Abstract
Induction of mannanase, xylanase, and cellulase (endoglucanase) synthesis in the plant-pathogenic basidiomycete Sclerotium rolfsii was studied by incubating noninduced, resting mycelia with a number of mono-, oligo-, and polysaccharides. The simultaneous formation of these three endoglycanases could be provoked by several polysaccharides structurally resembling the carbohydrate constituents of lignocellulose (e.g., mannan and cellulose), by various disaccharide catabolites of these lignocellulose constituents (e.g., cellobiose, mannobiose, and xylobiose), or by structurally related disaccharides (e.g., lactose, sophorose, and galactosyl-β-1,4-mannose), as well as by l-sorbose. Synthesis of mannanase, xylanase, and endoglucanase always occurred concomitantly and could not be separated by selecting an appropriate inducer. Various structurally different inducing carbohydrates promoted the excretion of the same multiple isoforms of endoglycanases, as judged from the similar banding patterns obtained in zymogram analyses of enzyme preparations obtained in response to these different inducers and resolved by analytical isoelectric focusing. Whereas enhanced xylanase and endoglucanase formation is strictly dependent on the presence of suitable inducers, increased levels of mannanase are excreted by S. rolfsii even under noninducing, derepressed conditions, as shown in growth experiments with glucose as the substrate. Significant mannanase formation commenced only when glucose was exhausted from the medium. Under these conditions, only very low, presumably constitutive levels of xylanase and endoglucanase were formed. Although the induction of the three endoglycanases is very closely related in S. rolfsii, it was concluded that there is no common, coordinated regulatory mechanism that controls the synthesis of mannanase, xylanase, and endoglucanase.
Lignocellulose, the most abundant renewable resource in nature, is composed of the three major structural polymers: cellulose (a homopolymer built of d-glucosyl residues), hemicellulose (a group of heteropolymers that includes xylans and mannans), and lignin (a complex polyphenolic polymer). The main carbohydrate constituents of lignocellulosic material, i.e., cellulose, mannan, and xylan, consist of main chains of β-1,4-linked pyranosyl units which can be variously substituted. These β-1,4-glycosidic bonds within the polysaccharide backbones are hydrolyzed by cellulases, mannanases, and xylanases, respectively, the synthesis of which is, in general, subject to induction and/or catabolic repression. Since the polysaccharides, by themselves, are far too large to pass through the cell membrane and trigger the response in the microbial cell leading to the enhanced synthesis of endoglycanases, it is generally accepted that low-molecular-mass soluble catabolites, which are released from the polymeric compounds by the action of low, constitutive amounts of these hydrolases and which can easily enter the cell, signal the presence of an extracellular substrate and provide the stimulus for the accelerated synthesis of the respective enzymes (5). In a number of fungi, these various endoglycanases can be quite specifically induced. During the growth of Trichoderma reesei and T. harzianum on xylan-based media, mainly xylanase activities with low levels of endoglucanase are formed, while growth on cellulose or on heterogeneous native substrates containing both xylan and cellulose results in the concomitant production of both endoglucanase and xylanase activities. This unspecific effect of cellulose could be explained by xylan impurities found in commercially available cellulose preparations (26, 38). Accordingly, the low-molecular-mass inducer xylobiose stimulated only the synthesis of xylanase in resting cells of T. reesei, whereas sophorose provoked the formation of both cellulase and xylanase activities. Analysis of the sophorose-induced enzyme system revealed, however, that most of the xylanase activity could be attributed to a nonspecific endoglucanase while specific xylanases were induced only in relatively small amounts (26). In a similar way, Penicillium kloeckeri specifically produced xylanases during growth on xylan, while mannanases were predominantly formed when mannans were used as the growth substrate (15). Obviously, this scheme cannot be generalized and there appears to be no generally applicable regulatory mechanism. Higher levels of xylanase activity are formed by a number of fungi during growth on cellulose than during growth on xylan (36, 41), while for Schizophyllum commune, production of xylanase is strictly linked to the presence of cellulose (7, 24). Trichoderma and several other organisms produce similar or even higher mannanase activities when grown on cellulose than when they are grown on mannan-based media (2, 25, 35).
While the regulation of the synthesis of both cellulase and xylanase in fungi has been well studied and a number of low-molecular-weight signal molecules promoting endoglycanase formation have been identified and described (5, 10, 13, 30), relatively little is known about the regulation of mannanase biosynthesis. Mannanases only recently attracted increased scientific and commercial attention due to potential applications in the pulp and paper industry for removal of hemicellulose from dissolving pulps (20) or for enhancement of the bleachability of pulp and, thus, reduction of the use of environmentally harmful bleaching chemicals (14, 19). A similar application of xylanases for pulp prebleaching is an already well-established technology and has greatly stimulated research on hemicellulases in the past decade (44). Further applications of mannanases which have been studied for a longer time can be found in food technology, where mannanases are used for the hydrolysis of high-molecular-weight mannans, e.g., in coffee pulp, as well as in the feed industry for increasing the digestibility of animal feed (45, 46).
It was the objective of this study to investigate the regulation of the synthesis of endoglycanases which are part of the lignocellulose-degrading enzyme system of the plant-pathogenic fungus Sclerotium rolfsii (or Athelia rolfsii, which is used for the teleomorph). This organism is known as an excellent producer of cellulolytic enzymes (32, 33), as well as of hemicellulolytic enzymes (21, 22). It was of special interest to examine whether by selecting an appropriate inducing substrate high levels of hemicellulases with only low levels of concurrently produced cellulase could be attained, since hemicellulase preparations free of cellulase activity have gained significant interest in recent years due to their application in the pulp and paper industry (4, 44).
MATERIALS AND METHODS
Inducers and chemicals.
