Abstract
As is the case for Saccharomyces boulardii, Saccharomyces cerevisiae W303 protects Fisher rats against cholera toxin (CT). The addition of glucose or dinitrophenol to cells of S. boulardii grown on a nonfermentable carbon source activated trehalase in a manner similar to that observed for S. cerevisiae. The addition of CT to the same cells also resulted in trehalase activation. Experiments performed separately on the A and B subunits of CT showed that both are necessary for activation. Similarly, the addition of CT but not of its separate subunits led to a cyclic AMP (cAMP) signal in both S. boulardii and S. cerevisiae. These data suggest that trehalase stimulation by CT probably occurred through the cAMP-mediated protein phosphorylation cascade. The requirement of CT subunit B for both the cAMP signal and trehalase activation indicates the presence of a specific receptor on the yeasts able to bind to the toxin, a situation similar to that observed for mammalian cells. This hypothesis was reinforced by experiments with 125I-labeled CT showing specific binding of the toxin to yeast cells. The adhesion of CT to a receptor on the yeast surface through the B subunit and internalization of the A subunit (necessary for the cAMP signal and trehalase activation) could be one more mechanism explaining protection against the toxin observed for rats treated with yeasts.
Viable yeast cells were recently used to improve the resistance of the intestinal ecosystem to bacterial infection (4, 16). The nonpathogenic yeast Saccharomyces boulardii (12), which is widely used in many countries for the treatment of antibiotic-induced gastrointestinal disorders (18) and Clostridium difficile-associated enterocolopathies (2), has been extensively studied. Controlled clinical trials have also demonstrated this activity in the treatment of various types of enteric syndromes, such as acute infantile gastroenteritis (3) and diarrhea associated with continuous-flow enteral nutrition (17).
Several possible mechanisms for the protective effect of S. boulardii against infections by either indigenous gastrointestinal microbiota or recently acquired exogenous microbes (1) have been proposed, mainly based on results from studies with experimental animals. One of these hypotheses is related to the inhibition of bacterial toxin production or action. Experimentally, S. boulardii inhibits C. difficile toxin A binding and enterotoxicity in rat ileum (14). The same yeast also inhibits or neutralizes the enterotoxicity of Escherichia coli toxins and Vibrio cholerae toxin (7, 11, 23). Recent results have shown that S. boulardii produces a 120-kDa protein able to neutralize the effect of cholera toxin (CT) (6). The mechanism of this toxin-neutralizing effect may be related to the ability of a protein from the yeast to bind to a receptor that in turn regulates intracellular adenylate cyclase levels. An additional mechanism may be specific adhesion of the toxin to the yeast.
The 84-kDa V. cholerae toxin, which is functionally, structurally, and immunologically similar to E. coli heat-labile enterotoxin, is composed of the catalytically active A subunit and five identical B subunits that constitute the binding region of the toxin. Binding of the CT to ganglioside receptors (GM1) of enterocyte microvilli is followed by the internalization of subunit A, which catalyzes the activation of adenylate cyclase, causing a rise in cyclic AMP (cAMP) levels that triggers active secretion of chloride and bicarbonate in crypt cells and inhibits chloride absorption in the villi. Since water flows passively with electrolytes in response to osmotic gradients, CT causes the cessation of the absorption of water through villi and the amplification of water secretion from crypt cells, resulting in copious diarrhea (9).
In the yeast Saccharomyces cerevisiae, the addition of rapidly fermented sugar to cells growing on a nonfermentable carbon source is known to trigger a cAMP-mediated protein phosphorylation cascade. The addition of glucose causes a rapid, transient increase in cAMP levels, followed by the activation of trehalase and other enzymes known to be regulated by cAMP-dependent protein phosphorylation. The effect of glucose can be mimicked by the addition of protonophores such as dinitrophenol (DNP) at a low external pH. The normal physiological function of the glucose-induced cAMP-mediated protein phosphorylation cascade is to switch metabolism from gluconeogenic-respirative to fermentative (22).
In the present study, we hypothesized that CT is able to adhere specifically to the surfaces of different yeasts, and we assessed in vivo some of the biochemical effects of this adhesion on microorganisms.
MATERIALS AND METHODS
Animals and treatments.
