Abstract
There has been an explosion in the number of papillomaviruses that have been identified and fully sequenced. Yet only a minute fraction of these has been studied in any detail. Most of our molecular research efforts have focused on the E6 and E7 proteins of “high-risk”, cancer-associated human papillomaviruses (HPVs). Interactions of the high-risk HPV E6 and E7 proteins with their respective cellular targets, the p53 and the retinoblastoma tumor suppressors, have been investigated in minute detail. Some have thus questioned if research on papillomaviruses remains an exciting and worthwhile area of investigation. However, fundamentally new insights on the biological activities and cellular targets of the high-risk HPV E6 and E7 proteins have been discovered and previously unstudied HPVs have been newly associated with human diseases. HPV infections continue to be an important cause of human morbidity and mortality and since there are no antivirals to combat HPV infections, research on HPVs should remain attractive to new investigators and biomedical funding agencies, alike.
Keywords: Human Papillomavirus, viral oncoproteins, signaling pathways, viral replication, persistent infection, viral oncogenesis
Introduction
In addition to the capsid proteins, all papillomaviruses express the E1 and E2 proteins that are essential for viral replication, viral genome partitioning during mitosis of the host cell, and transcriptional regulation of viral gene expression. In addition, most papillomaviruses also express a series of accessory proteins that function to enable the viral life cycle by rewiring important signaling circuits of the infected host cell. Amongst these, the E6 and E7 proteins of the “high-risk”, cancer-associated human papillomaviruses (HPVs) have been studied in detail. Despite what some say that we have arrived at a definitive mechanistic understanding of how these proteins and by extension the E6 and E7 proteins of other papillomaviruses function and that there is not much new to learn, many mysteries remain. Moreover, some papillomaviruses encode accessory proteins that remain essentially unstudied, and thus represent “unchartered territories”. The following is a biased and by no means complete selection of some of the mysteries and unchartered territories of papillomaviral accessory proteins that are ready for mechanistic investigation. For comprehensive discussions on the cellular targets of the E6 and E7 proteins, the role of HPVs in epithelial differentiation, and the link between papillomaviruses and epithelial stem cell dynamics, readers are referred to the following reviews1–5.
Knowns
Many of the early studies in the papillomavirus field focused on animal PVs. The bovine papillomavirus type 1 (BPV1) served as the go-to model for investigations on the mechanisms of viral replication, the regulation of viral gene transcription, and cellular transformation. The cotton tail rabbit papillomavirus (CRPV, now referred to as SfPV1) causes cutaneous papillomas, which can progress to frank carcinomas6 and thus provided the first animal model to study PV pathogenesis and carcinogenesis7,8.
PVs are small viruses with double-stranded, circular DNA genomes of less than 10,000 base pairs. Only one of the two strands is known to be transcribed. The genomic organization of these viruses is quite simple, and PV genomes consist of two regions that encode proteins and a non-coding regulatory region that contains various binding sites for viral and cellular transcription factors as well as the viral origin of replication. The early coding region contains two to nine open reading frames (ORFs) that encode nonstructural proteins whereas the late region encodes the major and minor capsid proteins, L1 and L2, respectively. All known PVs encode the core viral transcription and replication factors, E1 and E2. Most PVs also encode additional accessory proteins, including E4, E5, E6, E7, E9, and/or E109, which a colleague has referred to as “evolutionary embellishments”10.
Pioneering studies by Stefania Jablonska and Gerard Orth linked HPV infections to cutaneous squamous cell carcinomas (cSCCs) that emerge in individuals afflicted by a rare hereditary skin condition, epidermodysplasia verruciformis (EV)11. After the isolation of HPV16 sequences from a human cervical carcinoma by Harald zur Hausen’s group12, several other HPVs were isolated from cervical cancers13,14. It is now clear that infections with a small group of “high-risk” HPVs contribute to almost 5% of all human cancers worldwide. In addition to cervical carcinomas, high-risk HPV infections cause other anogenital tract cancers as well as a growing number of head and neck cancers, particularly oropharyngeal carcinomas15,16. Remarkably, HPV-associated cancers minimally express only two small viral proteins, E6 and E7, and the cancers remain “addicted” to expressing these two proteins17. Consequently, the biological activities of the high-risk HPV E6 and E7 proteins have been investigated most thoroughly.
An efficacious prophylactic vaccine became available within less than two decades after these HPVs were linked to cervical cancers18,19. Even a single administration shows great promise in preventing new infections by the specific HPV types that are targeted by the vaccine20. After the commercial launch of the HPV vaccine, a common sentiment (even by fellow virologists) was that mechanistic studies with HPVs were no longer needed because the cancer-causing HPVs were about to be eradicated. Sadly, this sentiment persists and, in the US, research grant applications to the National Institutes of Health (NIH) that focus on delineating oncogenic activities of HPVs are no longer routinely reviewed by virology-based review groups but are punted to general cancer panels where they often fail to generate enthusiasm. Consequently, there is a dearth of young investigators who dare to venture into studying HPV biology. This is even though the vaccine has not yet reached the medically underserved populations where the incidence of HPV-associated cancers is highest21 and vaccination rates remain low in many countries22. Moreover, many regions of the world currently experience an unabated, steep increase in HPV-associated oropharyngeal tumors23,24. Despite these sobering circumstances, there are no antivirals to combat HPV infections. Hence, it should be obvious that research on HPVs, even on the well-studied E6 and E7 proteins of the “high-risk” HPVs, remains necessary from a biomedical perspective. Moreover, mechanistic studies on HPV pathogenesis will continue to provide new and unexpected insights into the fascinating biology of these viruses and how they interact with and subvert their hosts.
How known are the “knowns” of the papillomavirus E6 and E7 proteins?
The sequences of >400 HPVs are available through the PAVE database25,26 and only a few mucosal “high-risk” alpha HPVs, mostly HPV16 and HPV18, have been studied in any detail. Mechanistic studies have mostly focused on their E6 and E7 proteins because they are known cancer drivers27,28. High-risk HPVs also encode E5 proteins, multi-pass transmembrane proteins that have been implicated in multiple biological processes, most frequently as activators of receptor tyrosine kinase signaling29. Many of these studies were likely motivated by results obtained with the BPV1 E5 protein. BPV1 E5 encodes a single-pass disulfide-linked dimeric transmembrane protein30, which forces the dimerization of receptor tyrosine kinases thereby activating them in the absence of ligand binding and is largely unrelated to high-risk HPV E5 proteins31. Unlike E6 and E7, expression of the high-risk HPV E5 proteins is not necessary for the maintenance of the transformed phenotype, but high-risk HPV E5 proteins may play important roles in blunting host cellular innate and adaptive immune responses32,33. Cutaneous beta and gamma HPVs do not encode E5 proteins, whereas the low-risk alpha HPVs as well as some animal PVs, can encode multiple E5 proteins that share only limited sequence similarities to the high-risk HPV E5 proteins (Figure 1; Table 1). Hence, much remains to be learned about the biological activities of the various PV E5 proteins34.
Figure 1: Examples of Papillomavirus E5 proteins.

The bovine papillomavirus 1 (BPV1) E5 protein has been studied in the greatest detail and encodes a single-pass transmembrane protein. The beta, delta, zeta, and some epsilon E5s are also predicted to encode single-pass membrane proteins but there is limited sequence similarity between the E5 proteins encoded by the different groups. Some epsilon E5 proteins do not have recognizable transmembrane domains. In contrast, the alpha and gamma E5s encode multi-pass transmembrane proteins. Potential transmembrane sequences as predicted by the CCTOP server (https://cctop.ttk.hu/) are highlighted in crimson. See Table 1 for a full listing of papillomaviruses that encode E5 proteins.
Table 1:
HPVs and animal PVs that encode E5 proteins.
| E5 Protein Family | Virus | Species | Host Common Name | GenBank ID | Notes |
|---|---|---|---|---|---|
| E5_Alpha | HPV16 | Alphapapillomavirus 9 | Human | K02718 | |
| HPV18 | Alphapapillomavirus 7 | Human | X05015 | ||
| HPV31 | Alphapapillomavirus 9 | Human | J04353 | ||
| HPV33 | Alphapapillomavirus 9 | Human | M12732 | ||
| HPV34 | Alphapapillomavirus 11 | Human | X74476 | ||
| HPV35 | Alphapapillomavirus 9 | Human | X74477 | ||
| HPV39 | Alphapapillomavirus 7 | Human | M62849 | ||
| HPV45 | Alphapapillomavirus 7 | Human | X74479 | ||
| HPV52 | Alphapapillomavirus 9 | Human | X74481 | ||
| HPV58 | Alphapapillomavirus 9 | Human | D90400 | ||
| HPV59 | Alphapapillomavirus 7 | Human | X77858 | ||
| HPV67 | Alphapapillomavirus 9 | Human | D21208 | ||
| HPV68 | Alphapapillomavirus 7 | Human | DQ080079 | ||
| HPV70 | Alphapapillomavirus 7 | Human | U21941 | ||
| HPV73 | Alphapapillomavirus 11 | Human | X94165 | ||
| HPV85 | Alphapapillomavirus 7 | Human | AF131950 | ||
| HPV97 | Alphapapillomavirus 7 | Human | DQ080080 | ||
| HPV177 | Alphapapillomavirus 11 | Human | KR816168 | ||
| E5_Beta | HPV2 | Alphapapillomavirus 4 | Human | X55964 | |
| HPV3 | Alphapapillomavirus 2 | Human | X74462 | ||
| HPV10 | Alphapapillomavirus 2 | Human | X74465 | ||
| HPV27 | Alphapapillomavirus 4 | Human | X74473 | ||
| HPV28 | Alphapapillomavirus 2 | Human | U31783 | ||
| HPV29 | Alphapapillomavirus 2 | Human | U31784 | ||
| HPV57 | Alphapapillomavirus 4 | Human | X55965 | ||
| HPV61 | Alphapapillomavirus 3 | Human | U31793 | ||
| HPV62 | Alphapapillomavirus 3 | Human | AY395706 | ||
| HPV71 | Alphapapillomavirus 14 | Human | AB040456 | ||
| HPV72 | Alphapapillomavirus 3 | Human | X94164 | ||
| HPV77 | Alphapapillomavirus 2 | Human | Y15175 | ||
| HPV78 | Alphapapillomavirus 2 | Human | KC138720 | ||
| HPV81 | Alphapapillomavirus 3 | Human | AJ620209 | ||
| HPV83 | Alphapapillomavirus 3 | Human | AF151983 | ||
| HPV84 | Alphapapillomavirus 3 | Human | AF293960 | ||
| HPV86 | Alphapapillomavirus 3 | Human | AF349909 | ||
| HPV87 | Alphapapillomavirus 3 | Human | AJ400628 | ||
| HPV89 | Alphapapillomavirus 3 | Human | AF436128 | ||
| HPV90 | Alphapapillomavirus 14 | Human | AY057438 | ||
| HPV94 | Alphapapillomavirus 2 | Human | AJ620211 | ||
| HPV102 | Alphapapillomavirus 3 | Human | DQ080083 | ||
| HPV106 | Alphapapillomavirus 14 | Human | DQ080082 | ||
