Abstract
This study aimed to identify fungal species associated with trunk diseases of sweet cherries (Prunus avium) in several commercial cherry orchards in Beijing, Guizhou and Shandong provinces, China. In total, eighteen fungal strains that fitted well into the species concept of Diaporthe were isolated. Based on both morphological and multi-locus phylogenetic analyses of internal transcribed spacer region (ITS), beta-tubulin (tub-2), calmodulin (Cal) and translation elongation factor 1-α (tef1–α) sequencing data, fourteen isolates were identified as Diaporthe eres, while four isolates were classified as D. hongkongensis. Here, we report D. hongkongensis causing sweet cherry branch dieback disease and, further, we confirmed the host association of D. eres with sweet cherries in China. A pathogenicity assay revealed the ability of both D. eres and D. hongkongensis to cause shoot necrosis and stem lesions on Prunus avium cv. ‘Brooks’ (mean lesion lengths of 1.86 cm and 1.56 cm, respectively). The optimal temperature for the growth of both Diaporthe species was tested. The optimal growth temperature for D. hongkongensis was 30 °C, and the 25–28 °C temperatures were the most favorable for the growth of D. eres strains. This research advances the understanding of fungal trunk diseases in fruit crops, particularly gummosis and branch dieback disease in Chinese cherry orchards, and will aid growers in making decisions about cultural practices and disease management.
Keywords: Diaporthaceae, trunk disease, Koch’s postulates, new record, phylogeny, Prunus, stone fruits
1. Introduction
Cherries belong to Prunus in the Rosaceae, and they are widely cultivated for their aromatic, bright-colored and tasty fruits [1,2]. Many Prunus species are grown worldwide, including black cherry (Prunus serotine), sour cherry/tart or pie cherry (Prunus cerasus), sweet cherry (Prunus avium) and West Indian cherry (Prunus myrtifolia) [2].
China is one of the largest cherry producers in the world. In 2018, a 163 kha planted area produced approximately 1.35 million tonnes of fruits in China [1,3,4,5]. Sweet cherries ripen earlier than other deciduous fruit trees that are planted in open fields in Northern China, and farmers can often obtain a high price for their production [3,4,5]. However, severe yield losses can occur due to many abiotic and biotic factors [1,6].
Plantation crops, especially pome fruits, stone fruits, nut crops, grapevines, citrus and olive, are threatened by pathogenic fungi [6,7,8,9,10]. Fungal trunk diseases are a significant problem in fruit crops because they can cause devastating tree damage, reduce fruit production and reduce the lifespan of the hosts [7,11]. These diseases primarily affect the trunk and branches of trees, frequently resulting in wood degradation, decreased vigor and, eventually, tree mortality [8,11]. Several fungal species, especially species of Botryosphaeriaceae, Diaporthaceae and Diatrypaceae, have been identified as fungal trunk disease pathogens [8,10,11]. Several Basidiomycota fungi have also been reported to cause fungal trunk diseases [11]. The majority of these fungi infect host wood, mostly through wounds and the subsequent colonization of vascular tissues. After entering through the wounds or natural openings, some of these fungi live as an endophyte and spread through asymptomatic plant materials. This process may increase concerns about the quarantine regulations of a particular country or region [11,12].
Abiotic stresses are also strongly involved, especially in fungal trunk diseases [11], and abiotic stresses can have a synergistic effect in fungal trunk diseases by compromising plant defenses and creating conditions favorable to fungal infection [11]. Understanding these interactions is also crucial for developing effective strategies to manage both abiotic stresses and fungal diseases in plants.
Cherry gummosis and branch dieback disease are two important diseases affecting sweet cherries worldwide [4,6], and due to the complexity of their etiologies, controlling these trunk diseases is difficult [11]. This study was carried out to identify and characterize the pathogens involved in cherry gummosis and branch dieback disease in Chinese cherry orchards.
2. Materials and Methods
2.1. Field Surveys and Isolation of Fungi
In the survey of fungal trunk disease pathogens in Chinese fruit crops conducted in April 2021, ten symptomatic diseased cherry branches with typical gummosis and branch dieback diseases were collected from three localities in China (Beijing, Guizhou and Shandong) (Figure 1). Samples were taken to the laboratory for further observations. Diseased branches were washed with water and air-dried. Tissue isolations were carried out as described in [13,14]. Tissue pieces (each about 5 mm2) were cut from the edge of the healthy and diseased areas that exhibited gummosis and dieback symptoms. Tissues were then surface sterilized using 1.5% NaOCl (sodium hypochlorite) for 1 min. Later, tissues were immersed in 75% ethyl alcohol for 1 min., and washed three times in sterilized distilled water. Then, the tissues were air-dried and placed on PDA plates (Potato dextrose agar). PDA plates were incubated at room temperature. Typical fungal colonies grown from plant tissue pieces were sub-cultured onto new PDA plates. Single hyphal tip isolation was carried out to obtain pure cultures, as illustrated in [13]. Newly sub-cultured PDA plates were observed under the microscope to reveal the septate hyphae and the hyphal tip was isolated using a sterilized needle and/or toothpick [15]. Purified cultures were saved at 4 °C.
Figure 1.
Field symptoms of sweet cherry branch dieback (A,B,D) and gummosis (E) and the cross-section of a diseased cherry branch (C).
All fungal isolates were deposited in the culture collection of the Beijing Academy of Agriculture and Forestry Sciences (JZB). Dry cultures were deposited as herbarium materials in the Herbarium at the Beijing Academy of Agriculture and Forestry Sciences (JZBH).
2.2. Molecular Identification of Fungi
2.2.1. Fungal DNA Sequencing
Total genomic DNA was extracted from 5–7-day-old pure cultures using the TIANcombi DNA Lyse and Det PCR Kit (Tiangen Biotech Co., Ltd., Beijing, China) following the manufacturer’s protocols. In total, four gene regions were sequenced and amplified (Table 1). The PCR mixture for all gene regions was as follows: 1 μL of genomic DNA, 45 μL of Golden Star T6 Super PCR mix (1.1×) (Tsingke Biotechnology Co., Ltd., Beijing, China), 2 μL of 10 μM of each forward and reverse primer up to a total volume of 50 μL. All gene regions with respective primer pairs and annealing temperatures are given in Table 1. The PCR was performed in a C1000 TouchTM thermal cycler (Bio-Rad Laboratories Inc., Hercules, CA, USA), and the results were observed on 1.5% agarose gel electrophoresis under ultraviolet light using GelDocXR+ (Bio-Rad Laboratories Inc., Hercules, CA, USA). All of the PCR products were sequenced by a commercial sequence provider (Sinogenomax Co., Ltd., Beijing, China).
Table 1.
