Abstract
Standing litter of emergent macrophytes often forms a major portion of the detrital mass in wetland habitats. Microbial assemblages inhabiting this detritus must adapt physiologically to daily fluctuations in temperature and water availability. We examined the effects of various environmental conditions on the concentrations of osmoregulatory solutes (polyols and trehalose) and the respiratory activities of fungal assemblages inhabiting standing litter of the freshwater emergent macrophyte Juncus effusus. Under field conditions, the concentrations of osmolytes (polyols plus trehalose) in fungal decomposers were negatively correlated with plant litter water potentials (r = −0.75, P < 0.001) and rates of microbial respiration (r = −0.66, P < 0.001). The highest concentration of osmolytes (polyols plus trehalose) occurred in standing litter exposed to desiccating conditions (range from wet to dry, 0.06 to 0.68 μmol · mg of fungal biomass−1). Similar fluctuations in polyol and trehalose concentrations were observed in standing litter wetted and dried under laboratory conditions and for four predominant fungal decomposers of J. effusus grown individually on sterilized Juncus leaves. These studies suggest that fungal inhabitants associated with standing litter of emergent macrophytes can adjust their intracellular solute concentrations in response to daily fluctuations in water availability.
In many emergent macrophytes, leaf abscission is absent and collapse of shoot material to the sediment surface does not occur until long after senescence. Large amounts of standing dead plant matter can accumulate in wetland and salt marsh habitats (9), where it begins to decay in an upright aerial position without detachment from the parent rhizomes (3, 43, 44, 51). Diverse assemblages of microorganisms, principally fungi, are known to colonize standing litter in freshwater wetland and salt marsh habitats (2, 6, 31).
Previous studies have established that water availability is a major factor affecting the activity of microbial decomposers in standing litter of emergent macrophytes (19, 21, 33, 45). Recently, Kuehn and Suberkropp (33) reported that the rate of CO2 evolution by microbial assemblages inhabiting standing litter of Juncus effusus L. increases (>100 μg of CO2-C · g of organic matter−1 · h−1) after exposure to wetting conditions and continues to be high until the plant litter becomes dry. In the absence of precipitation, microbial respiratory activity exhibits a distinct diel response, with respiration rates increasing during the evening following dew condensation on plant litter. Exposure of standing litter to increasing temperatures during the day contributes to the desiccation of litter, which leads to decreases in microbial respiration. Thus, the metabolic activity of microbial assemblages inhabiting standing litter changes rapidly in response to daily changes in water availability.
Since microorganisms possess no active cross-membrane transport mechanism for water, they must raise the intracellular water potential relative to the external environment to meet the physiological demands for cellular maintenance and growth (46). Intracellular turgor pressure is an important factor that affects the rate of hyphal extension growth and provides the driving force for invasive fungal growth through organic debris (40, 41). The magnitude of hyphal turgor is controlled by a complex osmoregulatory system that controls the internal cytoplasmic osmotic potential (14, 39). Osmotic adjustment is achieved by the uptake or export of inorganic ions (K+, Na+) across the cell membrane and by the biosynthesis or degradation of organic compounds (5). Acyclic sugar alcohols (i.e., polyols) and trehalose have been shown to be important carbohydrate storage products in fungi (27, 34, 54), as well as the dominant osmolytes produced in response to increased water stress (4, 5, 23, 27, 39). These solutes are often referred to as compatible solutes, because their accumulation in the cytoplasm does not interfere with or inhibit the normal physiological functions of the cell (4, 5).
Most investigations assessing the osmoregulatory responses of fungi to increased water stress have been laboratory-based studies in which pure fungal isolates, primarily yeast isolates, were grown in liquid media containing various concentrations of solutes (e.g., NaCl, KCl, polyethylene glycol) (4, 5, 23). Glycerol is consistently reported to be the main cytoplasmic polyol produced in fungi under conditions of increased solute-associated water stress (4, 5, 23). In addition, trehalose has been documented to be important as a general stress-protective solute in fungi during periods of increased desiccation, high and low temperatures, and toxic chemical (metal) exposure (16, 17, 24, 42, 49, 56). In contrast, few studies have assessed the osmotic responses of fungi to changes in matric potential water stress within naturally decaying substrates. Several researchers have reported that the dominant polyol pool in fungal cultures can vary depending on the age, state of growth, and specific stress solute used (22, 58).
The present study was conducted to examine the effect of increased desiccation on the osmoregulatory response of fungal decomposers inhabiting standing litter of the freshwater emergent macrophyte J. effusus under field and laboratory conditions. Changes in concentrations of fungal osmolytes (polyols and trehalose) were assessed in response to changing water availability within decaying standing litter. Rates of CO2 evolution from microbial assemblages inhabiting plant litter were also monitored to determine the relationship between fungal osmotic adjustment and metabolic activity.
MATERIALS AND METHODS
Field studies.
Diel field studies were conducted in a small freshwater wetland located in the Talladega National Forest, Hale County, Alabama (32°54′30"N, 87°26′30"W) (32). Standing dead leaves of J. effusus L. (8 to 10 leaves for each sample; upper three-quarters of each leaf blade) were collected from the peripheries of three plant tussocks at time intervals ranging from 1 to 12 h. The leaf samples were cut into 10-cm lengths, and the rates of CO2 evolution from 10 leaf pieces were monitored (see below). Plant litter samples were also cut into 2-cm leaf pieces to measure plant litter water potentials and litter polyol and trehalose contents (see below). Leaf litter samples were collected from replicate plant tussocks at the completion of each diel study to determine their ergosterol contents as an indicator of fungal biomass (see below). Meteorological conditions in the field were continually monitored by using a model CM6 meteorological station and a model CR-10 data logger (Campbell Scientific Inc.).