Ivory nut mannan (a β-mannan from Phytelephas macrocarpa), mannobiose, galactobiose, xylobiose, azo-carob galactomannan (covalently dyed with Remazol brilliant blue [RBB]), azo-carboxymethyl cellulose, and azo-xylan (birch wood) were purchased from Megazyme (Sydney, Australia). Lactose, l-sorbose, d-xylose, and carboxymethyl cellulose were from Fluka (Buchs, Switzerland); sophorose was from Serva (Heidelberg, Germany); and xylan from beechwood was from Lenzing AG (Lenzing, Austria). Konjac mannan, a glucomannan from Amorphophallus konjac with a mannose-to-glucose ratio of 1.8:1, was obtained from Arkopharma (Carros, France), and xylan from birchwood was from Roth (Karlsruhe, Germany). Xylooligosaccharides containing more than 95% xylobiose (xylooligo-95) were a kind gift from Suntory (Tokyo, Japan). Bacterial cellulose was produced by Acetobacter xylinum as previously described (16). All other chemicals, including locust bean gum (a galactomannan from Ceratonia siliqua with a mannose-to-galactose ratio of 4:1) and guar gum (a galactomannan from Cyamopsis tetragonoloba with a mannose-to-galactose ratio of 2:1), were obtained from Sigma (St. Louis, Mo.).
Microbial strain, mycelium preparation, and induction experiments.
S. (A.) rolfsii CBS 191.62 (Centraalbureau voor Schimmelcultures, Baarn, The Netherlands) was used throughout this study. Stock cultures were maintained on glucose-maltose Sabouraud agar and routinely subcultured every 4 weeks. Inoculated plates were incubated at 30°C for 4 to 6 days and then stored at 4°C.
Mycelial biomass was produced in medium containing the following (in grams per liter): glycerol, 20; peptone from meat, 20; NH4NO3, 2.5; KH2PO4, 1.2; MgSO4 · 7H2O, 1.5; KCl, 0.6; and a trace metal solution at 0.3 ml/liter (22). The pH was adjusted to 5.0 prior to sterilization; tap water was used for medium preparation. Each 1,000-ml Erlenmeyer flask containing 300 ml of medium was inoculated by adding mycelial mat (two pieces of approximately 1 cm2) from the actively growing part of stock cultures. To obtain a homogeneous preparation of the mycelia, media were homogenized with a laboratory homogenizer (Polytron; Kinematica, Kriens, Switzerland) at 9,500 rpm for 15 s after inoculation. The inoculated flasks were continuously shaken on an orbital shaker at 150 rpm, 30°C, and 60% relative humidity. Mycelia of S. rolfsii were harvested in the late exponential phase of growth by filtration through nylon cloth and successively washed with the basal medium (0.1 M sodium citrate buffer, pH 4.5, containing the following [in grams per liter]: KH2PO4, 1.2; MgSO4 · 7H2O, 1.5; and KCl, 0.6). They were then suspended in the basal medium, to which 2.5 g of NH4NO3 per liter and the respective inducers (3.0 mM for mono- or oligosaccharides and 1.0 mg/ml for polysaccharides, unless otherwise indicated) were added, so that the biomass concentration was approximately 2 mg (dry weight) per ml. The flasks were incubated at 30°C under continuous agitation (150 rpm) for various lengths of time. Blanks were prepared in the same way, except that no inducer was added.
Growth experiments.
Cultivations of S. rolfsii were performed in unbaffled 300-ml Erlenmeyer flask with 100 ml of medium as described above on a growth medium containing the following (in grams per liter): peptone from meat, 80; NH4NO3, 2.5; MgSO4 · 7H2O, 1.5; KH2PO4, 1.2; KCl, 0.6; and a trace element solution at 0.3 ml/liter. Carbon sources were added as indicated in Results at a concentration of 42.6 g/liter (22). Cultures were incubated for 13 days at 30°C as described above. Biomass was then separated by centrifugation, and the clear supernatant was used to estimate enzyme activities.
Enzyme activity assays.
All activity assays were carried out in 0.05 M sodium citrate buffer, pH 4.5, unless otherwise stated. Endo-β-1,4-d-xylanase (β-1,4-d-xylan xylanohydrolase [EC 3.2.1.8]) activity was assayed by using a 1% solution of xylan (4-O-methyl glucuronoxylan from birchwood; Roth) as the substrate (3). The release of reducing sugars in 5 min at 50°C was measured as xylose equivalents by the dinitrosalicylic acid (DNS) method (34). Endo-β-1,4-d-mannanase (1,4-β-d-mannan mannanohydrolase [EC 3.2.1.78]) activity was assayed in a manner similar to that used to determine xylanase activity, with a 0.5% solution of locust bean galactomannan in 0.05 M sodium citrate buffer, pH 4.0 as the substrate. Endo-β-1,4-d-glucanase (1,4-β-d-glucan glucanohydrolase [EC 3.2.1.4]) activity was determined in accordance with International Union of Pure and Applied Chemistry recommendations, with a 1% solution of carboxymethyl cellulose (sodium salt, ultralow viscosity) as the substrate (17). Reducing sugars were assayed as mannose or glucose by the DNS method. One unit of enzyme activity is defined as the amount of enzyme producing 1 μmol of xylose, mannose, or glucose equivalents per min under the given conditions and corresponds to 16.67 nkat.
Other analyses.
Protein concentrations were determined by the dye-binding method of Bradford (11) with bovine serum albumin (fraction V) as the standard.
Analytical IEF and activity stains.