Gene regions and primers used in this study.
| Gene Region | Primer Pairs | Sequence (5′—3′) | Reference |
|---|---|---|---|
| ITS | ITS1 ITS4 |
TCCGTAGGTGAACCTGCGG TCCTCCGCTTATTGATATGC |
[16] |
| Tef1-α | EF1-688F EF1-1251R EF1-728F EF1-986R |
CGGTCACTTGATCTACAAGTGC CCTCGAACTCACCAGTACCG CATCGAGAAGTTCGAGAAGG TACTTGAAGGAACCCTTACC |
[17,18] |
| tub-2 | Bt2a Bt2b TUB2Fd TUB4Rd |
GGTAACCAAATCGGTGCTGCTTTC ACCCTCAGTGTAGTGACCCTTGGC GTBCACCTYCARACCGGYCARTG CCRGAYTGRCCRAARACRAAGTTGTC |
[19,20] |
| Cal | CAL-228F CAL-737R |
GAGTTCAAGGAGGCCTTCTCCC CATCTTTCTGGCCATCATGG |
[18] |
2.2.2. Phylogenetic Analysis
Sequences generated in this study were checked for sequencing quality by checking chromatograms of sequences using BioEdit v.7.0.9.0. All sequences generated in this study are deposited in NCBI GenBank (Table 2).
Table 2.
Strains/isolates and GenBank numbers used in this study.
| Species | Culture No. | Origin | GenBank Number | |||
|---|---|---|---|---|---|---|
| ITS | Cal | tef1-α | tub-2 | |||
| D. acuta | PSCG 046 | China | MK626958 | MK691124 | MK654803 | MK691224 |
| D. acuta | PSCG 047 * | China | MK626957 | MK691125 | MK654802 | MK691225 |
| D. alleghaniensis | CBS 495.72 = ATCC 24097 * | Canada | KC343007 | KC343249 | KC343733 | KC343975 |
| D. apiculatum | CGMCC 3.17533 | China | KP267896 | - | KP267970 | KP293476 |
| D. arecae | CBS 161.64 * | India | KC343032 | KC343274 | KC343758 | KC344000 |
| D. arecae | CBS 535.75 | Suriname | KC343033 | KC343275 | KC343759 | KC344001 |
| D. australiana | BRIP 66145 * | Australia | MN708222 | - | MN696522 | MN696530 |
| D. australiana | BRIP 66147 | Australia | MN708224 | - | MN696523 | MN696532 |
| D. cercidis | CFCC 52565* | China | MH121500 | MH121424 | MH121542 | MH121582 |
| D. cercidis | CFCC 52566 | China | MH121501 | MH121425 | MH121543 | MH121583 |
| D. charlesworthii | BRIP 54884 m * | Australia | KJ197288 | - | KJ197250 | KJ197268 |
|
D. eres
D. eres |
AR5193 * CBS 101742 |
Germany Netherlands |
KJ210529 KC343073 |
KJ434999 KC343315 |
KJ210550 KC343799 |
KJ420799 KC344041 |
| D. eres (=D. biguttusis) | CGMCC 3.17081 * | China | KF576282 | - | KF576257 | KF576306 |
| D. eres (=D. castaneae-mollisimae) | DNP 128 * | China | JF957786 | JX197430 | JX275401 | JX275438 |
| D. eres (=D. castaneae-mollisimae) | DNP 129 | China | JQ619886 | JX197431 | JX275402 | JX275439 |
| D. eres (=D. cotoneastri) | DP0667 | - | KC843328 | KC843155 | KC843121 | KC843229 |
| D. eres (=D. ellipicola) | CGMCC 3.17084 * | - | KF576270 | - | KF576245 | KF576294 |
| D. eres (=D. nobilis) | CBS 200.39 | - | KC343151 | KC343393 | KC343877 | KC344119 |
| D. eres (=D. nobilis) | CBS 587.79 | - | KC343153 | KC343395 | KC343879 | KC344121 |
| D. eres | JZB320206 | Guizhou, China | OM980309 | OQ473424 | OQ513364 | ON152804 |
| D. eres | JZB320207 | Guizhou, China | OM980310 | OQ473425 | OQ513365 | ON152805 |
| D. eres | JZB320208 | Guizhou, China | OM980311 | OQ473426 | OQ513366 | ON152806 |
| D. eres | JZB320209 | Guizhou, China | OM980312 | OQ473427 | - | ON152807 |
| D. eres | JZB320210 | Guizhou, China | OM980313 | OQ473428 | - | ON152808 |
| D. eres | JZB320211 | Beijing, China | OM980314 | OQ473429 | OQ513367 | ON152809 |
| D. eres | JZB320212 | Beijing, China | OM980315 | OQ473430 | OQ513368 | ON152810 |
| D. eres | JZB320213 | Beijing, China | OM980316 | OQ473431 | OQ513369 | ON152811 |
| D. eres | JZB320214 | Beijing, China | OM980317 | OQ473432 | OQ513370 | ON152812 |
| D. eres | JZB320215 | Beijing, China | OM980318 | OQ473433 | OQ513371 | ON152813 |
| D. eres | JZB320216 | Beijing, China | OM980319 | OQ473434 | OQ513372 | ON152814 |
| D. eres | JZB320217 | Shandong, China | OM980320 | OQ473435 | OQ513373 | ON152815 |
| D. eres | JZB320218 | Shandong, China | OM980321 | OQ473436 | OQ513374 | ON152816 |
| D. eres | JZB320219 | Shandong, China | OM980322 | OQ473437 | OQ513375 | ON152817 |
| D. fusicola | CGMCC 3.17087 * | China | KF576281 | KF576233 | KF576256 | KF576305 |
| D. fusicola | CGMCC 3.17088 | China | KF576263 | KF576221 | KF576238 | KF576287 |
| D. hongkongensis | CBS 115448 * | China | KC343119 | KC343361 | KC343845 | KC344087 |
| D. hongkongensis | ZJUD74 | China | KJ490609 | - | KJ490488 | KJ490430 |
| D. hongkongensis (=D. lithocarpus) | CGMCC 3.15175 * | - | KC153104 | KF576235 | KC153095 | KF576311 |
| D. hongkongensis (=D. lithocarpus) | CGMCC 3.17098 | - | KF576276 | KF576228 | KF576251 | KF576300 |
| D. hongkongensis | JZB320202 | Guizhou, China | OM980305 | OQ473420 | OQ513360 | OL845879 |
| D. hongkongensis | JZB320203 | Guizhou, China | OM980306 | OQ473421 | OQ513361 | OL845880 |
| D. hongkongensis | JZB320204 | Guizhou, China | OM980307 | OQ473422 | OQ513362 | OL845881 |
| D. hongkongensis | JZB320205 | Guizhou, China | OM980308 | OQ473423 | OQ513363 | OL845882 |
| D. longispora | CBS 194.36 | - | MH855769 | KC343377 | KC343861 | KC344103 |
| D. padina | CFCC 52590 * | China | MH121525 | MH121443 | MH121567 | MH121604 |
| D. padina | PSCG 160 | - | MK626892 | MK691172 | MK654851 | MK691261 |
| D. penetriteum | CGMCC 3.17532 | China | KP267879 | - | KP267953 | KP293459 |
| D. sennicola | CFCC 51634 * | China | KY203722 | - | KY228883 | KY228889 |
| D. vaccinii | CBS 160.32 = IFO 32646 * | USA | KC343228 | KC343470 | KC343954 | KC344196 |
| D. viniferae | JZB320071 * | Guangxi, China | MK341551 | MK500119 | MK500107 | MK500112 |
| D. viniferae | JZB320072 | Guangxi, China | MK341552 | MK500120 | MK500108 | MK500113 |
| Diaporthella corylina | CBS 121124 * | China | KC343004 | KC343246 | KC343730 | KC343972 |
AR: Culture collection of Systematic Mycology and Microbiology Laboratory, USDA-ARS, Beltsville, Maryland, USA; BRIP: Queensland Plant Pathology’s herbarium/culture collection, Australia; CBS: Culture collection of the Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, Utrecht, The Netherlands; CFCC: China Forestry Culture Collection Center, China; CGMCC: China General Microbiological Culture Collection; JZB: Beijing Academy of Agriculture and Forestry Sciences’ culture collection. ZJUD: Zhejiang University, Hangzhou, China. Isolates obtained from this study are in bold. * = type strain.
