Abstract
The burden of cardiovascular diseases and the critical role of acute coronary syndrome (ACS) in their progression underscore the need for effective diagnostic and prognostic tools. Biomarkers have emerged as crucial instruments for ACS diagnosis, risk stratification, and prognosis assessment. Among these, high-sensitivity troponin (hs-cTn) has revolutionized ACS diagnosis due to its superior sensitivity and negative predictive value. However, challenges regarding specificity, standardization, and interpretation persist. Beyond troponins, various biomarkers reflecting myocardial injury, neurohormonal activation, inflammation, thrombosis, and other pathways are being explored to refine ACS management. This review article comprehensively explores the landscape of clinically used biomarkers intricately involved in the pathophysiology, diagnosis, and prognosis of ACS (i.e., troponins, creatine kinase MB (CK-MB), B-type natriuretic peptides (BNP), copeptin, C-reactive protein (CRP), interleukin-6 (IL-6), d-dimers, fibrinogen), especially focusing on the prognostic role of natriuretic peptides and of inflammatory indices. Research data on novel biomarkers (i.e., endocan, galectin, soluble suppression of tumorigenicity (sST2), microRNAs (miRNAs), soluble oxidized low-density lipoprotein receptor-1 (sLOX-1), F2 isoprostanes, and growth differentiation factor 15 (GDF-15)) are further analyzed, aiming to shed light on the multiplicity of pathophysiologic mechanisms implicated in the evolution of ACS. By elucidating the complex interplay of these biomarkers in ACS pathophysiology, diagnosis, and outcomes, this review aims to enhance our understanding of the evolving trajectory and advancements in ACS management. However, further research is necessary to establish the clinical utility and integration of these biomarkers into routine practice to improve patient outcomes.
Keywords: biomarker, acute coronary syndrome, coronary artery disease, myocardial injury, neurohormonal activation, inflammation, thrombosis
1. Introduction
Cardiovascular diseases continue to pose a significant burden on overall mortality worldwide [1,2] and acute coronary syndrome (ACS) is often the initial presentation. ACS, encompassing a spectrum of conditions from unstable angina (UA) to ST-segment elevation myocardial infarction (STEMI), presents a critical challenge in contemporary cardiology. ACS results from the disruption of atherosclerotic plaques within the coronary arteries, leading to myocardial ischemia, necrosis, and subsequent clinical presentations [3]. Early diagnosis and risk stratification are therefore critical for guiding timely interventions and improving patient outcomes [4].
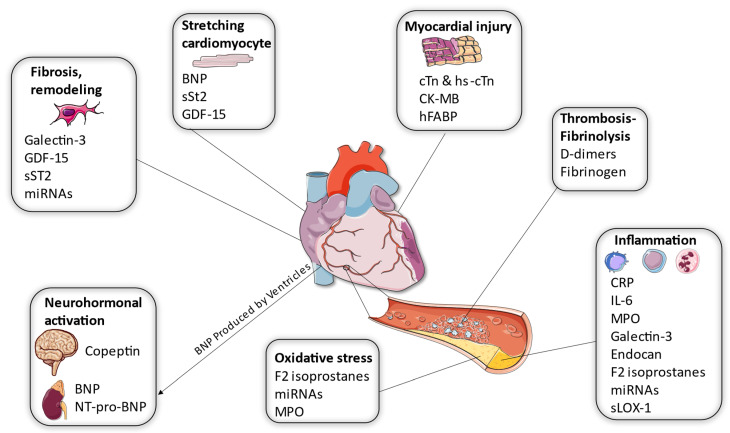
Biomarkers have emerged as essential endpoints for the diagnosis, risk stratification, and prognosis assessment of ACS. Among these biomarkers, troponin, particularly high-sensitivity troponin (hs-cTn), has revolutionized ACS diagnosis with its superior sensitivity and negative predictive value [5]. However, challenges such as specificity, standardization, and interpretation persist. Beyond troponins, a constellation of biomarkers reflecting myocardial injury, neurohormonal activation, inflammation, thrombosis, and other pathways are being explored to refine ACS management (Figure 1).
Figure 1.
Different roles of biomarkers in the pathophysiology of Myocardial Infarction. The biomarkers for MI can be categorized based on their mechanism of action. Some are secreted by the cardiomyocytes, while others are correlated with inflammation, clot formation, and neurohormonal pathways. It is interesting to note that some biomarkers (galectin-3, miRNAs, BNP, sST2, and GDF-15) are included in more than one category, proving the multiplicity of their pathophysiology (BNP: B type natriuretic peptide; CK-MB: Creatine Kinase- MB; CRP: reactive protein; cTn: Cardiac Troponin; GDF-15: Growth Differentiation Factor-15; hFABP: Heart- Type Fatty Acid Binding Protein; MI: Myocardial Infarction; miRNAs: micro-RNAs; MPO: Myeloperoxidase; NT-proBNP: N-terminal portion of the pro-BNP peptide; sLOX-1: Soluble oxidized low-density lipoprotein receptor-1; sST2: Soluble Suppression of Tumorigenicity) Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/) (accessed on 16 September 2023).
Therefore, in this review article, we focus on elucidating the elaborate landscape of biomarkers intricately involved in the pathophysiology, diagnostic process, and prognostic assessment of ACS, aiming to enhance our comprehension of the advancement of ACS as well as the clinical ramifications stemming from these biomarkers.
2. Biomarkers of Myocardial Injury
2.1. Troponin and High-Sensitivity Troponin
The troponin contractile apparatus consists of three isoforms (C, I, & T), which interact with tropomyosin, actomyosin, and Ca2+, regulate muscle contraction, and release from the necrotic myocardium (Table 1). Troponin C is not utilized in clinical practice as a biomarker since there is significant homology between the skeletal and cardiac isoforms, reducing cardiac specificity [6]. Since 2000, Troponin I and T has been a criterion ‘sine qua non’ for myocardial infarction (MI) diagnosis along with clinical and electrocardiography (ECG) conditions. Early conventional cTn assays, while specific, had a low sensitivity; analytical methods however have since evolved, with the hs-cTn assays (5th generation) prevailing as they offer higher diagnostic accuracy, an earlier detection of MI (within one hour), and a higher Negative Predictive Value (NPV) in order to rule out a MI (99.5–99.8%) [7,8]. These benefits in sensitivity and NPV come at the cost of specificity and Positive Predictive Value (PPV) compared to the conventional cTn (ruling in a MI), leading to more extensive testing [9,10]. Due to the high sensitivity, the ESC 2020 guidelines recommend the rapid ‘rule-out’ and ‘rule-in’ algorithms, especially the 0h/1h (alternatively 0 h/2 h) of serial drawing samples with a minimal sensitivity of 99% and a minimal PPV of 70%. In fact, Non-ST-Elevation Myocardial Infarction (NSTEMI) can be ruled out at baseline for a very low hs-cTn and/or lack of elevation within the first hour [7]. hs-cTn is also a quantitative biomarker in the diagnosis of MI as 5x Upper Reference Limit (URL) increases have a high PPV (>90%) for type 1 MI and this limit can differentiate from type 2 MI; at the same time, the increase in diagnosed MIs has led to a subtle reduction in the incidence of UA [7,11].
Table 1.
Biomarkers used and application in ACS.
| Biomarker | Mechanism of Action-Release | Clinical Application & Abilities |
|---|---|---|
| hs-Troponin | Regulates cardiac contraction. Released at myocardium necrosis. T & I isoforms cardiac specific |
Modification above 99th URL diagnostic for MI hs-cTn has high sensitivity & NPV at cost of specificity. 5x URL increase→high PPV (>90%) for type 1 MI ‘Rule-out’ & ‘rule-in’ MI algorithms Differentiate NSTEMI & UA Rises in 3–12 h, peak at 24 h. Prognosis of all-cause mortality |
| CK-MB | Released at myocardium necrosis. CK-MB isoenzyme mostly at cardiac muscle (low levels in skeletal muscle) |
Diagnosis: Rises after 4–6 h & peaks at 24 h Normal at 48–72 h→detects reinfarction. NPV 97% & sensitivity 91% at first 6 h Prognostic for infarct size, wall motion abnormalities, mortality, HF, possibly LV remodeling, CI-AKI |
| Cystatin C | Protease inhibitor secreted by nucleated cells. Filtered through glomerulus and catabolized in proximal tubule. Associated with Egfr |
Prognostic/risk stratification for all-cause mortality, HF hospitalization, CVD after ACS Peaks at 3rd day after ACS (vs. 6th Creatinine) Prognostic for NRP, MACE & mortality after PCI |
| H-FABP | Released from cytoplasm after cardiac injury and necrosis | High sensitivity at decreased cutoffs (4 μg/L) Early biomarker (<1 h), reinfarction detection Possible value in early ruling out MI |
| Endocan | Endothelial dysfunction & activation Inflammation |
Risk stratification and Prognosis of MACE, high SYNTAX score Possibly indicates reperfusion after PCI or CABG Possibly different levels in STEMI vs. NSTEMI/UA |
| Galectin | Cardiac remodeling and fibrosis (fibroblasts→myofibroblasts & collagen synthesis) Plaque Destabilization in CVD |
Prognosis of MI and HF Risk stratification (LVEF, MACE, mortality, HF, remodeling) Interrelated with atherosclerosis & inflammation. Possible therapeutic target |
| sST2 | Decoy receptor for sST2/IL-33 interaction cardiac fibrosis, hypertrophy and remodeling | Prognostic factor ADHF (>35 ng/mL) Therapeutic guidance in Type 1 & 2 MI (>35 ng/mL likely adverse remodeling & >70 ng/mL aggressive treatment) Prognostic for ACS (mortality, HF, remodeling) Prognostic for reperfusion & NRP after PCI |
| D-dimers | Breakdown of fibrin clot by plasmin at the site of coronary artery thrombosis | Possibly diagnostic for MI and differentiate from UA (>500 ng/mL) Prognostic for recurring MI, all-cause mortality, in hospital complications, NRP |
| CRP | Acute-phase inflammation | Prognostic factor of future myocardial infarction and stroke, levels > 3 mg/L upon discharge: increased risk of readmission within 1 year for recurrent cardiovascular instability or myocardial infarction |
| micro-RNA | Control of gene expression, oxidative stress, inflammation, apoptosis, fibrosis, and cardiac remodeling processes | Predictive factor for cardiovascular mortality and the development of heart failure |
| GDF-15 | Increases in tissue damage and inflammation | Risk predictor |
| Fibrinogen | Clot formation, platelet aggregation, fibrinolysis, inflammation | Induce coronary artery restenosis, baseline levels: increased risk of cardiovascular events within 2 years |
ACS: Acute Coronary Syndrome; ADHF: Acute Decompensated Heart Failure; CABG: Coronary Artery Bypass Grafting; CI-AKI: Contrast Induced Acute Kidney Injury; CK-MB: Creatine Kinase-MB; CVD: Cardiac Vascular Disease; eGFR: estimated Glomerular Filtration Rate; GDF-15: Growth Differentiation Factor-15; HF: Heart failure; hs-cTn: high sensitivity troponin; LV: Left Ventricle; MACE: Major Adverse Cardiac Events; MI: Myocardial Infarction; NSTEMI: non-ST-elevation Myocardial Infarction; NPV: Negative Predictive Value NRP: No Reflow Phenomenon; PCI: Percutaneous Coronary Intervention; PPV: Positive Predictive Value; UA: Unstable Angina; URL: Upper Reference Limit.
