Abstract
It is commonly believed that IL-12 produced by DCs in response to pathogens is the first signal that stimulates the production of IFN-γ by NK cells. However, IL-12 production by DCs in response to bacterial LPS depends on either engagement of CD40 by CD40L on activated T cells or IFN-γ from NK cells. This suggests that during the primary immune response, NK cells produce IFN-γ before IL-12 production by DCs. Here, using single-cell measurements, cell sorting and mouse lines deficient in IL-12, IL-23, type I IFN receptor and the IL-18 receptor, we show that a subset of BM-derived DCs characterized by low expression of MHC class II (MHCIIlow) stimulates IFN-γ production by NK cells. The expression of Toll-like Receptor (TLR) 4 on DCs but not NK cells was required for such NK-derived IFN-γ. In addition, soluble factor(s) produced by LPS-activated MHCIIlow DCs were sufficient to induce IFN-γ production by NK cells independent of IL-12, IL-23, and IL-18. This response was enhanced in the presence of a low dose of IL-2. These results delineate a previously unknown pathway of DC-mediated IFN-γ production by NK cells, which is independent of commonly known cytokines.
Keywords: dendritic cells, IFN-γ, IL-12, MHCIIlow DC, NK cells
Introduction
A rapid host response to microbial pathogens depends on the activation of a network of cells and the production of various soluble mediators, which constitute the first line of host defense commonly known as innate immunity. Natural killer (NK) cells are a subset of group 1 innate lymphoid cells (ILC1 s) [1] that play a critical role in eliminating pathogens through cytolytic activity and secretion of the potent effector cytokine IFN-γ [2]. NK cells are poised to make IFN-γ rapidly due to epigenetic modifications retaining the locus in an open configuration [3]. As initial mediators of innate immunity in inflamed tissues, NK cells can secrete IFN-γ within hours of infection [4], orchestrate the differentiation of monocytes into DCs [5], and prime monocytes for their regulatory function [6].
In mice and primates, systemic IFN-γ is detected within hours of bacterial infection or injection of LPS [7, 8]. The mechanism by which sensing pathogens translates into IFN-γ production by NK cells is not well understood. The prevailing view is that the detection of bacteria by TLRs on antigen presenting cells (APC) initiates the secretion of IL-12, which was originally identified as natural killer cell stimulatory factor (NKSF) [9]. IL-12 is a heterodimer of two subunits, p40 and p35 [10]. Accordingly, IL-12 produced by APCs activates NK cells to secrete IFN-γ [11], which in turn amplifies the polarization of naïve T cells toward Type 1 (TH1 and TC1) responses [12, 13]. In addition, NK cells regulate B-cell differentiation and Ig isotype switching [14]. Thus, NK cells have a central role early during infections by influencing which class of adaptive immune response follows.
This simple view that IL-12 is an upstream regulator of NK cells has been challenged by recent studies. For example, we have shown that IL-12 production by DCs is dependent on the prior presence of IFN-γ and therefore is not necessarily upstream of the IFN-γ required to induce TH1 responses [15, 16]. Similarly, studies of mice infected with Toxoplasma showed that NK cells are the major source of IFN-γ,which promotes IL-12 production by DCs [5]. In addition, stimulation of DCs by IFN-γ was shown to be critical for IL-12-dependent antitumor responses [17]. This leads to the question: which comes first, IL-12 or IFN-γ? Here, we provide a solution to this conundrum. We suggest that, similar to IL-4 production, a small amount of “priming” IFN-γ is needed early to stimulate a large amount later.
Using purified NK cells cocultured with BM-derived DCs obtained from cytokine-deficient mice, we measured IFN-γ produced at the single-cell level to answer this question. Here, we show that in the presence of LPS, DCs stimulate IFN-γ production by NK cells through signals that are dependent on TLR4 but not IL-12, IL-23, IL-18 or type I IFN. These data suggest that an alternative, IL-12-independent pathway operates for LPS-activated DCs to stimulate NK cells to become the early source of IFN-γ production. This IL-12-independent IFNγ (which comes first) is critical for subsequent IL-12 secretion and type 1 immunity.
Results and discussion
RAG−/− BM cells stimulated with LPS contain NK cells that make IFN-γ
We have reported that DCs generated from RAG−/− mice contain NK cells that secrete IFN-γ when stimulated for 18 h with LPS [18]. Following up on these observations, we determined that approximately 1 out of 1000 cells within freshly isolated BM from RAG−/− mice produce IFN-γ after stimulation with LPS from E. coli (Fig. 1A). A similar response was observed after stimulation with LPS from several bacterial species (Fig. S1). Furthermore, a similar low frequency of IFN-γ-producing cells was detected in the splenocytes of RAG−/− mice after stimulation with LPS (Fig. 1B). Magnetic bead-assisted depletion of NK cells from RAG−/− BM cells using antibodies against NK-cell markers resulted in a significant reduction in the number of IFN-γ-producing cells in response to LPS (Fig. 1C). Previous work has reported that NK cells stimulated with TLR agonists produce IFN-γ [19–21]. To test this possibility, NK1.1+ cells were directly sorted by flow cytometry into an ELISPOT plate. These highly purified NK cells (>99.99%) produced IFN-γ when stimulated with IL-12 plus IL-18 but not with LPS alone (Fig. 1D). We concluded that primary mouse NK cells do not respond directly to LPS and do not make IFN-γ unless other cells or signals are present. Therefore, we sought to further narrow down the nature of the cells and the signals involved.
Figure 1. BM cells from RAG−/− mice stimulated with LPS contain NK cells that make IFN-γ.
