Abstract
The ethical recruitment of participants with neurological disorders in clinical research requires obtaining initial and ongoing informed consent. The purpose of this study is to characterize barriers faced by research personnel in obtaining informed consent from research participants with neurological disorders and to identify strategies applied by researchers to overcome those barriers. This study was designed as a web-based survey of US researchers with an optional follow-up interview. A subset of participants who completed the survey were selected using a stratified purposeful sampling strategy and invited to participate in an in-depth qualitative interview by phone or video conference. Data were analyzed using a mixed methods approach, including content analysis of survey responses and thematic analysis of interview responses. Over 1 year, 113 survey responses were received from US research personnel directly involved in obtaining informed consent from participants in neurological research. Frequently identified barriers to informed consent included: cognitive and communication impairments (e.g. aphasia), unrealistic expectations of research participants, mistrust of medical research, time constraints, literacy barriers, lack of available social support, and practical or resource-related constraints. Strategies to enhance informed consent included: involving close others to support participant understanding of study-related information, collaborating with more experienced research personnel to facilitate training in obtaining informed consent, encouraging participants to review consent forms in advance of consent discussions, and using printed materials and visual references. Beyond conveying study-related information, researchers included in this study endorsed ethical responsibilities to support deliberation necessary to informed consent in the context of misconceptions about research, unrealistic expectations, limited understanding, mistrust, and/or pressure from close others. Findings highlight the importance of training researchers involved in obtaining informed consent in neurological research to address disease-specific challenges and to support the decision-making processes of potential research participants and their close others.
Keywords: Ethics, informed consent, recruitment, neurology, clinical trials
Introduction
National guidelines recommend implementing ethical safeguards within clinical research involving individuals with cognitive impairments rather than excluding these individuals from participation entirely; however, this population is often excluded from neurological and geriatric research without strong rationale, limiting the opportunity to become involved in research (Taylor et al., 2012; Trivedi and Humphreys, 2015). A 2019 position paper released by the National Institute on Aging (NIA, 2019) calls for the creation and dissemination of best practices and investment into the applied science of recruitment. Obtaining meaningful informed consent is integral to the ethical recruitment of clinical research participants. Informed consent in neurological research may be complicated by challenges affecting many areas of clinical research (Dickert et al., 2017; Grady, 2015) as well as disorder-specific barriers such as cognitive impairments or aphasia. Fluctuations in cognitive function over the course of study participation may detract from a participant’s ability to understand and appreciate complex study-related information, potentially necessitating dual consent or the involvement of a proxy or legally authorized representative (LAR) while affirming the participant’s assent to remain in a study. While some barriers to informed consent can be anticipated and planned for in advance of study enrollment, unexpected challenges may arise over the course of a clinical trial.
The US Department of Health and Human Services’ Federal Policy for the Protection of Human Subjects (45 CFR part 46, the “Common Rule”) and the Food and Drug Administration (FDA) CFR protection of human subjects (21 CFR parts 50, 56, 312, and 812) provide regulatory protections for human research subjects, including the requirement that informed consent be obtained for research participation. To elucidate what constitutes informed consent, 2018 updates to the Common Rule move from merely requiring that information be presented in a way that is understandable to research subjects (§46.116) to requiring that informed consent actually “facilitates the prospective subject’s or legally authorized representative’s understanding of the reasons why one might or might not want to participate” (§46.116(a)(5)). Neurological research involves both intrinsic and extrinsic considerations that may compromise the quality of decisions to participate, including disease-related impairments affecting decision-making capacity and the complexity of disease processes and study design (Janssen et al., 2019; Koopman et al., 2021). Guidance from the National Institutes of Health (NIH, 2009) calls for investigators and Institutional Review Boards (IRBs) to maintain “awareness of the ethical challenges associated with research involving this vulnerable population.”
Challenges arising in the informed consent process present barriers to the recruitment of individuals with neurological disorders in clinical research (Davis et al., 2002). This study examines challenges in obtaining and maintaining informed consent to participation in clinical research in neurological disorders and innovative approaches researchers apply to overcome these challenges. Respondents in this study characterize the ethical problems within their everyday recruitment and re-consent practices as research team members.
Methods
Research design
This study aimed to characterize shared experiences of US research personnel in obtaining informed consent for participation in research involving patients with neurological disorders and strategies employed to overcome these barriers. Web-based surveys and optional follow-up semi-structured interviews explored challenges in obtaining consent that were anticipated by the study protocol, unexpected challenges encountered during the study, and resources or approaches used to address unexpected challenges.
Survey and interview guide development
We developed a web-based survey through a review of relevant literature and with input from experienced investigators, study coordinators, and ethicists. To further refine the survey, we conducted cognitive interviews (Groves et al., 2011) with study coordinators to probe for opportunities for improvement in question wording, formatting, and survey structure. We then undertook a content validation process, as described by Lynn (1986). Six content experts representing expertise in the fields of bioethics, neurological research administration, and neurological research personnel (including principal investigators and study coordinators) were given information about survey aims and asked to rate each item based on relevance, clarity, and importance of each question. We included questions about respondents’ demography, background, and experiences obtaining informed consent from participants for neurological research. The final, validated survey comprises 12 categorical and openended questions about training, barriers, and strategies. Survey respondents had the option to agree to be contacted for a follow-up interview at the end of the survey. The semi-structured interview guide contained probes about specific challenges or strategies described in survey responses, and openended questions about challenges in obtaining consent and resources or approaches used to address challenges.
