Abstract
In glucose-grown cells of Saccharomyces cerevisiae IGC 4072, acetic acid enters only by simple diffusion of the undissociated acid. In these cells, ethanol and other alkanols enhanced the passive influx of labelled acetic acid. The influx of the acid followed first-order kinetics with a rate constant that increased exponentially with the alcohol concentration, and an exponential enhancement constant for each alkanol was estimated. The intracellular concentration of labelled acetic acid was also enhanced by alkanols, and the effect increased exponentially with alcohol concentration. Acetic acid is transported across the plasma membrane of acetic acid-, lactic acid-, and ethanol-grown cells by acetate-proton symports. We found that in these cells ethanol and butanol inhibited the transport of labelled acetic acid in a noncompetitive way; the maximum transport velocity decreased with alcohol concentration, while the affinity of the system for acetate was not significantly affected by the alcohol. Semilog plots of Vmax versus alcohol concentration yielded straight lines with negative slopes from which estimates of the inhibition constant for each alkanol could be obtained. The intracellular concentration of labelled acid was significantly reduced in the presence of ethanol or butanol, and the effect increased with the alcohol concentration. We postulate that the absence of an operational carrier for acetate in glucose-grown cells of S. cerevisiae, combined with the relatively high permeability of the plasma membrane for the undissociated acid and the inability of the organism to metabolize acetic acid, could be one of the reasons why this species exhibits low tolerance to acidic environments containing ethanol.
Acetic acid is a normal end product of fermentation by Saccharomyces cerevisiae, and additional amounts may be produced by contaminating lactic acid bacteria and/or acetic acid bacteria (for reviews see references 7 and 12).
S. cerevisiae is one of the yeast species containing strains that can use acetic acid as a sole carbon and energy source. The mechanisms that account for transport of the acid through the plasma membrane, the first step of its metabolism, have been elucidated in one strain, S. cerevisiae IGC 4072 (2, 3). There is evidence which indicates that cells of this strain grown in a medium containing acetic acid, lactic acid, or ethanol transport acetic acid mainly in the anionic form by secondary active transport systems which behave as proton symports specific for acetate and other monocarboxylates. These transporters are subject to glucose repression; therefore, no measurable acetate carrier activity was observed in glucose-grown cells. In these cells, the undissociated acid is the only form that crosses the plasma membrane by simple diffusion. If the extracellular pH is lower than the intracellular pH, the acid dissociates once it is inside the cell. Then, since the intracellular metabolism of the acid is also subject to glucose repression (7), the acid accumulates as a function of ΔpH and eventually acidifies the cytosol. It has been proposed that this process explains the negative effects of the acid on metabolic activity of S. cerevisiae that often occur in glucose-containing media, such as during grape must fermentation. Under these conditions, the acid may be toxic by itself and/or may enhance the apparent toxicity of ethanol to fermentation and viability of the fermentative yeast (1, 9–11).
The results described above contrast with what has been described for Zygosaccharomyces bailii, a food and beverage spoilage yeast that is typically characterized by its high tolerance to stressful acidic environments containing ethanol (for a review see reference 6). One of the peculiar traits of this species is that in acetic acid-, ethanol-, and glucose-grown cells, acetic acid enters mainly through a transporter, and simple diffusion of the undissociated acid contributes relatively little to overall acid uptake. In all of these cells, ethanol inhibits the transport of acetic acid. Hence, we hypothesized that under such growth conditions, Z. bailii can control the intracellular acid concentration so that it is compatible with the metabolic flux; this allows the yeast to tolerate environments containing acetic acid plus ethanol, where S. cerevisiae cannot survive (14). The objective of this study was to test this hypothesis in S. cerevisiae by examining the effects of ethanol and other alkanols on acetic acid uptake in strain IGC 4072 grown on different carbon sources.
MATERIALS AND METHODS
Microorganism, growth conditions, and cell suspension preparation.
S. cerevisiae IGC 4072 was supplied by the Portuguese Yeast Culture Collection, Faculty of Sciences and Technology, New University of Lisbon, Lisbon, Portugal. The strain was maintained on slants containing glucose (2%, wt/vol), peptone (1%, wt/vol), yeast extract (0.5%, wt/vol), and agar (2%, wt/vol). Cells were grown at 25°C in 1-liter Erlenmeyer flasks with mechanical shaking (150 rpm); each flask contained 200 ml of liquid mineral medium containing vitamins (15) and supplemented with glucose (2%, wt/vol), dl-lactic acid (0.5%, vol/vol), acetic acid (0.5%, vol/vol), or ethanol (1%, vol/vol). The pH of the medium was adjusted to 5.0 by adding 1 M sodium hydroxide. Cells were harvested in the mid-exponential phase (optical density at 640 nm, 0.5 to 0.6), centrifuged, washed twice with ice-cold distilled water, and suspended in distilled water to a final biomass concentration of 25 to 35 mg (dry weight) ml−1.
