Abstract
Understanding the physiological basis of physical resilience to clinical stressors is crucial for the well-being of older adults. This paper presents a novel framework to discover the biological underpinnings of physical resilience in older adults as part of the “Characterizing Resiliencies to Physical Stressors in Older Adults: A Dynamical Physiological Systems Approach” study, also known as The Study of Physical Resilience and Aging (SPRING). Physical resilience, defined as the capacity of a person to withstand clinical stressors and quickly recover or improve upon a baseline functional level, is examined in adults aged 55 years and older by studying the dynamics of stress response systems. The hypothesis is that well-regulated stress response systems promote physical resilience. The study employs dynamic stimulation tests to assess energy metabolism, the hypothalamic-pituitary-adrenal axis, the autonomic nervous system, and the innate immune system. Baseline characteristics influencing resilience outcomes are identified through deep phenotyping of physical and cognitive function, as well as of biological, environmental, and psychosocial characteristics. SPRING aims to study participants undergoing knee replacement surgery (n=100), bone and marrow transplantation (n=100), or anticipating dialysis initiation (n=60). Phenotypic and functional measures are collected pre-stressor and at multiple times after stressor for up to 12 months to examine resilience trajectories. By improving our understanding of physical resilience in older adults, SPRING has the potential to enhance resilient outcomes to major clinical stressors. The paper provides an overview of the study’s background, rationale, design, pilot phase, implementation, and implications for improving the health and well-being of older adults.
Keywords: Physical Resilience, Physiological Resilience, Resilience capacity, Clinical Procedures, stimulus-response testing
Introduction:
The term “resilience” has been in scientific use for at least 200 years, serving as an important concept in materials science1, ecology, engineering, and psychology. In Geriatric Medicine, the term describes the ability of an older adult to recover from the effects of social, psychological, and physical stressors, including common events such as loss of a loved one, chronic illnesses, acute injuries, and financial strain 2–4.
“Physical” resilience refers to the ability of individuals to overcome and quickly recover to equilibrium or homeostasis after physical rather than psychological or social stressors5; usually in the context of clinical stressors including medical or surgical procedures, physical injuries, or acute illnesses6. There is considerable evidence that older adults are generally less able to tolerate physical stressors and are therefore less physically resilient than younger adults. However, the specific biological, physiological, social, and psychological factors contributing to this difference remain poorly defined in part due to individual variations7,8. Given the rapid growth of the older adult population worldwide and the increasing numbers of high-risk medical procedures older adults will undergo, it is imperative that the basis of physical resilience be better understood and measures to assess it be developed. This will enable the development of treatments and preventive strategies to improve physical resilience and identify older adults who are most vulnerable during medical and surgical procedures.
To understand physical resilience and its biological basis, both traditional and newer conceptual frameworks are being utilized. Traditional epidemiological approaches in clinical and population studies are identifying risk factors for non-resilient phenotypes and clinical outcomes after different physical stressors 6–8, 9. Chronological age, disease burden, pre-stressor cognitive and functional status, along with biomarkers, have been identified as possible predictors of resilient patients. Epidemiologic studies have improved our understanding of the many factors that are associated with physical resilience, but they have not provided a mechanistic explanation of the biological and physiological pathways that promote a resilient response to specific stressors. These pathways have been hypothesized to involve dynamic interaction among physiological systems that maintain homeostasis, which is a balanced state of equilibrium that manages incoming stressors9. Importantly, these systems are dynamic and responsive to stimuli. Due to the dynamic interaction of physiologic systems, studying resilience under steady-state conditions is unlikely to yield useful insights. Buchner and Wagner observed that age-related declines in physiologic functioning are more pronounced during provocative tests than under resting conditions10. If so, then experiments, in which a controlled stimulus is applied and the subsequent temporal response of the relevant physiological system is measured, are needed to assess capacity to respond resiliently to stressors. Accordingly, a major National Institute on Aging consensus conference in 2016 strongly recommended the development of stimulus-response tests to better assess the physiology underlying a resilient (or lack of resilient) response to specific stressors and identify at-risk older adults facing clinical stressors.
The Study of Physical Resilience and Aging (SPRING) was designed to address this recommendation. This paper describes the SPRING study design, including the background and rationale for using stimulus-response testing to assess physiological functioning and physical resilience in older adults. It introduces the conceptual framework developed for assessing physical resilience with this study and concludes with pilot-phase data.
Background and Rationale
Querying the role of dynamic systems in the maintenance of physical resiliencies with increasing age.
SPRING aims to study older adults preparing for physically demanding clinical stressors. It examines the dynamic (sometimes termed dynamical in technical systems descriptions) functioning of key physiological systems that govern the response to stress exposure, using stimulus-response experiments performed before the clinical stressor. We hypothesize that this approach elicits differences in older adults’ ability to recover from and withstand clinical stressors. Its successful implementation should enable the identification of older adults at risk for adverse outcomes from impending stressors, define resilient phenotypes post-stressor, and improve understanding of the biological features underlying resilience. Translation of resulting knowledge will promise better outcomes following various clinical stressors in older adults, including surgical and medical procedures.
Our rationale for implementing pre-stressor tests in SPRING that probe dynamic, stress response systems derived in part from prior studies of the biological basis of physical frailty in older adults. Physical frailty is characterized as a heightened vulnerability to adverse health outcomes, following stressors, that has its biological basis in dynamic systems decline15,16. This physiological decline, described as a ‘transition from homeostatic symphony to a cacophony17, signifies an advanced state of vulnerability to non-resilient outcomes. Studies of physical frailty have identified specific homeostatic physiological systems for further study, including energy regulation systems, the autonomic nervous system, and the innate immune system18–20. Because these systems are dynamic, tightly regulated, and have subject-specific setpoints, their functionality is best ascertained by stimulus-induced stressors21,22.
Existing conceptual frameworks from which to study resilience.
Resilience has been commonly characterized as the intrinsic ability/capacity to respond effectively to stressors, or alternatively as the optimal outcome following a stressor.