The initial species were identified via the search against the sequences in the NCBI BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 4 April 2023), and relevant sequences were downloaded from NCBI following recent taxonomic treatments [21,22]. Sequences were aligned using MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server, accessed on 4 April 2023). Combined and single gene files were prepared and analyzed based on each genus/family, as given in previous studies. Phylogenetic analysis was inferred via maximum likelihood (ML) and Bayesian analysis (BI).
The evolutionary models for Bayesian and ML analyses were selected using jModelTest v. 2.0 [23,24]. Both ML and BI analyses were carried out using the CIPRES Science Gateway portal (www.phylo.org, accessed on 4 April 2023) [25]. Maximum likelihood (ML) analyses were conducted using RaxML-HPC2 on XSEDE v. 8.2.8 [26]. The GTR + I + G evolution model was used. Bayesian Inference (BI) analyses were performed using MrBayes v. 3.2.7 [27]. The Markov chain Monte Carlo sampling (BMCMC) analysis was conducted with six simultaneous Markov chains. The MCMC heated chain was set at a 05 “temperature” value and run for 1,000,000 generations. Trees were sampled at every 100th generation. From the 10,000 trees obtained, the first 2000 trees representing the burn-in phase were discarded. To calculate posterior probabilities, the remaining 8000 trees were used.
2.3. Morphological Characterization
Fungal colonies grown on PDA or PNA (pine needles + water agar) at 25 °C, and after seven days, they were used to examine the morphological characters. Colony morphology and conidial characteristics were examined. Images of morphological structures were taken using an Axio Imager Z2 photographic microscope (Carl Zeiss Microscopy, Oberkochen, Germany). Measurements were taken using ZEN PRO 2012 (Carl Zeiss Microscopy, Oberkochen, Germany). The conidial size was measured for each isolate, and the mean values were calculated.
2.4. Temperature Growth Studies
Two isolates from each species (Diaporthe eres: JZB320215, JZB320216 and D. hongkongensis: JZB320202, JZB320204) were tested to identify their optimal growth temperature conditions. One PDA plate with each isolate was incubated for 7 days at 25 °C. From these cultures, a 5-millimeter-wide plug was taken and placed in the center of the new PDA plates. Three replicate plates per isolate were incubated at 5, 10, 15, 20, 25, 28, 30 and 35 °C in the dark for three days.
2.5. Pathogenicity Study
One-year-old healthy shoots of sweet cherry cv. “Brooks”, about 30 cm long, were inoculated with two representative isolates (Diaporthe hongkongensis: JZB320204 and Diaporthe eres: JZB320215). In total, eighteen shoots per isolate were used. The shoots were initially washed with running tap water to remove the debris. Then, shoots were wiped with 75% ethanol and immersed in 1% NaOCl for 1 min and washed with sterilized distilled water for 1 min. Then, they were air-dried.
Wounds were made on each shoot using a cork borer (5 mm). Agar plugs with mycelium were placed on the wounds. Then, the wounds were sealed. Inoculated shoots were kept at 25 °C with a 12 h photoperiod. The shoots were covered to maintain a moist environment. Uncolonized PDA plugs were used as a negative control. Pots were arranged in a completely randomized design. The experiment was repeated once.
Five days after inoculation, the disease incidences were calculated (dividing the number of shoots showed the disease symptoms by the total number of shoots used to inoculated in each treatment), and the lesion lengths were measured. After the lesion measurements, shoots were subjected to surface sterilization and re-isolation. All fungal colonies resembling to Diaporthe were subjected to morpho-molecular identification to fulfill the Koch’s postulates. The mean lesion length (cm) was calculated, and ANOVA assumptions were verified. The analyses were performed using stats packages SPSS Statistics ver. 25 (IBM).
3. Results
During the survey of fungal trunk diseases causing pathogens on fruit crops in China, symptomatic sweet cherry branches were collected in several orchards in Beijing, Guizhou and Shandong provinces. As a result of mycological study of plant tissues, eighteen fungal isolates were isolated.
3.1. Molecular Identification and Phylogenetic Analysis
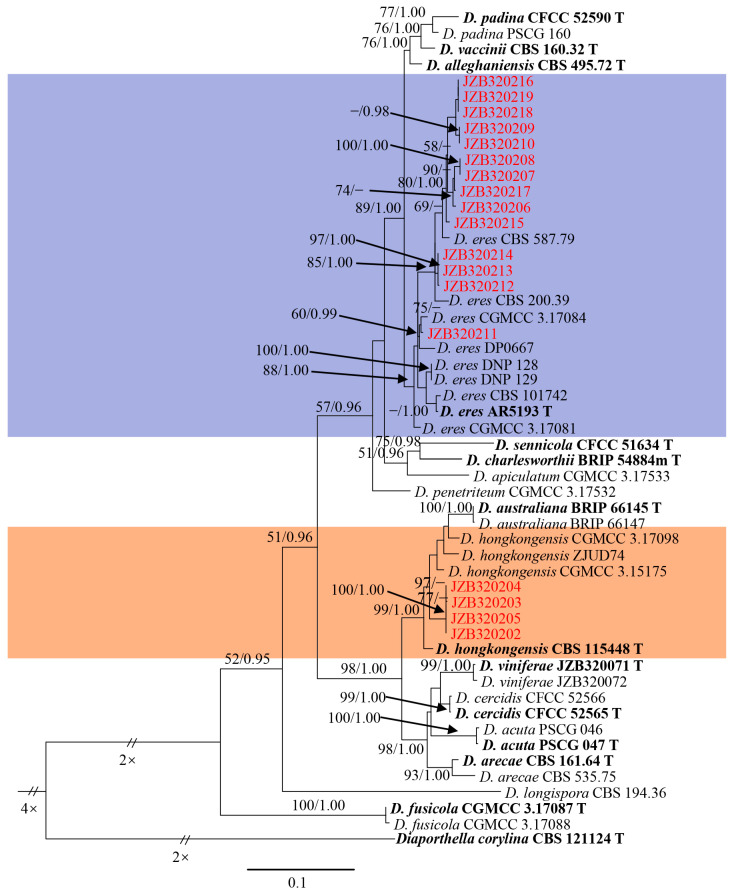
All fungal isolates were sequenced, and the combined ITS, tef1-α, Cal and tub-2 sequence data were analyzed based on Maximum Likelihood (ML) and Bayesian Inference (BI). Fourteen isolates were clustered with several reference strains of D. eres, including AR5193 (ex-type), and the ML bootstrap support value (BS) and BI posterior probabilities (PP) were 88 and 1.00, respectively (Figure 2). Four isolates were clustered with D. hongkongensis (CBS 115448, ex-type; ZJUD74; CGMCC 3.15175; CGMCC 3.17098) with a 99 ML-BS and 1.00 BI-PP value (Figure 2).