Isoelectric focusing (IEF) was carried out on a PhastSystem unit (Pharmacia, Uppsala, Sweden) with precast dry gels (PhastGel dry IEF; Pharmacia) rehydrated with carrier ampholytes (7.5 parts Pharmalyte [pH 2.5 to 5] and 2.5 parts Ampholine [pH 3.5 to 5]; Pharmacia) as described by the manufacturer. Alternatively, precast gels (PhastGel IEF; Pharmacia) at pH 4 to 6.5 or 3 to 9 were used. When necessary, the supernatants from the induction experiments were concentrated by ultrafiltration (Ultrafree-15 centrifugal filter device; Millipore, Bedford, Mass.; molecular size cutoff, 10 kDa). Mannanase, xylanase, and endoglucanase activities in IEF gels were detected by active staining (zymogram technique) with agar replicas containing the corresponding, covalently dyed polysaccharides as described by Biely (8). Cellobiohydrolase activity was located by using 4-methylumbelliferyl cellobioside as the substrate (43). The isoelectric points of the various isoenzymes were determined by comparison with marker proteins (Pharmacia low-pI kit; pH range, 2.8 to 6.5) which were run simultaneously and were visualized by silver staining as recommended by the manufacturer.
RESULTS
Influence of mycelial concentration.
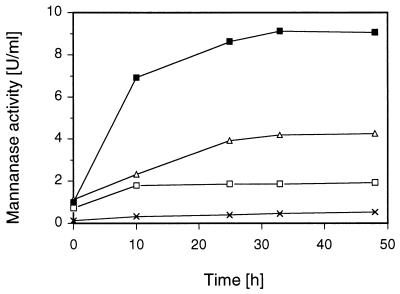
The replacement technique described by Sternberg and Mandels (39) was used to study the induction of mannanase, xylanase, and endoglucanase in S. rolfsii in detail. Mycelia were pregrown on a medium containing glycerol as the substrate, washed, and placed in fresh medium lacking a carbon and organic nitrogen source and to which the potential inducers were added in the concentrations indicated later in the text. To investigate the influence of the mycelial concentration on the levels of endoglycanases formed by S. rolfsii, an inducer solution containing 10 mM cellobiose, which was routinely used as the reference inducing carbohydrate, was tested with various amounts of mycelia. The results for the time-dependent synthesis of mannanase are shown in Fig. 1. An experimental blank contained no added carbohydrate. An increase in the mycelial concentration in the range considered in this experiment (0.5 to 2.0 mg/ml) resulted in an almost linear increase in the enzyme activities formed. The highest mannanase levels were reached within 24 h of incubation when the low-molecular-weight inducer cellobiose was used and thereafter remained constant for at least another 24 h. Very similar results were obtained for the induction of xylanase and endoglucanase by different mycelial concentrations with regard to both the effect of the biomass concentration and the time course of endoglycanase formation (data not shown).
FIG. 1.
Effect of mycelial biomass concentration and time course of extracellular mannanase formation in S. rolfsii CBS 191.62. Washed, glycerol-grown mycelia were transferred to basal medium containing 10 mM cellobiose. The initial pH was 4.5; incubation was done at 30°C and 150 rpm. Mycelium concentrations: □, 0.5 mg (dry weight) per ml; ▵, 1 mg/ml; ▪, 2 mg/ml. ×, blank (no carbohydrate added).
Influence of xylobiose concentration.
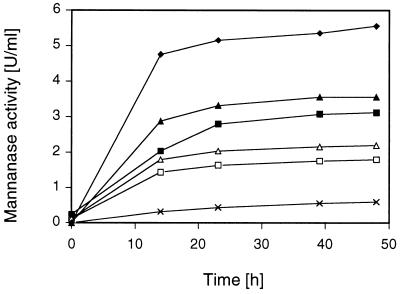
The effect of the concentration of the carbohydrate inducer on the synthesis of endoglycanases in S. rolfsii was studied by varying the concentration of xylobiose (Suntory) from 0 mM (blank) to 35.5 mM in the replacement experiments. Xylobiose was preferred over cellobiose in this experiment since it resulted in comparable levels of endoglycanases formed (see below). However, S. rolfsii forms significantly lower levels of β-xylosidase than β-glucosidase activity (37). This might result in prolonged availability of the inducing disaccharide and circumvent a possible repression caused by the monosaccharides released, especially at higher sugar concentrations. Results for the time course of mannanase formation are given in Fig. 2. Induction of mannanase is strongly dependent on the concentration of the inducer present in the medium. An increase in the inducer over a certain range of low concentrations resulted in an almost linear increase in mannanase activity secreted by the mycelia. This relationship, however, will be lost at higher inducer concentrations (6). This was also found for xylanase and endoglucanase activities when various concentrations of xylobiose were used as the inducer (data not shown).
FIG. 2.
Time course of mannanase secretion by washed, glycerol-grown mycelia of S. rolfsii CBS 191.62 in the presence of various amounts of xylobiose. The mycelial concentration was 1 mg (dry weight) per ml, the initial pH was 4.5, and incubation was done at 30°C and 150 rpm. Xylobiose concentrations: □, 0.5 mg/ml; ▵, 1.0 mg/ml; ▪, 3.0 mg/ml; ▴, 5.0 mg/ml; ⧫, 10 mg/ml; ×, blank (no carbohydrate added).
Effects of various inducers.
To obtain a more detailed insight into the induction of endoglycanase activities in S. rolfsii, various mono-, di-, and polysaccharides were studied as potential inducers of these enzyme activities. Several more easily metabolizable carbohydrates were added as a control. Results of these induction experiments obtained for mannanase, xylanase, and endoglucanase activities are given in Table 1. Interestingly, the cellulosic substrates α-cellulose and bacterial cellulose resulted in the highest activities not only of endoglucanase but also of mannanase and xylanase. The different mannan preparations and xylan from beechwood significantly induced the formation of all three of the enzyme activities investigated, while xylan from birchwood only gave results similar to those obtained with the blank. In accordance with the results obtained with the different polysaccharides, cellobiose was the best low-molecular-weight inducer of mannanase and endoglucanase, while the highest activities of xylanase resulted when lactose or xylobiose was used as the inducer. None of the enzyme activities investigated could be specifically induced by the end product of their action on the polymeric substrate. The β-1,4-linked disaccharides mannobiose, xylobiose, and cellobiose provoked the simultaneous formation of mannanase, xylanase, and endoglucanase, as did the structurally related disaccharides lactose (Gal-β-1,4-Glc) and galactosyl-β-1,4-mannose, as well as the positional isomers of cellobiose, i.e., sophorose (Glc-β-1,2-Glc), laminaribiose (Glc-β-1,3-Glc), and gentiobiose (Glc-β-1,6-Glc).