In the framing as intrinsic capacity, Resnick and others characterized resilience as the ability to bounce back from a physical challenge 7. Whitson et al. described it as a whole-person characteristic that determines ability to resist functional decline and to recover after a stressor 4. Varadhan et al characterized it as ability of a physiologic system to recover its essential identity and function after experiencing a major stressor which pushes the system into a state far from its original state 11.
In the framing as a phenotypic manifestation and outcome after a stressor, Rowe and Khan characterized resilience as the rapidity and completeness with which people recover from stressor episodes and return to meeting the criteria of success. They also posited that determination of resilience to a specific clinical stressor would require “assessment of relevant functions before the stressing challenge is encountered and subsequent monitoring to observe the initial decremental effect, the time required to regain stability of function, and the level of function regained” 12. Further, Mancini and Bonnanno stated “[a]lthough people may possess characteristics associated with resilience, whether people actually exhibit resilience can only be defined in terms of their level of adjustment after the stressor event.” They added that resilience cannot be defined in the abstract or applied to individuals in the absence of an extremely aversive experience 13.
To clarify these resilience characterizations, we propose the terms resilience capacity and resilience phenotype. The SPRING study aims to characterize: (i) resilience capacity using static and dynamic measures before a clinical/physical stressor, and its biological underpinnings, and (ii) the resilience phenotype through measurements before and after the clinical/physical stressor. Refer to Table 1 for key terms used in the description of the SPRING study.
Table 1.
Glossary table of relevant key terms
| Term | Definition |
|---|---|
| Resilience Capacity | The capacity of a physiologic system to recover to, or improve upon, a baseline level of health and function after experiencing a significant clinical stressor. |
| Resilience Phenotypes | Resilience phenotypes characterize both the immediate response to the stressor, and the medium and long-term recovery or decline thereafter, in key markers of health. These, therefore, are “trajectories” of key functional measures, beginning shortly before the stressor experience and continuing afterward. |
| Dynamic Indicator of Resilience / Stimulus-Response testing | An approach to examine the fitness of a physiologic system, where the system is stressed/stimulated in a controlled setting and its response over time is analyzed. The overall response (area under the response curve), timing and peak of response, and the rate of recovery to baseline are typically estimated. |
| Baseline Contributors to Resilience Capacity | Static health, functional, demographic, and biological measures that contribute to resilience capacity. |
| Clinical Outcomes | Clinically important consequences of stressors that include both favorable (e.g., survival) and unfavorable (e.g., hospitalization, death) outcomes. |
| Physical frailty | An age-related biologically driven phenotype that signals vulnerability to adverse health outcomes |
| System | A collection of interacting elements that forms an integrated whole. A physiological system is delineated and distinguished from its surroundings by motifs such as function (e.g., immune system), and structure (e.g., mitochondria). |
| Dynamical system (referred to as Dynamic system) | A system whose state changes over time. The adjective “dynamical” is traditionally used in the physics, engineering, and mathematics to denote systems that evolve over time rather than the popular adjective “dynamic.” |
| Stress response systems | A series of neurological, hormonal, and immune system communication mechanisms that are activated in times of stress and inactivated as the stressor resolves |
Research Strategy for SPRING Study:
Conceptual Framework and Design Overview
We have developed a new conceptual framework for the study of physical resilience. This builds on literature describing the interlinked decline in dynamic systems that accompanies physical frailty, conceptual frameworks developed by other investigators, and the NIA call to design studies that utilize stressors to facilitate the understanding of the biological and physiological basis of resilience. The conceptual framework for the SPRING study is detailed (Figure 1). At its heart is the oval labeled as “Physiologic Resilience Capacity” (see Table 1). We hypothesize that this is determined by the functionality of a specific dynamic system comprising the inter-connected physiological systems governing stress-response, energy production/metabolism, and musculoskeletal integrity. The state of these systems’ fitness shortly before experiencing a clinical stressor is a key element that our study aims to characterize; we hypothesize that this plays a major role in determining the stressor response and ultimately resilience capacity. We cannot observe this capacity directly, rather we must infer it through a collection of both static and stimulus-response (“dynamic”) measures detailed below. Given that resilience capacity is also influenced by other physical characteristics and health conditions as well as the social and environmental milieu in which one is embedded at the time of the stressor, an array of measures also are collected in these domains. Finally, the stressor magnitude and type are also measured because they partly determine the post-stressor response: Their effects also are hypothesized to be moderated by the resilience capacity.
Figure 1:
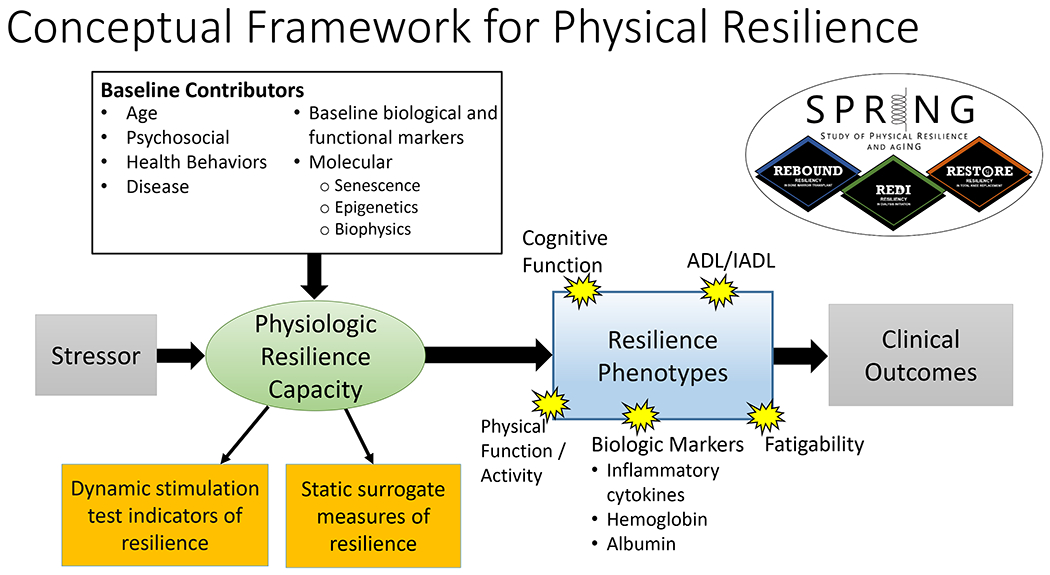
The conceptual organizing framework that guides measurement collection in the SPRING study. In this framework, a clinical stressor elicits a resilience response, measured by resilience phenotypes (blue box). Resilience phenotypes (Table 1) characterize both the initial response to the stressor, and the recovery or decline thereafter, in key markers of physical and cognitive function and health. These therefore are “trajectories” of key measures, beginning shortly before the stressor experience and continuing afterward up until a timeframe at which we may expect a resilient person to recover their pre-stressor status—operationalized in this study as one year. We hypothesize that physiologic resilience capacity is influenced by static baseline measures (white box) and can be elicited by measures assessing the functionality of specific dynamical systems that are measured before the stressor (left orange box). We also hypothesize that physical frailty and a measure of fatigue can function as surrogate measures of resilience capacity (right orange box). Both the resilience capacity and phenotypes potentially contribute to clinical outcomes (gray box).