However, in a study there was a significant discordance in the results of 3 different hs-cTn assays, showcasing a lack of standardization between different manufacturers. This inconsistency was especially pronounced in troponin levels below the limit of detection (LOD) and between the LOD and the 99th percentile, while the proportion of samples above the 99th percentile did not fluctuate significantly between the clusters. As a result, the patients with rule-out and observe recommendations significantly differed between groups, while there was no significant difference in the rule-in candidates across different assays and research teams. This discrepancy can be attributed to differences in sensitivity and LOD or differences in the reference biobank used by each assay to determine the 99th percentile [8]. Standardization and harmonization issues between hs-cTn I assays are especially pronounced compared to hs-cTn T as Troponin I is offered by a variety of manufacturers; results between companies are therefore not interchangeable [6]. Concerning the differences between the isoforms T and I, cTn- I has a higher diagnostic accuracy in early presenters, while cTn-T prevails later [12,13]. Finally, cTn-T is superior in the prognosis of all-cause mortality, but its clearance is more heavily impacted in renal failure [13,14].
2.2. Creatine Kinase MB (CK-MB)
Creatine Kinase MB (CK-MB) has constituted the gold standard for MI diagnosis in the past [15] although the current European Society of Cardiology (ESC) guidelines do not recommend its routine measurement for diagnostic purposes [7]. Creatine Kinase metabolizes Creatine and Adenosine Triphosphate (ATP) to Creatine Phosphate and Adenosine Diphosphate (ADP) in muscle cells, while the CK-MB isoenzyme constitutes 20% of the overall myocardium CK pool. CK-MB starts rising 4–6 h after an MI, attains its apex at 24 h, and declines in 48–72 h, providing the possibility of early detection of a re-infarction [7,16]. During the first 6 h after a MI, CK-MB has a NPV of 97% and a 91% sensitivity. As CK-MB is released from skeletal muscle as well, it is important to avoid false positives in diagnosis by paying attention to the regularity and amount of secretion; a CK-MB:CK ratio > 2.5% increases the likelihood of cardiac sources [16]. Apart from its diagnostic properties, CK-MB adds prognostic characteristics as well, since peak CK-MB strongly correlates with the width of the lesion and wall movement anomalies in smaller non-transmural [17] and larger infarcts [18] after reperfusion. Additionally, in a study of participants 4 months after a STEMI, peak CK-MB was considered as a very strong predictor of the end systolic volume index assisting in prognosis of Left Ventricle (LV) remodeling [19]. Other useful associations consist of peak CK-MB levels correlating with 3-year mortality in NSTEMI after percutaneous coronary intervention (PCI) [20] and with the rare incidence of Heart Failure (HF) after a STEMI [21]. Finally, among MI patients undergoing coronary angiography, those with a preprocedural log (CK-MB) > 4.7 have an amplified incidence of contrast-induced acute kidney injury (CI-AKI) independently of other risk factors [22].
3. Biomarkers of Neurohormonal Activation
3.1. B Type Natriuretic Peptide (BNP)
BNP, a hormone generated due to myocardial dysfunction, is produced predominantly by the ventricles. Its functions encompass the hindrance of the renin-angiotensin-aldosterone system (RAAS), promotion of renal sodium excretion, and reduction in vascular resistance [23].
The concentration of BNP increases significantly within the initial 24 h following an MI in patients with STEMI and subsequently reaches a relatively stable level. There might be a second peak in BNP levels around 5 days later, possibly indicating the ongoing remodeling process [24].
Extensive research has been conducted on BNP, revealing its significance as a prognostic indicator following an MI [24,25,26]. BNP has a relatively short half-life, but it is secreted alongside the N-terminal section of the pro-BNP peptide (NT-proBNP), a fragment with a longer half-life in plasma and therefore more conveniently measured [27].
In a case-control study involving patients with non-ST-elevation ACS, it was discovered that NT-proBNP concentration was elevated in subjects who experienced mortality compared to those who survived [28]. NT-proBNP elevation occurs in the initial stages of ACS, particularly when angina lasts for less than 4 h [29]. It also exhibits a close association with the extent of myocardial ischemia and predicts both short- and long-term mortality among ACS cases [30]. In another study, NT-proBNP served as a marker strongly linked to all-cause mortality [31].
The TACTICS-TIMI 18 study [32] involved the randomization of 1676 patients with non-ST-elevation ACS into conservative and early invasive therapy groups. The study measured patients’ BNP levels within 24 h and compared the results. The findings revealed that patients with BNP levels below the cut-off of 80 pg/mL had a six-month mortality rate of 1.4%, whereas those with BNP levels above the cut-off had a mortality rate of 8.4%. Additionally, the risk of mortality or congestive heart failure was 3.6% for patients below the cut-off, compared to 16.3% for those above the cut-off. However, similar to another study [33], the TACTICS-TIMI 18 study did not determine which patients would have better outcomes from early invasive revascularization based on BNP levels.
3.2. Copeptin
Copeptin is a portion of the vasopressin precursor hormone (CT-proAVP) secreted alongside vasopressin after the precursor is cleaved. Measuring copeptin appears to be a clinically valuable approach for assessing plasma concentrations of vasopressin, which cannot be directly measured due to demanding pre-analytical techniques [34,35]. Studies have indicated that it could serve as a prognostic factor for STEMI [36]. In recent findings, copeptin has emerged as both an independent surrogate of total mortality and a marker of overall vulnerability, affected by the infliction of HF, type 2 diabetes, female sex, and prior MI [37]. In a prospective trial of patients with acute myocardial infarction (AMI), it was demonstrated that incorporating copeptin alongside cTn I enabled a secure exclusion of AMI with a NPV exceeding 99% in patients presenting early with suspected ACS. Moreover, the study indicated that the presence of both aberrant copeptin and cTn I levels was an independent indicator of mortality within 6 months [38]. In summary, recent evidence indicates that copeptin may offer supplementary benefits to cTn in promptly ruling out patients with suspected ACS [39].
4. Inflammatory Biomarkers
4.1. C Reactive Protein (CRP)
Atherothrombosis, a major contributor to ACS [40], mainly results from inflammatory processes [41]. CRP has been extensively researched as an indicator of acute-phase inflammation and could possibly assess increased cardiovascular risk in individuals with pre-existing atherosclerosis. According to the literature, plasma levels of CRP can estimate the likelihood of future MI and stroke [42]. Importantly, statins have the potential to decrease CRP levels beyond their cholesterol-lowering effects [43], making CRP a valuable test to reassess individuals categorized as having an intermediate risk for future cardiovascular events.
Moreover, patients diagnosed with UA and CRP levels greater than 3 mg/L upon discharge face an increased risk of readmission within one year for reoccurring cardiovascular instability or MI [44]. Likewise, in a prospective study of individuals who went through early invasive therapy for NSTEMI, elevated CRP levels exceeding 10 mg/L during admission still presented with a higher likelihood of death over an average follow-up period of 20 months [45].
Although certain studies have yielded encouraging findings, CRP has not consistently demonstrated itself as a standalone predictor of events. Given the existing treatment approaches for ACS, which include dual antiplatelet, high-intensity statin treatment, and reperfusion, the significance of CRP in regular prognostic estimation for ACS remains uncertain [39].
4.2. Interleukin-6 (IL-6)
IL-6, like CRP, has a central role in the inflammatory cascade and has been used as an inflammatory biomarker that may play a significant role in diagnosis, risk stratification, and outcome prediction in patients with AMI. Elevated expression of IL-6 has been observed in induced MI by transcoronary ablation of septal hypertrophy, suggesting potential diagnostic significance [46]. Furthermore, IL-6 shows substantial upregulation in ACS [47] and its levels are linked to adverse cardiac events, highlighting its potential as a therapeutic target in unstable ischemic heart disease [48]. IL-6 receptor antagonists have been found to improve the inflammatory response and the release of cTn after PCI in patients with NSTEMI. This improvement is independent of the inhibition of endothelial cell activation [49].
4.3. Myeloperoxidase (MPO)
Myeloperoxidase (MPO) is an enzyme involved in the immune system response and is excreted from neutrophils and macrophages into the extracellular fluid during inflammation [50]. MPO is implicated in the pathophysiology of numerous diseases including atherosclerosis. Is associated with oxidation of LDL cholesterol particles and formation of foam cells; moreover, it has been also studied as a possible therapeutic target against cardiovascular diseases [50,51]. Interestingly, expression of MPO by macrophages is increased at the later stages of atherosclerosis contrary to the initial atherosclerotic lesions (i.e., fatty streaks) in a process controlled by proinflammatory mediators such as granulocyte macrophage colony-stimulating factor (GM-CSF) [52].
Regarding diagnosis of ACS, measurement of MPO at 6 h has displayed a significant diagnostic capability discriminating patients presenting with ACS from patients with other diagnoses of cardiovascular disease [53]. Furthermore, there is an association between MPO levels and the presence of unstable coronary artery plaques as well as the likelihood of future cardiovascular events [54]. Specifically, elevated serum levels of MPO in individuals experiencing ACS have been linked to future cardiovascular events and could identify those at risk for adverse events [55]. Accordingly high levels of MPO on admission could indicate the patients with ACS at risk for complications during their index hospitalization such as HF, arrhythmias and renal failure [56]. Actually, MPO levels have demonstrated an inverse association with left ventricular systolic function in patients hospitalized due to ACS [56]. Moreover, evidence from a meta-analysis of 13 studies has displayed that MPO could predict mortality of patients with ACS and especially for smokers whereas female gender depicted an inverse association with mortality and recurrence of MI; nevertheless, MPO had great prognostic capability irrespectively of other classic cardiovascular risk factors such as age, hypertension and diabetes [57]. Prognostic capability of MPO for future cardiovascular events has been also confirmed by a meta-analysis of 27 studies of patients with ACS [58]. What is more, higher expression of MPO could identify also patients with NSTEMI at risk of major adverse cardiovascular events (MACE) at the first year and particularly for patients > 65 years of age and NT-proBNP levels beyond 1000 pg/mL [59]. Better understanding of the diagnostic and prognostic capability of MPO for patients with ACS could facilitate the development of future clinical studies and possible therapeutic inhibition of the pathways of MPO-related inflammation and oxidative stress [51].
5. Biomarkers Associated with Thrombosis-Fibrinolysis
5.1. D-Dimers
Whereas the d-dimers have been traditionally established as the diagnostic marker for venous thromboembolism, recently the potential for the diagnosis and prognosis of ACS has been brought to attention due to the coronary artery thrombosis characterizing MI [60]. A recent study considered this biomarker as a potential diagnostic tool for patients with MI and UA; indeed, the determined cut-off to differentiate MI from UA was 548 ng/mL, with 91.2% sensitivity and 63.4% specificity [60]. This cut-off value is compatible with the one from the Bayes-Genis et al. study, where d-dimers > 500 μg/L were diagnostic for MI [61]. In contrast, a recent study found the diagnostic ability of dimers to differentiate between MI and chest pain of non-coronary aetiology to be moderate unless the values exceed the 95th percentile [62]. However, the same study proved the d-dimer’s prognostic ability for MI recurrence (p = 0.0333) and all-cause death (p < 0.0001) [62]. Finally, a meta-analysis of 5 studies with a mean follow-up of 13.2 months found high d-dimer levels associated with a higher hospital stay, more long-term adverse outcomes, and with the No Reflow Phenomenon (NRP) in STEMI patients after revascularization [63].
