Abstract
Lowering blood cholesterol levels is crucial for reducing the risk of cardiovascular disease in patients with familial hypercholesterolemia. To develop Perilla frutescens (L.) Britt. leaves as a functional food with a cholesterol-lowering effect, in this study, we collected P. frutescens (L.) Britt. leaves from different regions of China and Republic of Korea. On the basis of the extraction yield (all components; g/kg), we selected P. frutescens (L.) Britt. leaves from Hebei Province, China with an extract yield of 60.9 g/kg. After evaluating different concentrations of ethanol/water solvent for P. frutescens (L.) Britt. leaves, with luteolin 7-glucuronide as the indicator component, we selected a 30% ethanol/water solvent with a high luteolin 7-glucuronide content of 0.548 mg/g in Perilla. frutescens (L.) Britt. leaves. Subsequently, we evaluated the cholesterol-lowering effects of P. frutescens (L.) Britt. leaf extract and luteolin 7-glucuronide by detecting total cholesterol in HepG2 cells. The 30% ethanol extract lowered cholesterol levels significantly by downregulating 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression. This suggests that P. frutescens (L.) Britt leaves have significant health benefits and can be explored as a potentially promising food additive for the prevention of hypercholesterolemia-related diseases.
Keywords: Perilla frutescens (L.) Britt., luteolin-7-diglucuronide, hypercholesterolemia cholesterol-lowering effect
1. Introduction
Familial hypercholesterolemia is a genetic, lipid-related, monogenic, and autosomal dominant disorder [1]. It is characterized by elevated low-density lipoprotein cholesterol (LDL-C) levels, premature atherosclerotic cardiovascular disease (ASCVD), and high mortality. Hypercholesterolemia contributes to a higher risk of atherosclerotic cardiovascular disease than other causes of dyslipidemia at all LDL cholesterol levels [2]. Preventing ASCVD in individuals with hypercholesterolemia usually requires lifelong adherence to cholesterol-lowering therapies [3]. Biosynthesis of cholesterol in the liver is mainly controlled by sterol regulatory element-binding protein 2 in order to modulate the expression of HMG-CoA reductase, one of the main enzymes of cholesterol synthesis [4]. Therefore, controlling cholesterol metabolism by regulating the expression of HMG-CoA reductase is necessary to suppress secondary diseases (cardiovascular and cerebrovascular diseases) caused by high cholesterol.
Statins, among the most widely used drugs worldwide, reduce LDL-C by 30–50% on average [5]. Although statins are generally considered “very safe and well tolerated”, concerns have arisen regarding the management of certain patient groups owing to reports of muscular complications, increased risk of diabetes, and temporary elevation of liver aminotransferase levels. These concerns significantly affect patients’ quality of life, causing considerable inconvenience [6,7]. The utilization of natural medicines for the treatment of various diseases and disorders has a long history dating back to ancient times. Therefore, the prominent utilization of herbal drugs as a fundamental approach is essential for the management, prevention, and treatment of hypercholesterolemia. Therefore, there is an urgent need to discover bioactive substances from natural products that lower blood cholesterol while producing few side effects.
Perilla frutescens (L.) Britt., an annual herbal plant belonging to the mint family Lamiaceae, is extensively cultivated in various Asian countries, including China, Japan, South Korea, Vietnam, and India [8]. The traditional uses of P. frutescens (L.) Britt. include two aspects: culinary and medicinal uses. In terms of culinary uses, the utilization of P. frutescens (L.) Britt. leaves as a culinary aromatic for fish preparations has a historical trajectory spanning over 2000 years in China [9]. Furthermore, P. frutescens (L.) Britt. serves as a representative flavor in Japan and a spicy vegetable in Korea [10]. Additionally, the seeds of P. frutescens (L.) Britt. hold significant global importance as a primary reservoir of perilla oil, owing to their substantial omega-3 fatty acid content. In terms of medicinal uses, according to the Chinese Pharmacopeia 2015, different parts of P. frutescens (L.) Britt. have been utilized as natural herbal medicines to alleviate various symptoms. P. frutescens (L.) Britt. leaves are noted for their therapeutic attributes, including the capacity to disperse surface pathogenic factors, alleviate cold conditions, and promote gastric function. P. frutescens (L.) stems are recognized for their potential to promote qi circulation, alleviate pain, and assist in ensuring a safe pregnancy. Moreover, P. frutescens (L.) seeds are acknowledged for their efficacy in promoting qi circulation, resolving phlegm, alleviating coughs, easing respiratory distress, and facilitating intestinal regularity [11]. Contemporary famous doctors created multiple new prescriptions based on ancient classic prescriptions. On the basis of San-Zi-Yang-Qin decoction, Raphani semen, Sinapis semen, and P. frutescens (L.) Britt were added to a cure for nonalcoholic fatty liver disease [12]. Many studies have revealed the pharmacological properties of P. frutescens (L.) Britt., including its antioxidant, antibacterial, antifungal, antiallergic, antidepressant, anti-inflammatory, and antitumor effects [13,14,15,16,17]. Over 200 phytoconstituents have been isolated from P. frutescens (L.) Britt., including alkaloids, phenylpropanoids, terpenoids, polyphenolic compounds, and flavonoids [8]. Furthermore, studies have shown that the total flavonoid extract of P. frutescens mainly contains luteolin 7-glucuronide, caffeic acid, scutellarin, apigenin-7-glucuronide, and rosmarinic acid, which inhibited hyperlipidemia in rats fed with a high-fat diet [10,18,19,20]. Therefore, P. frutescens (L.) Britt. leaves are a promising source of new functional food ingredients to lower cholesterol levels and improve hypercholesterolemia treatments.
