., Richardt G., Hennersdorf M., Empen K., et al. Intra-Aortic Balloon Counterpulsation in Acute Myocardial Infarction Complicated by Cardiogenic Shock (IABP-SHOCK II): Final 12 Month Results of a Randomised, Open-Label Trial. Lancet. 2013;382:1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 31.Thiele H., Zeymer U., Thelemann N., Neumann F.J., Hausleiter J., Abdel-Wahab M., Meyer-Saraei R., Fuernau G., Eitel I., Hambrecht R., et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation. 2019;139:395–403. doi: 10.1161/CIRCULATIONAHA.118.038201. [DOI] [PubMed] [Google Scholar]
- 32.Hanlon-Pena P.M., Quaal S.J. Intra-Aortic Balloon Pump Timing: Review of Evidence Supporting Current Practice. Am. J. Crit. Care. 2011;20:323–334. doi: 10.4037/ajcc2011542. [DOI] [PubMed] [Google Scholar]
- 33.Kapur N.K., Alkhouli M.A., DeMartini T.J., Faraz H., George Z.H., Goodwin M.J., Hernandez-Montfort J.A., Iyer V.S., Josephy N., Kalra S., et al. Unloading the Left Ventricle Before Reperfusion in Patients With Anterior ST-Segment–Elevation Myocardial Infarction. Circulation. 2019;139:337–346. doi: 10.1161/CIRCULATIONAHA.118.038269. [DOI] [PubMed] [Google Scholar]
- 34.De Silva K., Lumley M., Kailey B., Alastruey J., Guilcher A., Asrress K.N., Plein S., Marber M., Redwood S., Perera D. Coronary and Microvascular Physiology during Intra-Aortic Balloon Counterpulsation. JACC Cardiovasc. Interv. 2014;7:631–640. doi: 10.1016/j.jcin.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Fuernau G., Ledwoch J., Desch S., Eitel I., Thelemann N., Jung C., de Waha-Thiele S., Pöss J., Feistritzer H.-J., Freund A., et al. Impact of Timing of Intraaortic Balloon Counterpulsation on Mortality in Cardiogenic Shock—A Subanalysis of the IABP-SHOCK II Trial. Eur. Heart J. Acute Cardiovasc. Care. 2020;10:54–61. doi: 10.1177/2048872620930509. [DOI] [PubMed] [Google Scholar]
- 36.Cheng J.M., van Leeuwen M.A.H., de Boer S.P.M., Wai M.C.G.T.J., den Uil C.A., Jewbali L.S.D., van Geuns R.-J., Kardys I., van Domburg R.T., Boersma E., et al. Impact of Intra-Aortic Balloon Pump Support Initiated before versus after Primary Percutaneous Coronary Intervention in Patients with Cardiogenic Shock from Acute Myocardial Infarction. Int. J. Cardiol. 2013;168:3758–3763. doi: 10.1016/j.ijcard.2013.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.