Litters of male Fisher rats weighing about 40 g (Department of Nutrition, Federal University of Ouro Preto, Ouro Preto, Brazil) were used. The animals were divided at random into experimental and control groups soon after weaning. S. cerevisiae W303 production was carried out with a bench-top fermentor (model MF 114; New Brunswick Scientific Co., Edison, N.J.). During the operation, aeration, agitation, and temperature were adjusted to 1 volume of air per volume of medium per min (vvm), 600 rpm, and 30°C, respectively. Stationary-phase cells were harvested by centrifugation and thoroughly washed with distilled water. The biomass was resuspended in saline to obtain about 2 × 108 CFU/ml, and aliquots of 0.5 ml were administered to the experimental group animals by gastric gavage three times a day. The animals in the control group received saline according to the same schedule. Five days after the beginning of these treatments, an 18-h culture of V. cholerae (recently isolated from a clinical case at Fundação Ezequiel Dias, Belo Horizonte, Brazil) that had been incubated at 37°C in brain heart infusion (108 CFU/ml) was inoculated by gastric intubation into both experimental and control group animals. After the bacterial challenge, treatments with yeast suspension or saline were continued for an additional 5 days. By days 2 and 5 of infection (corresponding to 7 and 10 days of treatments, respectively), five animals from each group were sacrificed by ether inhalation. Liver, spleen, mesenteric lymph node, small intestine (upper, middle, and lower), and colon (middle) samples were obtained and fixed in 10% neutral formalin. The fixed tissue was dehydrated, embedded in paraffin, cut into 7-μm-thick sections, and stained by the routine hematoxylin and eosin method. The slides were codified and examined by only one pathologist, who did not have access to the experimental conditions for each group. After the report had been written, the material was decodified.
Yeast strains and growth conditions.
S. boulardii (Floratil; Merck S.A., Rio de Janeiro, Brazil) and S. cerevisiae W303 strains were grown in a rotary incubator (200 rpm) at 30°C in YPG medium, containing 1% (wt/vol) yeast extract, 2% (wt/vol) peptone, and 3% (vol/vol) glycerol. Cells in the logarithmic phase were harvested by centrifugation at 3,000 × g for 5 min, washed three times with distilled water, and resuspended in 100 mM morpholineethanesulfonic acid–KOH buffer (pH 6.0). The cell concentration was 20 mg (wet mass)/ml in all experiments.
Incubation and extraction conditions.
For measurement of trehalase activity and intracellular cAMP levels, the cells were incubated in a shaking water bath at 30°C. Two samples were taken at 15-min intervals for the determination of basal trehalase activity and intracellular cAMP levels before the addition of 100 mM glucose, 2 mM DNP, different toxin concentrations, or toxin subunits A and B (Sigma Chemical Co., St. Louis, Mo.). Cells and crude extract preparations were sampled by the method of Thevelein and Beullens (20).
Determination of trehalase activity and cAMP and protein concentrations.
Trehalase activity and cAMP and protein concentrations in crude extracts were determined as described by Thevelein and Jones (19), Thevelein et al. (21), and Lowry et al. (10), respectively. All experiments were performed at least twice, with consistent results. Representative results are shown.
Specific binding of 125I-labeled CT to yeast cells.
The CT was iodinated by the chloramine-T method according to Cuatrecasas (5) with some modifications. Free 125I was separated by chromatography on a 3-ml Sephadex G-50 column. The total radioactivity incorporated into protein was determined with a gamma counter after paper chromatography (Whatman no. 1) by use of methanol saturated with KI.
S. boulardii cells (104 cells/ml) were incubated for 30 min with 0.1 nM 125I-CT (3.4 Ci/mmol) at room temperature in a mixture containing 500 μl of 140 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 1.8 mM CaCl2, 10 mM glucose, 25 mM HEPES (pH 7.4), and 0.2% bovine serum albumin. Bound and free radiolabeled ligands were separated after incubation by centrifugation at 12,000 × g for 10 min in an Eppendorf Microfuge. The pellets were collected and washed two times with 1 ml of cold buffer (same composition as the mixture described above but with 0.5% albumin), and the radioactivity was measured by gamma counting. Nonspecific binding was determined by incubation of the yeast cells with an excess of unlabeled CT (0.1 μM) before addition of the iodinated protein. Total and nonspecific binding experiments were done in triplicate. Specific binding indicates the amount of 125I-CT which could be displaced by unlabeled CT and was calculated as the difference between total and nonspecific binding values.
Statistics.
Data for the stimulation of trehalase activity were evaluated statistically by a one-way analysis of variance, and means were compared by use of the least significant difference. Statistical analysis was performed with EPISTAT software (T. L. Gustafson, Round Rock, Tex.).