Therefore, in this study, the extraction yields (all components; %) of P. frutescens (L.) Britt. leaves from China and Republic of Korea were evaluated, using luteolin 7-glucuronide as an indicator component to establish a quantitative method for luteolin 7-glucuronide; the extraction solvent was selected on the basis of the content of luteolin 7-glucuronide (mg/g in P. frutescens (L.) Britt. leaves). The cholesterol-lowering effect of P. frutescens (L.) Britt. leaf extract and luteolin 7-glucuronide was studied by detecting total cholesterol in HepG2 cells. This study indicated that P. frutescens (L.) Britt. leaf extract had good activity in terms of its cholesterol-lowering effect, which provides a theoretical basis for the development of P. frutescens (L.) Britt. leaves as a functional food for their cholesterol-lowering effect.
2. Results and Discussion
2.1. Method Validation
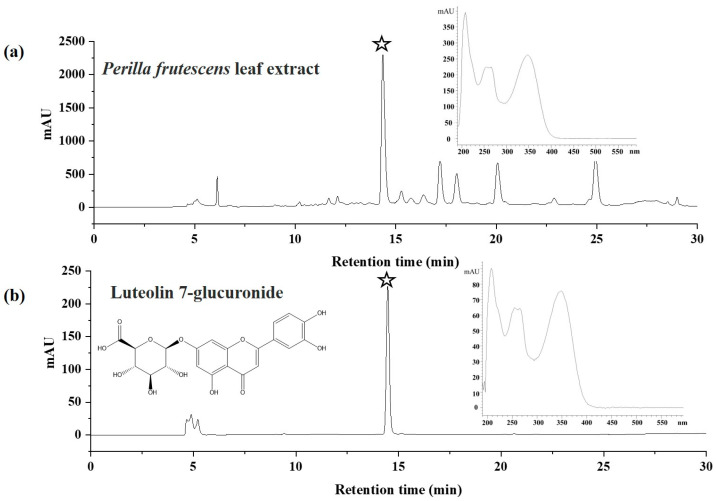
Luteolin 7-glucuronide had the same retention time (14.48 min) as the major compounds in the P. frutescens (L.) Britt. leaf extract, and when scanned using the UV pattern, these two peaks exhibited the same UV spectrum (Figure 1). This result was similar to that of a previous report by Fan [19]. Therefore, the major compound with retention time 14.48 min in the P. frutescens (L.) Britt. leaf extract was luteolin 7-glucuronide. As shown in Figure 2, P. frutescens (L.) Britt. leaf extract and luteolin 7-glucuronide had a good effect in terms of lowering cholesterol, so we used luteolin 7-glucuronide as the indicator component to optimize the extract solution.
Figure 1.
HPLC spectra of 30% ethanol extract of P. frutescens (L.) Britt. leaves from Hebei Province, China (a) and luteolin 7-glucuronide (b), stars in the figure are the target compound in the 30% ethanol extract of P. frutescens (L.) Britt. leaves.
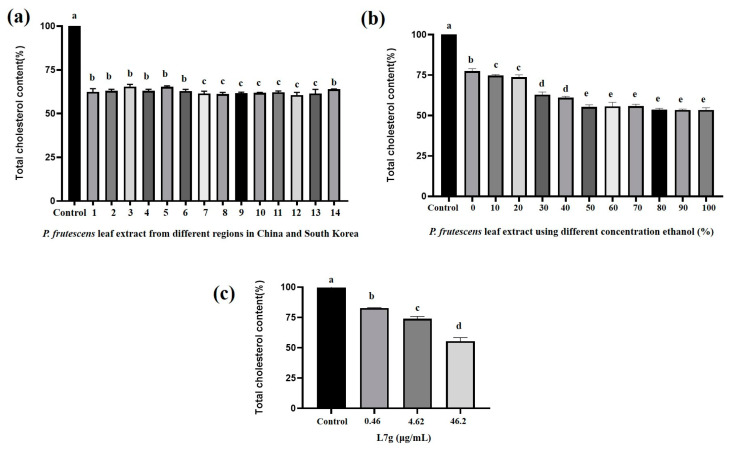
Figure 2.
Cholesterol-lowering effects of P. frutescens (L.) Britt. leaf extract from different regions in China and Republic of Korea (a); P. frutescens (L.) Britt. leaf extract using different concentrations of ethanol (b) (control used 50% DMSO; samples were 10 μg/mL in 50% DMSO); and luteolin 7-glucuronide (L7g) (c). Bars with different letters show significant differences between groups (p < 0.05) determined with the analysis of variance and Dunnett’s multiple comparison test.
According to the International Conference on Harmonization guidelines ICH Q2, we developed an HPLC method for the quantitative analysis of luteolin 7-glucuronide in P. frutescens (L.) Britt. leaf extract. Linear regression analysis for luteolin 7-glucuronide was performed by plotting the peak area (y) against the concentration (x, μg/mL) of luteolin 7-glucuronide standard solutions (Table 1). To assess the performance of the proposed method, analytical parameters were measured (Table 1). A satisfactory linearity was obtained in the range of 0.98–980 μg/mL with a determination coefficient of 0.999. The limit of quantification (LOQ) and limit of detection (LOD) for luteolin 7-glucuronide were determined to be three and ten times the signal-to-noise ratio, respectively. On the basis of these calculations, the LOD for luteolin 7-glucuronide was 6 μg/mL, whereas the LOQ was 17 μg/mL, indicating that the analytical method was acceptable with sufficient sensitivity. The relative standard deviation (RSD) values of the peak area of luteolin 7-glucuronide were 0.99–2.96% (intraday) and 2.03–2.74% (interday), which indicated that the precision of the instruments was good. To further validate the developed method, the spiked recoveries (30, 40, and 50 μg/mL) for the P. frutescens (L.) Britt. leaf extract ranged from 89.66 to 99.50%, and the RSD values were 0.77–4.62%, which indicated that the recovery of the method was good (Table 2). These parameters indicated that the HPLC method developed in this study has good precision, stability, repeatability, and accuracy and that it can be used to evaluate the luteolin 7-glucuronide content in P. frutescens (L.) Britt. leaves in different regions of China and Republic of Korea.