Laboratory experiments.
The effects of moisture on fungal osmoregulatory responses and total microbial respiratory activities were examined under controlled laboratory conditions. Dry standing litter (>100 leaves) was collected from the peripheries of three J. effusus tussocks (see above), placed on ice, and returned to the laboratory. Subsamples of plant litter (10 leaves) from each of the three replicate plant tussocks were immediately cut into 10-cm pieces, and the rate of CO2 evolution from 10 leaf pieces was monitored. Leaves were also cut into 2-cm pieces to measure plant litter water potentials, fungal biomass, and polyol and trehalose contents. After initial rates of CO2 evolution were recorded, plant litter was placed into a ventilated Plexiglas chamber (0.7 by 0.5 by 1.4 m) and wetted with deionized water by using a hand-held spray bottle. The plant litter was kept saturated with water with a mist-generating vaporizer (Kaz Inc.) placed in the chamber. Subsamples of plant litter were removed 5 min after the initial wetting and then at intervals over a 48-h period to measure microbial respiration rates, plant litter water potentials, fungal biomass, and polyol and trehalose contents. After 48 h of incubation, plant material was removed from the chamber and allowed to air dry under laboratory conditions (20 ± 3°C) for 24 h. Subsamples were also collected during this drying period to measure respiration rates, plant litter water potentials, fungal biomass, and polyol and trehalose contents.
Pure-culture experiments.
The responses of four fungi, isolated from standing J. effusus litter, to desiccating conditions were examined in the laboratory. These were among the most frequently isolated or observed fungi on decomposing standing litter of J. effusus (32). The isolates used were two hyphomycetes (Drechslera sp. and Conioscypha lignicola Höhnel), one coelomycete (Phoma sp.), and one basidiomycete (Panellus copelandii (Pat.) Burdsall & Miller). Standing plant litter was cut into 2-cm pieces and sterilized by autoclaving. Individual leaf pieces were placed on the surface of a mineral agar medium in petri dishes (60 by 15 mm). The medium contained (per liter of water) 0.25 g of K2HPO4, 0.25 g of KNO3, 20 g of agar, and 1 ml of a trace element solution; the trace element solution contained (per liter of water) 57 mg of H3BO3, 4.2 g of ZnCl2, 0.25 g of CuSO4 · 5H2O, 0.10 g of FeCl3 · 6H2O, 72 mg of MnCl2 · 4H2O, 42 mg of NaMoO4, and 1 ml of concentrated H2SO4. The leaf piece preparations were then inoculated with individual isolates. The leaf pieces were inoculated with pieces of agar (ca. 5 by 5 mm) obtained from the growing hyphal apical regions of fungal cultures. The agar pieces were placed adjacent to, but not touching, the sterilized leaf pieces. The leaf pieces were incubated at 22°C in the dark for 3 weeks. Leaf pieces were then removed from individual petri dishes and combined within species, and equal numbers (60 leaf pieces) were randomly divided and placed into two petri dishes (100 by 15 mm) containing Whatman no. 7 filter paper. The leaf pieces and filter paper in one petri dish were saturated with sterile deionized water (ca. 5 ml), the excess water was removed, and the dish was covered. The leaf pieces and filter paper in the remaining petri dish were not wetted and were allowed to dry uncovered. The petri dishes were incubated at 22°C in the dark for 24 h. After 24 h, replicate leaf piece samples were randomly removed to determine polyol and trehalose contents, fungal biomass, plant litter water potentials, and the organic mass of the leaf pieces.
Respiration rates.
In laboratory and field studies, rates of carbon dioxide (CO2) evolution from litter were measured by enclosing leaf litter samples in a Licor model Li-600-11 0.25-liter sample chamber connected to a model Li-6250 infrared gas analyzer (Licor Inc.) (33). The linear rate of CO2 evolution from litter was monitored over a 10-min period. After rate measurements, field samples were stored on ice, returned to the laboratory, and dried at 60°C to a constant weight, and the organic contents were determined following combustion overnight at 550°C. Laboratory samples were dried and ashed immediately.
Water potentials.
Plant litter water potentials were monitored by using a model HR-33T dew point microvoltmeter (Wescor Inc.). Five leaf pieces (length, 2 cm) were placed in each of three replicate type C-30 sample chambers and incubated for 3 h before reading. Measurements were made by using the dew point hygrometric mode (57) and were recorded when repeated readings were stable and reproducible.
Fungal biomass.
Fungal biomass was determined by extracting and quantifying ergosterol from plant litter samples (20). Ten leaf pieces (length, 2 cm) from each litter sample collected were preserved in 5 ml of methanol (high-performance liquid chromatography [HPLC] grade) and stored at −20°C in the dark until they were extracted. Additional replicate leaf pieces (10 pieces from each sample) were dried at 60°C and combusted overnight at 550°C to determine the organic mass of the leaf material in preserved samples. The ergosterol in plant litter samples was extracted by refluxing in alcoholic KOH (25 ml of methanol plus 5 ml of 4% KOH in 95% methanol) for 30 min (53). The resultant extract was partitioned into n-pentane and evaporated to dryness under a stream of nitrogen gas at 30°C. Dried ergosterol extracts were redissolved in 2 ml of methanol (HPLC grade), filtered, and stored tightly capped at −20°C in the dark until they were analyzed. Separation and analysis of ergosterol were performed by using a Whatman partisphere C18 reverse-phase column (0.46 by 12.5 cm, with a 20-μl sample loop) connected to a HPLC system (model LC-10A5 HPLC equipped with a model SPD-10A UV-VIS detector; Shimadzu Scientific Inc.). The mobile phase used was methanol (HPLC grade) at a constant flow rate of 1 ml/min. Ergosterol was detected at 282 nm and exhibited a retention time of ca. 6.5 min. Ergosterol was identified and quantified based on a comparison with known ergosterol standards (Fluka Chemical Co.). Fungal biomass was determined by using the following conversion factor: 5 μg of ergosterol/mg of organic fungal mass (20).
