Abstract
Grazing of Echinopogon spp. by livestock in Australia has caused symptoms similar to those of perennial ryegrass staggers. We observed an endophytic fungus in the intercellular spaces of the leaves and seeds of New Zealand and Australian specimens of Echinopogon ovatus. Culture of surface-sterilized seeds from New Zealand specimens yielded a slow-growing fungus. An examination in which immunoblotting and an enzyme-linked immunosorbent assay (ELISA) were used indicated that E. ovatus plants from Australia and New Zealand were infected with fungi serologically related to Neotyphodium lolii (the endophyte of perennial ryegrass) and other Epichloe and Neotyphodium spp. endophytic in pooid grasses. No lolitrems (the indole–diterpenoids implicated as the causative agents of perennial ryegrass staggers), peramine analogs, or ergot alkaloids were detected in the infected specimens by high-performance liquid chromatography or ELISA. However, in endophyte-infected E. ovatus plants from New Zealand, analogs of the indole–diterpenoid paxilline (thought to be a biosynthetic precursor of the lolitrems and related tremorgens) were detected by ELISA, and N-formylloline was detected by gas chromatography. Endophyte-free specimens of New Zealand E. ovatus did not contain detectable paxilline analogs or lolines and were more palatable than infected specimens to adults of the pasture pest Listronotus bonariensis (Argentine stem weevil). Hyphae similar to those of the E. ovatus endophyte were also found in herbarium specimens of Echinopogon nutans var. major, Echinopogon intermedius, Echinopogon caespitosus, and Echinopogon cheeli. This appears to be the first time that an endophytic Neotyphodium species has been identified in grasses endemic to New Zealand or Australia.
Echinopogon ovatus (G. Forst) P. Beauv. (Gramineae; Aveneae) is a tufted perennial grass often found in forested areas in New Zealand and Australia; all other members of the genus Echinopogon are confined to Australia (12, 24). E. ovatus has been reported to cause intoxication of stock in Australia (7, 12, 21, 23, 40, 41, 44), but the reported cases predate the revision of the genus Echinopogon by Hubbard (22). Prior to this revision, the genus was regarded as monotypic, and the toxic species was identified as E. ovatus. It is not now possible to ascertain which of the seven currently accepted Echinopogon species were responsible for outbreaks of intoxication (12).
The symptoms of Echinopogon intoxication, as described by Everist (12), are very similar to those of perennial ryegrass staggers (RGS) (9). Seeds from a toxic specimen of an Echinopogon sp. were reported to contain an intercellular endophytic fungus similar to the fungi that infect Lolium temulentum L. (7, 23) and Festuca hieronymi Hackel (Rivas and Zanolli (1909) [7]). It has since been demonstrated that RGS is caused by an endophytic fungus, Neotyphodium lolii (Latch, Christensen & Samuels) A. Glenn, C. W. Bacon & R. T. Hanlin (formerly Acremonium lolii), growing in the intercellular spaces of the aerial parts of perennial ryegrass (Lolium perenne L.) (13). The principal causative agent of RGS is thought to be the lolitrem family of neurotoxic indole–-diterpenoid alkaloids (15) produced by the fungus (28, 34).
Grasses infected with Neotyphodium endophytes are generally more resistant to herbivory and abiotic stresses than their uninfected counterparts (4, 8). Alkaloids produced by such endophytes, including peramine, lolines, indole–diterpenoids, and ergopeptines, play a major role in the resistance of infected grasses to a wide range of insect pests (35, 38). The extent of this effect is such that in some parts of New Zealand it can be uneconomic to plant endophyte-free Lolium perenne due to damage inflicted by insect pests, especially Argentine stem weevil (Listronotus bonariensis (Kuschel)) (36, 37).
The recognition that endophytic fungi are present in numerous grasses (45), together with the similarity of the symptoms of poisoning caused by Echinopogon spp. and Lolium perenne, prompted us to examine New Zealand and Australian Echinopogon specimens for Neotyphodium endophytes and for alkaloids known to be produced by such fungi.
Here we describe a Neotyphodium endophyte found in New Zealand specimens of E. ovatus, isolation of the endophyte from infected seeds, identification of the endophyte as a Neotyphodium sp. on the basis of its cultural and serological characteristics, and the presence of indole-diterpenoid and loline alkaloids in endophyte-infected E. ovatus but not in endophyte-free E. ovatus. The endophyte found in New Zealand E. ovatus was shown to decrease the plant’s palatability to Listronotus bonariensis. Similar endophytic fungi also were observed in Australian specimens of E. ovatus, Echinopogon nutans var. major C. E. Hubb., Echinopogon intermedius C. E. Hubb., Echinopogon caespitosus C. E. Hubb., and Echinopogon cheeli C. E. Hubb.
MATERIALS AND METHODS
Mycology. (i) New Zealand specimens.
Initially, herbarium specimens of E. ovatus (G. Forst.) P. Beauv. (WAIK11055, WAIK5423, WAIK9722, WAIK12346, and WAIK5357) were examined for the presence of endophytes. Subsequently, five flowering stems and five flowering plants of E. ovatus (WAIK15270; duplicate at the LandCare Herbarium, Christchurch, New Zealand [CHR]) were collected in January 1995 from Waingaro Forest Park and examined for endophytes. One plant (NZFRI22106) from Maungaharuru Ecological District and four plants from Whirinaki Ecological District (NZMS 260 Map V18 Grid Ref 313 665) were examined soon after collection in April 1996.