| HPV114 | Alphapapillomavirus 3 | Human | GQ244463 | ||
| HPV117 | Alphapapillomavirus 2 | Human | GQ246950 | ||
| HPV125 | Alphapapillomavirus 2 | Human | FN547152 | ||
| HPV160 | Alphapapillomavirus 2 | Human | AB745694 | ||
| CgPV1 | Alphapapillomavirus 14 | Colobus monkey | GU014532 | ||
| NasiPV1 | Unclassified | Narrow-ridged finless porpoise | OQ274126 | Encodes E5_Beta and E5_Unclassified | |
| E5_Delta | HPV6 | Alphapapillomavirus 10 | Human | X00203 | Encodes E5_Delta and E5_Gamma |
| HPV7 | Alphapapillomavirus 8 | Human | X74463 | ||
| HPV11 | Alphapapillomavirus 10 | Human | M14119 | Encodes E5_Delta and E5_Gamma | |
| HPV13 | Alphapapillomavirus 10 | Human | X62843 | Encodes E5_Delta and E5_Gamma | |
| HPV32 | Alphapapillomavirus 1 | Human | X74475 | ||
| HPV40 | Alphapapillomavirus 8 | Human | X74478 | ||
| HPV43 | Alphapapillomavirus 8 | Human | AJ620205 | ||
| HPV44 | Alphapapillomavirus 10 | Human | U31788 | Encodes E5_Delta and E5_Gamma | |
| HPV74 | Alphapapillomavirus 10 | Human | AF436130 | Encodes E5_Delta and E5_Gamma | |
| HPV91 | Alphapapillomavirus 8 | Human | AF419318 | ||
| PpPV1 | Alphapapillomavirus 10 | Pygmy chimpanzee | X62844 | Encodes E5_Delta and E5_Gamma | |
| E5_Epsilon | BPV32 | Unclassified | Domestic cow | MW401529 | |
| BPV40 | Unclassified | Domestic cow | MW428425 | ||
| MfPV3 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558839 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV4 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558841 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV5 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558843 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV6 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558840 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV7 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558838 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV8 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558842 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV9 | Alphapapillomavirus 12 | Cynomolgus macaque | EU490516 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV10 | Alphapapillomavirus 12 | Cynomolgus macaque | EU490515 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV11 | Alphapapillomavirus 12 | Cynomolgus macaque | GQ227670 | Encodes E5_Epsilon and E5_Zeta | |
| MfuPV1 | Unclassified Alphapapillomavirus | Japanese macaque | KT944080 | Encodes E5_Epsilon and E5_Zeta | |
| MmPV1 | Alphapapillomavirus 12 | Rhesus macaque | M60184 | Encodes E5_Epsilon and E5_Zeta | |
| MmPV6 | Unclassified Alphapapillomavirus | Rhesus macaque | MH745748 | ||
| NasiPV3 | Unclassified | Narrow-ridged finless porpoise | OQ274128 | ||
| PhPV1 | Alphapapillomavirus 12 | Hamadryas baboon | JF304764 | Encodes E5_Epsilon and E5_Zeta | |
| PtroPV1 | Unclassified | Chimpanzee | OP934207 | ||
| E5_Gamma | HPV6 | Alphapapillomavirus 10 | Human | X00203 | Encodes E5_Delta and E5_Gamma |
| HPV11 | Alphapapillomavirus 10 | Human | M14119 | Encodes E5_Delta and E5_Gamma | |
| HPV13 | Alphapapillomavirus 10 | Human | X62843 | Encodes E5_Delta and E5_Gamma | |
| HPV44 | Alphapapillomavirus 10 | Human | U31788 | Encodes E5_Delta and E5_Gamma | |
| HPV74 | Alphapapillomavirus 10 | Human | AF436130 | Encodes E5_Delta and E5_Gamma | |
| PpPV1 | Alphapapillomavirus 10 | Pygmy chimpanzee | X62844 | Encodes E5_Delta and E5_Gamma | |
| E5_Zeta | MfPV3 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558839 | Encodes E5_Epsilon and E5_Zeta |
| MfPV4 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558841 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV5 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558843 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV6 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558840 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV7 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558838 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV8 | Alphapapillomavirus 12 | Cynomolgus macaque | EF558842 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV9 | Alphapapillomavirus 12 | Cynomolgus macaque | EU490516 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV10 | Alphapapillomavirus 12 | Cynomolgus macaque | EU490515 | Encodes E5_Epsilon and E5_Zeta | |
| MfPV11 | Alphapapillomavirus 12 | Cynomolgus macaque | GQ227670 | Encodes E5_Epsilon and E5_Zeta | |
| MfuPV1 | Unclassified Alphapapillomavirus | Japanese macaque | KT944080 | Encodes E5_Epsilon and E5_Zeta | |
| MmPV1 | Alphapapillomavirus 12 | Rhesus macaque | M60184 | Encodes E5_Epsilon and E5_Zeta | |
| PhPV1 | Alphapapillomavirus 12 | Hamadryas baboon | JF304764 | Encodes E5_Epsilon and E5_Zeta | |
| E5_Unclassified | HPV54 | Alphapapillomavirus 13 | Human | U37488 | |
| AaPV1 | Deltapapillomavirus 1 | European elk | M15953 | ||
| BPV1 | Deltapapillomavirus 4 | Domestic cow | X02346 | ||
| BPV2 | Deltapapillomavirus 4 | Domestic cow | M20219 | ||
| BPV13 | Deltapapillomavirus 4 | Domestic cow | JQ798171 | ||
| BPV14 | Deltapapillomavirus 4 | Domestic cow | KP276343 | ||
| BgPV1 | Deltapapillomavirus 4 | Yak | JX174437 | ||
| CcaPV1 | Deltapapillomavirus 5 | Western roe deer | EF680235 | ||
| EbonPV2 | Unclassified | Dwarf bonneted bat | OL704824 | ||
| GcPV1 | Unclassified Deltapapillomavirus | Giraffe | KX954132 | ||
| MmPV2 | Unclassified Alphapapillomavirus | Rhesus macaque | MG837557 | ||
| NasiPV1 | Unclassified | Narrow-ridged finless porpoise | OQ274126 | Encodes E5_Beta and E5_Unclassified | |
| OaPV1 | Deltapapillomavirus 3 | Domestic sheep | U83594 | ||
| OaPV2 | Deltapapillomavirus 3 | Domestic sheep | U83595 | ||
| OaPV4 | Unclassified Deltapapillomavirus | Domestic sheep | KX954121 | ||
| OcPV1 | Kappapapillomavirus 1 | New Zealand white rabbit | AF227240 | ||
| OjohPV1 | Unclassified Deltapapillomavirus | Okapi | MH049343 | ||
| OvPV1 | Deltapapillomavirus 2 | American white-tailed deer | M11910 | ||
| RalPV1 | Unclassified Deltapapillomavirus | Visayan spotted deer | KT626573 | ||
| RtPV1 | Deltapapillomavirus 1 | Reindeer | AF443292 | ||
| SfPV1 | Kappapapillomavirus 2 | Cottontail rabbit | K02708 |
Much progress has been made in delineating the biological activities of the high-risk HPV E6 and E7 proteins. The high-risk HPV E7 proteins bind and destabilize the retinoblastoma tumor suppressor protein, pRB35,36, thus “liberating” the E2F transcriptional activator and inducing aberrant proliferation. The mechanism of pRB destabilization remains unknown for most high-risk HPV E7 proteins. E7 expression causes activation of the p53 tumor suppressor. This cellular defense response is short-circuited by the E6 protein and the associated E6-AP (UBE3A) ubiquitin ligase which target p53 for degradation through the proteasome37. Combined inhibition of the pRB and p53 tumor suppressors opens the gates for unchecked aberrant cellular proliferation17. E6 also activates telomerase and in combination with E7-mediated pRB inactivation causes an extension of cellular lifespan, thereby facilitating cellular immortalization38,39. Since E6 and E7 expression interfere with genomic stability, high-risk HPV E6/E7-expressing cells are prone to acquiring additional chromosomal aberrations that facilitate malignant progression40. Variations of this enticing and seductive model of HPV carcinogenesis are widely published in review articles and textbooks even though it is incomplete, at best41. In addition to regulating E2F transcription factor activity, pRB also has additional, less extensively studied “non-canonical” biological activities that are relevant to its tumor suppressor function42 and these will also be inhibited as a result of destabilization by the high-risk HPV E7 proteins.
Similarly, the assumption that there is “one best replication strategy” for HPVs and that the E6 and E7 proteins by different HPV genera share similar biological activities and target the same cellular proteins has not been proven correct43–45. The E7 proteins of the low-risk mucosal alpha HPVs and the cutaneous beta HPVs that were originally linked to cSCCs in EV patients bind pRB with lower efficiency than high-risk HPVs and they do not destabilize pRB46,47. The E6 proteins of these viruses do not target p53 for degradation48 and cannot efficiently stimulate telomerase activity to enable cellular immortalization27,49. Some cutaneous gamma HPVs encode E7 proteins that bind pRB through different sequences which does not result in efficient activation of E2F target genes50,51. The cellular targets of the E6 proteins of beta HPVs associated with cSCCs in EV patients are distinct from those of the high-risk alpha HPV E6 proteins and impact pathways that regulate keratinocyte differentiation and genomic stability43–45. Thus, there is still much that remains to be learned regarding the “known” biological activities of the E5, E6, and E7 proteins and how they enable the viral life cycle.
Persistent infections - a question of target cells, cell competition, and basal cell identity?
HPV infections of the anogenital tract are very frequent. Most spontaneously resolve within a few months52,53 but some result in long-term persistent infections. Persistent cervical infections with high-risk HPVs constitute the major risk factor for the development of high-grade premalignant lesions and cancers15,54. Studies aimed at identifying the factors that contribute to the establishment of long-term, persistent HPV infections have only recently been initiated54–57. The immune status of the host plays an important role, as evidenced by the increased incidence of high-risk HPV-associated cervical and anal cancers in HIV-positive individuals58 and the emergence of beta HPV-associated cSCCs in immunosuppressed organ transplant patients59. The E2, E5, E6, and E7 proteins have each been implicated in blinding the host’s innate and/or adaptive immune system33,60–62. Whether or not persistently infected tissues constantly shed infectious HPVs or whether virus production is only activated occasionally and if so, what the triggers are has not been elucidated. Additionally, whether there are differences between persistently infected males and females is also unknown.
Stratified epithelia are dynamic, and most keratinocytes commit to differentiation and are replaced every few weeks, with only a few long-lived cells retained in the basal layer of stratified epithelia for the long term63. Even so, a key hallmark of many HPVs is their ability to establish persistent, long-term infections in the proliferative basal layer5. How papillomaviruses promote or retain basal cell identity, which is necessary for the retention of infected cells in the basal layer, has not yet been fully elucidated.
One theory has been that HPVs specifically infect a stem cell population in basal epithelia and a specific cell population within the squamocolumnar transformation zone of the cervix that may be at a higher risk for the development of persistent high-risk HPV infections64. The HPV16 E6 and E7 proteins may cause aberrant stem cell mobilization4,65 and similar findings have been reported for the E6 and E7 proteins of some cutaneous HPVs66. It will be challenging to experimentally determine whether the cell initially infected by HPV is a stem cell, and it is hard to imagine that a virus that could only persistently infect a minuscule subset of basal cells would be as highly prevalent as HPV.