RESULTS
After challenge with V. cholerae, more severe histological changes were observed in animals not treated with S. cerevisiae W303 than in treated rats. Greater alterations were found in the small intestine than in other organs, i.e., severe degenerative and necrotic changes in the superficial epithelium, with karyorrhectic cells being found in the lumen. Significant reductions in the lymphoid component of the lamina propria and in mitotic activity were also noted in all portions of the small intestine. In treated animals, histological evidence of damage was minimal or absent, and a marked expansion of the lymphoid component of the intestinal lamina propria was observed.
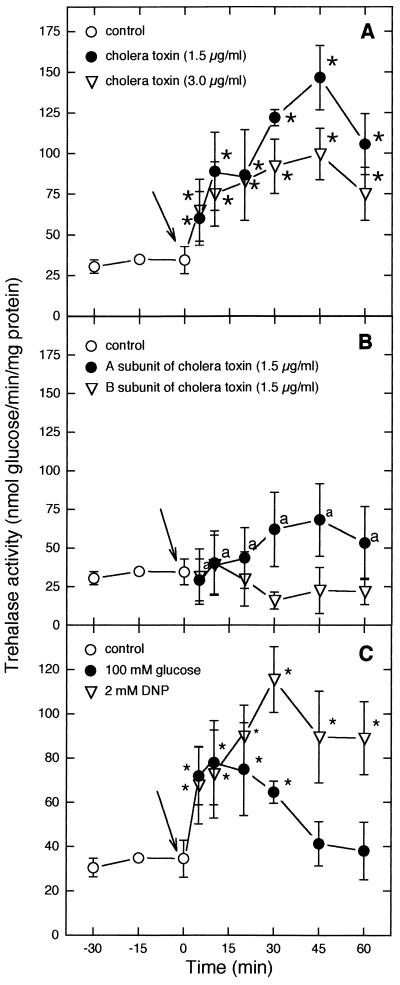
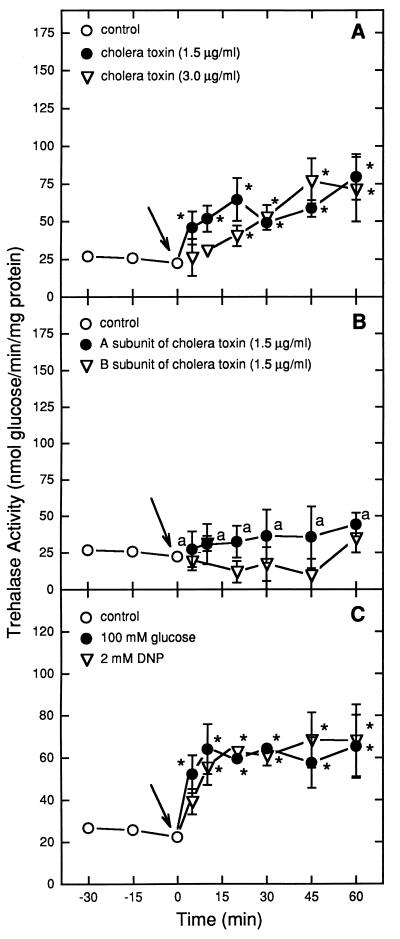
The results in Fig. 1 and 2 show that the addition of glucose or DNP to S. boulardii cells grown on a nonfermentable carbon source caused trehalase activation (Fig. 1C) in a manner similar to that observed for S. cerevisiae W303 (Fig. 2C). On the other hand, the addition of CT to S. boulardii or S. cerevisiae W303 cells obtained under the same conditions also resulted in the stimulation of trehalase activity (Fig. 1A and 2A). Greater stimulation of trehalase activity by CT was observed in S. boulardii than in S. cerevisiae W303 (P < 0.05). The separate addition of CT subunit A or B to S. boulardii or S. cerevisiae W303 cells did not cause trehalase activation (P > 0.05) (Fig. 1B and 2B).
FIG. 1.
Effects of 1.5 and 3.0 μg of CT per ml (A), of the A or B subunit of CT (B), or of 100 mM glucose and 2 mM DNP (C) on trehalase activity in S. boulardii. Specific activity is expressed as nanomoles of glucose released per minute per milligram of protein. Arrows indicate the time of addition of the tested compounds. Asterisks indicate significant differences for trehalase activities after the addition of CT (1.5 and 3.0 μg/ml), subunit (A and B), 100 mM glucose, or 2 mM DNP in comparison with basal trehalase activities (P < 0.05). a’s indicate significant differences for trehalase activities after the addition of CT subunit A in comparison with values after the addition of CT (1.5 μg/ml) and for the same incubation time (P < 0.05).
