Abstract
Minerals play a dynamic role in plant growth and development. However, most of these mineral nutrients are unavailable to plants due to their presence in fixed forms, which causes significant losses in crop production. An effective strategy to overcome this challenge is using mineral solubilizing bacteria, which can convert insoluble forms of minerals into soluble ones that plants can quickly assimilate, thus enhancing their availability in nutrient-depleted soils. The main objective of the present study was to isolate and characterize mineral solubilizing rhizobacteria and to assess their plant growth-promoting potential for Rhodes grass. Twenty-five rhizobacterial strains were isolated on a nutrient agar medium. They were characterized for solubilization of insoluble minerals (phosphate, potassium, zinc, and manganese), indole acetic acid production, enzymatic activities, and various morphological traits. The selected strains were also evaluated for their potential to promote the growth of Rhodes grass seedlings. Among tested strains, eight strains demonstrated strong qualitative and quantitative solubilization of insoluble phosphate. Strain MS2 reported the highest phosphate solubilization index, phosphate solubilization efficiency, available phosphorus concentration, and reduction in medium pH. Among tested strains, 75% were positive for zinc and manganese solubilization, and 37.5% were positive for potassium solubilization. Strain MS2 demonstrated the highest quantitative manganese solubilization, while strains MS7 and SM4 reported the highest solubilization of zinc and potassium through acidifying their respective media. The strain SM4 demonstrated the most increased IAA production in the presence and absence of L-tryptophan. The majority of strains were positive for various enzymes, including urease, catalase protease, and amylase activities. However, these strains were negative for coagulase activity except strains SM7 and MS7. Based on 16S rRNA gene sequencing, six strains, namely, SM2, SM4, SM5, MS1, MS2, and MS4, were identified as Bacillus cereus, while strains SM7 and MS7 were identified as Staphylococcus saprophyticus and Staphylococcus haemolyticus. These strains significantly improved growth attributes of Rhodes grass, such as root length, shoot length, and root and shoot fresh and dry biomasses compared to the uninoculated control group. The present study highlights the significance of mineral solubilizing and enzyme-producing rhizobacterial strains as potential bioinoculants to enhance Rhodes grass growth under mineral-deficient conditions sustainably.
Keywords: Bacillus, Chloris gayana, enzymatic activities, mineral solubilization, Staphylococcus, partial gene sequencing
1. Introduction
Rhodes grass (Chloris gayana Kunth), native to Africa [1,2], was named after the well-known Cecil Rhodes, who popularized its use. Its role is prominent in forage crops and is known for its versatility, adaptability to diverse environments and soil types, and remarkable drought resistance [3,4]. Furthermore, Rhodes grass boasts a high nutritional value, with a protein content ranging from 9% to 12%, rendering it a valuable feed source for livestock, particularly in tropical and subtropical regions [4,5]. Moreover, Rhodes grass is helpful for the rotation of grasslands and effective for soil conservation due to its extensive root system and ability to thrive in degraded soils. The hay form of Rhodes grass is much more prevalent around the globe, notably in Gulf countries such as UAE, Qatar, and Saudi Arabia [5]. In Pakistan, fodder production is a crucial energy source to feed the livestock during the lean time. According to previous research, large-scale cultivation of Rhodes grass started in 2008. Over 100,000 acres of Balochistan, Sindh, and Punjab are dedicated to cultivating Rhodes grass for fodder production [6,7]. Improved forage grasses are one of the primary feed sources for grazing animals. Therefore, to address the feed shortage and increase livestock production, it is crucial to enhance the production of high-quality forages with greater biomass [7].
Chemical fertilizers are utilized to sustain soil fertility, but the soil cannot hold the applied amount of fertilizer. The excessive and long-term use of synthetic fertilizers has undesirable impacts on soil, environment, plants, and human health [8]. A significant proportion of the applied fertilizer is transformed into insoluble forms and fixed in the soil [9]. There is a need to improve nutrient use efficiency in the crops in an economically reliable and ecologically safer manner [10]. Applying effective microbial bioinoculants as plant growth-promoting (PGP) agents is an alternative and promising biotechnological strategy against synthetic chemicals [11]. The bacterial bioinoculants consist of beneficial plant growth-promoting rhizobacteria (PGPR) that reside in the rhizosphere in association with roots to improve plant growth and development [12]. Microbial bioinoculants are safer, cost-effective, and eco-friendly than agrochemicals and can improve soil structure and fertility [13].
Mineral solubilizing bacteria (MSB) have the potential to solubilize insoluble mineral forms such as phosphate (P), potassium (K), and zinc (Zn) that can be absorbed by plants [14,15]. Among MSB, P-solubilizing bacteria were reported to solubilize various inorganic forms of insoluble minerals, including tricalcium phosphate, fluoroapatite, francolites, hydroxyapatite, augellite, barrandite, crandallite, strengite, variscite, wavellite [16]. K-solubilizing bacteria were involved in solubilizing insoluble minerals, including micas, muscovite, feldspar, biotite, illite, and orthoclase [17]. The insoluble Zn minerals, including zinc oxide, zincite, zinc silicates, willemite, sphalerite, smithsonite, and zinc sulfide, were reported to be solubilized by Zn solubilizing bacteria [18]. These MSBs adopted various mechanisms in nature to give plants nutrients, such as mineralization, solubilization, and mobilization [19]. Among these mechanisms, solubilization of minerals converts insoluble form into soluble form by producing organic acid secretion, chelation, acidification, exchange reaction, and secretion of protons [20,21]. Most effective MSBs include bacterial genera such as Azospirillum, Acinetobacter, Azotobacter, Arthobacter, Burkholderia, Bacillus, Erwinia, Enterobacter, Pseudomonas, Rhizobium, Rhodococcus, Thiobacillus, Klebsiella, Frankia, and Serratia [22,23,24,25,26,27].
Thus, present study aimed (i) to isolate potential plant growth-promoting rhizobacterial strains from the rhizosphere soil of Rhodes grass, (ii) to screen them for in vitro solubilization of insoluble minerals, (iii) in vitro screening of MSB strains for PGP attributes (iv) to identify the promising minerals solubilizing PGPR through 16S rRNA gene sequencing, and (v) to evaluate the impact of mineral solubilizing bacterial strains inoculation on Rhodes grass growth parameters and nutrients uptake under presence of insoluble mineral compounds.
2. Materials and Methods
2.1. Isolation of Rhizobacteria
Rhizosphere soil samples of Rhodes grass were collected from two different sites: site-I Rahim Yar Khan, Punjab, Pakistan (located at Latitude 28.49 N, Longitude 70.25 E, and elevation 83.0 m); and site-II Ghotki, Sindh, Pakistan (located at Latitude 27.96 N, Longitude 69.63 E, and elevation 69.04 m). The collected samples were separately placed in sterile polythene bags and transferred to the Laboratory of Microbial Biotechnology, Institute of Molecular Biology and Biotechnology (IMBB), The University of Lahore (UOL), Lahore, Pakistan for microbial isolation and stored at 4 °C. Bacterial isolation was carried out using the serial dilution method, with dilutions performed up to 10−7. The diluted samples were spread on Luria–Bertani (LB) media plates using a glass spreader and incubated at 30 ± 1 °C for 72 h [28]. The resulting bacterial growth was observed visually, and selected colonies were repeatedly sub-cultured on LB media plates until the purified bacteria cultures were obtained. The pure single colonies were preserved at –20 °C in 50% glycerol stock for further experiments [29].
2.2. Screening of Rhizobacterial Strains for Phosphate Solubilization
Isolated bacterial strains were screened for their ability to solubilize phosphate Pikovskaya (PVK) agar media [30]. Bacterial cultures were spot inoculated on PVK media in triplicate and incubated at 30 ± 1 °C for 7 days. After incubation, plates were observed for halo zones around bacterial colonies. Using a measuring scale, the bacterial colony and solubilization halo zone diameters were measured in millimeters. P solubilization index (PSI) and P solubilization efficiency (PSE) were calculated by using the formula described by Ahmad et al. [31]. Bacterial strains were inoculated in PVK broth to quantify available P concentration and incubated at 30 ± 1 °C for 7 days. Bacterial cells were harvested by filtering through Whatman filter paper 42, and the solubilized P concentration was determined by the colorimetric method through a spectrophotometer (Agilent Technologies, Santa Clara, CA, USA).
2.3. Potassium Solubilization Assay
For K solubilization, Aleksandrov agar media was spot inoculated by bacterial strains in triplicate [32]. After incubation for seven days at 30 ± 1 °C, the plates were flooded with iodine solution to observe the clear halo zones. K solubilization index (KSI) and K solubilizing efficiency (KSE) were determined by measuring the solubilization halo zone and bacterial colony diameter by using the formula described by Setiawati and Mutmainnah [33]. The solubilized concentration by bacterial strains was determined using a supernatant of Aleksandrov broth after one week and subjected to a flame photometer. The available K concentration was determined by plotting the standard curve.
2.4. Zinc Solubilization Assay
The Zn solubilizing ability of rhizobacterial strains was evaluated using Bunt and Rovira agar media [34]. The Bunt and Rovira agar medium amended with zinc oxide (1% of Zn) was spot inoculated with bacterial strains and incubated for seven days at 30 ± 1 °C. The appearance of a solubilization halo zone was considered positive for Zn solubilization. Zn solubilization efficiency (ZSE) and Zn solubilization index (ZSI) were calculated by measuring the diameter of bacterial growth and solubilization halo zones of bacterial isolates by using the formula as reported by Ahmad et al. [31]. Similarly, bacterial strains were inoculated in Bunt and Rovira broth amended with zinc oxide (1% of Zn). After one week of incubation at 30 ± 1 °C, broth cultures were filtered, and available Zn concentration was determined through an atomic absorption spectrophotometer.