Figure 2.
Phylogenetic tree generated via Maximum Likelihood-based combined ITS, tef1-α, Cal and tub-2 sequence alignments of Diaporthe. Diaporthella corylina (CBS 121124) was used as an outgroup taxon. The separate sequence lengths were 510 bp (ITS), 378 bp (tef1-α), 386 bp (Cal) and 432 bp (tub-2). Maximum Likelihood (ML) bootstrap support value (BS ≥ 50) and Bayesian Inference (BI) posterior probabilities (PP ≥ 0.95) were shown on each node. The type strains were indicated in ‘T’ and bold. The isolates in this study were marked in red.
The combined gene analyses comprising 1716 characters with gaps and the best RAxML tree with a final likelihood value of -10633.567676 are presented. The matrix had 694 distinct alignment patterns, with undetermined characters being 20.59%. Estimated base frequencies were as follows: A = 0.220258, C = 0.317917, G = 0.232470 and T = 0.229355; substitution rates AC = 1.203612, AG = 2.754249, AT = 1.180540, CG = 0.839140, CT = 4.894412 and GT = 1.000000; gamma distribution shape parameter α = 0.652442.
3.2. Morphological Characterization
Based on the phylogenetic analysis, all fungal isolates were identified as Diaporthe. All isolates showed two different morphological characteristics. A total of fourteen isolates of Diaporthe eres and four isolates of D. hongkongensis were obtained (Figure 3 and Figure 4). The morphological characteristics of JZB320204 (D. hongkongensis) and JZB320215 (D. eres) were further observed, and the two isolates were comparatively similar in terms of their alpha conidia size, shapes and growth rates. While JZB320204 showed relatively larger alpha conidia (L/W ratio = 2.93) than JZB320215 (L/W ratio = 2.64), the reverse view of the colony was white in JZB320204 and pale black in JZB320215.
3.3. Taxonomy
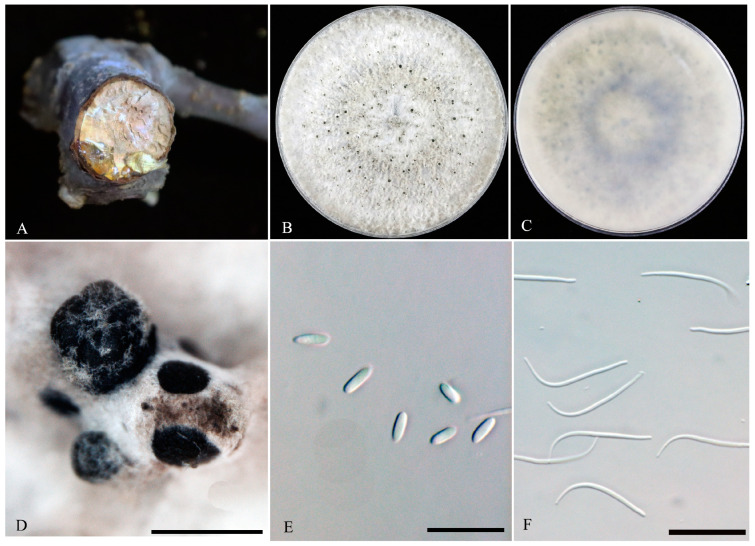
Diaporthe eres Nitschke (1870) (Figure 3).
Index Fungorum no: IF 172054.
Sexual morph: Not observed. Asexual morph: Conidiomata pycnidial, black, formed on PDA, superficial, solitary or aggregated. Conidiophores lining the inner cavity are hyaline and reduced to conidiogenous cells. Conidiogenous cells are hyaline and smooth. Alpha conidia hyaline, ellipsoid, fusiform, aseptate, 5–8.5 × 2–3 μm (mean ± SD = 6.9 ± 0.8 × 2.6 ± 0.3 μm, n = 40, L/W ratio = 2.64). Beta conidia hyaline, aseptate, filiform, 22–31 × 1–1.5 μm (mean ± SD = 27.7 ± 2 × 1.3 ± 0.1 μm, n = 40, L/W ratio = 21.19). Gamma conidia were not observed.
Culture characteristics: Colonies were flat, with sparse-to-moderate aerial mycelium, growing on a PDA plate reaches 60 mm after 2 days at 25 °C in 12 h light/dark, with a 27 mm/d growth rate. On PDA, pale grey to grey and reverse pale grey–smoke grey.
Material examined: China, Beijing, Tongzhou district, from diseased branches of Prunus avium with gummosis symptoms, 9 April 2021, P. Chen, dried culture: JZBH320215, living culture: JZB320215 = TZZG36-S1. Other isolates studied are listed in Table 2.
Notes: Diaporthe eres have been identified as a plant pathogen causing leaf spots and stem cankers in many economically important plant hosts [28,29]. This species was first identified from the twigs of Ulmus sp. in Germany and has a worldwide distribution in both temperate and tropical regions [28]. Diaporthe eres is listed as a pathogen with plant health inspection and quarantine significance [28,30]. We were able to isolate fourteen strains of D. eres (JZB320206–JZB320219), which clade within the Diaporthe eres species complex. All our D. eres isolates share similar morphology (black pycnidial conidomata, hyaline conidiophores and conidiogenous cells, ellipsoidal and aseptate alpha conidia) with the ex-epitype strain (AR5193), with minor dimensional differences [28,29]. These dimensional differences are probably due to variation in the host associations or the different culture media. The comparison of ITS, Cal, tef1-α and tub-2 regions of our D. eres isolates with ex-epitype strain (AR5193) reveals 81–89%, 73–76%, 88–92% and 87–93% base pair similarities, respectively.
Figure 3.
Diaporthe eres (JZB320215). (A) gummosis symptom of the sweet cherry branch. Surface (B) and reverse (C) colony on PDA at 25 °C after seven days. (D) Pycnidia on PDA, (E) alpha conidia and (F) beta conidia. Scale bars: (D) = 500 μm, (E,F) = 20 μm.
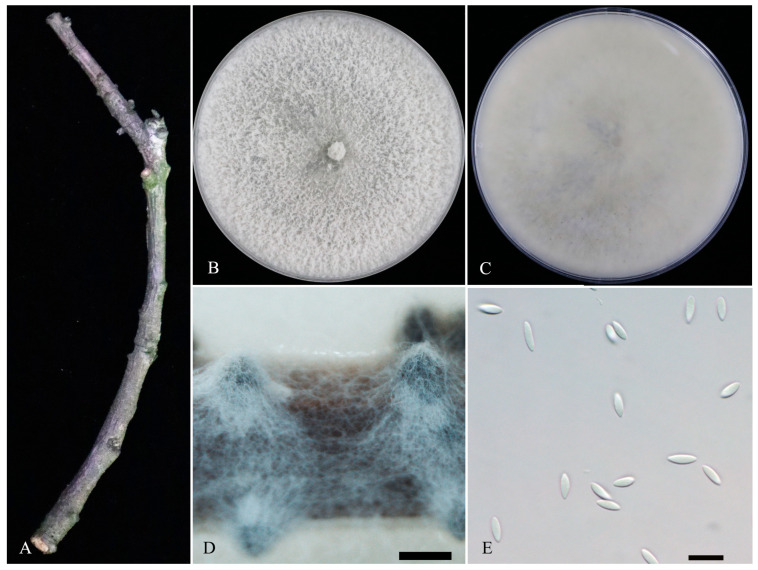
Diaporthe hongkongensis R.R. Gomes, Glienke and Crous (2013) (Figure 4).