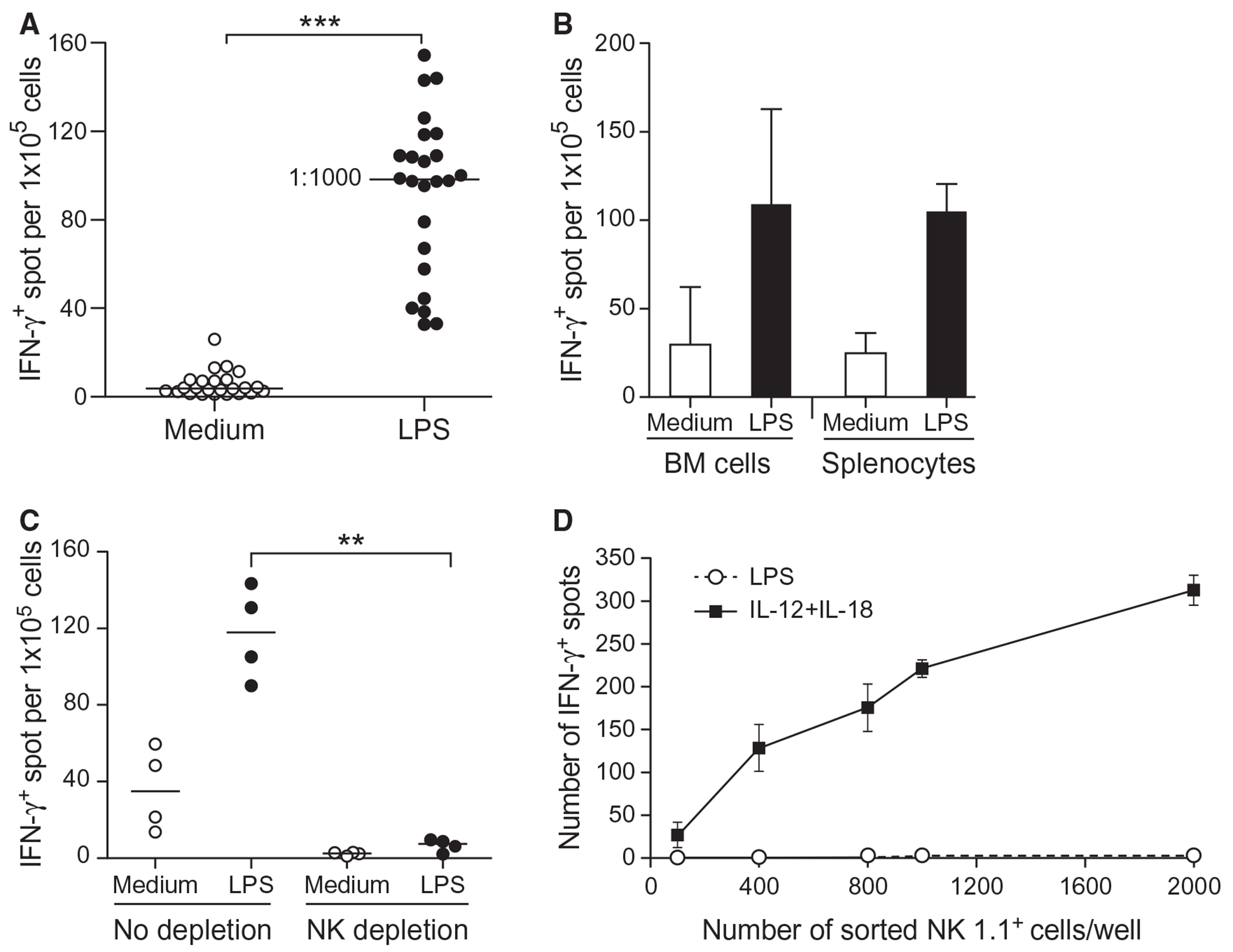
(A) Fresh BM cells from RAG−/− mice were cultured in a 96-well ELISPOT plate at 1 × 105 cells/well to detect IFN-γ–secreting cells in the presence or absence of 200 ng/ml LPS for 18 h at 37°C. Each dot represents an independent experiment (24 experiments). Statistical significance was assessed by Student’s t test. (B) Same as (A) except that BM cells and splenocytes from RAG−/− mice were used for comparison. Data represent the mean ± SD of four independent experiments. (C) Same as (A) except that BM cells were either NK-depleted using anti-NK1.1 plus -NKp46 antibodies or left untouched and cultured at 1 × 105 cells/well. Each dot represents an independent experiment. Statistical significance was assessed by one-way ANOVA. (D) NK cells from BM or splenocytes from RAG−/− mice were either preenriched by negative sorting for NK1.1+ cells or a Miltentyi NK-cell isolation kit. Various numbers of NK1.1+ cells were directly sorted into the ELISPOT plate using anti-NK1.1+ Ab and stimulated with 200 ng/ml LPS or a combination of ~125 pg/ml IL-12 plus ~600 ng/ml of IL-18. IFN-γ–secreting cells were counted after stimulation for 18 h at 37°C. Data represent the mean ± SD of three independent experiments. Statistical significance was assessed by **p<0.01, ***p<0.001.
IFN-γ production by NK cells is independent of IL-12 and IL-23
We used a standard sepsis model to measure IFN-γ in the serum of mice after intravenous (i.v.) injection of LPS. Serum was collected from WT, RAG−/−, and RAG−/− γC−/− mice 6 h after LPS injection. RAG−/− mice had more IFN-γ in their sera than WT mice (Fig. 2A), in agreement with the higher number of NK cells in the spleens of RAG−/− mice [22]. In contrast, no IFN-γ was detected in the serum of RAG−/− γC−/− mice (Fig. 2A), which lack NK cells [23]. We concluded that NK cells are the primary source of IFN-γ in this model, which is consistent with studies showing that activated NK cells are the main source of IFN-γ during sepsis [24], and depletion of NK cells in septic mice offers protection against LPS-induced shock [24, 25].
Figure 2. DCs help NK cells make IFN-γ in a TLR4-dependent but IL-2-, IL-12-, IL-23-, and IL-18-independent manner.