TABLE 1.
Induction of mannanase, xylanase, and endoglucanase activities in washed, glycerol-pregrown mycelia of S. rolfsii after 24 h of incubationa
| Compoundb | Activity (U/ml) of:
|
||
|---|---|---|---|
| Mannanase | Xylanase | Endoglucanase | |
| None (blank) | 0.63 | 0.22 | 0.08 |
| Monosaccharides | |||
| l-Arabinose | 0.63 | 0.18 | 0.08 |
| d-Xylose | 0.93 | 0.33 | 0.33 |
| d-Glucose | 0.63 | 0.27 | 0.06 |
| d-Galactose | 0.64 | 0.32 | 0.01 |
| d-Mannose | 0.69 | 0.24 | 0.05 |
| l-Sorbose | 3.00 | 0.80 | 1.68 |
| Disaccharides | |||
| Xylobiose (Xyl-β-1,4-Xyl) | 2.57 | 1.08 | 2.37 |
| Methyl-β-d-xyloside | 0.82 | 0.29 | 0.28 |
| Sophorose (Glc-β-1,2-Glc) | 3.26 | 0.73 | 2.11 |
| Laminaribiose (Glc-β-1,3-Glc) | 2.97 | 0.71 | 1.26 |
| Cellobiose (Glc-β-1,4-Glc) | 3.68 | 0.92 | 3.67 |
| Gentiobiose (Glc-β-1,6-Glc) | 2.39 | 0.58 | 1.02 |
| Galactobiose (Gal-β-1,4-Gal) | 1.16 | 0.51 | 0.17 |
| Mannobiose (Man-β-1,4-Man) | 1.58 | 0.53 | 0.89 |
| Galactosyl-β-1,4-mannose | 3.08 | 0.95 | 2.45 |
| Lactose (Gal-β-1,4-Glc) | 2.80 | 1.14 | 2.89 |
| Polysaccharides | |||
| Xylan beechwood | 2.90 | 1.24 | 2.91 |
| Xylan birchwood | 0.94 | 0.21 | 0.31 |
| Carboxymethyl cellulose | 3.69 | 1.45 | 4.03 |
| α-Cellulose | 7.57 | 9.70 | 23.82 |
| Bacterial cellulose | 9.15 | 11.93 | 29.04 |
| Ivory nut mannan | 3.33 | 0.34 | 1.10 |
| Konjac glucomannan | 3.73 | 0.98 | 4.22 |
| Guar galactomannan | 3.68 | 1.64 | 4.24 |
| Locust bean galactomannan | 3.27 | 1.18 | 3.33 |
The mycelium concentration was 2 mg (dry weight) per ml.
Concentrations: sugars, 3.0 mM; polysaccharides, 1.0 mg/ml. In the control (blank), no carbohydrate was added to the basal medium. In addition to the substrates shown here, the following carbohydrates were tested and resulted in enzyme activities corresponding to the values obtained in the control experiment: d-ribose, d-lyxose, d-fructose, d-mannitol, d-sorbitol, d-glucuronic acid, lactulose, lactitol, lactobionic acid, and sucrose.
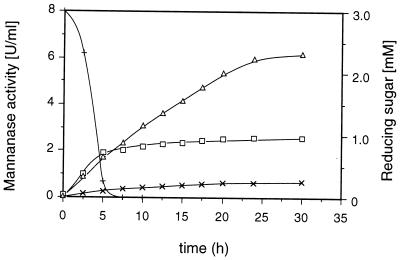
To determine whether prolonged availability of the low-molecular-weight inducer at low levels provides increased enzyme formation, the time courses of endoglycanase synthesis induced by 3 mM cellobiose added in a single dose or in 10 equal doses every 2.5 h were compared; the results obtained for mannanase activity are given in Fig. 3. In the initial phase of this experiment, mannanase formation was higher when the inducer was added in a single dose. However, enzyme synthesis stopped after approximately 5 h, when the cellobiose was depleted. In contrast, the phase of mannanase secretion lasted considerably longer when the inducer was added stepwise, resulting in a final mannanase activity that was significantly higher than that of the control. A similar effect of increased enzyme formation when the inducer was added sequentially was also observed for the induction of xylanase and endoglucanase activities (data not shown).
FIG. 3.
Induction of mannanase in S. rolfsii CBS 191.62 as a function of the rate of cellobiose addition. Washed, glycerol-grown mycelia were suspended in the basal medium (mycelial concentration, 2 mg [dry weight] per ml). Cellobiose (3.0 mM) was added in 1 dose at the beginning of the experiment (□) or in 10 0.3-mM doses every 2.5 h (▵). The control experiment contained no carbohydrate (×). In the single-dose experiment, cellobiose was measured as reducing sugar by the DNS method (+).
Analysis of mannanases, xylanases, and cellulases induced by different carbohydrates.