2.5. Manganese Solubilization Assay
Mn solubilization by rhizobacterial strains was carried out in nutrient agar media supplemented with 50 mM MnO2 [35]. After incubation of media plates at 30 ± 1 °C for 72 h, halo zone formation around the bacterial growth was determined visually by adding iodine solution as an indicator. Mn solubilization index (MSI) and solubilization efficiency (MSE) were determined according to the formula reported by Ijaz et al. [35]. The Mn solubilized concentration was determined by inoculating nutrient broth amended with MnO2 with rhizobacterial strains. After one week of incubation, culture filtrate was used to assess their solubilized concentration through the atomic absorption spectrophotometer.
2.6. Determination of Indole Acetic Acid
The selected bacterial strains were tested for indole-3-acetic acid (IAA) production using sulfide–indole-motility (SIM) media. Test tubes were incubated at 30 ± 1 °C for 48 h and observed for the production of a red-colored ring by adding 1 mL of KOVAC’S reagent into test tubes. To quantify IAA production, bacterial strains were inoculated in nutrient broth in the presence and absence of L-tryptophan (5 mg mL−1) [36]. The setup was incubated in triplicate at 30 ± 1 °C and 100 rpm for 72 h. The cultures were harvested by centrifugation up to 10,000× g, and 1 mL of supernatant was treated with 2 mL of Salkowski reagent. The optical density of the reaction mixture and IAA working standards were read at 530 nm through a spectrophotometer. The IAA concentration was calculated by drawing the standard curve.
2.7. Determination of Enzymatic Activities
Bacterial strains were assessed for their protease and amylase activities using methods reported by Cappuccino and Welsh [37]. The catalase, coagulase, and urease activities were determined by following the standard procedures described in Cheesbrough [38]. For protease activity, bacterial strains were screened for their ability to produce protease enzyme on skim milk agar (SMA) media. The bacterial colony was spot-inoculated on media using a sterile needle. The appearance of halo zones around bacterial colonies was checked after 48 h of incubation at 30 ± 1 °C. The amylase activity was determined by inoculating freshly grown bacterial cultures on media containing starch agar, and plates were incubated at 30 ± 1 °C for 48 h. Iodine solution was poured on bacterial colonies for a few seconds and recorded data. The catalase activity was determined by adding a few drops of hydrogen peroxide (H2O2) into the pure bacterial cells on a glass slide using a dropper. The slide was observed for bubble formation. For coagulase activity, distilled water drop was added on a glass slide, and the bacterial colony was emulsified to make a suspension. A few drops of plasma were placed on the bacterial colony, and the slide was examined for clump formation after 10 s. Christensen’s urea slant media was used to check the urease activity of bacterial strains. The slant media was incubated at 30 ± 1 °C and examined after 72 h.
2.8. Morphological and Microscopic Characterization
The LB agar plates were used to examine the morphological characteristics of bacterial strains. The colonies were illustrated based on shape, size, surface, color, opacity, elevation, margin, and consistency [37]. For the triple sugar iron (TSI) test, a pure colony of bacterial strain was streaked on TSI agar media and examined after 24 h of incubation. Production of gas, blackening of media, and changes in the color of slant and butt were observed [38]. Microscopic observation was conducted to determine the bacterial isolates’ form, Gram reactivity, and motility. Vincent and Humphrey’s [36] method was used for Gram staining. A bacterial colony was taken with the help of a sterile loop and emulsified in a water drop on a glass slide. The slide was heat fixed by passing through Bunsen flame three times. Crystal violet was added to the slide and washed with running water after a minute. After the primary stain, Gram iodine was added as a mordant. The slide was then flooded with ethanol for 30 s and washed with tap water. Afterward, safranin was added and allowed to stain for one minute. The slide was washed, air-dried, and examined under a microscope to study the color and shape of the colony [36].
2.9. Molecular Identification of Selected Bacterial Strains
The most promising rhizobacterial strains were chosen for 16S rRNA gene sequence analysis based on the mineral’s solubilizing ability and enzymatic activities. The genomic DNA of selected strains was extracted from the cell culture using proteinase K treatment [39]. The 16S rRNA region of 2.5 µL genomic DNA was amplified through thermocycler (Eppendorf, Enfield, Connecticut, United States) using the universal forward primers 785F (5′–GGATTAGATACCCTGGTA–3′) and reverse primers 907R (5′–CCGTCAATTCMTTTRAGTTT–3′). The size of the amplified 16S rRNA region was confirmed by running gel electrophoresis through 1% agarose gel along with GeneRuler 1 kb DNA (Fermentas; Thermo Scientific, Waltham, MA, USA). The PCR product was purified through a PCR purification kit (Favorgen, Taiwan), and the 16S rRNA partial gene was sequenced from the commercial service of Macrogen Seoul, Korea (https://dna.macrogen.com/, accessed on 10 July 2022). The consensus sequence from forward and reverse sequences was obtained using the software Bioedit version 7.2 (https://bioedit.software.informer.com/7.2/, accessed on 1 September 2022). The consensus sequences were analyzed on highly similar sequences (megablast) program of nucleotides blast (blastn) in NCBI web server (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 25 September 2023). The strains were identified based on the maximum homology found in the 16S ribosomal DNA sequence (bacteria and archaea) from the RNA database using the EzBiCloud web server (https://www.ezbiocloud.net/, accessed on 25 September 2023). The sequences of closely related type strains were obtained from the EzBioCloud web service (https://www.ezbiocloud.net/, accessed on 25 September 2023) using the 16S-based-ID database. Phylogenetic analyses based on the maximum likelihood method were performed with all closely related taxa using MEGA version X with kimura 2 parameter model, using 1000 bootstrap (https://www.megasoftware.net/downloads/dload_win_gui, accessed on 25 September 2023) [40]. The sequences used for bacterial identification were submitted to the NCBI GenBank database, and accession numbers were obtained.
2.10. Evaluation of Bacterial Strains for Promotion of Rhodes Grass Growth
The soil culture pot experiment was conducted in plastic pots to evaluate the potential of promising rhizobacterial strains on Rhodes grass growth attributes. Based on minerals solubilization, eight bacterial strains; SM2, SM4, SM5, SM7, MS1, MS2, MS4, and MS7 were selected for pot trial. The rhizobacterial cultures were freshly grown in LB broth under shaking conditions (100 rpm) at 30 ± 1 °C for 48 h. Before seed sowing, Rhodes grass seeds were soaked in bacterial cultures of 0.70 optical density at 600 nm for one hour. In the uninoculated control group, seeds were soaked in LB broth without bacterial inoculation. This experiment was performed under natural climatic conditions in IMBB, UOL, Pakistan greenhouse at 31.39 N latitude, 74.24 E longitude, and 206 m elevation. The sieved soil of 9 kg weight was added to each pot, and seeds were sown at a depth of 2 inches. Pots were arranged in a completely randomized design (CRD) with three replications for each treatment. Plants were watered on alternate days, and Hoagland solution was added once a week. After 4 weeks of seed germination, plants were harvested carefully, and data regarding root and shoot growth were recorded. Roots were washed with tap water and separated from shoots. Growth parameters like root length and shoot length were recorded through a measuring scale. Fresh and dry biomasses of roots and shoots were weighed through a top-loaded electrical weight balance.
2.11. Statistical Analysis
Statistical analysis was performed by using one-way analysis of variance (ANOVA) in completely randomized design (CRD) with Statistix 8.1 software to evaluate the effects of rhizobacterial strains on the growth parameters of Rhodes grass. Mean values from in vitro and in vivo assays were compared using the Tukey HSD test. At the same time, the pot experiment was analyzed through the least significant difference (LSD) test at a 5% probability level (p ≤ 0.05) [41]. Values presented in the tables are the mean of triplicates ± standard error (SE), which means sharing similar letters was not significantly different.
3. Results
A total of 25 bacterial strains were isolated from the Rhodes grass rhizosphere samples collected from two different locations, site I (Rahim Yar Khan, Punjab, Pakistan) and site II (Ghotki, Sindh, Pakistan. Of these 25 strains, 10 rhizobacterial strains were isolated from site I, and 15 rhizobacterial strains were isolated from site II.
3.1. Rhizobacterial Strains Demonstrated Phosphate Solubilization Potential
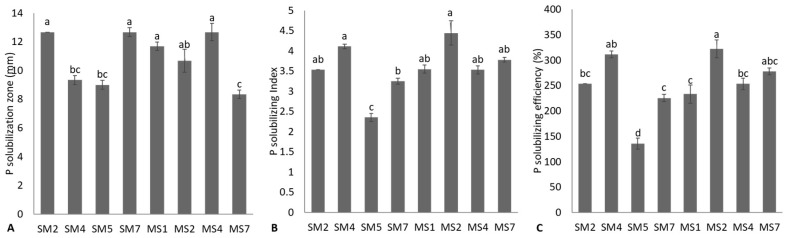
The isolated bacterial strains were assessed for their mineral solubilization potential of different insoluble minerals, such as P, K, Zn, and Mn solubilization. Out of 25 isolated strains, eight rhizobacterial strains were positive for P-solubilization coded as SM2, SM4, SM5, SM7, MS1, MS2, MS4 and MS7 (Figure 1). The maximum halo zone diameter was shown by rhizobacterial strains SM2, SM7, and MS4; these strains were statistically (p ≤ 0.05) similar to MS2. Strain MS7 was the lowest to report P-solubilization zone and bacterial growth zone diameters. The strains MS2 followed by SM4 strains reported the maximum PSI and PSE. These strains were statistically non-significant (p ≤ 0.05) with strains SM2, MS1, MS4, and MS7 in the case of PSI and with strain MS7 in the case of PSE. The strain SM5 was the lowest to report PSI and PSE. The quantitative P solubilization and reduction in pH were observed, and data are given in Table 1. Strain MS2 reported a maximum available P concentration of 27.45 ± 0.14 ppm and was statistically non-significant (p ≤ 0.05) to strains SM2 and SM4. The increase in available P concentration was observed due to the reduction in pH of the PVK medium. The highest pH reduction in the PVK medium was found by strain SM2 (4.24 ± 0.02) and SM4 (4.39 ± 0.03).
Figure 1.