Leaf sheaths from fresh material and stems from dried material were stained in heated lactophenol-aniline blue and examined for fungal hyphae by light microscopy (11). The Waingaro Forest Park plants (WAIK15270) were potted and kept in a controlled-environment room (26°C; 16 h of light and 8 h of dark; artificial illumination at a level of 110 μE m−2 s−1) until the seedheads were mature. Seeds were harvested from the plants, and some of the seeds were sectioned and stained with lactophenol-aniline blue for direct examination. To isolate the endophyte, seeds were surface sterilized (treated with 70% ethanol for 30 s and 10% commercial bleach [Janola] for 5 min and rinsed in sterile water), placed on water agar in petri plates, and incubated in the controlled-environment room (see above). After germination, which took up to 9 weeks, the seedlings were inoculated into broth (2% glucose, 1% peptone, 0.5% yeast extract) and incubated in the dark at the ambient temperature. When fungal growth was apparent, subcultures were transferred to Gibco potato dextrose agar, and growth from these subcultures was transferred to Difco cornmeal agar, malt agar (2% malt extract, 0.25% peptone, 1.5% agar), and modified Sabouraud agar (4% glucose, 1% peptone, 0.5% yeast extract, 1.5% agar).
(ii) Australian specimens (all lodged at the National Herbarium of New South Wales, Royal Botanic Gardens, Sydney, Australia [NSW]).
Initially, herbarium specimens of E. ovatus (Henderson f19, L. Simpson NSW253020, Pickard 2765, Melville 2248, Coveny 8982, Hosking 252) were examined for endophytic hyphae as described above.
Subsequently, samples of leaves and seeds of E. ovatus were collected in the summer of 1996-1997 from three sites in New South Wales (Jacobs 8162, Jacobs 8164, Jacobs 8172). Leaf sheaths from one collection (Jacobs 8162) were examined for endophytes as described above, and the endophyte status of the remaining collections (Jacobs 8164, Jacobs 8172) was assessed by examining six to eight seeds from each plant. The procedure used involved removal of the husks, after which the seeds were soaked in sodium hydroxide (5%, 3 h), stained with lactic acid-aniline blue, mounted in lactic acid-aniline blue-glycerol, squashed under a coverslip, and examined by light microscopy.
Leaf sheaths from herbarium specimens of the following Echinopogon species and varieties were also examined for endophytic fungi: Echinopogon phleoides C. E. Hubb. (NSW372699, NSW372700, NSW373061), E. nutans var. major C. E. Hubb. (Lloyd 0698), Echinopogon nutans var. nutans C. E. Hubb. (Lloyd 1171, Vickery 17.4.1953, White-.12.1917, NSW377741), Echinopogon mckiei C. E. Hubb. (NSW377744, NSW377751, NSW42726, NSW377755, McBarron 2827), E. intermedius C. E. Hubb. (NSW52870, Lloyd 1218, Cordingly 3.1.1975, Lloyd 0938, Mead 23.1.1962, Lloyd 9.1.1982), E. caespitosus C. E. Hubb. (Pickard 711 & Black, NSW16638, Lloyd 27.3.1980, Lloyd 0400, P.P.B. Inspector 9.1.37, Lloyd 0937), and E. cheeli C. E. Hubb. (Vickery 8.1.1940, Lloyd 0335, McKie 432, NSW377426, NSW377439, NSW377435).
Preparation of endophyte-free E. ovatus.
Seeds of endophyte-infected E. ovatus from Waingaro Forest Park were soaked in 1% sodium hypochlorite for 2 h and then rinsed in sterile water. The seeds were dried on sterile filter paper in a laminar flow cabinet and transferred to petri dishes, which were then stored for 3 weeks at 37°C above 1 cm of water in a bell jar. Treated and untreated seeds were sown in trays containing potting mixture, and the seedlings were grown in a glasshouse. When the plants were approximately 6 weeks old, endophyte infection was determined by peeling a strip of epidermis from the leaf sheath of each plant. This was mounted on a slide, stained with lactophenol-aniline blue, and examined by light microscopy for endophyte mycelium (11).
After we tested for endophytes, endophyte-free and endophyte-infected E. ovatus seedlings were transplanted into pots (diameter, 10 cm) containing potting mixture and kept in a shadehouse for approximately 4 weeks. All of the plants were again checked to confirm their endophyte infection status before experiments were performed.
Tissue print immunoblotting.
Two tillers of each plant were assayed by tissue print immunoblotting (19) for Neotyphodium infection. The serum used for analysis was highly specific for Epichloe species and their asexual relatives, the Neotyphodium species (1).
Endophyte ELISA.
E. ovatus specimens from New Zealand and Australia were tested for the presence of Neotyphodium antigens with a sandwich enzyme-linked immunosorbent assay (ELISA). Rabbit antibodies were raised against N. lolii as previously described (32), and goat antibodies were a gift from R. G. Keogh (AgResearch Grasslands). Each ELISA plate was washed two to four times with phosphate-buffered saline (PBS)–Tween 20 or PBS and aspirated between assay steps.