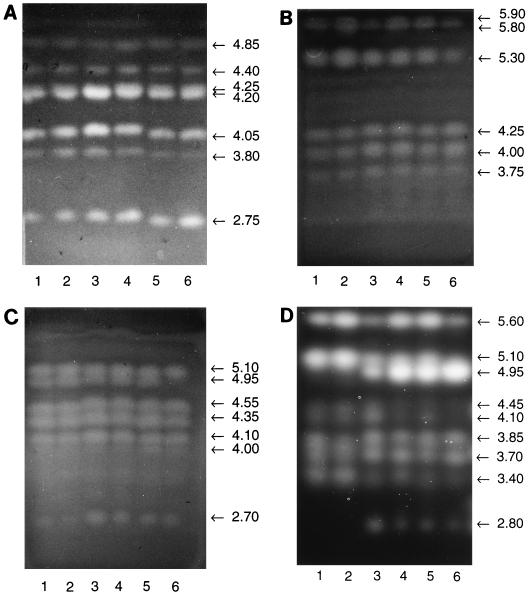
The proteins secreted in response to α-cellulose, bacterial cellulose, locust bean galactomannan, cellobiose, xylobiose, and l-sorbose were separated by analytic IEF on polyacrylamide gels and subsequently monitored for mannanase activity by active staining with RBB-mannan-containing agar overlays (Fig. 4A). At least seven bands showing mannanase activity with pI values of 2.75 to 4.85 were visible. All seven of these multiple isomeric forms of mannanase were excreted in the presence of the structurally different carbohydrate inducers investigated in the comparison. The same banding patterns were also obtained with these enzyme preparations when they were analyzed for xylanase and cellulase activities. At least six protein bands showing activity with RBB-xylan and pI values of 3.75 to 5.90 were detected (Fig. 4B), whereas seven isoforms of endoglucanase with pI values of 2.70 to 5.10 (Fig. 4C) and nine isoformic enzymes showing activity with MeUmb(Glc)2 and having pI values of 2.80 to 5.60 (Fig. 4D) were visualized by the zymogram analysis. The banding patterns of multiple endoglycanases visualized by the various zymogram analyses are very similar for the enzyme systems secreted by S. rolfsii in response to the different carbohydrate inducers. Obvious differences can only be seen for the activity with MeUmb(Glc)2 (Fig. 4D). Two of the isoformic enzymes showing pI values of 2.80 and 4.95 could not be detected in the activity staining for the enzyme preparations obtained in the presence of α-cellulose or bacterial cellulose. Presumably, these two enzymes completely bind to the cellulosic inducers and therefore were not detectable in the supernatant.
FIG. 4.
Endoglycanases induced in resting mycelia of S. rolfsii CBS 191.62 (2 mg [dry weight] per ml) by α-cellulose (lane 1), bacterial cellulose (lane 2), locust bean galactomannan (lane 3), cellobiose (lane 4), xylobiose (lane 5), and l-sorbose (lane 6) after 24 h of incubation. The concentrations of the inducers were 1.0 mg/ml for polysaccharides and 3.0 mM for soluble sugars. Proteins were resolved by IEF, and enzyme activities were detected by a zymogram technique using RBB-mannan (A), RBB-xylan (B), RBB-carboxymethyl cellulose (C), and methylumbelliferyl-β-d-cellobioside (D) as substrates. pI values are given for the main enzyme components next to the arrows.
Growth experiments.
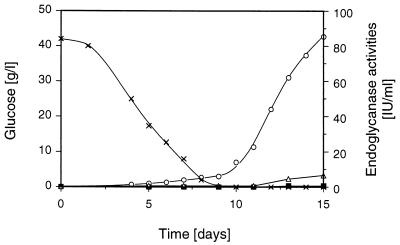
In excellent agreement with the results obtained in the induction experiments, α-cellulose was found to be an outstanding substrate for the simultaneous production of all three glycanases by S. rolfsii, resulting in elevated activities of these enzymes (Table 2). Similarly, cellobiose, when used as the growth substrate, provoked the formation of appreciable enzyme activities which, however, are significantly lower than those obtained with α-cellulose. As expected, very low levels of xylanase and endoglucanase activities were formed during growth on glucose, while surprisingly high levels of mannanase activity were assayed in the spent medium of this cultivation. The addition of a small amount of α-cellulose to a glucose-based medium was sufficient to produce a significant increase in all three enzyme activities. This increase was pronounced for both xylanase and endoglucanase activities, whereas the supplementation of the glucose-based medium with cellulose enhanced the formation of mannanase activity only slightly. To further study the unexpected marked formation of elevated mannanase activities on a more readily metabolized sugar, shaken-flask cultivation of S. rolfsii on a medium containing glucose as the only carbohydrate substrate was investigated in more detail. The time course of this growth experiment is shown in Fig. 5. During the initial phase of this cultivation, glucose was utilized by the fungus while only very low activities of all three endoglycanases were secreted. Significant formation of mannanase started only after complete depletion of the glucose. A maximum value of 85.5 U/ml was obtained after 15 days of cultivation. Simultaneously, very low xylanase and endoglucanase activities were formed under these growth conditions.
TABLE 2.
Formation of extracellular protein and endoglycanase activities by S. rolfsii CBS 191.62 grown in shaken flasks on different substratesa
| Growth substrate | Protein concn (mg/ml) | Activity (U/ml) of:
|
||
|---|---|---|---|---|
| Mannanase | Xylanase | Endoglucanase | ||
| Glucose | 0.62 | 81.5 | 0.59 | 6.84 |
| Glucose + α-cellulose (9:1) | 0.72 | 153 | 4.45 | 34.9 |
| Cellobiose | 1.12 | 286 | 11.0 | 127 |
| α-Cellulose | 3.83 | 675 | 228 | 1,090 |
All of the substrates were used at 42.6 g/liter. Flasks were incubated at 30°C and 150 rpm on a rotary shaker for 13 days.
FIG. 5.
Time course of shaken-flask cultivation of S. rolfsii CBS 191.62 on medium containing 42.6 g of glucose per liter. The fungus was grown at 30°C and 150 rpm on a rotary shaker. Symbols: ×, glucose; ○, mannanase; ▪, xylanase; ▵, endoglucanase.