An alternative and more likely model is that instead of targeting stem cells for initial infection, HPV-encoded proteins could reprogram infected keratinocytes to adopt a basal epithelial cell identity. Much like HPV proteins activating cell growth pathways, it is plausible that HPVs have evolved to act on pathways that control epithelial homeostasis to drive cells into a more stem-like state. Two such pathways that are central regulators of epithelial stemness are the Notch and Hippo/YAP signaling pathways.
Notch signaling is a well-known driver of keratinocyte differentiation67. There is already strong evidence that cutaneous HPV E6 proteins inactivate Notch. Several groups determined that cutaneous HPV E6 proteins inhibit differentiation by binding to the transcriptional co-regulator mastermind like 1 (MAML1), thereby repressing Notch signaling68–70. Like cutaneous HPV E6, the Mus musculus papillomavirus 1 (MmuPV1) E6, inhibits Notch signaling by binding MAML171, and it promotes basal identity in mouse lesions72. HPV16 E6 may also affect Notch signaling indirectly as a consequence of p53 inactivation. NOTCH1 is a transcriptional target of p53, and p53 inactivation downregulates NOTCH1 expression and downstream Notch signaling73,74. The role of HPV16 E6 in downregulating NOTCH1 mRNA levels is dependent on its ability to degrade p5375, but how well these effects on Notch are conserved in other mucosal HPV E6 proteins remains to be determined. Except for the MmuPV1 E6 experiments75, the studies that have tested the effects of E6 on Notch have mostly been performed in two-dimensional cell culture systems. It will be important to determine whether the Notch-suppressive effects of cutaneous HPV E6 proteins are associated with an ability to drive basal identity in lesions or three-dimensional tissue models.
The YAP1 oncoprotein is a potent driver of epithelial cell stemness and self-renewal76–81. YAP1 activation is sufficient to rapidly drive non-viral squamous epithelial cancers at mucosal sites in several mouse models82–85. YAP1 activity is suppressed by an active Hippo kinase cascade. Remarkably, the three mitogenic pathways that are most differentially altered with high mutation rates in HPV-negative HNSCC and low mutation rates in HPV-positive HNSCC are p53 (targeted by E6), cell cycle/RB1 (targeted by E7), and Hippo/YAP86,87. Recent mechanistic insight into how HPV oncoproteins activate YAP1 comes from research on the host cell targets of HPV E7 proteins. Highly conserved sequences in the C-terminus of HPV E7 enable E7 to bind directly and with nanomolar affinity to the tumor suppressor PTPN1488. PTPN14 is a suppressor of YAP189–92, and high-risk HPV E7 proteins activate YAP1 dependent on their ability to target PTPN14 for degradation86. Consistent with the findings that YAP1 drives an epithelial stemness gene expression program, high-risk HPV E7-expressing keratinocytes retain their basal cell identity as a consequence of PTPN14 degradation and YAP activation in engineered three-dimensional stratified epithelial tissues86. Independent confirmation of the concept that PTPN14 inactivation drives basal epithelial identity comes from an analysis that used large, genome-wide biobank sequencing datasets for unbiased identification of drivers of basal cell carcinoma. Remarkably, these analyses revealed that individuals with germline mutations in PTPN14 are highly predisposed to develop basal cell carcinoma, and likely, cervical cancer93. There are several reports that HPV E6 proteins can also activate YAP1 or promote its nuclear localization94–97 and it will be important to determine whether or how this contributes to the maintenance of basal cell identity.
Other recent experiments have used co-culture experiments to measure cell competition in two-dimensional cultures. In such experiments, high-risk HPV E6- but not E7-expressing basal keratinocytes have been shown to “outcompete” uninfected basal keratinocytes68,72,97. However, it is unclear whether the cell competition assays measure the same properties of keratinocytes that enable cells to be maintained in the basal layer. Induction of cell competition may allow for the lateral expansion of a pool of infected basal cells that are spawned from the initially infected cell. For the high-risk HPV16 E6 protein, induction of cell competition has been linked to p53 binding68 and E6AP activity97. These findings are consistent with reports that p53 promotes cell death in dense epithelial cultures98,99. In experiments with the MmuPV1 E6 proteins, an intact binding site for the transcriptional co-activator of Notch signaling, MAML1, was required for cell competition72.
It will be interesting to determine how intertwined these two activities of E6 and E7 are, which activities of E6 and/or E7 direct basal identity and cell competition, whether inhibiting these activities will block HPV persistence, and whether specific subsets of HPVs, such as high-risk HPVs, have a unique ability to promote basal identity and cell competition.
Why are HPV16 and other high-risk HPVs such successful, highly prevalent viruses?
Even though HPV16 has been more extensively studied than any other HPV, we know embarrassingly little about what makes it such a successful and thus frequently detected virus. HPV16 is the most prevalent HPV in cervical carcinoma and is even more predominant in other anogenital tract carcinomas and head and neck cancers. Because cancer formation often represents a dead end for the viral life cycle, it is not obvious why despite the frequent cancer association, HPV16 is such a “successful” and prevalent virus. The argument that cancer association should negatively affect evolutionary fitness can also be made for other high-risk HPVs.
Perhaps the high-risk HPVs and HPV16 in particular, are evolutionarily, nimbler, and thus they can more rapidly adapt to individual hosts. When Mirabello and colleagues sequenced HPV16 genomes from HPV16-infected individuals, they detected an astonishing number of sequence variants100. A subsequent large-scale sequencing study of HPV31-infected individuals yielded similar results101. HPV31 is a high-risk HPV102 and like HPV16 is a member of the alpha 9 species. In general, the detected variants represented a majority of the HPV species that were detected in a particular individual. Hence, they did not represent some minor, biologically irrelevant species, derived from a rare, abortive “accident” during genome replication100,101. The mechanisms by which these variants arise, however, have not been fully explored. Like other intracellular pathogens, some HPVs potently trigger the expression of members of the apolipoprotein B mRNA editing enzyme catalytic polypeptide 3 (APOBEC3) family of cytidine deaminases103. These are important components of the host cell’s arsenal of defense mechanisms and function as potent mutagens and garble the infecting pathogen’s genetic information104. Unlike many other pathogens, HPVs do not appear to mute this cellular defense response105, and many of the HPV16 variants detected in this study are consistent with APOBEC3 activity100,101. Those mutations that do not follow the APOBEC mutational signature may have arisen due to replication errors. HPV genomes are synthesized at sites of double-strand DNA breaks and may involve a more error-prone recombination-based replication mechanism, which could represent another important source for the generation of these variants106–108.
Some of the nucleotide changes present in these variants result in alterations of the coding sequence of the viral ORFs, including E6 and, remarkably to a far lesser extent, E7100,101. It will be interesting to determine whether some of these variants may be more or less carcinogenic109 and whether the presence of a specific variant may predict an increased or decreased risk for malignant progression. Moreover, it will be fascinating to investigate whether some of these HPV16 variants may have emerged and/or accumulated to be optimally compatible with the specific genetic makeup of an individual host. Consistent with this idea, it is well known that there are HPV16 variants that track with specific ethnic groups110,111.
Are there additional “high-risk” HPVs?
There are currently 12 mucosal alpha HPVs (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) that have been designated “carcinogenic to humans” by the World Health Organization/International Agency for Research on Cancer (WHO/IARC) as of 2012 with several other HPVs listed as “probably” or “possibly” carcinogenic112. Seven of the 12 carcinogenic HPVs (types 16, 18, 31, 33, 45, 52, and 58) are included in the current 9-valent prophylactic HPV vaccine113. As the transmission of HPV vaccine types is decimated in vaccinated populations, it will be important to determine whether other HPVs will fill the void, thus becoming more prevalent over time and potentially needing to be included in future vaccine preparations. This will necessitate the use of less biased survey mechanisms, such as next-generation sequencing (NGS), that can reliably detect any HPV type in a given specimen.
The existence of additional high-risk HPVs was demonstrated by the recent identification of HPV42 as the oncogenic driver of digital papillary adenocarcinoma. This was achieved by analyzing non-human reads from NGS data of human skin cancers114. HPV42 was isolated 30 years earlier from a vulvar papilloma and even though the authors noted that “Although associated with benign genital lesions, it shows characteristics so far found in HPVs associated with either invasive carcinomas or nongenital HPVs only”115 it was not further studied at that time.
There are likely other HPVs that deserve to be evaluated for their carcinogenic potential. These may include the gamma 6 HPVs, HPV101116, HPV103116, and HPV108117, and based on their genomic organization also HPV214118, HPV226119, and HPV-MW02C24ANR120. Most gamma HPVs infect cutaneous epithelia and have received almost no experimental attention because they are widely regarded as biomedically irrelevant normal skin commensals. The gamma 6 HPVs as well as HPV214 and 226, however, have all been isolated from the anogenital tract mucosa, with HPV101 and 108 having been isolated from high- and low-grade premalignant cervical lesions, respectively. Unlike all the other known >430 fully sequenced HPVs currently listed in the PAVE database, the gamma 6 HPVs do not contain E6 ORFs, but they encode in its place E10, a unique, 37 amino acid, hydrophobic, putatively helical transmembrane protein121 (Figure 2A).
Figure 2: E10 proteins encoded by HPVs and animal PVs.