5.2. Fibrinogen
Fibrinogen (FIB), an early-identified clotting factor, is produced in the liver, and affects processes such as clot formation, platelet aggregation, and the fibrinolysis system. It contributes to the inflammatory response via interactions with cytokines, impacting cardiovascular disease progression. Furthermore, FIB degradation products can induce coronary artery restenosis by promoting smooth muscle cell proliferation [64]. It was discovered that individuals with ACS following PCI who have high baseline fibrinogen levels are at increased risk of MACE within two years [65]. The pathways through which fibrinogen contributes to increased cardiovascular risk can be elucidated as follows: Firstly, fibrinogen stimulates the aggregation of platelets. Moreover, elevated fibrinogen concentrations force the creation of fibrin and elevate plasma viscosity. Lastly, fibrinogen actively engages in inflammatory responses, with its levels rising under inflammatory conditions [66].
6. Additional Biomarkers
Beyond the most well-studied biomarkers with clinical applications in the ACS, several other serum biomarkers are continuously evaluated for their diagnostic or prognostic ability in the setting of myocardial infarction (Table 2).
Table 2.
Studies on potential biomarkers regarding ACS.
| Biomarker | Study | Type | Population | Results | Concentrations |
|---|---|---|---|---|---|
| Cystatin C (CysC) | Brankovic et al., 2020 [67] | BIOMArCS prospective multicenter study | Case cohort of 844 patients after ACS for 1 year follow-up | CysC independent of GRACE score and associated with mortality, non-fatal MI & revascularization due to angina | CysC at any time associated with endpoint (HR [95% CI]: per 1SD increase of 2logCysC: 1.79 [1.21–2.63], p = 0.006) |
| Correa et al., 2018 [68] | Double- blind clinical trial | 4965 random, hospitalized for ACS patients from SOLID-TIMI 52 trial | Strong correlation with Creatinine & eGFR Elevated CysC- 89% higher risk of CVD, HF hospitalization, 44% of MACE, 28% of MI Q4: x5 risk of CVD or HF, >x2 MACE |
Quartiles of CysC: Q1 < 0.78, Q2 = 0.78–0.88, Q3 = 0.88–1.03, Q4 > 1.03 mg/mL |
|
| Sun et al., 2021 [69] | Meta-analysis | 10 studies | Significant correlation of high level CysC with all-cause mortality & MACE but not significant with recurrent MI | High Q4 and low Q1 quartiles from each study | |
| Chen et al., 2022 [70] | Meta-analysis | 8 studies with 7394 patients after PCI or CABG | ↑cystatin significant relation with MACE & mortality after PCI Non-significant after CABG |
- | |
| hFABP | Young et al., 2016 [71] | Feasibility study | 1079 patients, 248 with MI | hFABP + hs-cTn can identify up to 40% patients as low risk at presentation | hFABP < 4.3 ng/mL + hs-cTn I < 10.0 ng/L + (-) ECG (>99% sensitivity) |
| Van Hise et al., 2018 [72] | Cohort study | 1230 patients, 112 with MI | h-FABP, hs-cTn and ECG has high accuracy and can rule out more patients | hFABP + hs-cTn T (100% sensitivity + 32.4% specificity) hFABP and hs-cTn I (99.1% sensitivity + 43.4% specificity) hs-cTn I alone higher specificity 68.1% |
|
| Collinson et al., 2013 [73] | Randomized controlled trial | 850 patients with chest pain + (-) ECG sampled on admission + 90 min | Hs-cTn best single marker, further info on hFABP required | H-FABP + cTn T/ cTn I (sensitivity 0.78–0.92) at 2.5 μg/L cut-off (single troponin at 2 samples 0.78–0.95) | |
| Dupuy et al., 2015 [74] | Prospective cohort study | 181 patients, 47 with MI (31 NSTEMI) within 12 h | HFABP + hs-cTn T increased sensitivity (+13%) and NPV (+3%) for NSTEMI hFABP lower diagnostic accuracy than hs-cTn T |
5.8 ng/mL cutoff (sensitivity of 97% + NPV of 99%) | |
| Endocan | Ziaee et al., 2019 [75] | Cross- sectional and prospective | 320 patients with ACS: 160 with STEMI and 160 with UA/NSTEMI | Significant positive correlation between endocan levels and TIMI risk score and MACE. | Optimal cutoff values to predict clinical end points: 3.45 ng/mL in STEMI (80% sensitivity and 72% specificity) and 2.85 ng/mL in NSTEMI/UA (74% sensitivity and 67% specificity) |
| Kundi et al., 2017 [76] | Cross- sectional | 133 patients: 88 patients with STEMI and 45 patients with normal coronary arteries | Elevated in STEMI and positively correlated with hs-CRP and SYNTAX score | Cutoff value to predict STEMI: 1.7 ng/mL (76.1% sensitivity 73.6% specificity) | |
| Dogdus et al., 2021 [77] | Cross- sectional | 137 STEMI patients undergoing PCI: 45 NRP (+) & 92 NRP (-) | Endocan, initial troponin I, Triglyceride and high-grade thrombus burden were independent predictors of NRP |
Cutoff value to predict NRP: >2.7 ng/mL (89.6% sensitivity and 74.2% specificity) |
|
| Cimen et al., 2019 [78] | Cross-sectional | 35 ACS patients undergoing CABG | Significant decrease in serum hs-CRP and endocan levels (372.8 ng/mL vs. 320.2) after CABG (p < 0.05) |
- | |
| Qiu et al., 2016 [79] | Cross-sectional | 216 patients with ACS and 60 controls | Endocan significantly increased in ACS group. STEMI vs NSTEMI: (38.2 [14.4, 78.5] vs 10.5 [2.7, 32.6] ng/mL) |
- | |
| Galectin | Tian et al., 2019 [80] | Meta-analysis | 2809 patients (10 studies) | Significant negative correlation between galectin & LVEF Non-significant correlation between gal-3 & infarct size Galectin associated with high mortality |
- |
| Asleh et al., 2019 [81] | Population based cohort study | 1342 patients at time of MI | Tertile 2 & 3: 1.3 & 2.4 increased risk of death 1.4 & 2.3 risk of HF |
Gal-3 cut-offs in 3 tertiles: 1: <15.1 ng/mL 2: 15.1–22.4 ng/mL 3: >22.4 ng/ml |
|
| Gagno et al., 2019 [82] | Prospective cohort study | 469 patients with MI (60% STEMI) with 12 month follow up | Galectin associated with all-cause mortality. Gal-3bp correlated with risk of angina/MI |
Median Gal-3bp: 9.1 μg/mL Median Galectin: 9.8 ng/mL |
|
| Święcki et al., 2020 [83] | Controlled pilot study | 110 MI patients (66 STEMI & 44 NSTEMI) vs control | Galectin↓ at follow up if endpoint occurrence. Galectin > 9.2 ng/mL at discharge→x9 increase of risk of endpoint occurrence |
Galectin cut-off ≥9.2 ng/mL (91% specificity & 50% sensitivity) for MACE at follow-up | |
| Mitić et al., 2022 [84] | Cohort study | 89 patients undergoing PCI | Early galectin correlates with atherosclerosis. Day 30 galectin correlates with diastolic dysfunction and LV remodeling. |
- | |
| sST2 | Jenkins et al., 2017 [85] | Prospective longitudinal cohort | 1401 subjects with MI | Mortality increases at 5 yrs: 11.8%, 25.5% & 52% HF at 5 yrs: 11.4%, 23.6% & 44.8% in respective tertiles |
3 tertiles: T1: <37, T2: 37–72.3, T3: <72.3 ng/mL ST2 |
| Hartopo et al., 2018 [86] | Cohort study | 95 STEMI patients & 10 controls | Supramedian sST2 levels in STEMI patients 38.3% versus 12.5% higher incidence of MACE | STEMI vs controls: 152.1 ng/mL vs. 28.5 ng/mL, p < 0.01 | |
| Zhang et al., 2020 [87] | Meta-analysis | 16 studies | 3 groups:1. ischemic heart disease, 2. MI & 3 HF → No statistical significance between control and groups 1 & 2, significant only in 3. |
- | |
| D-dimers | Reihani et al., 2018 [60] | Cross- sectional study | 75 patients (34 with MI, 41 with UA) | Differentiation of MI (>548) from UA | Cut-off: 548 ng/mL (91.2% sensitivity & 63.4% specificity, p < 0.001) |
| Koch et al., 2022 [62] | Retrospective study | 435 patients with UA, 420 with NSTEMI, 22 NSTEMI, 2680 non coronary cause | PPV for final ACS diagnosis ↑ with d-dimer ↑ Unable to discriminate STEMI from non-coronary cause & UA. ↑d-dimer→↑risk of recurrent MI (esp. Q4) & all-cause mortality |
D-dimer concentrations (mg/L): 0.19–0.50 (Q1), 0.51–1.00 (Q2), 1.01–5.00 (Q3), and 5.01–35.00 (Q4). | |
| GDf-15 | Bonaca et al., 2010 [88] | Randomized control trial | 4162 patients with ACS, follow up for 2 years | significantly higher risk of death and MI | >1362 ng/L, higher rate of death or MI |
| Fibrinogen | Cetin et al., 2020 [65] | Observational study | 261 patients treated with PCI for ACS | FAR predictive of MACE | - |
| miR-483-5p | Zhao et al., 2023 [89] | Observational study | 118 patients with ACS and 75 healthy controls | Serum miR-483-5p levels were higher in ACS patients, high diagnostic value | Cut-off value of 1.292, demonstrated a feasible diagnostic value |
ACS: Acute Coronary Syndrome; CABG: Coronary Artery Bypass Grafting; CVD: Cardiac Vascular Disease; eGFR: estimated Glomerular Filtration Rate FAR: fibrinogen-to-albumin ratio; Gal-3bp: Galectin binding protein, GDF-15: Growth Differentiation Factor-15; HF: Heart Failure; LVEF: Left Ventricular Ejection Fraction; MACE: Major adverse cardiac events; MI: Myocardial Infarction; NRP: No-Reflow Phenomenon; MI: Myocardial Infarction; NPV: Negative Predictive Value; PCI: percutaneous coronary intervention; PPV: Positive Predictive Value; TIMI: Thrombolysis in Myocardial Infarction risk score.