Phosphate (P) solubilization potential of bacterial strains based on P solubilization zone formation (A), P solubilizing index (B), and P solubilizing efficiency (C) of bacterial strains; values are mean of triplicates ± standard error and mean sharing similar letters were not significantly different from each other.
Table 1.
The available concentration of P and reduction in pH of the media by rhizobacterial strains in response to insoluble tricalcium phosphate.
| Bacterial Strains | Available P Concentration (ppm) | pH Reduction in Response to Phosphate |
|---|---|---|
| Control | 3.05 ± 0.34 e | 6.87 ± 0.06 a |
| SM2 | 26.00 ± 0.65 abc | 4.51 ± 0.15 de |
| SM4 | 26.60 ± 0.15 ab | 4.39 ± 0.03 ef |
| SM5 | 24.35 ± 0.41 c | 4.82 ± 0.06 c |
| SM7 | 20.65 ± 0.25 d | 5.21 ± 0.02 b |
| MS1 | 24.85 ± 0.40 c | 4.65 ± 0.02 cd |
| MS2 | 27.45 ± 0.14 a | 4.24 ± 0.02 f |
| MS4 | 25.45 ± 0.55 bc | 4.58 ± 0.14 de |
| MS7 | 22.30 ± 0.61 d | 4.93 ± 0.05 b |
| CVC | 1.7496 | 0.2077 |
CVC stands for critical value for comparison at p ≤ 0.05; The means sharing the same alphabet letters were non-significant from each other.
3.2. Rhizobacterial Strains Demonstrated Zinc Solubilization Potential
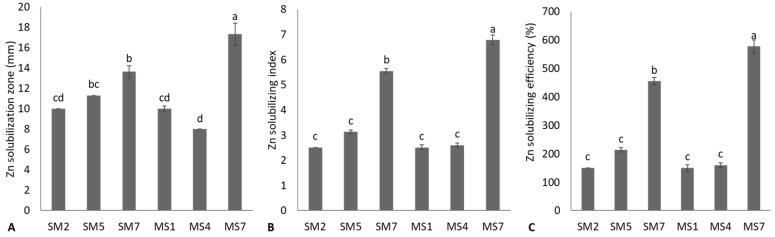
These P-solubilizing bacterial strains were tested for Zn-solubilization; six rhizobacterial strains were positive (Figure 2). Strain MS7, followed by SM7, showed the maximum Zn-solubilization zone diameter. The MS4 reported a minimum Zn-solubilization diameter and was statistically (p ≤ 0.05) similar to MSI and SM2. The strains MS7 and SM7 reported the highest ZSI and ZSE. Strains SM2, SM5, MSI, and MS4 reported the lowest and non-significant (p ≤ 0.05) ZSI and ZSE. The quantitative Zn solubilization and reduction in pH were observed, and data are given in Table 2. The highest available Zn was obtained from strain MS7 (21.72 ± 0.39 ppm) due to the highest reduction in pH (4.60 ± 0.08) of medium containing insoluble Zn. After that, strain SM7 showed a better increase in available Zn (19.81 ± 0.33 ppm) due to a reduced pH (4.79 ± 0.08) of the medium containing insoluble Zn.
Figure 2.
Zinc (Zn) solubilization potential of bacterial strains based on Zn solubilization zone formation (A), Zn solubilizing index (B), and Zn solubilizing efficiency (C) of bacterial strains; values are mean of triplicates ± standard error and mean sharing similar letters were not significantly different from each other.
Table 2.
The available concentration of Zn and reduction in pH of the media by rhizobacterial strains in response to zinc oxide.
| Bacterial Strains | Available Zn Concentration (ppm) | pH Reduction in Response to Zinc Oxide |
|---|---|---|
| Control | 2.16 ± 0.09 f | 6.78 ± 0.01 a |
| SM2 | 19.11 ± 0.29 bc | 5.32 ± 0.09 c |
| SM4 | NT | NT |
| SM5 | 17.96 ± 0.93 c | 5.18 ± 0.06 cd |
| SM7 | 19.81 ± 0.33 b | 4.79 ± 0.08 de |
| MS1 | 11.63 ± 0.29 e | 6.17 ± 0.4 b |
| MS2 | 15.33 ± 0.45 d | 5.01 ± 0.02 cd |
| MS4 | NT | NT |
| MS7 | 21.72 ± 0.39 a | 4.60 ± 0.08 e |
| CVC | 1.5441 | 0.2258 |
The symbol NT stands for not tested; CVC stands for critical value for comparison at p ≤ 0.05; The means sharing the same alphabet letters were non-significant from each other.
3.3. Rhizobacterial Strains Demonstrated Manganese and Potassium Solubilization Potential
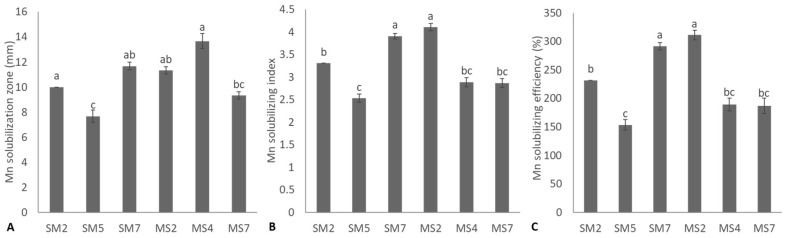
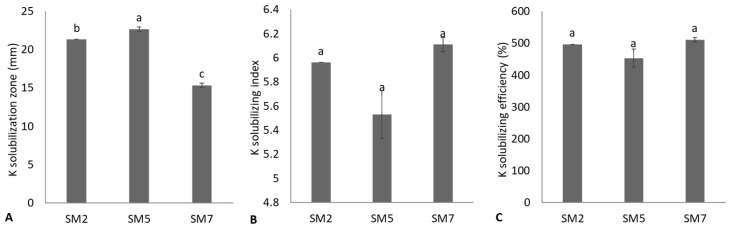
Six rhizobacterial strains were positive for Mn solubilization (Figure 3). Strain MS4 showed the maximum Mn solubilization zone diameter and was statistically (p ≤ 0.05) similar to strains SM2, SM7, MS2, and MS4. The strains SM7 and MS2 reported the highest MSI and MSE and were statistically (p ≤ 0.05) identical. The minimum Mn solubilization zone diameter, MSI, and MSE were observed from strain SM5. Three rhizobacterial strains (SM2, SM5, SM7) were positive for K-solubilization (Figure 4). Strain SM5 showed maximum halo zone diameter, while strain SM7 reported the lowest K-solubilization diameter. However, SM7 reported maximum KSI and KSE and was non-significant (p ≤ 0.05) to SM2 and SM5. Strain MS2 reported maximum available Mn concentration (11.07 ± 0.13 ppm) with the highest reduction in pH (4.12 ± 0.04) of the nutrient broth containing insoluble Mn (Table 3). The maximum available K concentration (21.6 ± 0.61 ppm) was obtained from SM4 due to the highest reduction in pH (4.60 ± 0.02) of medium containing mica (Table 3).
Figure 3.
Manganese (Mn) solubilization potential of bacterial strains based on Mn solubilization zone formation (A), Mn solubilizing index (B), and Mn solubilizing efficiency (C) of bacterial strains; values are mean of triplicates ± standard error and mean sharing similar letters were not significantly different from each other.
Figure 4.
Potassium solubilization potential of bacterial strains based on potassium solubilization zone formation (A), potassium solubilizing index (B), and potassium solubilizing efficiency (C) of bacterial strains; values are mean of triplicates ± standard error and mean sharing similar letters were not significantly different from each other.
Table 3.
The available concentration of Mn and K and reduction in pH of the media by rhizobacterial strains in response to their insoluble sources.
| Bacterial Strains | Available Mn Concentration (ppm) |
pH Reduction in Response to Manganese Oxide |
Available K Concentration (ppm) |
pH Reduction in Response to Zinc Oxide |
|---|---|---|---|---|
| Control | 3.01 ± 0.06 f | 6.90 ± 0.02 a | 2.99 ± 0.29 d | 6.92 ± 0.06 a |
| SM2 | 7.65 ± 0.29 b | 4.45 ± 0.04 c | 21.6 ± 0.61 a | 4.60 ± 0.02 d |
| SM4 | NT | NT | NT | NT |
| SM5 | 4.79 ± 0.13 e | 4.80 ± 0.03 b | 17.86 ± 0.72 b | 5.18 ± 0.02 c |
| SM7 | 5.15 ± 0.14 d | 5.08 ± 0.05 b | 11.54 ± 0.38 c | 6.18 ± 0.03 b |
| MS1 | NT | NT | NT | NT |
| MS2 | 11.07 ± 0.13 a | 4.12 ± 0.04 d | NT | NT |
| MS4 | 6.86 ± 0.14 c | 4.69 ± 0.04 b | NT | NT |
| MS7 | 9.87 ± 0.35 ab | 4.39 ± 0.03 c | NT | NT |
| CVC | 0.3154 | 0.2989 | 2.3377 | 0.2110 |
The symbol NT stands for not tested; CVC stands for critical value for comparison at p ≤ 0.05; The means sharing the same alphabet letters were non-significant from each other.
3.4. IAA Production by Rhizobacterial Strains
The rhizobacterial strains produced IAA in a medium without L-tryptophan; however, the bacterial ability for IAA production was more prominent in the presence of L-tryptophan (Table 4). Strain SM4 reported the highest production of IAA (26.87 ± 3.14 µg mL−1), followed by strains SM5 (22.39 ± 1.55 µg mL−1) and SM7 (23.74 ± 2.18 µg mL−1) in the presences of L-tryptophan. In the absence of L-tryptophan, strain SM4 also reported maximum IAA production (5.76 ± 1.61 µg mL−1) and was statistically (p ≤ 0.05) similar to strains SM2, SM5, and SM7.
Table 4.