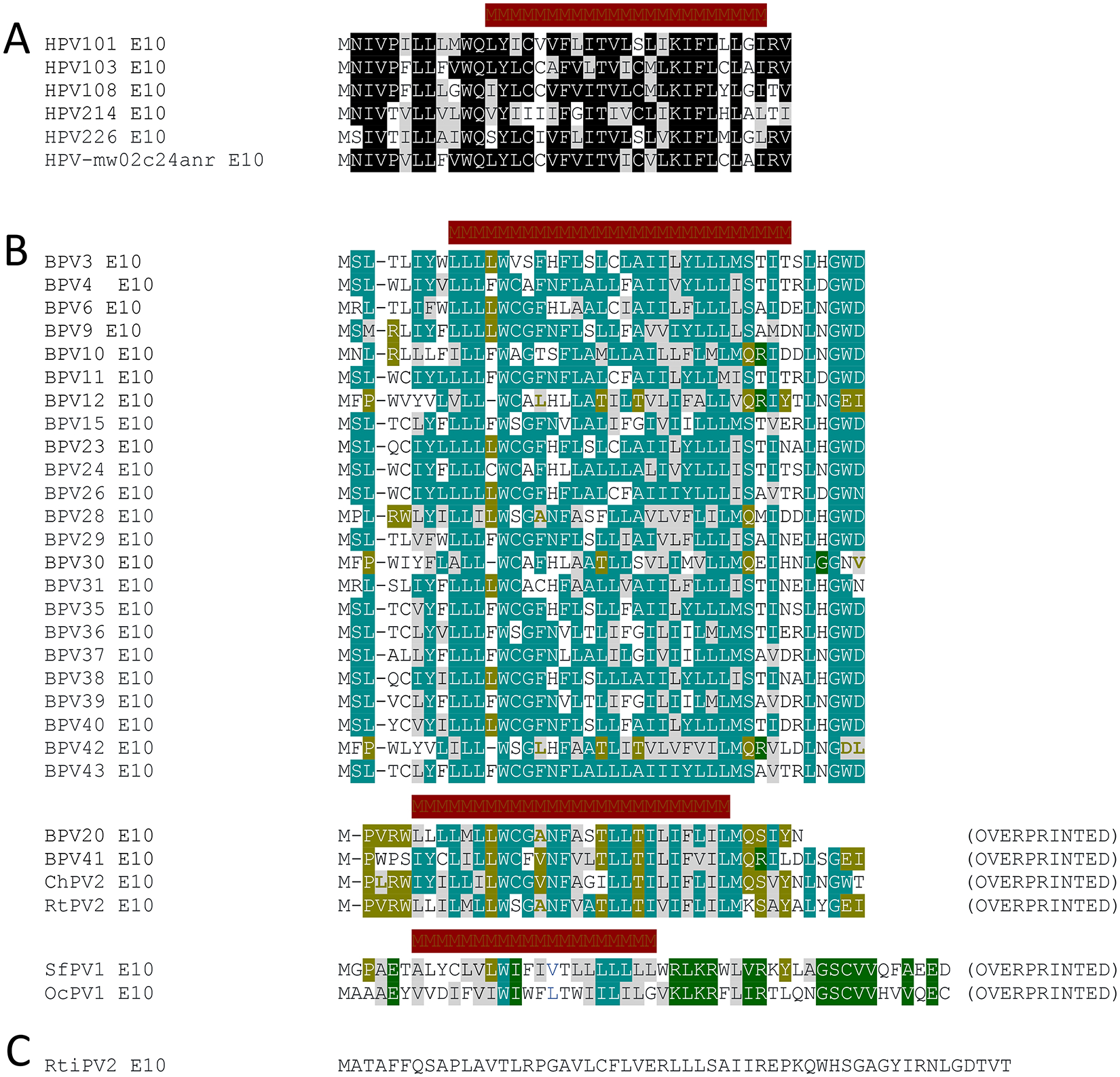
HPV101, HPV103 HPV108 are classified as gamma 6 PVs, whereas HPV214, HPV226, and HPV-mw02c24anr are currently unclassified gamma HPVs.
Identical residues are highlighted by black boxes and chemically similar residues by gray boxes. Potential transmembrane sequences as predicted by the CCTOP server (https://cctop.ttk.hu/) are indicated by crimson bars. (A). Many bovine papillomaviruses (BPVs) as well as a PV isolated from a domestic goat (Capra hircus Papillomavirus 2; ChPV2), raindeer (Rangifer tarandus Papillomavirus 2; RtPV2), cottontail rabbit (Sylvilagus floridanus Papillomavirus 1; SfPV1 aka CRPV), and New Zealand white rabbit (Oryctolagus cuniculus Papillomavirus 1; OcPV1) also encode E10 proteins. The E10s encoded by BPV20, BPV41, ChPV2, RtPV2, SfPV1 and OcPV1 are overprinted on the E6 ORF. Amino acid residues conserved amongst all the animal PV encoded E10s are highlighted by pale green boxes, overprinted E10 residues conserved amongst BPV20, BPV40, ChPV2, and RtPV2 are highlighted by olive-colored boxes, and residues conserved between SfPV1 and OcPV1 E10s by moss green boxes. Chemically similar amino acid residues are highlighted by gray boxes. Potential transmembrane sequences as predicted by the CCTOP server (https://cctop.ttk.hu/) are indicated by crimson bars. (B). A papillomavirus isolated from the Javan rusa (Rusa timorensis papillomavirus 2; RtiPV2) also encodes an E10 protein but it shares no extensive sequence similarity to other E10s and is not predicted to contain a transmembrane region (C).
Even though HPV5 and HPV8 were the first HPVs found to be associated with human cancers, the potential association of cutaneous beta and, potentially, some gamma HPVs with cSCCs in immune-competent, and not genetically predisposed patients remains tenuous. One provocative study has suggested the interesting possibility that infection with some cutaneous HPVs may in fact protect from human skin carcinogenesis122. It is currently unknown whether the cutaneous HPVs may also be classified as low- or high-risk similar to the mucosal HPVs. While some of the E6 and E7 proteins of the cutaneous beta HPVs have transforming activities in various cell- and animal-based model systems, the molecular mechanisms by which these viruses may contribute to cSCC development have not been fully delineated123,124. This matter is complicated by the fact that cutaneous HPVs are detected in bona fide precancerous lesions but not in most cancer cells, and hence they may only contribute to cancer initiation, but not to cancer maintenance. Such a “hit-and-run” carcinogenesis model is hard to prove, but a fascinating animal experiment has provided some experimental support for this concept. Transgenic mice where HPV38 E6 and E7 expression was targeted to the basal epithelial cells were much more highly susceptible to UV carcinogenesis than control mice, and the mutations in the lesions were similar to those seen in human cSCCs. Most importantly, the tumors persisted when the HPV 38 E6/E7 expression cassette was deleted using the cre-lox recombination system. This result is consistent with the model that at least some cutaneous HPVs can sensitize cells to UV-induced genomic instability and contribute to skin carcinogenesis through a “hit-and-run” mechanism125. Induction of genome instability is a well-recognized “enabling characteristic” that can facilitate the acquisition of cancer hallmarks,126 but is only one of the potential “hit-and-run” mechanisms by which viruses can contribute to cancer development127. Hence, it would not be surprising if there are additional HPVs that contribute to cancer initiation, but unlike the high-risk alpha HPVs, may not be necessary for tumor maintenance.
Are proteins the only important cellular targets and effectors of papillomaviruses?
Most of the research on papillomavirus-host interactions has focused on associated cellular proteins and modulation of protein-coding RNAs. However, most of the human transcriptome is noncoding128. RNA sequencing analyses have documented that HPVs can alter the expression of host cell non-coding RNAs. One class of non-coding RNA that has been investigated in some detail in the context of HPV infections are microRNAs (miRNAs). Whether some HPVs express miRNAs has been debated129–131. Modulation of cellular miRNA expression in HPV-expressing cells, however, has been extensively documented, but the effect of dysregulated miRNAs on viral and host cellular gene expression is not yet fully understood132,133. Moreover, miRNAs are also secreted in extracellular vesicles (EVs) and the miRNA content of EVs is altered in HPV oncogene-expressing cells134. Whether and how this may affect gene expression in neighboring, uninfected cells and/or tumor stromal cells will be an interesting area of future research.
In addition to miRNA, HPVs also affect the expression of other non-coding RNAs, including circular (circ)RNAs and long noncoding (lnc)RNAs. HPV16 produces a circRNA, which unlike most cellular circRNAs has coding capacity and can encode the E7 protein135. The level of expression and thus the biological relevance of this circRNA has been debated136,137, but the dysregulation of cellular circRNAs in human carcinogenesis has been well-documented138. LncRNAs are biochemically versatile molecules that can interact with other RNAs, as well as DNA and proteins, and have been implicated in many biological processes139. For example, the HPV16 E7 protein forms a complex with the lncRNA HOTAIR, which recruits the PRC2 chromatin remodeling complex to silence gene expression140. This suggests that HPV proteins may not only alter the expression of but also directly affect the biological activities of cellular lncRNAs. Studies on the functional interplay of HPV proteins with the various classes of non-coding RNAs will continue to yield fascinating insights into the strategies by which these viruses functionally reprogram their host cells.
Is there only a single papillomavirus that infects standard laboratory mice?
The species specificity has long been a major barrier to HPV pathogenesis research. The “classical” CRPV and BPV1 models have provided interesting insights, but rabbits and particularly cattle are not handled in typical academic animal facilities. Experiments with non-human primates are prohibitively expensive and difficult to justify ethically. The dog model provided key insights during the preclinical development of the prophylactic vaccine, but it has seen only limited use in molecular pathogenesis studies7. Although the multimammate mouse (Mastomys coucha) is not a traditional organism for animal work, it allows for experimental infections with wild-type and mutant PV genomes and serves as an excellent model of papillomavirus-induced skin carcinogenesis141.
The identification and isolation of MmuPV1 from cutaneous papillomas in a colony of nude mice provided a more convenient model in a genetically tractable, standard animal system142,143. It enables viral pathogenesis experiments not only by experimental infections with mutant MmuPV1 genomes but also by testing the effect of viral infection in genetically manipulated mice144. The MmuPV1 E6 and E7 proteins share some biological activities with certain cutaneous beta and gamma HPVs145,146. However, MmuPV1 does not only cause cutaneous lesions and tumors as it can also infect mucosal epithelia and cause anogenital tract lesions like the well-studied mucosal high-risk alpha HPVs147,148. Sexual transmission of MmuPV1 has been observed in laboratory settings149, opening the exciting possibility of investigating means to interfere with the sexual transmission of PVs.
The major advantage of mouse models, the ability to perform experiments in inbred mouse strains with well-defined genetic backgrounds, is also a major shortcoming in that such mice do not reflect the genetic diversity of human populations. This limits efforts to discover genetic modifiers that affect disease susceptibility. In recent years, populations of outbred mice have been generated by interbreeding of multiple well-defined, inbred founder strains150. MmuPV1 pathogenesis studies with such outbred mice promise to provide fascinating insights into host factors that determine disease outcomes.
Multiple papillomavirus types have been detected in most animal species. A total of 44 bovine PVs are listed in PAVE and they show a remarkable genetic diversity25,26. It is thus hard to believe that there is only a single PV that infects Mus musculus, the common house mouse. It will be interesting to determine whether non-laboratory-confined Mus musculus harbor other PVs and if they are associated with other disease manifestations.
Unchartered territory – is it worth studying some of the “strange” animal papillomaviruses?
The list of animal PVs in the PAVE database currently stands at >280 entries25,26. With a few exceptions that are mentioned in earlier sections, their life cycles and potential disease associations are mostly unknown. Yet the genomic organization of some of the animal PVs is fascinating. The currently unclassified PVs isolated from various fish species as well as from a toad, Rhinella marina (RmarPV1) do not encode E6 and E7 proteins, whereas the omega PVs (occurring in bears and seals) as well as the PVs isolated from cetaceans encode E6, but not E7 proteins. Others such as the unclassified PVs from geckos encode an E7, but not an E6 protein (Table 2). Furthermore, some of the animal PVs encode interesting variations of the E6 and E7 proteins. Avian PVs, for example, encode much larger E7 proteins and no or truncated E6 proteins151. The avian E7 proteins all contain the canonical LXCXE (L, leucine; C, cysteine; E, glutamic acid; X, any amino acid)-based binding motif for the retinoblastoma protein family members in their amino-terminal sequences and (CXXC)2 metal binding motifs in their carboxy termini. However, the spacing between the two CXXC motifs is smaller than in E7 proteins encoded by other PVs and there is overall poor amino acid sequence conservation between the different avian PV E7 proteins. The truncated versions of E6 that are encoded by some avian PVs consist of a single (CXXC)2-based metal binding site (Figure 3), and some contain an additional unique ORF, termed E9, that is overprinted on E1 (Figure 4; Table 3). Other animal PVs, including many bovine papillomaviruses (BPVs), contain E10 but no E6 ORFs. These E10s encode 42 amino acid hydrophobic polypeptides that share no significant amino acid similarity to the gamma 6 HPVs encoded E10s. Other animal PVs also contain E10 ORFs, but they are overprinted on E6 and can encode 42 to 50 amino acid hydrophobic polypeptides that are related to the non-overprinted BPV E10s but are distinct from the gamma 6 HPV E10s (Figure 2B; Table 4). SfPV1 and OcPV1, which contain E6-overprinted E10s also encode hydrophobic E5 ORFs (Figure 2B; Table 4). The E10 protein encoded by the Javan rusa PV2 (RtiPV2) is unique, lacks a predicted transmembrane domain, and shares no sequence similarity to the other E10s (Figure 2C; Table 4).