Index Fungorum no: IF 802934.
Sexual morph: Not observed. Asexual morph: Conidiomata pycnidial, black, formed on PNA after 20 days, superficial, solitary or aggregated. Conidiophores lining the inner cavity are hyaline and reduced to conidiogenous cells. Conidiogenous cells are hyaline and smooth. Alpha conidia fusiform, aseptate, 6–8.5 × 2–2.9 μm (mean ± SD = 7 ± 0.6 × 2.5 ± 0.1 μm, n = 40, L/W ratio = 2.93). Beta and gamma conidia were not observed.
Culture characteristics: Colonies growing on PDA reached 57–60 mm after 2 days in 12 h light/dark, with a 27 mm/d growth rate; after 7 days, white and reverse grey–white to white.
Material examined: China, Guizhou Province, Guiyang city, from diseased branches of Prunus avium with dieback symptoms, 9 April 2021, P. Chen, dried culture: JZBH320204, living culture: JZB320204 = GZZG2-S3. Other isolates studied are listed in Table 2.
Note: Diaporthe hongkongensis was initially reported from Dichroa febrifuga from Hong Kong as Phomopsis pittospori [31]. Later, this species was identified from Camelia sinensis, Citrus sp. and Vitis vinifera [32]. This species was reported as causing stem-end rot on kiwifruit [33], shoot blight and leaf spot on kiwifruit [34] and fruit rot disease on peach [35]. Further, this species was reported as causing top blight in Cunninghamia lanceolata [36] and shoot canker in pear [37]. Diaporthe hongkongensis belongs to Diaporthe arecae species complex [21,22], and in this study, we have recovered four isolates from sweet cherries in China. All four isolates share similar morphologies (superficial pycnidial conidiomata and hyaline, fusiform alpha conidia) to that of the ex-type strain of D. hongkongensis (CBS 115448), with minor dimensional differences [22,31]. Comparison of ITS, Cal and tef1-α and tub-2 regions of our D. hongkongensis isolates with ex-type strain (CBS 115448) reveals 87–91%, 64%, 86–88% and 90–92% base pair similarities, respectively.
Figure 4.
Diaporthe hongkongensis (JZB320204). (A) dieback symptom of sweet cherry branch. Surface (B) and reverse (C) colony on PDA at 25 °C after seven days. (D) conidiomata on PNA. (E) alpha conidia. Scale bars: (D) = 500 μm, (E) = 10 μm.
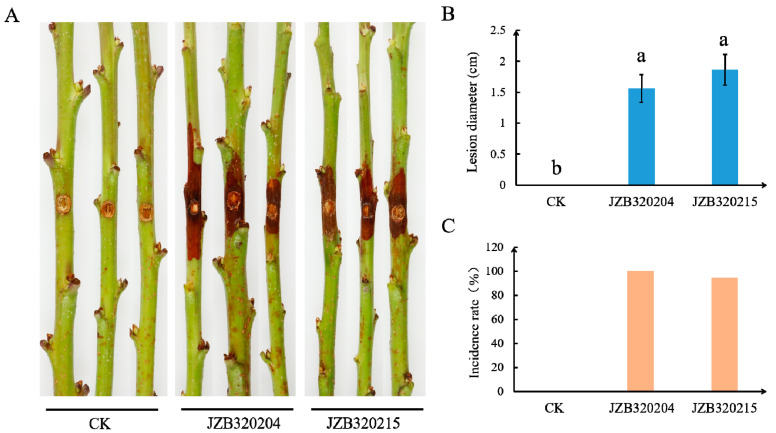
3.4. Pathogenicity Study
After five days of inoculation on wounded one-year-old detached shoots of sweet cherry (cv. ‘Brooks’), both species, namely Diaporthe hongkongensis (JZB320204) and D. eres (JZB320215), caused shoot browning and necrosis (Figure 5A). None of the control shoots showed any disease or symptoms (Figure 5A,C). Diaporthe hongkongensis (JZB320204) caused symptoms on all inoculated shoots (with disease incidence of 100%), and the isolate of D. eres (JZB320215) caused symptoms on 94.4% of the shoots. The average length of lesions was significantly larger than that of the control, reaching 1.56 cm and 1.86 cm, respectively (Figure 5B). Re-isolated fungi share similar morphological characteristics to primary inoculated isolates, and this observation was further confirmed via the sequencing of the ITS region.
Figure 5.
Pathogenicity of Diaporthe hongkongensis (JZB320204) and D. eres (JZB320215) isolates to Prunus avium cv. ‘Brooks’. (A) Control (CK) and the representative symptoms on one-year-old detached branches (5 dpi). (B) Mean lesion lengths on shoots inoculated in vitro after 5 d (bars indicates the standard errors (p < 0.05)). (C) Disease incidences of shoots inoculated in vitro.
3.5. Temperature Growth Studies
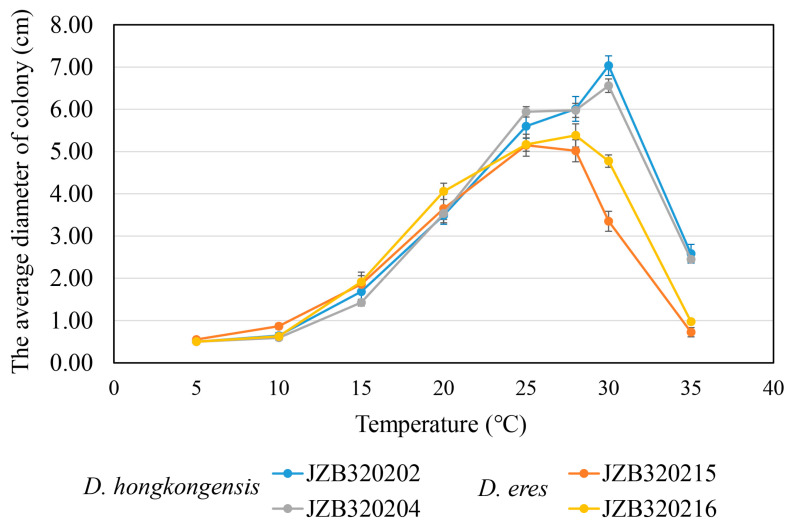
Two isolates derived from each species were used to test for the optimal growth temperature (Diaporthe eres: JZB320215, JZB320216 and D. hongkongensis: JZB320202, JZB320204), and the results are shown in Figure 6. The optimal growth temperature of D. hongkongensis was 30 °C, and the optimal growth temperatures of the two D. eres strains were 25 °C (JZB320215) and 28 °C (JZB320216), respectively. However, both Diaporthe hongkongensis and D. eres did not grow at 5 °C. During 5–20 °C temperatures, the colony size of both D. hongkongensis and D. eres is similar. At the temperature of 25–35 °C, the colony diameter of D. hongkongensis is significantly higher than that of D. eres, indicating that D. hongkongensis is more resistant to high temperatures than D. eres.