DISCUSSION
The plant-pathogenic fungus S. rolfsii has received considerable attention as an industrially attractive producer of plant cell wall-degrading enzymes, including cellulases and xylanases (18). Recently, it has also been identified as an outstanding producer of mannanolytic enzymes, including endo-β-mannanase activity (21, 22, 37). Based on the results of this study, it can be concluded that the formation of mannanase, xylanase, and cellulase (endoglucanase) is inducible in wild-type S. rolfsii CBS 191.62. This is in agreement with several other fungi that have been closely investigated with respect to the regulation of xylanase or cellulase biosynthesis (5, 13, 23, 30). This induction of the three endoglycanases is, however, closely related in S. rolfsii, which is contrary to the reports on most other filamentous fungi. When several polysaccharides structurally resembling the main carbohydrate constituents of lignocellulose and including mannan, xylan, and cellulose from different origins were investigated as potential inducers of mannanase, xylanase, and endoglucanase synthesis in resting, nongrowing mycelia of S. rolfsii, the increased formation of the three endoglycanase activities was always simultaneously stimulated by these different polysaccharides. The synthesis of only one of these enzyme activities could not be specifically provoked by the corresponding substrate of its action, as has been shown in growth and induction experiments for various cellulolytic fungi in which the formation of at least xylanases and cellulases is commonly under separate control (5). Interestingly, the highest levels of endoglucanase, as well as mannanase and xylanase, activity were secreted by S. rolfsii when cellulose was present as the inducer. By using bacterial cellulose, produced by the bacterium A. xylinum, as an inducer, the possibility can also be ruled out that contaminating xylan, which is always found in lignocellulose-derived commercial cellulose preparations, causes the elevated formation of xylanase activity, as has been shown for T. reesei and T. harzianum (26, 38).
This closely interrelated regulation of the synthesis of mannanase, xylanase, and endoglucanase in S. rolfsii was further corroborated when a number of mono- and oligosaccharides were used as potential inducers. Concurrent synthesis of these three enzyme activities was also observed in response to the positional isomers of cellobiose (Glc-β-1,4-Glc), i.e., sophorose (Glc-β-1,2-Glc), laminaribiose (Glc-β-1,3-Glc), and gentiobiose (Glc-β-1,6-Glc). It is worth noting that all of the β-linked glucobioses significantly provoked the formation of the endoglycanases, with cellobiose being the best inducer. This is in contrast to several other fungi, in which the β-1,2-linked or the β-1,3-linked disaccharides were found to be much more effective inducers than the disaccharide that directly arose from the hydrolysis of cellulose (27, 28, 31, 40, 42). In addition to these disaccharides, a significant stimulating effect on enzyme synthesis has also been identified for l-sorbose, which is a known inducer of cellulases in T. reesei (10, 29, 30). In this respect, the proposed regulatory effect of l-sorbose on endoglycanase synthesis certainly has to be reconsidered. The stimulating effect was explained by a change in the cell wall of the fungus due to inhibition of β-1,3-glucan synthetase, resulting in enhanced release of the enzymes into the extracellular environment and in the reduced uptake of inducers such as cellobiose due to the lowered amounts of wall-bound β-glucosidase (10). This model certainly does not explain our results, since l-sorbose was used with resting, nongrowing mycelia and no other inducers were added simultaneously.
Cellulose had the most pronounced effect on endoglycanase secretion by S. rolfsii and resulted in significantly higher enzyme activities than the soluble compounds tested. This can be explained by the prolonged availability of the inducing molecules that are slowly released from cellulose by the action of extracellular enzymes. This mechanism has also been suggested by Biely et al. (6) to explain the induction of xylanase in Cryptococcus albidus by xylobiose. This assumption has been proven by the increased formation of endoglycanases by mycelia exposed to the inducer cellobiose added in several doses and thereby available for a prolonged period. The kinetics of endoglycanase formation in this experiment with sequential addition of the inducer strongly resembled those obtained when a single initial dose of cellulose was used.
Although the nature of true in vivo inducers of endoglycanases in S. rolfsii cannot be elucidated from the results of our study, it is tempting to speculate that the regulatory macromolecules that finally react with the true inducer in the cell and trigger the increased formation of endoglycanases (5, 30) are quite unspecific in this fungus and seem to react with a number of structurally similar or related compounds. This is also corroborated by the results of the activity staining, which proved that S. rolfsii secreted the same isoformic multiple mannanases, xylanases, and endoglucanases in response to a number of structurally different signal molecules. This proposed unspecificity of induction could be of a certain advantage to the organism, since it would allow it a faster reaction to a changed environmental situation, i.e., the presence of a polymeric substrate that can be utilized as a carbon and energy source, by producing and secreting all three endoglycanases concurrently as a response to only one signal. It should be noted that in nature the polysaccharides mannan, xylan, and cellulose almost always occur in close association. Furthermore, this unspecificity of induction and the simultaneous formation of the endoglycanases could play a certain role in the plant pathogenicity of S. rolfsii, which has a marked ability to effect rapid destruction of cell walls in herbaceous plants during pathogenesis (12). Hemicellulases are believed to be important virulence factors for at least some pathogenic organisms (1, 9, 47).
Although the induction of mannanase, xylanase, and cellulase is very closely related in S. rolfsii, it can be concluded from our experimental data that there is no common, coordinated regulatory mechanism for all three endoglycanases since there exist certain significant differences in the regulatory control of these enzyme activities. The most obvious distinction certainly concerns the elevated formation of mannanase, which is synthesized in appreciable levels by the organism, even when it is cultivated on glucose as the main substrate. Under these growth conditions, mannanase formation commences only after complete depletion of the sugar substrate and therefore is caused by derepression of enzyme synthesis and not by induction. In contrast to this, xylanase and endoglucanase are formed in enhanced levels only when an appropriate inducer is present; otherwise, only very low, presumably constitutive synthesis occurred, as shown by cultivation on glucose-based medium.