Polyols and trehalose.
Polyols and trehalose were extracted from plant litter by using methods modified from those of Karsten et al. (28) and Richardson et al. (50). Twenty leaf pieces (length, 2 cm) from each sample were collected, placed into 10 ml of 70% ethanol, returned to the laboratory, and stored at −20°C until extracted. The leaf pieces were homogenized with a Polytron apparatus (Brinkman) at speed setting 5 for 15 s, and each sample was transferred to a 100-ml round-bottom flask along with an additional 10 ml of 70% ethanol. The homogenized plant tissue was extracted by refluxing for 2.5 h at 80°C. The resultant extract was cooled to room temperature and filtered (pore size, 0.7 μm; type GF/F; Whatman). An aliquot of the filtered extract was evaporated to dryness under a stream of nitrogen gas at 75°C. The polyols and trehalose in dried extracts were redissolved and converted to their oximes by adding 500 μl of hydroxylamine in pyridine (25 μg/ml) containing phenyl-β-d glucoside (200 μg/ml in pyridine) as an internal reference standard (8). The resultant mixture was heated for 30 min at 75°C with periodic vortexing. Sugars were derivatized by adding 500 μl of N,O-bis(trimethylsilyl)trifluoroacetamide–1% trimethylchlorosilane (Pierce Chemical Co.) and were heated for an additional 20 min at 75°C. A small amount (ca. 150 mg) of anhydrous sodium sulfate was added after derivatization reactions to absorb any water. Samples were stored tightly capped at −20°C in the dark until they were analyzed.
Separation and analysis of trimethylsilyl-derivatized polyols and trehalose were performed by injecting 1-μl samples into a type DB-1 fused-silica capillary column (0.25 mm by 15 m; film thickness, 2.5 μm; Alltech, Inc.) connected to a Hewlett-Packard model HP-5890 series II gas chromatograph equipped with a flame ionization detector. The chromatographic results were analyzed by using a model HP-3365 Chemstation (software version 3.5; Hewlett-Packard, Inc.). The injector and detector temperatures were maintained at 225 and 280°C, respectively. The oven temperature was initially kept at 125°C for 4 min and then was programmed to increase at three program levels. In level one, the oven temperature was increased from 125 to 166°C at a rate of 1°C min−1 and kept at 166°C for 1 min. In level two, the temperature was increased from 166 to 172°C at a rate of 0.5°C min−1 and kept at 172°C for 4 min. In level three, the temperature was increased from 172 to 300°C at a rate of 10°C min−1, kept at 300°C for 6 min, and then decreased to the initial temperature (125°C). Helium was used as the carrier gas at a flow rate of 1.9 ml/min. A split flow injection mode was used with a split flow rate of 100 ml/min. The air and hydrogen flow rates to the detector were 250 and 24 ml/min, respectively. Standard trimethylsilyl-derivatized polyols and trehalose (Sigma Chemical Co.) were used to determine retention times and to establish optimal chromatographic separations. Derivatized sugars in samples were identified and quantified based on a comparison with these known compounds.
Statistical analysis.
Statistical analysis of the data was performed by using SAS software (52). Data are presented below as means ± standard errors. Values were considered significant at P < 0.05.
RESULTS
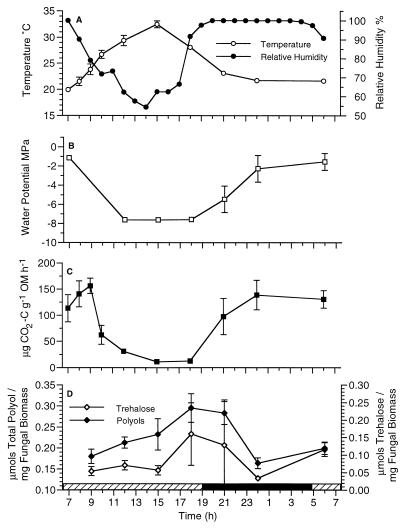
Increasing temperatures (Fig. 1A) during the day contributed to the desiccation of standing J. effusus litter, which led to decreases in plant litter water potentials (Fig. 1B). Both microbial respiratory activities (Fig. 1C) and the concentrations of fungal compatible solutes (polyols) (Fig. 1D) exhibited significant diel responses (P < 0.05, analysis of variance [ANOVA]). The rates of carbon dioxide evolution from plant litter decreased precipitously during these desiccation periods (Fig. 1C). In contrast, polyols and trehalose accumulated gradually during these dry periods (Fig. 1D) (polyol range, 0.139 to 341 μmol · mg of fungal biomass−1; trehalose range, 0.024 to 0.334 μmol · mg of fungal biomass−1). Mannitol was the predominant polyol identified (79% ± 1% of the total polyol concentration), followed by arabitol (12% ± 2%) and glycerol (7% ± 1%). At night, decreasing temperatures and increasing relative humidities (Fig. 1A) resulted in dew formation on standing litter, which led to increased plant litter water potentials (Fig. 1B) and decreased microbial water stress. The rates of CO2 evolution from plant litter subsequently increased (Fig. 1C), and the total polyol and trehalose contents decreased (Fig. 1D).
FIG. 1.
