Abstract
This systematic review aims to provide a comprehensive understanding of the current literature regarding gut microbiota composition in patients with Parkinson’s disease (PD) and Alzheimer’s disease (AD) compared to healthy controls. To identify the relevant studies, a thorough search of PubMed, Medline, and Embase was conducted following the PRISMA guidelines. Out of 5627 articles, 73 studies were assessed for full-text eligibility, which led to the inclusion of 42 studies (26 PD and 16 AD studies). The risk of bias assessment showed a medium risk in 32 studies (20 PD studies and 12 AD studies), a low risk in 9 studies (5 PD studies and 4 AD studies), and 1 PD study with a high risk. Among the PD studies, 22 out of 26 studies reported a different gut microbiota composition between the PD cases and the healthy controls, and 15 out of 16 AD studies reported differences in gut microbiota composition between the AD cases and the healthy controls. The PD and AD studies consistently identified the phyla Bacteroidetes, Firmicutes, and Proteobacteria as prevalent in the gut microbiota in both the healthy groups and the case groups. Microbial dysbiosis was specifically characterized in the PD studies by a high abundance of Akkermansia, Verrucomicrobiaceae, Lachnospiraceae, and Ruminococcaceae in the cases and a high abundance of Blautia, Coprococcus, Prevotellaceae, and Roseburia in the controls. Similarly, Bacteroides and Acidobacteriota were abundant in the AD cases, and Acidaminococcaceae, Firmicutes, Lachnospiraceae, and Ruminiclostridium were abundant in the AD controls. The microbial signature assessment showed the association of several microbial taxa, including Akkermansia, Lachnospiraceae, Verrucomicrobiaceae, Bifidobacterium, Ruminococcacea, and Verrucomicrobia with PD and Ruminococcaceae, Bacteroides, and Actinobacteria with AD. The microbial diversity evaluations in the PD and AD studies indicated comparable alpha diversity in some groups and distinct gut microbiota composition in others, with consistent beta diversity differences between the cases and the controls across multiple studies. The bacterial signatures identified in this study that are associated with PD and AD may offer promising prospects for efficient management and treatment approaches.
Keywords: gut microbiota, Parkinson’s disease, Alzheimer’s disease, gut–brain axis, dysbiosis
1. Introduction
Neurodegenerative disorders, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD), pose significant challenges to healthcare systems and influence people’s cognitive and physical abilities. PD is the second most common neurodegenerative disorder after AD, affecting approximately 1–2% of the population over the age of 65 [1]. Also, AD is the most common cause of dementia worldwide, accounting for 60–70% of all cases [2]. It is estimated that AD affects more than 50 million people globally, and this number is expected to triple by 2050 [3].
Despite the differences in clinical presentation and pathology, PD and AD share common features such as progressive neurodegeneration, cognitive decline, and decreased motor function [4,5]. The etiology of these disorders remains complex and multifactorial, involving a combination of genetic and environmental factors [6]. Recent findings suggest that the gut microbiota, a diverse community of microorganisms found in the gastrointestinal tract, may play a crucial role in the pathophysiology and development of neurodegenerative disorders [7].
The gut–brain axis, a bidirectional communication system between the gut and the central nervous system, is actively influenced by the gut microbiota and its metabolites. This axis represents a complex network through which the gut and brain can influence each other’s functions and activities [8]. Imbalanced gut microbiota can impact the brain function through various mechanisms, including: (a) activation of pro-inflammatory responses; (b) production of neuroactive compounds, such as short-chain fatty acids (SCFAs) like acetate, propionate, and butyrate; (c) initiation of immune responses triggered by microbial metabolites, such as lipopolysaccharides (LPS); (d) contribution to the formation and accumulation of abnormal brain proteins, such as amyloid; (e) disruption of neurotransmitter production in the gut (e.g., serotonin and dopamine) [9,10]; and (f) disruption of gastrointestinal integrity, leading to microbial translocation into the bloodstream and brain, as well as dysfunction of blood–brain barrier integrity [11,12].
Decreased levels of SCFAs as a result of a lesser abundance of a beneficial microbial population have been linked to intestinal barrier malfunction and neuroinflammation and to an increase in the susceptibility of neurons to injury in AD and PD cases [13,14,15]. Additionally, increased levels of LPS-producing bacteria (such as Baeroides) have been linked to the translocation of microbial substances into the brain and the promotion of neuroinflammation, a crucial pathological feature associated with a variety of neurological disorders, including AD and PD [16,17,18].
Studies have shown that an increased abundance of the Verrucomicrobiaceae family, particularly the Akkermansia genus (A. muciniphila) has been linked to increased mucin degradation in patients with PD and AD [17,18,19]. Mucin-degrading bacteria can destroy the protective mucus layer in the gut and lead to compromised gut barrier integrity, potentially contributing to increased permeability and the translocation of harmful substances into the bloodstream, triggering neuroinflammatory responses in PD and AD [17,20].
A decreased abundance of SCFA-producing bacteria, such as Faecalibacterium prausnitzii, Roseburia, and Coprococcus, has also been observed in PD cases, which may explain the altered gut barrier function and the increased inflammation observed in PD individuals [15].
Studies have also shown a decrease in beneficial bacteria such as Prevotellaceae and Lachnospiraceae [21,22,23] and reduced levels of SCFA producers [24], along with an increase in potentially pro-inflammatory bacteria such as Enterobacteriaceae in PD and AD cases [25,26,27,28]. Bardenhorst et al.’s study highlighted the significance of reduced abundance in butyrate-producing microbial communities like Roseburia and Faecalibacterium, as well as the increased mucus degradation activity by Akkermansia. These factors were associated with the promotion of intestinal inflammation, leaky gut, and the subsequent translocation of microbes and metabolites from the gut into the bloodstream and enteric nervous system in PD cases [29]. Another study showed the increasing trend of Proteobacteria and the decreased abundance of Firmicutes and Bifidobacteria in PD and AD cases [30].
Also, a recent systematic review on 11 studies showed lower microbial diversity in AD cases compared to healthy controls, with a high abundance of Proteobacteria, Bifidobacterium, and Phascolarctobacterium in AD cases. However, this study only included studies from the USA and China; this can significantly influence the interpretation of the gut microbiota findings [31].
Also, it is worth pointing out that in recent years, an emerging body of research has shown the complex association between nutrition, gut microbiota composition, and the development of several diseases in humans. For instance, studies have shown that adhering to a Mediterranean diet rich in fruits [32], vegetables [33], and omega-3 fatty acids [34] has been associated with a more diverse and beneficial microbial profile. Similarly, a diet high in dietary fiber can promote the growth of beneficial bacteria in the gut, reducing inflammation and potentially controlling the risk of neurodegenerative diseases [35]. These findings underscore the importance of dietary factors as a crucial component of the complex interplay between the gut microbiota and the development of AD and PD.
Although previous studies have provided valuable insights into gut microbiota composition, there remains a need to comprehensively assess and synthesize the existing body of knowledge in this area. This systematic review paper addresses this gap by presenting a rigorous evaluation of gut microbiota composition in patients with PD and AD, as well as in healthy controls. In contrast to prior research that may have been limited to specific timeframes or geographical locations, we performed an inclusive approach to include all the relevant studies available to date. This strategy enables us to not only consolidate previous findings but also to address the potential limitations identified in some of the previous research. Additionally, this review summarized bacterial communities based on the frequency with which they have been reported across the studies, thereby shedding light on the most consistently observed microbial communities. Through a thorough assessment of the risk of bias, adherence to the PRISMA guidelines, and the identification of the potential microbial signatures associated with these neurodegenerative disorders, this paper provides a deeper understanding of gut microbiota in both the PD and the AD cases and controls. Furthermore, we not only highlight the limitations identified by the original authors but also introduce additional unaddressed limitations, along with strategies to overcome them to increase the reliability and comparability of microbiota findings.
Understanding the link between gut microbiota composition and neurodegenerative disorders is critical as it may provide insights into disease etiology, progression, and potential therapeutic targets. Therefore, conducting a systematic review of the existing literature on gut microbiota composition in patients with PD and AD is vital to evaluate and synthesize the current evidence.
This systematic review aims to comprehensively evaluate the available literature on gut microbiota composition in patients with PD and AD, focusing on microbial alterations and their potential implications in disease pathogenesis. By providing details on the relationship between gut microbiota and neurodegenerative disorders, this review can potentially contribute to the development of novel strategies for diagnosis, prevention, and treatment through gut microbiota modulation.
2. Methodology
This review was conducted under the guidelines provided by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [36]. The protocol for this review was registered prospectively with PROSPERO under the registration number CRD42023422561.
2.1. Searching Strategy
Relevant research databases (PubMed, Medline, and Embase) were thoroughly searched from the beginning to 11 July 2023. The search was conducted using a combination of keywords along with various logical operators (AND, OR, (), “”). The keywords used included “microbiome”, “microbiota”, “intestinal flora”, “intestinal microbi”, “dysbiosis”, and “gut microbio*” combined with “Parkinson’s Disease” and “Alzheimer’s Disease”. The complete search strategy can be found in Supplementary Material S1. No specific restrictions regarding time, location, or study design were applied.
2.2. Selection Criteria and Screening
The studies were uploaded to Covidence (www.covidence.org, 1 May 2023) for eligibility screening by two authors (FSH, KN). Conflicts were resolved through discussion between the authors. The initial screening involved assessing the title and abstract, and further screening was conducted based on the full text of the studies. Inclusion (A) and exclusion criteria (B) were applied to determine the eligibility of the studies:
A: (i) Human studies; (ii) studies comparing the composition of gut microbiota between healthy individuals and patients with Parkinson’s or Alzheimer’s disease; (iii) English studies; and (iv) studies utilizing next-generation sequencing-based approaches to compare the gut microbiota composition between healthy individuals and patients with Alzheimer’s or Parkinson’s disease (such as 16S rRNA sequencing or shotgun metagenomics).
B: (i) Animal studies; (ii) studies without appropriate healthy and control groups; (iii) studies employed irrelevant methodology (such as culture-based or targeted PCR methods); and (iv) review articles.
2.3. Data Extraction
The data were extracted independently by two authors (FSH, KN) from the included studies. In the case of any conflicts, the authors resolved them through discussions. The following data were extracted whenever they were available: the year of publication, study location, recruitment period, population size, age range, gender, the total number of collected samples, number of samples per participant, sample type, inclusion and exclusion criteria, method of preserving samples before DNA extraction, the storage temperature of the samples, DNA extraction method, sequencing platform, specific regions of the 16S rRNA gene that were sequenced, primers, the total number of sequencing reads or sequencing reads per sample, availability of the data, reference database, significant findings related to gut microbiota, changes observed in the gut microbiota over time in both the healthy individuals and the cases, alpha (microbial diversity within individual samples) and beta diversity indices (microbial diversity between groups), methods utilized to determine differential abundance between the cases and the controls, and the limitations and strengths highlighted in the study.
2.4. Risk of Bias Assessment
Two authors (FSH, KN) conducted a risk of bias assessment, examining potential biases known to impact the findings across four main domains: (1) sampling, (2) comparability, (3) data reporting, and (4) outcome measurement biases. Each domain was also broken down into subdomains to assess the risk of bias with more details. A traffic light plot summarizing the risk of bias was generated using Robvis [37]. Additional information, including subdomains within each domain, can be found in Supplementary Material S2.
2.5. Evaluation of Findings
To comprehensively evaluate the gut microbiota composition in individuals with PD or AD, a narrative analysis approach was utilized. This involved conducting an extensive literature review to gather and compare relevant data on the core gut microbiota, prevalent microbial communities, comparisons of alpha and beta diversity in cases and controls, and bacterial taxa associated with PD and AD.
3. Results
3.1. Study Selection
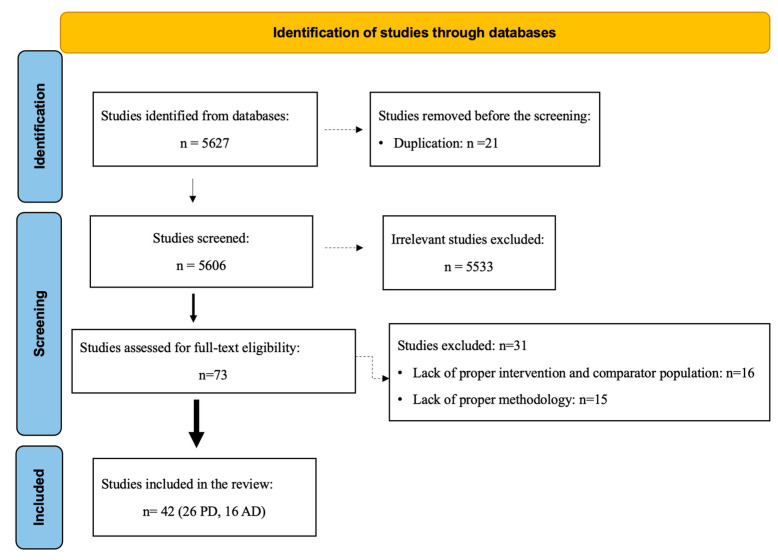
Through the initial database search, a total of 5627 articles were identified. After removing duplicate records (n = 21), 5606 unique records remained. Following the title and abstract screening, 5533 records were identified as irrelevant. The remaining 73 records underwent full-text screening to determine eligibility. Among these, 31 studies were excluded due to the absence of appropriate case and control groups (n = 16) or inappropriate methodology (such as culture-based or targeted PCR studies) (n = 15). Ultimately, a total of 42 eligible studies (26 focused on Parkinson’s disease and 16 on Alzheimer’s disease) were included in this review (Figure 1).
Figure 1.
PRISMA flow diagram of the literature search and article selection.
3.2. Risk of Bias Assessment
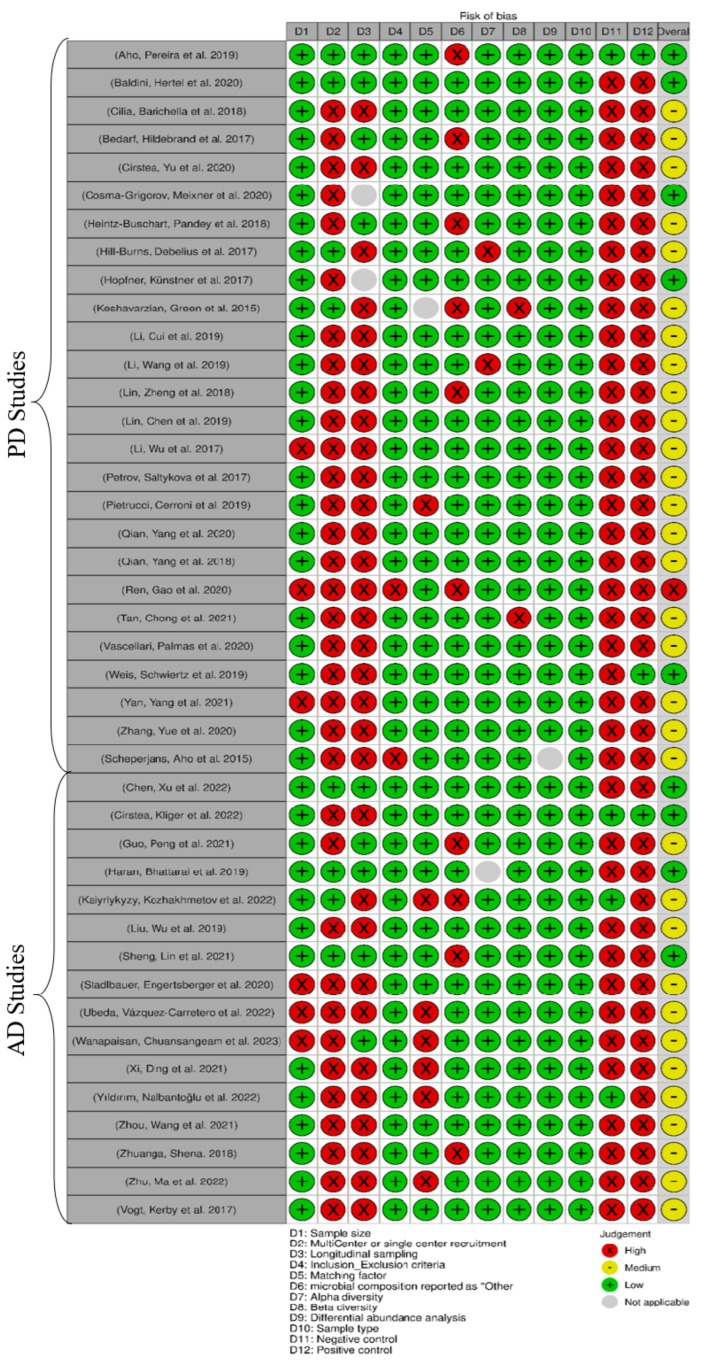
Of the articles, nine studies had the least risk of bias (five PD studies and four AD studies) [17,22,26,38,39,40,41,42,43], thirty-two studies had a medium risk (twenty PD studies and twelve AD studies) [14,18,19,20,21,23,24,25,27,28,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65], and one PD study had a high risk [66] (Figure 2). A lack of appropriate experimental controls was observed in 39 studies for positive control [14,17,18,19,20,21,22,23,24,25,27,28,39,40,41,42,44,45,46,47,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] and in 38 studies for negative control [14,17,18,19,20,21,22,23,24,25,27,28,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55,56,57,58,59,60,62,63,64,65,66]. Furthermore, 34 studies mainly recruited participants from a single center [18,19,20,21,22,23,25,26,27,28,41,43,44,45,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66], and 31 studies did not employ a longitudinal sampling method [14,18,21,23,24,25,26,27,28,43,44,46,47,48,49,50,51,52,53,54,55,56,57,59,60,61,62,63,64,65,66]. In 10 studies, the microbial composition was reported as “Other” without providing specific details [14,19,20,27,38,42,45,46,51,65,66]. Additionally, seven studies did not adequately address matching factors between the cases and the controls [18,46,51,58,59,61,64]. Also, the sample size was small in six studies (n < 49) [18,48,54,58,60,66]. Two studies did not specify their inclusion/exclusion criteria [25,66], while another two studies did not assess alpha [21,24] and beta diversity comparison [14,55], respectively (Supplementary Material S3).
Figure 2.
Risk of bias assessment with corresponding biases (D1 to D12) and risk indicators: green: low risk; yellow: medium risk; red: high risk; and gray: not applicable [14,17,18,19,20,21,22,23,24,25,26,27,28,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
3.3. General Characteristics of Included Studies
The included studies were mainly published between the years 2014 and 2022; they were conducted in various locations, encompassing a diverse range of locations. China emerged as the leading contributor to this field, with 17 studies [21,27,28,40,42,45,47,48,52,53,59,60,62,63,64,65,66], followed by Germany (5 studies) [19,20,22,41,43] and the USA (4 studies) [14,17,24,57]. Other countries made smaller contributions. The study populations varied between 20 and 350 participants (median: 98), with 10 to 237 cases and 10 to 162 controls. Stool samples were consistently the most prevalent sample type utilized across the studies.
DNA extraction predominantly relied on the QIAamp DNA Stool Mini Kit and PowerSoil DNA Isolation Kit. Among the sequencing platforms, Illumina MiSeq was the most commonly utilized platform and was utilized in 34 studies [18,20,21,22,23,24,26,28,38,39,40,41,42,43,44,45,46,49,50,51,53,54,55,56,57,58,59,60,61,63,64,65,66]. The V3–V4 region was the primary target region for sequencing in 22 studies [22,23,28,38,39,40,42,45,49,50,51,53,55,56,58,59,60,61,63,64,65,66]. Notably, the forward primers 341F1 (in 9 studies) [21,22,38,40,42,53,55,61,63] and 515F (in 7 studies) [18,20,24,26,44,47,66] and the reverse primers 806R (in 10 studies) [18,22,24,26,42,44,47,59,64,65] and 805R (in 6 studies) [20,21,40,55,61,63] were frequently used.
In this manuscript, a summarized data extraction table is provided (Table 1); however, a comprehensive data extraction table, including all the extracted details, is available in Supplementary Material S4.
Table 1.
Summary of included studies (see Supplementary Material S4 for complete data extraction table).
| Study | Year | Location | Population Size | Age | Gender | Sample Type | Matching Factor | Sample Preservative |
Extraction Method | Sequencing Platform | Sequenced Regions | Forward Primer | Reverse Primer | Data Availability |
Main Microbiota Finding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [38] | 2019 | Finland | 128 (64 case, 64 control) | case: 65.2 ± 5.52/control: 64.45 ± 6.9; (years, mean ±SD) | Female: case: 48.6%/control: 50.0% | stool | age, sex | DNA stabilizer PSP (Spin Stool DNA Plus Kit, STRATEC Molecular) | PSP Spin Stool DNA Plus Kit (STRATEC Molecular) | Illumina MiSeq | V3–V4 | 341F1–4 (5′ CCTACGGGNGGCWGCAG 3′) | 785R1–4 (5′ GACTACHVGGGTATCTAATCC 3′) | PRJEB27564 | Significant differences in gut microbiota between cases and controls. Disease progression did not influence gut microbiota. No difference in Firmicutes/Bacteroidetes ratio between cases and controls. |
| [39] | 2020 | Ireland | 309 (147 case, 162 control) | case: 69.3 ± 8.6/control: 63.3 ± 8.3; (mean ± SD) | Female: case: 31.5%/control: 35.8% | stool | age, sex | MNIgene.GUT® kit | Chemagic DNA blood protocol | Illumina MiSeq | V3–V4 | NR | NR | Available on request | Composition of the gut microbiome could potentially serve as a marker of disease severity in PD. Bilophila and Paraprevotella abundance were significantly associated with disease severity. |
| [23] | 2018 | Italy | 350 (237 cases, 113 control) | cases: 67.6 (9.7)/control: 65.9 (9.9); (y, mean (SD)) | cases: 115 (59.6)/control 47 (41.6): Male gender, n (%) | stool | age, nutritional status, geographical area | No preservative | QIAamp DNA Stool Mini Kit | Illumina MiSeq | V3–V4 | NR | NR | NR | Low Lachnospiraceae in cases. |
| [19] | 2017 | Germany | 59 (31 case, 28 control) | case: 64.8 ± 9.5/control 65.6 ± 10.4; (years, mean ± SD) | Male: all case and control | stool | age | NR | NR | Illumina Hiseq4000 | NR | NR | NR | ERP019674 | Case and controls had different gut microbiota composition, characterized by increased levels of Verrucomicrobiaceae (Akkermansia muciniphila) and unclassified Firmicutes, and decreased levels of Prevotellaceae (Prevotella copri) and Erysipelotrichaceae (Eubacterium biforme) in cases. |
| [44] | 2020 | Canada | 300 (197 case, 103 control) | case:66 (59–71)/control 66 (58–71); (years) | Female: case: 38.1%/control: 51.5% | feces | age | DNA stabilizer buffer | OMNIgeneGUT Kit | Illumina MiSeq | V4 | 515F | 806R | On publisher’s website | Increased abundance of Akkermansia and Bifidobacterium and decreased abundance of Faecalibacterium, Lachnospiraceae, and SCFA-producing bacteria in cases. |
| [22] | 2020 | Germany | 101 (71 case, 30 control) | case: 65.3 ± 10.2/controls: 64.3 ± 8.9; (years) | Female: case 45.7%/controls: 45.2% | feces | MS score, NMS constipation item, Wexner Constipation, coffee consumption | DNA stabilizing solution | PSP R Spin Stool DNA Plus Kit | Illumina MiSeq | V3–V4 | 341F (5′-ACTCCTACGGGAGGCAGCAG-3′) | 806R (5′-GGACTAC HVGGGTWTCTAAT-3′) | Available on request | High abundance of Faecalibacterium, Ruminococcus, Clostridia, Lachnospiraceae, Oscillospira, Betaproteaobacteria, Burkholderiales, Alcaligenaceae, and Sutterella in cases compared to controls. |
| [20] | 2017 | Germany | 154 (76 case, 78 control) | case: 68.0 6 9.7/control: 68.4 6 6.7; (year) | Male: case 66%/control 59% | stool | diabetes medication | Stool specimen collector (MedAuxil) | Qiagen AllPrep kit | Illumina MiSeq | V4 | 515F | 805R | PRJNA381395 | High abundance of Akkermansia in PD, different gut microbiota between cases and controls. |
| [24] | 2017 | USA | 327 (197 case, 130 control) | Total 69, 68.4, 9.2; (Median, Mean, SD) | Female: 65; 132 (67.0%), Male (% Male) | stool | PD medications, disease duration, spousal relationship, geographic site | Media-free swabs kit with DNA/RNA-free sterile swabs | According to the Earth Microbiome Project Protocol | Illumina MiSeq | V4 | 515F | 806R | ERP016332 | Significantly higher abundance of Bifidobacteriaceae, Christensenellaceae, Lactobacillaceae, Pasteurellaceae, and Verrucomicrobiaceae families in cases compared to controls/association of a high abundance of Akkermansia, Lactobacillus, Bifidobacterium, and reduced Lachnospiraceae (chain fatty acids producer) with disease development. |
| [41] | 2017 | Germany | 58 (29 case, 29 control) | case: 69.2, 6.5/control: 69.4, 6.7 (years; mean, SD) | case: 23 male, 6 female/control: 13 male, 16 female | stool | age | Shipment with no preservative, at room temp | PowerSoil Kit | Illumina MiSeq | V1–V2 | NR | NR | NR | High abundance of Lactobacillaceae, Barnesiellaceae, and Enterococcacea in cases. |
| [14] | 2015 | USA | 72 (38 case, 34 control) | case: 61.6 ± 9.4/control: 45.1 ± 14.4; (mean) | case: 24/14, control: 18/16; male/female | fecal samples and sigmoid mucosal biopsies | NR | Supporting info | Supporting info | NR | V4 | NR | NR | NR | Anti-inflammatory butyrate- producing bacteria from the genera Blautia, Coprococcus, and Roseburia were significantly more abundant in the feces of controls than PD patients/Faecalibacterium were significantly more abundant in the mucosa of controls than in PD/proinflammatory bacteria such as Proteobacteria were significantly more abundant in the mucosa of PD than controls/the ratio of Firmicutes-to-Bacteroidetes in fecal samples was not significantly different between PD and HC groups/positive correlation of Bacteroidetes and Proteobacteria with PD duration/negative correlation of Firmicutes with PD duration. |
| [47] | 2019 | China | 99 (51 case, 48 control) | case: 62.4 ± 8.2, control: 62.2 ± 9.2 (years, mean ± SD) | case: male: 32, female: 19/control: control: male: 19, female: 29 | stool | age | Sterilized tube | QIAamp Fast DNA Stool Mini Kit | HiSeq2500 PE250 | V4 | 515F | 806R | NR | Low alpha and beta diversity, high abundance of Akkermansia, P. copri Prevotella, Ruminococcaceae, Veillonellaceae Verrucomicrobiaceae, methanobrevibacter smithii, Ruminococcus callidus, Roseburia inulinivorans, Parabacteroides merde, Ruminococcus torques in cases and a high abundance of Bacteroidales, Lactobacillaceae, lactobacillus gasseri in controls. |
| [21] | 2019 | China | 20 (10 case, 10 control) | over 65 | case: male: 7 (70%)/control: male: 5 (50%) | feces | NR | DNA/RNA Shield | PSP SPIN Stool DNA plus kit | Illumina MiSeq | V1–V3 | 341F | 805R | NR | Slightly different gut microbiota between cases and controls, a significant abundance of Bacteroides and Prevotellaceae in healthy controls, a significant abundance of Ruminococcaceae, Verrucomicrobiaceae, Porphyromondaceae, Hydrogenoanaerobacterium, and Lachnospiraceae in PD cases. |
| [27] | 2018 | China | 120 (75 case, 45 control) | case: 60.48 ± 10.72/control; 63.20 ± 6.00 | case: male: 49, female: 26/control: male: 23, female: 22 | stool | age | Without preservation solution at room temperature during shipment | Huirui.® DNA kit | Illumina HiSeq PE250 | V4 | V4T9 (5′-GTGTGYCAGCMG-CCGCGG TAA-3′) | V4R19 (5′-CCGGACTACNVGGGTWTCTAAT-3′) | NR | Significant reductions in Tenericutes, Euryarchaeota, and Firmicutes, Lachnospiraceae in patients with PD. Veillonellaceae and Verrucomicrobiaceae showed marked increases but without statistical significance. Significant differences in alpha diversity (but not beta) between patients with PD who had a disease duration of greater than 5 years compared to those with a disease duration of fewer than 5 years. |
| [49] | 2019 | Taiwan | 157 (80 case, 77 control) | case: 62.3 ± 7.8/control: 61.8 ± 8.3 | case: 62.5% men/control: 60% men | stool | age and sex | Flash-frozen on dry ice, and stored at −80 °C | QIAamp Fast DNA Stool Mini Kit | Illumina MiSeq | V3–V4 | NR | NR | NR | Microbiota from patients with PD dominated by Verrucomicrobia, Mucispirillum, Porphyromonas, Lactobacillus, and Parabacteroides. In contrast, Prevotella was more abundant in controls. The abundances of Bacteroides were more increased in patients with non-tremor PD subtype than patients with tremor subtype. Bacteroides abundance was correlated with motor symptom severity defined by UPDRS part III motor scores. |
| [48] | 2017 | China | 38 (24 case, 14 control) | case: 73.75 ± 6.26/control: 74.64 ± 5.57 | case: 16 men/control: 6 men | stool | age and sex | NR | TIANamp stool DNA kit (Tiangen Biotech Co., Ltd., Beijing, China) | Illumina MiSeq | V3–V5 | 5′-CCTACGGRRBGCASCAGKVRVGAAT-3′ | e 5′-GGACTACNVGGGTWTCTAATCC-3′ | NR | The genera Blautia, Faecalibacterium, and Ruminococcus were significantly decreased in PD compared to healthy controls. The genera Escherichia-Shigella, Streptococcus, Proteus, and Enterococcus were significantly increased in PD subjects. |
| [50] | 2017 | Russia | 155 (89 case, 66 control) | case: 67/control: 63 | NR | stool | age and sex | NR | NR | Illumina MiSeq | V3–V4 | NR | NR | NR | Reduction in taxonomic diversity of gut microbiota in patients with PD. |
| [51] | 2019 | Italy | 152 (80 case, 72 control) | NR | NR | stool | age, sex, loss of weight | NR | PSP Spin Stool DNA Kit Plus (Stratec Molecular) | Illumina MiSeq | V3–V4 | NR | NR | PRJNA510730 | Significantly higher levels of Lactobacillaceae, Enterobacteriaceae, and Enterococcaceae families compared to healthy individuals. On the other hand, the levels of Lachnospiraceae were significantly reduced in PD patients. |
| [52] | 2020 | China | 80 (40 case, 40 control) | case: 66.6 ± 7.1/control: 66.3 ± 8.1 | case: 19 men/control: 21 men | stool | lifestyle, gender, age, and BMI | NR | QIAamp DNA Stool Mini Kit (Qiagen) | Illumina HiSeq X-ten | NR | NR | NR | PRJNA433459 | The diversity and community of gut microbial genes in PD patients differed from those of healthy control subjects. Thirty-six different taxa were enriched in the PD patients, and no taxon was enriched in the healthy controls. |
| [53] | 2018 | China | 90 (45 case, 45 control) | case: 68.1 ± 8.0/control: 67.9 ± 8.0 | case: 22 men/control: 23 men | stool | age, sex, BMI, constipation | NR | QIAamp DNA Stool Mini Kit (Qiagen) | Illumina MiSeq | V3–V4 | 341F | 806R | PRJNA391524 | Some bacteria were correlated with PD clinical characteristics, including disease duration, severity, medication, and non-motor symptoms. |
| [66] | 2020 | China | 40 (27 case, 13 control) | case: 62.1 ± 10.2/control: 63 ± 8.76 | case: 19 men/control: 3 men | stool | age | NR | QIAamp DNA Stool Mini Kit (Qiagen) | Illumina MiSeq | V3–V4 | 515F 5′-GTGCCAGCMGCCGCGGTAA-3′ | 926R 5′-CCGTCAATTCMTTTGAGTTT-3′ | PRJNA561023 | Compared with HC and patients with PD-NC, the gut microbiota of patients with PD-MCI was significantly altered, particularly manifesting in enriched genera from Porphyromonadaceae family and decreased abundance of genera Blautia and Ruminococcus. |
| [55] | 2021 | Malaysia | 200 (104 case, 96 control) | case: 65.4 ± 8.4/control: 62.4 ± 9.0 | case: 62.5% male/control: 37.5% male | stool | age, sex | Preservatives were not added | QIAamp DNA Stool Mini Kit (Qiagen) | Illumina MiSeq | V3–V4 | 341F (5′-CCTACGGGNGGCWGCAG-3′) | 805R (5′-GACTACHVGGGTATCTAATCC-3′) | PRJNA494620 | Ten bacterial taxa were significantly increased in PD; largest fold changes were observed for B. fragilis, Lactobacillus acidophilus, Megasphaera and Gammaproteobacteria. |
| [56] | 2020 | Italy | 115 (64 case, 51 control) | case: 71.39 ± 10.99/51.67 ± 12.42 | case: 44 men/control: 31 men | stool | sex, age, BMI, coffee consumption, smoking | NR | QIAamp DNA Stool Mini Kit (Qiagen) | Illumina MiSeq | V3–V4 | NR | NR | PRJEB30401 | The most significant changes within the PD group highlighted a reduction in bacterial taxa, which are linked to anti-inflammatory/neuroprotective effects, particularly in the Lachnospiraceae family and key members, such as Butyrivibrio, Pseudobutyrivibrio, Coprococcus, and Blautia. |
| [43] | 2019 | Germany | 59 (34 case, 25 control) | case: 67.9 ± 8.6/63.9 ± 5.8 | case: 23 men/control: 11 men | stool | age | Sterile containers | FastDNA SPIN kit | Illumina MiSeq | V4–V5 | 520 F (5′-AYTGGGYDTAAAGNG-3′) | 926 R (5′-CCGTCAATTCMTTTRAGTTT-3′) | PRJEB30615 | PD patients exhibit alterations in their gut microbiota composition, characterized by a decrease in beneficial bacteria and an increase in certain bacterial groups. A potential link between the gut microbiome and PD development, as well as the influence of PD medications on the gut microbiota. |
| [60] | 2021 | China | 40 (20 case, 20 control) | case: 63.65 ± 5.64/61.95 ± 4.73 | case: 10 men/control: 10 men | stool | age, sex, BMI | NR | DNeasy PowerSoil Kit (Qiagen) | Illumina MiSeq | V3–V4 | 343 F: 5′-TACGGRAGGCAGCAG-3′ | 798 R: 5′-AGGGTATCTAATCCT-3′ | NR | A greater abundance of Alistipes, Rikenellaceae_RC9_gut_group, Bifidobacterium, Parabacteroides, while Faecalibacterium was decreased in fecal samples from PD patients. |
| [62] | 2020 | China | 126 (63 case, 63 control) | case: 64.0 ± 7.4/63.9 ± 7.9 | case: 40 men/control: 23 men | stool | age | NR | QIAamp Fast DNA Stool Mini Kit (Qiagen) | Illumina HiSeq PE250 | V4 | NR | NR | CRA001938 | There were markedly different microbial compositions among PD, HS, and HP samples by alpha/beta diversity. They also found differential microbial compositions among Hoehn and Yahr stage/disease duration. |
| [25] | 2014 | Finland | 144 (72 case, 72 control) | case: 65.3 ± 6 5.5/64.5 ± 6.9 | case: 51.4% male/control: 50% men | stool | age, sex | NR | NR | NR | V1–V3 | NR | NR | NR | Reduced abundance of Prevotellaceae in PD patients and the positive association of Enterobacteriaceae abundance with PIGD symptoms. |
| [40] | 2022 | China | 172 (132 case (mild: 43, moderate: 89), 40 control) | 60 to 90 | M/F case: 15/28 (mild AD), 33/56 (moderate AD)/control: 16/24 | feces | age, sex | Special cytoprotective agent | E.Z.N.A. Soil DNA Kit | Illumina MiSeq | V3–V4 | CCTACGGGNGGCWGCAG | GACTACHVGGGTATCTAATCC | PRJNA855571 | Elevated abundance of certain bacteria in cases (moderate vs. control), including Proteobacteria, Verrucomicrobia, Actinobacteria, and Synergistetes. Reduced levels of Firmicutes and Bacteroidetes in cases. Controls, when compared to mild and moderate cases, showed higher levels of Firmicutes, Erysipelotrichia, Acidaminococcaceae, Ruminococcaceae, and Bacteroidetes. |
| [26] | 2022 | Canada | 99 (45 case, 54 control) | case: 74: (65–78)/control: 70 (66–74); (year) | Female: case 33.3%/control 33.3% | feces | age | OMNIgene® GUT | QIAamp PowerFecal DNA Kits | MiSeq | V4 | 515F (GTGCCAGC MGCCGCGGTAA) | 806R (GGACTACHVHHHTWTCTAAT) | PRJNA770746 | No significant different between AD patients and controls (beta diversity), lower alpha diversity in cases, higher abundance of Erysipelotrichaceae in cases. |
| [45] | 2021 | China | 64 (28 cases (18 AD), 18 control) | case: 63.5 (4.7)/control: 64.5 (4.5); (SD) | Male (%): case 2 (11)/control: 4 (22) | feces | age, sex | NR | PowerSoil DNA Isolation Kit | Illumina Miseq/Microseq | V3–V4 | NR | NR | NR | AD cases exhibited increased microbial diversity, decreased levels of Bacteroides, Lachnospira, and Ruminiclostridium, and increased Prevotella. |
| [17] | 2019 | USA | 108 | case: 84.7 (8.1)/control: 83 (10.2); (mean [SD]) (year) | Male: case: 4 (16.7)/control: 8 (15.7) | stool | age, sex | NR | PowerMag soil DNA isolation kit | NextSeq 500 | NR | NR | NR | Upon request | Significant increase in Bacteroides, Alistipes, Odoribacter, Barnesiella, Osplanchnicus, Odoribacter spp., K. pneumoniae, B. fragilis, E. lenta, and Desulfovibrio AD (sulfate-reducing bacteria). Conversely, there were significant decreases in bacteria, including Lachnoclostridium, Butyrivibrio, B. proteoclasticus, B. hungatei, Eubacterium, E. eligens, E. hallii, E. rectale, Clostridium sp. SY8519, R. hominis, and F. prausnitzii./significantly different beta diversity between controls and AD cases. |
| [46] | 2022 | Kazakhstan | 84 (41 case, 43 control) | case: 68 (62–74)/control 68 (61–75); (median (IQR)) | Female, n (%): case 30 (73.2%)/control 35 (81.4%) | feces | NR | special kit | QIAamp DNA stool Mini Kit | Illumina MiSeq | NR | NR | NR | PRJNA811324 | Increased abundance of Acidobacteriota, Verrucomicrobiota, Planctomycetota, and Synergistota and decreased abundance of Bifidobacterium, Clostridia bacterium, Castellaniella, Erysipelotrichaceae UCG-003, Roseburia, Tuzzerella, Lactobacillaceae and Monoglobus in AD patients. |
| [28] | 2019 | China | 97 (33 case (32 aMCI), 32 control) | case: 74.85 ± 11.37 (AD), 70.53 ± 11.0 (aMCI)/control: 76.88 ± 9.35; (years, mean ± SD) | Male: case: 14 (AD; 42.42%), 18 (56.25%) aMCI/control 16 (50%) | feces | age, sex | Sterile collection containers | QIAGEN | Illumina MiSeq | V3–V4 | 5′-CAAGCAGAAG ACGGCATACGAGATGTGACTGGAGTTCAGACGTGTGCTCTTCCGA TCT-3′ | 5′-AATGATACGGCGACCACCGAGATC TACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′ | PRJNA496408 | Decreased diversity in AD patients compared to controls. Different gut microbiota between healthy and cases/reduced abundance of Firmicutes, increased abundance of Proteobacteria in cases/a significant correlation between AD severity and the abundance of altered microbiomes/association of Enterobacteriaceae with AD. |
| [42] | 2021 | China | 105 (case: 53 (SCD) 14 (CI: MCI, n = 8; mild AD dementia, n = 6))/38 control) | case: 66.68 ± 6.32 (SCD), 73.21 ± 7.89 (CI)/control: 66.79 ± 5.13; (year) | M/F: case: 10/43 (SCD), 4/10 (CI)/control: 15/23 | feces | age, sex, educational years, other potential factors | Cytoprotective agents | QIAamp DNA Stool Mini Kit | Illumina Miseq PE250 | V3–V4 | 341F (CCTAC GGGRSGCAGCAG) | 806R (GGACTACVVGGGTATCTAATC) | NR | Decreasing abundance of Firmicutes, Clostridia, Clostridiales, Ruminococcaceae, and Faecalibacterium in cases. |
| [54] | 2020 | Austria | 41 (23 case, 18 control) | case: 88 (73, 85)/control: 75 (74, 76); (years) | F/M: case: 15/18, control: 11/7 | stool | age, sex | Collection tubes | MagnaPure LC DNA Isolation Kit III | Illumina MiSeq | V1–V2 | 27F (AGAGTT TGATCCTGGCTCAG) | R357 (CTGCTGCCTYCCGTA) | PRJNA608281 | Decreased abundance of Lachnospiraceae in cases, clear clustering of case and controls at different stages of dementia according to beta diversity, AD association with a reduction in bacteria producing short-chain fatty acids (SCFA) and increased biomarkers of gut permeability and inflammation. Increased abundance of C. clostridioforme and Eisenbergiella is associated with cognitive impairment. |
| [18] | 2022 | Spain | 22 (12 case, 10 control) | 60 to 70 | case: 2 men/control: 6 men | stool | NR | NR | QIAamp PowerFecal Pro DNA isolation kit (Qiagen, Madrid, Spain) | Illumina MiSeq | NR | 515F-Y (50 GTG YCA GCM GCC GCG GTA A 30 | 806R (50 GGA CTA CNV GGG TWT CTA AT 30 | NR | At a more advanced stage of AD, the gut microbiota and volatiles shifted towards a profile with increases in Ruminococcus and Blautia. |
| [58] | 2022 | Thailand | 40 (20 case, 20 control) | case: 72.8 ± 5.6/control: 69.4 ± 6.2 | case: 45.5% male/control: 38.5% male | stool | NR | Preservation System (Norgen Biotek Corp., Thorold, ON, Canada) | QIAamp Stool Mini kit (Qiagen, USA) | Illumina MiSeq | V3–V4 | NR | NR | NR | A significant difference at the operational taxonomic unit level. The altered gut microbiome could be potentially targeted for the early diagnosis of dementia and the reduction in AD risk. |
| [59] | 2021 | China | 65 (21 case, 44 control) | case: 76.2 ± 9.9/control: 78.4 ± 6.6 | case: 13 men/control: 20 men | stool | NR | Sterile fecal collection containers | E.Z.N.A.@ Stool DNA Kit (Omega Bio-Tek, Norcross, GA, USA) | Illumina Miseq | V3–V4 | 338 forward (5′-ACTCCTACGGGAGGCAGCAG-3′) | 806 reverse (5′-GGACTACHVGGGTWTCTAAT-3′) | SRP252374 | Gut microbial alterations and related metabolic output changes may be associated with pathogenesis of AD. Fecal markers might be used as a non-invasive examination to assist screening and diagnosis of AD. |
| [61] | 2022 | Turkey | 98 (47 case, 51 control) | case: 71.4 ± 5.1/control: 67 ± 5.3 | case: 24 men/control: 28 men | stool | NR | NR | QiaAmp DNA stool minikit (Qiagen, Germany) | Illumina MiSeq | V3–V4 | 341 F (59-CCTACGGGNGGCWGCAG-39) | 805 R (59-GACTACHVGGGTATCTAATCC-39) | NCBI BioProject database | A different gut microbiota composition in AD cases marked primarily by Prevotella and Bacteroides, but also subnetworks of other taxa exist in the community. |
| [63] | 2021 | China | 92 (60 case, 32 control) | case: 72.82 ± 7.25/control: 71.06 ± 5.92 | case: 24 men/control: 14 men | stool | age | NR | QIAamp® DNA Stool Mini Kit (Qiagen, Hilden, Germany) | Illumina MiSeq | V3–V4 | 5-CCTACGGGNGGCWGCAG-3 | 5-GACTACHVGGGTATCTAATCC-3 | NR | AD patients had gut microbiota alterations related to cognition, and differential taxa between AD patients with and without NPS associated differently with NPS domains. |
| [65] | 2018 | China | 86 (43 case, 43 control) | case: 70.12 ± 8.76/control: 69.72 ± 9.24 | case: 23 men/control: 23 men | stool | age, sex | NR | QIAamp® DNA Stool Mini Kit (Qiagen, Hilden, Germany) | Illumina MiSeq | V3–V4 | 338F | 806R | NR | Altered gut microbiota composition and diversity AD cases compared to cognitively normal controls. Several bacterial taxa, including Actinobacteria, Bacteroidales, Ruminococcaceae, Selenomonadales, and Lachnoclostridium, were found to contribute to these differences. |
| [64] | 2022 | China | 302 (125 MCI, 83 AD case, 94 control) | case: 71.8 ± 8.3/control: 74.3 ± 10.6 | case: 53 men/control: 58 men | stool | NR | Sterile containers | E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) | Illumina MiSeq | V3–V4 | 338F (50 -ACTCCTACGGGAGGCAGCAG-30) | 806R (50-GGACTACHV GGGTWTCTAAT-30) | NR | No significant difference in the alpha and beta diversity among groups. Patients with AD or MCI had increased bacterial taxa including Erysipelatoclostridiaceae, Erysipelotrichales, Patescibacteria, Saccharimonadales, and Saccharimonadia, compared with NC group. |
| [57] | 2017 | USA | 50 (25 case, 25 control) | case: 71.3 ± 7.3/control: 69.3 ± 7.5 | case: 8 men/control: 7 men | stool | age, sex | NR | NR | Illumina MiSeq | V4 | NR | NR | NR | Decreased microbial diversity in AD cases and compositionally distinct from controls. |
3.4. Gut Microbiota Comparison between Cases and Controls
Among the PD studies, 22 out of 26 studies reported a different gut microbiota composition between the PD cases and the healthy controls [19,20,21,22,23,24,25,38,39,44,47,48,49,50,51,52,53,55,56,60,62,66], supporting the association between PD and gut microbial alterations. Additionally, 15 out of 16 AD studies reported differences in gut microbiota composition between the AD cases and the healthy controls [17,18,28,40,42,45,46,54,57,58,59,61,63,64,65].
These studies utilized a variety of analytical methods, including OTU clustering, diversity analysis (richness and evenness measurements), taxonomic classification, and differential abundance analysis to compare the gut microbiota composition between cases and controls, as discussed further in this systematic review.
3.5. Core Gut Microbiota
The comparison of the PD studies has revealed a set of abundant bacteria that were consistently found in both the case and the control groups, regardless of the individuals’ health status.
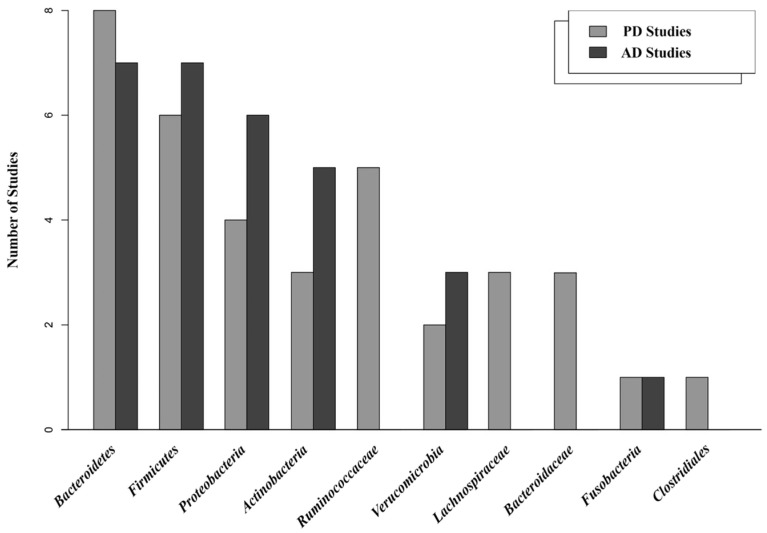
Bacteroidetes (eight studies) [21,25,48,49,56,60,62,66], Firmicutes (six studies) [21,25,48,56,60,62], Ruminococcaceae (five studies) [19,20,21,27,38], Proteobacteria (four studies) [21,25,56,60], Lachnospiraceae [20,27,38], Actinobacteria [25,56,60], and Bacteroidaceae [19,21,27] (each in three studies), Verrucomicrobia (two studies) [25,56], Clostridiales order [19] and Fusobacteria [25] (each in one study) were identified as common microbial communities in both the PD healthy groups and the case groups, regardless of their grouping.
Similarly, in the context of the AD studies, Bacteroidetes and Firmicutes (each in seven studies) [18,38,42,57,61,64,65] emerged as the most prevalent phyla. Proteobacteria (six studies) [38,42,57,61,64,65], Actinobacteria (five studies) [18,42,57,61,64], Verrucomicrobia (three studies) [40,57,61], and Fusobacteria (one study) [40] were also consistently identified in the AD studies as core gut microbiota in both the case and the control groups.
As a result, both the PD and the AD studies consistently identified the phyla Bacteroidetes, Firmicutes, and Proteobacteria as prevalent in the gut microbiota in both the healthy and the case groups (Figure 3).
Figure 3.
Predominant bacterial communities, at different taxonomic ranks, identified as core gut microbiota in PD and AD studies, irrespective of the participants’ health status.
3.6. Microbial Communities in the Healthy Group
Several prevalent bacterial taxa have been consistently identified within specific taxonomic ranks in the healthy control groups in both the PD and the AD studies. In the PD studies, the genera Blautia (three studies) [14,38,41], Coprococcus (two studies) [14,41], and Roseburia (two studies) [41,46], as well as the family Prevotellaceae (two studies) [21,38] were consistently found. Additionally, other bacterial communities such as those within the orders Bacteroidales, the genus Bacteroides, the family Clostridieceae, and the genus Faecalibacterium, among others, were identified in individual studies.
Similarly, Acidaminococcaceae and Lachnospiraceae (each in two studies) [40,45] were identified as prevalent bacteria in healthy individuals in the AD studies. Furthermore, several other bacterial communities, such as the family Bacteroidaceae, the genus Agathobacter, and the species Bacteroides fragilis were observed in single studies.
3.7. Microbial Communities in the Case Group
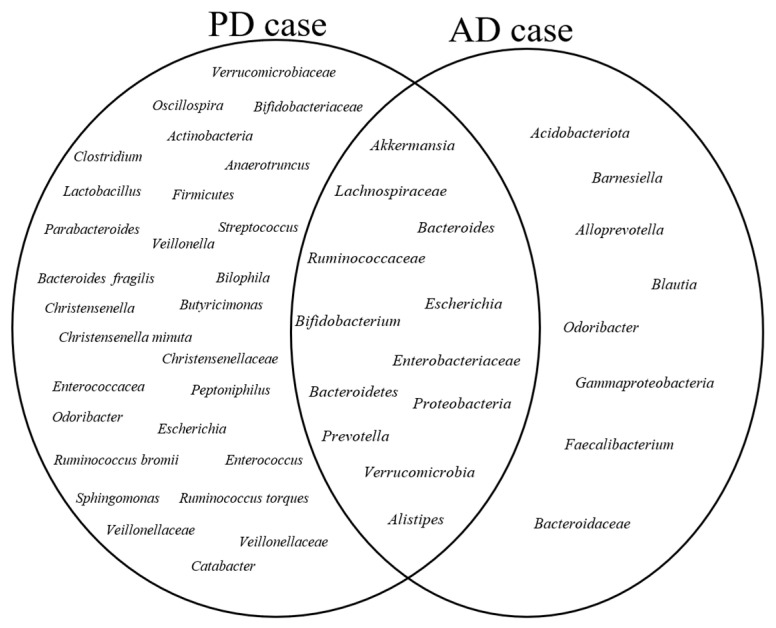
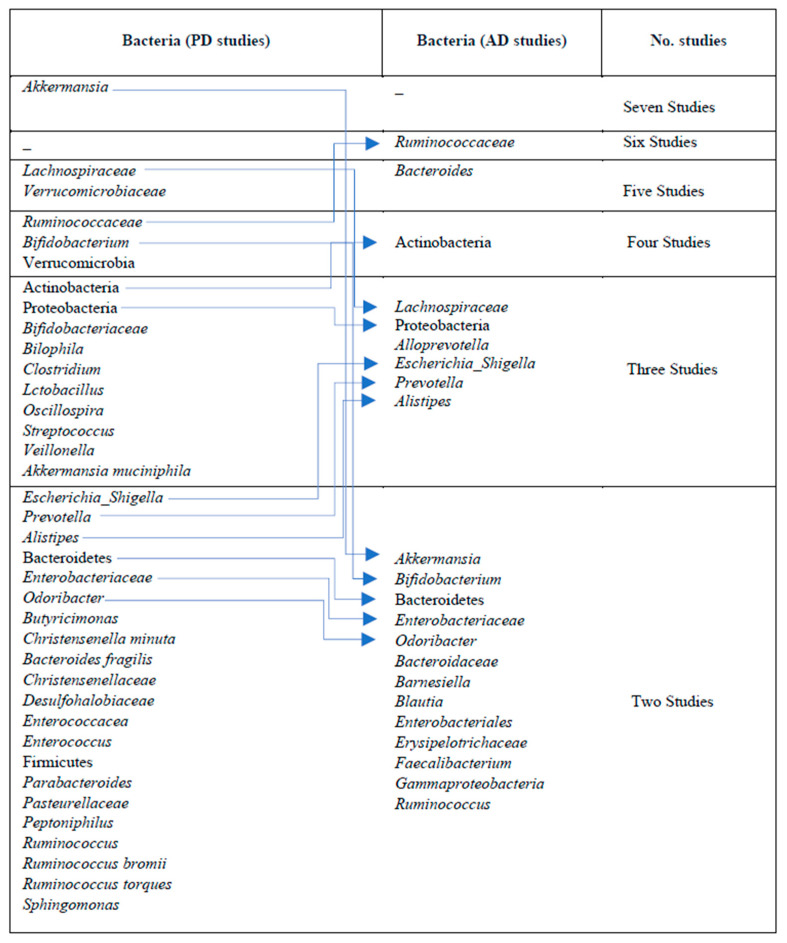
The comparison of highly abundant bacteria in the PD and AD case groups revealed several key findings at specific taxonomic ranks. In the PD studies, the genus Akkermansia was consistently identified in 10 studies [14,20,21,23,24,44,47,49,52,56], with Akkermansia muciniphila reported in 3 studies [19,39,52], highlighting its potential significance in PD pathogenesis. Additionally, the family Verrucomicrobiaceae (eight studies) [19,20,21,23,24,27,47,56], the family Lachnospiraceae (six studies) [21,22,23,24,41,51], and the genus Bifidobacterium (five studies) [21,22,47,60,66] were found to be highly abundant in the PD cases. Similarly, in the AD studies, the genus Bacteroides [17,18,46,58,61] and the phylum Acidobacteriota [18,26,46,65] were consistently found to be prevalent in the AD cases.
Other microbial communities reported in a lower number of studies in the AD and PD cases are shown in Figure 4.
Figure 4.
Highly abundant bacterial communities in PD and AD Cases: A Venn diagram representing bacterial communities reported as highly abundant in PD and AD cases across multiple included studies. The circles represent the bacterial taxa found in each disease group, while the overlapping sections denote the shared bacteria between PD and AD cases. These highly abundant microbial communities were consistently reported as such when compared to their respective healthy control groups in the included studies.
Figure 4 shows increased microbial communities in the AD and PD cases. A Venn diagram illustrates the highly abundant bacterial communities shared between the PD and AD case groups. The AD and PD circles show specific bacterial taxa that are abundant in each disease, with the left circle representing the PD cases and the right circle representing the AD cases. These shared microbial communities, represented by the overlapping sections of the diagram, suggest a potential link between the shared microbial communities and the pathophysiology of PD and AD.
Low abundant bacteria in the PD and AD cases provided further insights into the microbial composition of the gut microbiota in these groups. In the PD studies, the family Lachnospiraceae [14,23,24,27,44,51,56] and the genus Faecalibacterium [25,43,44,48,50,60] were identified in seven and six studies, respectively, suggesting their potential role as less abundant but still significant bacterial taxa in PD.
Similarly, in the AD studies, the phylum Firmicutes was found in low abundance in the AD cases across six studies [18,26,28,42,57,61], followed by the phylum Bacteroidetes [26,28,61,65] and the family Lachnospiraceae [28,42,54,65] in four studies (Figure 4). A comprehensive overview of the list of bacteria identified in lower numbers of studies is provided in Supplementary Material S5.
3.8. Bacterial Diversity in PD and AD Studies (Alpha and Beta Diversity)
In the PD and AD studies, a diverse range of alpha (microbial diversity within individual samples) and beta diversity indices (microbial diversity between groups) were utilized to evaluate the diversity and composition of microbial communities between the healthy individuals and the cases. Among the indices used, the most common alpha indices were Shannon, Chao1, and Simpson. In terms of beta diversity, the primary metrics utilized were Bray–Curtis dissimilarity, unweighted UniFrac distances, and weighted UniFrac distances.
In the PD studies, a total of 16 studies showed no difference in alpha diversity between the PD cases and the controls [14,20,22,23,25,27,28,38,39,41,43,44,51,55,56,60]. However, eight studies reported distinct differences in gut microbiota between the PD cases and the healthy controls [23,47,49,50,52,53,62,66]. Among these eight studies, six found higher alpha diversity in the PD cases [23,49,52,53,62,66], while two observed lower alpha diversity in the cases compared to the controls [47,51] (Table 2).
Table 2.
Summary of alpha and beta diversity findings between cases and controls in PD and AD studies.
| PD Studies | AD Studies | |
|---|---|---|
| Alpha Diversity (case vs. control) |
|
|
| Beta Diversity (case vs. control) |
|
|
Regarding beta diversity analysis in the PD studies, consistent dissimilarities were found between the PD cases and the controls in 20 studies [19,20,21,22,23,24,25,38,39,41,44,47,49,50,52,53,56,60,62,66], indicating a notable divergence in gut microbiota composition. However, four studies did not identify any differences in beta diversity between the two groups [27,48,51,60].
In the AD studies, eight studies indicated no significant difference in alpha diversity between the AD cases and the controls [45,46,54,58,59,61,63,64]. Conversely, six studies demonstrated a lower alpha diversity in the AD cases compared to the controls [18,26,28,40,42,57], suggesting a potential alteration in the gut microbiota composition associated with AD.
In terms of beta diversity, 11 studies identified a difference in gut microbiota composition between the AD cases and the controls [17,18,28,40,42,45,54,57,59,61,63], implying distinct microbial community structures. However, five studies did not observe any dissimilarities in beta diversity between the two groups [44,46,57,58,65] (Table 2).
3.9. Gut Microbiota Associated with PD and AD (Differential Abundance Analysis (DAA))
A variety of statistical methods were utilized to identify differentially abundant bacteria between the cases and the controls in PD and AD studies. Among the commonly employed methods, seven studies used ANOSIM (analysis of similarities) [14,50,52,53,60,62], four studies used PERMANOVA (permutational multivariate analysis of variance) [24,49,55,56], LEfSe (linear discriminant analysis effect size) [21,27,47,66], and ANCOM (analysis of composition of microbiomes) [19,20,24,38] in the PD studies. In the AD studies, LEfSe was used in eight studies [28,42,45,46,54,57,59,63], while PERMANOVA was used in six studies [17,40,58,59,61,63].
It is worth noting that each method has its own strengths and considerations. Some studies may combine different methods to gain a comprehensive understanding of the differential abundance of bacterial taxa associated with PD and AD. Conversely, certain studies lacked sufficient details regarding these analyses.
The comparison of the DAA findings revealed several bacterial taxa that exhibited differential abundance between the cases and the control groups in the PD and AD studies.
In the PD cases, differentially abundant bacteria were described as Akkermansia (seven studies) [20,44,47,49,51,52,56], Lachnospiraceae [21,22,24,41,51] and Verrucomicrobiaceae [19,20,24,47,56] (each in five studies), Bifidobacterium [44,50,56,60], and Verrucomicrobia (each in four studies) [14,21,49,56]. Other bacterial communities, such as Ruminococcaceae [21,47,60], Actinobacteria [56,60,66], Akkermansia muciniphila [19,38,52], Bifidobacteriaceae [24,27,56], Bilophila [38,44,49], lactobacillus [38,49,50], Oscillospira [14,22,50], streptococcus [38,48,56], and Veillonella [49,55,56], were reported in three studies each.
In the AD cases, the differentially abundant bacteria included Ruminococcaceae (six studies), Bacteroides (five studies), Actinobacteria (four studies), and Alistipes, Alloprevotella, Escherichia_Shigella, Lachnospiraceae, Prevotella, and Proteobacteria (each in three studies). There were also several bacteria identified in only one study. These bacteria have not been consistently reported across multiple studies but still may hold potential significance (Figure 5).
Figure 5.
Differentially abundant microbial communities associated with PD and AD. Microbial communities identified in both PD and AD are shown using connecting arrows.
In individuals with AD, certain microbial communities appear to be associated with potential mechanisms contributing to the disease: microbial communities such as Bacteroides and Prevotella may trigger inflammation and immune activation in the gut [67], while microbial groups like Ruminococcaceae, Faecalibacterium, Butyricimonas, and Odoribacter are associated with the production of metabolites, such as SCFAs, which can play a significant role in gut–brain communication [68]. Additionally, A. muciniphila, and Verrucomicrobiaceae are implicated in mucin degradation and the disruption of gut barrier integrity [69]. The diversity and balance of the gut microbiota may also play a role, with microbial communities like Lachnospiraceae, Actinobacteria, Alistipes, Alloprevotella, Christensenellaceae, and Ruminococcus contributing to this aspect.
Also, in the PD cases, different microbial communities are associated with similar mechanisms. Enterobacteriaceae, Escherichia_Shigella, and Gammaproteobacteria may contribute to inflammation and immune activation, while Bifidobacterium and Blautia are linked to metabolite production. Akkermansia and Verrucomicrobia may be involved in mucin degradation and gut barrier function. The importance of comprehending the gut–brain connection in the context of neurodegenerative diseases may be highlighted by these shared mechanisms involving particular bacterial communities and metabolites. These mechanisms may also open up opportunities for therapeutic interventions that target the gut microbiota to reduce the risk and progression of both AD and PD.
4. Discussion
This systematic review aims to comprehensively evaluate the existing literature on gut microbiota composition in patients diagnosed with Parkinson’s and Alzheimer’s disease.
In this study, the evaluation of the core gut microbiota observed in both the case and the healthy groups, irrespective of their health condition, showed the abundance of six main phyla, Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, Verrucomicrobia, and Fusobacteria, and three families, Ruminococcaceae, Bacteroidaceae, and Lachnospiraceae, which also play a crucial role in maintaining the overall human health and gut homeostasis through several pathways, such as carbohydrate metabolism, short-chain fatty acid (SCFAs) production [70], regulation of immune response [71], intestinal barrier maintenance, and vitamin production [72,73].
Microbial taxa, such as Akkermansia, Verrucomicrobiaceae, Lachnospiraceae, Ruminococcaceae, Bifidobacterium, and Proteobacteria, among others, were found to be predominant in the PD cases. These bacterial communities are associated with various functions and metabolites that could potentially contribute to the pathology of PD. For instance, the capacity of Akkermansia (a member of the Verrucomicrobiota phylum) to degrade the mucus of the gut barrier can result in increased gut permeability (leaky gut). This condition can potentially enable the translocation of specific molecules, such as lipopolysaccharides (LPS), from the gut into the bloodstream, thereby initiating immune responses and systemic inflammation, which are implicated in neurodegenerative diseases. Also, it may impact the vagus nerve, a major pathway connecting the brain and the gut, and disturb the gut–brain axis communication [15]. The family Lachnospiraceae is also known to produce SCFAs, such as butyrate. Dysregulation of SCFAs may impact the gut–brain axis and contribute to neuroinflammation and PD progression [11].
Similarly, in the AD cases, Acidobacteriota, Ruminococcaceae, Bacteroides, Proteobacteria, and Alistipes appear as highly abundant bacteria. An imbalanced abundance of these bacterial taxa can have detrimental consequences for gut barrier function and can induce neuroinflammation through the production of metabolites and promote amyloid-beta aggregation, all of which contribute to the development and progression of AD [74,75].
Future studies should investigate these mechanisms in more detail to provide a clearer understanding of how the gut microbiota influences disease development and progression.
It is also interesting to note that some bacteria were observed in both the PD and the AD cases, such as Alistipes, Bifidobacterium, Lachnospiraceae, and Proteobacteria, although their abundance may differ. This overlap may highlight the possibility of shared interactions or mechanisms in the gut microbiota in these disorders. Further research is required to explore these shared aspects and determine whether common pathways or dysregulated processes contribute to the similarities observed in gut microbiota composition.
The comparison of differentially abundant microbial taxa between the PD cases and the healthy controls, as well as the AD cases and the healthy controls, showed that the PD cases were enriched with Akkermansia, Lachnospiraceae, Verrucomicrobiaceae, Bifidobacterium, Ruminococcacea, Verrucomicrobia, Actinobacteria, and other bacterial taxa. Similarly, Ruminococcaceae, Actinobacteria, Bacteroides, Alloprevotella, Escherichia/Shigella, Prevotella, and Proteobacteria were differentially abundant between the AD cases and the AD controls. It is worth pointing out that although this association might suggest a microbial signature associated with PD and AD, it only indicates a correlation and does not establish a causal relationship between the identified bacterial taxa and the development of PD or AD.
Furthermore, emerging studies on the gut–brain axis have highlighted bidirectional communication between the gut and the dopaminergic system, involving the intricate interaction of neurotransmitters like dopamine with the gut microbiota [76]. Dysbiosis has been associated with disruptions in these signaling pathways, potentially impacting the regulation of dopamine levels. Some bacterial species have been found to produce metabolites that can influence the dopaminergic system, while others may trigger inflammatory responses affecting the brain. The increased abundance of bacteria like Ruminococcus, Enterobacteriaceae, and Clostridium, among others, along with their metabolites, has been linked to dopamine dysregulation and the development of PD [77]. Understanding the role of gut microbiota dysbiosis and its impact on dopamine activity in neurological diseases may offer opportunities for diagnosis, prevention, and treatment.
Also, recent investigations on the association of nutrition, gut microbiota, and the development of neurodegenerative diseases have yielded interesting results. Although our primary objective in this systematic review did not include this aspect, it is important to recognize the significance of nutritional factors and potential mechanistic pathways when investigating the gut microbiota’s role in neurodegenerative diseases. Dietary choices, including the administration of probiotics (such as strains of Lactobacillus and Bifidobacterium) [78] have demonstrated their ability to influence the composition of the gut microbiota profile. Furthermore, certain dietary patterns, such as adherence to the Mediterranean diet [79] or increased dietary fiber intake [80], have shown associations with a more stable and beneficial microbial profile among individuals diagnosed with PD or AD. These findings have significant potential for the development of personalized nutrition interventions aimed at preventing these neurological conditions.
The evaluation of alpha diversity (microbial diversity within a given sample) and beta diversity (microbial diversity between groups) showed variable results across the different studies. In 24 studies (16 PD and 8 AD studies), no alpha diversity difference was observed between the cases and the controls. However, 14 studies reported distinct alpha diversity between the cases and the controls, with lower alpha diversity detected in the cases: (8 studies: 2 PD and 6 AD) and higher alpha diversity observed in 6 PD studies. Lower alpha diversity, indicating a less diverse gut microbiota, has been consistently linked to numerous diseases, such as irritable bowel syndrome (IBS) and fibromyalgia, along with other conditions [81,82]. Reduced alpha diversity is believed to influence the regulation of immune responses, metabolic processes, gut barrier function, and neurotransmitter function [71].
In terms of beta diversity, 31 studies (20 PD and 11 AD) demonstrated a distinct community structure between the cases and the controls, while 9 studies (4 PD and 5 AD) showed comparable community structures with a high degree of similarity between the two groups.
These findings highlight the heterogeneity in the gut microbiota composition in the PD and AD studies. It is also possible that the gut microbiota alterations in PD and AD are not universally consistent, reflecting the complex nature of these diseases and the potential involvement of multiple factors (such as study design, sample size, geographical location, and patient characteristics) in their pathogenesis [83]. Additional analyses, such as functional profiling of the microbiota or the investigation of specific bacterial taxa, metabolites, or functional pathways, can provide further insights into the potential mechanisms underlying the associations between the gut microbiota and PD or AD.
4.1. Limitation Described in Included Studies as Claimed by the Authors
Lack of covariate consideration: Important covariates such as diet, exercise, smoking, drug treatment, and comorbidities were not adequately addressed in some studies (13 studies).
Lack of longitudinal data: Several studies lacked longer follow-up periods to capture the microbial community changes during disease progression. Also, the cross-sectional design of many the studies limited their ability to establish causal relationships between the gut microbiota and neurodegenerative disorders (nine studies).
Small sample size: Several studies reported small sample sizes, which may have limited the statistical power and generalizability of the findings (six studies).
Lack of host–microbiome interaction consideration: The studies often did not consider the interactions between the host metabolism and the gut microbiota, which could provide a more comprehensive understanding of the mechanisms underlying neurodegenerative disorders (six studies).
Lack of species/strain resolution: The use of the 16S rRNA sequencing method limited the ability to analyze microbial composition at the species or strain level, which is crucial for identifying the specific microorganisms associated with the diseases (five studies).
Lack of mucosal microbiota analysis: Although nearly all of the included studies only utilized stool samples, only two studies acknowledged the need for the analysis of mucosal microbiota composition using gastrointestinal biopsies. Such analysis provides a deeper understanding of the local host–microbiota interaction (two studies).
As the authors of the included studies identified these limitations in their studies, these should be considered when interpreting the results. Addressing these limitations in future research can enhance the understanding of the gut microbiota’s role in neurodegenerative disorders and provide more robust insights into their underlying mechanisms.
4.2. Strength and Limitation of This Systematic Review
This systematic review has several strengths. First, it was registered with PROSPERO, which highlights its transparency and adherence to pre-established protocols. Additionally, it includes the highest number of studies to date, enhancing the comprehensiveness and interpretation power of the review. It also involves the extraction and comparison of significant findings related to the gut microbiota in relation to PD and AD.
It is important to note that while bacteria are the predominant community in the gut, other forms of intestinal flora, such as mycobiota, archaeome, protozoa, and virome, also exist, although in lesser amounts. However, due to insufficient research on these communities, they were not considered in this review, thus representing a limitation of the study.
4.3. Future Recommendations
-
1:
Considering important covariates such as diet, exercise, smoking, comorbidities, and drug treatment and their potential influence on the gut microbiota as a fundamental step in the study design would provide a more comprehensive insight into the role of gut microbiota in AD and PD diseases.
-
2:
The design of longitudinal studies with longer follow-up periods to capture microbial community changes during disease progression is needed. Also, well-designed intervention studies, such as probiotic or prebiotic trials, can help to determine the therapeutic potential of modulating the gut microbiota in relation to disease symptoms and progression.
-
3:
Large-scale cohort studies involving diverse populations with larger sample sizes and frequent sampling to capture variations in gut microbiota composition associated with different backgrounds, geographical locations, and lifestyles are essential. This will help to identify potential factors influencing gut microbiota and allow for personalized approaches to managing diseases using gut microbiota markers. Furthermore, considering the heterogeneity among ethnic groups, which is reflected in the wide variation in microbiota, categorizing research studies according to their geographical location and subsequently comparing outcomes between regions could provide a valuable basis for a deeper understanding of gut microbiota profile in diverse human populations.
-
4:
Investigating the functional analysis of gut microbiota by exploring metabolomic and metagenomic approaches can provide insights into specific mechanisms underlying disease pathogenesis. Also, utilizing advanced sequencing techniques such as shotgun metagenomics allows species- and strain-level resolution, and other omics approaches, such as metratranscriptmics and metabolomics, allow the understanding of the mechanistic insight into host–microbiota interactions.
-
5:
Additionally, incorporating sigmoid mucosal biopsies and detailed characterization of microbial functions would enhance the understanding of host–microbiota interactions.
-
6:
Establishing standardized microbiota protocols from sample collection to data analysis to enhance the reliability and comparability of microbiota findings would lead to a better understanding of the dynamic relationship between the host and the gut microbiota [84].
5. Conclusions
In conclusion, this systematic review provided evidence indicating a link between gut microbiota dysbiosis and PD and AD. Additionally, it is important to consider the limitations of the included studies and to note that the findings have been inconsistent, suggesting that a single microbial community or factor may not fully explain the complexities of these diseases. Instead, it is likely that a network of microbial communities, along with multiple host and environmental factors, contribute to the development and progression of these disorders. Further research is needed to evaluate the host–gut microbiota interactions more effectively to provide novel personalized therapeutic interventions and preventive strategies targeting the gut–brain axis more effectively.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15204365/s1.
Author Contributions
F.S.H. designed the study; F.S.H. and K.N., data extraction, study screening; F.S.H. conducted the comparison of findings, data interpretation, and drafted the manuscript; K.N. and H.H. reviewed and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dorsey E., Sherer T., Okun M.S., Bloem B.R. The emerging evidence of the Parkinson pandemic. J. Park. Dis. 2018;8:S3–S8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monica Moore M., Díaz-Santos M., Vossel K. Alzheimer’s association 2021 facts and figures report. Alzheimer’s Assoc. 2021;17 [Google Scholar]
- 3.Nichols E., Steinmetz J.D., Vollset S.E., Fukutaki K., Chalek J., Abd-Allah F., Abdoli A., Abualhasan A., Abu-Gharbieh E., Akram T.T., et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105-25. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tysnes O.-B., Storstein A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 6.Ballatore C., Lee V.M.-Y., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 7.Cryan J.F., O’Mahony S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 8.Appleton J. The gut-brain axis: Influence of microbiota on mood and mental health. Integr. Med. A Clin. J. 2018;17:28. [PMC free article] [PubMed] [Google Scholar]
- 9.Abd Mutalib N., Syed Mohamad S.A., Jusril N.A., Hasbullah N.I., Mohd Amin M.C.I., Ismail N.H. Lactic Acid Bacteria (LAB) and Neuroprotection, What Is New? An Up-To-Date Systematic Review. Pharmaceuticals. 2023;16:712. doi: 10.3390/ph16050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghezzi L., Cantoni C., Rotondo E., Galimberti D. The Gut Microbiome–Brain Crosstalk in Neurodegenerative Diseases. Biomedicines. 2022;10:1486. doi: 10.3390/biomedicines10071486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 13.Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., Wilson K.E., Glover L.E., Kominsky D.J., Magnuson A. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 15.Unger M.M., Spiegel J., Dillmann K.-U., Grundmann D., Philippeit H., Bürmann J., Faßbender K., Schwiertz A., Schäfer K.-H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Gorecki A.M., Preskey L., Bakeberg M.C., Kenna J.E., Gildenhuys C., MacDougall G., Dunlop S.A., Mastaglia F.L., Akkari P.A., Koengten F. Altered gut microbiome in Parkinson’s disease and the influence of lipopolysaccharide in a human α-synuclein over-expressing mouse model. Front. Neurosci. 2019;13:839. doi: 10.3389/fnins.2019.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haran J.P., Bhattarai S.K., Foley S.E., Dutta P., Ward D.V., Bucci V., McCormick B.A. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio. 2019;10:e00632-19. doi: 10.1128/mBio.00632-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubeda C., Vázquez-Carretero M.D., Luque-Tirado A., Ríos-Reina R., Rubio-Sánchez R., Franco-Macías E., García-Miranda P., Calonge M.L., Peral M.J. Fecal Volatile Organic Compounds and Microbiota Associated with the Progression of Cognitive Impairment in Alzheimer’s Disease. Int. J. Mol. Sci. 2022;24:707. doi: 10.3390/ijms24010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedarf J.R., Hildebrand F., Coelho L.P., Sunagawa S., Bahram M., Goeser F., Bork P., Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9:1–13. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heintz-Buschart A., Pandey U., Wicke T., Sixel-Döring F., Janzen A., Sittig-Wiegand E., Trenkwalder C., Oertel W.H., Mollenhauer B., Wilmes P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018;33:88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F., Wang P., Chen Z., Sui X., Xie X., Zhang J. Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson’s disease. Neurosci. Lett. 2019;707:134297. doi: 10.1016/j.neulet.2019.134297. [DOI] [PubMed] [Google Scholar]
- 22.Cosma-Grigorov A., Meixner H., Mrochen A., Wirtz S., Winkler J., Marxreiter F. Changes in gastrointestinal microbiome composition in PD: A pivotal role of covariates. Front. Neurol. 2020;11:1041. doi: 10.3389/fneur.2020.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cilia R., Barichella M., Severgnini M., Cassani E., Bolliri C., Caronni S., Ferri V., Cancello R., Faierman S., Pinelli G. Proceedings of the Movement Disorders. WILEY; Hoboken, NJ, USA: 2018. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism; p. 1987. [DOI] [PubMed] [Google Scholar]
- 24.Hill-Burns E.M., Debelius J.W., Morton J.T., Wissemann W.T., Lewis M.R., Wallen Z.D., Peddada S.D., Factor S.A., Molho E., Zabetian C.P. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017;32:739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheperjans F., Aho V., Pereira P.A., Koskinen K., Paulin L., Pekkonen E., Haapaniemi E., Kaakkola S., Eerola-Rautio J., Pohja M. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 26.Cirstea M.S., Kliger D., MacLellan A.D., Yu A.C., Langlois J., Fan M., Boroomand S., Kharazyan F., Hsiung R.G., MacVicar B.A. The oral and fecal microbiota in a Canadian cohort of Alzheimer’s disease. J. Alzheimer’s Dis. 2022;87:247–258. doi: 10.3233/JAD-215520. [DOI] [PubMed] [Google Scholar]
- 27.Lin A., Zheng W., He Y., Tang W., Wei X., He R., Huang W., Su Y., Huang Y., Zhou H. Gut microbiota in patients with Parkinson’s disease in southern China. Park. Relat. Disord. 2018;53:82–88. doi: 10.1016/j.parkreldis.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Liu P., Wu L., Peng G., Han Y., Tang R., Ge J., Zhang L., Jia L., Yue S., Zhou K. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Kleine Bardenhorst S., Cereda E., Severgnini M., Barichella M., Pezzoli G., Keshavarzian A., Desideri A., Pietrucci D., Aho V.T., Scheperjans F. Gut microbiota dysbiosis in Parkinson disease: A systematic review and pooled analysis. Eur. J. Neurol. 2023;30:3581–3594. doi: 10.1111/ene.15671. [DOI] [PubMed] [Google Scholar]
- 30.Angoorani P., Ejtahed H.-S., Siadat S.D., Sharifi F., Larijani B. Is there any link between cognitive impairment and gut microbiota? A systematic review. Gerontology. 2022;68:1201–1213. doi: 10.1159/000522381. [DOI] [PubMed] [Google Scholar]
- 31.Hung C.-C., Chang C.-C., Huang C.-W., Nouchi R., Cheng C.-H. Gut microbiota in patients with Alzheimer’s disease spectrum: A systematic review and meta-analysis. Aging. 2022;14:477. doi: 10.18632/aging.203826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solfrizzi V., Custodero C., Lozupone M., Imbimbo B.P., Valiani V., Agosti P., Schilardi A., D’Introno A., La Montagna M., Calvani M. Relationships of dietary patterns, foods, and micro-and macronutrients with Alzheimer’s disease and late-life cognitive disorders: A systematic review. J. Alzheimer’s Dis. 2017;59:815–849. doi: 10.3233/JAD-170248. [DOI] [PubMed] [Google Scholar]
- 33.Trichopoulou A., Kyrozis A., Rossi M., Katsoulis M., Trichopoulos D., La Vecchia C., Lagiou P. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur. J. Nutr. 2015;54:1311–1321. doi: 10.1007/s00394-014-0811-z. [DOI] [PubMed] [Google Scholar]
- 34.Fiala M., Kooij G., Wagner K., Hammock B., Pellegrini M. Modulation of innate immunity of patients with Alzheimer’s disease by omega-3 fatty acids. FASEB J. 2017;31:3229. doi: 10.1096/fj.201700065R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris M.C., Tangney C.C., Wang Y., Sacks F.M., Barnes L.L., Bennett D.A., Aggarwal N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015;11:1015–1022. doi: 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 37.McGuinness L.A., Higgins J.P. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 38.Aho V.T., Pereira P.A., Voutilainen S., Paulin L., Pekkonen E., Auvinen P., Scheperjans F. Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707. doi: 10.1016/j.ebiom.2019.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldini F., Hertel J., Sandt E., Thinnes C.C., Neuberger-Castillo L., Pavelka L., Betsou F., Krüger R., Thiele I. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020;18:1–21. doi: 10.1186/s12915-020-00775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L., Xu X., Wu X., Cao H., Li X., Hou Z., Wang B., Liu J., Ji X., Zhang P. A comparison of the composition and functions of the oral and gut microbiotas in Alzheimer’s patients. Front. Cell. Infect. Microbiol. 2022;12:942460. doi: 10.3389/fcimb.2022.942460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopfner F., Künstner A., Müller S.H., Künzel S., Zeuner K.E., Margraf N.G., Deuschl G., Baines J.F., Kuhlenbäumer G. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017;1667:41–45. doi: 10.1016/j.brainres.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Sheng C., Lin L., Lin H., Wang X., Han Y., Liu S.-L. Altered gut microbiota in adults with subjective cognitive decline: The SILCODE study. J. Alzheimer’s Dis. 2021;82:513–526. doi: 10.3233/JAD-210259. [DOI] [PubMed] [Google Scholar]
- 43.Weis S., Schwiertz A., Unger M.M., Becker A., Faßbender K., Ratering S., Kohl M., Schnell S., Schäfer K.-H., Egert M. Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. NPJ Park. Dis. 2019;5:28. doi: 10.1038/s41531-019-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cirstea M.S., Yu A.C., Golz E., Sundvick K., Kliger D., Radisavljevic N., Foulger L.H., Mackenzie M., Huan T., Finlay B.B. Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov. Disord. 2020;35:1208–1217. doi: 10.1002/mds.28052. [DOI] [PubMed] [Google Scholar]
- 45.Guo M., Peng J., Huang X., Xiao L., Huang F., Zuo Z. Gut microbiome features of Chinese patients newly diagnosed with Alzheimer’s disease or mild cognitive impairment. J. Alzheimer’s Dis. 2021;80:299–310. doi: 10.3233/JAD-201040. [DOI] [PubMed] [Google Scholar]
- 46.Kaiyrlykyzy A., Kozhakhmetov S., Babenko D., Zholdasbekova G., Alzhanova D., Olzhayev F., Baibulatova A., Kushugulova A.R., Askarova S. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 2022;12:15115. doi: 10.1038/s41598-022-19393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C., Cui L., Yang Y., Miao J., Zhao X., Zhang J., Cui G., Zhang Y. Gut microbiota differs between Parkinson’s disease patients and healthy controls in Northeast China. Front. Mol. Neurosci. 2019;12:171. doi: 10.3389/fnmol.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W., Wu X., Hu X., Wang T., Liang S., Duan Y., Jin F., Qin B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 2017;60:1223–1233. doi: 10.1007/s11427-016-9001-4. [DOI] [PubMed] [Google Scholar]
- 49.Lin C.-H., Chen C.-C., Chiang H.-L., Liou J.-M., Chang C.-M., Lu T.-P., Chuang E.Y., Tai Y.-C., Cheng C., Lin H.-Y. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019;16:1–9. doi: 10.1186/s12974-019-1528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrov V., Saltykova I., Zhukova I., Alifirova V., Zhukova N., Dorofeeva Y.B., Tyakht A., Kovarsky B., Alekseev D., Kostryukova E. Analysis of gut microbiota in patients with Parkinson’s disease. Bull. Exp. Biol. Med. 2017;162:734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 51.Pietrucci D., Cerroni R., Unida V., Farcomeni A., Pierantozzi M., Mercuri N.B., Biocca S., Stefani A., Desideri A. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Park. Relat. Disord. 2019;65:124–130. doi: 10.1016/j.parkreldis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Qian Y., Yang X., Xu S., Huang P., Li B., Du J., He Y., Su B., Xu L.-M., Wang L. Gut metagenomics-derived genes as potential biomarkers of Parkinson’s disease. Brain. 2020;143:2474–2489. doi: 10.1093/brain/awaa201. [DOI] [PubMed] [Google Scholar]
- 53.Qian Y., Yang X., Xu S., Wu C., Song Y., Qin N., Chen S.-D., Xiao Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018;70:194–202. doi: 10.1016/j.bbi.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Stadlbauer V., Engertsberger L., Komarova I., Feldbacher N., Leber B., Pichler G., Fink N., Scarpatetti M., Schippinger W., Schmidt R. Dysbiosis, gut barrier dysfunction and inflammation in dementia: A pilot study. BMC Geriatr. 2020;20:1–13. doi: 10.1186/s12877-020-01644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan A.H., Chong C.W., Lim S.Y., Yap I.K.S., Teh C.S.J., Loke M.F., Song S.L., Tan J.Y., Ang B.H., Tan Y.Q. Gut microbial ecosystem in Parkinson disease: New clinicobiological insights from multi-omics. Ann. Neurol. 2021;89:546–559. doi: 10.1002/ana.25982. [DOI] [PubMed] [Google Scholar]
- 56.Vascellari S., Palmas V., Melis M., Pisanu S., Cusano R., Uva P., Perra D., Madau V., Sarchioto M., Oppo V. Gut microbiota and metabolome alterations associated with Parkinson’s disease. Msystems. 2020;5:e00561-20. doi: 10.1128/mSystems.00561-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., Carlsson C.M., Asthana S., Zetterberg H., Blennow K. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wanapaisan P., Chuansangeam M., Nopnipa S., Mathuranyanon R., Nonthabenjawan N., Ngamsombat C., Thientunyakit T., Muangpaisan W. Association between Gut Microbiota with Mild Cognitive Impairment and Alzheimer’s Disease in a Thai Population. Neurodegener. Dis. 2023;22:43–54. doi: 10.1159/000526947. [DOI] [PubMed] [Google Scholar]
- 59.Xi J., Ding D., Zhu H., Wang R., Su F., Wu W., Xiao Z., Liang X., Zhao Q., Hong Z. Disturbed microbial ecology in Alzheimer’s disease: Evidence from the gut microbiota and fecal metabolome. BMC Microbiol. 2021;21:1–13. doi: 10.1186/s12866-021-02286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan Z., Yang F., Cao J., Ding W., Yan S., Shi W., Wen S., Yao L. Alterations of gut microbiota and metabolome with Parkinson’s disease. Microb. Pathog. 2021;160:105187. doi: 10.1016/j.micpath.2021.105187. [DOI] [PubMed] [Google Scholar]
- 61.Yıldırım S., Nalbantoğlu Ö.U., Bayraktar A., Ercan F.B., Gündoğdu A., Velioğlu H.A., Göl M.F., Soylu A.E., Koç F., Gülpınar E.A. Stratification of the gut microbiota composition landscape across the alzheimer’s disease continuum in a Turkish cohort. Msystems. 2022;7:e00004–e00022. doi: 10.1128/msystems.00004-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F., Yue L., Fang X., Wang G., Li C., Sun X., Jia X., Yang J., Song J., Zhang Y. Altered gut microbiota in Parkinson’s disease patients/healthy spouses and its association with clinical features. Park. Relat. Disord. 2020;81:84–88. doi: 10.1016/j.parkreldis.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Y., Wang Y., Quan M., Zhao H., Jia J. Gut microbiota changes and their correlation with cognitive and neuropsychiatric symptoms in Alzheimer’s disease. J. Alzheimer’s Dis. 2021;81:583–595. doi: 10.3233/JAD-201497. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Z., Ma X., Wu J., Xiao Z., Wu W., Ding S., Zheng L., Liang X., Luo J., Ding D. Altered Gut Microbiota and Its Clinical Relevance in Mild Cognitive Impairment and Alzheimer’s Disease: Shanghai Aging Study and Shanghai Memory Study. Nutrients. 2022;14:3959. doi: 10.3390/nu14193959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhuang Z.-Q., Shen L.-L., Li W.-W., Fu X., Zeng F., Gui L., Lü Y., Cai M., Zhu C., Tan Y.-L. Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2018;63:1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 66.Ren T., Gao Y., Qiu Y., Jiang S., Zhang Q., Zhang J., Wang L., Zhang Y., Wang L., Nie K. Gut microbiota altered in mild cognitive impairment compared with normal cognition in sporadic Parkinson’s disease. Front. Neurol. 2020;11:137. doi: 10.3389/fneur.2020.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borsom E.M., Conn K., Keefe C.R., