Abstract
The effect of low temperatures on the survival, structure, and metabolism of Campylobacter coli SP10, a virulent strain, was investigated. C. coli became nonculturable rapidly at 20 and 10°C and slightly later at 4°C. Incubation in a microaerobic atmosphere improved survival, but after day 8, campylobacters were detectable by direct-count procedures only. The increase in the number of coccoid cells was most pronounced at 37°C but also was noticeable at 20 and 10°C. Two forms of coccoid cells were seen electron microscopically, but only one (20 and 10°C) seemed to be a degenerative form. The flagella were shorter at 20 and 10°C, a result which correlates well with the observed slight changes in the 62-kDa protein band. The fatty acid composition of bacterial cells was influenced significantly by low temperatures. An increase in the short-chain and unsaturated acids was noted; above all, a drastic increase in C19:0 cyc at 20°C with a concomitant decrease in C18:1 trans9,cis11 was seen. The concentrations of excreted metabolites were analyzed to obtain information on metabolic activity. Depending on the magnitude of the temperature downshift, the production of organic acids decreased, but it was always observable after a temperature-specific lag phase and regardless of ability to be cultured. Under optimal conditions, succinate, lactate, and acetate were the main metabolites, other acids being of less importance. The pattern changed significantly at lower temperatures. Succinate was never detected at 20°C and was only occasionally detected at 10 and 4°C. At the same time, fumarate concentrations, which are normally not detectable at 37°C, were highest at 20°C and reduced at 10 and 4°C. Inactivation of fumarate reductase was considered to be a possible explanation.
Campylobacters are playing an increasingly important role in gastrointestinal disease all over the world, and in some countries they are even more frequent than salmonellae. In industrialized countries, the annual incidence of enteritis caused by campylobacters is estimated to be 1%. In underdeveloped countries it is much higher, affects mainly small children and, in contrast to the situation in developed countries, is caused more often by Campylobacter coli than by C. jejuni (12, 43, 44). The natural reservoir of campylobacters is the intestine of warm-blooded animals; 24 to 82% of examined chicken populations, 58 to 95% of Scandinavian pigs, and 23% of slaughter cattle were campylobacter positive, to name only a few animal species (1, 9, 19, 41). Campylobacters, like other intestinal pathogens, are released into the environment via the feces of infected animals or humans. On average, raw sewage contains log 2 to 4 CFU of campylobacters per 100 ml, but concentrations can rise significantly if abattoirs or chicken farms are connected to the sewer system (18). Treated sewage may contain campylobacters at log 1 to 2 CFU/100 ml, and fresh, undisinfected sewage sludge, which is used as a fertilizer in some countries, may contain campylobacters at up to log 6 CFU/100 ml (2, 17). Sewage discharge into surface water and agricultural runoff can thus lead to the contamination of bathing water areas and drinking water reservoirs and can pose a significant health risk for the population. Not surprisingly, large waterborne outbreaks of campylobacter-induced enteritis have been reported (31, 45, 48).
Various surface waters have been examined in the past, and 25 to 95% were shown to be campylobacter positive, with campylobacter concentrations ranging from log 0.04 to log 2 CFU/100 ml. Detection rates were higher during the winter months, suggesting enhanced survival at low temperatures, although, apart from starvation, temperature downshift is one of the greatest stress factors that bacteria experience when they are released into the environment. While psychrophilic and psychrotrophic bacteria have adapted evolutionarily to life at low temperatures, human pathogens, being mesophilic bacteria, can be severely inhibited. In nutrient-rich media, reactions such as intracellular (p)ppGpp (guanosine 5′-triphosphate-3′-diphosphate and guanosine 5′-diphosphate-3′-diphosphate) synthesis or production of cold shock proteins have been shown to be dependent on the temperature shift (22). Changes in the composition of the cell membrane and inhibition of enzyme activities and membrane transport systems are further conceivable reactions of mesophilic bacteria to temperature downshift (29). To study the effect of low temperatures on campylobacters, a virulent C. coli strain was subjected to temperatures normally encountered in Central European aquatic systems. The influence on growth, morphology, membrane fluidity, proteins, and metabolism was examined.
MATERIALS AND METHODS
Test strain and culture conditions.
The test strain, C. coli SP10, originated from the feces of a diarrheic pig and was kindly provided by W. Bär, Medical University of Hannover, Hannover, Germany. With as few passages as possible, a stock culture was prepared, divided into aliquots, and maintained at −70°C in Schaedler broth (BBL; pancreatic digest of casein [8.1 g/liter], peptic digest of animal tissue [2.5 g/liter], papaic digest of soybean meal [1 g/liter], dextrose [5.82 g/liter], yeast extract [5 g/liter], sodium chloride [1.7 g/liter], dipotassium phosphate [0.82 g/liter], hemin [0.01 g/liter], l-cystine [0.4 g/liter], Tris [3 g/liter]). Vials were freshly thawed for each experiment. Unless otherwise stated, cultures were incubated microaerobically at 37°C in jars (Anaerocult C; Merck) or in appropriate incubators flushed with gas.
Survival curves for C. coli.
Thawed stock culture (0.1 ml) was plated on Schaedler agar (Pasteur Diagnostics; tryptic soy broth [10 g/liter], Polypeptone [5 g/liter], dextrose [5 g/liter], yeast extract [5 g/liter], Tris buffer [3 g/liter], hemin [0.01 g/liter], l-cystine [0.4 g/liter], agar [13.5 g/liter]). After 48 h, Schaedler broth was inoculated and incubation was continued for another 16 h. For the experiments, 0.1 ml was transferred to tubes containing 10 ml of Schaedler broth, and the tubes were incubated in the dark at 4, 10, 20, and 37°C. At regular intervals, colony counts were determined by plating decimal dilutions onto Schaedler agar. For each temperature and each incubation period, test tubes were inoculated in duplicate. In addition, according to the expected cell density, 1, 0.1, 0.01, or 0.001 ml was filtered through black polycarbonate filters (pore size, 0.2 μm; Millipore). Small volumes were diluted with 10 ml of 0.9% NaCl, and the total volume was filtered to ensure a homogeneous distribution of bacteria on the filters. The filters were stained with 0.01% acridine orange solution (Sigma) for 5 min and immediately analyzed, and the cell counts were recorded. The experiment was repeated with minor variations, i.e., 1 ml of preculture samples in bottles with tightly closing caps containing 200 ml of broth. The rest of the broth culture was used for electron microscopy.
Electron microscopy.
Broth cultures were centrifuged (20 min; 3,700 × g), and the supernatants were discarded. The pellets were negatively stained with 1% ammonium molybdate (pH 5.0) according to the method of Doane and Anderson (8) and examined with an Elmiskop I microscope (Siemens).
Fatty acid analysis.
To determine the fatty acid composition, C. coli was passaged twice in Schaedler broth and then incubated microaerobically at 4, 10, 20, and 37°C. Samples were analyzed after 0, 24, 48, and 72 h. For each temperature and each incubation period, five samples were examined in parallel and the experiment was repeated. To obtain data for shorter incubation times, the experiment was rerun and samples were analyzed in duplicate at hourly intervals during the first 8 h and then after 24, 48, and 72 h. Cells were harvested by centrifugation, and supernatants were discarded. Fatty acids from the cells were methylated and extracted as described by Miller and Berger (32). The system consists of a Hewlett-Packard HP 5890 II gas chromatograph with an Ultra 2 HP fused-silica capillary column, helium as a carrier gas, a flame ionization detector, and an automatic sampler. Integration of data was performed with the software package Maxima 3.2 (Waters), and fatty acids were identified by comparing their retention times with those of a known standard mixture (bacterial acid methylester CP mix; Matreya). Schaedler broth is a complex medium that contains small amounts of fatty acids. Blanks were examined in parallel, and corrections were made accordingly.
Analysis of OMPs.
Preculturing and subsequent passages were performed as described above (see fatty acid analysis). Cells were harvested by centrifugation, and the pellet was washed three times in 0.01 M Tris buffer. Due to experience gained in preliminary experiments, this suspension was passed three times through a French press (14,000 lb/in2; American Instruments Co., Inc.) and treated further according to the method of Blaser et al. (6). Separation of membranes was checked by analyzing the samples for the concentration of 2-keto-3-deoxyoctonate. The method of Osborn (38) was applied. The concentration of proteins was determined by the bicinchoninic acid test (Pierce), and the samples were adjusted to a protein concentration of 5 to 7 μg/ml. The outer membrane proteins (OMPs) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis according to the Laemmli method as modified by Jany (20). A two-step procedure was used for staining. Gels were immersed first in Serva-Blue G solution and then in Serva Brilliant Blue R 250 solution on a rotating platform.
Analysis of organic acids.
Metabolically active bacterial cells produce organic acids and subsequently excrete them into broth cultures. The organic acids can be analyzed by high-pressure liquid chromatography (HPLC). Preculturing, passages, and experimental setup were performed as described above (see fatty acid analysis). For each temperature and each incubation period, six samples were examined in parallel. In addition, colony counts were recorded. The HPLC method of Guerrant et al. (13), simplified by Krausse and Ullmann (25), was further modified. Briefly, broth cultures were acidified with 0.125 ml of 50% sulfuric acid, and organic acids were extracted twice with 1 ml of ether. The ether was evaporated in a vacuum centrifuge, and the organic acids were redissolved in 1.5 ml of water of analytical grade. These samples (0.25 ml) were injected by an automatic sampler (WISP 712; Waters), and 0.2% phosphoric acid was used as an eluent (flow rate, 1 ml/min). After passage through a guard column (Ionpak KC-810P; Shodex), the organic acids were separated on a cationic exchange column (Ionpak KC-811; Shodex) heated to 30°C. UV detection (210 nm) of acids followed; they were identified by comparison of their retention times with those of a known standard mixture prepared in-house. Blanks were examined in parallel, and corrections were made for the organic acid content of Schaedler broth.
Statistical analysis.
The gas chromatography and HPLC data were analyzed by a multivariate analysis of variance. The influence of temperature on each fatty acid and organic acid was determined with a multiple comparison and a Bonferroni adjustment (α = 0.05). Some data were additionally analyzed with the U test. The statistical calculations were performed with the software package Systat 5.2 (Systat, Inc.).
RESULTS
Effect of low temperatures on the survival of C. coli SP10.
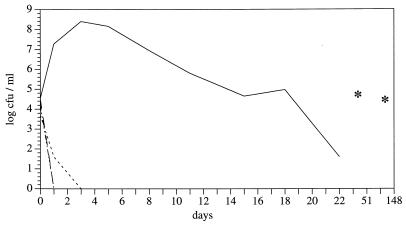
In microaerobically incubated batch cultures, a rapid decrease in colony counts was observed at all temperatures below 37°C. The effect was most pronounced at 20 and 10°C, where the colony counts were below the detection limit after incubation for 4 days. At 4°C, the decrease was slightly slower, and inability to be cultured was observed after 8 days. Similar data were obtained with other C. coli strains (data not shown). In batch cultures without gaseous interchange, the transition to the nonculturable state was even faster, but it was again more retarded at 4°C (Fig. 1). Cultures incubated at 37°C showed a slow decrease in colony counts to log 1.60 CFU/ml on day 22 but a slight increase later on to log 4.99 and log 4.71 CFU/ml on days 51 and 148, respectively. Direct cell counts corresponded fairly well to CFU at 37°C. They were significantly higher, however, than colony counts at low incubation temperatures. At the end of the experiment, the cell counts ranged between log 5.13 and log 5.93 CFU/ml, regardless of the incubation atmosphere.
FIG. 1.
Survival of C. coli SP10 at different incubation temperatures without gaseous interchange. ——, 37°C; —–—–, 20°C; –··–, 10°C, ––––, 4°C. Asterisks indicate log colony-forming units per milliliter (37°C) after 51 and 148 days.
Electron microscopy.
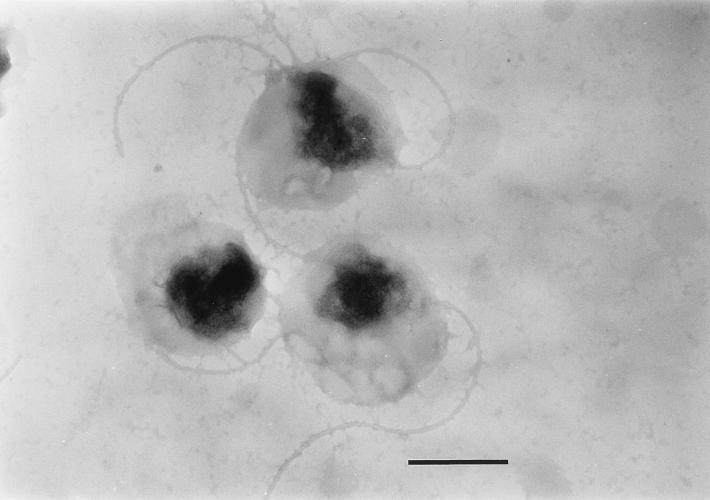
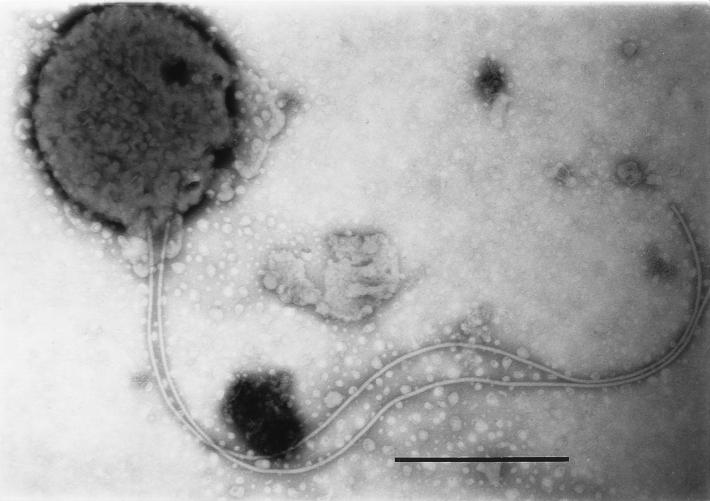
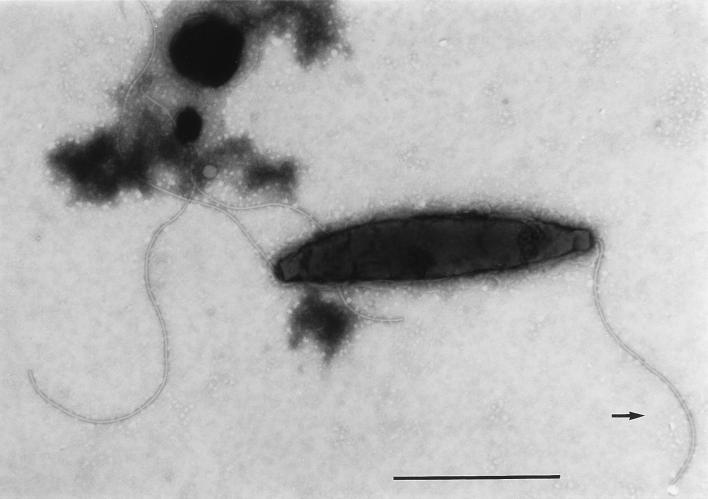
At the beginning of the experiments, nearly all campylobacters had their normal straight or spiral rod structure. During incubation, transition to the coccoid form occurred most rapidly in cultures incubated at 37°C. Depending on the magnitude of the temperature downshift, this transition was less pronounced at lower temperatures. At day 51, 200 bacterial cells were counted by shape with a light microscope, and the percentages of coccoid cells were 98% (37°C), 94% (20°C), 71% (10°C) and 4% (4°C). Electron microscopy revealed two kinds of coccoid cells. Large cells that were not easily stained were predominant at 10 and 20°C, whereas small, dense coccoid cells were typical for incubation at 4°C (Fig. 2 and 3). In addition to the different coccoid forms, assay mixtures that were incubated at 10 and 20°C contained many cells with shortened flagella (Fig. 4).
FIG. 2.
C. coli SP10 degenerative coccoid forms. Incubation was done at 20°C for 72 h. Bar, 0.5 μm.
FIG. 3.
C. coli SP10 dense coccoid form with long flagellum. Incubation was done at 4°C for 48 h. Bar, 0.5 μm.
FIG. 4.
C. coli SP10 with shortened flagellum (arrow). Incubation was done at 20°C after 48 h. Bar, 1 μm.
Fatty acid analysis.
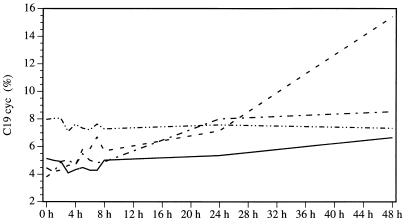
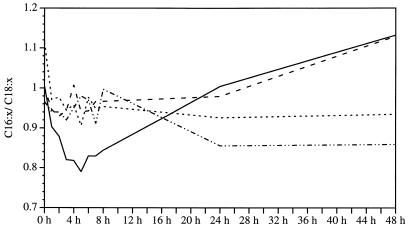
The normal fatty acid composition of C. coli SP10 is as follows: C18:1 trans9,cis11, 39 to 48%; C16:0, 35 to 45%; C19:0 cyc, 3 to 7%; C14:0, 2 to 5%; C18:0, 2 to 3%; and C16:1 cis9, 1 to 2%. The amounts of the other saturated, unsaturated, or branched fatty acids were below 1% and thus negligible. Stress due to low temperatures, i.e., 20°C, altered this pattern dramatically. The level of C19:0 cyc, normally one of the less important acids, increased significantly during incubation (Fig. 5). At just 1 h of incubation, the percentage had risen from 3.81 to 4.19%, and it reached 20.59% after 72 h. At 10°C, a doubling of the C19:0 cyc amount was observed during the first 24 h, but after that, as in all assay mixtures incubated at 4°C, only a few changes were seen. Similarly, incubation at 37°C had no effect on the C19:0 cyc concentration. In the other experimental setup, the observed increase at 20°C was from 1.14% (1 h) to 12.77% (72 h) and consequently was also highly significant. The relationship between saturated and unsaturated acids (Cx:0/Cx:x) shifted at 4 and 10°C in favor of the unsaturated acids. At 20°C, the level of C18:1 trans9,cis11 decreased, thus leading to a higher level of the other main fatty acid, C16:0 (Fig. 6). These findings were confirmed in other experiments.
FIG. 5.
Percentages of C19:0cycl in C. coli SP10 cultures at different incubation temperatures. Lines are as defined in the legend to Fig. 1.
FIG. 6.
Ratio of shorter- to longer-chain fatty acids (C16:x/C18:x) in C. coli SP10 cultures at different incubation temperatures. Lines are as defined in the legend to Fig. 1.
Analysis of membrane proteins.
The total protein concentration was reduced in cultures incubated at low temperatures. The number of bands increased over time at 37°C, but decreased at low temperatures. The main OMP with a molecular mass of 40 to 42 kDa and the 62-kDa protein were present in all samples, although the typically diffuse 62-kDa band was less pronounced and narrower at low temperatures. At 37°C, proteins with molecular masses of 20, 23, 30, 54, and 70 kDa were always observable; at lower temperatures, their appearance was irregular. A clear temperature-dependent pattern could not be detected. There was, however, a tendency for high-molecular-mass proteins (>70 kDa) to appear predominantly after incubation at 10 and 4°C.
Production of organic acids.
At 37°C, i.e., under optimum growth conditions, C. coli produces a characteristic pattern of organic acids. The main acids are succinate, lactate, and acetate. Depending on the duration of incubation, their concentrations range from 2 to 7 μmol/ml. 2-Oxoglutarate, malonate, methylmalonate, pyruvate, propionate, butyrate, isobutyrate, and 2- and 4-methylvalerate appear at concentrations of less than 1 μmol/ml. If fumarate and formate are detected at all, which is extremely seldom, they are detected only at the beginning of incubation.