FIG. 2.
Effect of 1.5 and 3.0 μg of CT per ml (A), of the A or B subunit of CT (B), or of 100 mM glucose and 2 mM DNP (C) on trehalase activity in S. cerevisiae W303. Specific activity is expressed as nanomoles of glucose released per minute per milligram of protein. Arrows indicate the time of addition of the tested compounds. Asterisks indicate significant differences for trehalase activities after the addition of CT (1.5 and 3.0 μg/ml), subunit (A and B), 100 mM glucose, or 2 mM DNP in comparison with basal trehalase activities (P < 0.05). a’s indicate significant differences for trehalase activities after the addition of CT subunit A in comparison with values after the addition of CT (1.5 μg/ml) and for the same incubation time (P < 0.05).
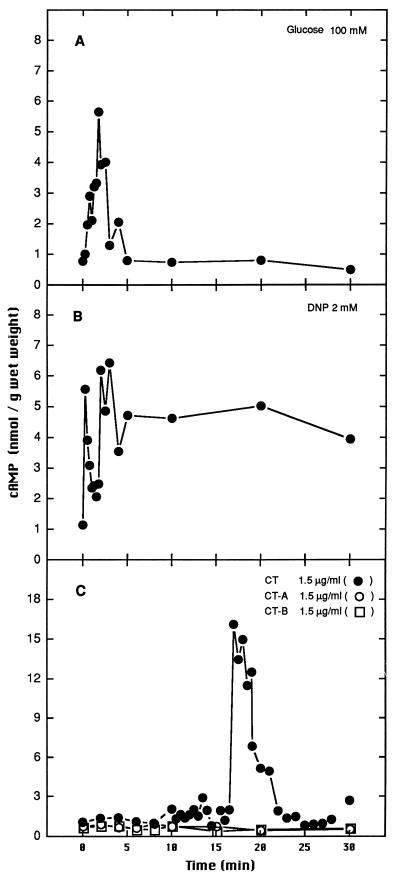
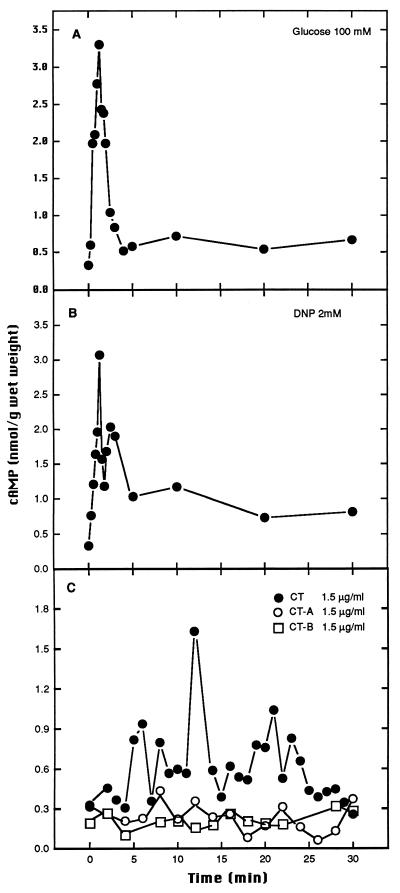
The addition of glucose, DNP, or CT to S. boulardii or S. cerevisiae W303 cells induced a transient increase in the cAMP level (Fig. 3 and 4). However, this increase in the cAMP level was observed at different times, depending on the inducting agent added (0 to 5 min for glucose and DNP or 15 to 20 min for CT). As was observed for trehalase stimulation, a greater transient increase in cAMP levels was induced by CT in S. boulardii than in S. cerevisiae W303. cAMP levels were not altered when subunit A or B was added individually (Fig. 3C and 4C).
FIG. 3.
cAMP signalling induced by 100 mM glucose (A), by 2 mM DNP (B), or by 1.5 μg of CT or of the A or B subunit of CT per ml (C) in S. boulardii.
FIG. 4.
cAMP signalling induced by 100 mM glucose (A), by 2 mM DNP (B), or by 1.5 μg of CT or of the A or B subunit of CT per ml (C) in S. cerevisiae W303.
When CT was iodinated, about 60% of the radioactivity was incorporated into protein, and the specific radioactivity of the toxin was 3.4 Ci/mmol. Binding to the Eppendorf tubes in the absence of yeast cells was less than 2% of the assay values. Specific binding of CT represented 48% of total binding.