Figure 6.
The average diameter of D. hongkongensis and D. eres cultured under different temperatures (3 dpi). The bar indicates the standard deviation (SD) (n = 3).
4. Discussion
During our survey, the branch samples of Prunus avium that showed dieback and gummosis disease symptoms were collected from three locations in China. The surveyed orchards situated in Beijing and Shandong shared similar climatic conditions (temperate monsoon climate), while orchards in Guizhou belonged to the humid subtropical monsoon climate. The incidence of dieback disease in Beijing was 10%, while orchards in Shandong showed a 15–25% incidence rate. A total of eighteen fungal isolates were obtained, and all fungal isolates fitted well with the species concept of Diaporthe [21,22]. Phylogenetic analyses based on the four combined loci (ITS, tef1-α, Cal and tub-2) coupled with morphology revealed two Diaporthe species (viz. D. eres and D. hongkongensis) associated with sweet cherry die-back and gummosis disease in China. We do not observe the mixed fungal infection in the same branch sample. But we observe the two-disease occurring on the same orchard. In most cases, Diaporthe hongkongensis was isolated from dieback samples, and D. eres was isolated from both dieback and gummosis samples.
Diaporthe (Diaporthales, Pezizomycotina, Ascomycota) species are endophytic, saprophytic or parasitic, being widely distributed throughout the world and reported in different host plants [21,22,29,38,39]. Many pathogenic Diaporthe species cause diseases in many important crops, including grape (Vitis vinifera) [40], pear (Pyrus pyrifolia) [37], apple (Malus domestica) [41], blueberry (Vaccinium corymbosum) [42] and Toxicodendron vernicifluum [43], and cause symptoms such as dieback, canker, rot and leaf spots [44]. Diaporthe hongkongensis and D. eres have also been reported to cause diseases in woody plants, especially causing branch dieback and canker. Diaporthe hongkongensis identified on Actinidia deliciosa (Kiwifruit) [33], Castanopsis eyrei [45], Citrus sinensis [46], Cyclobalanopsis glauca [45], Dichroa febrifuga [31], Herba Patriniae [45], Llex Latifolia [45], Loropetalum chinensis [45], Miscanthus sinensis [45], Smilax china [45], Ternstroemia gymnanthera [45], Vitis vinifera [32] and D. eres reported a wide variety of host plants [21,22,29].
The Rosaceae family comprises a large number of plant species, and it extends from herbaceous and ornamental plants to fruit trees [47]. Most of the species in Rosaceae are regarded as economically important crops, such as strawberry, apple, cherry, almond, peach, plum, pear, blackberry and raspberry [47]. Even though D. eres is a well-known fungus, there are few studies of their pathogenicity on the above-mentioned hosts [47]. However, this species is identified as a pathogen and cause of canker and shoot blight in peaches, cane blight in blackberry, trunk canker and death in apple trees and wilting of shoots in Cotoneaster species [47]. In China, D. perniciosa (as Phomopsis perniciosa) has previously been reported to cause cherry stem canker disease in plants in Shandong Peninsula [48]. They have observed symptoms of canker and branch dieback, including cracks, black pycnidia and cirri containing α-conidia [48]. However, according to [21] and [22], based on their ITS phylogeny, D. perniciosa has been grouped with D. asheicola (CBS 136967) and other reference isolates of D. eres. Therefore, we also suspected that D. perniciosa is a species of D. eres, and through this study, we confirmed its host association and the pathogenicity of sweet cherries in China. Interestingly, in this study, we isolated D. eres from symptomatic branches of both dieback and gummosis disease. However, we were unable to observe the gummosis symptoms when we artificially inoculated one-year-old cherry shoots during the pathogenicity experiments. Several other pathogens have also been reported to cause gummosis disease in sweet cherries, including Botyosphaeria dothidea [49], Valsa mali [50], Pseudomonas syringae pv. syringae [51] and P. syringae pv. morsprunorum R1 (Race 1) and R2 (Race 2). Further, this study is the first study to report that D. hongkongensis is associated with sweet cherries and can cause branch dieback in cherries.
A study on the temperature range of fungal growth showed that the optimal temperature for D. hongkongensis is 30 °C, and for D. eres it is 25–28 °C. Proper cultural practices, such as maintaining optimal irrigation, managing proper spacing, nutrient management and providing protective measures during extreme weather events, can help to mitigate the impacts of abiotic stresses on plant health and reduce susceptibility to fungal trunk diseases [11]. According to [52], while almond production has been expanding in Spain, the incidence of fungal diseases is increasing and compromises crop productivity. According to the authors, the prevalence of shoot cankers and blight caused by Diaporthe species is higher in coastal areas with higher humidity and milder temperatures [51]. Even though the cherries are often planted in open fields where temperature control is challenging, understanding the temperature requirements of fungal pathogens will helps farmers to check for signs of disease or the development and progression of fungal diseases.
5. Conclusions
This study represents the investigation of 18 fungal isolates of Diaporthe species causing on gummosis and branch dieback diseases of sweet cherries in China. According to molecular and morphological data fourteen isolates were identified as Diaporthe eres and other four as D. hongkongensis. Our result demonstrated that both D. eres and D. hongkongensis are pathogenic to sweet cherry shoots, but the optimal growth temperatures for these fungi differ. Further studies are necessary to understand the factors that determine the pathogenicity of these Diaporthe strains towards fruit trees, especially stone fruits. An extensive collection of disease samples of sweet cherries with expanded investigations throughout China is also recommended.
Acknowledgments
The authors would like to thank the commercial cherry growers in Beijing, Guizhou and Shandong provinces for their support during the survey and disease specimen collection stage. Tongzhou Experimental Station for Cherries, Beijing Academy of Forestry and Pomology Sciences, Beijing, China, is thanked for providing healthy one-year-old cherry shoots for the pathogenicity experiment. Pranami D. Abeywickrama thanks the Beijing Academy of Agriculture and Forestry Sciences (BAAFS), Beijing, China, for her postdoctoral fellowship (Postdoctoral number: 349000).
Author Contributions
Conceptualization, P.C., P.D.A. and W.Z.; methodology, P.C., P.D.A., S.J., Y.Z., X.L. and W.Z.; formal analysis, P.C., P.D.A., S.J. and Y.Z.; investigation, P.C., P.D.A., S.J., X.L. and W.Z.; writing—original draft preparation, P.C. and P.D.A.; writing—review and editing, P.C., P.D.A., J.Y. and W.Z.; supervision, J.Y. and W.Z.; project administration, J.Y. and W.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequence data obtained in this study are openly available in NCBI GenBank and the accession numbers are stated in the article.