Indole acetic acid (IAA) production by bacterial strains in the presence and absence of L-tryptophan.
| Bacterial Strains | IAA Production without L-Tryptophan (µg mL−1) |
IAA Production with L-Tryptophan (µg mL−1) |
|---|---|---|
| SM2 | 5.12 ± 1.91 ab | 17.89 ± 2.51 c |
| SM4 | 5.76 ± 1.61 a | 26.87 ± 3.14 a |
| SM5 | 4.96 ± 0.43 ab | 22.39 ± 1.55 b |
| SM7 | 3.60 ± 1.66 bcd | 13.94 ± 1.40 de |
| MS1 | 2.37 ± 1.71 d | 7.38 ± 0.36 f |
| MS2 | 2.92 ± 1.86 cd | 12.48 ± 1.75 e |
| MS4 | 4.07 ± 1.09 bc | 15.87 ± 2.90 cd |
| MS7 | 5.02 ± 0.55 ab | 23.74 ± 2.18 b |
| CVC | 1.6539 | 2.5692 |
CVC stands for critical value for comparison at p ≤ 0.05; The means sharing the same alphabet letters were non-significant from each other.
3.5. Enzymatic Activities by Rhizobacterial Strains
Enzymatic activities of rhizobacterial strains include catalase, coagulase, urease, amylase, and protease activities are presented in Table 5. All tested rhizobacterial strains showed catalase production. Half of the tested strains were positive for urease activity in terms of change in color from yellow to pink. Only two rhizobacterial strains, SM7 and MS7, were positive for the coagulase activity. Comparatively, the other six rhizobacterial strains were coagulase-negative, as clumping in blood plasma was absent. Five rhizobacterial strains were protease-positive, while six were amylase-positive. The urease, protease, and amylase experiment results can be viewed in Figures S1–S3. The TSI results are also provided in Table S1 and Figure S4.
Table 5.
Enzymatic activities of rhizobacterial strains.
| Bacterial Strains | Urease Activity | Catalase Activity | Protease Activity | Coagulase Activity | Amylase Activity |
|---|---|---|---|---|---|
| SM2 | −ve | +ve | +ve | −ve | +ve |
| SM4 | +ve | +ve | −ve | −ve | −ve |
| SM5 | −ve | +ve | +ve | −ve | +ve |
| SM7 | +ve | +ve | −ve | +ve | +ve |
| MS1 | −ve | +ve | +ve | −ve | +ve |
| MS2 | +ve | +ve | −ve | −ve | +ve |
| MS4 | −ve | +ve | +ve | −ve | +ve |
| MS7 | +ve | +ve | +ve | +ve | −ve |
+ve indicates the trait’s presence, and −ve shows the absence of the trait.
3.6. Morphological Characteristics of Rhizobacterial Strains
The morphological characteristics of eight tested mineral solubilizing rhizobacterial strains varied widely (Table 6). These bacterial colonies have circular shapes except strain SM5, which showed an irregular shape. Most colonies were small; however, strains SM4, SM5, and MS4 showed medium colony sizes. Bacterial colonies have different colors: off-white (SM5 and MS1); pure white (SM7 and MS4); skin (SM2 and MS7); yellow (SM2); and mustard (MS2). They also showed variable colony surfaces of smooth (SM2, SM4, SM78, MS7), shiny (MS5 and MS2), dull (MS1), and wrinkled (MS4). More than 62% of colonies were translucent, and 38% were opaque, while flat elevation colonies were observed in 50% of strains, and the rest were of raised and umbonate surface elevation. Most strain colonies were of wavy margin (75%), and the rest were even margin (25%). Their consistency was butyrous (62.5%), dry (12.5%), and hard (25%). All the tested strains were of Gram-positive nature (Table 7). The microscopic observation showed that seven bacterial isolates were rod-shaped, and one was cocci. Six strains were motile, and two strains were non-motile.
Table 6.
Colony characteristics of bacterial strains.
| Strains | Shape | Size | Surface | Color | Opacity | Elevation | Margin | Consistency |
|---|---|---|---|---|---|---|---|---|
| SM2 | Circular | Small | Smooth | Skin | Opaque | Flat | Even | Butyrous |
| SM4 | Circular | Medium | Smooth | Yellow | Opaque | Raised | Even | Butyrous |
| SM5 | Irregular | Medium | Shiny | Off white | Translucent | Raised | Wavy | Butyrous |
| SM7 | Circular | Small | Smooth | Pure white | Translucent | Flat | Even | Butyrous |
| MS1 | Circular | Small | Dull | Off white | Translucent | Flat | Even | Dry |
| MS2 | Circular | Small | Shiny | Mustard | Translucent | Raised | Even | Butyrous |
| MS4 | Circular | Medium | Wrinkle | Pure white | Opaque | Umbonate | Wavy | Hard |
| MS7 | Circular | Small | Smooth | Skin | Translucent | Flat | Even | Hard |
Table 7.
Microscopic observation of mineral solubilizing bacteria.
| Strains | Form | Gram Reaction | Color | Motility |
|---|---|---|---|---|
| SM2 | Rod | Gram +ve | Purple | Motile |
| SM4 | Rod | Gram +ve | Purple | Motile |
| SM5 | Rod | Gram +ve | Purple | Motile |
| SM7 | Cocci | Gram +ve | Purple | Non-motile |
| MS1 | Rod | Gram +ve | Purple | Motile |
| MS2 | Rod | Gram +ve | Purple | Motile |
| MS4 | Rod | Gram +ve | Purple | Motile |
| MS7 | Cocci | Gram +ve | Purple | Non-motile |
3.7. Effect of Rhizobacterial Strains on Growth Promotion of Rhodes Grass
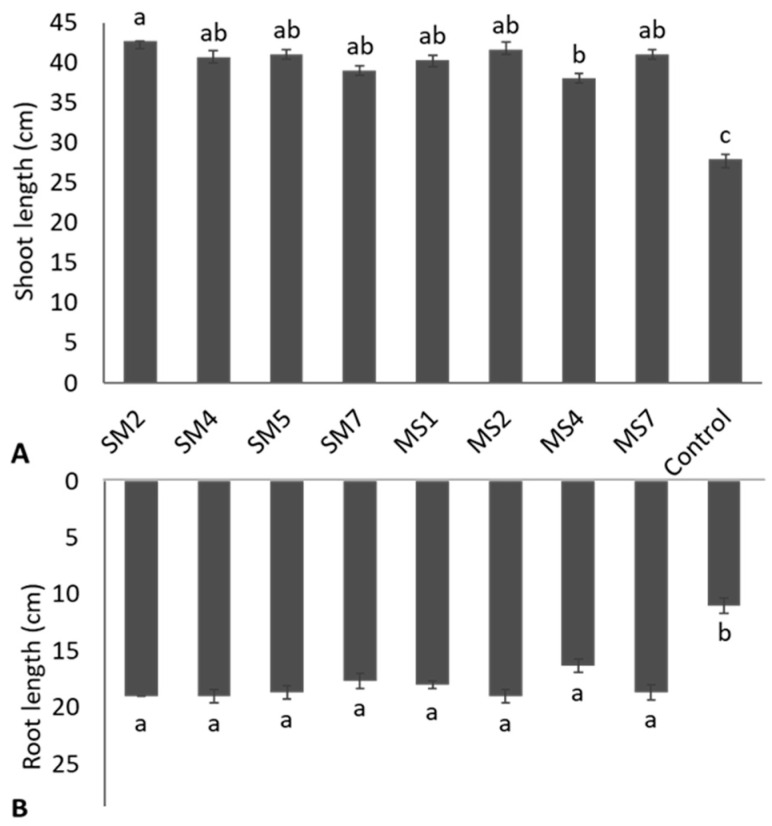
Bacterial inoculation significantly (p ≤ 0.05) enhanced Rhodes grass growth parameters regarding root length, shoot length, and fresh and dry biomasses of root and shoot (Figure S5). The shoot length and root length due to bacterial inoculation were non-significant (p ≤ 0.05) to each other but were statistically significant (p ≤ 0.05) to the uninoculated control (Figure 5). The strain SM2, followed by MS2, SM5, and MS7, reported the highest increase of 52.4%, 48.8%, 46.4%, and 46.4%, respectively, in shoot length over the uninoculated control (Figure 5A). The maximum gain of 72.7% in the root length of Rhodes grass was observed due to strains SM2, SM4, and MS2 application (Figure 5B).
Figure 5.
Effect of bacterial strains on shoot length (A) and root length (B) on seedlings of Rhodes grass. Values are mean of triplicates ± standard error and mean sharing similar letters were not significantly different from each other.
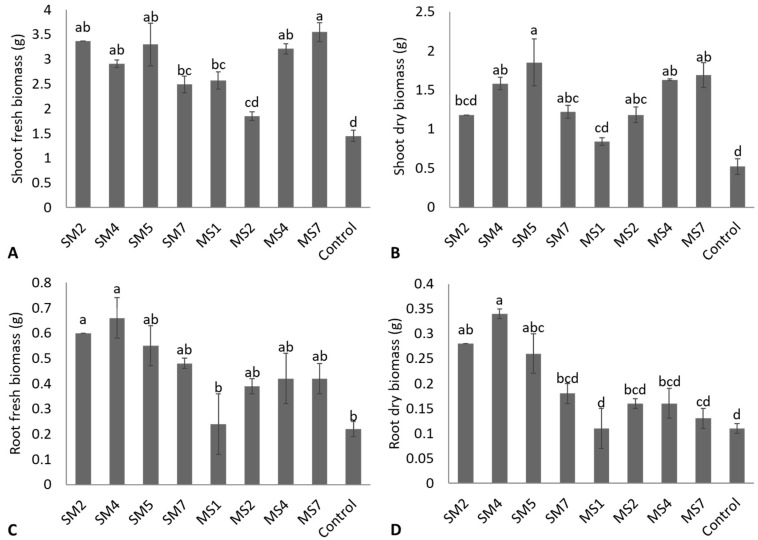
Strain MS7 reported the maximum increase of 144.8% in shoot fresh biomass and was statistically non-significant (p ≤ 0.05) to strains SM4, SM5, and MS4; however, these strains were statistically (p ≤ 0.05) different from the uninoculated control (Figure 6A). The lowest increase of 27.6% was obtained from strain MS2 and was statistically non-significant (p ≤ 0.05) to the uninoculated control. The highest shoot dry biomass was reported by strain SM5, with an increase of 255.8% over the uninoculated control (Figure 6B). The shoot dry biomass due to strain SM5 was statistically non-significant (p ≤ 0.05) to strains SM4, SM7, MS2, MS4, and MS7; however, these strains showed statistically (p ≤ 0.05) highest shoot dry biomass over uninoculated control. The increase in root fresh biomass due to applied bacterial strains was statistically non-significant (p ≤ 0.05) to uninoculated except strains SM2 and SM4, which reported the maximum increase of 172.7% and 200% in root fresh biomass (Figure 6C). The bacterial strains also reported an increase in root dry biomass; however, it was statistically non-significant (p ≤ 0.05) to the uninoculated control except SM2, SM4, and SM5 (Figure 6D). The highest gain of 209% was reported by strains SM4, over uninoculated control, and was statistically (p ≤ 0.05) similar to SM2 and SM5. The uninoculated control showed minimum root length, shoot length, shoot and root fresh and dry biomass (Figure 5 and Figure 6).