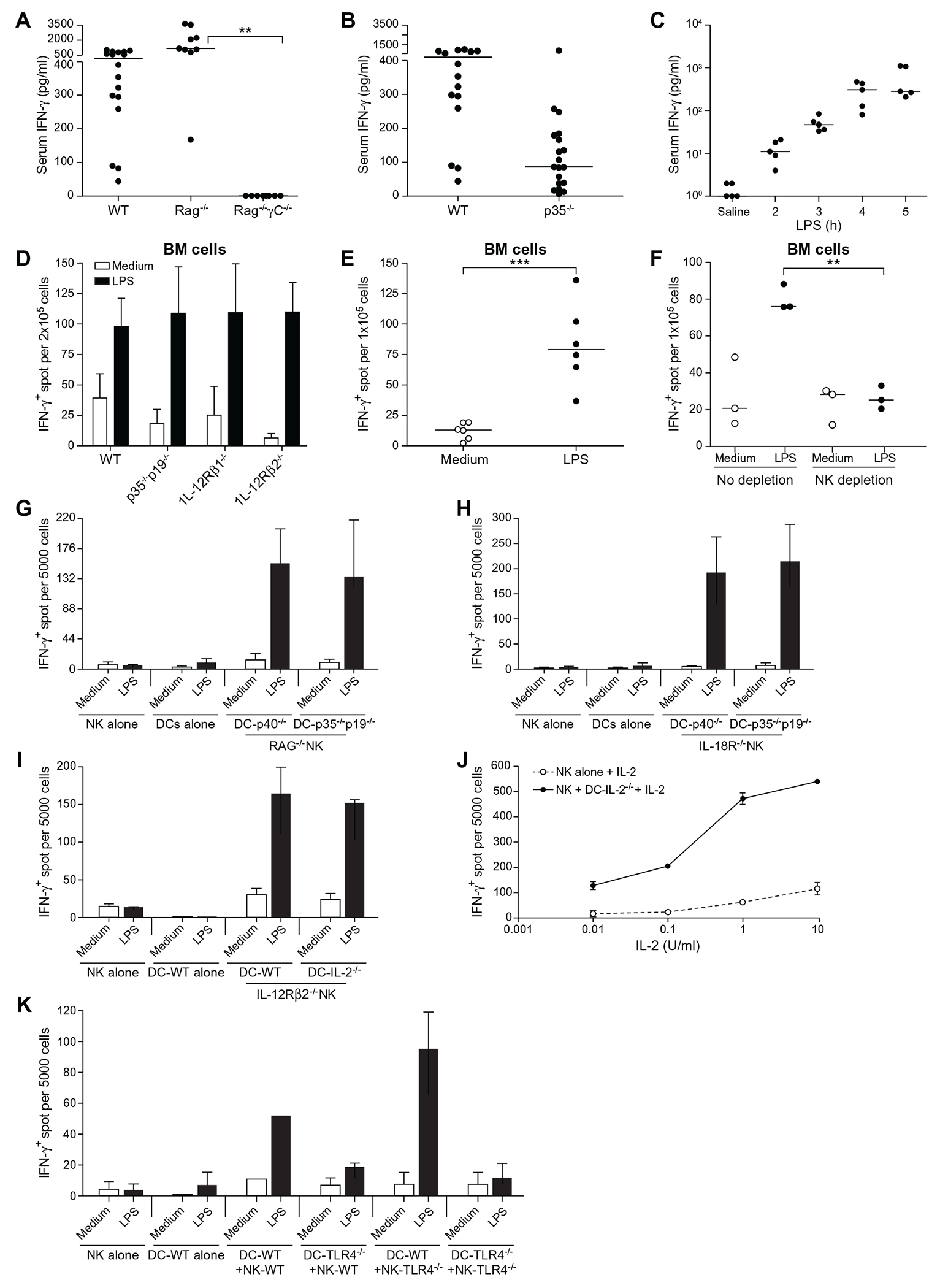
(A) Serum IFN-γ measured 6 h post-i.v. injection with 10 μg LPS in WT, RAG−/−, and RAG−/−γC−/− mice. (B) Same as (A), except that WT and IL-12p35−/− mice were compared. (C) Same as (A) except that p35−/−p19−/− double knockout mice were used and that serum IFN-γ was measured at various time points after LPS injection. Each dot represents a different mouse. Statistical significance was assessed by one-way ANOVA. The bars indicate the median. (D) Fresh BM cells from the indicated mice were cultured in ELISPOT plates at 2 × 105 cells/well to detect IFN-γ–secreting cells in the presence or absenceof 200 ng/ml LPS for 18 h at 37°C. Data represent the mean ± SD of three independent experiments. (E) Same as (D) except that the BM cells were from p35−/−p19−/− mice. Each dot represents an independent experiment. Statistical significance was assessed by Student’s t test. (F) Same as (E) except that BM cells were either NK-depleted using anti-NK1.1 plus -NKp46 antibodies or left untouched and cultured at 1 × 105 cells/well. Each dot represents an independent experiment. Statistical significance was assessed by one-way ANOVA. (G) 5 × 103 NK cells from RAG−/− mice, which had been preenriched by negative sorting for NK cells and directly sorted into the IFN-γ ELISPOT plate using anti-NK1.1 Ab and cocultured with 1 × 104 from p40−/− or -p35−/−p19−/− mice in the presence or absence of LPS for 18 h at 37°C. Data represent the mean ± SD of four independent experiments. (H) Same as (G) except that NK cells were from IL-18R1−/− mice. Data represent the mean ± SD of four independent experiments. (I) Same as (G) except that NK cells were from IL-12Rβ2−/− and that DCs were generated from IL-2−/− mice. Data represent the mean ± SD of at least two or more experiments. (J) The same as (I) except that NK cells were cocultured with DC-IL-2−/− or cultured alone in the presence of various doses of IL-2 plus LPS. Data represent the mean ± SD of at least two or more experiments. (K) Same as (I) except that NK cells and DCs were from WT or TLR4−/− mice, as indicated. Data represent the mean ± SD of two independent experiments. **p<0.01, ***p<0.001.
IL-12 has been shown to be required for IFN-γ production in LPS-induced shock in mice [26]. To test whether IL-12 was needed, we measured serum IFN-γ in WT or IL-12p35−/− (p35−/−) mice 6 h after LPS injection. IFN-γ was produced in p35−/− mice, albeit at far lower levels than in WT controls (Fig. 2B). These results show that there is an IL-12-independent pathway for IFN-γ production in mice injected with LPS. Such IL-12-independent IFN-γ production in p35−/− mice is also consistent with the IFN-γ-dependent protection against infections including Listeria [27] Salmonella [28] and Mycobacterium [29].
IL-23, which is composed of the IL-12p40 subunit and the p19 protein, enhances IL-17 production in T cells [30] and compensates for the lack of IL-12 in p35−/− mice to support antigen-specific IFN-γ responses to Mycobacterium [31] and limited resistance to Toxoplasma [32]. To examine whether IFN-γ detected in p35−/− mice required IL-23, we injected mice doubly deficient for both p35 and p19 (p35−/−p19−/−) with LPS. Serum IFN-γ was still detected as early as 2 to 3 h after LPS challenge (Fig. 2C). These results were confirmed in a direct comparison of freshly isolated BM cells from mice missing either the cytokines IL-12 and IL-23 or their receptors. Mice that were either WT, p35−/−p19−/−, IL-12Rβ1−/−, or IL-12Rβ2−/− mice all produced IFN-γ in response to LPS (Fig. 2D). We concluded that IFN-γ can be produced in the absence of IL-12 and IL-23 in these in vitro cultures (Fig. 2E). Next, we asked if NK cells are still the source of IFN-γ in p35−/−p19−/− BM cells. NK-cell depletion from freshly isolated p35−/−p19−/− BM cells caused a significant reduction in the number of IFN-γ-producing cells (Fig. 2F). These results confirmed that NK cells contribute to IFN-γ production by LPS-stimulated p35−/−p19−/− BM cells.