Measurement of initial uptake rates.
The rates of uptake of labelled monocarboxylic acids were estimated by using 10-ml conical centrifuge tubes containing 30 μl of 0.1 M potassium phosphate buffer at the desired pH and 10 μl of yeast suspension. After 4 min of incubation at 25°C in a water bath, the reaction was started by adding 10 μl of an aqueous solution of labelled acid (3,000 to 3,300 dpm/nmol) at the desired concentration and pH. The reaction was stopped by diluting the preparation with 5 ml of ice-cold water. The sampling times were 0, 5, and 10 s for ethanol- or monocarboxylic acid-grown cells and 0, 30, and 60 s for glucose-grown cells; during these periods of time the rates of uptake of labelled acids were linear. The reaction mixtures were filtered immediately through Whatman type GF/C membrane filters, and the filters were washed with 10 ml of ice-cold water and transferred to scintillation fluid (Opti-Phase HiSafe II; LKB FSA Laboratory Supplies, Loughgorough, United Kingdom). Radioactivity was measured with a Packard Tri-Carb 2200 CA liquid scintillation spectrophotometer, with correction for disintigrations per minute. When the uptake rates were measured in the presence of alkanol, the cells were preincubated for 2 min with the alkanol before the labelled substrate was added. The results were corrected for nonspecific 14C adsorption to the filters and/or the cells, which was determined by diluting the cells with 5 ml of ice-cold distilled water before the labelled carboxylic acid was added. The estimated values represented less than 10% of the total incorporated radioactivity.
Measurement of intracellular acid concentration.
Propionic acid was used to estimate the accumulative capacity of the monocarboxylate transport systems without interference from metabolism (2, 3). Acetic acid-, dl-lactic acid-, or ethanol-grown cells (25 μl of cell suspension prepared as described above) were added to 75-μl portions of 0.1 M potassium phosphate buffer with and without alkanol at the desired concentration, and the preparations adjusted to the experimental pH and incubated at 25°C with magnetic stirring. Each reaction was started by adding 25 μl of labelled propionic acid (3,000 to 3,300 dpm/nmol). At appropriate times, 10-μl aliquots were taken from the reaction mixture, placed in 5 ml of ice-cold water, and filtered immediately through Whatman type GF/C membrane filters. The filters were washed with 10 ml of ice-cold water, and the radioactivity was counted. In glucose-repressed cells accumulation measurements were obtained by using the same method except that labelled acetic acid (3,000 to 3,300 dpm/nmol) was used. The intracellular volume was measured as previously described (5, 13). When glucose- and acetic acid-grown cells were used, the estimated intracellular volumes of water were 2.1 ± 0.2 and 0.9 ± 0.2 μl per mg (dry weight) of yeast, respectively.
Concentration of carboxylic acids as a function of pH.
The concentration of the ionization form of carboxylic acids was calculated by using the Henderson-Hasselbalch equation and the following pKa values: propionic acid, 4.88; and acetic acid, 4.76 (4).
Chemicals.
The radioactively labelled acids utilized were [U-14C]acetic acid (sodium salt; catalog no. CFA229; Amersham) and [2-14C]propionic acid (sodium salt; catalog no. CFA88; Amersham). All other chemicals were reagent grade and were obtained from commercial sources.
Reproducibility of the results.
All experiments were repeated at least three times, and the data reported below are averages.
RESULTS
Effects of alcohols on passive diffusion of undissociated acetic acid by glucose-grown cells.