DISCUSSION
Although the antidiarrheic properties of S. boulardii are widely recognized, this yeast has been prescribed on an empirical basis, and the exact mechanism of its protective effect is unknown. Recent results have shown that other yeasts can also be used for the treatment of enteric disorders (4, 16). The pharmacodynamics of S. boulardii involve three different hypothetical aspects: (i) a direct antagonistic effect (15); (ii) a trophic effect, with stimulation of enzymatic expression (2) and of intestinal defense mechanisms (12, 13); and (iii) an antisecretory effect, with action on the binding of toxins to intestinal receptors. As an example of the last aspect, S. boulardii significantly reduced the liquid secretion and permeability for mannitol caused by toxin A of C. difficile in rat ileum (compared with a control group). This effect could be explained by the production of a 54-kDa protease which digested both toxin A and its receptor on enterocytes in vitro (14). S. boulardii also produces a 120-kDa protein which does not have proteolytic activity and which reduces the formation of cAMP by intestinal cells in a medium to which CT or E. coli thermolabile toxin has been added (6). Specific toxin adhesion to the yeast surface may be another mechanism responsible for this phenomenon; this mechanism was proposed in this work with CT as a toxin model. If specific receptors for CT existed on the S. boulardii membrane, they probably would be structurally and functionally similar to the enterocyte GM1 system. In this case, CT fixation would trigger the same intracellular signal as for enterocytes through the cAMP-mediated protein phosphorylation cascade. As a consequence, stimulation of cAMP-dependent enzymatic systems, such as trehalase, would be expected. If this mechanism is present in S. boulardii, it should be found in other yeasts, such as S. cerevisiae.
The protective effect of S. boulardii treatment against CT in rats (7) was also observed when S. cerevisiae W303 was used. These experimental results, taken together with clinical data (4, 16), suggest that this protective property is shared by yeasts in general.
The addition of CT to S. boulardii and S. cerevisiae W303 cells resulted in trehalase activation (Fig. 1A and 2A) and a cAMP signal (Fig. 3C and 4C). Moreover, this induction depended on CT integrity. The requirement of subunit B could be explained by the presence in the yeasts of a specific receptor able to bind the toxin, i.e., a situation similar to that observed in mammalian cells. Preliminary data obtained with 125I-labeled CT demonstrated the presence of a specific binding site on yeast cell surfaces. This similarity of CT-specific binding on mammalian and yeast cells was also reinforced by the observation of a kinetically correlated delay in both trehalase activation and cAMP signal induction by CT in comparison with glucose and DNP. For intestinal epithelial cells and after CT binding, there is also a lag of 15 to 60 min before adenylate cyclase is activated. This time lag is necessary to allow the A1 peptide to translocate through the membrane and come into contact with the G proteins (8). Higher levels of trehalase activity stimulation and higher cAMP levels triggered by CT in S. boulardii than in S. cerevisiae may be related to more numerous or active CT receptors on the surface of the former yeast. If such a situation is confirmed, more efficient CT inactivation by S. boulardii would be expected.
Taken together, these results suggest that CT (and probably other toxins) could be neutralized by binding to the yeast surface when the A subunit is internalized, triggering the stimulation of different biochemical systems, such as a cAMP signal and trehalase activity. The data show that trehalase stimulation by CT probably occurs through part of the cAMP-mediated protein phosphorylation cascade. The surface receptor for CT and the biochemical pathway for its activation of trehalase in yeasts are currently being investigated in our laboratories.
ACKNOWLEDGMENTS
This research was supported by Merck S.A. (Amadeu Gonçalves, Rio de Janeiro, Brazil), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (CBS 172/92), Conselho Nacional de Desenvolvimento Científico e Tecnológico (522177/94-8), and Pró-Reitoria da Universidade Federal de Minas Gerais (23072.040800/95-50).