Figure 6.
Effect of bacterial strains on shoot fresh biomass (A), shoot dry biomass (B), root fresh biomass (C), and root dry biomass (D) on seedlings of Rhodes grass. Values are mean of triplicates ± standard error and mean sharing similar letters were not significantly different from each other.
3.8. Identification of Rhizobacterial Strains
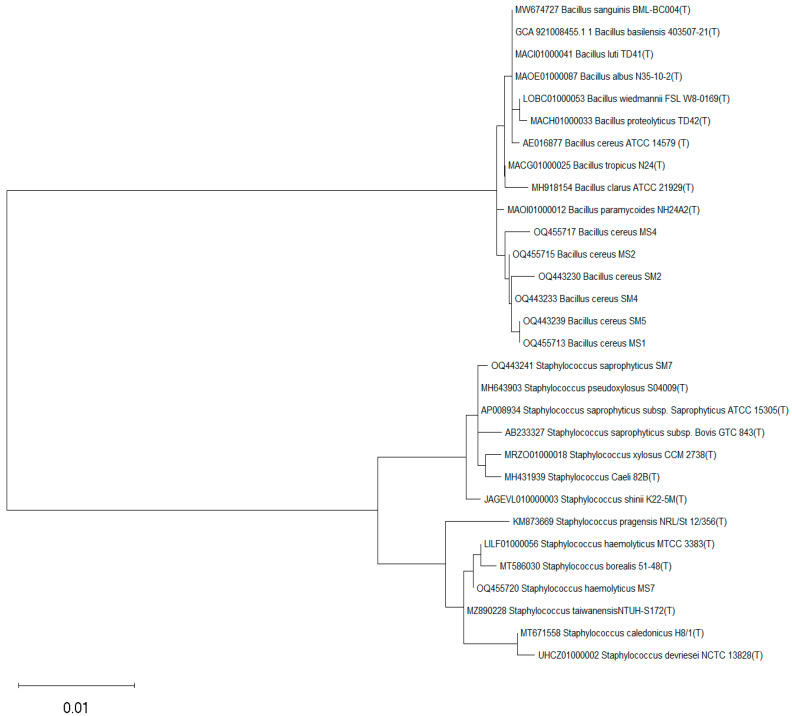
The rhizobacterial strains SM2, SM4, SM5, MS1, MS2, and MS4 were identified as Bacillus cereus, while strains SM7 and MS7 were identified as Staphylococcus saprophyticus and Staphylococcus haemolyticus, respectively through 16S rRNA gene sequencing (Table 8). The sequences of identified strains were submitted in the gene bank of NCBI, and obtained accession numbers are given in Table 8. The retrieved sequences were analyzed using different bioinformatics tools such as DNA Star, BLAST, and MEGA X. All the strains showed an excellent blend of microflora belonging to different bacterial species. The neighbor-joining method was used to construct the phylogenetic tree. The phylogenetic tree of identified Bacillus cereus strains SM2 (OQ443230), SM4 (OQ443233), SM5 (OQ443239), MS1 (OQ455713), MS2 (OQ455715), and MS4 (OQ455717), while, SM7 (OQ443241) and MS7 (OQ455720) were identified as Staphylococcus saprophyticus and Staphylococcus haemolyticus with their closest matching strains (Figure 7).
Table 8.
Identification of bacterial strains based on their 16S rRNA sequencing.
| Strains | Identification by 16S rRNA Gene Sequencing | Similarity with Type Strains (%) | Size (bp) | Accession No. |
|---|---|---|---|---|
| SM2 | Bacillus cereus ATCC 14579T (AE016877) | 99.66 | 1466 | OQ443230 |
| SM4 | Bacillus cereus ATCC 14579T (AE016877) | 99.86 | 1486 | OQ443233 |
| SM5 | Bacillus cereus ATCC 14579T (AE016877) | 99.86 | 1501 | OQ443239 |
| SM7 | Staphylococcus saprophyticus subsp. Saprophyticus ATCC 15305T (AP008934) | 99.93 | 1512 | OQ443241 |
| MS1 | Bacillus cereus ATCC 14579T (AE016877) | 99.86 | 1463 | OQ455713 |
| MS2 | Bacillus cereus ATCC 14579T (AE016877) | 99.86 | 1475 | OQ455715 |
| MS4 | Bacillus cereus ATCC 14579T (AE016877) | 99.86 | 1479 | OQ455717 |
| MS7 | Staphylococcus haemolyticus MTCC3383T (LILF01000056) | 99.93 | 1544 | OQ455720 |
Figure 7.
The phylogenetic tree of strains with their closet matching strains; SM2 (OQ443230), SM4 (OQ443233), SM5 (OQ443239), MS1 (OQ455713), MS2 (OQ455715), and MS4 (OQ455717) were identified as Bacillus cereus, while strains SM7 (OQ443241) and MS7 (OQ455720) were identified as Staphylococcus saprophyticus and Staphylococcus haemolyticus, respectively.
4. Discussion
Plants often face a significant limitation in mineral availability, as minerals exist in insoluble forms that plants cannot take up despite their importance in proper plant growth and functioning. Plant growth-promoting bacteria offer a promising alternative to traditional agrochemicals, as they enhance the bioavailability of nutrients in the soil and encourage plant growth and development [42,43,44]. Applying rhizobacteria as biofertilizers can improve plant growth and yield and is crucial for maintaining sustainable agriculture and soil fertility [45]. Therefore, the present study aimed to isolate mineral solubilizing bacteria from the rhizosphere soil of Rhodes grass and evaluate their potential on plant growth attributes. The selected strains were identified as Bacillus and Staphylococcus spp. through 16S rRNA gene sequence analysis. A limited number of studies reported the rhizobacterial strain’s interaction with Rhodes grass. Gupta et al. [46] reported the highest abundance of diverse diazotroph communities containing the NifH gene in the plant’s stem and root parts.
Among tested bacterial strains, eight bacterial strains showed strong P-solubilization in solid and liquid mediums. Rhizobacterial strains B. cereus MS2, B. cereus SM4, and S. haemolyticus MS7 reported the highest P solubilizing index and efficiency, available P concentration, and maximum reduction in pH of the medium. Similarly, Zaheer et al. [47] reported P solubilization by Pseudomonas sp. AZ5 and Bacillus sp. AZ17. They reported an increase in plant growth, yield, and nutrient uptake in chickpeas through the application of Pseudomonas sp. AZ5 and Bacillus sp. AZ17 [47]. Ahmad et al. [48] also reported predominant P solubilization by Bacillus subtilis IA6, Paenibacillus polymyxa IA7, Bacillus sp. IA16, and Bacillus aryabhattai IA20. These strains also demonstrated their potential to promote cotton growth under nutrient-depleted conditions [48]. Numerous studies have also reported similar P solubilization by diverse microorganisms [49,50,51,52,53]. Such bacterial strain uses various strategies to solubilize insoluble phosphate, which might be the production of low molecular mass organic acid, including acetic, butyric, citric, fumaric, gluconic, glucuronic, lactic, maleic, malic, oxalic, propionic, succinic, tartaric, and valeric acids [49,51,54]. These organic are produced by many bacterial strains in the natural environment and in vitro conditions and play an important role in the solubilization of phosphate through chelating mineral ions and reducing the pH of the medium [50,52,55].
In the current study, six rhizobacterial strains demonstrated qualitative and quantitative Zn solubilization. The maximum Zn solubilization index, Zn solubilization efficiency, available Zn concentration, and reduction in pH of the medium were obtained from strains S. saprophyticus SM7 and S. haemolyticus MS7. Previously, similar findings were reported in various studies [56,57,58,59,60,61]. Mumtaz et al. [57] isolated thirteen rhizobacterial strains and reported Zn and phosphate solubilization by promising Bacillus strains, including Bacillus sp. (ZM20), B. aryabhattai ZM31, and B. subtilis (ZM63). Similarly, Bhatt and Maheshwari [59] reported Zn solubilization by B. megaterium CDK25 through phytase activities. Such bacterial strains might be accomplished by various mechanisms for Zn solubilization, like proton extrusion, production of organic acids, and chelating agents [62,63,64,65]. In the current study, six bacterial strains showed their potential to solubilize Mn on nutrient agar media amended with insoluble MnO2, while only three rhizobacterial strains were positive for K solubilization. Strains B. cereus MS2 and S. haemolyticus MS7 reported maximum qualitative and quantitative Mn solubilization, while S. saprophyticus SM7 reported highest qualitative and quantitative K solubilization. Previously, Mn solubilization by microbial strains was reported by various researchers [36,66]. Solubilization of Mn and K insoluble minerals by rhizobacterial strains may involve multiple mechanisms, including organic acid production and protons by acidolysis [67,68]. The primary process for mineral solubilization by these rhizobacterial strains might be due to organic acid production, which reduces the soil pH and solubilizes minerals [66,69]. These organic acids include 2-keto gluconic, gluconic, butyric, lactic, glyoxylic, malic, fumaric, propionic, pyruvic, succinic, tartaric, citric, fumaric, and acetic acids [21,47,70,71,72,73,74,75]. Most rhizobacterial strains tested positive for enzymatic activities such as catalase, coagulase, urease, protease, and amylase. These enzymes could play an important role in plant growth promotion by acting as biocontrol agents against various plant pathogens and affecting characteristics of biochemical processes [76].