Table 1.
Analytical performances of the developed method for luteolin 7-glucuronide from Perilla frutescens (L.) Britt. leaf extract.
| Analyte | Linearity (μg/mL) |
Regression Equation |
R2 | LOD (μg/mL) |
LOQ (μg/mL) |
RSD (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 mg/mL | 5.0 mg/mL | 10 mg/mL | |||||||||
| Intra | Inter | Intra | Inter | Intra | Inter | ||||||
| luteolin 7- glucuronide |
0.98–980 | Y = 22,850 X + 185.59 | 0.999 | 6 | 17 | 0.99 | 2.03 | 2.96 | 2.39 | 1.54 | 2.74 |
Table 2.
Recovery of luteolin 7-glucuronide using the developed method for the spiked P. frutescens (L.) Britt. leaf extract.
| Analyte | 30 μg/mL | 40 μg/mL | 50 μg/mL | |||
|---|---|---|---|---|---|---|
| Recovery (%) |
RSD (%) |
Recovery (%) |
RSD (%) |
Recovery (%) |
RSD (%) |
|
| luteolin 7- glucuronide |
89.66 | 4.62 | 94.79 | 3.05 | 99.50 | 0.77 |
2.2. Selection of Origin of Perilla frutescens (L.) Britt. Leaves and Optimization of Extraction Conditions
P. frutescens (L.) Britt. leaves are mainly produced in China, Japan, North Korea, and South Korea. Due to the epidemic, we only collected 14 types of P. frutescens (L.) Britt. leaves from different regions of China (2 types) and Republic of Korea (12 types). After drying, all P. frutescens (L.) Britt. leaves were extracted using the maceration method. The extraction yield (all components; g/kg) and luteolin 7-glucuronide content (mg/g in P. frutescens (L.) Britt. leaves; dry weight) are shown in Table 3. The extraction yield of P. frutescens (L.) Britt. leaves from different regions and the luteolin 7-glucuronide content in the extract ranged from 23.6 to 60.9 g/kg and 1.10 to 29.73 mg/g, respectively. The highest extraction yield (60.9 g/kg) was observed in P. frutescens (L.) Britt. leaves from Hebei Province, China. According to the highest extraction yield (g/kg) of the final product and the cost and continuous supply of P. frutescens (L.) Britt. leaves, leaves from Hebei Province, China were chosen for subsequent experiments.
Table 3.
Extract yield (%) and luteolin 7-glucuronide (mg/g) content in P. frutescens (L.) Britt. leaves from different regions in China and Republic of Korea.
| Sample No. | Location | Extract Yield (g/kg) |
Luteolin 7-Glucuronide (mg/g in P. frutescens (L.) Britt. Leaves) |
|---|---|---|---|
| 1 | Goesan-gun, Chungcheongbuk-do, Republic of Korea | 31.7 | 0.539 ± 0.001 |
| 2 | Jecheon-si, Chungcheongbuk-do, Republic of Korea | 34.2 | 0.549 ± 0.005 |
| 3 | Taean-gun, Chungcheongnam-do, Republic of Korea | 34.5 | 0.325 ± 0.001 |
| 4 | Haenam-gun, Jeollanam-do, Republic of Korea | 36.2 | 0.689 ± 0.008 |
| 5 | Gokseong-gun, Jeollanam-do, Republic of Korea | 35.2 | 0.285 ± 0.003 |
| 6 | Jangsu-gun, Jeollabuk-do, Republic of Korea | 47.2 | 0.391 ± 0.026 |
| 7 | Yeongcheon-si, Gyeongsangbuk-do, Republic of Korea | 26.3 | 0.406 ± 0.013 |
| 8 | Gapyeong-gun, Gyeonggi-do, Republic of Korea | 31.4 | 0.059 ± 0.026 |
| 9 | Uiseong-gun, Gyeongsangbuk-do, Republic of Korea | 29.7 | 0.758 ± 0.076 |
| 10 | Nonsan-si, Chungcheongnam-do, Republic of Korea | 44.9 | 1.335 ± 0.076 |
| 11 | Namwon-si, Jeollabuk-do, Republic of Korea | 22.8 | 0.127 ± 0.007 |
| 12 | Jangheung-gun, Jeollanam-do, Republic of Korea | 27.6 | 0.293 ± 0.012 |
| 13 | Anguo City, Hebei Province, China | 60.9 | 0.445 ± 0.001 |
| 14 | Guangzhou City, Guangdong Province, China | 23.6 | 0.026 ± 0.001 |
Studies have shown that the total flavonoid extract of P. frutescens, which mainly contains apigenin and luteolin, inhibited hyperlipidemia in rats fed with a high-fat diet [10]. However, the main ingredient capable of lowering cholesterol was ignored. Our research showed that luteolin 7-glucuronide was the main compound in P. frutescens (L.) Britt. leaf extract, so we chose luteolin 7-glucuronide as the index component to optimize the extraction conditions of P. frutescens (L.) Britt. leaves. As established knowledge dictates, solvent selection, extraction temperature, and extraction time affected the yield of extraction processes. Nevertheless, it was imperative to underscore that the solvent’s inherent characteristics loom as the predominant determinants influencing extraction efficiency, owing to the proclivity of secondary metabolites within plant materials to be preferentially extracted by solvents possessing congruent chemical attributes [21]. Considering the need for the development of functional foods, we opted to extract P. frutescens (L.) Britt. leaves using an ethanol/water solution which was less toxic to the human body as the extraction solvent.