The SPRING study was designed to implement this conceptual framework, operationalize measures of its key elements, and facilitate testing specific biological and physiological hypotheses related to physical resilience capacity to clinical stressors. Ultimately, if the conceptual framework is valid, the dynamic system function (or dysfunction) as ascertained by the dynamic stimulation tests, along with baseline contributors, will facilitate 1) the determination of the biological features that characterize resilience capacity, 2) the further development of measures that will allow early identification of older adults at risk from impending stressors, and 3) the development of interventions to improve resilience capacity to physical stressors in older adults.
Three Clinical Stressors Define the Substudies for SPRING:
We identified 3 clinical procedures that serve as major stressors for this study – allogenic bone and marrow transplantation (alloBMT) for hematologic malignancy, initiation of dialysis for chronic kidney failure; and knee replacement surgery for degenerative joint disease. These were chosen because they evoke a broad range of resilience phenotypes and clinical outcomes that can be captured in the year after the stressor. Three study protocols were developed, approved by Johns Hopkins Institutional Review Boards, and conducted according to the Declaration of Helsinki. For each study, eligibility criteria included the ability to walk without human assistance, English-speaking and ability to understand plus willingness to sign a written informed consent document. The general structure of each study protocol is: 1) identify and contact potential participants during their initial evaluation for clinical procedures; 2) gauge interest and eligibility by phone; 3) consent participants and complete in-person pre-stressor baseline visit(s) ideally approximately one month before the stressor experience; 4) complete follow-up visits aiming to assess short-term impact (1 or 3 months), potential initial recovery (6 months), and longer-term recovery (12 months) post-stressor. A 2-year pilot study informed the design of each of the main observational studies, including recruitment strategies, measurement capture, and analytical planning. Multiple baseline visits were needed to accommodate the full range of dynamic stimulus tests we administered, as described below:
REBOUND: REsiliency in older adults UNDergoing BOne marrow transplant is a study of patients ages 60 and older undergoing alloBMT for hematologic malignancy at the Johns Hopkins Hospital in Baltimore, Maryland or the City of Hope in Duarte, California. Exclusions include unwillingness or inability to return at 6 and 12 months after transplantation for repeated evaluation. The baseline assessments for the study are allocated across three visits, closely aligning with clinical appointments scheduled in preparation for the transplantation. They follow a period of chemotherapy conditioning to reduce malignancy burden before the alloBMT is initiated. Follow-up assessments occur at 1, 6 & 12 months (Figure 2, panel A).
Figure 2:
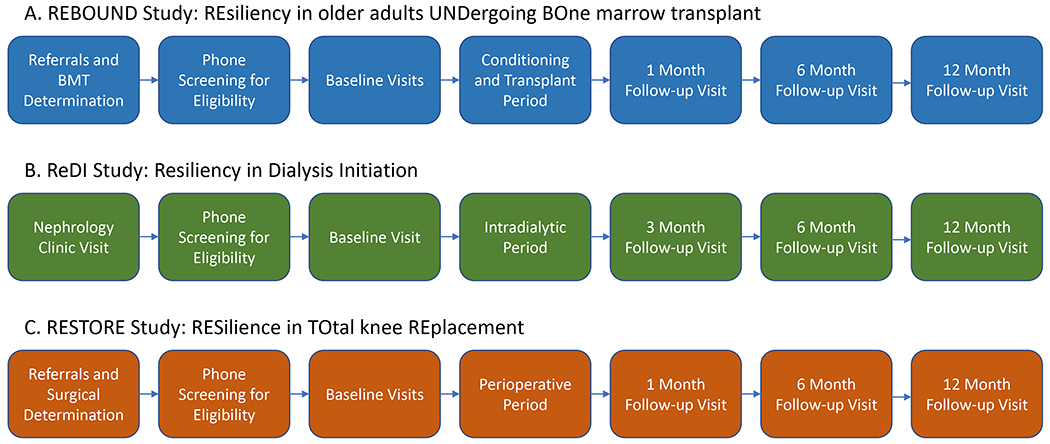
Panel A depicts the study sequence for REBOUND study recruitment and study visit flow, including 3 baseline visits to collect: 1) oral glucose tolerance test, 2) ACTH stim test, and 3) physical function testing. Panel B depicts the ReDI study recruitment and study visit flow, including 1 baseline visit consisting of ACTH stimulation test and physical function assessment. Panel C depicts RESTORE (total knee replacement) study protocol, with 2 baseline visits that include 1) oral glucose tolerance test, 2) ACTH stim test.
ReDI: Resiliency in Dialysis Initiation
Adults ages 55 and older with advanced chronic kidney disease of any etiology, estimated glomerular filtration rate (eGFR) of 20 ml/min/1.73 m2 or lower, and who are anticipated to initiate dialysis, are recruited. Recruitment takes place at the Johns Hopkins Bayview Medical Center and the Good Samaritan Hospital in Baltimore, Maryland. Because a subset of dynamic stimulus tests is contraindicated in this cohort (described below), the baseline assessment can be completed in a single visit. For those who initiate dialysis, follow-up visits occur at 3, 6, and 12 months following dialysis initiation as illustrated in Figure 2, panel B.