TLR4 in DCs but not in NK cells is required for IFN-γ production
The central question of this study is the early triggering of NK-cell-IFN-γ, as it may happen in the context of a microbial insult. Based on earlier literature and the known positioning of cells, this process can operate through DCs (which sense PAMPs and then secrete IFN-γ-inducing factors such as IL-12, IL-23, and IL-18) or through bidirectional exchanges between DCs and NK cells [33–36]. To study these two possibilities, we first tested the ability of p35−/−p19−/− DCs to directly stimulate IFN-γ production by NK cells in a coculture assay. Five thousand RAG−/− NK cells were sorted into wells of an ELISPOT and combined with 10,000 BM-derived DCs from either p40−/− (i.e., lacking the subunit common to IL-12 and IL-23) or p35−/−p19−/− mice in the presence or absence of LPS. IFN-γ production was detected in the presence but not absence of LPS (Fig. 2G), confirming that NK cells can be triggered by activated DCs to make IFN-γ in the absence of IL-12 and IL-23.
NK cells express IL-18R, and it is possible that IL-18 secreted by DCs in response to LPS in these coculture assays might contribute to NK-IFN-γ production [37–39]. We therefore sorted NK cells from IL-18R1−/− mice into an ELISPOT plate and cocultured them with p40−/− DCs or p35−/−p19−/− DCs in the presence or absence of LPS. IFN-γ-producing cells were still present in the coculture assay (Fig. 2H). We concluded that NK cells could make IFN-γ independent of IL-12, IL-23, and IL-18.
In addition, IL-2 secreted by DCs in response to LPS has also been implicated in the induction of IFN-γ production by NK cells [40]. Although subsequent studies have shown that DCs generated in the presence of IL-4 (as done here) lose their capacity to secrete IL-2 [41, 42], we examined whether IL-2 might play a role in our assay. DCs from IL-2-deficient mice (DC-IL-2−/−) were cocultured with sorted NK cells missing the IL-12 receptor in an ELISPOT plate in the presence or absence of LPS. Fig. 2I shows that the absence of IL-2 did not prevent the production of NK-derived IFN-γ. Similar experiments showed that IL-15, which is trans-presented by DCs to NK cells and plays an essential role in NK-cell differentiation and survival [43, 44], was not required for NK-cell production of IFN-γ (Fig. S2). We concluded that neither IL-2 nor IL-15 is required for IFN-γ production by NK cells in the coculture assay. We did, however, notice that the addition of IL-2, even in low amounts to the cocultures of IL-2−/− DCs with IL-12R−/− NK cells, increased the number of NK cells producing IFN-γ (Fig. 2J). Indeed, activation of NK cells by IL-2 is a well-established paradigm [45]. This result revealed a synergy between IL-2 and the signals exchanged in these coculture assays.
Previous data have also suggested a role for type I IFNs in triggering IFN-γ production by NK cells [46]. Consistent with this, we also found that the addition of IFN-β to highly pure primary NK cells induced IFN-γ production from these cells (Fig. S3A). Furthermore, even in the presence of blocking antibodies to IFN-β in the coculture of DCs and NK cells, IFN-γ-positive spots could still be detected (Fig. S3B). Moreover, LPS-activated DCs induced IFN-γ production by IFNAR1−/− NK cells, even after blocking any IL-12-dependent signal with a p40 homodimer (p40HD, a potent IL-12 antagonist) (Fig. S3C). Taken together, although the prevailing view is that IL-12 secreted by DCs has a central role in the stimulation of NK cells for IFN-γ production [35], our data revealed the existence of an additional pathway of IFN-γ production by NK cells that is independent of IL-2, IL-12, IL-23, type I IFN and IL-18.
Finally, to determine whether NK cells and DCs both respond independently to LPS and then collaborate, we isolated DCs and NK cells from WT and TLR4−/− mice. Consistent with previous studies [47], TLR4 on DCs, but not on NK cells, was essential for IFN-γ production by NK cells (Fig. 2K). These results provide clear evidence that LPS-activated DCs can stimulate primary NK cells to make IFN-γ.
An MHCIIlow subset of DCs is responsible for LPS-dependent stimulation of NK cells
By elimination, our results imply the existence of other triggers made by DCs that activate the production of IFN-γ by NK cells. BM-derived DC cultures consist of two distinct populations based on the expression of MHC class II: an MHCIIlow subset and an MHCIIhi subset (Fig. 3A). Ten thousand DCs of each subset from p40−/− mice were sorted into an ELISPOT plate and cocultured with 5000-sorted IL-18R1−/− NK cells in the presence or absence of LPS. Remarkably, IFN-γ-inducing activity was associated mainly with MHCIIlow cells but not MHCIIhi subsets of CD11c+ DCs (Fig. 3B). As observed with total DCs (Fig. 2J), the addition of a low dose of IL-2 increased the number of IFN-γ-producing cells compared to cultures stimulated with LPS alone (Fig. 3C).
Figure 3. MHC-IIlow but not MHC-IIhi DCs are responsible for the stimulation of IFN-γ secretion.
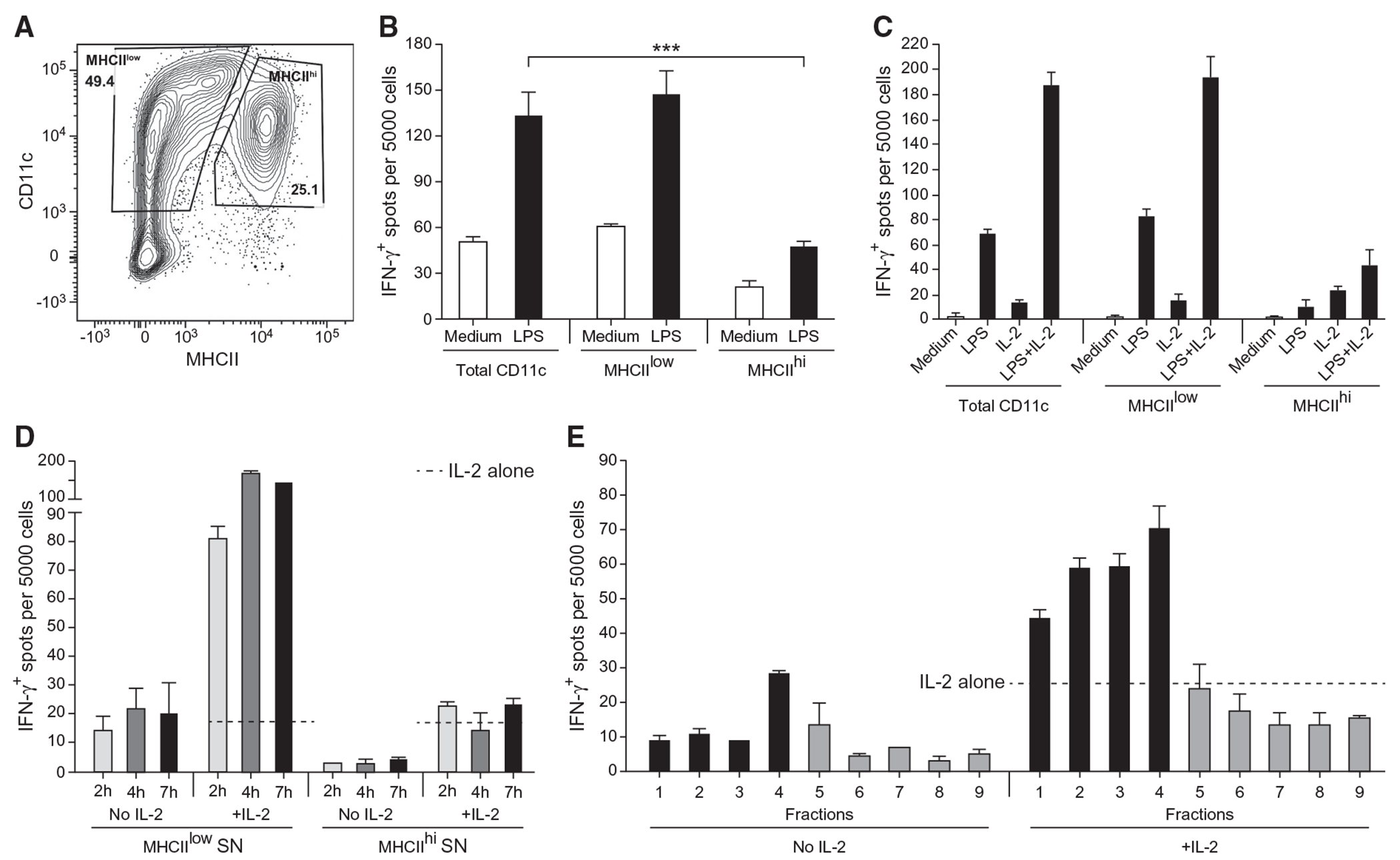