(A) Diel changes in air temperature and relative humidity above decomposing J. effusus during field studies conducted on 7 and 8 September 1994. (B) Diel changes in water potential of J. effusus plant litter. (C) Rates of CO2 evolution. (D) Total polyol and trehalose concentrations extracted from plant litter. The relative humidity data are from single measurements; all other data are means ± standard errors (n = 3). The solid horizontal bar in panel D indicates nighttime, and the cross-hatched horizontal bars indicate daytime. OM, organic matter.
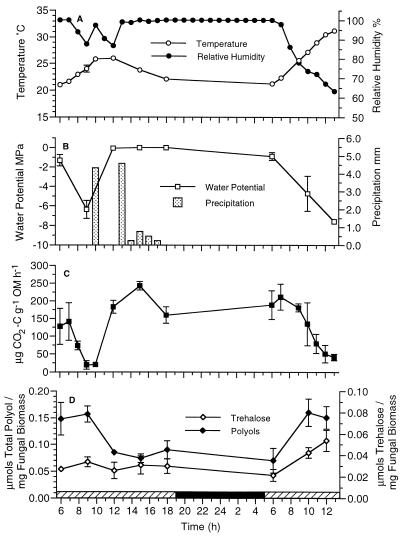
The results of field studies conducted in June 1994 illustrate the effect of rain and prolonged daytime water saturation of standing plant litter on the metabolic status of microbial inhabitants (Fig. 2). In the morning hours, before rainfall, the plant litter was exposed to increasing temperatures (Fig. 2A). These environmental conditions contributed to desiccation of the plant litter, which resulted in significant decreases in plant litter water potentials (Fig. 2B) and the respiration rates of the microbial inhabitants (Fig. 2C). However, following rainfall, both plant litter water potentials and rates of CO2 evolution from standing litter increased significantly (P < 0.0001, ANOVA) (Fig. 2B and C). High rates of CO2 evolution continued throughout the day. Significant decreases in polyol concentrations in litter were noted following precipitation (Fig. 2D) (P < 0.05, ANOVA), and the concentrations remained low until the litter was exposed to drying conditions the following morning (polyol concentration range, 0.031 to 0.21 μmol · mg of fungal biomass−1). Mannitol was the predominant polyol identified during these studies (72% ± 8% of the total polyol concentration), followed by glycerol (15% ± 7%) and arabitol (8% ± 1%). The trehalose concentrations in standing litter (Fig. 2D) remained low throughout the day and night until the litter was exposed to drying conditions the following morning (trehalose concentration range, 0.013 to 0.066 μmol · mg of fungal biomass−1). When combined data from both field studies were used, the concentrations of fungal osmotic solutes (polyols and trehalose) in standing litter were negatively correlated with the rates of carbon dioxide evolution and plant litter water potentials (Table 1).
FIG. 2.
(A) Diel changes in air temperature and relative humidity above decomposing J. effusus during field studies conducted on 14 to 16 June 1994. (B) Diel changes in precipitation and water potential of J. effusus plant litter. (C) Rates of CO2 evolution. (D) Total polyol and trehalose concentrations extracted from plant litter. The relative humidity data are from single measurements; all other data are means ± standard errors (n = 3). The values for precipitation (bars) indicate the amounts accumulated over 1-h periods. The solid horizontal bar in panel D indicates nighttime; and the cross-hatched horizontal bars indicate daytime. OM, organic matter.
TABLE 1.
Spearman correlation matrix showing the relationships among rates of CO2 evolution, plant litter water potentials, polyol and trehalose concentrations, and environmental conditions during field studies
| Parameter | Spearman correlation coefficienta
|
||||
|---|---|---|---|---|---|
| Temp | Relative humidity | Water potential | Trehalose concn | Polyol concn | |
| Carbon dioxide evolution | −0.52 (75)b | 0.44 (75)b | 0.86 (45)b | −0.40 (45) | −0.66 (45)b |
| Temp | −0.81 (75)b | −0.61 (45)b | 0.38 (45) | 0.42 (45) | |
| Relative humidity | 0.63 (45)b | −0.45 (45) | −0.43 (45) | ||
| Water potential | −0.53 (45)b | −0.75 (45)b | |||
| Trehalose concn | 0.62 (45)b | ||||
The values in parentheses are the numbers of samples examined.
P < 0.05. P values were adjusted for multiple comparisons (15) by using the Bonferroni adjustment method.