RESTORE: RESilience in TOtal knee Replacement
Adults 60 and older who are scheduled to undergo elective total knee replacement surgery for degenerative joint disease at the Johns Hopkins Bayview Medical Center or at the University of Maryland Medical System in Baltimore, Maryland are recruited into this study. Exclusion factors include severe visual or hearing impairments, intubation pre-surgery, severe medical comorbidities including acute congestive heart failure or cardiac ischemia, and infection requiring IV antibiotics. There are two baseline visits and follow-ups at 1, 6 & 12 months as illustrated in Figure 2, panel C.
Study Protocols and Measures
To operationalize the SPRING conceptual framework, measures to characterize each element in Figure 1 were developed. Table 2 summarizes these, including magnitude and type of clinical stressor, baseline medical, psychosocial, and biological baseline contributors; dynamic stimulation measures that are hypothesized to reflect the biological and physiological basis of resilience capacity; surrogate (static) measures that may allow for estimation of resilience capacity based on simple clinical measures; resilience phenotypes that evolve after the clinical stressor; and clinical outcomes measures. Preceding and during the 2-year pilot phase, measures were identified through structured elicitation in our investigator team, which has expertise in each clinical area under investigation as well as functional, cognitive, psychological, biological, dynamic physiological, and molecular measurement. Dynamic measures were highly prioritized, and customized assessments and visit timings were fit to existing clinic schedules of patients set to undergo stressor procedures.
Table 2:
Common and Study-specific Measurements in SPRING
| Resilience Measurement Categories | Common Measures across studies | Bone Marrow Transplant (REBOUND) study only | Dialysis Initiation (ReDI) study only | Knee Replacement (RESTORE) study only |
|---|---|---|---|---|
| Baseline Contributors | Age, demographics, SES, psychosocial measures, health behaviors, disease meas-ures, medicat-ions, biological measures: immune cell phenotypes, 24-hour catechol-amines, metabol-omic markers | DNA methylation / methylation clock, malignancy type, cytogenetic risk group | Pre-dialysis course, cause of end-stage renal disease, type of dialysis access | Senescent cell markers |
| Stressor characteristics | n/a | Dose and type of chemotherapy, total body dose of radiation, conditioning regimen | Interdialytic weight gain, volume removed, dialysis treatment time, dialysate temperature, electrolyte composition, treatment adherence | Anesthesia type & dose, time in surgery, blood loss, fluids administered, transfusion and/or pressor use, use of cement, use of tourniquet, need for intubation, intraoperative temperature, postoperative opioid use |
| Static Surrogate Measures * | Phenotypic frailty, SF-36, nutrition (albumin, total cholesterol, vitamin D), Karnofsky performance measure 14 | ECOG15 performance measure, HCT-specific comorbidity index | KDQOL, NYHA self-reported symptoms | Knee Injury and Osteoarthritis Outcome Score (KOOS) |
| Dynamic Stimulation Indicator Measures | ACTH stimulat-ion, 24-hour salivary cortisol profile, dynamic ex-vivo response of immune cells, Orthostatic Blood Pressure, Holter monitoring*; Observed Fatigability treadmill test | Oral glucose tolerance test | n/a | Oral glucose tolerance test |
| Resilience Phenotype Measures * | Physical function (SPPB, mobility function), accelerometry, cognitive function (MOCA, DSST, NIH Cognition Toolbox), ADLs/IADLs, Pittsburgh fatigability scale, sleep, physiologic markers including inflammatory markers, hormones, clotting factors | |||
| Clinical Outcomes | Hospitalization/re-hospitalization, falls, morbidity, mortality | Graft versus host disease, relapse, infection, rehab or skilled nursing facility, new malignancies, post-transplant therapies | Kidney transplantation, transfer to home dialysis modality, uremic symptoms (pruritus, fatigue, anorexia, pain, restless legs), health-related quality of life | NSQIP post-op 30-day complic-ations, LOS until discharge, delirium, dis-charge home, rehab, ambulate unaided 60 days later, independ-ent living; CCI change, patient satisfaction with surgery, post-operative pain |
measured pre and post clinical stressor
Abbreviations: SES=Socioeconomic status; ACTH= Adrenocorticotropic hormone; SPPB=short physical performance battery; MOCA=Montreal cognitive assessment; DSST=Digital symbol substitution test; NIH=National Institute of Health; ADL=Activities of daily living; IADL=instrumental activities of daily living; NSQIP= National Surgical Quality Improvement Program; LOS=Length of stay; KDQOL=Kidney Disease Quality of Life; NYHA=New York Heart Association; HCT=Hematopoietic cell transplantation; DNA=Deoxyribonucleic acid; ECOG=Eastern Cooperative Oncology Group; CCI=Charlson Comorbidity Index. The NIH Cognitive Toolbox tests included in SPRING are Flanker Inhibitory Control and Attention Test; Auditory Verbal Learning Test; and Pattern Comparison Test.
Key Dynamic Stimulation Measures:
Key to this study are measures that probe the functionality of stress response systems hypothesized to play an important role in maintaining physical resilience in the face of major clinical/ physical stressors. These systems include energy metabolism (EM), hypothalamic pituitary adrenal (HPA) axis, autonomic nervous system (ANS), and innate immune system activation (IIS). Each of the tests utilized to probe these stress response systems are described below.
Oral Glucose Tolerance Test: Older adults with insulin resistance and abnormal energy regulation are generally at high risk for adverse outcomes 16,17. Optimal response to glucose challenge may signal resilience in the face of a stressor; dysregulation may signal lower resilience capacity. Participants fast overnight and are given the oral 75-gram glucose load after arrival at the study visit in the form of a sweet beverage. Blood draws occur at 0 (prior to the administration of the beverage), and then again at 30, 60, and 120 minutes after administration, enabling assessment of hormonal regulation (insulin, ghrelin, adiponectin, resistin, leptin) of energy metabolism in response to a caloric load. Because of known energy dysregulation, those with pre-existing diabetes mellitus or with known chronic kidney disease, including all ReDI participants, are excluded from this test.