(A) Flow cytometry analysis of unstimulated BM-derived DCs from p40−/− mice stained with antibodies against CD11c and MHCII. The contour plot depicts the surface expression of CD11c and MHCII by live DAPI−Ter119−NK1.1−CD3−C19− cells. Gating is shown for the sorting of CD11c+MHCIIlow and CD11c+MHCIIhi. Data represent one of at least 15 experiments. (B) A total of 5 × 103 NK cells from IL-18R1−/− mice preenriched by negative sorting were directly sorted into IFN-γ ELISPOT plates using anti-NK1.1 Ab and cocultured with the indicated subsets of DC-p40−/− at 1 × 104/well in the presence or absence of LPS for 18 h at 37°C. Data represent the mean ± SD representative of at least 4 experiments. (C) Same as (B), except that 5 U/ml IL-2 was added to the cocultures of NK cells and DCs in combination with LPS as indicated. Data represent the mean ± SD representative of at least 3 or more experiments. (D) Same as (B), except that the supernatant (SN) from DC-p40−/− cells that were previously sorted and stimulated with LPS was harvested at various time points and added to sorted IL-18R1−/− NK cells in the presence or absence of 5 U/ml IL-2. The frequency of IFN-γ+ cells in response to IL-2 alone (no SN) is indicated by a dotted line. Data represent the mean ± SD representative of at least 2 or more experiments. (E) Same as (B) except that the supernatant (SN) from sorted MHCIIlow DC-p40−/− that were stimulated with LPS was fractionated over a size exclusion column. Individual fractions were added to sorted IL-18R1−/− NK cells in the presence or absence of 5 U/ml IL-2. The frequency of IFN-γ+ cells in response to IL-2 alone (no fraction) is indicated by a dotted line. Data represent the mean ± SD representative of at least 2 experiments. Gray color represents later fractions that did not have IFN-γ-stimulating activities.
We then investigated whether DCs and NK cells had to be cocultured to elicit IFN-γ production by the latter or whether soluble factor(s) secreted by DCs stimulated with LPS would be sufficient to induce IFN-γ production by NK cells. Supernatants (SN) of DC cultures were collected after sorting MHCIIlow and MHCIIhi subsets of p40−/− DCs and stimulating them with LPS. SN was added to the IL-18R1−/− NK cells sorted into an ELISPOT plate and incubated for 18 h at 37°C in the presence or absence of 5 U/ml IL-2. SN from the MHCIIlow subset of p40−/− DCs collected as early as 2 h after LPS stimulation was sufficient to induce IFN-γ production by IL-18R1−/− NK cells (Fig. 3D). The number of responding NK cells was slightly greater after stimulation with SN collected at 4 h and 7 h, and the addition of 5 U/ml IL-2 to NK cells with SN increased their number by 5- to 7-fold (Fig. 3D). In contrast, even in the presence of IL-2, the response of NK cells to SN of MHCIIhi DCs was negligible (Fig. 3D). We concluded that LPS-stimulated DCs can produce a soluble factor other than IL-12, IL-23, type I IFN, IL-2 or IL-18 that induces NK cells to produce IFN-γ. This does not eliminate a contribution by any of those soluble factors, or by other contact-dependent interactions, to the stimulation of NK-cell IFN-γ production, but it does show that they were not necessary in our system.
To be rigorous, we also stimulated MHCIIlow p40−/− DCs with LPS in the absence of fetal bovine serum (FBS) to eliminate any possible contribution of bovine serum proteins to the NK stimulation assay (and facilitate future proteomic studies). SN of five independent experiments were pooled and fractionated by size exclusion chromatography. Fractions were collected and added to IL-18R1−/− NK cells that had been sorted into an IFN-γ ELISPOT plate. IFN-γ-inducing activity was detected in fractions 1–4, which correspond to molecular weights of ~55 kDa to ~130 kDa and was enhanced by low-dose IL-2 (Fig. 3E). Size exclusion chromatography fractions obtained from unstimulated DCs (medium alone) did not have much stimulatory activity (Fig. S4). Our results underscore a previously unappreciated pathway leading to IFN-γ production by NK cells in response to DCs and demonstrate that soluble factor(s) released by LPS-stimulated DCs act on NK cells to produce IFN-γ in the absence of IL-12, IL-23, and IL-18. Furthermore, this response is greatly enhanced in the presence of low-dose IL-2. The identification of soluble factor(s) secreted by MHCIIlow p40−/− DCs that stimulate NK cells for IFN-γ production will be the focus of future studies.
Concluding remarks
Collectively, these results show that DCs stimulated with LPS induce the production of IFN-γ by NK cells, independent of the commonly known and expected cytokines, thereby revealing a previously unsuspected pathway for an early source of IFN-γ. These findings may also be relevant to the production of IFN-γ by NK cells during early inflammatory responses [5, 6], serving as a prerequisite signal for IL-12 production by DCs and a pivotal step in TH1 polarization [15, 16]. This notion is supported by a study showing that recruitment of NK cells to the lymph node in response to LPS-matured DCs provides the early IFN-γ signal necessary to promote differentiation of naïve T cells toward a TH1 pathway [13].