The tested rhizobacterial strains in the present study demonstrated the production of IAA in the presence and absence of L-tryptophan. These strains showed significant variation in IAA production, which could be due to the utilization ability of L-tryptophan by various rhizobacterial strains. Such variation in IAA production by bacterial strains was also reported by many authors [77,78,79,80]. In the current study, strains B. cereus SM2, B. cereus MS4, B. cereus SM5, and S. haemolyticus MS7 yielded the highest IAA production in the presence and absence of L-tryptophan. Similarly, Mumtaz et al. [57] reported the production of IAA by multiple trait mineral solubilizing Bacillus strains ZM20, ZM27, ZM63, and S10, both in the presence and absence of L-tryptophan. In the presence of L-tryptophan, IAA production was boosted by rhizobacterial strains. Batista et al. [81] demonstrated the complete set of genomes of B. thuringiensis RZ2MS9 required in indole-3-pyruvate and tryptamine pathways for IAA production. The L-tryptophan is a precursor of IAA and stimulates its production by bacteria and responds as a phytostimulatory effect in plants [82]. Our previous findings reported an increase in plant growth due to the production of IAA, especially in the presence of L-tryptophan [31,35,57,72,83]. In the current study, the inoculation with rhizobacterial strains promoted Rhodes grass seedlings, which could be due to phytostimulation of IAA. Under natural soil conditions, bacteria can utilize L-tryptophan released from root exudates and degrading roots, and the auxin biosynthesis in the rhizosphere is promoted [84]. Although IAA production was highest in all tested strains in the presence of L-tryptophan; however, strain MS1 showed the lowest IAA concentration up to 7.38 ± 0.36 µg mL−1, which might be due to the activity of IAA oxidase and peroxidase activities that degrade auxin [85].
The inoculation of Rhodes grass seedlings with bacterial strains significantly promoted plant growth attributes such as shoot length, root length, root and shoot dry, and fresh biomasses over the uninoculated control. The present study’s findings are consistent with previously reported studies [31,86,87,88,89,90,91]. Ahmad et al. [48] reported that cotton seeds inoculated with Bacillus strains having the ability to solubilize P and Zn significantly promoted growth attributes over uninoculated control. PGPRs such as Staphylococcus pasteuri, Bacillus cereus, and Bacillus velezensis have been reported in earlier studies as promising mineral solubilizers with multifarious plant growth-promoting activities [92,93,94]. Among these plant growth activities, production of IAA, siderophores, biofilm formation, and ACC-deaminase activities play important roles in promoting plant growth, which might be true in the current study. An increase in growth parameters could be associated with the potential of bacterial strains to perform various enzymatic activities, solubilize insoluble minerals, including P, Zn, K, and Mn, and increase their uptake plants. These beneficial rhizobacterial strains could enhance nutrient availability and promote their uptake under nutrient-deficient soils [95]. Such bacterial strains are economically and ecologically more significant and help to overcome mineral deficiency. Inoculation with such mineral-solubilizing bacterial strains could enhance nutrient availability and significantly promote Rhodes grass growth attributes.
5. Conclusions
The present study concluded that the Bacillus and Staphylococcus strains demonstrated in vitro mineral solubilization, enzymatic activities, and IAA production. Among tested strains, B. cereus SM4, S. saprophyticus SM7, B. cereus MS2, and S. haemolyticus MS7 demonstrated maximum solubilization of insoluble phosphate, zinc, manganese, and potassium minerals. Strain B. cereus SM2 and B. cereus SM4 reported maximum production of IAA in the presence and absence of its precursor. The inoculation of Rhodes grass with tested rhizobacterial strains significantly promoted seedling growth. Maximum shoot growth was obtained from inoculation with B. cereus SM5 and S. haemolyticus MS7, while maximum root growth was reported by strains B. cereus SM2 and B. cereus SM4. These strains could be attractive bioinoculants to enhance Rhodes grass production because they possess multiple PGP attributes and enzymatic activities and can address the problem of nutrient deficiencies. These strains could be more efficient and eco-friendly alternatives to chemical fertilizers, leading to a promising approach to sustainable agriculture.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2023R123), King Saud University, Riyadh, Saudi Arabia.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms11102543/s1, Figure S1: Urease activity of rhizobacterial strains; Figure S2. Protease activity of rhizobacterial strains; Figure S3. Amylase activity of rhizobacterial bacteria; Figure S4. View of triple sugar iron due to inoculation with rhizobacterial strains; Figure S5. Pictorial view of a pot experiment conducted to evaluate the effect of various bacterial strains on seedlings of Rhodes grass. Table S1. The results of the triple sugar iron test of rhizobacterial strains from Rhodes grass rhizosphere.
Author Contributions
Conceptualization, S.J. and G.R.; methodology, S.M., M.A. and U.M.; software, S.M. and S.J.; validation, S.J., G.R. and T.N.; formal analysis, H.M.A., G.A. and L.L.; investigation, M.F.-U.-Z.A.; resources, M.Z.M.; data curation, S.M.; writing—original draft preparation, S.M. and S.J.; writing—review and editing, M.Z.M.; visualization, M.Z.M.; supervision, S.J.; project administration, G.A.; funding acquisition, H.M.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The partial gene sequences of Bacillus cereus strains SM2 (OQ443230), SM4 (OQ443233), SM5 (OQ443239), MS1 (OQ455713), MS2 (OQ455715), and MS4 (OQ455717), Staphylococcus saprophyticus strain SM7 (OQ443241) and Staphylococcus haemolyticus strain MS7 (OQ455720) can be availed using Gen Bank Accession Numbers, which were deposited in the NCBI database.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Researchers Supporting Project number (RSP2023R123), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ponsens J., Hanson J., Schellberg J., Moeseler B.M. Characterization of phenotypic diversity, yield and response to drought stress in a collection of Rhodes grass (Chloris gayana Kunth) accessions. Field Crops Res. 2010;118:57–72. doi: 10.1016/j.fcr.2010.04.008. [DOI] [Google Scholar]
- 2.Faji M., Abebe A., Ahmad K. Forage biomass yield performance of Rhodes grass (Chloris gayana) accessions in benishngul-gumuz region of western ethopia. J. Ecol. Environ. 2021;13:35–41. [Google Scholar]
- 3.Loch D.S., Rethman N.F., Van Niekerk W.A. Rhodesgrass. In: Moser L., Burson B., Sollenberger L., editors. Warm-Season (C4) Grasses. Volume 45. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2004. pp. 833–872. [Google Scholar]
- 4.Negawo A.T., Muktar M.S., Assefa Y., Hanson J., Sartie A.M., Habte E., Jones C.S. Genetic diversity and population structure of a Rhodes grass (Chloris gayana) collection. Genes. 2021;12:1233. doi: 10.3390/genes12081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnusdei M.G., Di Marco O.N., Nenning F.R., Aello M.S. Leaf blade nutritional quality of rhodes grass (Chloris gayana) as affected by leaf age and length. Crop Pasture Sci. 2012;62:1098–1105. doi: 10.1071/CP11164. [DOI] [Google Scholar]
- 6.Arshad I., Medani K.M., Khan Z.A. Effect of manual and artificial application of NPK fertilizers on the growth and yield of Rhodes Grass (Chloris gayana L. Kunth.) by using central pivot irrigation technology. Int. J. Res. 2014;1:2348–6848. [Google Scholar]
- 7.Arshad I., Ali W., Khan Z.A., Bhayo W.A. Effect of water stress on the growth and yield of Rhodes grass (Chloris gayana L. Kunth.) PSM Biol. Res. 2016;1:58–61. [Google Scholar]
- 8.Zhang L., Yan C., Guo Q., Zhang J., Ruiz-Menjivar J. The impact of agricultural chemical inputs on environment: Global evidence from informetrics analysis and visualization. Int. J. Low Carbon Technol. 2018;13:338–352. doi: 10.1093/ijlct/cty039. [DOI] [Google Scholar]
- 9.Zeng Q., Ding X., Wang J., Han X., Iqbal H.M., Bilal M. Insight into soil nitrogen and phosphorus availability and agricultural sustainability by plant growth-promoting rhizobacteria. Environ. Sci. Pollut. Res. 2022;29:45089–45106. doi: 10.1007/s11356-022-20399-4. [DOI] [PubMed] [Google Scholar]
- 10.Abbas M., Shah J.A., Irfan M., Memon M.Y. Remobilization and utilization of phosphorus in wheat cultivars under induced phosphorus deficiency. J. Plant Nutr. 2018;41:1522–1533. doi: 10.1080/01904167.2018.1458871. [DOI] [Google Scholar]
- 11.Khalid M., Hassani D., Bilal M., Asad F., Huang D. Influence of bio-fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Bot. Stud. 2017;58:35. doi: 10.1186/s40529-017-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benidire L., Madline A., Pereira S.I.A., Castro P.M.L., Boularbah A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere. 2021;262:127803. doi: 10.1016/j.chemosphere.2020.127803. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya P.N., Jha D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 14.Khan N., Ali S., Shahid M.A., Mustafa A., Sayyed R.Z., Curá J.A. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells. 2021;10:1551. doi: 10.3390/cells10061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oteino N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J., Dowling D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sims J.T., Pierzynski G.M. Chemistry of phosphorus in soils. In: Tabatabai A.M., Sparks D.L., editors. Chemical Processes in Soil. SSSA; Madison, WI, USA: 2005. pp. 151–192. (SSSA Book Series 8). [Google Scholar]