6.1. Cystatin C (CysC)
Cystatin C (CysC) is a small 13 kDa molecular peptide and a cysteine protease inhibitor secreted by cells with a nucleus into the bloodstream. It is then streamed freely through the kidney glomerulus and almost thoroughly catabolized into amino acids in the proximal tubule without being secreted. A decline in estimated Glomerular Filtration Rate (eGFR) correlates with a decreased CysC, appointing CysC as a possible biomarker along with creatinine for kidney disease [90]. In fact, CysC interrelation with a worsening renal function as a predictor for mortality has actually been studied in the case of ACS: CysC peaked on the 3rd day after the ACS, whereas creatinine peaked on the 6th day. CysC also was significantly higher than creatinine during the 11.5-month follow-up, therefore predicting mortality or ACS repetition independently of the GRACE score [67]. Correa et al. emphasized a significant association between CysC and long-term risk (2.5 years) of Coronary Vascular Disease (CVD), HF hospitalization or MI for ACS patients [68]. Further, a recent meta-analysis established a significant link between CysC and all-cause death in ACS and MACE but claimed no significant prognostic value for recurrent MI (HR = 1.71 [95%CI: 0.99–2.97]) [69]. This was corroborated in another study, where CysC was prognostic for all-cause death in a 4-year observation interval, but no differences were noted regarding the incidence of non-fatal MI, stroke, UA, and unplanned revascularization [91]. Finally, regarding the prognosis after coronary revascularization in ACS patients, a meta-analysis claimed that higher CysC levels after a PCI were significantly related to mortality and MACE [70], while a different article acknowledged CysC as a possible predictor for the NRP after PCI [92]. Interestingly, high CysC levels before and after Coronary Artery By-Pass Graft Operation (CABG) could significantly relate to renal and cardiovascular effects postoperatively [93], an observation rebutted in the beforementioned meta-analysis with no statistically significant correlation between CysC levels after a CABG with MACE and mortality [70].
6.2. Heart-Type Fatty Acid Binding Protein (hFABP)
hFABP, a small soluble molecule normally found in the cardiomyocyte cytoplasm without being cardiac-specific, is responsible for transporting fatty acids [94]. hFABP is released in large concentrations into the plasma after injury, (usual cut-off 5–7 ng/mL), starts rising within 1 h of injury and peaks at 6–8 h (whereas cTn rises after 4–6 h), implicating a possible value for early MI and reinfarction detection. Multiple studies claim that hFABP has a higher sensitivity than single cTn (esp. at 3–6 h after MI), although there is a large heterogeneity between results, probably due to small patient pools, variable cut-off values, different assays, and time of testing [94,95]. In particular, a study has emphasized the ability of hFABP to rule out MI early and characterize up to 40% of patients as low-risk when combined with hs-cTn T [71]. This finding was supported by Van Hise et al.; however, the necessity of an additional biomarker was questioned, as hs-cTn I alone had similar sensitivity and higher specificity than the hFABP and troponin combination [72]. Similarly, in another study, whereas hFABP combined with cTnT at admission improved sensitivity, the same results were reproduced with the serial sampling of troponin at 0 and 90 min, securing the superiority of hs-cTn as a single biomarker for MI [73]. Therefore, the most promising diagnostic ability of hFABP agreed upon in various studies is the possibility of an early MI rule out, which under the results of hs-cTn I is questioned [71,72,74]. Finally, there is discordance between studies regarding the prognosis of mortality by the biomarker, possibly due to different sampling times and populations requiring further research [94,96].
6.3. Endocan
Endocan, otherwise endothelial cell-specific molecule 1, is a soluble dermatan sulfate proteoglycan excreted by the activated endothelial cells of vessels. Endocan is upregulated by a variety of proinflammatory cytokines (Tumor Necrosis Factor-a (TNF-α), Interleukin-1β (IL-1β), Vascular Endothelial Growth Factor-a (VEGF-a)), whereas it is implicated in the binding of white blood cells to the endothelium and in inflammatory processes by inducing the levels of Vascular Cell Adhesion Molecule (VCAM), Intercellular Adhesion Molecule (ICAM), and E-selectin [97]. As a result of these mechanisms, endocan has been considered a potential marker for many pathologies, namely sepsis, lung and kidney diseases, preeclampsia, and chronic heart failure [98]. Regarding cardiovascular disease, endocan can potentially act as a surrogate marker for hypertension [99], coronary artery disease (CAD), coronary slow flow [100], angina, and subclinical atherosclerosis [101,102]. Ziaee et al. [75] assessed endocan after a STEMI and UA/NSTEMI and concluded that endocan is independently correlated with MACE, increased thrombolysis in MI (TIMI) risk score and is found in higher levels in STEMI compared to the NSTEMI/UA group. The prognostic role of endocan was also examined in another study, where endocan levels were elevated in the presence of STEMI compared to controls and were associated with increased cardiovascular risk and a high SYNTAX score [76]. Interestingly, due to its pro-inflammatory properties, endocan could possibly assist in predicting the NRP following PCI in STEMI patients, as its levels were significantly increased in the NRP (+) group compared to the control [77]. In contrast, patients undergoing CABG after an ACS had diminished endocan serum levels upon successful reperfusion [78].
6.4. Galectin
Galectin-3, a β-galactoside-binding lectin, is predominantly secreted by macrophages and is expressed in a variety of tissues, such as cardiac, renal, hepatic, pulmonary, and vascular tissues. Galectin induces healing and fibrosis by differentiating fibroblasts into myofibroblasts, synthesizing collagen I and III, and contributing to scar formation [103,104]. Galectin-3 is additionally implicated in atherosclerosis as it is upregulated in unstable plaques and attracts monocytes, therefore propagating inflammation [105]. Galectin-3 has been linked to CAD [106] and has been proposed as a prognostic biomarker for HF [104]. A recent meta-analysis examined the interrelation of galectin-3 with MACE following a MI, concluding on: a. a significant negative association between galectin and Left Ventricular Ejection Fraction (LVEF) during and after the MI; b. a non-significant inverted interrelation between galectin and the infarct size; and c. a significant prediction of MACE and all cause-mortality with higher galectin-3 levels [80]. A multitude of studies have emphasized the possible use of galectin as a risk stratification biomarker for MI outcomes. In a study, increased levels sampled 2 days after a MI translated to increased mortality in 5-year follow-up as well as a higher incidence of HF; all associations were found independent of troponin T levels [81]. Similarly, elevated galectin 3 binding protein during a MI correlated with markers of inflammation (IL-1β, fibrinogen, and high-sensitivity CRP), and in a 12-month follow-up, galectin 3 binding protein was associated with increased risk of angina or reinfarction and galectin-3 with all-cause mortality [82]. Another study observed no differences in galectin levels between STEMI and NSTEMI at baseline; however, a positive correlation with hyperlipidemia and carotid atherosclerosis was observed, and during follow-up only patients who did not have a subsequent MI, PCI, CABG, or stroke had a decline in galectin-3 [83]. Finally, galectin couldn’t differentiate between stable angina, NSTEMI, and STEMI in a group of patients; however, in an early stage (1–5 days), it was correlated with atherosclerotic factors (hypertension history and triglycerides), and on the 30-day follow-up, galectin-3 was predictive of diastolic dysfunction and LV remodeling [84].
6.5. Soluble Suppression of Tumorigenicity (sST2)
Soluble Suppression of Tumorigenicity 2 factor (sST2), an isoform of ST2, is a portion of the IL-1 receptor family, with its levels increasing in inflammatory diseases as well as in several heart diseases. sST2 is produced by stretching cardiomyocytes and fibroblasts and binds its ligand interleukin-33 (IL-33), not allowing for the desired anti-inflammatory and antifibrotic interaction of IL-33 and the STL2 ligand (STL2L) isoform (transmembrane receptor) [107,108]. ST2 therefore acts as a decoy receptor, and the sST2/IL33 complex may be associated with cardiomyocyte hypertrophy, fibrosis, and remodeling [87,107]. Numerous studies have already proven the strength of sST2 as a prognostic marker for HF, with a proposed cutoff for life-threatening cardiovascular events of 35 ng/mL [107,109]. Aleksova et al., propose specific algorithms both for HF and MI: a. Diagnosis of acute decompensated HF (ADHF) is fairly common (40%) for sST2 > 35ng/mL with values > 70 ng/mL requiring hospitalization; b. Prognosis of Type 1 MI: sST2 can assist with treatment decisions as for <35 ng/mL standard care is proposed, 35–70 ng/mL adverse remodeling is likely and for >70 ng/mL fibrosis is commonly activated and aggressive therapies are needed; and c. Prognosis of Type 2 MI: similar cutoffs and clinical decisions to Type 1 MI [110]. Regarding the ACS, Jenkins et al. suggested that sST2 is associated with age, female sex, and co-morbidities and categorized the sST2 values into 3 tertiles, with patients in the 3rd tertile presenting with a sixfold hazard of mortality and heart failure at 5 years. sST2 in their study did not have a significant relationship with troponin and ECG; therefore, it can be assumed that ST2 as a biomarker expresses different pathophysiologic processes from myocardial injury and ischemia/infarcted burden [85]. Similarly, in a cohort study with 95 STEMI patients enrolled, the mean sST2 was significantly higher in MI patients than in controls whereas for values above the medium, significantly more cardiac adverse effects, and especially acute heart failure, occurred [86]. These findings are, however, challenged in a recent meta-analysis of 16 studies: out of 3 assigned groups (1. ischemic heart disease; 2. MI; and 3. HF), only the HF group had a significant increase in sST2 levels compared to healthy subjects [87]. Finally, high sST2 values have a significant predictive role in the NRP or impaired myocardial reperfusion after a PCI in STEMI patients [111,112].
6.6. Micro-RNA
MicroRNAs (miRNAs) are a group of non-coding Ribonucleic Acids (RNAs) that play a significant role in controlling gene expression. Dysregulated miRNA expression is linked to numerous diseases. Interestingly, miRNAs can be released into extracellular fluids, where they can act as potential biomarkers for various conditions and also function as signaling molecules, facilitating cell-to-cell communication [113]. Many research investigations have demonstrated the role of miRNAs in cardiovascular diseases, controlling a wide range of processes such as cardiomyocyte death, cell growth, inflammation, and blood vessel formation [114]. The initial molecules demonstrated to hold prognostic relevance concerning mortality were miRNA-133a and miRNA-208b. These microRNAs were associated with a significant rise in all-cause mortality at 6 months following AMI [115]. Research has demonstrated that miRNA-145 can serve as a prognostic biomarker for cardiovascular mortality and the progression of heart failure [116]. MiRNAs deriving from the cardiac cells, namely miR-1, miR-195, miR-133, miR-126, miR-16, miR-590, miR-199, miR-143, miR-208a, miR-499, miR-27-b, miR-497, miR-126, miR-30-d, miR-208b, miR-15a/b, and miR-16-1/2, play crucial roles in supervising the growth of the cardiac system. Of particular interest are miR-1 and miR-133, which are transcribed together in high amounts specifically in the heart but have contrasting functions in the tissue by promoting cell proliferation while inhibiting cardiac differentiation. Conversely, miR-499 and miR-208, expressed at lower levels in the heart, demonstrate greater specificity for cardiac injury compared to skeletal muscle. miRNAs can enhance genome expression by attaching to promoter sequences and targeted regions; as a result, numerous miRNA transcription compositions are implicated in key pathways related to ACS. These miRNAs are involved in oxidative stress, inflammation, apoptosis, fibrosis, and cardiac remodeling processes that contribute to the pathophysiology of ACS [117]. Serum miR-483-5p, has been recently studied as a potential diagnostic marker for ACS and for its ability to predict major adverse cardiac events after PCI. It was shown that higher levels of miR-483-5p were present in ACS subjects and were linked to the severity of the condition. The miR-483-5p test effectively distinguished ACS patients from healthy controls and differentiated AMI patients from ACS patients. Patients with elevated miR-483-5p had a higher likelihood of experiencing MACE, and miR-483-5p was a strong indicator of MACE incidence after PCI [89].