In our study, we used water as a solvent with different concentrations of ethanol (0–100%) to obtain P. frutescens (L.) Britt. leaf extract. We calculated the extraction yield (all components; g/kg) and luteolin 7-glucuronide content (mg/g in P. frutescens (L.) Britt. leaves; Table 4) obtained using solvents with varying ethanol concentrations. The extraction yield (g/kg) was 24.0–62.7 g/kg, whereas the luteolin 7-glucuronide content was 0.013–0.548 mg/g. When the extraction solvent was 30% ethanol, P. frutescens (L.) Britt. leaf extract obtained the maximum extraction yield (62.7 g/kg) and the maximum luteolin 7-glucuronide content (0.548 mg/g). As the ethanol concentration increased, the solvent polarity decreased. Solvents with ethanol proportions less than 30% exhibited stronger polarity, whereas those with ethanol proportions greater than 30% exhibited weaker polarity. As reported in the literature, flavonoids and phenolic compounds were easily extracted from highly polar solvents [11,22]. To obtain a higher luteolin 7-glucuronide content and more flavonoid active ingredients, the cholesterol-lowering effect of the extract of P. frutescens (L.) Britt. leaves in 30% ethanol was investigated.
Table 4.
Luteolin 7-glucuronide (mg/g) content in P. frutescens (L.) Britt. leaves extracted using different ethanol concentrations.
| Sample No. | Ethanol Concentration (%) |
Extract Yield (g/kg) | Luteolin 7-Glucuronide (mg/g in P. frutescens (L.) Britt. Leaves) |
|---|---|---|---|
| 1 | 0 | 47.1 | 0.171 ± 0.017 |
| 2 | 10 | 62.1 | 0.154 ± 0.006 |
| 3 | 20 | 59.9 | 0.250 ± 0.005 |
| 4 | 30 | 62.7 | 0.548 ± 0.009 |
| 5 | 40 | 57.1 | 0.548 ± 0.005 |
| 6 | 50 | 59.4 | 0.540 ± 0.016 |
| 7 | 60 | 56.9 | 0.513 ± 0.016 |
| 8 | 70 | 56.3 | 0.251 ± 0.028 |
| 9 | 80 | 52.2 | 0.046 ± 0.020 |
| 10 | 90 | 35.2 | 0.017 ± 0.001 |
| 11 | 100 | 24.0 | 0.013 ± 0.001 |
2.3. Cholesterol-Lowering Effects of Luteolin 7-Glucuronide and P. frutescens (L.) Britt. Leaf Extract
Figure 2a presents the cholesterol-lowering effects of P. frutescens (L.) Britt. leaf extracts from different regions of China and Republic of Korea. Compared with the control group, the groups treated with P. frutescens (L.) Britt. leaf extracts exhibited statistically significant activity in terms of displaying a cholesterol-lowering effect; however, no significant difference was observed in the cholesterol-lowering effects of extracts from different countries or regions. Figure 2b shows the cholesterol-lowering effects of P. frutescens (L.) Britt. leaf extracts obtained using solvents with different ethanol concentrations. Compared with the cholesterol-lowering effect in the control group, the cholesterol-lowering effects of P. frutescens (L.) Britt. leaf extracts increased as the ethanol concentration increased, and the difference was statistically significant. The P. frutescens (L.) Britt. leaf extract showed a significant cholesterol-lowering effect compared with the control treatment. With the increase in ethanol concentration (%), the cholesterol-lowering effect of P. frutescens (L.) Britt. leaf extracts was significantly enhanced and entered a plateau at 50% ethanol. Figure 2c shows the cholesterol-lowering effects of different concentrations of luteolin 7-glucuronide. Compared with the control treatment, luteolin 7-glucuronide exhibited a statistically significant cholesterol-lowering effect. The cholesterol-lowering effect of luteolin 7-glucuronide was dose-dependent.
Although luteolin 7-glucuronide exhibited good activity in terms of lowering cholesterol, luteolin 7-glucuronide was not the only compound in P. frutescens (L.) Britt. leaf extract that exhibited cholesterol-lowering effects, since the luteolin 7-glucuronide content in P. frutescens (L.) Britt. leaf extracts and the cholesterol-lowering effect did not show the same trend. Starting from 70% ethanol extract, the content of luteolin 7-glucuronide in P. frutescens (L.) Britt. leaves decreased, but their cholesterol-lowering effect did not change significantly. This result showed that there are other components in P. frutescens (L.) Britt. leaves that have a cholesterol-lowering effect. In our prior investigations, we identified eleven compounds in P. frutescens (L.) Britt. leaf extract, specifically protocatechuic acid, chlorogenic acid, caffeic acid, 4-methoxycinnamic acid, oleanolic acid, kaempferol-3-O-rutinoside, rosmarinic acid, luteolin, methyl-rosmarinic acid, apigenin, and 4′,5,7-trimethoxyflavone [23]. Notably, protocatechuic acid, chlorogenic acid, and caffeic acid have demonstrated their cholesterol-lowering effects through the inhibition of HMG-CoA reductase [24,25,26]. In the study conducted by Feng et al., it was substantiated that P. frutescens (L.) Britt. leaves contain apigenin and its analogues. Administration of oral dosages ranging from 50 to 200 mg/kg demonstrated their capacity to mitigate blood lipid levels and lipid accumulation within adipose tissues in experimental rodents. Moreover, it manifested the inhibition of the formation of lipid peroxidation products, amelioration of disturbances in lipoprotein metabolism, enhancement of antioxidant enzyme activity, and attenuation of hyperlipidemia incidence [18]. While previous investigations have established the cholesterol-lowering properties of apigenin, chlorogenic acid, and caffeic acid in P. frutescens (L.) Britt. leaves, it is noteworthy that luteolin 7-glucuronide is the main compound in P. frutescens (L.) Britt. leaf extract, and it has a good cholesterol-lowering effect. We continue to investigate the cholesterol-lowering effect mechanism of luteolin 7-glucuronide and P. frutescens (L.) Britt. leaf extract in this study.