Adrenocorticotropic hormone (ACTH) Stimulation test: The HPA axis is a critical component of stress response and undergoes changes in aging and in specific medical conditions 18,19. Indeed, prior studies have demonstrated an abnormal cortisol response to ACTH in aging, with a delayed return of cortisol to baseline, suggesting improperly tuned feedback responses, which could lead to prolonged exposure to elevated cortisol levels 20–23. The test assesses the reactivity of HPA axis to a physiological stimulus. After an intravenous port is placed and a non-fasting baseline blood serum sample is taken, 1 mcg (low dose) Cosyntropin (synthetic ACTH) is injected intravenously over 1-2 minutes. Serum samples are then collected from the ports at 30- and 60-minute times. Those taking anti-inflammatory drugs related to cortisol or with illnesses caused by adrenal or other cortisol abnormalities are excluded from this test.
Physical Fatigability: Fatigability refers to fatigue in relation to a standardized task. To measure dynamic fatigability, participants are asked to walk on a treadmill for five minutes (1.5 mph/0.67 m/s/0% grade), supervised by the research coordinator. At completion, participants are asked to rate their perceived exertion using the Borg Rating of Perceived Exertion Scale (RPE) 24.
Orthostatic Blood Pressure: Orthostatic blood pressure is a simple, feasible dynamic test of changes in blood pressure and heart rate from sitting to standing position. After 2 minutes of sitting rest, blood pressure (BP) and heart rate (HR) are measured in the non-dominant arm with automated cuff. The participant is then instructed to slowly stand. Once the participant is standing comfortably, BP and HR are measured as before, preferably between 15 and 45 seconds after rising from the chair. For safety purposes, we ensure that the participant is asymptomatic following orthostatic measurements before proceeding to the next test or measurement. This is a surrogate measure for the autonomic nervous system (ANS).
Holter monitoring for heart rate variability: Holter monitoring measures heart rate and rhythm and rate variability during activities of daily life. As some individuals age, variability in response to stressors lessens, perhaps in part to increased sympathetic nervous system activity 25–27. We hypothesize that those with lower levels of heart rate variability have less resilience capacity to withstand stressors. The Holter monitor is a 2x3 inch thin rectangular recording device attached to an adhesive patch that is placed on the central chest during a baseline visit. It is worn at home for 24 hours and then mailed back by the participant in a padded, pre-labeled, pre-stamped envelope. Data are then downloaded from the device for interpretation. Heart rate variability is a widely accepted measure of ANS stability 28,29.
Diurnal Salivary Cortisol Profile: This test aims to elucidate how the diurnal activity of the HPA axis may relate to resiliencies in older adults encountering a physical stressor event. Subjects are asked to chew on a cotton tipped swab at: 1) wake up time before breakfast, 2) 30 minutes after wake-up; 3) 11 AM, 4) 4 PM and 5) just before bedtime. Each swab, once soaked with saliva, is placed in a plastic, pre-labeled sleeve. The swabs are mailed back to the laboratory office using a pre-addressed, stamped envelope. Cortisol is measured from the saliva from these 5 time points, which provides a window into diurnal cortisol secretion. This is a measure of HPA axis activity.
Dynamic ex-vivo response of immune cells to stimuli: Prior studies have identified exaggerated responses of immune system cells to lipopolysaccharide (LPS) in frail compared to nonfrail older adults. 28,30 We therefore hypothesized that LPS stimulation leads to exaggerated cytokine responses in non-resilient older adults. Peripheral blood mononuclear cells (PBMCs) are separated from whole blood and stored at −80C. Cells are later thawed, reconstituted and subjected to low dose LPS stimulation. Supernatant is collected from the cells at 4 times over 48 hours, and cytokines are measured and compared between subjects. This is a surrogate marker of innate immune system activity, and the system’s potential dysregulation.
Statistical Modeling Plan:
We hypothesize that: (A) dynamic measures are key indicators of resilience capacity, (B) resilience capacity is a primary driver of response to stressors, and (C) the dynamic functioning of key physiologic systems, in addition to pre-stressor biologic, health, and psychosocial characteristics, determines resilience capacity. We have identified four specific statistical modeling goals: Approaches to address each are outlined in this section. Please see Supplementary File S1 for more details.
(1). To measure physiologic resilience capacity (PRC) using the baseline data from static and dynamic assessments.
Our conceptual framework hypothesizes physical resilience capacity (oval in Figure 1) as a physiological system property that is not directly quantifiable, but can be implicitly described through a combination of dynamic stimulus test metrics and static surrogate measures. We will implement three approaches to combine the data into a categorization or score that can be used to evaluate PRC association with resilience phenotypes and clinical outcomes. First, we will derive a: “PRC-deficit” by developing a simple sum (index) of deficits. Second, we will generate a “PRC-scale” which implements a simplified version of our theoretical framework, using latent variable models. Third, a machine learning (ML)-based “PRC-ML” will be generated.
(2). To characterize resilience phenotypes of stressor-response:
Table 2 lists multiple assessments identified as promising resilience phenotype candidates. We will develop phenotypes of stressor response by characterizing trajectories of phenotypic measures before-to-after the stressor, allowing heterogeneity between individuals and for curvilinear shape (notably, an initial decline followed by a rebound). Mixed effects models and functional principal components analysis adapted for sparsely repeated data (fPCA) 36,37 are two methods we will employ to achieve this.
(3). To characterize the association and predictive accuracy/precision of physical resilience capacity measures for resilience phenotypes and outcomes following the stressor.
We will assess the degree to which PRC-deficit and PRC-scale from (1) independently predict the phenotypic measures developed in (2), after adjusting for determinants already in common clinical use, including age, sex, multimorbidity and BMI. We will also assess the degree to which PRC may moderate the stress-response by studying the interaction between resilience capacity and stressor magnitude, and evaluate which of the PRC estimates most accurately predicts resilience phenotypes and longer-term outcomes.