This significant difference in the regulation of mannanase synthesis in S. rolfsii could be of technological relevance. By selecting an appropriate inducing substrate, e.g., lactose or cellulosic material, an enzyme preparation containing elevated activities of all three endoglycanases can be obtained, while a mannanase preparation with only very low endoglucanase and xylanase activities can be produced on cheap, easily metabolized, noninducing substrates such as glucose. Such an enzyme preparation could find wide applications in the pulp and paper industry (45, 46).
ACKNOWLEDGMENTS
We thank Marianne Prebio for linguistic corrections. José Fontana is thanked for his precious samples of bacterial cellulose, and Takashi Yasuda from Suntory is thanked for the generous gift of xylooligosaccharides.
This work was supported by grant P10753-MOB from the Austrian Science Foundation (Fonds zur Förderung der wissenschaftlichen Forschung) to D.H.
REFERENCES
- 1.Apel-Birkhold P C, Walton J D. Cloning, disruption, and expression of two endo-β1,4-xylanase genes, XYL2 and XYL3, from Cochliobolus carbonum. Appl Environ Microbiol. 1996;62:4129–4135. doi: 10.1128/aem.62.11.4129-4135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arisan-Atac I, Hodits R, Kristufek D, Kubicek C P. Purification and characterization of a β-mannanase of Trichoderma reesei C-30. Appl Microbiol Biotechnol. 1993;39:58–62. doi: 10.1128/aem.59.5.1347-1353.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey M J, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. [Google Scholar]
- 4.Biely P. Biotechnological potential and production of xylanolytic systems free of cellulases. ACS Symp Ser. 1991;460:408–416. [Google Scholar]
- 5.Biely P. Biochemical aspects of the production of microbial hemicellulases. In: Coughlan M P, Hazlewood G P, editors. Hemicellulose and hemicellulases. London, England: Portland Press; 1993. pp. 29–51. [Google Scholar]
- 6.Biely P, Krátky Z, Vrsanská M, Urmanicová D. Induction and inducers of endo-1,4-β-xylanase in the yeast Cryptococcus albidus. Eur J Biochem. 1980;108:323–329. doi: 10.1111/j.1432-1033.1980.tb04726.x. [DOI] [PubMed] [Google Scholar]
- 7.Biely P, MacKenzie C R, Schneider H. Production of acetyl xylan esterase by Trichoderma reesei and Schizophyllum commune. Can J Microbiol. 1988;34:767–772. [Google Scholar]
- 8.Biely P, Markovic O, Mislovicová D. Sensitive detection of endo-1,4-β-glucanases and endo-1,4-β-xylanases in gels. Anal Biochem. 1985;144:147–151. doi: 10.1016/0003-2697(85)90096-x. [DOI] [PubMed] [Google Scholar]
- 9.Binz T, Canevascini G. Xylanases from the Dutch elm disease pathogens Ophiostoma ulmi and Ophiostoma novo-ulmi. Physiol Mol Plant Pathol. 1996;49:159–175. [Google Scholar]
- 10.Bisaria V S, Mishra S. Regulatory aspects of cellulase biosynthesis and secretion. Crit Rev Biotechnol. 1989;9:61–103. doi: 10.3109/07388558909040616. [DOI] [PubMed] [Google Scholar]
- 11.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Cole A L J, Bateman D F. Arabanase production by Sclerotium rolfsii and its role in tissue maceration. Phytopathology. 1969;59:1750–1753. [PubMed] [Google Scholar]
- 13.Coughlan M P, Hazelwood G P. β-1,4-d-Xylan-degrading enzyme systems: biochemistry, molecular biology and applications. Biotechnol Appl Biochem. 1993;17:259–289. [PubMed] [Google Scholar]
- 14.Cuevas W A, Kantelinen A, Tanner P, Bodie B, Leskinen S. Purification and characterization of novel mannanases used in pulp bleaching. In: Srebotnik E, Messner K, editors. Biotechnology in the pulp and paper industry. Vienna, Austria: Facultas-Universitätsverlag; 1996. pp. 123–126. [Google Scholar]
- 15.Farrell R L, Biely P, McKay D L. Production of hemicellulase by the fungus Penicillium kloeckeri. In: Srebotnik E, Messner K, editors. Biotechnology in the pulp and paper industry. Vienna, Austria: Facultas-Universitätsverlag; 1996. pp. 485–489. [Google Scholar]
- 16.Fontana J D, Franco V C, de Souza S J, Lyra I N, de Souza A M. Nature of plant stimulators in the production of Acetobacter xylinum (“tea fungus”) biofilm used in skin therapy. Appl Biochem Biotechnol. 1991;28–29:341–352. doi: 10.1007/BF02922613. [DOI] [PubMed] [Google Scholar]
- 17.Ghose T K. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. [Google Scholar]
- 18.Goyal A, Ghosh B, Eveleigh D. Characteristics of fungal cellulases. Bioresource Technol. 1991;36:37–50. [Google Scholar]
- 19.Gübitz G M, Haltrich D, Latal B, Steiner W. Mode of depolymerisation of hemicellulose by various mannanases and xylanases in relation to their ability to bleach softwood pulp. Appl Microbiol Biotechnol. 1997;47:658–662. [Google Scholar]
- 20.Gübitz G M, Lischnig T, Stebbing D, Saddler J N. Enzymatic removal of hemicellulose from dissolving pulps. Biotechnol Lett. 1997;19:491–495. [Google Scholar]
- 21.Gübitz G M, Steiner W. Simultaneous production of xylanase and mannanase by several hemicellulolytic fungi. ACS Symp Ser. 1995;618:319–331. [Google Scholar]