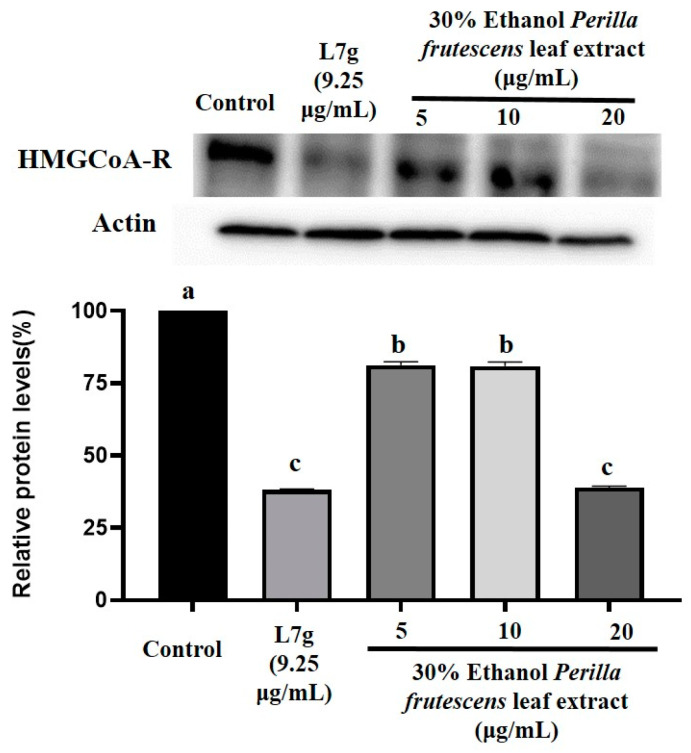
The liver is widely recognized as the primary organ responsible for cholesterol synthesis [27]. HMG-CoA reductase plays a crucial role in the synthesis of cholesterol in the liver [28]. Additionally, HepG2 cells primarily regulate the expression of HMG-CoA reductase and hepatic glycerolipid lipase [29]. Consequently, HepG2 cells can be used to assess the effectiveness of cholesterol-lowering treatments. In recent years, HepG2 cells have been widely used by researchers to assess cholesterol-lowering effects. For instance, Shuming Kou et al. employed HepG2 cells and high-cholesterol hamsters to investigate the synergistic cholesterol-lowering effect of five major alkaloids [30]. Similarly, Yunying Huang et al. utilized HepG2 cells to explore the cholesterol-lowering mechanism of bergamot extract [31]. To better understand the cholesterol-lowering mechanism of P. frutescens (L.) Britt. leaf extract and luteolin 7-glucuronide, we evaluated the expression of HMG-CoA reductase (Figure 3). Compared with the control group treatment, luteolin 7-glucuronide and the P. frutescens (L.) Britt. leaf extract significantly downregulated the expression of HMG-CoA reductase, and the difference was statistically significant. Moreover, when the concentration of P. frutescens (L.) Britt. leaf extract was 20 μg/mL, its downregulation effect on HMG-CoA reductase expression was equivalent to that of luteolin 7-glucuronide (9.25 μg/mL), with no statistical difference. P. frutescens (L.) Britt. leaf extract and luteolin 7-glucuronide significantly downregulated the expression of HMG-CoA reductase in HepG2 cells. Mansoureh Tavan’s research showed that after L929 cells were treated with different concentrations of P. frutescens (L.) Britt. leaf water extract (37.5–600 μg/mL), the cell survival rate was still greater than 90%, which indicated that P. frutescens (L.) Britt. leaf water extract had no toxicity to normal cells [32]. Puchadapirom’s research showed that after V79 cells were treated with different concentrations of P. frutescens (L.) Britt. leaf extract (100–250 μg/mL), the cell survival rate was still greater than 90% [33]. Lapatrada Mungmai’s results demonstrated the straightforward antimelanogenic effects of P. frutescens (L.) Britt. leaf extract at the optimum concentration (1.25–40 μg/mL) on B16F10 cells without inducing cytotoxicity or death of cells [34]. According to current research, there are currently no studies showing the cytotoxicity of P. frutescens (L.) Britt. leaf extract. Therefore, we concluded that P. frutescens (L.) Britt. leaf extract and luteolin 7-glucuronide could reduce cholesterol levels by downregulating the expression of HMG-CoA reductase. P. frutescens (L.) Britt. leaves have the potential to be developed as a functional food with a cholesterol-lowering effect. This study provides a theoretical basis for the further development of P. frutescens (L.) Britt. leaves.
Figure 3.
Effects of P. frutescens (L.) Britt. leaf extract (30% ethanol) from Hebei Province, China and luteolin 7-glucuronide (L7g) on lowering HMG-CoA reductase content in HepG2 cells. Control used 50% DMSO. Bars with different letters show significant differences between groups (p < 0.05) determined with analysis of variance and Dunnett’s multiple comparison test.
3. Materials and Methods
3.1. Materials and Reagents
Cell lysis buffer, chemiluminescence kit, fetal bovine serum, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase) antibody, luteolin 7-glucuronide, L-glutamine, NP-40, penicillin-streptomycin, phosphate-buffered saline with 0.05% TWEEN® 20, pH 7.4 (PBST), SDS polyacrylamide, trifluoroacetic acid, and trypsin were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MI, USA). HepG2 (human hepatocellular carcinoma) cells were purchased from American Type Culture Collection. The EZ Total Cholesterol Assay Kit was purchased from Abcam Co. (Cambridge, UK). Acetonitrile, chloroform, ethanol, and isopropanol were purchased from J. T. Baker Co. (Phillipsburg, NJ, USA). Ultrapure water used in this study was obtained from a Milli-Q water purification system from Millipore Co. (Bedford, MA, USA).
P. frutescens (L.) Britt. leaves were collected from Goesan-gun, Chungcheongbuk-do, Jecheon-si, Chungcheongbuk-do, Taean-gun, Chungcheongnam-do, Haenam-gun, Jeollanam-do, Gokseong-gun, Jeollanam-do, Jangsu-gun, Jeollabuk-do, Yeongcheon-si, Gyeongsangbuk-do, Gapyeong-gun, Gyeonggi-do, Uiseong-gun, Gyeongsangbuk-do, Nonsan-si, Chungcheongnam-do, Namwon-si, Jeollabuk-do, Jangheung-gun, and Jeollanam-do in Republic of Korea and Hebei Province and Guangdong Province in China in September 2022. The specimens were authenticated by Emeritus Professor H. J. Chi, Seoul National University, Republic of Korea. Dried P. frutescens (L.) Britt. leaves (L-2022-PF1-14) and voucher sample (RIC-2012-5) were stored at the Center for Efficacy Assessment and Development of Functional Foods and Drugs (Room 8510) at Hallym University.
3.2. Preparation of Perilla frutescens Leaf Extract
Dried P. frutescens (L.) Britt. leaves (1 g) from different regions (China and Republic of Korea) were successively extracted three times with 20 mL of 30% ethanol at 70 °C for 7 h using the maceration method. Dried P. frutescens (L.) Britt. leaves (1 g) from Hebei Province, China were extracted in 20 mL of different concentrations of ethanol at 70 °C for 7 h. The extract solution was filtered with filter paper and evaporated to dryness via rotary evaporation at 37 °C. Every sample was extracted three times. The yield was calculated using the following formula:
| Extract yield (g/kg) = extract weight (g)/sample weight (g) × 1000 |
3.3. HPLC Analysis of Luteolin 7-Glucuronide
The P. frutescens (L.) Britt. leaf extract (2 mg) was dissolved in methanol to a concentration of 2 mg/mL and filtered through a 0.2 μm polyvinyl difluoride (PVDF) syringe filter. Luteolin 7-glucuronide was prepared at 1 μg/mL in methanol. Agilent 1100 series HPLC/UV–Vis/MSD (Santa Clara, CA, USA) was used for analyzing the samples and standard solutions. The HPLC system was equipped with an auto-degasser, quaternary pump, autosampler, column thermostat, and diode array detector (DAD). The HPLC mobile phases used were acidic water (0.1% trifluoroacetic acid (95%) + acetonitrile (5%); (A) and acetonitrile (95%) + 0.1% trifluoroacetic acid (5%) (B). The P. frutescens (L.) Britt. leaf extract and luteolin 7-glucuronide solutions were analyzed at 254 nm and separated with a flow rate of 0.7 mL/min using a CAPCELL PAK DD Type C8 column (250 × 4.6 mm, 5 μm). The separation process was as follows: 0–15% B for 0–5 min, 15–25% B for 5–20 min, 25–100% B for 20–30 min, and 100% B for 30–35 min. The spectra of luteolin 7-glucuronide were analyzed at a working wavelength range of 190–400 nm.
3.4. Method Validation
To evaluate the quality of the analytical method, validation studies were performed using the optimized HPLC method according to the International Conference on Harmonization guidelines ICH Q2 (R2) [35]. The method was validated for precision, stability, repeatability, accuracy, LOQ, LOD, and calibration curves of luteolin 7-glucuronide.
3.5. Cell Culture
HepG2 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, and 4 mM L-glutamine. The cells were cultured at 37 °C in a humidified atmosphere of 95% air to 5% CO2 [31].
3.6. Cellular Cholesterol Content Analysis
HepG2 cells were seeded in 6-well plates and incubated in Dulbecco’s modified Eagle’s medium in the absence or presence of P. frutescens (L.) Britt. leaf extract (different regions and different ethanol concentration extracts from Hebei Province, China; 10 μg/mL) and different concentrations of luteolin 7-glucuronide (0.46, 4.62, and 46.2 μg/mL) for 24 h. Cholesterol was measured using the EZ Total Cholesterol Assay Kit according to the manufacturer’s instructions. The cells were isolated using trypsin, washed with PBS, and centrifuged. Subsequently, 200 μL of chloroform: isopropanol: NP-40 (7:11:0.1) solution was added to the cell pellet, and the cells were homogenized on ice before being centrifuged at 15,000× g for 10 min in a microcentrifuge. The liquid (organic phase) was transferred to another tube, leaving the pellet, and dried at 50 °C to remove the chloroform. The samples were then vacuum-dried for 30 min to remove any trace organic solvents. Next, 200 μL of cholesterol assay buffer was added to the dried lipid and dissolved with sonication until the solution became turbid. The sample was subsequently transferred to a 96-well plate, and the absorbance was measured at 570 nm. All determinations were performed via replicate experiments with triplicate analysis.
3.7. Western Blotting
HepG2 cells were seeded in 6-well plates and incubated in Dulbecco’s modified Eagle’s medium in the absence or presence of different concentrations (5, 10, and 20 μg/mL) of P. frutescens (L.) Britt. leaf extract from Hebei Province, China and luteolin 7-glucuronide (9.25 μg/mL) for 24 h, and Western blot analysis was performed on the cells. Luteolin 7-glucuronide was used as the positive control, and the control group remained untreated. The cells were separated using trypsin, washed with PBS, and centrifuged. The cell pellet was then treated with a cell lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 0.25% sodium deoxycholate; and 1% NP-40; supplemented with a protease inhibitor cocktail) to lyse the cells. After centrifugation, the cell extracts were prepared. The protein extract (25 μg) was separated via electrophoresis on an SDS polyacrylamide gel. The proteins in the gel were then electrotransferred to an immunoblot PVDF membrane, which was subsequently incubated with HMG-CoA reductase antibody and washed with PBST. Horseradish peroxidase-conjugated secondary antibodies were added and incubated before washing, and the protein bands were removed. Finally, the bands were visualized using an enhanced chemiluminescence kit. Band intensity was quantified using ImageJ software (https://ij.imjoy.io/, accessed on 7 October 2023) to determine the protein concentration of HMG-CoA reductase. All determinations were performed via replicate experiments with triplicate analysis.
3.8. Statistical Analysis
All data are expressed as the mean values ± standard deviation (SD). Differences between groups were compared using the Statistical Package for Social Science (SPSS 25.0) with one-way analysis of variance, and post hoc comparisons were evaluated using Dunnett’s test. All statistical tests were two-sided, and the significance level was set at p < 0.05.
4. Conclusions
In this study, the source of P. frutescens (L.) Britt. leaves, that is, Hebei Province, China, was selected on the basis of the extraction yield (%), and 30% ethanol was selected as the solvent for the extraction of P. frutescens (L.) Britt. leaves on the basis of the content of luteolin 7-glucuronide (mg/g in P. frutescens (L.) Britt. leaves) as the indicator component. The cholesterol-lowering effects of P. frutescens (L.) Britt. leaf extract and luteolin 7-glucuronide were evaluated, and the results show that the 30% ethanol extract was effective in reducing cholesterol levels by downregulating the expression of HMG-CoA reductase. This suggests that P. frutescens (L.) Britt leaves have significant health benefits and can be explored as a potentially promising food additive for the prevention of hypercholesterolemia-related diseases.
Author Contributions
Conceptualization, J.L. and S.L. (Soonsung Lim); methodology, S.L. (Sangyoun Lee) and B.K.; software, Z.W.; validation, S.L. (Sangyoun Lee), Z.W. and K.K.; formal analysis, S.L. (Sangyoun Lee) and S.L. (Sookyeong Lee); investigation, Z.W., S.L. (Sangyoun Lee) and B.K.; resources, S.L. (Soonsung Lim); data curation, S.L. (Soonsung Lim); writing—original draft preparation, Z.W.; writing—review and editing, S.L. (Soonsung Lim); visualization, S.L. (Soonsung Lim); supervision, J.L.; project administration, J.L. and S.L. (Soonsung Lim); funding acquisition, J.L. and S.L. (Soonsung Lim). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
All the samples are available from the authors.
Funding Statement
This research was funded by the Collabo R&D between Industry, Academy, and Research Institute funded by the Korea Ministry of SMEs and Startups, grant number S3248829, 2022; and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, grant number 2021R1A6A1A03044501.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jones L.K., Sturm A.C., Seaton T.L., Gregor C., Gidding S.S., Williams M.S., Rahm A.K. Barriers, facilitators, and solutions to familial hypercholesterolemia treatment. PLoS ONE. 2020;15:e0244193. doi: 10.1371/journal.pone.0244193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khera A.V., Won H.H., Peloso G.M., Lawson K.S., Bartz T.M., Deng X., van Leeuwen E.M., Natarajan P., Emdin C.A., Bick A.G., et al. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J. Am. Coll. Cardiol. 2016;67:2578–2589. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordestgaard B.G., Chapman M.J., Humphries S.E., Ginsberg H.N., Masana L., Descamps O.S., Wiklund O., Hegele R.A., Raal F.J., Defesche J.C., et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J.S., Sung J.H., Lee S.K. Inhibition of Cholesterol Synthesis in HepG2 Cells by GINST-Decreasing HMG-CoA Reductase Expression Via AMP-Activated Protein Kinase. J. Food Sci. 2017;82:2700–2705. doi: 10.1111/1750-3841.13828. [DOI] [PubMed] [Google Scholar]
- 5.Ruscica M., Ferri N., Banach M., Sirtori C.R., Corsini A. Side effects of statins: From pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc. Res. 2023;118:3288–3304. doi: 10.1093/cvr/cvac020. [DOI] [PubMed] [Google Scholar]
- 6.Bruckert E., Hayem G., Dejager S., Yau C., Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc. Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 7.Cederberg H., Stancakova A., Yaluri N., Modi S., Kuusisto J., Laakso M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: A 6 year follow-up study of the METSIM cohort. Diabetologia. 2015;58:1109–1117. doi: 10.1007/s00125-015-3528-5. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules. 2018;24:102. doi: 10.3390/molecules24010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian S. Perilla frutescens detoxifies the toxicity of fish and crab. Zhong Hua Yang Sheng Bao Jian. 2012;9:71. [Google Scholar]
- 10.Hou T., Netala V.R., Zhang H., Xing Y., Li H., Zhang Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules. 2022;27:3578. doi: 10.3390/molecules27113578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H., Qiu J.F., Ma L.J., Hu Y.J., Li P., Wan J.B. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Pt BFood Chem. Toxicol. 2017;108:375–391. doi: 10.1016/j.fct.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Liu S., Jin X., Shang Y., Wang L., Du K., Chen S., Li J., He J., Fang S., Chang Y. A comprehensive review of the botany, ethnopharmacology, phytochemistry, pharmacology, toxicity and quality control of Perillae Fructus. J. Ethnopharmacol. 2023;304:116022. doi: 10.1016/j.jep.2022.116022. [DOI] [PubMed] [Google Scholar]
- 13.Shin T.Y., Kim S.H., Kim S.H., Kim Y.K., Park H.J., Chae B.S., Jung H.J., Kim H.M. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by Perilla frutescens. Immunopharmacol. Immunotoxicol. 2000;22:489–500. doi: 10.3109/08923970009026007. [DOI] [PubMed] [Google Scholar]
- 14.Chang H.H., Chen C.S., Lin J.Y. Dietary perilla oil lowers serum lipids and ovalbumin-specific IgG1, but increases total IgE levels in ovalbumin-challenged mice. Food Chem. Toxicol. 2009;47:848–854. doi: 10.1016/j.fct.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Kim D.H., Kim Y.C., Choi U.K. Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against Staphylococcus aureus using evolutionary operation factorial design technique. Int. J. Mol. Sci. 2011;12:2395–2407. doi: 10.3390/ijms12042395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao G., Zang S.Y., Jiang Z.H., Chen Y.Y., Ji X.H., Lu B.F., Wu J.H., Qin G.W., Guo L.H. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J. Nutr. Biochem. 2011;22:929–936. doi: 10.1016/j.jnutbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X.J., Yan L.L., Yin P.P., Shi L.L., Zhang J.H., Liu Y.J., Ma C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014;164:150–157. doi: 10.1016/j.foodchem.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 18.Feng L.-J., Yu C.-H., Ying K.-J., Hua J., Dai X.-Y. Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res. Int. 2011;44:404–409. doi: 10.1016/j.foodres.2010.09.035. [DOI] [Google Scholar]
- 19.Fan Y., Cao X., Zhang M., Wei S., Zhu Y., Ouyang H., He J. Quantitative Comparison and Chemical Profile Analysis of Different Medicinal Parts of Perilla frutescens (L.) Britt. from Different Varieties and Harvest Periods. J. Agric. Food Chem. 2022;70:8838–8853. doi: 10.1021/acs.jafc.2c03104. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Guo L., Yang G., Yang A., Zheng Y., Wang L. Metabolomic profiling of developing perilla leaves reveals the best harvest time. Front. Plant Sci. 2022;13:989755. doi: 10.3389/fpls.2022.989755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaves J.O., de Souza M.C., da Silva L.C., Lachos-Perez D., Torres-Mayanga P.C., Machado A., Forster-Carneiro T., Vazquez-Espinosa M., Gonzalez-de-Peredo A.V., Barbero G.F., et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020;8:507887. doi: 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu M., Ran L., Chen N., Fan X., Ren D., Yi L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019;297:124970. doi: 10.1016/j.foodchem.2019.124970. [DOI] [PubMed] [Google Scholar]
- 23.Kwon S.H., Wang Z., Hwang S.H., Kang Y.-H., Lee J.-Y., Lim S.S. Comprehensive evaluation of the antioxidant capacity of Perilla frutescens leaves extract and isolation of free radical scavengers using step-wise HSCCC guided by DPPH-HPLC. Int. J. Food Prop. 2017;20((Suppl. 1)):921–934. doi: 10.1080/10942912.2017.1318289. [DOI] [Google Scholar]
- 24.Heera R., Chandra K., Karishma S., Anita S., Jaykaran C., Paras S., Rajsekhar R., Surajit G. In-vitro and in-silico determinations of HMG-CoA reductase inhibition potential of caffeic acid for therapeutics of hypercholesterolemia. J. Appl. Pharm. Sci. 2022;12:190–198. doi: 10.7324/JAPS.2021.120119. [DOI] [Google Scholar]
- 25.Alexsandra M.H.-R., July A.L.-G., Carmen R.S.-C., William Antonio S.-G., Cesar D.G.-S. In silico Analysis of the Polyphenolic Metabolites of Zea mays L. “Purple Corn” on HMG-CoA Reductase. Pharmacogn. J. 2022;14:549–558. doi: 10.5530/pj.2022.14.70. [DOI] [Google Scholar]
- 26.Hao S., Xiao Y., Lin Y., Mo Z., Chen Y., Peng X., Xiang C., Li Y., Li W. Chlorogenic acid-enriched extract from Eucommia ulmoides leaves inhibits hepatic lipid accumulation through regulation of cholesterol metabolism in HepG2 cells. Pharm. Biol. 2016;54:251–259. doi: 10.3109/13880209.2015.1029054. [DOI] [PubMed] [Google Scholar]
- 27.Kruit J.K., Groen A.K., van Berkel T.J., Kuipers F. Emerging roles of the intestine in control of cholesterol metabolism. World J. Gastroenterol. 2006;12:6429–6439. doi: 10.3748/wjg.v12.i40.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang N., Li Y.M., He Z., Hao W., Zhao Y., Liu J., Zhu H., Kwek E., Ma K.Y., He W.S., et al. Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration. Molecules. 2021;26:3766. doi: 10.3390/molecules26123766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busch S.J., Barnhart R.L., Martin G.A., Flanagan M.A., Jackson R.L. Differential regulation of hepatic triglyceride lipase and 3-hydroxy-3-methylglutaryl-CoA reductase gene expression in a human hepatoma cell line, HepG2. J. Biol. Chem. 1990;265:22474–22479. doi: 10.1016/S0021-9258(18)45729-8. [DOI] [PubMed] [Google Scholar]
- 30.Kou S., Han B., Wang Y., Huang T., He K., Han Y., Zhou X., Ye X., Li X. Synergetic cholesterol-lowering effects of main alkaloids from Rhizoma Coptidis in HepG2 cells and hypercholesterolemia hamsters. Life Sci. 2016;151:50–60. doi: 10.1016/j.lfs.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y., Tocmo R., Nauman M.C., Haughan M.A., Johnson J.J. Defining the Cholesterol Lowering Mechanism of Bergamot (Citrus bergamia) Extract in HepG2 and Caco-2 Cells. Nutrients. 2021;13:3156. doi: 10.3390/nu13093156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavan M., Hanachi P., Mirjalili M.H., Dashtbani-Roozbehani A. Comparative assessment of the biological activity of the green synthesized silver nanoparticles and aqueous leaf extract of Perilla frutescens (L.) Sci. Rep. 2023;13:6391. doi: 10.1038/s41598-023-33625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puchadapirom P., Suttajit M., Thongpraditchote S., Kitphati W., Tammasakchai A. GenotoxicityEvaluationofEthanolicLeafExtractofThaiPerillaPerillafrutescensL.Britt.usingMicronucleusAssayinV79Cell Line. Thai J. Pharm. Sci. 2020;42:5–16. [Google Scholar]
- 34.Mungmai L., Preedalikit W., Pintha K., Tantipaiboonwong P., Aunsri N. Collagenase and Melanogenesis Inhibitory Effects of Perilla Frutescens Pomace Extract and Its Efficacy in Topical Cosmetic Formulations. Cosmetics. 2020;7:69. doi: 10.3390/cosmetics7030069. [DOI] [Google Scholar]
- 35.International Council for Harmonisation of Technnical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline. [(accessed on 6 October 2022)]. Available online: https://database.ich.org/sites/default/files/ICH_Q2R2_Document_Step2_Guideline_2022_0324.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.