Each ELISA plate was coated with purified goat anti-N. lolii antibodies (2 μg ml−1 in 0.05 M NaHCO3 [pH 9.6], 100 μl well−1) for 6 h at 20°C and then blocked with 110 μl of 1% (wt/vol) bovine serum albumin (BSA) in PBS. Freeze-dried, milled herbage was extracted with PBS containing 0.05% Tween 20 (20 mg ml−1) for 3 h at 20°C, and the extracts were applied to the ELISA plate (100 μl well−1) and incubated overnight at 4°C. Rabbit anti-N. lolii antiserum (2 μg/ml−1 in BSA-PBS) was then added (100 μl well−1), and the ELISA plate was incubated for 3 h at 20°C. The presence of Neotyphodium antigens was revealed by incubation for 3 h at 20°C with goat anti-rabbit antibody conjugated to horseradish peroxidase (Dako, Glostrup, Denmark) diluted 2,000-fold in BSA-PBS (100 μl well−1), followed by addition of a substrate solution (prepared by adding 3,3′,5,5′-tetramethylbenzidime in dimethyl sulfoxide [10 mg ml−1] to 10 ml of 0.1 M sodium acetate buffer [pH 5.5] containing 0.005% H2O2). The plates were incubated for 15 min, the reaction was stopped by adding 50 μl of 2 M H2SO4, and the A450 was determined.
Alkaloid analyses.
Lolitrem B (16) and peramine (2, 43) analyses were performed by using standard high-performance liquid chromatography (HPLC) methods. Paxilline analogs, ergot alkaloids, and peramine analogs were analyzed by ELISAs (17, 18).
N-Acetylloline and N-formylloline were analyzed by using a method modified from the method of Kennedy and Bush (25) and Yates et al. (48). To 1 g of dry, powdered plant tissue 0.3 g of NaHCO3 and 2 ml of water were added, and the mixture was ground in a mortar with glasperlen (1 g). The resultant paste was suspended in 8 ml of dichloromethane-methanol (19:1) containing 250 μg of 4-phenylmorpholine as an internal standard. After 15 min at 4°C, the suspension was centrifuged, and 1 μl of the supernatant was injected into a gas chromatograph equipped with a poly(dimethylsiloxane) SPB1 column (0.5-μm film; 15 m by 0.53 mm [inside diameter]; Supelco) and flame ionization detector; the temperature was programmed to increase from 80°C (2-min hold) to 212°C at a rate of 4°C min−1.
Argentine stem weevil feeding preferences.
Freshly harvested leaves (length, 3 cm) were presented, adaxial surface up, to Listronotus bonariensis adults on moist filter paper in petri dishes in a “leaf comb choice feeding test” (3). Each test (10 replicates) consisted of one E. ovatus leaf and two Lolium perenne cv. Yatsyn 1 leaves whose endophyte status was known. Two Lolium perenne leaves were used per test so that the leaf surface areas available to Listronotus bonariensis were approximately the same for the two grass species.
Listronotus bonariensis adults were collected from local pastures, and 10 weevils (mixed sexes) were placed in each petri dish and allowed to feed at an average ambient temperature of 24°C with a photoperiod consisting of 16 h of light and 8 h of darkness. Feeding damage was assessed after 18 h by estimating the area of each leaf blade consumed. Data were analyzed by performing an analysis of variance.
RESULTS
New Zealand E. ovatus.
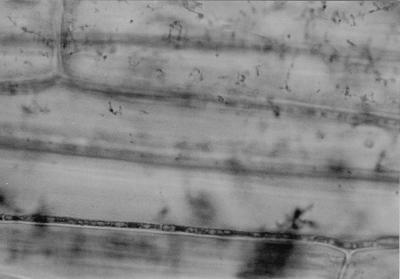
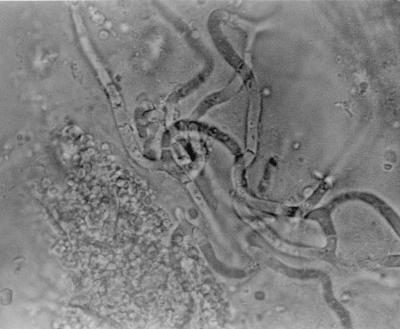
Septate convoluted hyaline hyphae typical of endophytic Neotyphodium infection were seen in the stem of a herbarium specimen of E. ovatus (WAIK11055). When the Waingaro Forest Park material (WAIK15270) was examined directly, septate fungal hyphae ca. 2 μm wide were seen in leaf sheaths from all five plants and from four of the five flowering stems. The hyphae were straight to undulating and rarely branched and were parallel to the length of the sheath in the intercellular spaces (Fig. 1). No lesions were apparent in the plant tissue. Convoluted branching hyphae were also visible in the aleurone layer of the seeds (Fig. 2). Hyphae were seen in the stems of all four plants from Whirinaki Ecological District, but were not observed in the specimen from Maungaharuru Ecological District. These results are summarized in Table 1.
FIG. 1.
Photomicrograph of endophyte mycelium in the leaf sheath of E. ovatus.
FIG. 2.
Photomicrograph of endophyte mycelium in the aleurone layer of a seed of E. ovatus.
TABLE 1.
Summary of Echinopogon species and varieties examined, their sources, and their endophyte status
| Species or variety | Country | Specimen type | No. of collection sites | No. of plants examined | Apparent infection rate (%) |
|---|---|---|---|---|---|
| E. ovatus | New Zealand | Herbarium | 5 | 5 | 20 |
| E. ovatus | New Zealand | Fresh | 3 | 10 | 90 |
| E. ovatus | Australia | Herbarium | 6 | 6 | 17 |
| E. ovatus | Australia | Fresh | 3 | 23 | 87 |
| E. phleoides | Australia | Herbarium | 3 | 3 | 0 |
| E. nutans var. major | Australia | Herbarium | 1 | 1 | 100 |
| E. nutans var. nutans | Australia | Herbarium | 4 | 4 | 0 |
| E. mckiei | Australia | Herbarium | 5 | 5 | 0 |
| E. intermedius | Australia | Herbarium | 6 | 6 | 17 |
| E. caespitosus | Australia | Herbarium | 6 | 6 | 17 |
| E. cheeli | Australia | Herbarium | 6 | 6 | 50 |
There was restricted fungal growth from 29 of 30 seedlings (from WAIK15270) 2 to 3 weeks after inoculation into broth. In potato dextrose agar subcultures, the colonies were circumscribed, raised, cerebriform, and tan, and they either were waxy or had slight white aerial mycelium. After 5 weeks of incubation at 25°C, the colony diameters were 7 to 10 mm on potato dextrose agar, 3 mm on cornmeal agar, 3 to 4 mm on malt agar, and 6 to 7 mm on modified Sabouraud agar. Sporulation was observed on one occasion with each of two isolates but could not be induced reproducibly. The phialides were tapered, produced singly, ca. 2 μm wide at the base, and 20 to 50 μm long; longer phialides were sometimes swollen (width at the midpoint, 2.5 μm), and septation was not discernable.