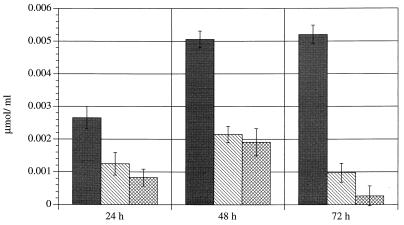
As was seen previously with long-chain fatty acids, the temperature downshift altered this pattern significantly. In comparison with the samples incubated at 37°C, the low-temperature cultures always contained higher 2-oxoglutarate concentrations and lower amounts of succinate, lactate, and acetate. The differences were almost all significant. Some organic acids were not detected at all temperatures. Fumarate and formate were not produced at 37°C after only a short incubation period, and succinate was never produced at 20°C. The mean concentrations of some organic acids (incubation period, 48 h) are listed in Table 1. These results were confirmed when the experiment was repeated, with the exception that trace amounts of succinate were detected after an incubation period of 48 h at 10 and 4°C, but again never at 20°C. While the concentrations of some acids (propionate, butyrate, and isobutyrate) varied in relation to each other, the relationships of the main acids as well as fumarate remained constant. The results for fumarate are shown in Fig. 7. The influence of temperature can be clearly seen. The differences between 20 and 10°C and between 20 and 4°C were significant. The overall production of organic acids was highest in cultures incubated at 37°C but still ranged from 1 to 7% at low temperatures.
TABLE 1.
Concentrations of some organic acids of C. coli SP10 at different incubation temperaturesa
| Organic acid | Incubation temp (°C) | Concn
|
|
|---|---|---|---|
| μmol/ml | SD | ||
| Succinate | 37 | 10.20 | 0.64 |
| 20 | 0.00 | 0.00 | |
| 10 | 0.00 | 0.00 | |
| 4 | 0.00 | 0.00 | |
| Lactate | 37 | 7.77 | 0.63 |
| 20 | 0.18 | 0.14 | |
| 10 | 0.36 | 0.10 | |
| 4 | 0.67 | 0.36 | |
| Acetate | 37 | 3.62 | 0.11 |
| 20 | 0.00 | 0.00 | |
| 10 | 0.14 | 0.08 | |
| 4 | 0.30 | 0.08 | |
| 4-Methylvalerate | 37 | 1.57 | 0.23 |
| 20 | 0.09 | 0.10 | |
| 10 | 0.28 | 0.27 | |
| 4 | 0.58 | 0.47 | |
Incubation was done for 48 h. Results are the means of six parallel experiments.
FIG. 7.
Production of fumarate at different incubation temperatures. Symbols: ▪, 20°C; ▧, 10°C; ▩, 4°C. At 37°C, fumarate was not detectable.
DISCUSSION
Although C. jejuni is more widespread in human intestinal disease in Europe, C. coli was chosen as the test organism. Apart from its epidemiological importance in developing countries, C. coli seems to be more sensitive to adverse environmental effects (10), as was confirmed in our survival experiments. C. coli SP10 was rapidly transformed into the nonculturable state; in other studies, it had been possible to maintain the culturability of C. jejuni for up to 4 weeks (5, 15, 46). Korhonen and Martikainen (24), who compared the lengths of survival of both species, obtained similar results, but their C. coli strain survived longer than C. coli SP10. One reason certainly is strain-specific reactions, because in experiments with environmental isolates (data not shown), culturability at low temperatures was retained for a few more days. Furthermore, the choice of nutrient-rich Schaedler broth most probably influenced C. coli SP10 negatively, as has been observed for C. jejuni (15, 39). Inability to be cultured cannot, however, be equated with cell death. Direct cell counts determined in parallel showed that at least the integrity of the cells was maintained over a long period. Interestingly, this was not the case with the samples incubated microaerobically, for which cell counts decreased slowly. It is possible that these culture conditions led to higher metabolic activity and consequently that temperature-stressed bacteria were more sensitive to hydrogen peroxide or superoxide anions (16, 34).
At 37°C, transformation into the coccoid form was most rapid, a feature noted earlier (40). The questions of how rod-shaped bacteria are changed into coccoid bacteria and what significance they have for the transmission of infection are still unsolved. Both active and passive processes have been considered responsible for the morphological alterations (15, 37). In accordance with the results of Moran and Upton (33), Hazeleger et al. (14) demonstrated that the DNA concentration was lower in coccoid than in rod-shaped campylobacters when the coccoid forms were generated at 37 and 25°C. At 12 and 4°C, the DNA concentration and intracellular ATP levels remained constant, whereupon the authors assumed not only that different sorts of coccoid cells exist but also that those formed at low temperatures might be important in the transmission of infection. The plump rod-shaped and large coccoid cells detected predominantly at 20°C and to a lesser degree at 10°C and the smaller coccoid cells detected at 4°C support some of these assumptions; however, studies with animal models would be necessary to clarify the role of different coccoid cells in the infection process. Jones et al. (21) regarded the large cells, not the small ones, as degenerative forms, but this conclusion is inconsistent with energetics and with observations for other bacterial species (4, 37).
For optimum function, the lipid double layer of biomembranes has to be nearly fluid to ensure lateral and rotating movements of embedded proteins. A temperature downshift that would lead to solidification and impairment of membrane function could be counteracted by a shortening of the chain length, increasing the amounts of single or polyunsaturated fatty acids, increasing the amounts of branched and cyclic fatty acids, and transforming the trans into the cis configuration. The necessary mechanisms are understood only incompletely, but it seems that for unsaturated fatty acid amounts to increase and for chain length to become shortened, the bacteria must be living or growing, however little (7). At all low temperatures, increases in the amounts of unsaturated acids as well as shorter-chain fatty acids were observed and are signs of adaptation of C. coli SP10 to a temperature downshift. The significant increase in C19:0 cyc amounts in cultures incubated at 20°C coincided with a decrease in the amounts of its precursor, C18:1 trans9,cis11 (49). Transformation of unsaturated acids into cyclic acids is a postsynthetic mechanism, but the physiological impact is still unclear. We could not confirm an increase over time as a sign of aging cultures (27). In continuous-culture experiments with nutrient limitation, Leach et al. (26) observed a growth-independent increase in C19:0 cyc amounts in C. jejuni and attributed it to a general reaction to stress. A comparison of coccoid and rod-shaped C. jejuni led to different and, with regard to a normal bacterial reaction to stress from low temperatures, unexplainable results (15). Leakiness of membranes was assumed, but contradicting data concerning coccoid forms have been reported (23, 28, 35).
The influence of suboptimal incubation temperatures has been investigated for other bacterial species, and no changes in membrane protein patterns, slight changes, and significant changes have been observed. Incubation at 37 and 42°C did not influence the OMP pattern of C. jejuni, but the total amount of protein was reduced at 37°C (6). C. coli SP10 cultures that had been stressed by low temperatures contained significantly less protein than did control cultures at 37°C. To a great extent, this result can be explained by the reduced cell volume of coccoid cells and a constant or decreasing cell count; degradation of intracellular substances may be another reason. The main OMP (40 to 42 kDa) was detectable and predominant in all samples; a relative decrease at low incubation temperatures, as has been described for aeromonads (47), was not observed. The 62-kDa protein, which consists of two flagellar proteins lying close to each other, was the second most important protein. Normally, more FlaA protein than FlaB protein is produced and the typical long flagella are produced. Spontaneously, but also due to environmental influences, the flaB gene can be activated and a short, fragile flagellum is the result. The electron microscopic observations, i.e., shortened flagella at 20 and 10°C, in addition to the less pronounced, typically diffuse appearance and the narrower band at 20 and 10°C indicate that energy-intensive processes are switched off under stress (36). The fact that cold shock proteins are involved in transcription and that flagellar expression is regulated at the transcriptional level strengthen this assumption (42).
Relatively little is known about the metabolism of campylobacters, especially at low temperatures. The excreted organic acids, which are determined by the main metabolic pathways, have been used for the identification of campylobacters (25). Differences in the observed acid pattern can be ascribed to methodological differences, e.g., column temperature and ether evaporation. The production of organic acids was most pronounced at 37°C and decreased in a temperature-dependent manner. The lag phase (4 h at 37°C) increased to 7 h at low temperatures before an increase in organic acid concentration could be seen. However, metabolic activity was retained long after nonculturability had begun. In cultures incubated at 10 and 4°C without gaseous interchange, acid production was noted even at day 22. This result confirms those of other studies in which similar data were obtained (11, 15).
The total amount of organic acids produced was not the only aspect influenced significantly by the incubation temperature. More importantly, the metabolic pattern changed as a function of the temperature downshift. Succinate, one of the main metabolites at optimal incubation temperatures, was never produced at 20°C and was only occasionally produced at 10 or 4°C. At the same time, fumarate production increased, with maximum amounts in the 20°C assays. Several explanations are possible: temperature-dependent injury of transport proteins, inactivation of formate dehydrogenase or fumarate reductase, enhanced catabolism of amino acids, or inactivation of fumarate reductase by excessive production of fumarate.
Specific transport proteins react sensitively to changes in temperature, because the velocity of many processes depends on membrane fluidity. Glucose transport of Helicobacter pylori has been shown to be linear at temperatures between 12 and 37°C (30). The existence of specific transport proteins for succinate and lactate is known (3), so that if reduced export were important, succinate would have been detectable at 20°C and not in the cultures incubated at lower temperatures. Temperature-dependent inactivation of formate dehydrogenase is also unlikely, because the formate in Schaedler broth was degraded at 20°C. We believe that the lack of succinate production and the concomitantly augmented export of fumarate were most probably caused by the inactivation of fumarate reductase. To what extent this enzyme inactivation was caused by the direct inhibitory effect of low temperatures cannot be answered at present. Perhaps the cause lies in the enhanced catabolism of amino acids, which are a main energy source for campylobacters. If other metabolic activity were simultaneously already limited at 20°C, the result would be high fumarate concentrations and possibly substrate-induced inhibition of the enzyme. A combination of both effects is also conceivable.
In conclusion, low temperatures had a significant effect on C. coli SP10. The injury was greatest at 20°C, as was demonstrated in all experiments. Survival was shortest at 10 and 20°C, and bacterial cells reacted with distinct morphological and physiological alterations to the temperature downshift. Fatty acid composition and production of metabolites were influenced significantly at intermediate temperatures, whereas incubation at 4°C seemed to stress the bacteria least.
REFERENCES
- 1.Aho M, Hirn J. Prevalence of campylobacteria in the Finnish broiler chicken chain from the producer to the consumer. Acta Vet Scand. 1988;29:451–462. doi: 10.1186/BF03548642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arimi S M, Fricker C R, Park R W A. Occurrence of ‘thermophilic’ campylobacters in sewage and their removal by treatment processes. Epidemiol Infect. 1988;101:279–286. doi: 10.1017/s0950268800054194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axe D D, Bailey J E. Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol Bioeng. 1995;47:8–19. doi: 10.1002/bit.260470103. [DOI] [PubMed] [Google Scholar]
- 4.Beveridge T J. The bacterial surface: general considerations towards design and function. Can J Microbiol. 1988;34:363–372. doi: 10.1139/m88-067. [DOI] [PubMed] [Google Scholar]
- 5.Blaser M J, Hardesty H L, Powers B, Wang W-L. Survival of Campylobacter fetus subsp. jejuniin biological milieus. J Clin Pathol. 1980;11:309–313. doi: 10.1128/jcm.11.4.309-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser M J, Hopkins J A, Berka R M, Vasil M L, Wang W L. Identification and characterization of Campylobacter jejuniouter membrane proteins. Infect Immun. 1983;42:276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diefenbach R, Heipieper H J, Keweloh H. The conversion of cis into trans unsaturated fatty acids in Pseudomonas putidaP8—evidence for a role in the regulation of membrane fluidity. Appl Microbiol Biotechnol. 1992;38:382–387. [Google Scholar]
- 8.Doane F W, Anderson A N. Electron microscopy in diagnostic virology. Cambridge, England: Cambridge University Press; 1987. [Google Scholar]
- 9.Garcia M M, Lior H, Stewart R B, Ruckerbauer G M, Trudel J R, Skljarevski A. Isolation, characterization, and serotyping of Campylobacter jejuni and Campylobacter colifrom slaughter cattle. Appl Environ Microbiol. 1985;49:667–672. doi: 10.1128/aem.49.3.667-672.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gondrosen B. Survival of thermophilic campylobacters in water. In: Pearson A D, editor. Campylobacter II. London, England: Public Health Laboratory Service; 1983. p. 190. [Google Scholar]
- 11.Gribbon L T, Barer M R. Oxidative metabolism in nonculturable Helicobacter pylori and Vibrio vulnificuscells studied by substrate-enhanced tetrazolium reduction and digital image processing. Appl Environ Microbiol. 1995;61:3379–3384. doi: 10.1128/aem.61.9.3379-3384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffiths P L, Park R W A. Campylobacters associated with human diarrhoeal disease. J Appl Microbiol. 1990;69:281–301. doi: 10.1111/j.1365-2672.1990.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 13.Guerrant G O, Lambert M A, Moss C W. Analysis of short-chain acids from anaerobic bacteria by high-performance liquid chromatography. J Clin Microbiol. 1982;16:355–360. doi: 10.1128/jcm.16.2.355-360.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazeleger W C, Arkesteijn C, Toorop-Bouma A, Beumer R R. Detection of the coccoid form of Campylobacter jejuniin chicken products with the use of the polymerase chain reaction. Int J Food Microbiol. 1994;24:273–281. doi: 10.1016/0168-1605(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 15.Hazeleger W C, Janse J D, Koenraad P M, Beumer R R, Rombouts F M, Abee T. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl Environ Microbiol. 1995;61:2713–2719. doi: 10.1128/aem.61.7.2713-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman P S, George H A, Krieg N R, Smibert R M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979;25:8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- 17.Höller C. Long-term study of occurrence, distribution and reduction of Campylobacter sp.in the sewage system and wastewater treatment plant of a big town. Water Sci Technol. 1988;20:529–531. [Google Scholar]
- 18.Höller C. Quantitative und qualitative Untersuchungen an Campylobacter in der Kanalisation einer Großstadt. Zentralbl Bakteriol Hyg Abt 1 Orig B. 1988;185:307–325. [PubMed] [Google Scholar]
- 19.Jacobs-Reitsma W F, Bolder N M, Mulder R W. Caecal carriage of Campylobacter and Salmonella in Dutch broiler flocks at slaughter: a one year survey. Poult Sci. 1994;73:1260–1266. doi: 10.3382/ps.0731260. [DOI] [PubMed] [Google Scholar]
- 20.Jany K D, editor. Polyacrylamid-Gelelektrophoresen für Proteine. Stuttgart, Germany: Gustav Fischer Verlag; 1991. [Google Scholar]
- 21.Jones D M, Curry A, Sutcliffe E M. Coccal forms of campylobacters and helicobacter are generated differently. Microb Ecol Health Dis. 1991;4:S76. [Google Scholar]
- 22.Jones P G, Cashel M, Glaser G, Neidhardt F C. Function of a relaxed-like state following temperature downshifts in Escherichia coli. J Bacteriol. 1992;174:3903–3914. doi: 10.1128/jb.174.12.3903-3914.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieft T L, Ringelberg D B, White D C. Changes in ester-linked phospholipid fatty acid profiles of subsurface bacteria during starvation and desiccation in a porous medium. Appl Environ Microbiol. 1994;60:3292–3299. doi: 10.1128/aem.60.9.3292-3299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korhonen L K, Martikainen P J. Comparison of survival of Campylobacter jejuni and Campylobacter coliin culturable form in surface water. Can J Microbiol. 1991;37:530–533. doi: 10.1139/m91-089. [DOI] [PubMed] [Google Scholar]
- 25.Krausse R, Ullmann U. Studies on the identification of Campylobacter speciesusing biochemical tests and HPLC. Zentralbl Bakteriol Hyg Abt 1 Orig A. 1985;260:342–360. doi: 10.1016/s0176-6724(85)80023-7. [DOI] [PubMed] [Google Scholar]
- 26.Leach S, Harvey P, Wait R. Changes with growth rate in the membrane lipid composition of and amino acid utilisation by continuous cultures of Campylobacter jejuni. J Appl Microbiol. 1997;82:631–640. doi: 10.1111/j.1365-2672.1997.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 27.Lechevalier M P. Lipids in bacterial taxonomy—a taxonomist’s view. Crit Rev Microbiol. 1977;5:109–210. doi: 10.3109/10408417709102311. [DOI] [PubMed] [Google Scholar]
- 28.Linder K, Oliver J D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989;55:2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margesin R, Schinner F. Properties of cold-adapted microorganisms and their potential role in biotechnology. J Biotechnol. 1994;33:1–14. [Google Scholar]
- 30.Mendz G L, Burns B P, Hazell S L. Characterisation of glucose transport in Helicobacter pylori. Biochim Biophys Acta. 1995;1244:269–276. doi: 10.1016/0304-4165(95)00018-7. [DOI] [PubMed] [Google Scholar]
- 31.Mentzing L-O. Waterborne outbreaks of Campylobacter enteritis in central Sweden. Lancet. 1981;ii:352–354. doi: 10.1016/s0140-6736(81)90658-9. [DOI] [PubMed] [Google Scholar]
- 32.Miller L, Berger T. Bacteria identification by gas chromatography of whole cell fatty acids. Application note 228-41. Palo Alto, Calif: Hewlett-Packard; 1985. [Google Scholar]
- 33.Moran A P, Upton M E. A comparative study of the rod and coccoid forms of Campylobacter jejuniATCC 29428. J Appl Bacteriol. 1986;60:103–110. doi: 10.1111/j.1365-2672.1986.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 34.Moran A P, Upton M E. Effect of medium supplements, illumination and superoxide dismutase on the production of coccoid forms of Campylobacter jejuniATCC 29428. J Appl Bacteriol. 1987;62:43–51. doi: 10.1111/j.1365-2672.1987.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 35.Morgan J A, Cranwell P A, Pickup R W. Survival of Aeromonas salmonicidain lake water. Appl Environ Microbiol. 1991;57:1777–1782. doi: 10.1128/aem.57.6.1777-1782.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuijten P J, Wassenaar T M, Newell D G, van der Zeijst B A. Molecular characterization and analysis of Campylobacter jejuni flagellin genes and proteins. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 282–296. [Google Scholar]
- 37.Oliver J D. Formation of viable but non-culturable cells. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 239–272. [Google Scholar]
- 38.Osborn M J. Studies on the gram-negative cell wall. I. Evidence for the role of 2-keto-3-deoxyoctonate in the lipopolysaccharide of Salmonella typhimurium. Biochemistry. 1963;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pokorny J. Überleben von Campylobacter jejuniin Wasser. Acta Hydrochim Hydrobiol. 1989;17:341–344. [Google Scholar]
- 40.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuniand its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosef O. Isolation of Campylobacter fetus subsp. jejunifrom the gallbladder of normal slaughter pigs, using an enrichment procedure. Acta Vet Scand. 1981;22:149–151. doi: 10.1186/BF03547219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahamat M, Mai U, Paszkokolva C, Kessel M, Colwell R R. Use of autoradiography to assess viability of Helicobacter pyloriin water. Appl Environ Microbiol. 1993;59:1231–1235. doi: 10.1128/aem.59.4.1231-1235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skirrow M B, Blaser M J. Clinical and epidemiologic considerations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 3–8. [Google Scholar]
- 44.Taylor D N. Campylobacter infections in developing countries. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 20–30. [Google Scholar]
- 45.Taylor D N, Brown M, McDermott K T. Waterborne transmission of Campylobacter enteritis. Microb Ecol. 1982;8:347–354. doi: 10.1007/BF02010674. [DOI] [PubMed] [Google Scholar]
- 46.Terzieva S I, McFeters G A. Survival and injury of Escherichia coli, Campylobacter jejuni, and Yersinia enterocoliticain stream water. Can J Microbiol. 1991;37:785–790. doi: 10.1139/m91-135. [DOI] [PubMed] [Google Scholar]
- 47.Tso M D, Dooley J S G. Temperature-dependent protein and lipopolysaccharide expression in clinical Aeromonas isolates. J Med Microbiol. 1995;42:32–38. doi: 10.1099/00222615-42-1-32. [DOI] [PubMed] [Google Scholar]
- 48.Vogt R S, Sours H E, Barrett T, Feldman R A, Dickinson R J, Witherell L. Campylobacter enteritis associated with contaminated water. Ann Intern Med. 1982;96:292–296. doi: 10.7326/0003-4819-96-3-292. [DOI] [PubMed] [Google Scholar]
- 49.Wait R, Hudson M J. The use of picolinyl esters for the characterization of microbial lipids: application to the unsaturated and cyclopropane fatty acids of Campylobacter species. Lett Appl Microbiol. 1985;1:95–99. [Google Scholar]