Measuring plasma miR-22 shortly after admission may serve as a diagnostic tool for MI and a predictor of left ventricular remodeling. However, the reliability of this approach may be influenced by factors like diabetic status and blood parameters, paving the way for further research to enhance patient care and interventions [118].
Due to their ability to control numerous genes through various signaling pathways, miRNAs hold significant promise as innovative therapeutic tools, with miRNA-based approaches extensively applied in areas such as angiogenesis, atherosclerosis, ischemic injury, vascular remodeling, hypertrophy, and fibrosis [119]. MiR-195-3p may play a pivotal role in the development of cardiac fibrosis and dysfunction following a heart attack. Inhibiting miR-195-3p might be a valuable approach for preventing cardiac fibrosis and maintaining heart function after a heart attack [120]. A recent study examined potential gene signatures associated with cardioprotection. The analysis identified 91 differentially expressed genes that may have relevance to AMI. Furthermore, the analysis highlighted the involvement of miR-660 and STAT1, known to impact AMI severity. These genes and miRNA could be pivotal in rescuing cardiomyocytes from severe damage, offering potential insights for the development of therapeutic strategies in AMI management [121].
While miRNAs show promise as potential key players in the early diagnosis of MI, further research is imperative to establish their potency as cardiac biomarkers and their potential implementation in everyday practice [39]. Lastly, long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) can also shed light on the diagnosis and treatment of cardiovascular diseases [122].
6.7. F2 Isoprostanes
F2 isoprostanes are byproducts of arachidonic acid metabolism and are expressed in various cells, including monocytes, during atherosclerotic procedures due to oxidative stress. Research has revealed elevated levels of these compounds in the urine of patients with UA. As potential biomarkers, F2 isoprostanes hold promise in predicting complications in nonfatal MI as well as the progression of HF and mortality [123].
6.8. Soluble Oxidized Low-Density Lipoprotein Receptor-1 (sLOX-1)
Soluble oxidized low-density lipoprotein receptor-1 (sLOX-1) was initially identified in 1997 as a scavenger receptor for modified low-density lipoprotein (LDL) found on the endothelium of blood vessels [124].
The research focused on the relationship between sLOX-1 and ACS in individuals with atherosclerotic CVD. ACS patients presented with augmented sLOX-1 levels compared to chronic coronary syndrome patients and healthy controls. Elevated sLOX-1 levels were independently interrelated with a heightened mortality hazard at both 30 days and 1 year. The link between sLOX-1 and cardiovascular mortality was particularly strong. In ACS patients receiving intracoronary imaging and statin therapy, those with coronary plaque regression at 1 year showed a significant reduction in sLOX-1 levels, and sLOX-1 demonstrated good predictive ability for plaque progression. In conclusion, increased plasma sLOX-1 levels during an ACS are associated with mortality in individuals with CVD, and continually high sLOX-1 is associated with coronary plaque progression in those with a history of atherosclerotic CVD [125].
6.9. Growth Differentiation Factor 15
Growth Differentiation Factor-15 (GDF-15), formerly recognized as a Non- Steroidal Anti-Inflammatory Drug (NSAID)—activated gene 1 (NAG-1) and macrophage inhibitory cytokine 1 (MIC-1), belongs to the Transforming Growth Factor β (TGF-β) family but stands apart. It is linked to cardiovascular disease and a range of other conditions such as inflammation, oxidative stress, and cellular stress [126]. Elevated GDF-15 levels have been associated with an increased occurrence of cardiovascular events observed in both cases of ST-segment- elevation MI and non-ST-elevation ACS [127]. Moreover, its levels increase with tissue damage and inflammatory conditions. Another study investigated the correlation between GDF-15 concentrations and the likelihood of repeated cardiovascular events in patients who had achieved stabilization after experiencing ACS. It was found that elevated GDF-15 levels were autonomously associated with a heightened probability of recurrent events, suggesting its viability as an indicator for evaluating forthcoming risk [88].
7. Conclusions
In conclusion, biomarkers play a pivotal role in diagnosing, stratifying risk, and assessing the prognosis of ACS. In this review article, we systematically discuss the role of several biomarkers, categorizing them based on their mechanisms of action and involved pathways, such as myocardial injury, neurohormonal activation, inflammation, and thrombosis. While this approach provides valuable pathophysiological insights, it’s important to note that the diagnostic and prognostic significance, as well as the clinical utility of most of the investigated biomarkers, is not well established. Accordingly, hs-cTn stands out, revolutionizing ACS diagnosis due to its exceptional sensitivity and negative predictive value underscoring the importance of ongoing research for the establishment and development of biomarkers with added prognostic or diagnostic value in ACS settings.
Author Contributions
M.K., I.K., V.T., P.T., G.C., G.M., I.G., K.Z., A.A., E.K., K.K. and O.K. conception and Drafting the work. E.O., M.V., G.S. and D.T. conception and reviewing it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Collaborators G.B.D.C.o.D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmis A., Vardas P., Townsend N., Torbica A., Katus H., De Smedt D., Gale C.P., Maggioni A.P., Petersen S.E., Huculeci R., et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022;43:716–799. doi: 10.1093/eurheartj/ehab892. [DOI] [PubMed] [Google Scholar]
- 3.Crea F., Libby P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation. 2017;136:1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne R.A., Rossello X., Coughlan J.J., Barbato E., Berry C., Chieffo A., Claeys M.J., Dan G.-A., Dweck M.R., Galbraith M., et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023:ehad191. doi: 10.1093/eurheartj/ehad191. [DOI] [Google Scholar]
- 5.Shah A.S.V., Anand A., E Strachan F., Ferry A.V., Lee K.K., Chapman A.R., Sandeman D., Stables C.L., Adamson P.D., Andrews J.P.M., et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: A stepped-wedge, cluster-randomised controlled trial. Lancet. 2018;392:919–928. doi: 10.1016/S0140-6736(18)31923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saenger A., Korpi-Steiner N. Advances in Cardiac Biomarkers of Acute Coronary Syndrome. Adv. Clin. Chem. 2017;78:1–58. doi: 10.1016/bs.acc.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Collet J.-P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 8.Karády J., Mayrhofer T., Ferencik M., Nagurney J.T., Udelson J.E., Kammerlander A.A., Fleg J.L., Peacock W.F., Januzzi J.L., Koenig W., et al. Discordance of High-Sensitivity Troponin Assays in Patients With Suspected Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2021;77:1487–1499. doi: 10.1016/j.jacc.2021.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta V., Paranzino M., Alnabelsi T., Ayoub K., Eason J., Mullis A., Kotter J.R., Parks A., May L., Nerusu S., et al. 5th generation vs 4th generation troponin T in predicting major adverse cardiovascular events and all-cause mortality in patients hospitalized for non-cardiac indications: A cohort study. PLoS ONE. 2021;16:e0246332. doi: 10.1371/journal.pone.0246332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipinski M.J., Baker N.C., Escárcega R.O., Torguson R., Chen F., Aldous S.J., Christ M., Collinson P.O., Goodacre S.W., Mair J., et al. Comparison of conventional and high-sensitivity troponin in patients with chest pain: A collaborative meta-analysis. Am. Hear. J. 2015;169:6–16.e6. doi: 10.1016/j.ahj.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Mueller C. Biomarkers and acute coronary syndromes: An update. Eur. Heart J. 2013;35:552–556. doi: 10.1093/eurheartj/eht530. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.-C., Huang S.-S., Yeo Y.H., Hou Y.-T., Park J.Y., Inoue K., Hsu W.-T. High-sensitivity-cardiac troponin for accelerated diagnosis of acute myocardial infarction: A systematic review and meta-analysis. Am. J. Emerg. Med. 2020;38:1402–1407. doi: 10.1016/j.ajem.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Gimenez M.R., Twerenbold R., Reichlin T., Wildi K., Haaf P., Schaefer M., Zellweger C., Moehring B., Stallone F., Sou S.M., et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur. Heart J. 2014;35:2303–2311. doi: 10.1093/eurheartj/ehu188. [DOI] [PubMed] [Google Scholar]
- 14.Artunc F., Mueller C., Breidthardt T., Twerenbold R., Peter A., Thamer C., Weyrich P., Haering H.-U., Friedrich B. Sensitive Troponins–Which Suits Better for Hemodialysis Patients? Associated Factors and Prediction of Mortality. PLoS ONE. 2012;7:e47610. doi: 10.1371/journal.pone.0047610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loria V., Leo M., Biasillo G., Dato I., Biasucci L.M. Biomarkers in Acute Coronary Syndrome. Biomark Insights. 2008;3:453–468. doi: 10.4137/BMI.S588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aydin S., Ugur K., Aydin S., Sahin İ., Yardim M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019;15:1–10. doi: 10.2147/VHRM.S166157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pöyhönen P., Kylmälä M., Vesterinen P., Kivistö S., Holmström M., Lauerma K., Väänänen H., Toivonen L., Hänninen H. Peak CK-MB has a strong association with chronic scar size and wall motion abnormalities after revascularized non-transmural myocardial infarction—A prospective CMR study. BMC Cardiovasc. Disord. 2018;18:27. doi: 10.1186/s12872-018-0767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohi T., Maehara A., Brener S.J., Généreux P., Gershlick A.H., Mehran R., Gibson C.M., Mintz G.S., Stone G.W. Utility of Peak Creatine Kinase-MB Measurements in Predicting Myocardial Infarct Size, Left Ventricular Dysfunction, and Outcome After First Anterior Wall Acute Myocardial Infarction (from the INFUSE-AMI Trial) Am. J. Cardiol. 2015;115:563–570. doi: 10.1016/j.amjcard.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Hendriks T., Hartman M.H.T., Vlaar P.J.J., Prakken N.H.J., van der Ende Y.M.Y., Lexis C.P.H., van Veldhuisen D.J., van der Horst I.C.C., Lipsic E., Nijveldt R., et al. Predictors of left ventricular remodeling after ST-elevation myocardial infarction. Int. J. Cardiovasc. Imaging. 2017;33:1415–1423. doi: 10.1007/s10554-017-1131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndrepepa G., Colleran R., Braun S., Xhepa E., Hieber J., Cassese S., Fusaro M., Kufner S., Laugwitz K.-L., Schunkert H., et al. Comparative prognostic value of postprocedural creatine kinase myocardial band and high-sensitivity troponin T in patients with non-ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2018;91:215–223. doi: 10.1002/ccd.27105. [DOI] [PubMed] [Google Scholar]