Conflicts of Interest
The authors declare no conflict of interest, and the funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
Funding Statement
This research was funded by the Beijing Academy of Agriculture and Forestry Sciences, grant number ‘KJCX20210403’, and the Beijing Innovation Team of the Modern Agricultural Research System, grant number ‘BAIC08-2023’.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chethana K.W.T., Jayawardene R.S., Zhang W., Zhou Y.Y., Liu M., Hyde K.D., Li X.H., Wang J., Zhang K.C., Yan J.Y. Molecular characterization and pathogenicity of fungal taxa associated with cherry leaf spot disease. Mycosphere. 2019;10:490–530. doi: 10.5943/mycosphere/10/1/8. [DOI] [Google Scholar]
- 2.Kumar A., Sharma M.K., Wani T.F., Sharma A., Nyorak G. Prunus-Recent Advances. IntechOpen; Rijeka, Croatia: 2021. Varietal Wealth of Prunus Species. [Google Scholar]
- 3.Zhang Q.J., Gu D.J., Yu K.H., Zhou Z.H. A model system for off season cherry production in northern China. Acta Hortic. 2018;1208:221–226. doi: 10.17660/ActaHortic.2018.1208.29. [DOI] [Google Scholar]
- 4.Zhang K., Yan G., Zhang X., Wang J., Duan X. Sweet cherry growing in China. Acta Hortic. 2019;1235:133–140. doi: 10.17660/ActaHortic.2019.1235.17. [DOI] [Google Scholar]
- 5.Chen H., Zhang K.C., Zhou J.Z., Zhang Y.Y., Ding C.X., Zhang W. Global cherry production status and suggestions for cherry industry development of Beijing. Deciduous Fruits. 2020;52:27–30. [Google Scholar]
- 6.Uyemoto J.K., Ogawa J.M., Jaffee B.A. Common Names of Plant Diseases: Diseases of Sweet Cherry (Prunus avium L.) and Sour Cherry (P. cerasus L.) The American Phyto-Pathological Society; St. Paul, MN, USA: 2018. [Google Scholar]
- 7.Cooke T., Persley D., House S. Diseases of Fruit Crops in Australia. Csiro Publishing; Collingwood, Australia: 2009. [Google Scholar]
- 8.Gramaje D., Agustí-Brisach C., Pérez-Sierra A., Morale-jo E., Olmo D., Mostert L., Damm U., Armengol J. Fungal trunk pathogens associated with wood decay of almond trees on Mallorca (Spain) Pers. Mol. Phylogeny Evol. 2012;28:1–13. doi: 10.3767/003158512X626155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espargham N., Mohammadi H., Gramaje D. Survey of trunk disease pathogens within citrus trees in Iran. Plants. 2020;9:754. doi: 10.3390/plants9060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohrabi M., Mohammadi H., León M., Armengol J., Banihashemi Z. Fungal pathogens associated with branch and trunk cankers of nut crops in Iran. Eur. J. Plant Pathol. 2020;157:327–351. doi: 10.1007/s10658-020-01996-w. [DOI] [Google Scholar]
- 11.Guarnaccia V., Kraus C., Markakis E., Alves A., Armengol J., Eichmeier A., Compant S., Gramaje D. Fungal trunk diseases of fruit trees in Europe: Pathogens, spread and future directions. Phytopathol. Mediterr. 2022;61:563–599. doi: 10.36253/phyto-14167. [DOI] [Google Scholar]
- 12.Slippers B., Wingfield M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007;21:90–106. doi: 10.1016/j.fbr.2007.06.002. [DOI] [Google Scholar]
- 13.Senanayake I.C., Rathnayaka A.R., Marasinghe D.S., Calabon M.S., Gentekaki E., Lee H.B., Hurdeal V.G., Pem D., Dissanayake L.S., Wijesinghe S.N., et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere. 2020;11:2678–2754. doi: 10.5943/mycosphere/11/1/20. [DOI] [Google Scholar]
- 14.Abeywickrama P.D., Zhang W., Li X., Jayawardena R.S., Hyde K.D., Yan J. Campylocarpon fasciculare (Nectriaceae, Sordariomycetes); Novel Emergence of Black-Foot Causing Pathogen on Young Grapevines in China. Pathogens. 2021;10:1555. doi: 10.3390/pathogens10121555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thesiya M.R., Rakholiya K.B., Lokesh R. Isolation, Cultural and Morphological Characterization of Phomopsis vexans (Sacc. and Syd.) Harter, causing Stem Blight and Fruit Rot of Brinjal. Int. J. Curr. Microbiol. App. Sci. 2020;9:2851–2859. doi: 10.20546/ijcmas.2020.907.337. [DOI] [Google Scholar]
- 16.White T.J., Bruns T., Lee S., Taylor J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols. Volume 38. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 17.Alves A., Crous P.W., Correia A., Phillips A.J.L. Morphological and molecular data reveal cryptic species in Lasiodiplodia theobromae. Fungal Diver. 2008;28:1–13. [Google Scholar]
- 18.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;93:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 19.Glass N.L.U.O., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woudenberg J.H.C., Aveskamp M.M., de Gruyter J., Spiers A.G., Crous P.W. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia. 2009;22:56–62. doi: 10.3767/003158509X427808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norphanphoun C., Gentekaki E., Hongsanan S., Jayawardena R., Senanayake I.C., Manawasinghe I.S., Abeywickrama P.D., Bhunjun C.S., Hyde K.D. Diaporthe: Formalizing the species-group concept. Mycosphere. 2022;13:752–819. doi: 10.5943/mycosphere/13/1/9. [DOI] [Google Scholar]
- 22.Hongsanan S., Norphanphoun C., Senanayake I.C., Jayawardena R.S., Manawasinghe I.S., Abeywickrama P.D., Khuna S., Suwannarach N., Senwanna C., Monkai J., et al. Annotated notes on Diaporthe species. Mycosphere. 2023;14:918–1189. [Google Scholar]
- 23.Guindon S., Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 24.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller M.A., Pfeiffer W., Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; Proceedings of the Gateway Computing Environments Workshop (GCE); New Orleans, LA, USA. 14 November 2010; pp. 1–8. [Google Scholar]
- 26.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. Mrbayes 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Udayanga D., Castlebury L.A., Rossman A.Y., Chukeatirote E., Hyde K.D. Insights into the genus Diaporthe: Phylogenetic species delimitation in the D. eres species complex. Fungal Divers. 2014;67:203–229. doi: 10.1007/s13225-014-0297-2. [DOI] [Google Scholar]
- 29.Abeywickrama P.D., Camporesi E., Jayawardena R.S., Hyde K.D., Yan J., Zhang W., Li X. Novel and surprising host associations of Diaporthe (Diaporthaceae, Diaporthales) species from Italy. Chiang Mai J. Sci. 2022;49:223–247. doi: 10.12982/CMJS.2022.028. [DOI] [Google Scholar]