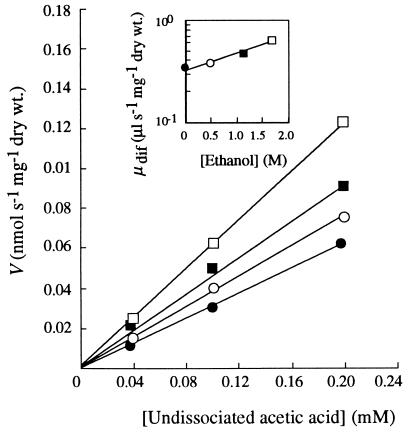
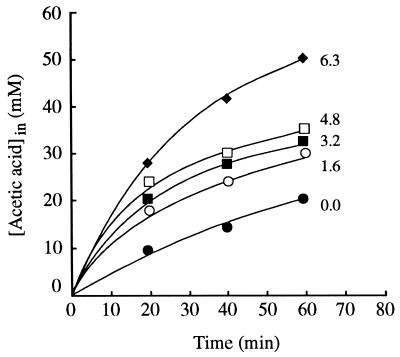
Plots of the initial rates of uptake of labelled acetic acid in the absence and presence of ethanol by glucose-grown cells as a function of the concentration of the undissociated acid at pH 6.0 (Fig. 1), pH 4.0 (data not shown), and pH 3.0 (data not shown) were linear. These results indicate that uptake kinetics are independent of the presence of ethanol in the extracellular medium and follow first-order kinetics. The effects of alcohols were mainly expressed by enhancement of the uptake of acetic acid. The diffusion constants, estimated from the slopes of the plots, increased exponentially with the ethanol concentration (Fig. 1, insert). Isopropanol, propanol, and butanol in the same pH range (pH 3.0 to 6.0) also enhanced the passive influx of labelled acetic acid, and the diffusion constants increased exponentially with the alkanol concentration (data not shown). The exponential enhancement constants (kdifenh) for each alkanol were not significantly affected by pH. The average values at pH 3.0, 4.0, and 6.0 were as follows: ethanol, 0.34 ± 0.04 liter mol−1; isopropanol, 0.65 ± 0.06 liter mol−1; propanol, 1.89 ± 0.94 liters mol−1; and butanol, 7.63 ± 0.56 liters mol−1. Accumulation of labelled acetic acid at pH 3.0 was also enhanced by ethanol (Fig. 2), propanol, and butanol (data not shown) during the first 60 min. Similar results were obtained at pH 5.0 (data not shown). The intracellular acetic acid concentration, estimated at the maximum accumulation level, increased exponentially with alkanol concentration (Fig. 3).
FIG. 1.
Initial rates of uptake (V) of undissociated acetic acid (as a function of concentration) by glucose-grown cells of S. cerevisiae IGC 4072 at pH 6.0 in the absence of ethanol (•) or in the presence of ethanol at a concentration of 0.52 M (○), 1.19 M (▪), or 1.72 M (□). (Insert) Dependence on ethanol concentration of the diffusion constants (μdif) calculated from the slopes, which increased exponentially in accordance with the following equation: μdifx = μdif0ekdifenh[x], where μdifx and μdif0 are the values of the diffusion constants at ethanol concentrations of x and zero, respectively, and kdifenh is an exponential constant that expresses the exponential enhancement of acid influx by the alcohol.
FIG. 2.
Intracellular concentration of total labelled acetic acid in glucose-grown cells of S. cerevisiae IGC 4072 measured in the absence and presence of ethanol at pH 3.0. The numbers next to the lines indicate the extracellular ethanol concentrations (expressed as percentages, weight/volume). The initial extracellular concentration of total acetic acid was 1 mM.
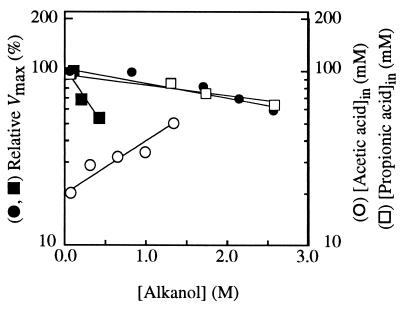
FIG. 3.
Effects of alcohols on the acid transport. Semilog plots of the relative maximum rates of uptake of labelled acetic acid (12 mM) at pH 3.0 by acetic acid-grown cells of S. cerevisiae IGC 4072 as a function of the concentration of ethanol (•) and butanol (▪). The Vmax values decreased exponentially in accordance with the following equation (16): Vmaxx = Vmax0e−ki[x], where Vmaxx and Vmax0 are the maximum initial uptake rates at external alcohol concentrations of x and zero, respectively. The dependence on ethanol concentration of the intracellular concentration of total labelled acetic acid calculated at the maximum level of accumulation by glucose-grown cells of S. cerevisiae IGC 4072 was also determined (○); the values were extrapolated from data shown in Fig. 2. In addition, the dependence on ethanol concentration of the intracellular concentration of total labelled propionic acid calculated at the maximum level of accumulation by acetic acid-grown cells of S. cerevisiae IGC 4072 was determined (□); the values were extrapolated from data shown in Fig. 4.