Table 2:
Animal PVs that do not encode E6 and/or E7 proteins.
| Virus | Species | Host Common Name | GenBank ID | Notes |
|---|---|---|---|---|
| AmPV2 | Omegapapillomavirus | Giant panda | MF327535 | No E7 |
| CstrPV1 | Unclassified | Sea bass | MZ570863 | No E6 or E7 |
| CstrPV2 | Unclassified | Sea bass | MZ570864 | No E6 or E7 |
| CstrPV3 | Unclassified | Sea bass | MZ570865 | No E6 or E7 |
| DdPV1 | Upsilonpapillomavirus 1 | Common short-beaked dolphin | GU117620 | No E7 |
| DleuPV1 | Unclassified | Beluga whale | OP856597 | No E7 |
| EaPV1 | Dyochipapillomavirus 1 | Donkey | KF741371 | No E6; E7 does not contain LXCXE motif |
| EaPV3 | Unclassified | African wild ass | OP699054 | No E6; E7 does not contain LXCXE motif |
| HfrePV1 | Unclassified | Common house gecko | MK207055 | No E6 |
| HfrePV2 | Unclassified | Common house gecko | MN194600 | No E6 |
| LcamPV1 | Unclassified | Red snapper | MH617579 | No E6 or E7 |
| LwPV6 | Omegapapillomavirus | Weddell seal | MG571091 | No E7 |
| LwPV7 | Omegapapillomavirus | Weddell seal | MG571092 | No E7 |
| MaegPV1 | Unclassified | Haddock | MH616908 | No E6 or E7 |
| MaegPV2 | Unclassified | Haddock | MH617143 | No E6 or E7 |
| MaegPV3 | Unclassified | Haddock | MZ570859 | No E6 or E7 |
| MaegPV4 | Unclassified | Haddock | MZ570860 | No E6 or E7 |
| MaegPV5 | Unclassified | Haddock | MZ570861 | No E6 or E7 |
| MrPV1 | Unclassified | Ricketts big-footed bat | JQ814847 | No E7 |
| NasiPV1 | Unclassified | Narrow-ridged finless porpoise | OQ274126 | No E7 |
| NasiPV2 | Unclassified | Narrow-ridged finless porpoise | OQ274127 | No E7 |
| NasiPV3 | Unclassified | Narrow-ridged finless porpoise | OQ274128 | No E7 |
| NasiPV4 | Unclassified | Narrow-ridged finless porpoise | OQ274129 | No E7 |
| NasiPV5 | Unclassified | Narrow-ridged finless porpoise | OQ274130 | No E7 |
| NasiPV6 | Unclassified | Narrow-ridged finless porpoise | OQ274131 | No E7 |
| OmykPV1 | Unclassified | Rainbow trout | MH510267 | No E6 or E7 |
| PphPV1 | Omikronpapillomavirus 1 | Harbor porpoise | GU117621 | No E7 |
| PphPV2 | Upsilonpapillomavirus 3 | Harbor porpoise | GU117622 | No E7 |
| PphPV4 | Dyopipapillomavirus 1 | Harbor porpoise | GU117623 | No E7 |
| PsPV1 | Omikronpapillomavirus 1 | Burmeisters porpoise | AJ238373 | No E7 |
| PV-fi100-fish | Unclassified | Fish | OP933686 | No E6 or E7 |
| PV-wesgulfec-fish | Unclassified | Unidentified | MZ602149 | No E6 or E7 |
| RmarPV1 | Unclassified | Cane toad | MW582900 | No E6 or E7 |
| SaPV1 | Unclassified | Gilt-head bream | KX643372 | No E6 or E7 |
| SglaPV1 | Unclassified | Wels catfish | MN515404 | No E6 or E7 |
| SsPV1 | Dyodeltapapillomavirus 1 | Domestic pig | EF395818 | No E7 |
| TberPV1 | Unclassified | Emerald rockcod | MZ447865 | No E6 |
| TtPV1 | Upsilonpapillomavirus 1 | Bottlenosed dolphin | EU240894 | No E7 |
| TtPV2 | Upsilonpapillomavirus 2 | Bottlenosed dolphin | AY956402 | No E7 |
| TtPV3 | Upsilonpapillomavirus 1 | Bottlenosed dolphin | EU240895 | No E7 |
| TtPV4 | Upsilonpapillomavirus 1 | Bottlenosed dolphin | JN709469 | No E7 |
| TtPV5 | Omikronpapillomavirus 1 | Bottlenosed dolphin | JN709470 | No E7 |
| TtPV6 | Omikronpapillomavirus 1 | Bottlenosed dolphin | JN709471 | No E7 |
| TtPV7 | Upsilonpapillomavirus 1 | Bottlenosed dolphin | JN709472 | No E7 |
| TtPV8 | Dyopipapillomavirus | Bottlenosed dolphin | MG744508 | No E7 |
| TtPV9 | Omikronpapillomavirus | Bottlenosed dolphin | MG905161 | No E7 |
| UmPV1 | Omegapapillomavirus 1 | Polar bear | EF536349 | No E7 |
Figure 3: Small E6 proteins encoded by avian papillomaviruses.
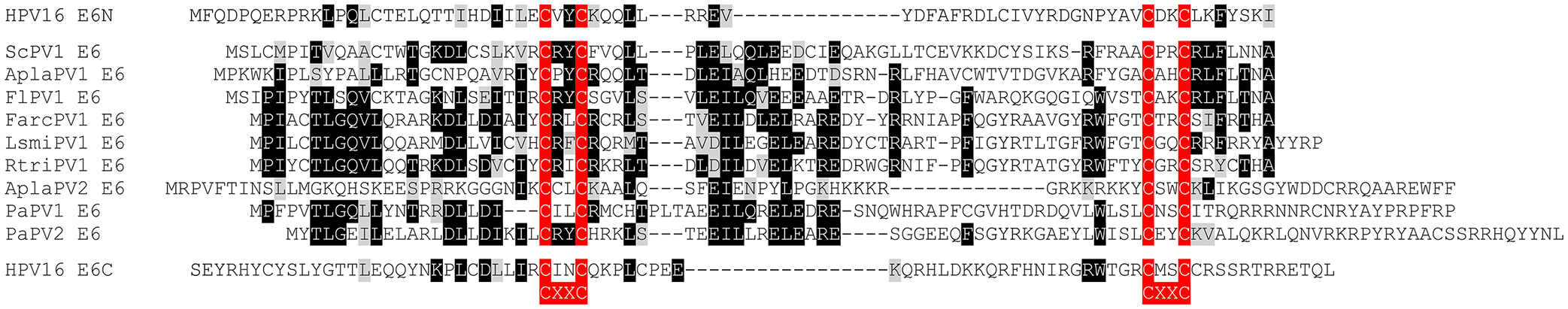
The amino acid sequences of E6 proteins encoded by papillomaviruses isolated from the Atlantic canary (Serinus canaria papillomavirus 1; ScPV1), mallard (Anas platyrhynchos Papillomavirus 1 and 2; AplaPV1 and AplaPV2), yellow-necked francolin (Francolinus leucoscepus Papillomavirus 1; FlPV1), Atlantic puffin (Fratercula arctica Papillomavirus 1; FarcPV1), American herring gull (Larus smithsonianus Papillomavirus 1; LsmiPV1), black-legged kittiwake (Rissa tridactyla Papillomavirus 1, RtriPV1), and Adelie penguin (Pygoscelis adeliae papillomavirus 1 and 2; PaPV1 and PaPV2) are shown and compared to the amino (E6N)- and carboxyl-terminal (E6C) regions of HPV16 E6. Identical residues are highlighted by black boxes and chemically similar residues by gray boxes. The CXXC (C=cysteine, X=any amino acid) metal binding motifs are highlighted in red.
Figure 4. Unique E9 proteins encoded by avian papillomaviruses.
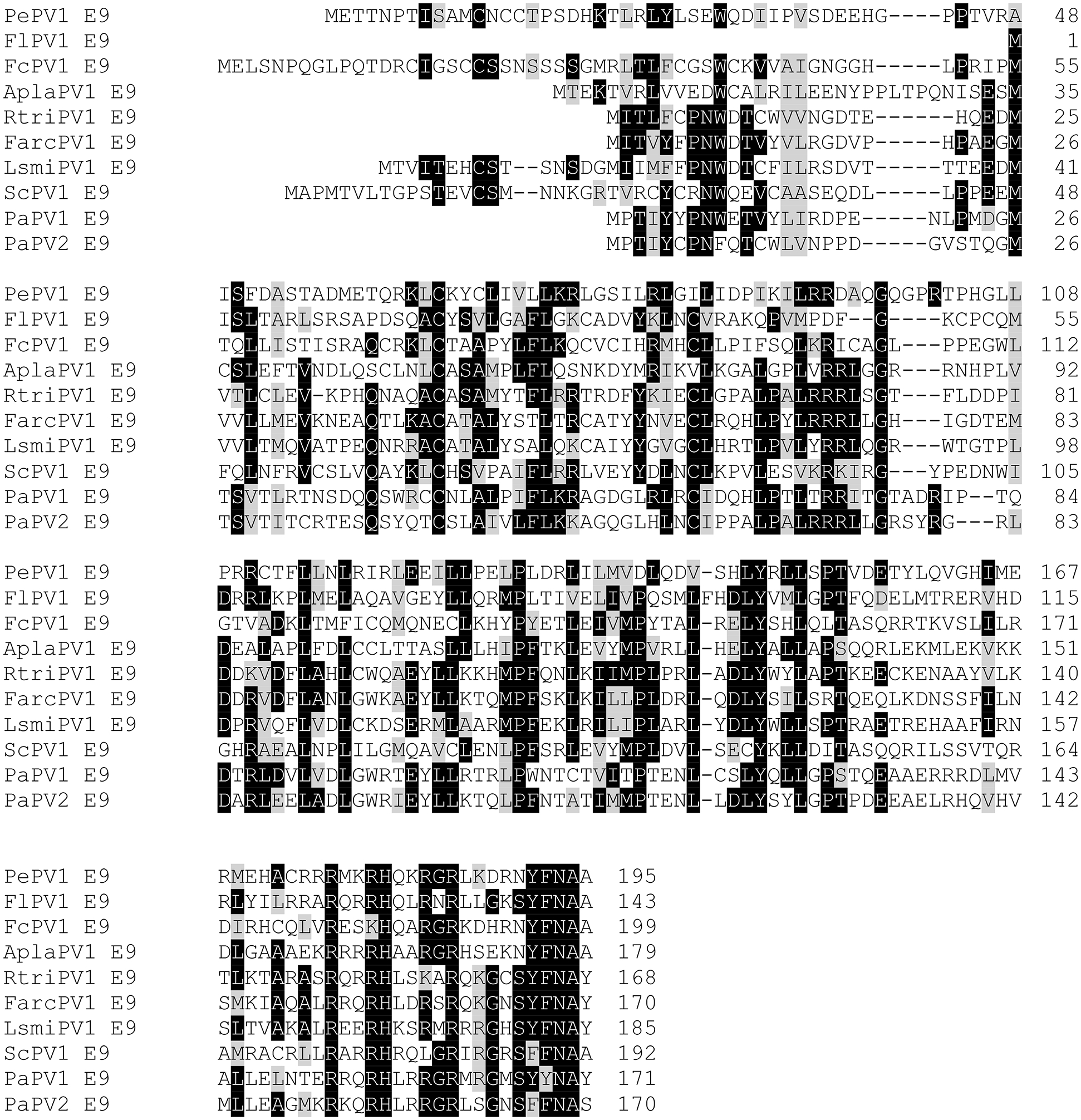
The amino acid sequences of E9 proteins encoded by papillomavirus isolated from the gray parrot (Psittacus erithacus Papillomavirus 1; PePV1), yellow-necked francolin (Francolinus leucoscepus Papillomavirus 1; FlPV1), common chaffinch (Fringilla coelebs Papillomavirus 1; FcPV1), mallard (Anas platyrhynchos Papillomavirus 1; AplaPV1), black-legged kittiwake (Rissa tridactyla Papillomavirus 1, RtriPV1), Atlantic puffin (Fratercula arctica Papillomavirus 1; FarcPV1), American herring gull (Larus smithsonianus Papillomavirus 1; LsmiPV1), Atlantic canary (Serinus canaria papillomavirus 1; ScPV1), and Adelie penguin (Pygoscelis adeliae papillomavirus 1 and 2; PaPV1 and PaPV1), are shown. Identical residues are highlighted by black boxes and chemically similar residues by gray boxes. The E9 ORFs are overprinted on the E1 ORF. See Table 3 for a full list of papillomaviruses with E9 ORFs.