- 30.Cline E.T., Farr D.F. Synopsis of fungi listed as regulated plant pests by the USDA Animal and Plant Health Inspection Service: Notes on nomenclature, disease, plant hosts, and geographic distribution. [(accessed on 15 May 2023)];Plant Health Prog. 2006 7:1. doi: 10.1094/PHP-2006-0505-01-DG. Available online: http://www.plantmanagementnetwork.org/php/ [DOI] [Google Scholar]
- 31.Gomes R., Glienke C., Videira S., Lombard L., Groenewald J.Z., Crous P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Pers. Mol. Phylogeny Evol. 2013;31:1–41. doi: 10.3767/003158513X666844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dissanayake A.J., Phillips A.J.L., Hyde K.D., Yan J.Y., Li X.H. The current status of species in Diaporthe. Mycosphere. 2017;8:1106–1156. doi: 10.5943/mycosphere/8/5/5. [DOI] [Google Scholar]
- 33.Erper I., Turkkan M., Ozean M., Luongo L., Belisario A. Characterization of Diaporthe hongkongensis species causing stem-end rot on kiwifruit in turkey. J. Plant Pathol. 2017;99:779–782. [Google Scholar]
- 34.Du Y., Wang X., Guo Y., Xiao F., Peng Y., Hong N., Wang G. Biological and molecular characterization of seven Diaporthe species associated with kiwifruit shoot blight and leaf spot in China. Phytopathol. Mediterr. 2021;60:177–198. doi: 10.36253/phyto-12013. [DOI] [Google Scholar]
- 35.Zhang Z., Zhang Z.B., Huang Y.T., Wang F.X., Hu W.H., Dai L.Y., Zhong J., Liu Y., Zhu J.Z. First report of Diaporthe hongkongensis causing fruit rot on peach (Prunus persica) in China. Plant Dis. 2021;105:2017. doi: 10.1094/PDIS-07-20-1505-PDN. [DOI] [Google Scholar]
- 36.Liao Y.C.Z., Sun J.W., Li D.W., Nong M.L., Zhu L.H. First report of top blight of Cunninghamia lanceolata caused by Diaporthe unshiuensis and Diaporthe hongkongensis in China. Plant Dis. 2023;107:962. doi: 10.1094/PDIS-06-22-1467-PDN. [DOI] [Google Scholar]
- 37.Guo Y.S., Crous P.W., Bai Q., Fu M., Yang M.M., Wang X.H., Du Y.M., Hong N., Xu W.X., Wang G.P. High diversity of Diaporthe species associated with pear shoot canker in China. Pers. Mol. Phylogeny Evol. 2020;45:132–162. doi: 10.3767/persoonia.2020.45.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udayanga D., Liu X., Crous P.W., McKenzie E.H.C., Chukeatirote E., Hyde K.D. A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis) Fungal Divers. 2012;56:157–171. doi: 10.1007/s13225-012-0190-9. [DOI] [Google Scholar]
- 39.Abeywickrama P.D., Wanasinghe D.N., Karunarathna S.C., Jayawardena R.S., Hyde K.D., Zhang W., Li X., Yan J. A new host report of Diaporthe manihotia (Diaporthales, Ascomycota) from Camellia sp. in Yunnan province, China. Asian J. Mycol. 2020;3:473–489. doi: 10.5943/ajom/3/1/17. [DOI] [Google Scholar]
- 40.Manawasinghe I.S., Dissanayake A.J., Li X., Liu M., Wanasinghe D.N., Xu J., Zhao W., Zhang W., Zhou Y., Hyde K.D. High Genetic Diversity and Species Complexity of Diaporthe Associated with Grapevine Dieback in China. Front. Microbiol. 2019;10:1936. doi: 10.3389/fmicb.2019.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Havenga M., Gatsi G.M., Halleen F., Spies C.F.J., van der Merwe R., Mostert L. Canker and Wood Rot Pathogens Present in Young Apple Trees and Propagation Material in the Western Cape of South Africa. Plant Dis. 2019;103:3129–3141. doi: 10.1094/PDIS-04-19-0867-RE. [DOI] [PubMed] [Google Scholar]
- 42.Hilario S., Amaral I.A., Goncalves M., Lopes A., Santos L., Alves A. Diaporthe species associated with twig blight and dieback of Vaccinium corymbosum in Portugal, with description of four new species. Mycologia. 2020;112:293–308. doi: 10.1080/00275514.2019.1698926. [DOI] [PubMed] [Google Scholar]
- 43.Ando Y. Diaporthe toxicodendri sp. nov., a causal fungus of the canker disease on Toxicodendron vernicifluum in Japan. Mycosphere. 2017;8:1157–1167. doi: 10.5943/mycosphere/8/5/6. [DOI] [Google Scholar]
- 44.van Rensburg J.C.J., Lamprecht S.C., Groenewald J.Z., Castlebury L.A., Crous P.W. Characterisation of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Stud. Mycol. 2006;55:65–74. doi: 10.3114/sim.55.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao Y., Liu F., Cai L. Unravelling Diaporthe species associated with Camellia. Syst. Biodivers. 2016;14:102–117. doi: 10.1080/14772000.2015.1101027. [DOI] [Google Scholar]
- 46.Huang F., Udayanga D., Wang X., Hou X., Mei X., Fu Y., Hyde K.D., Li H. Endophytic Diaporthe associated with Citrus: A phylogenetic reassessment with seven new species from China. Fungal Biol. 2015;119:331–347. doi: 10.1016/j.funbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Santos L., Phillips A.J.L., Crous P., Alves A. Diaporthe species on Rosaceae with descriptions of D. pyracanthae sp. nov. and D. malorum sp. nov. Mycosphere. 2017;8:485–511. doi: 10.5943/mycosphere/8/5/1. [DOI] [Google Scholar]
- 48.Wang C.X., Li B.H., Dong X.L., Li G.F. First Report of Stem Canker on Cherry Caused by Phomopsis perniciosa in Shandong Peninsula, Eastern China. Plant Dis. 2011;95:1316. doi: 10.1094/PDIS-04-11-0341. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L.Z., Zhang Q., Yang P., Niu Y., Niu W. First Report of Gummosis Disease of Sweet Cherry Caused by Botryosphaeria dothidea in China. Plant Dis. 2019;103:3283. doi: 10.1094/PDIS-07-19-1418-PDN. [DOI] [Google Scholar]
- 50.Dong X.L., Li B.H., Chen L.M., Zhang F.X. Valsa mali, an important fungus pathogen causes cherry gummosis; Proceedings of the 10th International Congress of Plant Pathology; Beijing, China. 25–30 August 2013; Beijing, China: Chinese Society of Plant Pathology; 2013. p16.024. [Google Scholar]
- 51.Xu L., Wang J.W., Chen X., Wei H.R., Zong X.J., Liu Q.Z. Identification and pathogenicity detection of the cherry gummosis pathogen. Acta Phytopathol. Sin. 2015;45:350–355. [Google Scholar]
- 52.León M., Berbegal M., Rodríguez-Reina J.M., Elena G., Abad-Campos P., Ramón-Albalat A., Olmo D., Vicent A., Luque J., Miarnau X., et al. Identification and Characterization of Diaporthe spp. Associated with Twig Cankers and Shoot Blight of Almonds in Spain. Agronomy. 2020;10:1062. doi: 10.3390/agronomy10081062. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequence data obtained in this study are openly available in NCBI GenBank and the accession numbers are stated in the article.