The endophyte in the E. ovatus seeds was killed by storage under warm, humid conditions. The viability of the seeds was not affected by this treatment, and propagation of treated and untreated seeds provided endophyte-infected and endophyte-free specimens of E. ovatus for comparison by chemical and immunochemical methods. The endophyte status of treated and untreated plants was assessed individually by microscopic examination, and none of the plants grown from treated seeds was endophyte infected.
Compared to endophyte-free material grown from heat-treated seeds (mean absorbance, 0.000; standard error of the mean [SEM], 0.010), plants grown from endophyte-infected seeds of New Zealand E. ovatus gave positive responses (mean absorbance, 0.092; SEM, 0.012) in the ELISA for endophytes. Eight of eight plants grown from endophyte-infected seeds of New Zealand E. ovatus also gave strong positive responses when they were analyzed for endophytes by immunoblotting.
Lolitrem B, peramine, and ergovaline were not detected by HPLC in endophyte-free or endophyte-infected plants (limit of detection, ca. 0.05 mg kg−1). The results of the ELISA analyses for ergot alkaloids and peramine analogs were negative (limit of detection, 0.05 mg kg−1) for both endophyte-infected and uninfected plants. However, paxilline analogs were detected in the endophyte-infected plants but not in the endophyte-free plants by ELISA (limit of detection, 0.05 mg of paxilline equivalents kg−1). Endophyte-infected E. ovatus from New Zealand contained the aminopyrrolizidine N-formylloline, but no detectable N-acetylloline (limit of detection, 30 mg kg−1). Neither aminopyrrolizidine was detected in endophyte-free E. ovatus (Table 2).
TABLE 2.
Alkaloid concentrations in E. ovatus specimens from New Zealand and Australia
| Alkaloid(s) | Analytical method | Concn (mg kg−1) in:
|
||
|---|---|---|---|---|
| New Zealand specimensa
|
Endophyte-infected Australian specimens | |||
| Endophyte infected | Endophyte free | |||
| Lolitrem B | HPLC | NDb | ND | NDc |
| Paxilline analogs | ELISA | 108 | ND | NDc,d |
| Ergot alkaloids | ELISA | ND | ND | NDc |
| Ergovaline | HPLC | ND | ND | NDd |
| Peramine analogs | ELISA | ND | ND | NDd |
| Peramine | HPLC | ND | ND | NDd |
| N-Acetylloline | Gas chromatography | ND | ND | NDd |
| N-Formylloline | Gas chromatography | 1,060 | ND | NDd |
Data from an analysis of plants grown from endophyte-infected and endophyte-free seeds from WAIK15270 specimens.
ND, not detected.
Data from an analysis of herbage from Jacobs 8162 specimens.
Data from an analysis of plants grown from endophyte-infected seeds from Jacobs 8162 specimens.
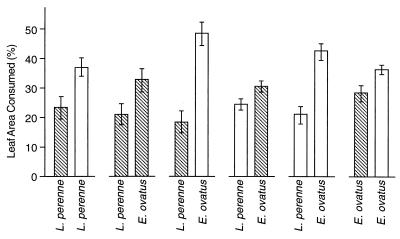
In the feeding experiments performed with Listronotus bonariensis (Fig. 3), adults preferentially fed on E. ovatus rather than Lolium perenne, regardless of whether the plants were infected with the endophyte. When offered a choice of endophyte-infected or endophyte-free leaves of the same grass species, weevils preferentially fed on endophyte-free leaves.
FIG. 3.
Feeding by adult Listronotus bonariensis on endophyte-free (open bars) and endophyte-infected (cross-hatched bars) E. ovatus (WAIK15270) and Lolium perenne in a leaf comb choice feeding test. Error bars indicate the SEM. Pairs of bars represent the results of paired choice feeding tests.
Australian E. ovatus.
Endophyte hyphae similar in appearance to the hyphae in the New Zealand plants were observed in the leaf sheath of one of the herbarium specimens of E. ovatus (Hosking 252). An examination of leaf sheaths from recently collected E. ovatus (Jacobs 8162) revealed endophyte infection in seven of eight plants. Seed squash tests also revealed the presence of endophytes in six of eight plants (Jacobs 8164) and seven of seven plants (Jacobs 8172) collected at other sites in New South Wales (Table 1). Six of six plants grown from seeds from endophyte-infected Australian E. ovatus specimens (Jacobs 8162) gave strong positive responses when they were analyzed for endophytes by immunoblotting. Herbage from such plants also gave a positive response (mean absorbance, 0.214; SEM, 0.008) compared to endophyte-free New Zealand E. ovatus (mean absorbance, 0.000; SEM, 0.010) in the endophyte ELISA. None of the alkaloids assayed for were detected in Australian endophyte-infected E. ovatus (Table 2).