Table 3:
Animal PVs that encode E9 proteins.
| Virus | Species | Host Common Name | GenBank ID | Notes |
|---|---|---|---|---|
| AplaPV1 | Unclassified | Mallard | MK620303 | Overprinted on E1 |
| FarcPV1 | Unclassified | Atlantic puffin | MK620302 | Overprinted on E1 |
| FcPV1 | Etapapillomavirus 1 | Common chaffinch | AY057109 | Overprinted on E1 |
| FlPV1 | Dyoepsilonpapillomavirus 1 | Yellow-necked francolin | EU188799 | Overprinted on E1 |
| LsmiPV1 | Unclassified | American herring gull | MK620304 | Overprinted on E1 |
| PaPV1 | Treisepsilonpapillomavirus 1 | Adelie penguin | KJ173785 | Overprinted on E1 |
| PaPV2 | Unclassified Treisepsilonpapillomavirus | Adelie penguin | MF168943 | Overprinted on E1 |
| PePV1 | Thetapapillomavirus 1 | Gray parrot | AF502599 | Overprinted on E1 |
| RtriPV1 | Unclassified | Black-legged kittiwake | MK620305 | Overprinted on E1 |
| ScPV1 | Unclassified Etapapillomavirus | Atlantic canary | MF564196 | Overprinted on E1 |
Table 4:
HPVs and animal PVs that encode E10 proteins.
| Virus | Species | Host Common Name | GenBank ID | Notes |
|---|---|---|---|---|
| HPV101 | Gammapapillomavirus 6 | Human | DQ080081 | Does not encode E6 |
| HPV103 | Gammapapillomavirus 6 | Human | DQ080078 | Does not encode E6 |
| HPV108 | Gammapapillomavirus 6 | Human | FM212639 | Does not encode E6 |
| HPV214 | Unclassified Gammapapillomavirus | Human | MF509819 | Does not encode E6 |
| HPV226 | Unclassified Gammapapillomavirus | Human | MG813996 | Does not encode E6 |
| HPV-mw02c24a | Unclassified Gammapapillomavirus | Human | MF588696 | Does not encode E6 |
| BPV3 | Xipapillomavirus 1 | Domestic cow | AF486184 | Does not encode E6 |
| BPV4 | Xipapillomavirus 1 | Domestic cow | X05817 | Does not encode E6 |
| BPV6 | Xipapillomavirus 1 | Domestic cow | AJ620208 | Does not encode E6 |
| BPV9 | Xipapillomavirus 1 | Domestic cow | AB331650 | Does not encode E6 |
| BPV10 | Xipapillomavirus 1 | Domestic cow | AB331651 | Does not encode E6 |
| BPV11 | Xipapillomavirus 1 | Domestic cow | AB543507 | Does not encode E6 |
| BPV12 | Xipapillomavirus 2 | Domestic cow | JF834523 | Does not encode E6 |
| BPV15 | Xipapillomavirus 1 | Domestic cow | KM983393 | Does not encode E6 |
| BPV20 | Unclassified Xipapillomavirus | Domestic cow | KU519395 | Overprinted on E6 |
| BPV23 | Unclassified Xipapillomavirus | Domestic cow | KX098515 | Does not encode E6 |
| BPV24 | Unclassified Xipapillomavirus | Domestic cow | MG602223 | Does not encode E6 |
| BPV26 | Unclassified Xipapillomavirus | Domestic cow | MG281846 | Does not encode E6 |
| BPV28 | Unclassified Xipapillomavirus | Domestic cow | LC500686 | Does not encode E6 |
| BPV29 | Unclassified Xipapillomavirus | Domestic cow | LC514113 | Does not encode E6 |
| BPV30 | Unclassified | Domestic cow | OL672227 | Does not encode E6 |
| BPV31 | Unclassified | Domestic cow | MW390885 | Does not encode E6 |
| BPV35 | Unclassified | Domestic cow | MW404256 | Does not encode E6 |
| BPV36 | Unclassified | Domestic cow | MW404257 | Does not encode E6 |
| BPV37 | Unclassified | Domestic cow | MW404258 | Does not encode E6 |
| BPV38 | Unclassified | Domestic cow | MW404259 | Does not encode E6 |
| BPV39 | Unclassified | Domestic cow | MW404260 | Does not encode E6 |
| BPV40 | Unclassified | Domestic cow | MW428425 | Encodes E5_Epsilon |
| BPV41 | Unclassified | Domestic cow | MW428427 | Overprinted on E6 |
| BPV42 | Unclassified | Domestic cow | MW428428 | Does not encode E6 |
| BPV43 | Unclassified | Domestic cow | MW428429 | Does not encode E6 |
| ChPV2 | Unclassified | Domestic goat | MN148899 | Overprinted on E6 |
| OcPV1 | Kappapapillomavirus 1 | New Zealand white rabbit | AF227240 | Overprinted on E6; Encodes E5_Unclassified |
| RtPV2 | Xipapillomavirus 3 | Reindeer | KC810012 | Overprinted on E6 |
| RtiPV2 | Unclassified | Javan rusa | KT852571 | |
| SfPV1 | Kappapapillomavirus 2 | Cottontail rabbit | K02708 | Overprinted on E6; Encodes E5_Unclassified |
One might argue that studies on these accessory proteins encoded by animal PVs are difficult to justify since there are no obvious biomedical applications. If the past has taught us something, however, then studying obscure phenomena in non-human model organisms can lead to surprising and transformative discoveries such as RNA interference and CRISPR-based gene editing.
Concluding Remarks
It appears obvious that the gloomy predictions of the upcoming demise of papillomavirus research are greatly exaggerated. The field will continue to flourish as long as investigators continue to upend some of the “knowns”, start addressing some of the “mysteries”, and also dare to boldly explore the “unchartered territories”. In the current era of low funding rates and an apparent general lack of interest in cultivating viral oncology research with funding agencies, this may be a tall order. Funders may realize that the availability of a prophylactic vaccine, however efficacious it may be, has not and will not put an immediate end to the human suffering caused by HPV infections. As a community, we can start this process by embracing new, “disruptive” ideas and approaches and support and inspire young investigators to enter and established researchers to remain in this field. Continued research into the “curious” life cycle of various papillomaviruses will provide us with important insights into epithelial cell biology and point to signaling circuits and mechanisms that are generally relevant to our mechanistic understanding of diseases and cancers even those that are not caused by papillomaviruses.
Acknowledgments
Many of the ideas mentioned here arose during discussions with colleagues at recent conferences. We thank Dr. Elizabeth White for her valuable insights and comments on this manuscript. The research efforts in the authors’ research group are supported by grants from the National Institutes of Health (AI170633; CA228543; AI166786) and the Mary Kay Foundation (06–21). K.M. is dedicating this article to the memory of Dr. Massimo Tommasino.
Footnotes
Conflict of Interest Statement:
The authors declare that they have no financial or other conflict of interest that might be construed to influence the contents of the manuscript.
Data Availability Statement
The sequence data that support the findings of this study are available in PAVE at https://pave.niaid.nih.gov/.