We propose that during the early phase of an immune response, DCs secrete soluble factor(s) that stimulate IFN-γ production by NK cells. This initial NK-derived IFN-γ primes DCs to produce IL-12, creating a milieu during which naïve T cells (which do not express IL-12Rβ2) receive signals one and two. This will lead to activation and transcription of T-bet and remodeling of the IFN-γ locus, which precedes that of IL-12, STAT4 and upregulation of IL-12Rβ2 [48]. This will ensure the differentiation and commitment of T cells toward the TH1 pathway, leading to the establishment of a positive feedback loop between IL-12 and IFN-γ that sustains the IFN-γ response (Fig. 4). Finally, we deduce from our data that the initiation of the IFN-γ response and polarization of T cells toward the TH1 pathway is dependent on preexisting IFN-γ rather than IL-12. This is analogous to TH2 responses, in which the production of IL-4 by TH2 cells requires the presence of preexisting IL-4 [49]. Understanding how initial IFN-γ expression is regulated will improve vaccine design and protocols for cancer immunotherapy.
Figure 4. Development of TH1 cells: the need for IFN-γ to turn on IL-12 and more IFN-γ.
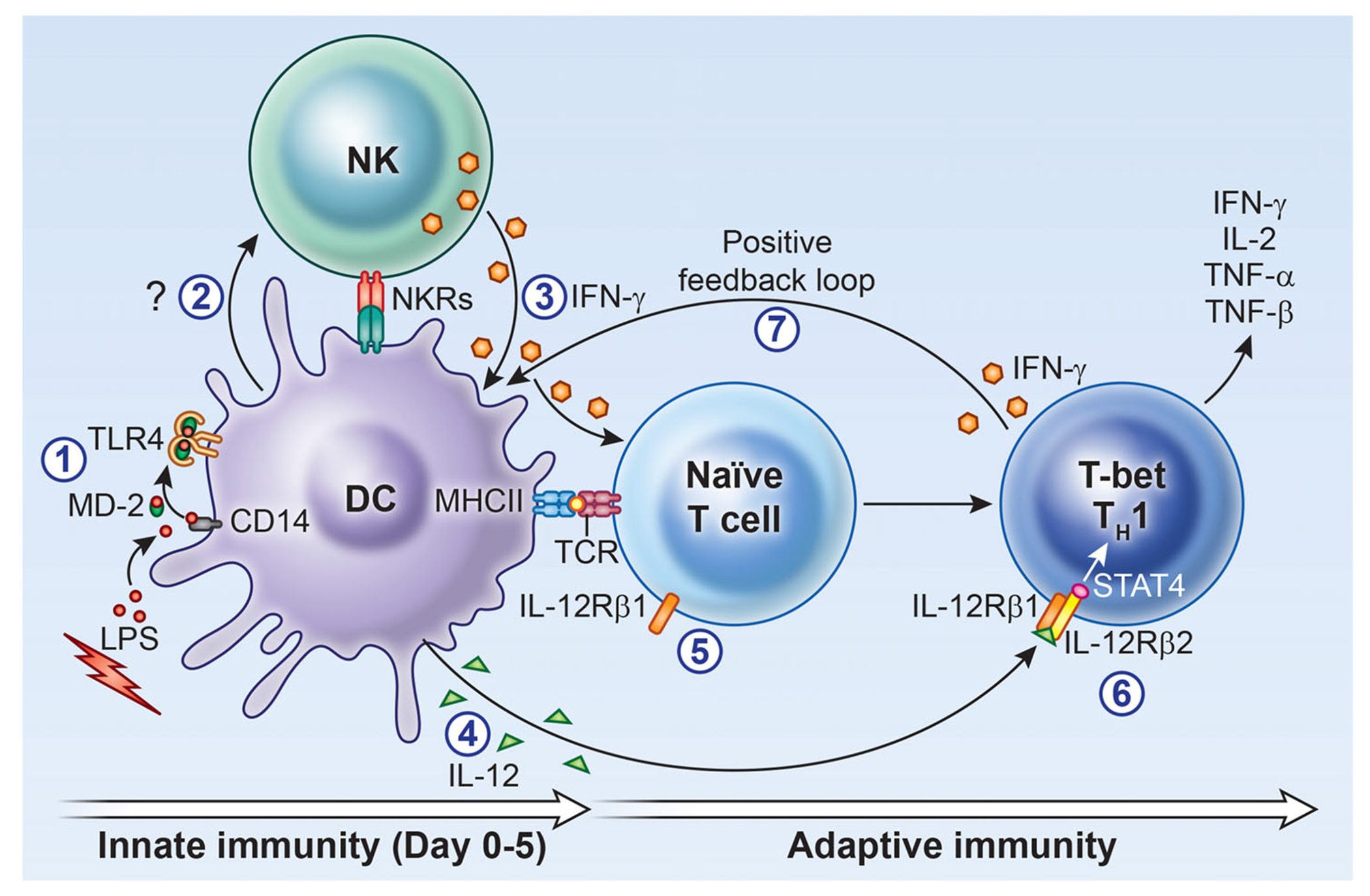
During the early innate immune response to pathogens (1), soluble factor(s) produced by activated DCs, independent of IL-12, IL-23, and IL-18 (2), stimulate NK cells to produce IFN-γ (3); this early IFN-γ in addition to priming DCs for IL-12 production (4) will play a critical role in inducing the differentiation of naïve T cells toward the TH1 pathway (5) through activation of transcription factor T-bet, upregulation of IL-12Rβ2 for signaling by IL-12 (6) and STAT4 for the secretion of IFN-γ, initiating a positive feedback loop (7) and generating more IFN-γ production, assuring the continuity of an ongoing TH1 response.
Materials and methods
Mice
Eight- to fourteen-week-old male and female C57BL/6J, B6-tlr4−/−, B6-il18R1−/−, B6-il12rb1−/−, B6-il12rb2−/− and B6-il12p35−/− mice were obtained from Jackson Laboratory. B6-il12p35−/−p19−/− was a generous gift from Dr. Andrea Cooper, Trudeau Institute; B6-rag2−/−γC−/−, B6-rag1−/−, B6-il12p40−/−, and type-I IFNAR1−/− mice were generated at the NIAID/Taconic Farms, Inc. All animal studies were carried out and approved in accordance with the Institutional Animal Care and Use Committee (IACUC) of the NIH. The manuscript does not contain human studies.