A microscopic examination subsequently revealed that similar endophyte hyphae were also present in some herbarium specimens of E. nutans var. major (Lloyd 0698), E. intermedius (Lloyd 1218), E. caespitosus (P.P.B. Inspector 9.1.37), and E. cheeli (Vickery 8.1.1940, McKie 432, NSW377435) (Table 1).
DISCUSSION
An endophyte similar in appearance to the endophytes belonging to the genus Neotyphodium was detected in a herbarium specimen of E. ovatus collected at Whangaehu, New Zealand. An examination of leaves (Fig. 1) and seeds (Fig. 2) from freshly collected specimens from several sites in New Zealand revealed high degrees of endophyte infection; five of five plants from Waingaro Forest Park and four of four plants from Whirinaki Ecological District were infected, and only the single specimen from Maungaharuru Ecological District was apparently endophyte-free. Similar endophytic hyphae were also seen in 85% of recently collected Australian specimens of E. ovatus from three sites. In addition, endophytic hyphae were observed in herbarium specimens of E. nutans var. major, E. intermedius, E. caespitosus, and E. cheeli (Table 1). However, contamination by saprophytic fungi prevented positive identification of endophyte hyphae in many of the herbarium specimens, and this may have been responsible for the apparent absence of endophytes in herbarium specimens of E. mckiei, E. nutans var. nutans, and E. phleoides and for the apparently low infection rate in herbarium specimens of E. ovatus (18%) compared to the infection rate of freshly collected specimens (88%) (Table 1). Our observations are in accord with those of Cleland (7), who reported that the “seed coats” of an Echinopogon sp. implicated in an outbreak of staggers contained endophytic fungal hyphae similar in appearance to those seen in Lolium temulentum and F. hieronymi.
The endophyte in New Zealand E. ovatus was isolated from seeds and, when it was grown in culture, its morphological and growth characteristics were similar to those of other endophytic Neotyphodium species. Endophyte-infected E. ovatus specimens from New Zealand and Australia gave strong positive responses when they were examined by tissue print immunoblotting with antiserum specific for Epichloe and Neotyphodium endophytes. Although the antibodies used in the immunoblotting analysis were raised against Neotyphodium coenophialum, they have strong cross-reactivities with all other Epichloe species sensu stricto and the Neotyphodium species that are asexual derivatives of Epichloe species (1, 39a). The antiserum used does not cross-react with other fungi expected to be present as contaminants or pathogens of grasses, including nonclavicipitaceous endophytes (1). Similarly, the endophyte ELISA showed that there was immunological recognition of endophyte-infected E. ovatus from New Zealand and Australia and did not recognize endophyte-free E. ovatus from New Zealand. The antibodies used in the ELISA were raised against N. lolii and cross-react strongly with several Neotyphodium endophytes (25a, 32). Thus, the serological test results also support the hypothesis that the endophyte of E. ovatus is related to the Epichloe–Neotyphodium group of grass endophytes.
Specimens of E. ovatus were analyzed for alkaloids commonly associated with infection of grasses by Neotyphodium endophytes (Table 2). Neither ergovaline nor peramine was detected by HPLC in specimens of E. ovatus. However, analogs of both of these compounds have been identified in the endophyte-infected grass Achnatherum inebrians in the absence of ergovaline and peramine (18a, 32, 42), so an analysis of E. ovatus in which we used ELISAs with broad cross-reactivities to analogs of ergovaline and peramine was undertaken. The results of these ELISAs were also negative, indicating that E. ovatus does not contain immunoreactive peramine analogs or ergot alkaloids.
No lolitrems were detected in HPLC analyses of endophyte-infected or endophyte-free E. ovatus. However, an ELISA revealed high levels of paxilline analogs in endophyte-infected E. ovatus from New Zealand, whereas no paxilline analogs were detected in either infected Australian E. ovatus or endophyte-free E. ovatus from New Zealand (Table 2). The antibodies used in this ELISA are sensitive to the indole–diterpenoid neurotoxin paxilline, as well as to many structurally related indole–diterpenoids (17, 31) of the type commonly associated with tremorgen biosynthesis by fungi (26, 33). Paxilline is a much less potent tremorgen than lolitrem B, the toxin thought to be principally responsible for causing RGS (when lolitrem B is administered intravenously to sheep, its time-weighted potency is ca. 400 times that of paxilline [20]). It is therefore unlikely that paxilline alone could be responsible for the staggers that characterize Echinopogon intoxication. It is, however, possible that large quantities of tremorgenic paxilline analogs with low cross-reactivities to the anti-paxilline antibody were present.
Paxilline and its analogs may be biosynthetic precursors of more potent tremorgens, such as the lolitrems, janthitrems, and penitrems (26, 28). Indeed, it has been proposed that ELISA analysis for paxilline production should be the first screening method used in the search for non-tremorgen-producing endophytes for use in ryegrass breeding programs (17, 34). Thus, the paxilline analogs in endophyte-infected E. ovatus could indicate that more potent, possibly novel, tremorgenic indole–diterpenoids are present. Similar conclusions were reached after ELISA and HPLC analyses of South African (Melica decumbens) (29) and South American (Poa huecu) (30) endophyte-infected grasses that cause staggers syndromes similar to RGS.
Gas chromatography analysis of foliage revealed high levels of N-formylloline in endophyte-infected E. ovatus from New Zealand but not in endophyte-free plants. Lolines are unique to grass-endophyte (Neotyphodium or Epichloe spp.) associations (5). Thus, the data from the chemical analysis of endophyte-infected E. ovatus, as well as the available mycological and serological data, indicate that the endophyte is a Neotyphodium sp.