- 17.Prajapati K., Modi H. Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil. CIBTech J. Microbiol. 2012;1:8–14. [Google Scholar]
- 18.Saravanan V.S., Kumar M.R., Sa T.M. Bacteria in Agrobiology: Plant Nutrient Management. Springer; Berlin/Heidelberg, Germany: 2011. Microbial zinc solubilization and their role on plants; pp. 47–63. [Google Scholar]
- 19.Jiang Y., Ge F., Li F., Zhang D., Deng S., Tian J. Intracellular metabolomics switching alters extracellular acid production and insoluble phosphate solubilization behavior in Penicillium oxalicum. Metabolites. 2020;10:441. doi: 10.3390/metabo10110441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alori E.T., Glick B.R., Babalola O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017;8:971. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouphael Y., Colla G. Biostimulants in Agriculture. Front. Plant Sci. 2020;11:40. doi: 10.3389/fpls.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Z., Zhang X., Wang Z., Cao B., Deng S., Bi M., Zhang Y. Enhanced biodegradation of atrazine by Arthrobacter sp. DNS10 during co-culture with a phosphorus solubilizing bacteria: Enterobacter sp. P1. Ecotoxicol. Environ. Saf. 2019;172:159–166. doi: 10.1016/j.ecoenv.2019.01.070. [DOI] [PubMed] [Google Scholar]
- 23.Thomas S., Mathew L., Rishad K. Isolation and molecular identification of phosphate solubilizing bacteria, Bacillus licheniformis UBPSB-07 capable of enhancing seed germination in Vigna radiata L. Phytomorphology. 2018;68:13–18. [Google Scholar]
- 24.Behera B.C., Yadav H., Singh S.K., Mishra R.R., Sethi B.K., Dutta S.K., Thatoi H.N. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017;15:169–178. doi: 10.1016/j.jgeb.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korir H., Mungai N.W., Thuita M., Hamba Y., Masso C. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front. Plant Sci. 2017;8:141. doi: 10.3389/fpls.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu A., Prasad P., Das S.N., Kalam S., Sayyed R.Z., Reddy M.S., El Enshasy H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability. 2021;13:1140. doi: 10.3390/su13031140. [DOI] [Google Scholar]
- 27.Bertani G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 2004;186:595–600. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somasegaran P., Hoben H.J. Counting Rhizobia by a Plant Infection Method. In: Somasegaran P., Hoben H.J., editors. Handbook for Rhizobia. Springer; New York, NY, USA: 1994. pp. 58–64. [Google Scholar]
- 29.Saegeman V.S., Ectors N.L., Lismont D., Verduyckt B., Verhaegen J. Short-and long-term bacterial inhibiting effect of high concentrations of glycerol used in the preservation of skin allografts. Burns. 2008;34:205–211. doi: 10.1016/j.burns.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Pikovskaya R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- 31.Ahmad M., Naseer I., Hussain A., Mumtaz M.Z., Mustafa A., Hilger T.H., Xu M. Appraising endophyte–plant symbiosis for improved growth, nodulation, nitrogen fixation and abiotic stress tolerance: An experimental investigation with chickpea (Cicer arietinum L.) Agronomy. 2019;9:621. doi: 10.3390/agronomy9100621. [DOI] [Google Scholar]
- 32.Parmar P., Sindhu S.S. Potassium solubilization by rhizosphere bacteria: Influence of nutritional and environmental conditions. J. Microbiol. Res. 2013;3:25–31. [Google Scholar]
- 33.Setiawati T.C., Mutmainnah L. Solubilization of potassium containing mineral by microorganisms from sugarcane rhizosphere. Agri. Agri. Sci. Procedia. 2016;9:108–117. doi: 10.1016/j.aaspro.2016.02.134. [DOI] [Google Scholar]
- 34.Bunt J.S., Rovira A.D. Microbiological studies of some subantarctic soils. J. Soil Sci. 1955;6:119–128. doi: 10.1111/j.1365-2389.1955.tb00836.x. [DOI] [Google Scholar]
- 35.Ijaz A., Mumtaz M.Z., Wang X., Ahmad M., Saqib M., Maqbool H., Zaheer A., Wang W., Mustafa A. Insights into manganese solubilizing Bacillus spp. for improving plant growth and manganese uptake in maize. Front. Plant Sci. 2021;12:719504. doi: 10.3389/fpls.2021.719504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V. Laboratory Manual of Microbiology. Scientific Publishers; Jodhpur, India: 2012. [Google Scholar]
- 37.Cappuccino J.G., Welsh C.T. Microbiology: A Laboratory Manual. 12th ed. Pearson; San Francisco, CA, USA: 2019. p. 560. [Google Scholar]
- 38.Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. 2nd ed. Cambridge University Press; New York, NY, USA: 2005. p. 464. [Google Scholar]
- 39.Kate W. Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 2001;56:2–4. doi: 10.1002/0471142727.mb0204s56. [DOI] [PubMed] [Google Scholar]
- 40.Zaheer A., Mirza B.S., Mclean J.E., Yasmin S., Shah T.M., Malik K.A., Mirza M.S. Association of plant growth-promoting Serratia spp. with the root nodules of chickpea. Res. Microbiol. 2016;167:510–520. doi: 10.1016/j.resmic.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Steel R.G.D., Torrie J.H., Dickey D.A. Principles and Procedures of Statistics. A Biometrical Approach. 3rd ed. McGraw Hill Book Co.; New York, NY, USA: 2007. [Google Scholar]
- 42.Dasgupta D., Sengupta C., Paul G. Screening and identification of best three phosphate solubilizing and IAA producing PGPR inhabiting the rhizosphere of Sesbania bispinosa. Screening. 2015;4:3968–3979. [Google Scholar]
- 43.Liu M., Liu X., Cheng B.S., Ma X.L., Lyu X.T., Zhao X.F., Ju Y., Min Z., Zhang Z.-W., Fang Y. Selection and evaluation of phosphate-solubilizing bacteria from grapevine rhizospheres for use as biofertilizers. Span. J. Agric. Res. 2016;14:e1106. doi: 10.5424/sjar/2016144-9714. [DOI] [Google Scholar]
- 44.Richardson A.E., Barea J.M., McNeill A.M., Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321:305–339. doi: 10.1007/s11104-009-9895-2. [DOI] [Google Scholar]
- 45.Tsegaye Z., Assefa F., Beyene D. Properties and application of plant growth promoting rhizobacteria. Int. J. Curr. Trends Pharmacobiol. Med. Sci. 2017;2:30–43. [Google Scholar]
- 46.Gupta V.V., Zhang B., Penton C.R., Yu J., Tiedje J.M. Diazotroph diversity and nitrogen fixation in summer active perennial grasses in a Mediterranean region agricultural soil. Front. Mol. Biosci. 2019;6:115. doi: 10.3389/fmolb.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaheer A., Malik A., Sher A., Qaisrani M.M., Mehmood A., Khan S.U., Rasool M. Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J. Biol. Sci. 2019;26:1061–1067. doi: 10.1016/j.sjbs.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad I., Ahmad M., Hussain A., Jamil M. Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: A promising approach for improving cotton growth. Folia Microbiol. 2020;66:115–125. doi: 10.1007/s12223-020-00831-3. [DOI] [PubMed] [Google Scholar]
- 49.Wei Y., Zhao Y., Shi M., Cao Z., Lu Q., Yang T., Wei Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018;247:190–199. doi: 10.1016/j.biortech.2017.09.092. [DOI] [PubMed] [Google Scholar]
- 50.Zhan Y., Zhang Z., Ma T., Zhang X., Wang R., Liu Y., Li J. Phosphorus excess changes rock phosphate solubilization level and bacterial community mediating phosphorus fractions mobilization during composting. Bioresour. Technol. 2021;337:125433. doi: 10.1016/j.biortech.2021.125433. [DOI] [PubMed] [Google Scholar]
- 51.Wang S., Walker R., Schicklberger M., Nico P.S., Fox P.M., Karaoz U., Brodie E.L. Microbial phosphorus mobilization strategies across a natural nutrient limitation gradient and evidence for linkage with iron solubilization traits. Front. Microbiol. 2021;12:572212. doi: 10.3389/fmicb.2021.572212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z.W., Zhang J., Yu Y., Li H.R., Du Z.J. Pontibacter arcticus sp. nov., isolated from rhizosphere soil of Saxifraga oppositifolia. Int. J. Syst. Evol. Microbiol. 2019;69:3609–3615. doi: 10.1099/ijsem.0.003668. [DOI] [PubMed] [Google Scholar]
- 53.Suleman M., Yasmin S., Rasul M., Yahya M., Atta B.M., Mirza M.S. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE. 2018;13:e0204408. doi: 10.1371/journal.pone.0204408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan M.S., Zaidi A., Ahmad E. Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology. Springer; Berlin/Heidelberg, Germany: 2014. Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms; pp. 31–62. [Google Scholar]
- 55.Trivedi P., Sa T. Pseudomonas corrugata (NRRL B-30409) mutants increased phosphate solubilization, organic acid production, and plant growth at lower temperatures. Curr. Microbiol. 2008;56:140–144. doi: 10.1007/s00284-007-9058-8. [DOI] [PubMed] [Google Scholar]
- 56.Dinesh R., Srinivasan V., Hamza S., Sarathambal C., Gowda S.A., Ganeshamurthy A.N., Divya V.C. Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma. 2018;321:173–186. doi: 10.1016/j.geoderma.2018.02.013. [DOI] [Google Scholar]