Media and reagents
Bacterial LPS was purchased from Sigma. Recombinant cytokines were purchased from Peprotech Inc. The culture medium used throughout was Iscove’s modified Dulbecco’s medium (IMDM: Gibco BRL). Recombinant mouse IL-2 (PeproTech), IFN beta and anti-mouse IFN-β were purchased (pbl Assay Science), and the mouse p40 homodimer was purchased from R&D Systems. The following antibodies were purchased from Biolegend: NK1.1-biotin (PK136), NKp46-biotin (29A1.4), CD3-biotin (17A2), CD19-biotin (6D5), MHC-II PerCP/Cy5.5 (AF6-120.1 & M5/114), CD11c BV785 (N418), CD3 FITC, CD19 FITC, Ter119 FITC, NK1.1 647 (PK136), -NKp46 PE/Dazzle (29A1.4), DAPI and -CD16/32 (Ultra-LEAF Purified clone 93).
Generation of NK cells
Freshly isolated BM cells or splenocytes were first preenriched for NK cells by negative selection using FACS sorting or a mouse NK isolation kit (Miltenyi). Unless otherwise stated, negatively selected NK cells were directly sorted into IFN-γ ELISPOT plates at 5 × 103 cells/well using anti-NK1.1 Ab.
Generation of BM-derived DCs
BM cells were flushed out of the femurs and tibias of various mice and magnetically depleted using a cocktail of biotinylated anti-CD3, -CD19, -Ter119, -NK1.1, and -NKp46 antibodies as previously described [18]. BM cells were cultured at 1 × 106 cells/well in a 24-well plate in medium supplemented with GM-CSF and IL-4 as described [16].
DC and NK-cell cocultures
A total of 5 × 103 sort-purified NK1.1+ cells were cocultured with 1 × 104 sort-purified DCs in an IFN-γ ELISPOT plate ± 200 ng/ml LPS for 18 h at 37°C. The ELISPOT plate was analyzed as previously described [16].
Cytokine measurements
The concentration of IFN-γ in the mouse serum was determined using an ELISA kit (Quantikine) or the SearchLight Multiplex cytokine array (Aushon Biosystems, Billerica, MA).
Detection of IFN-γ ELISPOT
The mouse IFN-γ ELISpotPLUS kit was from MABTECH (Cincinnati, OH). BM cells were incubated overnight at 1 × 105 cells/well (or as indicated) in the presence or absence of 200 ng/ml LPS for 18 h at 37°C in a final volume of 200 μl/welL. The spots were enumerated by ZellNet Consulting Inc. (FortLee, NJ).
Size exclusion chromatography
Cell-free supernatants (SN) were passed through a 0.45 μm syringe filter and fractionated using standard gel filtration chromatography by injecting the samples into a 5 ml loop. The protein fractions were separated on an AKTA FPLC using a HiPrep 16/60 Sephacryl S200 HR column (GE Healthcare). The column was eluted using PBS as a buffer at 0.5 ml per min. We collected 12 fractions, each containing 3 ml. The fractions were concentrated to 200 μl using a Vivaspin 3000 molecular weight (Sartorius Stedim). A total of 25–50 μl of each fraction was used to stimulate NK1.1+ cells that were previously sorted into the IFN-γ ELISPOT plate in the presence or absence of 5 U/ml IL-2.
In vivo model of sepsis
Mice were injected with 100 μl of saline or 10 μg of LPS (in saline) as described [15].
Flow cytometry
Flow cytometry data were collected on BD LSR-II and SONY. The data were analyzed using Flow Jo v9 (FLowJo, LLC).
Statistical analysis
Student’s t test and analysis of variance (ANOVA) were performed using GraphPad Prism software.
Supplementary Material
Acknowledgments:
We thank Giorgio Trinchieri, Polly Matzinger and Michael Bowman for discussions and comments, Elina Stregevsky and George McGrady for help with flow cytometry, and Alan Hoofring and Ethan Tyler for data illustrations. This work is supported by The Intramural Research Program at the NIH, NIAID.
Abbreviations:
- NK
natural killer
- SN
supernatant
Footnotes
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of interest: The authors declare no conflicts of interest.
Disclosures
The authors have no commercial or financial conflicts of interest.
Data availability statement:
The data that supports the findings of this study are available in the supplementary material of this article.
References
- 1.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S et al. , Innate lymphoid cells–a proposal for uniform nomenclature. Nat. Rev. Immunol 2013. 13: 145–149. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G, Biology of natural killer cells. Adv. Immunol, 1989. 47: 187–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sciumè G, Mikami Y, Jankovic D, Nagashima H,Villarino AV, Morrison T,Yao C et al. ,Rapid Enhancer Remodeling and Transcription Factor Repurposing Enable High Magnitude Gene Induction upon Acute Activation of NK Cells. Immunity, 2020. 53: 745–758 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama WM, Kim S and French AR, The dynamic life of natural killer cells. Annu. Rev. Immunol 2004. 22: 405–429. [DOI] [PubMed] [Google Scholar]
- 5.Goldszmid RS, Caspar P, Rivollier A,White S, Dzutsev A, Hieny S, Kelsall B et al. , NK-cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation ofmonocytes into dendritic cells at the site of infection. Immunity 2012. 36: 1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Askenase MH, Han S-J, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, Konkel JE, et al. , Bone-Marrow-Resident NK Cells Prime Monocytes for Regulatory Function during Infection. Immunity 2015. 42: 1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinzel FP, Rerko RM, Ling P, Hakimi J and Schoenhaut DS, Interleukin 12 is produced in vivo during endotoxemia and stimulates synthesis of gamma interferon. Infect. Immun 1994. 62: 4244–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen PM, Van Der Pouw Kraan TC, De Jong IW, Van Mierlo G, Wijdenes J, Chang AA, Aarden LA et al. , Release of interleukin-12 in experimental Escherichia coli septic shock in baboons: relation to plasma levels of interleukin-10 and interferon- gamma. Blood 1996. 87: 5144–5151. [PubMed] [Google Scholar]
- 9.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R. et al. , Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine withmultiple biologic effects on human lymphocytes. J. Exp. Med 1989. 170: 827–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trinchieri G, Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigenspecific adaptive immunity. Annu. Rev. Immunol 1995. 13: 251–276. [DOI] [PubMed] [Google Scholar]
- 11.Trinchieri G. and Sher A, Cooperation of Toll-like receptor signals in innate immune defense. Nat. Rev. Immunol 2007. 7: 179–190. [DOI] [PubMed] [Google Scholar]
- 12.Scharton TM and Scott P, Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T-cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med, 1993. 178: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F, Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol 2004. 5: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 14.Wilder JA, Koh CY and Yuan D, The role of NK cells during in vivo antigen-specific antibody responses. J. Immunol 1996. 156: 146–152. [PubMed] [Google Scholar]
- 15.Abdi K, Singh N and Matzinger P, T-cell control of IL-12p75 production. Scand. J. Immunol 2006. 64: 83–92. [DOI] [PubMed] [Google Scholar]