- 22.Haltrich D, Laussamayer B, Steiner W. Xylanase formation by Sclerotium rolfsii: effect of growth substrates and development of a culture medium using statistically designed experiments. Appl Microbiol Biotechnol. 1994;42:522–530. [Google Scholar]
- 23.Haltrich D, Nidetzky B, Kulbe K D, Steiner W, Zupancic S. Production of fungal xylanases. Bioresource Technol. 1996;58:137–161. [Google Scholar]
- 24.Haltrich D, Sebesta B, Steiner W. Induction of xylanase and cellulase in Schizophyllum commune. ACS Symp Ser. 1995;618:305–318. [Google Scholar]
- 25.Haltrich D, Steiner W. Formation of xylanase by Schizophyllum commune: effect of medium components. Enzyme Microb Technol. 1994;16:229–235. [Google Scholar]
- 26.Hrmová M, Biely P, Vrsanská M. Specificity of cellulase and β-xylanase induction in Trichoderma reesei QM 9414. Arch Microbiol. 1986;144:307–311. [Google Scholar]
- 27.Hrmová M, Biely P, Vrsanská M. Cellulose- and xylan-degrading enzymes of Aspergillus terreus and Aspergillus niger. Enzyme Microb Technol. 1989;11:610–616. [Google Scholar]
- 28.Hrmová M, Petráková E, Biely P. Induction of cellulose- and xylan-degrading enzyme systems in Aspergillus terreus by homo- and heterodisaccharides composed of glucose and xylose. J Gen Microbiol. 1991;137:541–547. doi: 10.1099/00221287-137-3-541. [DOI] [PubMed] [Google Scholar]
- 29.Kawamori M, Morikawa Y, Takasawa S. Induction and production of cellulases by l-sorbose in Trichoderma reesei. Appl Microbiol Biotechnol. 1986;24:449–453. [Google Scholar]
- 30.Kubicek C P, Messner R, Gruber F, Mach R L, Kubicek-Pranz E M. The Trichoderma cellulase regulatory puzzle: from the interior life of a secretory fungus. Enzyme Microb Technol. 1993;15:90–99. doi: 10.1016/0141-0229(93)90030-6. [DOI] [PubMed] [Google Scholar]
- 31.Kurasawa T, Yachi M, Suto M, Kamagata Y, Takao S, Tomita F. Induction of cellulase by gentiobiose and its sulfur-containing analog in Penicillium purpurogenum. Appl Environ Microbiol. 1992;58:106–110. doi: 10.1128/aem.58.1.106-110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurosawa K, Hosoguchi M, Hariantono J, Sasaki H, Takao S. Degradation of tough materials by cellulase from Corticium rolfsii. Agric Biol Chem. 1989;53:931–937. [Google Scholar]
- 33.Lachke A H, Deshpande M V. Sclerotium rolfsii: status in cellulase research. FEMS Microbiol Rev. 1988;54:177–194. [Google Scholar]
- 34.Miller G L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 35.Reese E T, Shibata Y. β-Mannanases of fungi. Can J Microbiol. 1965;11:167–183. doi: 10.1139/m65-023. [DOI] [PubMed] [Google Scholar]
- 36.Royer J C, Nakas J P. Xylanase production by Trichoderma longibrachiatum. Enzyme Microb Technol. 1989;11:405–410. [Google Scholar]
- 37.Sachslehner A, Haltrich D, Nidetzky B, Kulbe K D. Production of hemicellulose- and cellulose-degrading enzymes by various strains of Sclerotium rolfsii. Appl Biochem Biotechnol. 1997;63–65:189–201. doi: 10.1007/BF02920424. [DOI] [PubMed] [Google Scholar]
- 38.Senior D J, Mayers P R, Saddler J N. Xylanase production by Trichoderma harzianum E58. Appl Microbiol Biotechnol. 1989;32:137–142. [Google Scholar]
- 39.Sternberg D, Mandels G R. Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol. 1979;139:761–769. doi: 10.1128/jb.139.3.761-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson J A. Molecular biology of xylan degradation. FEMS Microbiol Rev. 1993;104:65–82. doi: 10.1111/j.1574-6968.1993.tb05864.x. [DOI] [PubMed] [Google Scholar]
- 41.Tuohy M G, Coughlan M P. Production of thermostable xylan-degrading enzymes by Talaromyces emersonii. Bioresource Technol. 1992;39:131–137. [Google Scholar]
- 42.Vaheri M, Leisola M, Kauppinen V. Transglycosylation products of cellulase system of Trichoderma reesei. Biotechnol Lett. 1979;1:41–46. [Google Scholar]
- 43.van Tilbeurgh H, Claeyssens M, de Bruyne C K. The use of 4-methylumbelliferyl and other chromophoric glycosides in the study of cellulolytic enzymes. FEBS Lett. 1982;149:152–156. [Google Scholar]
- 44.Viikari L, Kantelinen A, Sundquist J, Linko M. Xylanases in bleaching: from an idea to the industry. FEMS Microbiol Rev. 1994;13:335–350. [Google Scholar]
- 45.Viikari L, Tenkanen M, Buchert J, Rättö M, Bailey M, Siika-aho M, Linko M. Hemicellulases for industrial applications. In: Saddler J N, editor. Bioconversion of forest and agricultural plant residues. Wallingford, England: C.A.B. International; 1993. pp. 131–182. [Google Scholar]
- 46.Wong K K Y, Saddler J N. Applications of hemicellulases in the food, feed, and pulp and paper industries. In: Coughlan M P, Hazlewood G P, editors. Hemicellulose and hemicellulases. London, England: Portland Press; 1993. pp. 127–143. [Google Scholar]
- 47.Wu S-C, Kauffmann S, Darvill A G, Albersheim P. Purification, cloning and characterization of two xylanases from Magnaporthe grisea, the rice blast fungus. Mol Plant-Microbe Interact. 1995;8:506–514. doi: 10.1094/mpmi-8-0506. [DOI] [PubMed] [Google Scholar]