Effects of alcohols on the acetate carrier of acetic acid-, lactic acid-, and ethanol-grown cells.
In acetic acid-grown cells, ethanol and butanol inhibited the transport of labelled acetic acid in a noncompetitive way; i.e., the affinity constant for the acid (Km) was not significantly affected by the alcohol, while the transport capacity (Vmax) decreased with increasing alcohol concentration. A semilog plot of Vmax versus alcohol concentration yielded a straight line with a negative slope (Fig. 3). The exponential inhibition constant (ki) values for ethanol and butanol at pH 3.0 were 0.23 and 2.07 liters mol−1, respectively. At pH 5.0 and 6.0, the effects of ethanol and butanol on the initial rates of uptake of labelled acetic acid were similar to the effects at pH 3.0 (data not shown). In ethanol- or lactic acid-grown cells, ethanol and butanol also inhibited the transport of acetic acid. The inhibition was noncompetitive, and the inhibition of the maximum velocity followed exponential kinetics (data not shown). At pH 3.0 the ki values for ethanol and butanol were 0.37 and 1.42 liters mol−1, respectively, for lactic acid-grown cells and 0.33 and 3.43 liters mol−1, respectively, for ethanol-grown cells. The ki value for butanol was always much higher than the ki value determined for ethanol.
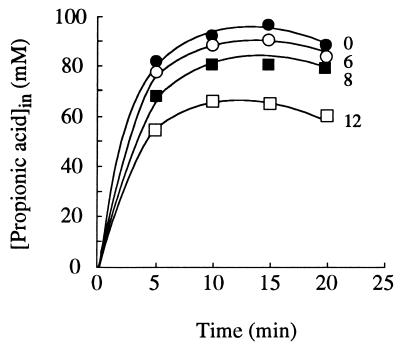
Propionic acid behaves like a nonmetabolizable analog of acetate for the strain which we studied (2, 3) and accumulated at pH 3.0 in acetic acid-grown cells (Fig. 4). Under these conditions, ethanol or butanol, at least during the first 20 min, reduced the accumulation of labelled propionic acid, and the effect increased exponentially with the alcohol concentration (Fig. 3).
FIG. 4.
Intracellular concentration of total labelled propionic acid in acetic acid-grown cells of S. cerevisiae IGC 4072, measured in the absence and presence of ethanol, at pH 3.0. The numbers next to the lines indicate the extracellular ethanol concentrations (expressed as percentages, weight/volume). The initial extracellular concentration of total propionic acid was 1.0 mM.
DISCUSSION
In glucose-grown cells of the strain of S. cerevisiae which we studied, acetic acid crosses the plasma membrane by simple diffusion and, once inside the cell, dissociates and can accumulate as a function of the ΔpH (2). In these cells, ethanol and butanol affect diffusion of undissociated acetic acid across the plasma membrane in an exponential manner, and the values of the exponential enhancement constants correlate with the lipid partition coefficients (8) of the alkanols.
In contrast, when the cells are grown in a medium supplemented with acetic acid, lactic acid, or ethanol, transport of acetic acid and other monocarboxylic acids occurs by means of monocarboxylate-proton symports (2, 3). This implies that transport of monocarboxylates is active and is driven by the substrate gradient across the plasma membrane and by the proton motive force. Our results show that ethanol and other alkanols inhibit acetic acid transport in a manner similar to the mechanism that inhibits other permeases for sugars, ammonium, and amino acids (16). The inhibition is noncompetitive, inhibition of the maximum velocity follows exponential kinetics, and the degree of inhibition increases with the lipid solubility of the alkanols. We think that the plasma membrane is the cellular target site of the inhibitory effects induced by alkanols. Alkanols could interfere with the transport proteins, probably by altering the conformation of the permeases and/or their lipid environment or by acting as uncouplers. Our results also show that the presence of a carrier for acetate is associated with very low diffusion of the undissociated acid. Even at pH 3.0, when simple diffusion of the undissociated acid could be significant, ethanol or butanol inhibited uptake (Fig. 3).