- 16.Abdi K, Singh NJ and Matzinger P, Lipopolysaccharide-activated dendritic cells: “exhausted” or alert and waiting? J. Immunol 2012. 188: 5981–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, et al. , Successful Anti-PD-1 CancerImmunotherapy Requires T-Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity 2018. 49: 1148–1161 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdi K, Thomas LM, Laky K, Abshari M, Matzinger P, Long EO Bone Marrow-Derived Dendritic cell cultures from RAG(−/−) Mice include IFN-gamma-producing NK Cells. Immunohorizons 2020. 4: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivori S, Falco M, Chiesa MD, Carlomagno S, Vitale M, Moretta L, Moretta A, CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A 2004. 101: 10116–10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, Wang F. et al. , APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J. Immunol 2004. 172: 138–143. [DOI] [PubMed] [Google Scholar]
- 21.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM, TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J. Immunol 2005. 175: 1636–1642. [DOI] [PubMed] [Google Scholar]
- 22.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J. et al. , RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992. 68: 855–867. [DOI] [PubMed] [Google Scholar]
- 23.Colucci F, Soudais C, Rosmaraki E, Vanes L, Tybulewicz VL, Di Santo JP, Dissecting NK-cell development using a novel alymphoid mouse model: investigating the role of the c-abl protooncogene in murine NK-cell differentiation. J. Immunol 1999. 162: 2761–2765. [PubMed] [Google Scholar]
- 24.Heremans H, Dillen C, Van Damme J. and Billiau A, Essential role for natural killer cells in the lethal lipopolysaccharideinduced Shwartzman-like reaction in mice. Eur. J. Immunol 1994. 24: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 25.Carson WE, Yu H, Dierksheide J, Pfeffer K, Bouchard P, Clark R, Durbin J. et al. , A fatal cytokine-induced systemic inflammatory response reveals a critical role for NK cells. J. Immunol 1999. 162: 4943–4951. [PubMed] [Google Scholar]
- 26.Wysocka M, Kubin M, Vieira LQ, Ozmen L, Garotta G, Scott P. and Trinchieri G, Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol 1995. 25: 672–676. [DOI] [PubMed] [Google Scholar]
- 27.Brombacher F, Dorfmüller A, Magram J, Dai WJ, Köhler G, Wunderlin A, Palmer-Lehmann K. et al. , IL-12 is dispensable for innate and adaptive immunity against low doses of Listeriamonocytogenes. Int. Immunol 1999. 11: 325–332. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann J, Bellmann S, Werner C, Schröder R, Schütze N, Alber G, IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J. Immunol 2001. 167: 5304–5315. [DOI] [PubMed] [Google Scholar]
- 29.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM, Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol 2002. 168: 1322–1327. [DOI] [PubMed] [Google Scholar]
- 30.Gaffen SL, Jain R, Garg AV, Cua DJ, The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol 2014. 14: 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N. et al. , IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFNgamma responses if IL-12p70 is available. J. Immunol 2005. 175: 788–795. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, Hunter CA et al. , IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J. Immunol 2004. 173: 1887–1893. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M. et al. , Dendritic cells directly trigger NK-cell functions: cross-talk relevant in innate antitumor immune responses in vivo. Nat. Med 1999. 5: 405–411. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka Y, Nishimura N, Suzuki Y, Sone S, Human monocytederived and CD83(+) blood dendritic cells enhance NK-cell-mediated cytotoxicity. Eur. J. Immunol 2001. 31: 2633–2641. [DOI] [PubMed] [Google Scholar]
- 35.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G, Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med 2002. 195: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moretta A, The dialog between human natural killer cells and dendritic cells. Curr. Opin. Immunol 2005. 17: 306–311. [DOI] [PubMed] [Google Scholar]
- 37.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H. et al. , Defective NK-cell activity and Th1 response in IL-18-deficient mice. Immunity 1998. 8: 383–390. [DOI] [PubMed] [Google Scholar]
- 38.Akira S, The role of IL-18 in innate immunity. Curr. Opin. Immunol 2000. 12: 59–63. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi K, Yoshimoto T, Tsutsui H. and Okamura H, Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol 2001. 19: 423–474. [DOI] [PubMed] [Google Scholar]
- 40.Granucci F, Zanoni I, Pavelka N, Van Dommelen SLH, Andoniou CE, Belardelli F, Degli Esposti MA et al. , A contribution of mouse dendritic cell-derived IL-2 for NK-cell activation. J. Exp. Med 2004. 200: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauma D, Michea P, Lennon-Dumenil A-M, Fierro A, Morales J, Rosemblatt M, Bono MR et al. , Interleukin-4 selectively inhibits interleukin-2 secretion by lipopolysaccharide-activated dendritic cells. Scand. J. Immunol 2004. 59: 183–189. [DOI] [PubMed] [Google Scholar]
- 42.Terme M, Tomasello E, Maruyama K, Crépineau F, Chaput N, Flament C, Marolleau J-P et al. , IL-4 confers NK stimulatory capacity to murine dendritic cells: a signaling pathway involving KARAP/DAP12-triggering receptor expressed on myeloid cell 2 molecules. J. Immunol 2004. 172: 5957–5966. [DOI] [PubMed] [Google Scholar]
- 43.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA et al. , CD56bright natural killer cells are present in human lymph nodes and are activated by T-cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 2003. 101: 3052–3057. [DOI] [PubMed] [Google Scholar]
- 44.Cooper MA, Bush JE, Fehniger TA, Vandeusen JB, Waite RE, Liu Y, Aguila HL et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 2002. 100: 3633–3638. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg S, Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J. Natl. Cancer Inst 1985. 75: 595–603. [PubMed] [Google Scholar]
- 46.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei X-Q, Liew FY, Caligiuri MA et al. , Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK-cell responses to viral infection. J. Immunol 2002. 169: 4279–4287. [DOI] [PubMed] [Google Scholar]
- 47.Zanoni I, Spreafico R, Bodio C, Di Gioia M, Cigni C, Broggi A, Gorletta T. et al. , IL-15 cis presentation is required for optimal NK-cell activation in lipopolysaccharide-mediated inflammatory conditions. Cell Rep. 2013. 4: 1235–1249. [DOI] [PubMed] [Google Scholar]
- 48.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL et al. , Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 2001. 292: 1907–1910. [DOI] [PubMed] [Google Scholar]
- 49.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE, Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J. Exp. Med 1990. 172: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