4). To explore age-related biological mechanisms potentially contributing to physiologic resilience capacity.
SPRING will collect measurements on important biological domains known to change with aging, including molecular (senescence cell surface markers, metabolomic measures, epigenetic markers), physiological (baseline inflammatory cytokines, urine catecholamines, ghrelin and other hormones) and clinical laboratory measurements (complete blood count, metabolic panel). We will assess the associations of these biological variables with stimulus-response data. Given the early stage of discovery, analyses will appropriately account for multiplicity, for example, using multiple comparisons corrections and penalized regression approaches. These analyses will help generate – hypothesis-driven analyses in future studies.
Pilot Phase:
During phase 1 pilots, the SPRING study was implemented to assess feasibility of recruitment and retention of human subjects, static and dynamic measurement collection, molecular assay development, early efforts to identify resilience phenotypes from our own data and from data collected in other studies, and outcomes through this pilot and through other studies.
Pilot cohort characteristics
During the pilot phase, a total of 77 participants were enrolled across the three study populations. Out of 23 enrollees who completed baseline assessments in the RESTORE study, 21 (91%) had total knee replacement surgery and 19 (83%) completed a 12-month post-surgery study visit. Twenty-seven (84%) of the 32 REBOUND study enrollees underwent alloBMT; 16 (50%) participants completed 12-month follow-up. Ten participants (31%) died from non-study related BMT complications before study completion. In the ReDI study, 7 (32%) of the 22 enrollees initiated hemodialysis following baseline assessment. This low transition rate was informative for our phase 2 study design.
Participant characteristics varied across the clinical populations as displayed in Table 3.
Table 3:
Characteristic comparisons between studies in the pilot phase.
| Bone Marrow Transplant (REBOUND) Pilot Study (n=32) | Dialysis Initiation (ReDI) Pilot Study (n=22) | Knee Replacement (RESTORE) Pilot Study (n=23) | |
|---|---|---|---|
| Gender: % (n) female | 34.4% (11) | 22.7% (5) | 69.7% (16) |
| Race: % (n) white | 93.8% (30) | 36.4% (8) | 65.2% (15) |
| Age: mean (sd) | 68.1 (4.0) | 69.9 (8.7) | 72.2 (4.8) |
| (min-max) | (60-76) | (55-86) | (65-81) |
| Number of diseases * : % (n) | |||
| 0 | 0% (0) | 0% (0) | 0% (0) |
| 1 | 27.6% (8) | 0% (0) | 9.1% (2) |
| 2 | 20.7% (6) | 13.6% (3) | 13.6% (3) |
| >=3 | 51.7% (15) | 86.4% (19) | 77.3% (17) |
| Baseline Frailty ** : % (n) | |||
| Non-frail | 46.9% (15) | 27.3% (6) | 21.7% (5) |
| Prefrail | 40.6% (13) | 63.6% (14) | 60.9% (14) |
| Frail | 3.1% (1) | 9.1% (2) | 13.0% (3) |
| Gait speed (m/s): | |||
| Mean (sd) | 1.12 (0.21) | 0.84 (0.15) | 0.77 (0.19) |
| Range | 0.77-1.66 | 0.55-1.13 | 0.33-1.10 |
Out of: MI, CHF, Angina, COPD, Asthma, Liver disease, Kidney disease, Stroke, TIA, Peripheral Neuropathy, Diabetes, Hypertension, Cancer, Arthritis, Spinal Stenosis, Osteoporosis, RA, Phlebitis, Intermittent Claudication, Parkinson’s (20 diseases). Max=6 except REDI=12.
RESTORE: 1 missing; REBOUND: 3 missing.
Legends: Figure legends should be presented in a list at the end of the manuscript file and should NOT appear in the figure file itself. Legends should explain the figure sufficiently so that a reader is able to interpret the figure without referencing the text. All abbreviations must be spelled out on the figure legend. Indicate normal range for instruments or scales.
Pilot Development Efforts:
The phase 1 pilot studies were not developed to detect statistical differences between groups. Rather the pilot period was utilized to inform data collection in the main study and to help to prioritize measurements for the ongoing second phase of our study. In that regard, we utilized population studies to analyze data in ways that helps to inform the development of resilience phenotypes, stressor characterization, and clinical outcomes for the clinical stressors. For example, a series of measures were identified in the Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR) cohort population that helped to inform the collection of data for the RESTORE post-stressor resilience phenotypes and outcomes 42. In the CHOICE population, REDI investigators developed a number of physical resilience phenotype trajectories that will be utilized in the main study 43 In the alloBMT population, outcomes of older patients depend on energy metabolism and maintenance of body weight 44.
In addition to these efforts, refinement of resilience-related biological measures that will be incorporated into phase 2 of the study continued. These included work on cellular senescence, circulating cell free DNA fragment measurement, and metabolomic measurements relevant to energy regulation 45,46.
Implementation of Phase 2 of SPRING and the Impact of COVID 19 pandemic:
The development phase of SPRING provided invaluable insights into the implementation and feasibility of conducting novel pilot research in distinct clinical populations in our main study. Valuable lessons learned in the pilot regarding recruitment and enrollment strategies, study population differences, infrastructure development and research navigation, distributions and overlap of measures, and the need for partnerships with additional sites for recruitment all greatly informed the design and measures of the ongoing phase 2 study. Some of these changes meant to address challenges learned in the pilot phase included: 1) lowering of age eligibility in REDI and REBOUND; 2) enacting MyChart recruitment for REDI and removal of the 8-week, baseline-to-dialysis initiation window; 3) offering car service to participants, and 4) protocol adjustments meant to reduce assessment time and participant burden. The COVID pandemic mandated the closure of clinical and research programs at Johns Hopkins University from mid-March 2020 until November of 2020.