REFERENCES
- 1.Berg R D. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 2.Buts J P, Corthier G, Delmee M. Saccharomyces boulardii for Clostridium difficile-associated enteropathies in infants. J Pediatr Gastroenterol Nutr. 1993;16:419–425. doi: 10.1097/00005176-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Chapoy P. Traitement des diarrhées aigües infantiles: essai controlé de Saccharomyces boulardii. Ann Pediatr. 1985;32:561–563. [PubMed] [Google Scholar]
- 4.Chia J K S, Chan S M, Goldstein H. Baker’s yeast as adjunctive therapy for relapses of Clostridium difficile diarrhea. Clin Infect Dis. 1995;20:1581. doi: 10.1093/clinids/20.6.1581. [DOI] [PubMed] [Google Scholar]
- 5.Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973;12:3547–3557. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- 6.Czerucka D, Roux I, Rampal P. Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3′,5′-cyclic monophosphate induction in intestinal cells. Gastroenterology. 1994;106:65–72. doi: 10.1016/s0016-5085(94)94403-2. [DOI] [PubMed] [Google Scholar]
- 7.Dias R S, Bambirra E A, Silva M E, Nicoli J R. Protective effect of Saccharomyces boulardii against the cholera toxin in rats. Braz J Med Biol Res. 1995;28:323–325. [PubMed] [Google Scholar]
- 8.Gill D M, King C A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975;250:6424–6432. [PubMed] [Google Scholar]
- 9.Kaper J B, Fasano A, Trucksis M. Toxins of Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: American Society for Microbiology; 1994. pp. 145–176. [Google Scholar]
- 10.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 11.Massot J, Desconclois M, Astoin J. Protection par Saccharomyces boulardii de la diarrhée àEscherichia coli du souriceau. Ann Pharm Fr. 1982;40:13–15. [PubMed] [Google Scholar]
- 12.McFarland L V, Bernasconi P. Saccharomyces boulardii: a review of an innovative biotherapeutic agent. Microb Ecol Health Dis. 1993;6:157–171. [Google Scholar]
- 13.Peret Filho, L. A., F. J. Penna, E. A. Bambirra, and J. R. Nicoli. Dose effect of oral Saccharomyces boulardii treatments on morbidity and mortality in immunosuppressed mice. J. Med. Microbiol., in press. [DOI] [PubMed]
- 14.Pouthoulakis C, Kelly C P, Joshi M A, Gao N, O’Keane C J, Castiglioulo I, Lamont J T. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology. 1993;104:1108–1115. doi: 10.1016/0016-5085(93)90280-p. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues A C P, Nardi R M, Bambirra E A, Vieira E C, Nicoli J R. Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol. 1996;81:251–256. doi: 10.1111/j.1365-2672.1996.tb04325.x. [DOI] [PubMed] [Google Scholar]
- 16.Schellenberg D, Bonington A, Champion C M, Lancaster R, Webb S, Main J. Treatment of Clostridium difficile diarrhoea with brewers yeast. Lancet. 1994;343:171. doi: 10.1016/s0140-6736(94)90960-1. [DOI] [PubMed] [Google Scholar]
- 17.Schlotterer M, Bernasconi P, Lebreton F, Touraine J L. Intérêt de Saccharomyces boulardii dans la tolérance digestive de la nutrition entérale à débit continu chez de brulé. Nutr Clin Metab. 1987;1:31–34. [Google Scholar]
- 18.Surawicz C M, Elmer G W, Speelman P, McFarland L V, Chinn J, Van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 19.Thevelein J M, Jones K A. Reversibility characteristics of the glucose-induced trehalase activation associated with the breaking of dormancy in the yeast ascospores. Eur J Biochem. 1983;136:583–587. doi: 10.1111/j.1432-1033.1983.tb07780.x. [DOI] [PubMed] [Google Scholar]
- 20.Thevelein J M, Beullens M. Cyclic AMP and stimulation of trehalase activity in yeast Saccharomyces cerevisiae by carbon sources, nitrogen sources and inhibitor of protein synthesis. J Gen Microbiol. 1985;131:3199–3209. doi: 10.1099/00221287-131-12-3199. [DOI] [PubMed] [Google Scholar]
- 21.Thevelein J M, Beullens M, Honsshoven F, Hobeeck G, Detremerie K, Den Hollander J A, Jans S W H. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: intracellular pH and the effect of membrane depolarizing compounds. J Gen Microbiol. 1987;133:2191–2196. doi: 10.1099/00221287-133-8-2191. [DOI] [PubMed] [Google Scholar]
- 22.Thevelein J M. Fermentable sugars and intracellular acidification as specific activators of the RAS-adenylate cyclase signalling pathway in yeast: the relationship to nutrient-induced cell cycle control. Mol Microbiol. 1991;5:1301–1307. doi: 10.1111/j.1365-2958.1991.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 23.Vidon N, Huchet B, Rambaud J C. Influence de Saccharomyces boulardii sur la sécrétion induite chez le rat par la toxine cholérique. Gastroenterol Clin Biol. 1986;10:13–16. [PubMed] [Google Scholar]