Data collection
We collected publicly available email addresses from contact information listed under “Contacts” on clinicaltrials.gov for neurological studies actively recruiting participants in the United States. Between November 2020 and February 2021, we disseminated email invitations to participate in this study. In an effort to oversample study coordinators, we also advertised the survey at a virtual Society for Clinical Research Associates (SOCRA) conference in September 2020. Any research personnel directly involved in obtaining informed consent from participants in neurological research were eligible to participate. The 12-question web-based survey was administered through REDCap. A subset of participants who completed the survey were selected using a purposeful sampling strategy (Palinkas et al., 2015) to maximize diverse perspectives on the bases of academic training, role on the research team, and racial/ethnic background. In-depth qualitative interview were conducted by telephone or video conferencing technology to further inquire into any openended responses they provided. Two authors, LS and MZ, conducted 15 follow-up interviews for which each participant was compensated $50. Interviews were transcribed verbatim by members of the research team.
Ethical considerations
The Cleveland Clinic Institutional Review Board approved all research activities. Participants acknowledged receipt and review of an information sheet prior to indicating voluntary agreement to participate in the survey. Interview participants signed an audio recording release. Confidentiality was protected by removing identifying details from transcribed data. As research personnel might be vulnerable to coercion into participation by their institutions or superiors, eligible research personnel were invited to participate only by individuals who do not have a supervisory role in relation to the participant. Research personnel did not need to communicate their involvement to supervisors unless they choose to do so.
Data analysis
We used descriptive statistics to report basic demographic data, research experience, training, and responses to Likert-type scale and categorical survey questions. We analyzed free-text responses to survey questions using content analysis (Bengtsson, 2016) to better understand how research personnel approach informed consent for neurological research. We coded surveys using latent analysis (coding for meaning underlying the text) in order to describe how research personnel experience challenges and implement strategies to overcome them. We utilized methods described by Graneheim and Lundman (2004) to build credibility, dependability, and transferability. Two independent coders worked through each survey response to extract codes related to barriers and challenges, generating a coding dictionary that was refined over the course of data analysis. We coded in vivo (using the respondents’ exact words) as often as possible to avoid losing meaning. Our study team met frequently to discuss codes and reconcile differences in coding. A third coder was included in discussions to resolve disagreements about codes. Coders completed memos and audit trails after each coding session to streamline data analysis meetings and to maintain a record of coding decisions. During the categorization process, coders initially condensed codes into broad content areas that address specific topics related to barriers and strategies. As coding progressed, we refined content areas to focus on barriers and strategies relevant to the stages of informed consent that we identified.
We analyzed interview data using thematic analysis, (Braun and Clarke, 2006) a process of intensive review of responses to identify thematic domains. Qualitative data analysis generated a coding scheme which was developed further through a constant comparison of participant responses. The constant comparative method was applied throughout data analysis to compare new data with thematic domains that emerged in previously-collected data. We continued holding regular meetings to review the coding process and resolve discrepancies. Data from survey responses were compared to interview transcript data to enrich and reconcile codes that were vague in survey responses and to explore different perspectives on the same theme. Because participants were probed about specific responses in follow-up interviews, we used interview transcript data to reconcile codes and support analysis. Interview transcripts were also analyzed for latent themes related to perceptions of the nature and goals of the informed consent process, as these themes explicitly and implicitly arose. COVID-19 related barriers to consent were analyzed separately and will be discussed in greater detail separately.
Results
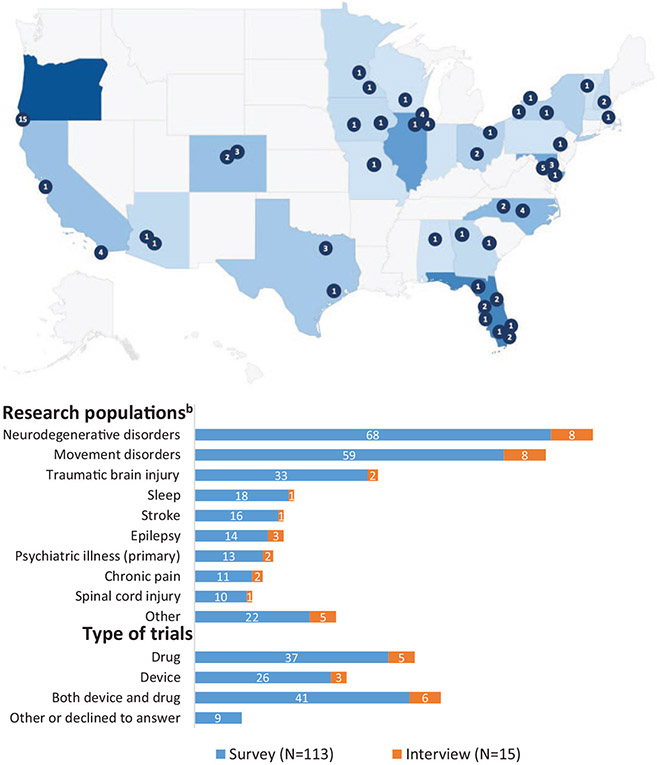
We received 113 completed REDCap surveys and conducted 15 follow-up interviews. The majority of participants in this study were study coordinators (75.2%). In addition to demographic information (Table 1), we collected information related to the research populations and types of clinical research respondents had experience conducting (Figure 1). Findings were grouped into the following domains: (1) Barriers to Informed Consent; (2) Ongoing Consent and Unanticipated Challenges; (3) Conceptualization and Goals of the Informed Consent Process; and (4) Strategies and Training. We coded survey and interview data for major barriers experienced by research personnel and strategies they employed relevant to each of these stages. Analysis of survey and interview data pointed to a shared emphasis on the informed consent process as an opportunity to establish rapport, trust, and expectations for research participation. Risks and discomfort were frequently identified among the most important considerations about which potential participants should be fully informed, followed by voluntariness and the expected duration of research participation.
Table 1.
Core categories, conceptual categories, frequencies, and coding examples.
| Categories and codes | Frequency | Example(s) | |||
|---|---|---|---|---|---|
| Survey | Interview | Total | |||
| Screening and preparation | |||||
| Barriers | Determining capacity or who has authority to consent | 5 | 1 | 6 | “When working in neurological diseases, it’s difficult to determine if someone is capable of providing consent for themselves or if an LAR is needed.” (34, survey) |
| Insufficient time or resources for consent discussion | 3 | 2 | 5 | “Inadequate time available during visit.” (90, survey) | |
| Participant unprepared for consent discussion | 4 | 0 | 4 | “Potential participants who obviously did not read the consent document.” (42, survey) | |
| Strategies | Encourage participant to review consent in advance | 12 | 6 | 18 | “Giving the ICFs to the subject before the informed consent process so the subject can read it over and come up with their own understanding and questions.” (28, survey) |
| Speak with participant more prior to consent | 5 | 6 | 11 | “I have learned to speak with the patient more prior to the consent … so that I can get a better idea of their cognitive status.” (57, survey) | |
| Plan for ample time and eliminate time constraints | 7 | 3 | 10 | “Adequate time is allotted for the consenting process in case there are issues/so participants are able to consider their options.” (39, survey) | |
| Secure proper space and prepare environment for consent | 4 | 2 | 6 | “Modified environment to accommodate for decline in motor function” (83, survey) | |
| Discuss participant with neurologist prior to consent | 2 | 2 | 4 | “Discussing the participant more in-depth with their neurologist before meeting them” (106, survey) | |
| Conveying study information | |||||
| Barriers | Informed consent is overwhelming | 20 | 7 | 27 | “Explaining everything involved with the study during the consent process seemed to be overwhelming for some participants.” (91, survey) |
| Difficulty simplifying research concepts (e.g. randomization) | 9 | 4 | 13 | “Explaining the studies in a clear, accurate, and unabridged manner is hard to do sometimes in layman language.” (20, survey) | |
| Participants become frustrated, fatigued, or distracted | 9 | 2 | 11 | “Patients with cognitive impairments have difficulty staying focused during a lengthy consent discussion.” (18, survey) | |
| Language barriers | 8 | 2 | 10 | “Language barriers are a big hurdle (we don’t have funding for translators)” (107, survey) | |
| Participant reluctant to go through consent process | 6 | 3 | 9 | “Some subjects want to skim over the consent and sign, they baulk at the detailed requirements needed for the consent process.” (22, survey) | |
| Participants’ questions require specialty knowledge | 2 | 3 | 5 | “One of the main challenges I have experienced is when consenting for studies that involves novel surgically invasive procedures. Potential subjects are apprehensive and want more in-depth clinically informative discussion, which is somewhat beyond regular consenting process.” (11, survey) | |
| Strategies | Use printed, visual, or other educational resources | 9 | 6 | 15 | “Providing additional educational materials” (71, survey) |
| Spend more time with participants | 8 | 4 | 12 | “Really spending time and having a discussion around consent.” (35, survey) | |
| Break down consent form based on cognitive needs | 4 | 6 | 10 | “Explain the document at different levels of understanding, to meet the patient where they were cognitively” (48, survey) | |
| Go through consent process at a slow pace | 5 | 4 | 9 | “Learning to slow down during the consent process to make sure the participant is absorbing all the information.” (91, survey) | |
| Involve PI or physician in discussion | 2 | 4 | 6 | “Make sure yourself as well as the PI is available during the ICF process to field those questions in case they ask some that you don’t know the answers to.” (34, survey) | |
| Orient participant (e.g. explain purpose of consent) | 4 | 2 | 6 | “I often will give a brief overview of the study itself before getting into the consent, so that way a patient goes into the consent with at least a level of understanding of the study.” (19, survey) | |
| Encourage participants to actively engage (e.g. take notes) | 1 | 1 | 2 | “Allowing participants to write on their consent forms/take notes” (37, survey) | |
| Assessing comprehension | |||||
| Barriers | Difficulty assessing comprehension (unclear whether participant understands) | 11 | 2 | 13 | “Ensuring understanding of the information for this population is also difficult at times.” (24, survey) |
| Strategies | Ask participants to repeat back information (“teach-back method”) | 4 | 3 | 7 | “The teach-back method is great with getting participants to understand the consent.” (37, survey) |
| Use a short questionnaire or quiz | 4 | 1 | 5 | “Using a short questionnaire to confirm understanding of the protocol” (15, survey) | |
| Supporting deliberation | |||||
| Barriers | Misconceptions or lack of knowledge about research | 7 | 5 | 12 | “One subject claimed that I was going to try and clone him (he was being serious).” (69, survey) |
| Participant holds unrealistic expectations | 6 | 6 | 12 | “Subjects feel that the treatment will be a magic cure.” (50, survey) | |
| Mistrust in research | 5 | 3 | 8 | “One of the greatest challenges I have incurred is the lack of representation on our study team … I believe this may sometimes lead to further mistrust of the scientific and medical community.” (94, survey) | |
| Participant consents without understanding | 6 | 1 | 7 | “I also encountered patients who did not fully understand the study or the risks due to cognitive decline but signed the consent form anyway.” (17, survey) | |
| Close others disagree or influence decision-making | 5 | 2 | 7 | “There may be challenges when you are consenting a patient who also has a caregiver or friend present … [they] may have different motives and aspirations that can impact a patient’s willingness to enroll.” (40, survey) | |
| Participant doesn’t fully consider risks | 2 | 1 | 3 | “It’s also common that participants will be so eager for relief from some new intervention that they will not properly consider the potential risks or practical downsides to joining a trial.” (20, survey) | |
| Lack of knowledge about/coming to terms with diagnosis | 1 | 1 | 2 | “I feel it’s difficult to present information fairly and concisely when people do not have a good understanding of their disease.” (106, survey) | |
| Strategies | Involve close others to support understanding | 15 | 6 | 21 | “Having a spouse/caregiver present helped to ensure that the study was understood.” (21, survey) |
| Encourage participant to speak with others (e.g. family) | 5 | 4 | 9 | “They are also encouraged to discuss the process prior to consenting with family/friends/caregivers/providers.” (39, survey) | |
| Honesty/transparency | 3 | 2 | 5 | “I find stark honesty works well for participants to recognize the challenges of being in a research trial.” (44, survey) | |
| Encourage participants to join support groups | 1 | 1 | 2 | “For people who have been newly diagnosed or seem to be lacking information about their condition … we encourage subjects and caregivers to join local support groups.” (31, survey) | |
| Existing knowledge about research aids understanding | 0 | 2 | 2 | “Often the populations we are recruiting from found out about the study through patient support groups … so we didn’t have to start from scratch with them.” (90, interview) | |
Shading was used only to create visual contrast to separate barriers and strategies.
Figure 1.
Participant demographics, research experience, and geographic location and description of trials participants have been involved in.
Barriers to informed consent
In survey responses and interviews, we observed that participants cited to barriers prevalent across all areas of research (Supplemental Figure 2); however, respondents identified a subset of these barriers as unique to or more pronounced in neurological research. According to respondents, cognitive or motor impairments of research participants can lead to quality control difficulties, pose communication challenges, hinder participant understanding of study information, and increase participant reliance on close others or study partners, which can result in conflicts during enrollment. Research personnel most often identified cognitive impairments of participants as the cause of several major barriers during the process of obtaining informed consent (56% of surveys and 87% of interviews made at least one reference to cognitive impairments). In the screening and preparation stage, cognitive impairments of participants lead to challenges related to assessing capacity and determining whether a proxy is needed. Additionally, research personnel often described running out of time during informed consent processes due to the additional effort required for consenting individuals with cognitive impairments. Research personnel noted increased challenges in informed consent processes for complex studies with lengthy informed consent forms that need to be simplified for participants with cognitive impairments who lose focus more quickly.
Research personnel perceived increased difficulties obtaining informed consent due to lower health or research literacy, as one participant stated “poor education level of our population” (47, survey) is a challenge when obtaining informed consent (41% of surveys and 60% of interviews). Research personnel in trials involving brain surgery highlighted that the informed consent process is more challenging due to the increased informational needs of participants who are apprehensive about surgery or, inversely, are over-eager and do not fully consider the risks of surgery. Several research personnel also noted that this population tends to have unrealistic expectations about the benefits of investigational therapies; as one respondent described, “The biggest challenge with our patient population is ensuring that they are not personally motivated or expected to be ‘cured’ from our intervention” (78, survey).
This research population is also more likely to rely on caregivers, LARs, or study partners during informed consent processes. Although respondents endorsed having close others present as a strategy for optimizing informed cosnet, challenges arise when participants’ close others have different goals. Notably, research personnel described uncertainty about what to do in situations where a participant only consents to appease a family member:
I have had a few participants have their spouse urge them to consent to the study when they themselves had no interest in being a research participant. I had to tell them both about the risks and benefits of the study but the subjects’ wife continued to urge him to participate even when he expressed several times that he did not want to participate. It was difficult to know what to say/do because one party was really excited about the study and its potential benefits for her husband but he only consented to make his wife happy.