Asymptomatic Neotyphodium-like grass endophytes are thought to be transmitted solely through the seed, and endophyte-free plants do not normally become infected by such endophytes (6, 46). However, infected grasses do not always transmit endophyte infection to all of their offspring (11, 39, 47), so our finding that a high proportion of E. ovatus plants are infected with a Neotyphodium endophyte suggests that infection by the endophyte confers advantages on E. ovatus. Among the advantages that similar endophytes confer on other host grasses are enhanced resistance to herbivory and drought and increased rates of growth and seed production (6, 46).
Listronotus bonariensis adults preferred E. ovatus to Lolium perenne as a food source, regardless of the endophyte status of the plants (Fig. 3). However, weevils preferred endophyte-free plants to endophyte-infected E. ovatus and Lolium perenne (both P ≤ 0.001) in choice feeding tests (Fig. 3). Thus, endophyte-infected E. ovatus appears to have a selective advantage over its endophyte-free counterparts due to reduced insect feeding. This effect may be due to the paxilline analogs and N-formylloline present in the infected grass, as N-formylloline is broadly toxic to insects (10) and adults and larvae of Listronotus bonariensis are very sensitive to paxilline (37). However, Listronotus bonariensis was first recorded in New Zealand in ca. 1927 (27) and is not indigenous to Australia, so although endophyte infection increases the resistance of E. ovatus to feeding by Listronotus bonariensis, other selection pressures must previously have been responsible for maintaining the endophyte infection in this grass in both Australia and New Zealand. It would, therefore, be interesting to determine whether endophyte infection of E. ovatus affects the indigenous herbivores to which the grass was exposed prior to European settlement of New Zealand. Further study of endophyte-infected Australian E. ovatus might also be instructive, as the ability of the endophyte to persist in this host does not appear to be associated with known endophyte alkaloids (Table 2).
Plant breeders have attempted to select endophytes that, when introduced into Lolium perenne, do not cause RGS but retain the beneficial effects of endophyte infection. Selection has been based principally on the ability of the endophyte to produce lolitrem B, peramine and, more recently, ergovaline in plants as determined by HPLC. However, of the four species of endophyte-infected grasses associated with staggers syndromes (E. ovatus, Lolium perenne, P. huecu, and M. decumbens), only Lolium perenne has been found to contain lolitrems. This finding and the data of Fletcher et al. (14) indicate that Neotyphodium endophytes (including N. lolii) are capable of producing potent tremorgens other than lolitrems and that it would not be advisable to use lolitrem B as the sole marker for the potential of a grass-endophyte combination to cause staggers.
Unfortunately, specimens of Echinopogon spp. from outbreaks of intoxication were not available for analysis. It is possible that the toxicity of the specimens of E. ovatus that we analyzed was low, that the alkaloid profiles of these specimens were different from those of plants from toxic Australian pastures, or that Echinopogon spp. other than E. ovatus were responsible for the reported incidents of Echinopogon intoxication. Nevertheless, the presence of a tremorgen-producing Neotyphodium endophyte provides a plausible explanation for the Echinopogon staggers reported in Australia. This hypothesis is based on the similarity of the symptoms of Echinopogon staggers (12) to those of RGS (9), on the fact that Echinopogon spp. from Australia and New Zealand are commonly infected with a Neotyphodium endophyte similar to the endophyte responsible for RGS, and on the fact that tremorgenic alkaloids (and their precursors) known to be associated with RGS are sometimes present in infected E. ovatus but not in uninfected E. ovatus. Further investigation is required to test this hypothesis.
ACKNOWLEDGMENTS
We thank B. D. Clarkson of Manaaki Whenua Landcare Research, C. E. Ecroyd of the New Zealand Forest Research Institute, C. M. Beard of the Waikato Herbarium, and P. D. Champion of the National Institute of Water and Atmospheric Research for assistance in obtaining specimens of E. ovatus and W. Hollin of the University of Kentucky for assistance with the immunoblot assay. We also thank the following members of the staff of the New Zealand Pastoral Agriculture Research Institute: J. M. Sprosen, K. M. Ross, E. Davies, and J. A. Armstrong, who assisted with the alkaloid analyses; J. G. Baltus, who helped with the insect feeding experiments; R. G. Keogh, who provided goat anti-N. lolii antibodies; and C. A. Cameron, who performed the statistical analyses.
This work was supported by USDA National Research Initiative grant 96-35303-3578 to H.H.W. and by grant DEB-9707427 from the National Science Foundation to C.L.S.