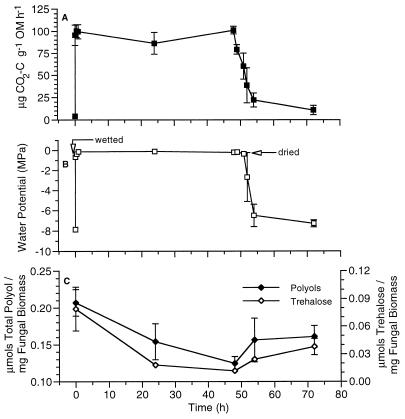
The results of experiments conducted under controlled laboratory conditions were similar to the results obtained during field studies. Microbial assemblages in field-collected samples responded rapidly when dry standing litter was wetted in the laboratory, with significant increases in the rates of CO2 evolution occurring within 5 min (from 4 to 96 μg of CO2-C · g of organic matter−1 · h−1) (P < 0.001, ANOVA) (Fig. 3A). Carbon dioxide evolution continued at high rates for up to 48 h after initial wetting with no significant fluctuations in the rates of CO2 release (P > 0.05, ANOVA, Tukey). When plant litter was exposed to drying conditions, the rates of CO2 evolution declined significantly (P < 0.05, ANOVA, Tukey). Plant litter water potentials (Fig. 3B) were positively correlated with microbial respiratory activities (r = 0.65 and P < 0.001, Spearman), rising from −7.9 to −0.7 MPa in 5 min after the litter was wetted and decreasing from −0.2 to −6.5 MPa after the litter was exposed to drying conditions. The concentrations of fungal osmolytes in standing litter also changed in response to wetting conditions (Fig. 3C). A significant decrease (P < 0.05, ANOVA) in trehalose content was observed after exposure of plant litter to water-saturating conditions. This decrease was followed by a slight but significant increase in trehalose concentration after exposure to drying conditions (P < 0.05, ANOVA, Tukey). The total polyol concentrations in standing plant litter followed a pattern similar to the trehalose pattern, but differences in polyol concentrations were not significant (P = 0.15, ANOVA). Mannitol was the predominant polyol identified from plant litter during these studies (47% ± 7% of the total polyol concentration), followed by glycerol (29% ± 5%) and arabitol (19% ± 3%). The combined accumulation patterns of both polyols and trehalose were negatively correlated with both rates of CO2 evolution and plant litter water potentials (r = −0.65 and −0.72, respectively; P < 0.01, Spearman). The fluctuations in ambient temperatures (17 to 23°C) were smaller than those observed during field studies. Fungal biomass ranged between 47 and 78 mg/g of organic mass (237 to 388 μg of ergosterol/g of organic mass), and no significant differences among samples were observed (P > 0.05, ANOVA, Tukey).
FIG. 3.
Changes in rates of CO2 evolution (A), plant litter water potentials (B), and total polyol and trehalose concentrations (C) in decomposing standing litter of J. effusus after wetting and drying under controlled conditions in the laboratory. The data are means ± standard errors (n = 3). OM, organic matter.
The four fungal isolates examined exhibited significant increases in the total concentration of osmolytes (polyols plus trehalose) when they were exposed to drying conditions in the laboratory at a constant temperature of 22°C (P < 0.05, Student’s t test) (Table 2). However, considerable variation in the level of polyols and trehalose was observed among isolates. Only two of the isolates (Drechslera sp. and P. copelandii) exhibited polyol concentrations within the range observed in laboratory and field samples. Phoma sp. and C. lignicola had polyol concentrations that were considerably lower. All of the isolates except P. copelandii had trehalose concentrations that were similar to those obtained for laboratory and field samples; however, the concentration of trehalose in P. copelandii was nearly four times the concentration of trehalose in the laboratory and field samples (Table 2).
TABLE 2.
Changes in polyol and trehalose concentrations in fungi colonizing J. effusus leaves when they were exposed to wetting and drying conditions in the laboratory (water potentials of the corresponding leaf pieces are also indicated)
| Fungus | Concn (μmol · mg [dry wt] of fungus−1)
|
Water potential (MPa)
|
||||
|---|---|---|---|---|---|---|
| Total polyols
|
Trehalose
|
Wetting conditions | Drying conditions | |||
| Wetting conditions | Drying conditions | Wetting conditions | Drying conditions | |||
| P. copelandii | 0.095 (0.004)a | 0.191 (0.034) | 0.473 (0.017) | 0.683 (0.063) | 0.00 (0.00) | −7.87 (0.23) |
| Drechslera sp. | 0.083 (0.002) | 0.150 (0.003) | 0.087 (0.010) | 0.108 (0.013) | −0.44 (0.44) | −7.51 (0.44) |
| C. lignicola | 0.031 (0.003) | 0.063 (0.002) | 0.104 (0.009) | 0.134 (0.014) | −0.20 (0.04) | −7.55 (0.31) |
| Phoma sp. | 0.048 (0.005) | 0.067 (0.007) | 0.010 (0.002) | 0.032 (0.004) | 0.00 (0.00) | −7.06 (0.13) |
The values are means ± standard errors (n = 3).
DISCUSSION
Results obtained in the present study provide evidence that fungi associated with standing J. effusus litter are physiologically adapted to the cyclic desiccation periods experienced in the standing dead phase. During periods of decreased water availability, the fungi adapted by accumulating intracellular organic solutes (polyols and trehalose). Fluctuations in the concentrations of polyols and trehalose, in response to litter drying and wetting conditions, were observed in litter under both field and laboratory conditions, indicating that fungal inhabitants can adjust their internal solute concentrations in response to changes in external water availability. Fungal respiratory activities in standing litter decreased concomitantly, as indicated by the significant negative correlation of total polyol and trehalose contents with rates of microbial respiration and plant litter water potentials (Table 1).
The total polyol and trehalose concentrations reported in the present study are within the range reported previously for other species of filamentous fungi (1, 18, 36–38, 58), even though the type of water stress experienced by microbiota in the present study differed markedly from the type of water stress in majority of the investigations cited above. During the present study, the increased water stress of microbial assemblages inhabiting J. effusus resulted from a decrease in the plant litter matric water potential. Previous studies have focused primarily on solute-induced water stress, and the water availability in liquid growth media was manipulated by varying the osmotic potential. In addition, in the prior studies the authors described polyol accumulation patterns in growing cultures of single species, which contained high concentrations of labile carbon (e.g., glucose) and other nutrients (N plus P). The values reported in the present study reflect the polyol and trehalose concentrations of a mixed assemblage of fungi inhabiting plant litter that is considerably more recalcitrant (i.e., lignocellulose) and nutrient poor (N, <1.0%; P, <0.05%) than most culture media (32).