(69, survey)
Research personnel also recounted situations in which family members misled potential participants in order to get them enrolled, with one respondent stating:
We had a study partner who was interested in the study for their loved one and confirmed that the participant was interested. On the day of screening, we found out that the participant had no idea they were there for a research study (they were told it was a normal doctor appointment) and did not consent to being enrolled.
(81, survey)
Some research personnel were asked by potential study participants to assess whether it was a good idea for them to join the trial and provide other forms of decision support. Respondents described unmet decisional needs, including potential decisional conflict and pressure participants may feel from close others:
One instance of [a] potential subject being pressured into consenting by family member. Subject withdrew prior to study participation when they were alone and asked to confirm willingness to participate in study.
(23, survey)
Others described challenges due to uncertainty about identifying a participant’s LAR when multiple close others are involved, disagreement between close others about the participant’s decision, and logistical difficulties in contacting, scheduling, and communicating with LARs that place an additional burden on research personnel. In general, research personnel pointed to unexpected difficulties consenting individuals with close others present or involved.
One interviewee specifically called for efforts to increase the availability of information about research on news and social media to promote “research literacy” and mitigate mistrust in research by dispelling misconceptions:
We can only umm dispel the rumors one person at a time… If we had more community feedback just in general, if people knew more about the kinds of studies that are being engaged in, if they knew why we’re trying to get these answers… So it is always about almost oversharing the information because we want people to know we’re not hiding anything.
(39, interview)
Ongoing consent and unanticipated challenges
Research team members most frequently identified changes in personal or social circumstances as an obstacle to ongoing consent informed consent (Supplemental Figure 2) (48% of surveys and 27% of interviews). This was followed closely by changes in cognitive or emotional health (44% of surveys and 27% of interviews).
When asked about unexpected challenges experienced when obtaining consent, survey respondents described unexpected decline in participants’ decision-making capacity (N = 4), participant anxiety about randomization (N = 3), participant apprehensiveness about research participation (N = 3), and low literacy (N = 4). In describing unexpected challenges related to literacy, one survey respondent described a situation where this was only discovered during consent: “Subject unable to read that was only discovered when consenting.” Also described as unexpected in survey responses were challenges related to re-consent (N = 2), conflict between a participant’s caregivers (N = 2), and explaining a participant’s ineligibility for enrollment (N = 2). Several research personnel described changes in a participants’ LAR or the sudden need for an LAR during re-consent as a significant barrier. Challenges related to COVID-19 and remote consent processes necessitated by physical distancing requirements will be reported separately.
Conceptualization and goals of the informed consent process
We identified four core process steps representing major stages of the informed consent process: 1) Screening and preparation; 2) Conveying study information; 3) Assessing comprehension; and 4) Supporting deliberation (see Table 1). In a latent analysis, we reviewed interview transcripts, corresponding survey responses, and memos to generate impressions about how interviewees conceptualized the consent process and what they alluded to as the goal of informed consent. Out of 15 interviewees, most appeared to view informed consent as an ongoing process (N = 10). Others implied informed consent consists of a single discussion (N = 3) or is simply having the participant sign the consent form (N = 2). Most interviewees implied that the goal of informed consent is to facilitate participants’ understanding of what research participation entails (N = 12); however, some suggested that the goal of informed consent is to obtain participants’ signatures (N = 3).
Strategies and training
In a survey question, we asked participants to rate how prepared they felt to obtain informed consent for the first time and how prepared they currently feel to obtain informed consent on a scale from 1 (not prepared at all) to 10 (completely prepared). Prior to obtaining consent for the first time, most participants rated their subjective preparedness as 7 (N = 17) or 8 (N = 34), with a median response of 7. Most participants rated their current preparedness as 9 (N = 28) or 10 (N = 77), with a median response of 10. Working with experienced research personnel, seeking advice of senior colleagues, and PI support were strategies described most frequently in both survey responses (N = 13) and interviews (N = 10). Providing the consent form to prospective research participants in advance (N = 12) and spending time with the participant (N = 8) were endorsed as strategies to support participant preparedness for the consent discussion and understanding of study information.
Discussion
Normative guidelines for informed consent contained in the 1979 Belmont report, Collaborative Institutional Training Initiative (CITI Program) training, and other training in Good Clinical Practice (GCP) outline the basic tenets of informed consent, including voluntariness and free, informed decision-making, but do not clarify the practical steps that must be undertaken to facilitate informed consent to research participation. Responsibility for obtaining informed consent for research participation varies across research teams and may fall to principal investigators (PIs), study coordinators, research assistants, and others. Boden-Albala et al. (2015) identify distinctive barriers to recruitment and retention in neurological clinical research using a survey, focus group, and interviews with investigators and senior research personnel. Investigators surveyed by Boden-Albala et al. ranked study coordinators (study team members responsible for assisting with recruitment of research subjects and development of research data under the supervision of a lead researcher or a research supervisor) as the “best” members of the research team to obtain consent, followed by principal investigators and attending physicians. Despite the vital role of study coordinators in obtaining informed consent to facilitate recruitment and retention of research participants, study coordinators remain “invisible players” in clinical research and a frequently overlooked stakeholder in the development of best ethical practices in clinical research (Davis et al., 2002). Focus group studies conducted by Davis et al. (2002) examine ethical challenges research personnel face in balancing advocacy roles and responsibilities to patients, patients-turned-subjects, and the study team or research enterprise. The potential for conflict between these roles complicates the process of obtaining consent to research participation. Haley et al. (2017) elicit neurological clinical research coordinators’ perspectives on barriers and strategies specific to recruitment of racial and ethnic minorities, calling for additional research on barriers facing research personnel, including “further identification of how and when barriers manifest.”
We found that research personnel surveyed and interviewed in this study viewed the informed consent process as an opportunity to establish rapport, trust, and expectations related to study participation. Common challenges in obtaining informed consent to participation in research into neurological disorders centered around conveying study-related information and supporting deliberation necessary to provide informed consent in the context of misconceptions about research, unrealistic expectations, limited understanding, mistrust, or pressure from close others. Key strategies to address barriers to consent in neurological research include involvement of social support, working with experienced research team members, and encouraging participants to review consent materials in advance. Despite these commonalities, our findings suggest neurological research personnel have highly variable perspectives about informed consent practices, including lack of consensus about: 1) the research team’s role in supporting a participant’s decision to enroll, 2) appropriate (formal and informal) evaluations of participant understanding of study-related information, and 3) features of participants or their situations that promote suitability for enrollment in a particular study.
Conceptualizing the informed consent processes
Consistent with recommendations from NIH guidance (National Institutes of Health, 2009), research personnel in this study described consent as an ongoing process. Participants in this study described a longitudinal process for obtaining informed consent, beginning as early as screening and preparation and extending to supporting deliberation regarding the decision to enroll in a research study. Interactions with research participants during the screening process provides an opportunity to prime the participant for a subsequent informed consent conversation and to help research personnel prepare a personalized informed consent process. Barriers to informed consent arose more frequently in relation to conveying study information and supporting deliberation than during screening and preparation, assessing comprehension, and ongoing communication. The strategies research personnel describe employing to overcome barriers to informed consent more frequently targeted screening and preparation for consent and conveying study information, suggesting there may be more opportunities for interventions to enhance informed consent at earlier stages of the consent process.
Researcher perceptions of disease-specific barriers to informed consent
Many of the barriers to informed consent described by respondents are broadly applicable to many areas of clinical research (e.g. insufficient time or resources for consent discussion, participant unprepared for consent discussion, language barriers, specialty knowledge required to address participant questions, misconceptions about research, mistrust, and unrealistic expectations). A study by Fortun et al. (2008) described the limited recall of trial information with healthy participants. This suggests that barriers to retention of study information are not limited to a neurological patient population. However, barriers associated with decision-making capacity, participant frustration, fatigue, difficulty assessing comprehension, and other challenges appeared to be population-specific to the extent they are attributable to cognitive, motor, or communication impairments and other symptoms of neurological disorders. Research personnel also identified disease-specific strategies for obtaining consent, such as spending more time with research participants, slowing the pace of the consent process, and breaking down information based on cognitive needs. Our respondents frequently endorsed a teach-back method to assess understanding which aligns with previously published recommendations for assessing comprehension of informed consent and privacy information in low-literacy populations (Kripalani et al., 2008).
Promoting research literacy and recruitment of underrepresented populations
Research personnel provided important insights about the most difficult topics to educate participants about during informed consent that could be overcome with enhancements in how research-related information is presented. Consistent with published findings that trial participants fail to understand equipoise and randomization (King et al., 2005; Robinson et al., 2004, 2005), many respondents in this study highlighted the difficulty of conveying research concepts such randomization and group assignments. Respondents advocated for efforts to enhance research literacy and address misconceptions about research, consistent with established knowledge that individuals with lower health literacy are less interested in participating in research (Kripalani et al., 2019). Efforts to promote “research literacy” have aimed to increase recruitment of underrepresented communities in research (Press, 2021). Barriers to engaging and recruiting members of underrepresented populations identified in this study resonate with findings from published studies of difficulties in engaging and recruiting members of racial minorities underrepresented in clinical research (Clark et al., 2019; Davis et al., 2019; Geller et al., 2018; Heller et al., 2014; Nature Medicine, 2018; Schmotzer, 2012). Safeguards and strategies identified through this research are responsive to recent calls for increasing diversity in clinical research (Commissioner of the Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration, 2020) and efforts to address past misconduct in research that has led to mistrust (Scherr et al., 2019). Our findings also emphasize that training for research personnel involved in obtaining informed consent needs to prioritize competencies in engaging with underrepresented communities (Niranjan et al., 2019).
Need for training and decision support interventions
Consistent with obligations outlined in the 2018 revisions to the “Common Rule,” respondents frequently described a sense of obligation to provide decision support to prospective research participants, but expressed uncertainty about the ways in which they can help. Advocating too strongly for enrollment could be misconstrued (or appropriately construed) as attempting to advance recruitment goals at the cost of voluntariness. Research personnel highlighted the importance of supporting participants in individualized assessments of the risks and benefits of study participation. Importantly, our data suggests that research personnel perceive risks differently than participants, and it affects their evaluation of the participants’ understanding and suitability for the trial. Strategies such as providing the consent form to prospective participants in advance, spending time with participants, stopping often to allow for questions, and planning for ample time to obtain consent support the goal of alleviating time pressure to support deliberation. Researchers’ endorsement of allowing time for potential participants to discuss the study with close others and/or their physician aligns with FDA guidance about establishing a waiting period to enhance participant decision-making and involving family members or friends during the informed consent discussion. While often cited as a strategy to support participants’ understanding and assist in decision-making, several instances of family pressure and conflicting goals recounted by research personnel in this study call for increased awareness of the potential for coercion when close others are involved in informed consent.
Barriers to supporting deliberation are not well-addressed by strategies identified in this domain, suggesting this may be an area in which additional decision support interventions are needed. Our findings suggest that complementary informed consent tools should support research personnel in emphasizing information related to study related risks, voluntariness, and study duration, and assessing participant comprehension of these aspects of the research protocol.
Limitations
This study of a cross section of research does not allow for an exhaustive representation of the frequency of barriers in a specific neurological condition but identifies points of commonality and shared experiences of researchers obtaining consent across populations of research participants with neurological disorders. Recruitment was limited to US research personnel, so findings may not be generalizable to research conducted in other jurisdictions. Survey and interview questions did not probe for challenges and experiences specific to conducting research with subpopulations of patients with neurological disorders, limiting the degree of specificity to which findings may be applicable. We are unable to calculate survey response rate due to the sharing of this survey invitation and advertisement of the opportunity to participate in this study at a SOCRA conference. The non-probability sampling strategy utilized limits the generalizability of findings from this survey study. Self-selection bias, social desirability bias, and participant awareness that the PI of this study is an ethics researcher may have influenced survey and interview responses. As this study was limited to a sample of neurological research personnel, potential discrepancies between perceptions of research personnel and participants could not be directly explored with this data. We also were unable to draw comparisons between research personnel experiences obtaining consent from patients with neurological disorders and experiences conducting research in other patient populations or with healthy participants, particularly as many respondents exclusively reported experience conducting research into neurological disorders. However, the current study provides baseline data on which future comparison can be done and that starts to fill a needed gap.
Conclusion
This study of research personnel involved in clinical research into neurological disorders advances efforts to optimize the ethical recruitment of potentially vulnerable research participants. Respondents highlighted the importance of training researchers involved in obtaining informed consent in neurological research to support and assess participant understanding of study-related risks and appreciation for what research participation entails. Findings from this study serve to increase awareness of ethical challenges associated with the recruitment and retention of participants in neurological research and bolster researchers’ efforts to address these challenges.
Supplementary Material
Funding
All articles in Research Ethics are published as open access. There are no submission charges and no Article Processing Charges as these are fully funded by institutions through Knowledge Unlatched, resulting in no direct charge to authors. For more information about Knowledge Unlatched please see here: http://www.knowledgeunlatched.org. Research reported in this publication was supported by the National Institute Neurological Disorders And Stroke (NINDS) and National Institute on Aging (NIA) of the National Institutes of Health under Award Numbers 3P30AG062428-02S2 and 3UH3NS100543-03S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Ethical approval
Ethical approval to conduct the described research was approved by the Cleveland Clinic Institutional Review Board (Research Protocol #20-634).
Supplemental material
Supplemental material for this article is available online.
Contributor Information
Lauren R Sankary, Cleveland Clinic, USA.
Megan E Zelinsky, Cleveland Clinic, USA.
Paul J Ford, Cleveland Clinic, USA.
Eric C Blackstone, Case Western Reserve University, USA.
Robert J Fox, Cleveland Clinic, USA.