REFERENCES
- 1.An Z-Q, Siegel M R, Hollin W, Tsai H-F, Schmidt D, Schardl C L. Relationships among non-Acremonium sp. fungal endophytes in five grass species. Appl Environ Microbiol. 1993;59:1540–1548. doi: 10.1128/aem.59.5.1540-1548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker D J, Davies E, Lane G A, Latch G C M, Nott H M, Tapper B A. Effect of water deficit on alkaloid concentrations in perennial ryegrass endophyte associations. In: Hume D E, Latch G C M, Easton H S, editors. Proceedings of the Second International Symposium on Acremonium/Grass Interactions. Palmerston North, New Zealand: AgResearch; 1993. pp. 67–71. [Google Scholar]
- 3.Barker G M, Pottinger R P, Addison P J, Prestidge R A. Effect of Lolium endophyte fungal infections on behaviour of adult Argentine stem weevil. N Z J Agric Res. 1984;27:271–277. [Google Scholar]
- 4.Breen J P. Acremonium endophyte interactions with enhanced plant resistance to insects. Annu Rev Entomol. 1994;39:401–423. [Google Scholar]
- 5.Bush L P, Wilkinson H H, Schardl C L. Bioprotective alkaloids of grass-fungal symbioses. Plant Physiol. 1997;114:1–7. doi: 10.1104/pp.114.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clay K. Fungal endophytes of grasses: a defensive mutualism between plants and fungi. Ecology. 1988;69:10–16. [Google Scholar]
- 7.Cleland J B. Third report of the Bureau of Microbiology. Australia: New South Wales Government; 1912. Plants poisonous to stock in Australia; pp. 187–217. [Google Scholar]
- 8.Clement S L, Kaiser W J, Eichenseer H. Acremonium endophytes in germplasms of major grasses and their utilization for insect resistance. In: Bacon C W, White J F Jr, editors. Biotechnology of endophytic fungi of grasses. Ann Arbor, Mich: CRC Press; 1994. pp. 185–199. [Google Scholar]
- 9.Connor H E. The poisonous plants in New Zealand. Wellington, New Zealand: E. C. Keating, Government Printer; 1977. pp. 88–89. [Google Scholar]
- 10.Dahlman D L, Eichenseer H, Siegel M R. Chemical perspectives of endophyte-grass interactions and their implications to insect herbivory. In: Barbosa P, Krischick V A, Jones C G, editors. Microbial mediation of plant-herbivore interactions. New York, N.Y: John Wiley; 1991. pp. 227–252. [Google Scholar]
- 11.di Menna M E, Waller J E. Visual assessment of seasonal changes in amount of mycelium of Acremonium loliae in leaf sheaths of perennial ryegrass. N Z J Agric Res. 1986;29:111–116. [Google Scholar]
- 12.Everist S L. Poisonous plants of Australia. Sydney, Australia: Angus & Robertson Publishers; 1981. pp. 316–318. [Google Scholar]
- 13.Fletcher L R, Harvey I C. An association of a Lolium endophyte with ryegrass staggers. N Z Vet J. 1981;29:185–186. doi: 10.1080/00480169.1981.34839. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher L R, Garthwaite I, Towers N R. Ryegrass staggers in the absence of lolitrem B. In: Hume D E, Latch G C M, Easton H S, editors. Proceedings of the Second International Symposium on Acremonium/Grass Interactions. Palmerston North, New Zealand: AgResearch; 1993. pp. 119–121. [Google Scholar]
- 15.Gallagher R T, Hawkes A D, Steyn P S, Vleggaar R. Tremorgenic neurotoxins from perennial ryegrass causing ryegrass staggers disorder of livestock: structure elucidation of lolitrem B. J Chem Soc Chem Commun. 1984;1984:614–616. [Google Scholar]
- 16.Gallagher R T, Hawkes A D, Stewart J M. Rapid determination of the neurotoxin lolitrem B in perennial ryegrass by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1985;321:217–226. doi: 10.1016/s0021-9673(01)90438-8. [DOI] [PubMed] [Google Scholar]
- 17.Garthwaite I, Miles C O, Towers N R. Immunological detection of indole-diterpenoid tremorgenic mycotoxins. In: Hume D E, Latch G C M, Easton H S, editors. Proceedings of the Second International Symposium on Acremonium/Grass Interactions. Palmerston North, New Zealand: AgResearch; 1993. pp. 77–80. [Google Scholar]
- 18.Garthwaite I, Sprosen J, Briggs L, Collin R, Towers N. Food quality on the farm: immunological detection of mycotoxins in New Zealand pastoral farming. Food Agric Immunol. 1994;6:123–129. [Google Scholar]
- 18a.Garthwaite, I., C. O. Miles, G. J. Stevenson, and M. R. Prinsep. Unpublished data.
- 19.Gwinn K D, Collins-Shepard M H, Reddick B B. Tissue print-immunoblot, an accurate method for the detection of Acremonium coenophialum in tall fescue. Phytopathology. 1991;81:747–748. [Google Scholar]
- 20.Hawkes A D, Embling P P, Towers N R. Intravenous administration of tremorgens. In: Garthwaite L L, editor. Toxinology & food safety research report. Hamilton, New Zealand: AgResearch; 1995. pp. 8–9. [Google Scholar]
- 21.Henry M, Massey A E. Some neglected sheep diseases of New South Wales. Agric Gaz NSW. 1911;22:109–117. [Google Scholar]
- 22.Hubbard C E. Hooker’s Icones Plantarum vol. 33, tab. 3261. Kew Gardens, United Kingdom: Bentham Trustees; 1935. [Google Scholar]
- 23.Hurst E. The poison plants of New South Wales. Sydney, Australia: New South Wales Poison Plants Committee; 1942. pp. 15–17. [Google Scholar]
- 24.Jacobs S W L, Hastings S M. Echinopogon. In: Harden G J, editor. Flora of New South Wales. Vol. 4. Kensington, New South Wales, Australia: NSW University Press; 1990. pp. 584–586. [Google Scholar]
- 25.Kennedy C W, Bush L P. Effect of environment and management factors on the accumulation of N-acetyl and N-formyl loline alkaloids in tall fescue. Crop Sci. 1983;23:547–552. [Google Scholar]
- 25a.Keogh, R. G. Personal communication.