In the present study, laboratory experiments revealed an increase in total polyol and trehalose concentrations in plant litter upon exposure to drying conditions. However, the changes in the concentrations of these solutes were not as pronounced as the changes observed under natural field conditions. In addition, there was considerable variation in the concentrations of polyols and trehalose in the isolates examined, suggesting that some fungal species may rely on either polyols, trehalose, or possibly other organic solutes as a means of osmoregulation. Amino acids can also play a role in fungal osmoregulation (15, 47); however, these specific solutes were not measured in the present study. Furthermore, it should be noted that the temperature fluctuations during laboratory studies were smaller (17 to 23°C) than those recorded during field studies (19 to 33°C). It is possible that increasing temperatures along with desiccation stress may have a synergistic effect on the accumulation of trehalose and polyols in fungi inhabiting standing litter, as has been demonstrated previously for several fungal species (24, 55, 56). Hottiger et al. (24) reported that large amounts of trehalose accumulated in cells of Saccharomyces cerevisiae in response to heat shock and that changes in trehalose concentrations were closely correlated with fluctuations in thermotolerance and desiccation tolerance. In the present study, higher concentrations of total polyols and trehalose in plant litter were observed during periods of increased temperatures (Fig. 1 and 2), suggesting that exposure of microbial inhabitants to higher temperatures may result in greater intracellular accumulation of these solutes.
Although there is clear evidence of the importance of glycerol in fungal osmoregulation, other polyols have also been implicated as important osmolytes (22, 30, 38, 58). Hocking (22) reported that glycerol contents declined significantly in five species of filamentous fungi as cultures aged and sporulation increased. Wethered et al. (58) found that growing cultures of Dendryphiella salina (G.K. Sutherland) Pugh & J. Nicot accumulated either glycerol, arabitol, or mannitol in response to increased solute concentrations in the growth media and that the predominance of any specific polyol was dependent on the specific stress solute used. Furthermore, Wethered et al. (58) also confirmed previous findings of Jennings (26), who found that in nongrowing mycelia of D. salina, only mannitol and arabitol were produced in response to increased salinity. The presence and predominance of mannitol in fungal mycelia that are not actively growing are noteworthy, since in the present study mannitol was the predominant polyol identified and was observed only during periods of low microbial respiratory activity. The low respiratory rates exhibited by fungal assemblages during these desiccation periods suggest that there is no active mycelial growth in litter. During these dry periods, fungal inhabitants apparently remain in a state of interrupted growth, in which metabolic activities proceed only at a level that maintains cellular integrity and viability. Since the accumulation of glycerol appears to be associated only with actively growing (metabolizing) mycelia under conditions of increased water stress, this may explain the predominance of mannitol compared with glycerol in this study.
The polyol and trehalose concentrations reported in the present study reflect the amounts of these solutes per unit of fungal dry mass. However, since the total cell water contents of fungi have been reported to decrease in response to solute-induced water stress, these concentrations may be underestimates (18, 48 [but see references 10 and 29]). If fungi inhabiting standing litter experience a similar dehydration pattern, then even a slight change in the amount of polyols or trehalose per unit of fungal biomass could be equivalent to a significant change in concentration on a molar basis.
In addition to increasing the cytoplasmic solute concentration, the presence of polyols, trehalose, and other sugars has been shown to increase the physical stability of cellular structures to the adverse effects of dehydration and thermal denaturation (11, 12). These osmolytes can interact and replace water around the polar groups of membrane phospholipids and proteins, which maintains integrity and increases the stability of membranes during thermal desiccation (11, 12). Additional studies have also documented the role of trehalose and polyols in stabilizing soluble cytoplasmic enzymes from both thermal and desiccation denaturation (7, 12, 13, 25, 35). Therefore, the production and accumulation of these compounds by fungal assemblages inhabiting standing litter may play a role in both osmotic solute adjustment and protection of cellular components during periods of exposure to increased desiccation and high temperature. The ability of fungi to synthesize and accumulate these solutes appears to represent a key adaptive strategy that facilitates the survival of these organisms and their predominance in the environmentally harsh standing-dead habitat.
ACKNOWLEDGMENTS
We thank R. G. Wetzel for the use of the LiCor instruments and G. M. Ward for meteorological station field data. In addition, we thank B. Pramanik for advice on gas chromatographic separation. We also express gratitude to R. G. Wetzel, M. O. Gessner, S. Y. Newell, and Colin Jackson for their comments on an earlier version of the manuscript.
This research was supported by grant OSR 9108761 from the National Science Foundation and by a Sigma Xi grant-in-aid of research to K.A.K.