- 21.Gho J.M.I.H., Postema P.G., Conijn M., Bruinsma N., de Jong J.S.S.G., Bezzina C.R., Wilde A.A.M., Asselbergs F.W. Heart failure following STEMI: A contemporary cohort study of incidence and prognostic factors. Open Hear. 2017;4:e000551. doi: 10.1136/openhrt-2016-000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei W., Zhang L., Zhang Y., Tang R., Zhao M., Huang Z., Liu J., Xu D., He Y., Wang B., et al. Predictive value of creatine kinase MB for contrast-induced acute kidney injury among myocardial infarction patients. BMC Cardiovasc. Disord. 2021;21:337. doi: 10.1186/s12872-021-02155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Tao Y., Zhang L., Xu W., Zhou X. Diagnostic and prognostic value of biomarkers in acute myocardial infarction. Postgrad. Med. J. 2019;95:210–216. doi: 10.1136/postgradmedj-2019-136409. [DOI] [PubMed] [Google Scholar]
- 24.Morita E., Yasue H., Yoshimura M., Ogawa H., Jougasaki M., Matsumura T., Mukoyama M., Nakao K. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation. 1993;88:82–91. doi: 10.1161/01.CIR.88.1.82. [DOI] [PubMed] [Google Scholar]
- 25.De Lemos J.A., Morrow D.A., Bentley J.H. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl. J. Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 26.Omland T., Aakvaag A., Bonarjee V.V.S., Caidahl K., Lie R.T., Nilsen D.W.T., Sundsfjord J.A., Dickstein K. Plasma Brain Natriuretic Peptide as an Indicator of Left Ventricular Systolic Function and Long-term Survival After Acute Myocardial Infarction. Circulation. 1996;93:1963–1969. doi: 10.1161/01.CIR.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 27.Mueller T., Gegenhuber A., Dieplinger B., Poelz W., Haltmayer M. Long-term stability of endogenous B-type natriuretic peptide (BNP) and amino terminal proBNP (NT-proBNP) in frozen plasma samples. Clin. Chem. Lab. Med. 2004;42:942–944. doi: 10.1515/CCLM.2004.153. [DOI] [PubMed] [Google Scholar]
- 28.Omland T., A de Lemos J., A Morrow D., Antman E.M., Cannon C.P., Hall C., Braunwald E. Prognostic value of N-terminal pro-atrial and pro-brain natriuretic peptide in patients with acute coronary syndromes. Am. J. Cardiol. 2002;89:463–465. doi: 10.1016/S0002-9149(01)02271-8. [DOI] [PubMed] [Google Scholar]
- 29.Salama R.H., El-Moniem A.E., El-Hefney N., Samor T. N-TerminaL PRO-BNP in Acute Coronary Syndrome Patients with ST Elevation Versus Non ST Elevation in Qassim Region of Saudi Arabia. Int. J. Health Sci. 2011;5:136–145. [PMC free article] [PubMed] [Google Scholar]
- 30.Hermans W.R., Foley D.P., Rensing B.J., Rutsch W., Heyndrickx G.R., Danchin N., Mast G., Hanet C., Lablanche J.-M., Rafflenbeul W., et al. Usefulness of quantitative and qualitative angiographic lesion morphology, and clinical characteristics in predicting major adverse cardiac events during and after native coronary balloon angioplasty. CARPORT and MERCATOR Study Groups. Am. J. Cardiol. 1993;72:14–20. doi: 10.1016/0002-9149(93)90211-T. [DOI] [PubMed] [Google Scholar]
- 31.Lindholm D., James S.K., Gabrysch K., Storey R.F., Himmelmann A., Cannon C.P., Wallentin L. Faculty Opinions recommendation of Association of Multiple Biomarkers With Risk of All-Cause and Cause-Specific Mortality After Acute Coronary Syndromes: A Secondary Analysis of the PLATO Biomarker Study. J. AMA Cardiol. 2018;3:1160–1166. doi: 10.3410/f.734439722.793556985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrow D.A., de Lemos J.A., Sabatine M.S., Murphy S.A., Demopoulos L.A., DiBattiste P.M., McCabe C.H., Gibson C., Cannon C.P., Braunwald E. Evaluation of B-type natriuretic peptide for risk assessment in unstable Angina/Non–ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J. Am. Coll. Cardiol. 2003;41:1264–1272. doi: 10.1016/S0735-1097(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 33.Jernberg T., Lindahl B., Siegbahn A., Andren B., Frostfeldt G., Lagerqvist B., Stridsberg M., Venge P., Wallentin L. N-terminal pro-brain natriuretic peptide in relation to inflammation, myocardial necrosis, and the effect of an invasive strategy in unstable coronary artery disease. J. Am. Coll. Cardiol. 2003;42:1909–1916. doi: 10.1016/j.jacc.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Struck J., Morgenthaler N.G., Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26:2500–2504. doi: 10.1016/j.peptides.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Refardt J., Winzeler B., Christ-Crain M. Copeptin and its role in the diagnosis of diabetes insipidus and the syndrome of inappropriate antidiuresis. Clin. Endocrinol. 2019;91:22–32. doi: 10.1111/cen.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roczek-Janowska M., Kacprzak M., Dzieciol M., Zielinska M., Chizynski K. Prognostic value of copeptin in patients with acute myocardial infarction treated with percutaneous coronary intervention: A prospective cohort study. J. Thorac. Dis. 2021;13:4094–4103. doi: 10.21037/jtd-21-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smaradottir M.I., Andersen K., Gudnason V., Näsman P., Rydén L., Mellbin L.G. Copeptin is associated with mortality in elderly people. Eur. J. Clin. Investig. 2021;51:e13516. doi: 10.1111/eci.13516. [DOI] [PubMed] [Google Scholar]
- 38.Maisel A., Mueller C., Neath S.X., Christenson R.H., Morgenthaler N.G., McCord J., Peacock W.F. Copeptin helps in the early detection of patients with acute myocardial infarction: Primary results of the CHOPIN trial (Copeptin Helps in the early detection Of Patients with acute myocardial INfarction) J. Am. Coll. Cardiol. 2013;62:150–160. doi: 10.1016/j.jacc.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 39.del Val Martin D., Sanmartín Fernández M., Zamorano Gómez J.L. Biomarkers in acute coronary syndrome. IJC Metab. Endocr. 2015;8:20–23. doi: 10.1016/j.ijcme.2015.04.003. [DOI] [Google Scholar]
- 40.Khandkar C., Madhavan M.V., Weaver J.C., Celermajer D.S., Galougahi K.K. Atherothrombosis in Acute Coronary Syndromes—From Mechanistic Insights to Targeted Therapies. Cells. 2021;10:865. doi: 10.3390/cells10040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crea F., Liuzzo G. Pathogenesis of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2013;61:1–11. doi: 10.1016/j.jacc.2012.07.064. [DOI] [PubMed] [Google Scholar]
- 42.Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P. Hennekens CH. Inflammation, Aspirin, and the Risk of Cardiovascular Disease in Apparently Healthy Men. N. Engl. J. Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 43.Ridker P.M., Cannon C.P., Morrow D., Rifai N., Rose L.M., McCabe C.H., Pfeffer M.A., Braunwald E. C-Reactive Protein Levels and Outcomes after Statin Therapy. New Engl. J. Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 44.Biasucci L.M., Liuzzo G., Grillo R.L., Caligiuri G., Rebuzzi A.G., Buffon A., Summaria F., Ginnetti F., Fadda G., Maseri A. Elevated Levels of C-Reactive Protein at Discharge in Patients With Unstable Angina Predict Recurrent Instability. Circulation. 1999;99:855–860. doi: 10.1161/01.CIR.99.7.855. [DOI] [PubMed] [Google Scholar]
- 45.Mueller C., Buettner H.J., Hodgson J.M., Marsch S., Perruchoud A.P., Roskamm H., Neumann F.-J. Inflammation and Long-Term Mortality After Non–ST Elevation Acute Coronary Syndrome Treated With a Very Early Invasive Strategy in 1042 Consecutive Patients. Circulation. 2002;105:1412–1415. doi: 10.1161/01.CIR.0000012625.02748.62. [DOI] [PubMed] [Google Scholar]
- 46.Liebetrau C., Hoffmann J., Dörr O., Gaede L., Blumenstein J., Biermann H., Pyttel L., Thiele P., Troidl C., Berkowitsch A., et al. Release Kinetics of Inflammatory Biomarkers in a Clinical Model of Acute Myocardial Infarction. Circ. Res. 2015;116:867–875. doi: 10.1161/CIRCRESAHA.116.304653. [DOI] [PubMed] [Google Scholar]
- 47.Kamińska J., Koper O.M., Siedlecka-Czykier E., Matowicka-Karna J., Bychowski J., Kemona H. The utility of inflammation and platelet biomarkers in patients with acute coronary syndromes. Saudi J. Biol. Sci. 2018;25:1263–1271. doi: 10.1016/j.sjbs.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fanola C.L., Morrow D.A., Cannon C.P., Jarolim P., Lukas M.A., Bode C., Hochman J.S., Goodrich E.L., Braunwald E., O’Donoghue M.L., et al. Interleukin-6 and the Risk of Adverse Outcomes in Patients After an Acute Coronary Syndrome: Observations From the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib—Thrombolysis in Myocardial Infarction 52) Trial. J. Am. Hear. Assoc. 2017;6:e005637. doi: 10.1161/JAHA.117.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleveland O., Kunszt G., Bratlie M., Ueland T., Broch K., Holte E., Michelsen A.E., Bendz B., Amundsen B.H., Espevik T., et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: A double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 2016;37:2406–2413. doi: 10.1093/eurheartj/ehw171. [DOI] [PubMed] [Google Scholar]
- 50.Frangie C., Daher J. Role of myeloperoxidase in inflammation and atherosclerosis (Review) Biomed. Rep. 2022;16:53. doi: 10.3892/br.2022.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramachandra C.J., Ja K.M.M., Chua J., Cong S., Shim W., Hausenloy D.J. Myeloperoxidase As a Multifaceted Target for Cardiovascular Protection. Antioxidants Redox Signal. 2020;32:1135–1149. doi: 10.1089/ars.2019.7971. [DOI] [PubMed] [Google Scholar]