References
- 1.McBride AA. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol. 2022;20(2):95–108. [DOI] [PubMed] [Google Scholar]
- 2.White EA. Manipulation of Epithelial Differentiation by HPV Oncoproteins. Viruses. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iacovides D, Michael S, Achilleos C, Strati K. Shared mechanisms in stemness and carcinogenesis: lessons from oncogenic viruses. Front Cell Infect Microbiol. 2013;3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strati K Changing Stem Cell Dynamics during Papillomavirus Infection: Potential Roles for Cellular Plasticity in the Viral Lifecycle and Disease. Viruses. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doorbar J, Zheng K, Aiyenuro A, et al. Principles of epithelial homeostasis control during persistent human papillomavirus infection and its deregulation at the cervical transformation zone. Curr Opin Virol. 2021;51:96–105. [DOI] [PubMed] [Google Scholar]
- 6.Syverton JT. The pathogenesis of the rabbit papilloma-to-carcinoma sequence. Ann N Y Acad Sci. 1952;54(6):1126–1140. [DOI] [PubMed] [Google Scholar]
- 7.Christensen ND, Budgeon LR, Cladel NM, Hu J. Recent advances in preclinical model systems for papillomaviruses. Virus Res. 2017;231:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cladel NM, Xu J, Peng X, et al. Modeling HPV-Associated Disease and Cancer Using the Cottontail Rabbit Papillomavirus. Viruses. 2022;14(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harden ME, Munger K. Human papillomavirus molecular biology. Mutat Res Rev Mutat Res. 2017;772:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBride AA. Mechanisms and strategies of papillomavirus replication. Biol Chem. 2017;398(8):919–927. [DOI] [PubMed] [Google Scholar]
- 11.Orth G, Jablonska S, Favre M, Croissant O, Jarzabek-Chorzelska M, Rzesa G. Characterization of two types of human papillomaviruses in lesions of epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1978;75(3):1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983;80(12):3812–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheurlen W, zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3(5):1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaudenon S, Kremsdorf D, Croissant O, Jablonska S, Wain-Hobson S, Orth G. A novel type of human papillomavirus associated with genital neoplasias. Nature. 1986;321(6067):246–249. [DOI] [PubMed] [Google Scholar]
- 15.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. [DOI] [PubMed] [Google Scholar]
- 16.Taberna M, Mena M, Pavon MA, Alemany L, Gillison ML, Mesia R. Human papillomavirus-related oropharyngeal cancer. Ann Oncol. 2017;28(10):2386–2398. [DOI] [PubMed] [Google Scholar]
- 17.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. [DOI] [PubMed] [Google Scholar]
- 18.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116(5):1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oliveira CM, Fregnani J, Villa LL. HPV Vaccine: Updates and Highlights. Acta Cytol. 2019;63(2):159–168. [DOI] [PubMed] [Google Scholar]
- 20.Barnabas RV, Brown ER, Onono MA, et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid. 2022;1(5):EVIDoa2100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 22.Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399. [DOI] [PubMed] [Google Scholar]
- 23.Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010;16(11):1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2015;33(29):3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Doorslaer K, Li Z, Xirasagar S, et al. The Papillomavirus Episteme: a major update to the papillomavirus sequence database. Nucleic Acids Res. 2017;45(D1):D499–D506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Doorslaer K, Tan Q, Xirasagar S, et al. The Papillomavirus Episteme: a central resource for papillomavirus sequence data and analysis. Nucleic Acids Res. 2013;41(Database issue):D571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vande Pol SB, Klingelhutz AJ. Papillomavirus E6 oncoproteins. Virology. 2013;445(1–2):115–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman A, Munger K. The papillomavirus E7 proteins. Virology. 2013;445(1–2):138–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilahi NE, Bhatti A. Impact of HPV E5 on viral life cycle via EGFR signaling. Microb Pathog. 2020;139:103923. [DOI] [PubMed] [Google Scholar]
- 30.Schlegel R, Wade-Glass M, Rabson MS, Yang YC. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986;233(4762):464–467. [DOI] [PubMed] [Google Scholar]
- 31.DiMaio D, Petti LM. The E5 proteins. Virology. 2013;445(1–2):99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campo MS, Graham SV, Cortese MS, et al. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology. 2010;407(1):137–142. [DOI] [PubMed] [Google Scholar]
- 33.Miyauchi S, Kim SS, Jones RN, et al. Human papillomavirus E5 suppresses immunity via inhibition of the immunoproteasome and STING pathway. Cell Rep. 2023;42(5):112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basukala O, Banks L. The Not-So-Good, the Bad and the Ugly: HPV E5, E6 and E7 Oncoproteins in the Orchestration of Carcinogenesis. Viruses. 2021;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56(20):4620–4624. [PubMed] [Google Scholar]
- 36.Gonzalez SL, Stremlau M, He X, Basile JR, Munger K. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J Virol. 2001;75(16):7583–7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75(3):495–505. [DOI] [PubMed] [Google Scholar]
- 38.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396(6706):84–88. [DOI] [PubMed] [Google Scholar]
- 39.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380(6569):79–82. [DOI] [PubMed] [Google Scholar]
- 40.Duensing S, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002;62(23):7075–7082. [PubMed] [Google Scholar]
- 41.Munger K, Jones DL. Human papillomavirus carcinogenesis: an identity crisis in the retinoblastoma tumor suppressor pathway. J Virol. 2015;89(9):4708–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dick FA, Goodrich DW, Sage J, Dyson NJ. Non-canonical functions of the RB protein in cancer. Nat Rev Cancer. 2018;18(7):442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozenblatt-Rosen O, Deo RC, Padi M, et al. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature. 2012;487(7408):491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White EA, Kramer RE, Tan MJ, Hayes SD, Harper JW, Howley PM. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J Virol. 2012;86(24):13174–13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White EA, Sowa ME, Tan MJ, et al. Systematic identification of interactions between host cell proteins and E7 oncoproteins from diverse human papillomaviruses. Proc Natl Acad Sci U S A. 2012;109(5):E260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989;8(13):4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita T, Segawa K, Fujinaga Y, Nishikawa T, Fujinaga K. Biological and biochemical activity of E7 genes of the cutaneous human papillomavirus type 5 and 8. Oncogene. 1993;8(9):2433–2441. [PubMed] [Google Scholar]
- 48.White EA, Walther J, Javanbakht H, Howley PM. Genus beta human papillomavirus E6 proteins vary in their effects on the transactivation of p53 target genes. J Virol. 2014;88(15):8201–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egawa N, Doorbar J. The low-risk papillomaviruses. Virus Res. 2017;231:119–127. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Zhou D, Prabhu A, Schlegel R, Yuan H. The canine papillomavirus and gamma HPV E7 proteins use an alternative domain to bind and destabilize the retinoblastoma protein. PLoS Pathog. 2010;6(9):e1001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grace M, Munger K. Proteomic analysis of the gamma human papillomavirus type 197 E6 and E7 associated cellular proteins. Virology. 2017;500:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syrjanen K, Hakama M, Saarikoski S, et al. Prevalence, incidence, and estimated life-time risk of cervical human papillomavirus infections in a nonselected Finnish female population. Sex Transm Dis. 1990;17(1):15–19. [PubMed] [Google Scholar]
- 53.Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Della Fera AN, Warburton A, Coursey TL, Khurana S, McBride AA. Persistent Human Papillomavirus Infection. Viruses. 2021;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maglennon GA, Doorbar J. The biology of papillomavirus latency. Open Virol J. 2012;6:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doorbar J The human Papillomavirus twilight zone - Latency, immune control and subclinical infection. Tumour Virus Res. 2023;16:200268. [DOI] [PubMed] [Google Scholar]
- 57.Warburton A, Della Fera AN, McBride AA. Dangerous Liaisons: Long-Term Replication with an Extrachromosomal HPV Genome. Viruses. 2021;13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palefsky JM. Human papillomavirus-associated anal and cervical cancers in HIV-infected individuals: incidence and prevention in the antiretroviral therapy era. Curr Opin HIV AIDS. 2017;12(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sichero L, Rollison DE, Amorrortu RP, Tommasino M. Beta Human Papillomavirus and Associated Diseases. Acta Cytol. 2019;63(2):100–108. [DOI] [PubMed] [Google Scholar]
- 60.Sunthamala N, Thierry F, Teissier S, et al. E2 proteins of high risk human papillomaviruses down-modulate STING and IFN-kappa transcription in keratinocytes. PLoS One. 2014;9(3):e91473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moody CA. Regulation of the Innate Immune Response during the Human Papillomavirus Life Cycle. Viruses. 2022;14(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westrich JA, Warren CJ, Pyeon D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017;231:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzales KAU, Fuchs E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev Cell. 2017;43(4):387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109(26):10516–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michael S, Lambert PF, Strati K. The HPV16 oncogenes cause aberrant stem cell mobilization. Virology. 2013;443(2):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hufbauer M, Biddle A, Borgogna C, et al. Expression of betapapillomavirus oncogenes increases the number of keratinocytes with stem cell-like properties. J Virol. 2013;87(22):12158–12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rangarajan A, Talora C, Okuyama R, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20(13):3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brimer N, Vande Pol S. Human papillomavirus type 16 E6 induces cell competition. PLoS Pathog. 2022;18(3):e1010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meyers JM, Spangle JM, Munger K. The human papillomavirus type 8 E6 protein interferes with NOTCH activation during keratinocyte differentiation. J Virol. 2013;87(8):4762–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan MJ, White EA, Sowa ME, Harper JW, Aster JC, Howley PM. Cutaneous beta-human papillomavirus E6 proteins bind Mastermind-like coactivators and repress Notch signaling. Proc Natl Acad Sci U S A. 2012;109(23):E1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyers JM, Uberoi A, Grace M, Lambert PF, Munger K. Cutaneous HPV8 and MmuPV1 E6 Proteins Target the NOTCH and TGF-beta Tumor Suppressors to Inhibit Differentiation and Sustain Keratinocyte Proliferation. PLoS Pathog. 2017;13(1):e1006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saunders-Wood T, Egawa N, Zheng K, Giaretta A, Griffin HM, Doorbar J. Role of E6 in Maintaining the Basal Cell Reservoir during Productive Papillomavirus Infection. J Virol. 2022;96(5):e0118121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lefort K, Mandinova A, Ostano P, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007;21(5):562–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yugawa T, Handa K, Narisawa-Saito M, Ohno S, Fujita M, Kiyono T. Regulation of Notch1 gene expression by p53 in epithelial cells. Mol Cell Biol. 2007;27(10):3732–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kranjec C, Holleywood C, Libert D, et al. Modulation of basal cell fate during productive and transforming HPV-16 infection is mediated by progressive E6-driven depletion of Notch. J Pathol. 2017;242(4):448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beverdam A, Claxton C, Zhang X, James G, Harvey KF, Key B. Yap controls stem/progenitor cell proliferation in the mouse postnatal epidermis. J Invest Dermatol. 2013;133(6):1497–1505. [DOI] [PubMed] [Google Scholar]