- 57.Mumtaz M.Z., Ahmad M., Jamil M., Hussain T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017;202:51–60. doi: 10.1016/j.micres.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Min L.J., Wu X.Q., Li D.W., Zhang L.Q., Ye J.R. Burkholderia pyrrocinia strain JK-SH007 affects zinc (Zn) accumulation and translocation in tomato. Arch. Agron. Soil Sci. 2020;67:447–458. doi: 10.1080/03650340.2020.1735628. [DOI] [Google Scholar]
- 59.Bhatt K., Maheshwari D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech. 2020;10:36. doi: 10.1007/s13205-019-2033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hussain A., Arshad M., Zahir Z.A., Asghar M. Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pak. J. Agric. Sci. 2015;52:915–922. [Google Scholar]
- 61.Bhakat K., Chakraborty A., Islam E. Characterization of zinc solubilization potential of arsenic tolerant Burkholderia spp. isolated from rice rhizospheric soil. World J. Microbiol. Biotechnol. 2021;37:39. doi: 10.1007/s11274-021-03003-8. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y., Lambers H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil. 2019;447:135–156. doi: 10.1007/s11104-019-03972-8. [DOI] [Google Scholar]
- 63.Masood F., Ahmad S., Malik A. Role of rhizobacterial bacilli in zinc solubilization. In: Khan S.T., Malik A., editors. Microbial Biofertilizers and Micronutrient Availability. Springer; Cham, Switzerland: 2022. pp. 361–377. [Google Scholar]
- 64.Fasim F., Ahmed N., Parsons R., Gadd G.M. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol. Lett. 2002;213:1–6. doi: 10.1111/j.1574-6968.2002.tb11277.x. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh S., Gandhi M., van Hullebusch E.D., Das A.P. Proteomic insights into Lysinibacillus sp.-mediated biosolubilization of manganese. Environ. Sci. Pollut. Res. 2021;28:40249–40263. doi: 10.1007/s11356-020-10863-4. [DOI] [PubMed] [Google Scholar]
- 66.Batool S., Iqbal A. Phosphate solubilizing rhizobacteria as alternative of chemical fertilizer for growth and yield of Triticum aestivum (Var. Galaxy 2013) Saudi J. Biol. Sci. 2019;26:1400–1410. doi: 10.1016/j.sjbs.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meena V.S., Maurya B.R., Verma J.P., Aeron A., Kumar A., Kim K., Bajpai V.K. Potassium solubilizing rhizobacteria (KSR): Isolation, identification, and K-release dynamics from waste mica. Ecol. Eng. 2015;81:340–347. doi: 10.1016/j.ecoleng.2015.04.065. [DOI] [Google Scholar]
- 68.Yadav A.N. Microbiomes of wheat (Triticum aestivum L.) endowed with multifunctional plant growth promoting attributes. Mol. Microbiol. 2019;15:1–6. [Google Scholar]
- 69.Hocking P.J. Organic acids exuded from roots in phosphorus uptake and aluminum tolerance of plants in acid soils. Advan. Agron. 2001;74:63–97. [Google Scholar]
- 70.Rawat P., Das S., Shankhdhar D., Shankhdhar S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021;21:49–68. doi: 10.1007/s42729-020-00342-7. [DOI] [Google Scholar]
- 71.Vyas P., Gulati A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009;9:174. doi: 10.1186/1471-2180-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mumtaz M.Z., Barry K.M., Baker A.L., Nichols D.S., Ahmad M., Zahir Z.A., Britz M.L. Production of lactic and acetic acids by Bacillus sp. ZM20 and Bacillus cereus following exposure to zinc oxide: A possible mechanism for Zn solubilization. Rhizosphere. 2019;12:100170. doi: 10.1016/j.rhisph.2019.100170. [DOI] [Google Scholar]
- 73.Kumar A., Maurya B.R., Raghuwanshi R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.) Biocatal. Agric. Biotechnol. 2014;3:121–128. doi: 10.1016/j.bcab.2014.08.003. [DOI] [Google Scholar]
- 74.Spohn M., Zeißig I., Brucker E., Widdig M., Lacher U., Aburto F. Phosphorus solubilization in the rhizosphere in two saprolites with contrasting phosphorus fractions. Geoderma. 2020;366:114245. doi: 10.1016/j.geoderma.2020.114245. [DOI] [Google Scholar]
- 75.Su M., Han F., Wu Y., Yan Z., Lv Z., Tian D., Li Z. Effects of phosphate-solubilizing bacteria on phosphorous release and sorption on montmorillonite. Appl. Clay Sci. 2019;181:105227. doi: 10.1016/j.clay.2019.105227. [DOI] [Google Scholar]
- 76.Piotrowska-Długosz A., Charzyński P. The impact of the soil sealing degree on microbial biomass, enzymatic activity, and physicochemical properties in the Ekranic Technosols of Toruń (Poland) J. Soils Sediments. 2015;15:47–59. doi: 10.1007/s11368-014-0963-8. [DOI] [Google Scholar]
- 77.Jiang Y., Wu Y., Hu N., Li H., Jiao J. Interactions of bacterial-feeding nematodes and indole-3-acetic acid (IAA)-producing bacteria promotes growth of Arabidopsis thaliana by regulating soil auxin status. Appl. Soil Ecol. 2020;147:103447. doi: 10.1016/j.apsoil.2019.103447. [DOI] [Google Scholar]
- 78.Choudhury A.R., Choi J., Walitang D.I., Trivedi P., Lee Y., Sa T. ACC deaminase and indole acetic acid producing endophytic bacterial co-inoculation improves physiological traits of red pepper (Capsicum annum L.) under salt stress. J. Plant Physiol. 2021;267:153544. doi: 10.1016/j.jplph.2021.153544. [DOI] [PubMed] [Google Scholar]
- 79.Saleem S., Iqbal A., Ahmed F., Ahmad M. Phytobeneficial and salt stress mitigating efficacy of IAA producing salt tolerant strains in Gossypium hirsutum. Saudi J. Biol. Sci. 2021;28:5317–5324. doi: 10.1016/j.sjbs.2021.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waday Y.A., Aklilu E.G., Bultum M.S., Ancha V.R. Optimization of soluble phosphate and IAA production using response surface methodology and ANN approach. Heliyon. 2022;8:e12224. doi: 10.1016/j.heliyon.2022.e12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Batista B.D., Dourado M.N., Figueredo E.F., Hortencio R.O., Marques J.P.R., Piotto F.A., Bonatelli M.L., Settles M.L., Azevedo J.L., Quecine M.C. The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom) Arch. Microbiol. 2021;203:3869–3882. doi: 10.1007/s00203-021-02361-z. [DOI] [PubMed] [Google Scholar]
- 82.Spaepen S., Vanderleyden J., Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 83.Mumtaz M.Z., Ahmad M., Jamil M., Asad S.A., Hafeez F. Bacillus strains as potential alternate for zinc biofortification of maize grains. Int. J. Agric. Biol. 2018;20:1779–1786. [Google Scholar]
- 84.Olanrewaju O.S., Glick B.R., Babalola O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017;33:197. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hrubcová M., Cvikrová M., Eder J., Zoń J., Macháčková I. Effect of inhibition of phenylpropanoid biosynthesison peroxidase and IAA-oxidase activities and auxin contentin alfalfa suspension cultures. Plant Physiol. Biochem. 2000;38:949–956. doi: 10.1016/S0981-9428(00)01208-0. [DOI] [Google Scholar]
- 86.Dubey R.C., Khare S., Kumar P., Maheshwari D.K. Combined effect of chemical fertilisers and rhizosphere-competent Bacillus subtilis BSK17 on yield of Cicer arietinum. Arch. Phytopathol. Pflanzenschutz. 2014;47:2305–2318. doi: 10.1080/03235408.2013.876744. [DOI] [Google Scholar]
- 87.Pereira S.I., Castro P.M. Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol. Eng. 2014;73:526–535. doi: 10.1016/j.ecoleng.2014.09.060. [DOI] [Google Scholar]
- 88.Ramesh A., Sharma S.K., Sharma M.P., Yadav N., Joshi O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014;73:87–96. doi: 10.1016/j.apsoil.2013.08.009. [DOI] [Google Scholar]
- 89.Santos R.M., Kandasamy S., Rigobelo E.C. Sugarcane growth and nutrition levels are differentially affected by the application of PGPR and cane waste. MicrobiologyOpen. 2018;7:e00617. doi: 10.1002/mbo3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kusale S.P., Attar Y.C., Sayyed R.Z., Malek R.A., Ilyas N., Suriani N.L., El Enshasy H.A. Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecules. 2021;26:1894. doi: 10.3390/molecules26071894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nithyapriya S., Lalitha S., Sayyed R.Z., Reddy M.S., Dailin D.J., El Enshasy H.A., Herlambang S. Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability. 2021;13:5394. doi: 10.3390/su13105394. [DOI] [Google Scholar]
- 92.Bhattacharyya C., Banerjee S., Acharya U., Mitra A., Mallick I., Haldar A., Ghosh A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020;10:15536. doi: 10.1038/s41598-020-72439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.López-Hernández J., García-Cárdenas E., López-Bucio J.S., Jiménez-Vázquez K.R., de la Cruz H.R., Ferrera-Rodríguez O., López-Bucio J. Screening of Phosphate Solubilization Identifies Six Pseudomonas Species with Contrasting Phytostimulation Properties in Arabidopsis Seedlings. Microb. Ecol. 2022;86:431–445. doi: 10.1007/s00248-022-02080-y. [DOI] [PubMed] [Google Scholar]
- 94.Akhtar N., Ilyas N., Yasmin H., Sayyed R.Z., Hasnain Z., Elsayed E.A., El Enshasy H.A. Role of Bacillus cereus in improving the growth and phytoextractability of Brassica nigra (L.) K. Koch in chromium contaminated soil. Molecules. 2021;26:1569. doi: 10.3390/molecules26061569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swarnalakshmi K., Yadav V., Tyagi D., Dhar D.W., Kannepalli A., Kumar S. Significance of plant growth promoting rhizobacteria in grain legumes: Growth promotion and crop production. Plants. 2020;9:1596. doi: 10.3390/plants9111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The partial gene sequences of Bacillus cereus strains SM2 (OQ443230), SM4 (OQ443233), SM5 (OQ443239), MS1 (OQ455713), MS2 (OQ455715), and MS4 (OQ455717), Staphylococcus saprophyticus strain SM7 (OQ443241) and Staphylococcus haemolyticus strain MS7 (OQ455720) can be availed using Gen Bank Accession Numbers, which were deposited in the NCBI database.