Conclusions:
Millions of older adults will face critical illnesses, injuries, surgical and medical procedures in the coming years. A subset of those individuals will go on to have serious complications, and may never recover to a ‘normal’ baseline. We and others have sought over the past many years to identify vulnerable older adults at high risk of adverse health outcomes in order to design new treatments or modify care plans that keep older patients safe. As part of this effort, we have worked to identify the highly complex and heterogeneous biological underpinnings of vulnerability in older adults. We have leveraged this knowledge to develop a study that is designed to identify specific physiological and biological components of resilience capacity in the face of large clinical stressors in older adults. This study uniquely incorporates the measurement of dynamic systems function before the actual clinical stressor in order to better assess the system’s functionality and in order to determine if normal functionality will predict resilience phenotypes and ultimately clinical outcomes.
Through the measurement of these dynamic systems, and more static measures before a clinical stressor, and the clinical outcomes after a stressor, we seek to identify crucial physiological signals that represent resilience capacity. If such signals are identified, we will seek to translate findings into other clinical stressors that impact older adults, and eventually develop more precise clinical implementation strategies that better prepare older adults for clinical stressors. The resilience capacity related findings of the SPRING study may change the paradigm of care for many older adults facing important decisions regarding procedures or treatments with moderate or higher risk. For example, if individuals are found to have specific dysregulated dynamic stress responses known to impact resilience outcomes before a given procedure, they and their care providers may work to improve the function of individual stress response systems before the procedure, or perhaps make different decisions about moving ahead at all with the procedure. Beyond this, the SPRING study will further the understanding of altered dynamic stress response systems and how they impact health outcomes in older adults. In summary, the SPRING study holds great potential to improve the understanding of how these dynamic systems act in concert or separately to influence health and well-being in older adults undergoing clinical procedures, and to inform the development of future practice patterns that will help to ensure improvements in resilience capacity and ultimately resilience outcomes.
Supplementary Material
Supplementary File S1:
Modeling Plans and Analytic Aims
Key Points
The SPRING study is designed to identify specific physiological and biological components of resilience capacity in the face of large clinical stressors among older adults.
This study uniquely incorporates the measurement of dynamic systems function before the actual clinical stressor to better assess the system’s functionality and to determine if normal functionality will predict resilience phenotypes and ultimately clinical outcomes.
Why Does this Paper Matter?
The SPRING study holds great potential to improve the understanding of how these dynamic systems act in concert or separately to influence health and well-being in older adults undergoing clinical procedures, and potential to facilitate the development of strategies that improve physical resilience in older adults before procedures.
ACKNOWLEDGMENTS
This work was supported by This work was funded by the National Institute on Aging of the National Institutes of Health under award numbers UH2AG056933, UH3AG056933, and P30AG021334.
Funding Sources:
This work was supported by NIH grants UH2AG056933, UH3AG056933, and P30AG021334.
Sponsor’s Role:
The sponsor had no role in the design, methods, subject recruitment, data collections, analysis or preparation of the paper.
Conflict of Interest:
The authors report no relevant conflicts of interest, excepting: AA is a consultant for AbbVie and Magenta Therapeutics; DC receives research grant support from Somatus and Baxter; JO is a consultant for DePuy and Zimmer; JS is a consultant for Edwards Lifesciences.
References:
- 1.Tredgold MT. XXXVII. On the transverse strength and resilience of timber. Philos Mag. 1818;51(239):214–216. doi: 10.1080/14786441808637536 [DOI] [Google Scholar]
- 2.Hadley EC, Kuchel GA, Newman AB. Report: NIA Workshop on Measures of Physiologic Resiliencies in Human Aging. J Gerontol A Biol Sci Med Sci. 2017;72(7):980–990. doi: 10.1093/gerona/glx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong AD, Bergeman CS, Boker SM. Resilience comes of age: defining features in later adulthood. J Pers. 2009;77(6):1777–1804. doi: 10.1111/j.1467-6494.2009.00600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical Resilience in Older Adults: Systematic Review and Development of an Emerging Construct. J Gerontol A Biol Sci Med Sci. 2016;71(4):489–495. doi: 10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston MC, Porteous T, Crilly MA, et al. Physical disease and resilient outcomes: a systematic review of resilience definitions and study methods. Psychosomatics. 2015;56(2):168–180. doi: 10.1016/j.psym.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gijzel SMW, Whitson HE, van de Leemput IA, et al. Resilience in Clinical Care: Getting a Grip on the Recovery Potential of Older Adults. J Am Geriatr Soc. 2019;67(12):2650–2657. doi: 10.1111/jgs.16149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resnick B, Galik E, Dorsey S, Scheve A, Gutkin S. Reliability and validity testing of the physical resilience measure. Gerontologist. 2011;51(5):643–652. doi: 10.1093/geront/gnr016 [DOI] [PubMed] [Google Scholar]
- 8.Franceschi C, Garagnani P, Morsiani C, et al. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med. 2018;5:61. doi: 10.3389/fmed.2018.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varadhan R, Walston JD, Bandeen-Roche K. Can a Link Be Found Between Physical Resilience and Frailty in Older Adults by Studying Dynamical Systems? J Am Geriatr Soc. 2018;66(8):1455–1458. doi: 10.1111/jgs.15409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8(1):1–17. [PubMed] [Google Scholar]
- 11.Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129(11):666–670. doi: 10.1016/j.mad.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe JW, Kahn RL. Successful Aging1. Gerontologist. 1997;37(4):433–440. doi: 10.1093/geront/37.4.433 [DOI] [PubMed] [Google Scholar]