In Z. bailii, a yeast species much more resistant than S. cerevisiae to acidic media containing ethanol, cells grown with either glucose or acetic acid had a mediated transport system for acetic acid that was inhibited when ethanol was present (14). We hypothesize that such interactions of ethanol with acid membrane transport, associated with the ability to metabolize the acid, could be correlated with the high resistance of this yeast species to acidic media containing ethanol. Our results with S. cerevisiae are consistent with this hypothesis. In acetic acid-, lactic acid-, or ethanol-grown cells of S. cerevisiae, ethanol and other alkanols inhibited the transport capacity and the accumulation of acetic acid, as observed in Z. bailii. In glucose-grown cells of S. cerevisiae, ethanol or butanol neither inhibited the initial rates of uptake of the labelled acid nor prevented accumulation. Instead, enhancement of both initial uptake and accumulation was observed, which reinforced the idea that the acid enters the cell by simple diffusion. In S. cerevisiae acetic acid is well-known for its negative effects on the metabolic activity of the yeast (1, 9–11). These effects may be intensified by ethanol and occur when cells are growing in media containing glucose (e.g., during wine fermentation). Under these conditions ethanol in the medium enhances the passive influx of acetic acid, which leads to an increase in the acid concentration inside the cell and to the toxic effects of the acid. Thus, it appears that the absence of an operational carrier for acetic acid in glucose-grown cells of S. cerevisiae, combined with the relatively high permeability of the plasma membrane to the undissociated acid and the inability to metabolize acetic acid, could underlie the low tolerance of S. cerevisiae to environments containing the two fermentation end products, ethanol and acetic acid.
ACKNOWLEDGMENT
This study was supported by a European Union research grant under contract AIR CT93-0830.
REFERENCES
- 1.Cardoso H, Leão C. Mechanisms underlying the low and high enthalpy death induced by short-chain monocarboxylic acids and ethanol in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1992;38:388–392. [Google Scholar]
- 2.Casal M, Cardoso H, Leão C. Mechanisms regulating the transport of acetic acid in Saccharomyces cerevisiae. Microbiology. 1996;142:1385–1390. doi: 10.1099/13500872-142-6-1385. [DOI] [PubMed] [Google Scholar]
- 3.Cássio F, Leão C, van Uden N. Transport of lactate and other short-chain monocarboxylates in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol. 1987;53:509–513. doi: 10.1128/aem.53.3.509-513.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson R M C, Elliot D C, William H E, Jones K M. Data for biochemical research. 3rd ed. Oxford, United Kingdom: Clarendon Press; 1989. [Google Scholar]
- 5.De la Peña P, Barros F, Gascon S, Lazo P S, Ramos S. Effect of yeast killer toxin on sensitive cells of Saccharomyces cerevisiae. J Biol Chem. 1981;256:10420–10425. [PubMed] [Google Scholar]
- 6.Fleet G. Spoilage yeasts. Crit Rev Biotechnol. 1992;12:1–44. doi: 10.3109/07388559209069186. [DOI] [PubMed] [Google Scholar]
- 7.Gancedo C, Serrano R. Energy yielding metabolism. In: Rose A H, Harrison J S, editors. The yeasts. Vol. 3. New York, N.Y: Academic Press; 1989. pp. 205–251. [Google Scholar]
- 8.Leo A, Hansh C, Elkins D. Partition coefficients and their uses. Chem Rev. 1971;71:525–616. [Google Scholar]
- 9.Pampulha M E, Loureiro-Dias M C. Combined effects of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl Microbiol Biotechnol. 1989;31:547–550. [Google Scholar]
- 10.Pampulha M E, Loureiro-Dias M C. Activity of glycolytic enzymes of Saccharomyces cerevisiae in the presence of acetic acid. Appl Microbiol Biotechnol. 1990;34:375–380. [Google Scholar]
- 11.Pinto I, Cardoso H, Leão C, van Uden N. High enthalpy and low enthalpy death in Saccharomyces cerevisiae induced by acetic acid. Biotechnol Bioeng. 1989;33:1350–1352. doi: 10.1002/bit.260331019. [DOI] [PubMed] [Google Scholar]
- 12.Radler F. Microbial biochemistry. Experimentia. 1986;42:884–893. [Google Scholar]
- 13.Rottenberg H. The measurement of membrane potential and pH in cells, organelles and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 14.Sousa M J, Miranda L, Côrte-Real M, Leão C. Transport of acetic acid in Zygosaccharomyces bailii: effects of ethanol and their implications on the resistance of the yeast to acidic environments. Appl Environ Microbiol. 1996;62:3152–3157. doi: 10.1128/aem.62.9.3152-3157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Uden N. Transport-limited fermentation and growth of Saccharomyces cerevisiae and its competitive inhibition. Arch Microbiol. 1967;58:155–168. doi: 10.1007/BF00406676. [DOI] [PubMed] [Google Scholar]
- 16.van Uden N. Alcohol toxicity in yeasts and bacteria. Boca Raton, Fla: CRC Press; 1989. [Google Scholar]