REFERENCES
- 1.Adler L, Pedersen A, Tunblad-Johansson I. Polyol accumulation by two filamentous fungi grown at different concentrations of NaCl. Physiol Plant. 1982;56:139–142. [Google Scholar]
- 2.Apinis A E, Chesters C G C, Taligoola H K. Microfungi colonizing nodes and internodes of aerial standing dead culms of Phragmites communis Trin. Nova Hedwigia. 1975;26:495–507. [Google Scholar]
- 3.Bärlocher F, Biddiscombe N R. Geratology and decomposition of Typha latifolia and Lythrum salicaria in a freshwater marsh. Arch Hydrobiol. 1996;136:309–325. [Google Scholar]
- 4.Blomberg A, Adler L. Physiology of osmotolerance in fungi. Adv Microb Physiol. 1992;33:145–212. doi: 10.1016/s0065-2911(08)60217-9. [DOI] [PubMed] [Google Scholar]
- 5.Brown A D. Microbial water stress physiology: principles and perspectives. Chichester, United Kingdom: John Wiley and Sons; 1990. [Google Scholar]
- 6.Cantrell S A, Hanlin R T, Newell S Y. A new species of Lachnum on Spartina alterniflora. Mycotaxon. 1996;57:479–485. [Google Scholar]
- 7.Carpenter J F, Crowe J H. Modes of stabilization of protein by organic solutes during desiccation. Cryobiology. 1988;25:459–470. [Google Scholar]
- 8.Chapman G W, Horvat R J. Determination of nonvolatile acids and sugars from fruits and sweet potato extracts by capillary GLC and GLC/MS. J Agric Food Chem. 1989;37:947–950. [Google Scholar]
- 9.Christian R R, Bryant W L, Brinson M M. Juncus roemerianus production and decomposition along gradients of salinity and hydroperiod. Mar Ecol Prog Ser. 1990;68:137–145. [Google Scholar]
- 10.Clipson N J W, Hajibagheri M A, Jennings D H. Ion compartmentation in the marine fungus Dendryphiella salina in response to salinity: X-ray microanalysis. J Exp Bot. 1990;41:199–202. [Google Scholar]
- 11.Crowe J H, Crowe L M, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 12.Crowe J H, Crowe L M, Carpenter J F, Rudolph A S, Wistrom C A, Spargo B J, Anchordoguy T J. Interactions of sugars with membranes. Biochim Biophys Acta. 1988;947:367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.De Cordt S, Hendrickx M, Maesmans G, Tobback P. The influence of polyalcohols and carbohydrates on thermostability of alpha-amylase. Biotechnol Bioeng. 1994;43:107–114. doi: 10.1002/bit.260430202. [DOI] [PubMed] [Google Scholar]
- 14.Eamus D, Jennings D H. Water, turgor and osmotic potentials of fungi. In: Ayres P G, Boddy L, editors. Water, plants and fungi. Cambridge, United Kingdom: Cambridge University Press; 1986. pp. 27–48. [Google Scholar]
- 15.El-Abyad M S, Attaby H, Abu-Taleb A M. Impact of salinity stress on the free amino acid pools of some phytopathological fungi. Microbiol Res. 1994;149:309–315. [Google Scholar]
- 16.Eleutherio E C A, de Araujo P S, Panek A D. Role of the trehalose carrier in dehydration resistance of Saccharomyces cerevisiae. Biochim Biophys Acta. 1993;1156:263–266. doi: 10.1016/0304-4165(93)90040-f. [DOI] [PubMed] [Google Scholar]
- 17.Gadd G M, Chalmers K, Reed R H. The role of trehalose in dehydration resistance of Saccharomyces cerevisiae. FEMS Microbiol Lett. 1987;48:249–254. [Google Scholar]
- 18.Gadd G M, Chudek J A, Foster R, Reed R H. The osmotic responses of Penicillium ochro-chloron: changes in internal solute levels in response to copper and salt stress. J Gen Microbiol. 1984;130:1969–1975. [Google Scholar]
- 19.Gallagher J L, Kibby H V, Skirvin K W. Community respiration of decomposing plants in Oregon estuarine marshes. Estuar Coast Shelf Sci. 1984;18:421–431. [Google Scholar]
- 20.Gessner M O, Newell S Y. Bulk quantitative methods for the examination of eukaryotic organoosmotrophs in plant litter. In: Hurst C J, Knudsen G R, Melueruey M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C: American Society for Microbiology; 1997. pp. 295–308. [Google Scholar]
- 21.Halupa P J, Howes B L. Effects of tidally mediated litter moisture content on decomposition of Spartina alterniflora and S. patens. Mar Biol. 1995;123:379–391. [Google Scholar]
- 22.Hocking A D. Effects of water activity and culture age on the glycerol accumulation patterns of five fungi. J Gen Microbiol. 1986;132:269–275. [Google Scholar]
- 23.Hocking A D. Responses of xerophytic fungi to changes in water activity. In: Jennings D H, editor. Stress tolerance of fungi. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 233–256. [Google Scholar]
- 24.Hottiger T, Boller T, Wiemken A. Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in Saccharomyces cerevisiae cells subjected to temperature shifts. FEBS Lett. 1987;220:113–115. doi: 10.1016/0014-5793(87)80886-4. [DOI] [PubMed] [Google Scholar]
- 25.Hottiger T, De Virgilio C, Hall M N, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur J Biochem. 1994;219:187–193. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]
- 26.Jennings D H. Cations and filamentous fungi: invasion of the sea and hyphal functioning. In: Anderson W P, editor. Ion transport in plants. London, United Kingdom: Academic Press; 1973. pp. 323–335. [Google Scholar]
- 27.Jennings D H. The physiology of fungal nutrition. Cambridge, United Kingdom: Cambridge University Press; 1995. [Google Scholar]
- 28.Karsten U, Thomas D N, Weykam G, Daniel C, Kirst G O. A simple and rapid method for extraction and separation of low molecular weight carbohydrates from macroalgae using high-performance liquid chromatography. Plant Physiol Biochem. 1991;29:373–378. [Google Scholar]
- 29.Kelly D J A, Budd K. Osmotic adjustment in the mycelia ascomycete Neocosmospora vasinfecta (E.F. Smith) Exp Mycol. 1990;14:136–144. [Google Scholar]
- 30.Kelly D J A, Budd K. Polyol metabolism and osmotic adjustment in the mycelia ascomycete Neocosmospora vasinfecta (E.F. Smith) Exp Mycol. 1991;15:55–64. [Google Scholar]
- 31.Kohlmeyer J, Kohlmeyer E. Marine mycology, the higher fungi. New York, N.Y: Academic Press; 1979. [Google Scholar]
- 32.Kuehn K A. Standing dead decomposition of the freshwater emergent macrophyte Juncus effusus L. Ph.D. thesis. Tuscaloosa: University of Alabama; 1997. [Google Scholar]
- 33.Kuehn, K. A., and K. Suberkropp. Diel fluctuations in microbial activity associated with standing dead leaf litter of the emergent macrophyte Juncus effusus. Aquat. Microb. Ecol., in press.