- 26.Mantle P G, Weedon C M. Biosynthesis and transformation of tremorgenic indole-diterpenoids by Penicillium paxilli and Acremonium lolii. Phytochemistry. 1994;36:1209–1217. [Google Scholar]
- 27.Marshall G A K. New Curculonidae collected from New Zealand. Trans N Z Inst. 1937;67:316–340. [Google Scholar]
- 28.Miles C O, Wilkins A L, Gallagher R T, Hawkes A D, Munday S C, Towers N R. Synthesis and tremorgenicity of paxitriols and lolitriol: possible biosynthetic precursors of lolitrem B. J Agric Food Chem. 1992;40:234–238. [Google Scholar]
- 29.Miles C O, di Menna M E, Kellerman T S, Garthwaite I, Ball O J-P. Melica decumbens. In: Garthwaite L L, editor. Toxinology & food safety research report. Hamilton, New Zealand: AgResearch; 1995. p. 20. [Google Scholar]
- 30.Miles C O, Uzal F, Garthwaite I, Munday-Finch S C, di Menna M E. Poa huecu and Festuca argentina. In: Garthwaite L L, editor. Toxinology & food safety research report. Hamilton, New Zealand: AgResearch; 1995. p. 20. [Google Scholar]
- 31.Miles C O, Wilkins A L, Garthwaite I, Ede R M, Munday-Finch S C. Immunochemical techniques in natural products chemistry: isolation and structure determination of a novel indole-diterpenoid aided by TLC-ELISAgram. J Org Chem. 1995;60:6067–6069. [Google Scholar]
- 32.Miles C O, Lane G A, di Menna M E, Garthwaite I, Piper E L, Ball O J-P, Latch G C M, Allen J M, Hunt M B, Min F K, Fletcher I, Harris P S. High levels of ergonovine and lysergic acid amide in toxic Achnatherum inebrians accompany infection by an Acremonium-like endophytic fungus. J Agric Food Chem. 1996;44:1285–1290. [Google Scholar]
- 33.Munday-Finch S C, Wilkins A L, Miles C O. Isolation of paspaline B, an indole-diterpenoid from Penicillium paxilli. Phytochemistry. 1996;41:327–332. [Google Scholar]
- 34.Penn J, Garthwaite I, Christensen M J, Johnson C M, Towers N R. The importance of paxilline in screening for potentially tremorgenic Acremonium isolates. In: Hume D E, Latch G C M, Easton H S, editors. Proceedings of the Second International Symposium on Acremonium/Grass Interactions. Palmerston North, New Zealand: AgResearch; 1993. pp. 88–92. [Google Scholar]
- 35.Popay A J, Rowan D D. Endophytic fungi as mediators of plant-insect interactions. In: Bernays E A, editor. Insect-plant interactions. V. Ann Arbor, Mich: CRC Press; 1994. pp. 83–103. [Google Scholar]
- 36.Prestidge R A, Pottinger R P, Barker G M. Proceedings of the 35th New Zealand Weed and Pest Control Conference. Palmerston North, New Zealand: New Zealand Weed and Pest Control Society Inc.; 1982. An association of Lolium endophyte with ryegrass resistance to Argentine stem weevil; pp. 119–122. [Google Scholar]
- 37.Prestidge R A, Ball O J-P. The role of endophytes in alleviating plant biotic stress in New Zealand. In: Hume D E, Latch G C M, Easton H S, editors. Proceedings of the Second International Symposium on Acremonium/Grass Interactions: plenary papers. Palmerston North, New Zealand: AgResearch; 1993. pp. 141–151. [Google Scholar]
- 38.Rowan D D, Latch G C M. Utilization of endophyte-infected perennial ryegrasses for increased insect resistance. In: Bacon C W, White J F Jr, editors. Biotechnology of endophytic fungi of grasses. Ann Arbor, Mich: CRC Press; 1994. pp. 169–183. [Google Scholar]
- 39.Sampson K. The presence and absence of an endophytic fungus in Lolium temulentum and L. perenne. Trans Br Mycol Soc. 1935;19:337–343. [Google Scholar]
- 39a.Schardl, C. L. Unpublished data.
- 40.Seddon H R, Carne H R. New South Wales Department of Agriculture Bulletin no. 26, Veterinary Research Report no. 2. Australia: New South Wales Department of Agriculture; 1926. Staggers in stock due to rough-bearded grass (Echinopogon ovatus) pp. 34–40. [Google Scholar]
- 41.Seddon H R, Carne H R. Staggers in stock due to rough-bearded grass (Echinopogon ovatus) Agric Gaz N S W. 1926;37:684–690. [Google Scholar]
- 42.Stevenson G J. Isolation of endophyte-produced metabolites using immunological detection methods. M.Sc. thesis. Hamilton, New Zealand: The University of Waikato; 1996. [Google Scholar]
- 43.Tapper B A, Rowan D D, Latch G C M. Detection and measurement of the alkaloid peramine in endophyte-infected grasses. J Chromatogr. 1989;463:133–138. [Google Scholar]
- 44.Turner F. Notes and exhibits. Proc Linn Soc N S W. 1913;38:107–108. [Google Scholar]
- 45.White J F., Jr Widespread distribution of endophytes in the Poaceae. Plant Dis. 1987;71:340–342. [Google Scholar]
- 46.White J F., Jr Endophyte-host associations in forage grasses. XI. A proposal concerning origin and evolution. Mycologia. 1988;80:442–446. [Google Scholar]
- 47.Wilson S M, Easton H S. International Neotyphodium/Grass Interactions Symposium, May 28–31, Athens, Ga. New York, N.Y: Plenum Press; 1998. Seed transmission of an exotic endophyte in tall fescue, abstr. 41. [Google Scholar]
- 48.Yates S G, Petroski R J, Powell R G. Analysis of loline alkaloids in endophyte-infected tall fescue by capillary gas chromatography. J Agric Food Chem. 1990;38:182–185. [Google Scholar]