- 13.Mancini AD, Bonanno GA. Predictors and parameters of resilience to loss: toward an individual differences model. J Pers. 2009;77(6):1805–1832. doi: 10.1111/j.1467-6494.2009.00601.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.JH KDB. NThe Clinical Evaluation of Chemotherapeutic Agents in Cancero Title. In: Valuation of Chemotherapeutic Agents. Columbia University Press; 1919:196. [Google Scholar]
- 15.Zubrod CG, Schneiderman M, Frei E, et al. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11(1):7–33. doi: 10.1016/0021-9681(60)90137-5 [DOI] [Google Scholar]
- 16.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67(12):1300–1306. doi: 10.1093/gerona/glr141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging. 2012;16(8):679–686. doi: 10.1007/s12603-012-0066-4 [DOI] [PubMed] [Google Scholar]
- 18.Gupta D, Morley JE. Hypothalamic-Pituitary-Adrenal (HPA) Axis and Aging. In: Comprehensive Physiology. ; 2014:1495–1510. doi: 10.1002/cphy.c130049 [DOI] [PubMed] [Google Scholar]
- 19.Goncharova ND. The HPA Axis under Stress and Aging: Individual Vulnerability is Associated with Behavioral Patterns and Exposure Time. BioEssays. 2020;42(9):2000007. doi: 10.1002/bies.202000007 [DOI] [PubMed] [Google Scholar]
- 20.Yiallouris A, Tsioutis C, Agapidaki E, et al. Adrenal Aging and Its Implications on Stress Responsiveness in Humans. Front Endocrinol . 2019;10. 10.3389/fendo.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le NP, Varadhan R, Fried LP, Cappola AR. Cortisol and Dehydroepiandrosterone Response to Adrenocorticotropic Hormone and Frailty in Older Women. J Gerontol A Biol Sci Med Sci. 2021;76(5):901–905. doi: 10.1093/gerona/glaa134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30(1):80–91. doi: 10.1016/j.psyneuen.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 23.Giordano R, Di Vito L, Lanfranco F, et al. Elderly subjects show severe impairment of dehydroepiandrosterone sulphate and reduced sensitivity of cortisol and aldosterone response to the stimulatory effect of ACTH(1–24). Clin Endocrinol (Oxf). 2001;55(2):259–265. doi: 10.1046/j.1365-2265.2001.01317.x [DOI] [PubMed] [Google Scholar]
- 24.Borg G Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16 Suppl 1:55–58. doi: 10.5271/sjweh.1815 [DOI] [PubMed] [Google Scholar]
- 25.Chaves PHM, Varadhan R, Lipsitz LA, et al. Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J Am Geriatr Soc. 2008;56(9):1698–1703. doi: 10.1111/j.1532-5415.2008.01858.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varadhan R, Chaves PHM, Lipsitz LA, et al. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J Gerontol A Biol Sci Med Sci. 2009;64(6):682–687. doi: 10.1093/gerona/glp013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji H, Venditti FJJ, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878 [DOI] [PubMed] [Google Scholar]
- 28.Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16(3):249–252. doi: 10.1007/BF03327392 [DOI] [PubMed] [Google Scholar]
- 29.Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63(2):190–195. doi: 10.1093/gerona/63.2.190 [DOI] [PubMed] [Google Scholar]
- 30.Qu T, Walston JD, Yang H, et al. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130(3):161–166. doi: 10.1016/j.mad.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009;12(6):403–410. doi: 10.1089/rej.2009.0883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinkley TE, Hsu FC, Beavers KM, et al. Total and abdominal adiposity are associated with inflammation in older adults using a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2012;67(10):1099–1106. doi: 10.1093/gerona/gls077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartholomew DJ, Knott M MI. Latent Variable Models and Factor Analysis: A Unified Approach, 3rd Edition. Wiley; 2011. [Google Scholar]
- 34.Bollen KA. Structural Equations with Latent Variables. Vol 210. John Wiley & Sons; 1989. [Google Scholar]
- 35.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 36.Yao F, Müller HG, Wang JL. Functional data analysis for sparse longitudinal data. J Am Stat Assoc. 2005;100(470):577–590. [Google Scholar]
- 37.Rice JA, Silverman BW. Estimating the Mean and Covariance Structure Nonparametrically When the Data are Curves. J R Stat Soc Ser B. 1991;53(1):233–243. http://www.jstor.org/stable/2345738 [Google Scholar]
- 38.Grimm KJ. Multivariate longitudinal methods for studying developmental relationships between depression and academic achievement. Int J Behav Dev. 2007;31(4):328–339. [Google Scholar]
- 39.Chiou JM, Chen YT, Yang YF. Multivariate functional principal component analysis: A normalization approach. Stat Sin. Published online 2014:1571–1596. [Google Scholar]
- 40.Leoutsakos JMS, Forrester SN, Corcoran CD, et al. Latent classes of course in Alzheimer’s disease and predictors: the Cache County Dementia Progression Study. Int J Geriatr Psychiatry. 2015;30(8):824–832. doi: 10.1002/gps.4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai D, Xu H, Koller D, Foroud T, Gao S. A MULTIVARIATE FINITE MIXTURE LATENT TRAJECTORY MODEL WITH APPLICATION TO DEMENTIA STUDIES. J Appl Stat. 2016;43(14):2503–2523. doi: 10.1080/02664763.2016.1141181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laskow T, Zhu J, Buta B, et al. Risk Factors for Non-Resilient Outcomes in Older Adults after Total Knee Replacement in the FORCE-TJR Cohort. J Gerontol A Biol Sci Med Sci. Published online September 2021. doi: 10.1093/gerona/glab257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hladek MD, Zhu J, Crews DC, et al. Physical Resilience Phenotype Trajectories in Incident Hemodialysis: Characterization and Mortality Risk Assessment. Kidney Int reports. 2022;7(9):2006–2015. doi: 10.1016/j.ekir.2022.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imus PH, Tsai HL, Luznik L, et al. Haploidentical transplantation using posttransplant cyclophosphamide as GVHD prophylaxis in patients over age 70. Blood Adv. 2019;3(17):2608–2616. doi: 10.1182/bloodadvances.2019000155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westbrook R, Abadir PM. Metabolomics Captures the Biological Signatures of Aging and Healthspan and Identifies Pathway Targets for Intervention. J Gerontol A Biol Sci Med Sci. Published online August 2022. doi: 10.1093/gerona/glac176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nidadavolu LS, Feger D, Wu Y, et al. Circulating Cell-Free Genomic DNA Is Associated with an Increased Risk of Dementia and with Change in Cognitive and Physical Function. J Alzheimers Dis. Published online August 2022. doi: 10.3233/JAD-220301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File S1:
Modeling Plans and Analytic Aims