References
- Bengtsson M (2016) How to plan and perform a qualitative study using content analysis. NursingPlus Open 2: 8–14. [Google Scholar]
- Boden-Albala B, Carman H, Southwick L, et al. (2015) Examining barriers and practices to recruitment and retention in stroke clinical trials. Stroke 46(8): 2232–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V and Clarke V (2006) Using thematic analysis in psychology. Qualitative Research in Psychology 3(2): 77–101. [Google Scholar]
- Clark LT, Watkins L, Piña IL, et al. (2019) Increasing diversity in clinical trials: Overcoming critical barriers. Current Problems in Cardiology 44(5): 148–172. [DOI] [PubMed] [Google Scholar]
- Commissioner of the Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration (2020) Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. FDA. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial (accessed 14 October 2021). [Google Scholar]
- Davis AM, Hull SC, Grady C, et al. (2002) The invisible hand in clinical research: The study coordinator’s critical role in human subjects protection. The Journal of Law, Medicine & Ethics: A Journal of the American Society of Law, Medicine & Ethics 30(3): 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TC, Arnold CL, Mills G, et al. (2019) A qualitative study exploring barriers and facilitators of enrolling underrepresented populations in clinical trials and biobanking. Frontiers in Cell and Developmental Biology 7: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickert NW, Eyal N, Goldkind SF, et al. (2017) Reframing consent for clinical research: A function-based approach. The American Journal of Bioethics 17(12): 3–11. [DOI] [PubMed] [Google Scholar]
- Fortun P, West J, Chalkley L, et al. (2008) Recall of informed consent information by healthy volunteers in clinical trials. QJM: Monthly Journal of the Association of Physicians 101(8): 625–629. [DOI] [PubMed] [Google Scholar]
- Geller SE, Koch AR, Roesch P, et al. (2018) The more things change, the more they stay the same: A study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Academic Medicine: Journal of the Association of American Medical Colleges 93(4): 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C (2015) Enduring and emerging challenges of informed consent. New England Journal of Medicine 372(9): 855–862. [DOI] [PubMed] [Google Scholar]
- Graneheim UH and Lundman B (2004) Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse Education Today 24(2): 105–112. [DOI] [PubMed] [Google Scholar]
- Groves RM, Fowler FJ Jr, Couper MP, et al. (2011) Evaluating survey questions. In: Survey Methodology. New York, NY: John Wiley & Sons, 263–265. [Google Scholar]
- Haley SJ, Southwick LE, Parikh NS, et al. (2017) Barriers and strategies for recruitment of racial and ethnic minorities: Perspectives from neurological clinical research coordinators. Journal of Racial and Ethnic Health Disparities 4(6): 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller C, Balls-Berry JE, Nery JD, et al. (2014) Strategies addressing barriers to clinical trial enrollment of underrepresented populations: A systematic review. Contemporary Clinical Trials 39(2): 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PM, Chalos V, van den Berg SA, et al. (2019) Neurological deficits in stroke patients that may impede the capacity to provide informed consent for endovascular treatment trials. Journal of Stroke and Cerebrovascular Diseases 28(12): 104447. [DOI] [PubMed] [Google Scholar]
- King M, Nazareth I, Lampe F, et al. (2005) Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials. Health Technology Assessment 9(35): 1–186, 3–4. [DOI] [PubMed] [Google Scholar]
- Koopman I, Verbaan D, Vandertop WP, et al. (2022) Deferred consent in an acute stroke trial from a patient, proxy, and physician perspective: A cross-sectional survey. Neurocritical Care 36: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripalani S, Bengtzen R, Henderson LE, et al. (2008) Clinical research in low-literacy populations: Using teach-back to assess comprehension of informed consent and privacy information. IRB: Ethics & Human Research 30(2): 13–19. [PubMed] [Google Scholar]
- Kripalani S, Heerman WJ, Patel NJ, et al. (2019) Association of health literacy and numeracy with interest in research participation. Journal of General Internal Medicine 34(4): 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn MR (1986) Determination and quantification of content validity. Nursing Research 35(6): 382–386. [PubMed] [Google Scholar]
- National Institute on Aging (2019) National strategy for recruitment and participation in Alzheimer’s and related dementias clinical research. Available at: http://www.nia.nih.gov/research/recruitment-strategy (accessed 27 October 2021).
- National Institutes of Health (2009) Research involving individuals with questionable capacity to consent: Points to consider. Available at: https://grants.nih.gov/grants/policy/questionablecapacity.htm (accessed 22 October 2021).
- Nature Medicine (2018) Diversifying clinical trials. 24(12): 1779. [DOI] [PubMed] [Google Scholar]
- Niranjan SJ, Durant RW, Wenzel JA, et al. (2019) Training needs of clinical and research professionals to optimize minority recruitment and retention in cancer clinical trials. Journal of Cancer Education 34(1): 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinkas LA, Horwitz SM, Green CA, et al. (2015) Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Administration and Policy in Mental Health 42(5): 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press VG (2021) Is ‘research literacy’ needed to increase diversity among participants in research studies? Mayo Clinic Proceedings 96(2): 280–281. [DOI] [PubMed] [Google Scholar]
- Research C for DE and (2020) Enhancing the diversity of clinical trial populations — Eligibility criteria, enrollment practices, and trial designs guidance for industry. FDA. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial (accessed 14 October 2021).
- Robinson EJ, Kerr C, Stevens A, et al. (2004) Lay conceptions of the ethical and scientific justifications for random allocation in clinical trials. Social Science & Medicine (1982) 58(4): 811–824. [DOI] [PubMed] [Google Scholar]
- Robinson EJ, Kerr CEP, Stevens AJ, et al. (2005) Lay public’s understanding of equipoise and randomisation in randomised controlled trials. Health Technology Assessment 9(8): 1–192, 3–4. [DOI] [PubMed] [Google Scholar]
- Scherr CL, Ramesh S, Marshall-Fricker C, et al. (2019) A review of African Americans’ beliefs and attitudes about genomic studies: Opportunities for message design. Frontiers in Genetics 10: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmotzer GL (2012) Barriers and facilitators to participation of minorities in clinical trials. Ethnicity & Disease 22(2): 226–230. [PubMed] [Google Scholar]
- Taylor JS, DeMers SM, Vig EK, et al. (2012) The disappearing subject: Exclusion of people with cognitive impairment and dementia from geriatrics research. Journal of the American Geriatrics Society 60(3): 413–419. [DOI] [PubMed] [Google Scholar]
- Trivedi RB and Humphreys K (2015) Participant exclusion criteria in treatment research on neurological disorders: Are unrepresentative study samples problematic? Contemporary Clinical Trials 44: 20–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.