- 34.Lewis D H, Smith D C. Sugar alcohols (polyols) in fungi and green plants. I. Distribution, physiology and metabolism. New Phytol. 1967;66:143–184. [Google Scholar]
- 35.Lozano P, Combes D, Iborra J L. Effect of polyols on alpha-chymotrypsin thermostability: a mechanistic analysis of the enzyme stabilization. J Biotechnol. 1994;35:9–18. doi: 10.1016/0168-1656(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 36.Luard E. Accumulation of intracellular solutes by two filamentous fungi. J Gen Microbiol. 1982;128:2563–2574. [Google Scholar]
- 37.Luard E. Effect of osmotic shock on some intracellular solutes in two filamentous fungi. J Gen Microbiol. 1982;128:2575–2581. [Google Scholar]
- 38.Luxo C, Nobre M F, Da Costa M S. Intracellular polyol accumulation by yeast like fungi of the genera Geotrichum and Endomyces in response to water stress (NaCl) Can J Microbiol. 1993;39:868–873. [Google Scholar]
- 39.Money N P. Osmotic adjustment and the role of turgor in mycelial fungi. In: Wessels J G H, Meinhardt F, editors. The Mycota. I. Growth, differentiation and sexuality. Berlin, Germany: Springer-Verlag; 1994. pp. 67–88. [Google Scholar]
- 40.Money, N. P. 1995. Turgor pressure and the mechanics of fungal penetration. Can. J. Bot. 73(Suppl. 1):S96–S102.
- 41.Money N P. Wishful thinking of turgor revisited: the mechanics of fungal growth. Fungal Genet Biol. 1997;21:173–187. [Google Scholar]
- 42.Neves M J, Jorge J A, Francois J M, Terenzi H F. Effects of heat shock on the level of trehalose and glycogen, and on the induction of thermotolerance in Neurospora crassa. FEBS Lett. 1991;283:19–22. doi: 10.1016/0014-5793(91)80544-d. [DOI] [PubMed] [Google Scholar]
- 43.Newell S Y. Decomposition of shoots of a saltmarsh grass: methodology and dynamics of microbial assemblages. Adv Microb Ecol. 1993;13:301–326. [Google Scholar]
- 44.Newell S Y. Established and potential impacts of eukaryotic mycelial decomposers in marine/terrestrial ecotones. J Exp Mar Biol Ecol. 1996;200:187–206. [Google Scholar]
- 45.Newell S Y, Fallon R D, Cal Rodriguez R M, Groene L C. Influence of rain, tidal wetting and relative humidity on release of carbon dioxide by standing-dead salt-marsh plants. Oecologia (Berlin) 1985;68:73–79. doi: 10.1007/BF00379477. [DOI] [PubMed] [Google Scholar]
- 46.Papendick R I, Mulla D J. Basic principles of cell and tissue water relations. In: Ayres P G, Boddy L, editors. Water, plants and fungi. Cambridge, United Kingdom: Cambridge University Press; 1986. pp. 1–26. [Google Scholar]
- 47.Ravishankar J P, Muruganandam V, Suryanarayanan T S. Effect of salinity on amino acid composition of the marine fungus Cirrenalia pygmea. Curr Sci (Bangalore) 1996;70:1086–1087. [Google Scholar]
- 48.Reed R H, Chudek J A, Foster R, Gadd G M. Osmotic significance of glycerol accumulation in exponentially growing yeast. Appl Environ Microbiol. 1987;53:2119–2123. doi: 10.1128/aem.53.9.2119-2123.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro M J S, Silva J T, Panek A D. Trehalose metabolism in Saccharomyces cerevisiae during heat shock. Biochim Biophys Acta. 1994;1200:139–147. doi: 10.1016/0304-4165(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 50.Richardson M D, Chapman G W, Hoveland C S, Bacon C W. Sugar alcohols in endophyte-infected tall fescue under drought. Crop Sci. 1991;32:1060–1061. [Google Scholar]
- 51.Samiaji J, Bärlocher F. Geratology and decomposition of Spartina alterniflora Loisel. in a New Brunswick saltmarsh. J Exp Mar Biol Ecol. 1996;201:233–252. [Google Scholar]
- 52.SAS Institute. SAS user’s guide: statistics. Cary, N.C: SAS Institute; 1989. [Google Scholar]
- 53.Suberkropp K, Weyers H. Application of fungal and bacterial production methodologies to decomposing leaves in streams. Appl Environ Microbiol. 1996;62:1610–1615. doi: 10.1128/aem.62.5.1610-1615.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thevelein J M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984;48:42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trollmo C, André L, Blomberg A, Adler L. Physiological overlap between osmotolerance and thermotolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1988;56:321–326. [Google Scholar]
- 56.Van Laere A. Trehalose, reserve and/or stress metabolite. FEMS Microbiol Rev. 1989;63:201–210. [Google Scholar]
- 57.Wescor Inc. Instruction/service manual: HR-33T dew point microvoltmeter. Logan, Utah: Wescor Inc.; 1986. [Google Scholar]
- 58.Wethered J M, Metcalf E C, Jennings D H. Carbohydrate metabolism in the fungus Dendryphiella salina. VIII. The contribution of polyols and ions to mycelial solute potential in relation to external osmoticum. New Phytol. 1985;101:631–649. [Google Scholar]