- 52.Sugiyama S., Okada Y., Sukhova G.K., Virmani R., Heinecke J.W., Libby P. Macrophage Myeloperoxidase Regulation by Granulocyte Macrophage Colony-Stimulating Factor in Human Atherosclerosis and Implications in Acute Coronary Syndromes. Am. J. Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calmarza P., Lapresta C., Martínez M., Lahoz R., Povar J. Utility of myeloperoxidase in the differential diagnosis of acute coronary syndrome. Arch. Cardiol. Mex. 2018;88:391–396. doi: 10.1016/j.acmx.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Loria V., Dato I., Graziani F., Biasucci L.M. Myeloperoxidase: A New Biomarker of Inflammation in Ischemic Heart Disease and Acute Coronary Syndromes. Mediat. Inflamm. 2008;2008:135625. doi: 10.1155/2008/135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldus S., Heeschen C., Meinertz T., Zeiher A.M., Eiserich J.P., Münzel T., Hamm C.W. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 56.Govindarajan S., Raghavan V.M., Rao A.C.V. Plasma Myeloperoxidase and Total Sialic Acid as Prognostic Indicators in Acute Coronary Syndrome. J. Clin. Diagn. Res. 2016;10:BC09-13. doi: 10.7860/JCDR/2016/20715.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolodziej A.R., Abo-Aly M., Elsawalhy E., Campbell C., Ziada K.M., Abdel-Latif A. Prognostic Role of Elevated Myeloperoxidase in Patients with Acute Coronary Syndrome: A Systemic Review and Meta-Analysis. Mediat. Inflamm. 2019;2019:2872607. doi: 10.1155/2019/2872607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yiu J.Y.T., Hally K.E., Larsen P.D., Holley A.S. Neutrophil-Enriched Biomarkers and Long-Term Prognosis in Acute Coronary Syndrome: A Systematic Review and Meta-analysis. J. Cardiovasc. Transl. Res. 2023:1–22. doi: 10.1007/s12265-023-10425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang N., Wang J.-X., Wu X.-Y., Cui Y., Zou Z.-H., Liu Y., Gao J. Correlation Analysis of Plasma Myeloperoxidase Level With Global Registry of Acute Coronary Events Score and Prognosis in Patients With Acute Non-ST-Segment Elevation Myocardial Infarction. Front. Med. 2022;9:828174. doi: 10.3389/fmed.2022.828174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reihani H., Shamloo A.S., Keshmiri A. Diagnostic Value of D-Dimer in Acute Myocardial Infarction Among Patients With Suspected Acute Coronary Syndrome. Cardiol. Res. 2018;9:17–21. doi: 10.14740/cr620w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayes-Genis A., Mateo J., Santaló M., Oliver A., Guindo J., Badimon L., Martínez-Rubio A., Fontcuberta J., Schwartz R.S., de Luna A.B. D -Dimer is an early diagnostic marker of coronary ischemia in patients with chest pain. Am. Hear. J. 2000;140:379–384. doi: 10.1067/mhj.2000.108823. [DOI] [PubMed] [Google Scholar]
- 62.Koch V., Booz C., Gruenewald L.D., Albrecht M.H., Gruber-RouhMD T., Eichler K., Yel I., Mahmoudi S., Scholtz J.-E., Martin S.S., et al. Diagnostic performance and predictive value of D-dimer testing in patients referred to the emergency department for suspected myocardial infarction. Clin. Biochem. 2022;104:22–29. doi: 10.1016/j.clinbiochem.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Biccirè F.G., Farcomeni A., Gaudio C., Pignatelli P., Tanzilli G., Pastori D. D-dimer for risk stratification and antithrombotic treatment management in acute coronary syndrome patients: A systematic review and metanalysis. Thromb. J. 2021;19:102. doi: 10.1186/s12959-021-00354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stătescu C., Anghel L., Tudurachi B.-S., Leonte A., Benchea L.-C., Sascău R.-A. From Classic to Modern Prognostic Biomarkers in Patients with Acute Myocardial Infarction. Int. J. Mol. Sci. 2022;23:9168. doi: 10.3390/ijms23169168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Çetin M., Erdoğan T., Kırış T., Özer S., Yılmaz A.S., Durak H., Aykan A., Şatıroğlu Ö. Predictive value of fibrinogen-to-albumin ratio in acute coronary syndrome. Herz. 2019;45:145–151. doi: 10.1007/s00059-019-4840-5. [DOI] [PubMed] [Google Scholar]
- 66.He D., Jiao Y., Yu T., Song J., Wen Z., Wu J., Duan W., Sun N., Sun Z., Sun Z. Prognostic value of fibrinogen-to-albumin ratio in predicting 1-year clinical progression in patients with non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention. Exp. Ther. Med. 2020;18:2972–2978. doi: 10.3892/etm.2019.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brankovic M., Kardys I., Berg V.v.D., Oemrawsingh R., Asselbergs F.W., van der Harst P., Hoefer I.E., Liem A., Maas A., Ronner E., et al. Evolution of renal function and predictive value of serial renal assessments among patients with acute coronary syndrome: BIOMArCS study. Int. J. Cardiol. 2020;299:12–19. doi: 10.1016/j.ijcard.2019.07.052. [DOI] [PubMed] [Google Scholar]
- 68.Correa S., Morrow D.A., Braunwald E., Davies R.Y., Goodrich E.L., Murphy S.A., Cannon C.P., O’Donoghue M.L. Cystatin C for Risk Stratification in Patients After an Acute Coronary Syndrome. J. Am. Hear. Assoc. 2018;7:e009077. doi: 10.1161/JAHA.118.009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun Y., Lu Q., Cheng B., Tao X. Prognostic value of cystatin C in patients with acute coronary syndrome: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2021;51:e13440. doi: 10.1111/eci.13440. [DOI] [PubMed] [Google Scholar]
- 70.Chen J., Yang Y., Dai C., Wang Y., Zeng R., Liu Q. Serum cystatin C is associated with the prognosis in acute myocardial infarction patients after coronary revascularization: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2022;22:156. doi: 10.1186/s12872-022-02599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young J.M., Pickering J.W., George P.M., Aldous S.J., Wallace J., Frampton C.M., Troughton R.W., Richards M.A., Greenslade J.H., Cullen L., et al. Heart Fatty Acid Binding Protein and cardiac troponin: Development of an optimal rule-out strategy for acute myocardial infarction. BMC Emerg. Med. 2016;16:34. doi: 10.1186/s12873-016-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Hise C.B., Greenslade J.H., Parsonage W., Than M., Young J., Cullen L. External validation of heart-type fatty acid binding protein, high-sensitivity cardiac troponin, and electrocardiography as rule-out for acute myocardial infarction. Clin. Biochem. 2018;52:161–163. doi: 10.1016/j.clinbiochem.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Collinson P., Gaze D., Thokala P., Goodacre S. Randomised Assessment of Treatment using Panel Assay of Cardiac markers–Contemporary Biomarker Evaluation (RATPAC CBE) Heal. Technol. Assess. 2013;17:v–vi, 1–122. doi: 10.3310/hta17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dupuy A.M., Cristol J.P., Kuster N., Reynier R., Lefebvre S., Badiou S., Jreige R., Sebbane M. Performances of the heart fatty acid protein assay for the rapid diagnosis of acute myocardial infarction in ED patients. Am. J. Emerg. Med. 2015;33:326–330. doi: 10.1016/j.ajem.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Ziaee M., Mashayekhi S., Ghaffari S., Mahmoudi J., Sarbakhsh P., Garjani A. Predictive Value of Endocan Based on TIMI Risk Score on Major Adverse Cardiovascular Events After Acute Coronary Syndrome. Angiology. 2019;70:952–959. doi: 10.1177/0003319718815241. [DOI] [PubMed] [Google Scholar]
- 76.Kundi H., Balun A., Cicekcioglu H., Karayigit O., Topcuoglu C., Kilinckaya M.F., Kiziltunc E., Cetin M., Ornek E. Admission Endocan Level may be a Useful Predictor for In-Hospital Mortality and Coronary Severity Index in Patients With ST-Segment Elevation Myocardial Infarction. Angiology. 2017;68:46–51. doi: 10.1177/0003319716646932. [DOI] [PubMed] [Google Scholar]
- 77.Dogdus M., Yenercag M., Ozyasar M., Yilmaz A., Can L.H., Kultursay H. Serum Endocan Levels Predict Angiographic No-Reflow Phenomenon in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Coronary Intervention. Angiology. 2021;72:221–227. doi: 10.1177/0003319720961954. [DOI] [PubMed] [Google Scholar]
- 78.Cimen A., Emet S., Elitok A. Endocan: A biomarker predicting successful reperfusion after coronary artery by-pass surgery of acute coronary syndrome patients. Eur. Rev. Med Pharmacol. Sci. 2019;23:338–342. doi: 10.26355/eurrev_201901_16781. [DOI] [PubMed] [Google Scholar]
- 79.Qiu C.R., Fu Q., Sui J., Zhang Q., Wei P., Wu Y., Zong B. Serum Endothelial Cell–Specific Molecule 1 (Endocan) Levels in Patients With Acute Myocardial Infarction and Its Clinical Significance: A Pilot Study. Angiology. 2017;68:354–359. doi: 10.1177/0003319716651349. [DOI] [PubMed] [Google Scholar]
- 80.Tian L., Chen K., Han Z. Correlation between Galectin-3 and Adverse Outcomes in Myocardial Infarction Patients: A Meta-Analysis. Cardiol. Res. Pr. 2020;2020:7614327. doi: 10.1155/2020/7614327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asleh R., Enriquez-Sarano M., Jaffe A.S., Manemann S.M., Weston S.A., Jiang R., Roger V.L. Faculty Opinions recommendation of Galectin-3 Levels and Outcomes After Myocardial Infarction: A Population-Based Study. J. Am. Coll. Cardiol. 2019;73:2286–2295. doi: 10.1016/j.jacc.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gagno G., Padoan L., Stenner E., Beleù A., Ziberna F., Hiche C., Paldino A., Barbati G., Biolo G., Fiotti N., et al. Galectin 3 and Galectin 3 Binding Protein Improve the Risk Stratification after Myocardial Infarction. J. Clin. Med. 2019;8:570. doi: 10.3390/jcm8050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Święcki P., Sawicki R., Knapp M., Kamiński K.A., Ptaszyńska-Kopczyńska K., Sobkowicz B., Lisowska A. Galectin-3 as the Prognostic Factor of Adverse Cardiovascular Events in Long-Term Follow up in Patients after Myocardial Infarction—A Pilot Study. J. Clin. Med. 2020;9:1640. doi: 10.3390/jcm9061640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitić B., Jovanović A., Nikolić V.N., Stokanović D., Andrejić O.M., Vučić R.M., Pavlović M., Ignjatović A., Momčilović S. Trend of Galectin-3 Levels in Patients with Non-ST-Elevation and ST-Elevation Myocardial Infarction. Medicina. 2022;58:286. doi: 10.3390/medicina58020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jenkins W.S., Roger V.L., Jaffe A.S., Weston S.A., AbouEzzeddine O.F., Jiang R., Manemann S.M., Enriquez-Sarano M. Prognostic Value of Soluble ST2 After Myocardial Infarction: A Community Perspective. Am. J. Med. 2017;130:1112.e9–1112.e15. doi: 10.1016/j.amjmed.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartopo A.B., Sukmasari I., Puspitawati I. The Utility of Point of Care Test for Soluble ST2 in Predicting Adverse Cardiac Events during Acute Care of ST-Segment Elevation Myocardial Infarction. Cardiol. Res. Pr. 2018;2018:3048941. doi: 10.1155/2018/3048941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang T., Xu C., Zhao R., Cao Z. Diagnostic Value of sST2 in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021;8:697837. doi: 10.3389/fcvm.2021.697837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonaca M.P., Morrow D.A., Braunwald E., Cannon C.P., Jiang S., Breher S., Wollert K.C. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: Observations from PROVE IT-TIMI 22. Arterioscler. Thromb. Vasc. Biol. 2011;31:203–210. doi: 10.1161/ATVBAHA.110.213512. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Y., Song X., Ma Y., Liu X., Peng Y. Circulating mir-483-5p as a novel diagnostic biomarker for acute coronary syndrome and its predictive value for the clinical outcome after PCI. BMC Cardiovasc. Disord. 2023;23:360. doi: 10.1186/s12872-023-03387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferguson T.W., Komenda P., Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr. Opin. Nephrol. Hypertens. 2015;24:295–300. doi: 10.1097/MNH.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 91.Saito T., Arashi H., Yamaguchi J., Mori F., Ogawa H., Hagiwara N. Elevated Cystatin-C Levels Are Associated with Increased Mortality in Acute Coronary Syndrome Patients: An HIJ-PROPER Sub-Analysis. Cardiorenal Med. 2022;12:20–28. doi: 10.1159/000522412. [DOI] [PubMed] [Google Scholar]
- 92.Cheng C., Liu X.-B., Bi S.-J., Lu Q.-H., Zhang J. Serum cystatin C levels relate to no-reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. PLoS ONE. 2019;14:e0220654. doi: 10.1371/journal.pone.0220654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shafranskaya K.S., Kashtalap V.V., Gruzdeva O.V., Kutikhin A.G., Barbarash O.L., Barbarash L.S. The Role of Cystatin C in the Prognosis of Adverse Outcomes after the Coronary Artery Bypass Graft Surgery During Hospitalisation. Hear. Lung Circ. 2015;24:193–199. doi: 10.1016/j.hlc.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 94.Bivona G., Agnello L., Bellia C., Sasso B.L., Ciaccio M. Diagnostic and prognostic value of H-FABP in acute coronary syndrome: Still evidence to bring. Clin. Biochem. 2018;58:1–4. doi: 10.1016/j.clinbiochem.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 95.Goel H., Melot J., Krinock M.D., Kumar A., Nadar S.K., Lip G.Y.H. Heart-type fatty acid-binding protein: An overlooked cardiac biomarker. Ann. Med. 2020;52:444–461. doi: 10.1080/07853890.2020.1800075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones J.D., Chew P.G., Dobson R., Wootton A., Ashrafi R., Khand A. The Prognostic Value of Heart Type Fatty Acid Binding Protein in Patients with Suspected Acute Coronary Syndrome: A Systematic Review. Curr. Cardiol. Rev. 2017;13:189–198. doi: 10.2174/1573403X13666170116121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J., Jiang L., Yu X.-H., Hu M., Zhang Y.-K., Liu X., He P., Ouyang X. Endocan: A Key Player of Cardiovascular Disease. Front. Cardiovasc. Med. 2022;8:798699. doi: 10.3389/fcvm.2021.798699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kosir G., Jug B., Novakovic M., Mijovski M.B., Ksela J. Endocan Is an Independent Predictor of Heart Failure-Related Mortality and Hospitalizations in Patients with Chronic Stable Heart Failure. Dis. Markers. 2019;2019:9134096. doi: 10.1155/2019/9134096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Behnoush A.H., Khalaji A., Bahiraie P., Alehossein P., Shobeiri P., Peisepar M., Cannavo A. Endocan as a marker of endothelial dysfunction in hypertension: A systematic review and meta-analysis. Hypertens. Res. 2023;46:1–12. doi: 10.1038/s41440-023-01402-y. [DOI] [PubMed] [Google Scholar]
- 100.Kundi H., Gok M., Kiziltunc E., Topcuoglu C., Cetin M., Cicekcioglu H., Ugurlu B., Ulusoy F.V. The Relationship Between Serum Endocan Levels With the Presence of Slow Coronary Flow: A Cross-Sectional Study. Clin. Appl. Thromb. 2017;23:472–477. doi: 10.1177/1076029615618024. [DOI] [PubMed] [Google Scholar]
- 101.Bessa J., Albino-Teixeira A., Reina-Couto M., Sousa T. Endocan: A novel biomarker for risk stratification, prognosis and therapeutic monitoring in human cardiovascular and renal diseases. Clin. Chim. Acta. 2020;509:310–335. doi: 10.1016/j.cca.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 102.Zhao T., Kecheng Y., Zhao X., Hu X., Zhu J., Wang Y., Ni J. The higher serum endocan levels may be a risk factor for the onset of cardiovascular disease: A meta-analysis. Medicine. 2018;97:e13407. doi: 10.1097/MD.0000000000013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li M., Yuan Y., Guo K., Lao Y., Huang X., Feng L. Value of Galectin-3 in Acute Myocardial Infarction. Am. J. Cardiovasc. Drugs. 2020;20:333–342. doi: 10.1007/s40256-019-00387-9. [DOI] [PubMed] [Google Scholar]
- 104.Meijers W.C., van der Velde A.R., Pascual-Figal D.A., de Boer R.A. Galectin-3 and post-myocardial infarction cardiac remodeling. Eur. J. Pharmacol. 2015;763:115–121. doi: 10.1016/j.ejphar.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 105.Papaspyridonos M., McNeill E., de Bono J.P., Smith A., Burnand K.G., Channon K.M., Greaves D.R. Galectin-3 Is an Amplifier of Inflammation in Atherosclerotic Plaque Progression Through Macrophage Activation And Monocyte Chemoattraction. Arter. Thromb. Vasc. Biol. 2008;28:433–440. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- 106.Kusaka H., Yamamoto E., Hirata Y., Fujisue K., Tokitsu T., Sugamura K., Sakamoto K., Tsujita K., Kaikita K., Hokimoto S., et al. Clinical significance of plasma galectin-3 in patients with coronary artery disease. Int. J. Cardiol. 2015;201:532–534. doi: 10.1016/j.ijcard.2015.08.099. [DOI] [PubMed] [Google Scholar]
- 107.Homsak E., Gruson D. Soluble ST2: A complex and diverse role in several diseases. Clin. Chim. Acta. 2020;507:75–87. doi: 10.1016/j.cca.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J., Chen Z., Ma M., He Y. Soluble ST2 in coronary artery disease: Clinical biomarkers and treatment guidance. Front. Cardiovasc. Med. 2022;9:924461. doi: 10.3389/fcvm.2022.924461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’gara P.T., Kushner F.G., Ascheim D.D., Casey D.E., Chung M.K., De Lemos J.A., Zhao D.X. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 110.Aleksova A., Paldino A., Beltrami A.P., Padoan L., Iacoviello M., Sinagra G., Emdin M., Maisel A.S. Cardiac Biomarkers in the Emergency Department: The Role of Soluble ST2 (sST2) in Acute Heart Failure and Acute Coronary Syndrome—There is Meat on the Bone. J. Clin. Med. 2019;8:270. doi: 10.3390/jcm8020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bai S., Liu H., Wu H., Wang X., Li R., Li X., Li X., Zhang L., Chen T., Du R. Predictive value of soluble suppression of tumourigenicity 2 on myocardial reperfusion. Intern. Med. J. 2020;50:985–992. doi: 10.1111/imj.14639. [DOI] [PubMed] [Google Scholar]
- 112.Somuncu M.U., Akgun T., Cakır M.O., Akgul F., Serbest N.G., Karakurt H., Can M., Demir A.R. The Elevated Soluble ST2 Predicts No-Reflow Phenomenon in ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Atheroscler. Thromb. 2019;26:970–978. doi: 10.5551/jat.48413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kansakar U., Varzideh F., Mone P., Jankauskas S.S., Santulli G. Functional Role of microRNAs in Regulating Cardiomyocyte Death. Cells. 2022;11:983. doi: 10.3390/cells11060983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Widera C., Gupta S.K., Lorenzen J.M., Bang C., Bauersachs J., Bethmann K., Kempf T., Wollert K.C., Thum T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J. Mol. Cell. Cardiol. 2011;51:872–875. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 116.Dong Y.-M., Liu X.-X., Wei G.-Q., Da Y.-N., Cha L., Ma C.-S. Prediction of long-term outcome after acute myocardial infarction using circulating miR-145. Scand. J. Clin. Lab. Investig. 2015;75:85–91. doi: 10.3109/00365513.2014.981855. [DOI] [PubMed] [Google Scholar]
- 117.Tanase D.M., Gosav E.M., Ouatu A., Badescu M.C., Dima N., Ganceanu-Rusu A.R., Popescu D., Floria M., Rezus E., Rezus C. Current Knowledge of MicroRNAs (miRNAs) in Acute Coronary Syndrome (ACS): ST-Elevation Myocardial Infarction (STEMI) Life. 2021;11:1057. doi: 10.3390/life11101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maries L., Moatar A.I., Chis A.R., Marian C., Luca C.T., Sirbu I.O., Gaiță D. Plasma hsa-miR-22-3p Might Serve as an Early Predictor of Ventricular Function Recovery after ST-Elevation Acute Myocardial Infarction. Biomedicines. 2023;11:2289. doi: 10.3390/biomedicines11082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Varzideh F., Kansakar U., Donkor K., Wilson S., Jankauskas S.S., Mone P., Wang X., Lombardi A., Santulli G. Cardiac Remodeling After Myocardial Infarction: Functional Contribution of microRNAs to Inflammation and Fibrosis. Front. Cardiovasc. Med. 2022;9:863238. doi: 10.3389/fcvm.2022.863238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carvalho A., Ji Z., Zhang R., Zuo W., Qu Y., Chen X., Tao Z., Ji J., Yao Y., Ma G. Inhibition of miR-195-3p protects against cardiac dysfunction and fibrosis after myocardial infarction. Int. J. Cardiol. 2023;387:131128. doi: 10.1016/j.ijcard.2023.131128. [DOI] [PubMed] [Google Scholar]
- 121.Kumar S., Shih C.-M., Tsai L.-W., Dubey R., Gupta D., Chakraborty T., Sharma N., Singh A.V., Swarup V., Singh H.N. Transcriptomic Profiling Unravels Novel Deregulated Gene Signatures Associated with Acute Myocardial Infarction: A Bioinformatics Approach. Genes. 2022;13:2321. doi: 10.3390/genes13122321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh D.D., Kim Y., Choi S.A., Han I., Yadav D.K. Clinical Significance of MicroRNAs, Long Non-Coding RNAs, and CircRNAs in Cardiovascular Diseases. Cells. 2023;12:1629. doi: 10.3390/cells12121629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.LeLeiko R.M., Vaccari C.S., Sola S., Merchant N., Nagamia S.H., Thoenes M., Khan B.V. Usefulness of Elevations in Serum Choline and Free F2-Isoprostane to Predict 30-Day Cardiovascular Outcomes in Patients With Acute Coronary Syndrome. Am. J. Cardiol. 2009;104:638–643. doi: 10.1016/j.amjcard.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 124.Sawamura T., Kume N., Aoyama T., Moriwaki H., Hoshikawa H., Aiba Y., Tanaka T., Miwa S., Katsura Y., Kita T., et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 125.Kraler S., A Wenzl F., Georgiopoulos G., Obeid S., Liberale L., von Eckardstein A., Muller O., Mach F., Räber L., Losdat S., et al. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts premature death in acute coronary syndromes. Eur. Heart J. 2022;43:1849–1860. doi: 10.1093/eurheartj/ehac143. [DOI] [PubMed] [Google Scholar]
- 126.Li M., Duan L., Cai Y.-L., Li H.-Y., Hao B.-C., Chen J.-Q., Liu H.-B. Growth differentiation factor-15 is associated with cardiovascular outcomes in patients with coronary artery disease. Cardiovasc. Diabetol. 2020;19:1–12. doi: 10.1186/s12933-020-01092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ullah A., Sajid S., Qureshi M., Kamran M., Anwaar M.A., Naseem M.A., Zaman M.U., Mahmood F., Rehman A., Shehryar A., et al. Novel Biomarkers and the Multiple-Marker Approach in Early Detection, Prognosis, and Risk Stratification of Cardiac Diseases: A Narrative Review. Cureus. 2023;15:e42081. doi: 10.7759/cureus.42081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.