- 77.Heng BC, Zhang X, Aubel D, et al. Role of YAP/TAZ in Cell Lineage Fate Determination and Related Signaling Pathways. Front Cell Dev Biol. 2020;8:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hicks-Berthet J, Ning B, Federico A, et al. Yap/Taz inhibit goblet cell fate to maintain lung epithelial homeostasis. Cell Rep. 2021;36(2):109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap. Dev Cell. 2015;34(3):283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157(6):1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao R, Fallon TR, Saladi SV, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30(2):151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He C, Lv X, Huang C, et al. A Human Papillomavirus-Independent Cervical Cancer Animal Model Reveals Unconventional Mechanisms of Cervical Carcinogenesis. Cell Rep. 2019;26(10):2636–2650 e2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maehama T, Nishio M, Otani J, Mak TW, Suzuki A. The role of Hippo-YAP signaling in squamous cell carcinomas. Cancer Sci. 2021;112(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishio M, To Y, Maehama T, et al. Endogenous YAP1 activation drives immediate onset of cervical carcinoma in situ in mice. Cancer Sci. 2020;111(10):3576–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Omori H, Nishio M, Masuda M, et al. YAP1 is a potent driver of the onset and progression of oral squamous cell carcinoma. Sci Adv. 2020;6(12):eaay3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hatterschide J, Castagnino P, Kim HW, et al. YAP1 activation by human papillomavirus E7 promotes basal cell identity in squamous epithelia. Elife. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. 2018;173(2):321–337 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yun HY, Kim MW, Lee HS, et al. Structural basis for recognition of the tumor suppressor protein PTPN14 by the oncoprotein E7 of human papillomavirus. PLoS Biol. 2019;17(7):e3000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knight JF, Sung VYC, Kuzmin E, et al. KIBRA (WWC1) Is a Metastasis Suppressor Gene Affected by Chromosome 5q Loss in Triple-Negative Breast Cancer. Cell Rep. 2018;22(12):3191–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mello SS, Valente LJ, Raj N, et al. A p53 Super-tumor Suppressor Reveals a Tumor Suppressive p53-Ptpn14-Yap Axis in Pancreatic Cancer. Cancer Cell. 2017;32(4):460–473 e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W, Huang J, Wang X, et al. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26(17):1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poernbacher I, Baumgartner R, Marada SK, Edwards K, Stocker H. Drosophila Pez acts in Hippo signaling to restrict intestinal stem cell proliferation. Curr Biol. 2012;22(5):389–396. [DOI] [PubMed] [Google Scholar]
- 93.Olafsdottir T, Stacey SN, Sveinbjornsson G, et al. Loss-of-Function Variants in the Tumor-Suppressor Gene PTPN14 Confer Increased Cancer Risk. Cancer Res. 2021;81(8):1954–1964. [DOI] [PubMed] [Google Scholar]
- 94.Wu SC, Grace M, Munger K. The HPV8 E6 protein targets the Hippo and Wnt signaling pathways as part of its arsenal to restrain keratinocyte differentiation. mBio. 2023:e0155623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He C, Mao D, Hua G, et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med. 2015;7(11):1426–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Webb Strickland S, Brimer N, Lyons C, Vande Pol SB. Human Papillomavirus E6 interaction with cellular PDZ domain proteins modulates YAP nuclear localization. Virology. 2018;516:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yin W, Egawa N, Zheng K, et al. HPV E6 inhibits E6AP to regulate epithelial homeostasis by modulating keratinocyte differentiation commitment and YAP1 activation. PLoS Pathog. 2023;19(6):e1011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kozyrska K, Pilia G, Vishwakarma M, et al. p53 directs leader cell behavior, migration, and clearance during epithelial repair. Science. 2022;375(6581):eabl8876. [DOI] [PubMed] [Google Scholar]
- 99.Wagstaff L, Goschorska M, Kozyrska K, et al. Mechanical cell competition kills cells via induction of lethal p53 levels. Nat Commun. 2016;7:11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mirabello L, Yeager M, Yu K, et al. HPV16 E7 Genetic Conservation Is Critical to Carcinogenesis. Cell. 2017;170(6):1164–1174 e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinheiro M, Harari A, Schiffman M, et al. Phylogenomic Analysis of Human Papillomavirus Type 31 and Cervical Carcinogenesis: A Study of 2093 Viral Genomes. Viruses. 2021;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldsborough MD, DiSilvestre D, Temple GF, Lorincz AT. Nucleotide sequence of human papillomavirus type 31: a cervical neoplasia-associated virus. Virology. 1989;171(1):306–311. [DOI] [PubMed] [Google Scholar]
- 103.Warren CJ, Santiago ML, Pyeon D. APOBEC3: Friend or Foe in Human Papillomavirus Infection and Oncogenesis? Annu Rev Virol. 2022;9(1):375–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris RS, Dudley JP. APOBECs and virus restriction. Virology. 2015;479–480: 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wallace NA, Munger K. The curious case of APOBEC3 activation by cancer-associated human papillomaviruses. PLoS Pathog. 2018;14(1):e1006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gillespie KA, Mehta KP, Laimins LA, Moody CA. Human papillomaviruses recruit cellular DNA repair and homologous recombination factors to viral replication centers. J Virol. 2012;86(17):9520–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McKinney CC, Hussmann KL, McBride AA. The Role of the DNA Damage Response throughout the Papillomavirus Life Cycle. Viruses. 2015;7(5):2450–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khurana S, Markowitz TE, Kabat J, McBride AA. Spatial and Functional Organization of Human Papillomavirus Replication Foci in the Productive Stage of Infection. mBio. 2021;12(6):e0268421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lou H, Boland JF, Li H, et al. HPV16 E7 Nucleotide Variants Found in Cancer-Free Subjects Affect E7 Protein Expression and Transformation. Cancers (Basel). 2022;14(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ho L, Chan SY, Burk RD, et al. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J Virol. 1993;67(11):6413–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ho L, Chan SY, Chow V, et al. Sequence variants of human papillomavirus type 16 in clinical samples permit verification and extension of epidemiological studies and construction of a phylogenetic tree. J Clin Microbiol. 1991;29(9):1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.IARC/WHO. Biological Agents, a review of human carcinogens. Vol 100 Lyon B, France: IARC; 2012. [Google Scholar]
- 113.Kamolratanakul S, Pitisuttithum P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines (Basel). 2021;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leiendecker L, Neumann T, Jung PS, et al. Human Papillomavirus 42 Drives Digital Papillary Adenocarcinoma and Elicits a Germ Cell-like Program Conserved in HPV-Positive Cancers. Cancer Discov. 2023;13(1):70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Philipp W, Honore N, Sapp M, Cole ST, Streeck RE. Human papillomavirus type 42: new sequences, conserved genome organization. Virology. 1992;186(1):331–334. [DOI] [PubMed] [Google Scholar]
- 116.Chen Z, Schiffman M, Herrero R, Desalle R, Burk RD. Human papillomavirus (HPV) types 101 and 103 isolated from cervicovaginal cells lack an E6 open reading frame (ORF) and are related to gamma-papillomaviruses. Virology. 2007;360(2):447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nobre RJ, Herraez-Hernandez E, Fei JW, et al. E7 oncoprotein of novel human papillomavirus type 108 lacking the E6 gene induces dysplasia in organotypic keratinocyte cultures. J Virol. 2009;83(7):2907–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murahwa AT, Meiring TL, Mbulawa ZZA, Williamson AL. Discovery, characterisation and genomic variation of six novel Gammapapillomavirus types from penile swabs in South Africa. Papillomavirus Res. 2019;7:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Latsuzbaia A, Arbyn M, Dutta S, et al. Complete Genome Sequence of a Novel Human Gammapapillomavirus Isolated from a Cervical Swab in Luxembourg. Genome Announc. 2018;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pastrana DV, Peretti A, Welch NL, et al. Metagenomic Discovery of 83 New Human Papillomavirus Types in Patients with Immunodeficiency. mSphere. 2018;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Doorslaer K, McBride AA. Molecular archeological evidence in support of the repeated loss of a papillomavirus gene. Sci Rep. 2016;6:33028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strickley JD, Messerschmidt JL, Awad ME, et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature. 2019;575(7783):519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Howley PM, Pfister HJ. Beta genus papillomaviruses and skin cancer. Virology. 2015;479–480: 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meyers JM, Munger K. The viral etiology of skin cancer. J Invest Dermatol. 2014;134(e1):E29–32. [DOI] [PubMed] [Google Scholar]
- 125.Viarisio D, Muller-Decker K, Accardi R, et al. Beta HPV38 oncoproteins act with a hit-and-run mechanism in ultraviolet radiation-induced skin carcinogenesis in mice. PLoS Pathog. 2018;14(1):e1006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 127.Gaglia MM, Munger K. More than just oncogenes: mechanisms of tumorigenesis by human viruses. Curr Opin Virol. 2018;32:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Consortium EP, Birney E, Stamatoyannopoulos JA, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai X, Li G, Laimins LA, Cullen BR. Human papillomavirus genotype 31 does not express detectable microRNA levels during latent or productive virus replication. J Virol. 2006;80(21):10890–10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gu W, An J, Ye P, Zhao KN, Antonsson A. Prediction of conserved microRNAs from skin and mucosal human papillomaviruses. Arch Virol. 2011;156(7):1161–1171. [DOI] [PubMed] [Google Scholar]
- 131.Qian K, Pietila T, Ronty M, et al. Identification and validation of human papillomavirus encoded microRNAs. PLoS One. 2013;8(7):e70202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gunasekharan V, Laimins LA. Human papillomaviruses modulate microRNA 145 expression to directly control genome amplification. J Virol. 2013;87(10):6037–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harden ME, Prasad N, Griffiths A, Munger K. Modulation of microRNA-mRNA Target Pairs by Human Papillomavirus 16 Oncoproteins. MBio. 2017;8(1): e02170–02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harden ME, Munger K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology. 2017;508:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao J, Lee EE, Kim J, et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat Commun. 2019;10(1):2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu L, Zheng ZM. Human Papillomavirus Type 16 Circular RNA Is Barely Detectable for the Claimed Biological Activity. mBio. 2022;13(1):e0359421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang RC, Lee EE, Zhao J, Kim J. Assessment of the Abundance and Potential Function of Human Papillomavirus Type 16 Circular E7 RNA. mBio. 2022;13(3):e0041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pisignano G, Michael DC, Visal TH, Pirlog R, Ladomery M, Calin GA. Going circular: history, present, and future of circRNAs in cancer. Oncogene. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rinn JL, Chang HY. Long Noncoding RNAs: Molecular Modalities to Organismal Functions. Annu Rev Biochem. 2020;89:283–308. [DOI] [PubMed] [Google Scholar]
- 140.Sharma S, Mandal P, Sadhukhan T, et al. Bridging Links between Long Noncoding RNA HOTAIR and HPV Oncoprotein E7 in Cervical Cancer Pathogenesis. Sci Rep. 2015;5:11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hasche D, Rosl F. Mastomys Species as Model Systems for Infectious Diseases. Viruses. 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ingle A, Ghim S, Joh J, Chepkoech I, Bennett Jenson A, Sundberg JP. Novel laboratory mouse papillomavirus (MusPV) infection. Vet Pathol. 2011;48(2):500–505. [DOI] [PubMed] [Google Scholar]
- 143.Joh J, Jenson AB, King W, et al. Genomic analysis of the first laboratory-mouse papillomavirus. J Gen Virol. 2011;92(Pt 3):692–698. [DOI] [PubMed] [Google Scholar]
- 144.Uberoi A, Yoshida S, Lambert PF. Development of an in vivo infection model to study Mouse papillomavirus-1 (MmuPV1). J Virol Methods. 2018;253:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meyers JM, Grace M, Uberoi A, Lambert PF, Munger K. Inhibition of TGF-beta and NOTCH Signaling by Cutaneous Papillomaviruses. Front Microbiol. 2018;9:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Romero-Masters JC, Grace M, Lee D, et al. MmuPV1 E7’s interaction with PTPN14 delays Epithelial differentiation and contributes to virus-induced skin disease. PLoS Pathog. 2023;19(4):e1011215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cladel NM, Budgeon LR, Cooper TK, Balogh KK, Hu J, Christensen ND. Secondary infections, expanded tissue tropism, and evidence for malignant potential in immunocompromised mice infected with Mus musculus papillomavirus 1 DNA and virus. J Virol. 2013;87(16):9391–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spurgeon ME, Uberoi A, McGregor SM, Wei T, Ward-Shaw E, Lambert PF. A Novel In Vivo Infection Model To Study Papillomavirus-Mediated Disease of the Female Reproductive Tract. mBio. 2019;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spurgeon ME, Lambert PF. Sexual transmission of murine papillomavirus (MmuPV1) in Mus musculus. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bogue MA, Churchill GA, Chesler EJ. Collaborative Cross and Diversity Outbred data resources in the Mouse Phenome Database. Mamm Genome. 2015;26(9–10):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Terai M, DeSalle R, Burk RD. Lack of canonical E6 and E7 open reading frames in bird papillomaviruses: Fringilla coelebs papillomavirus and Psittacus erithacus timneh papillomavirus. J Virol. 2002;76(19):10020–10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequence data that support the findings of this study are available in PAVE at https://pave.niaid.nih.gov/.


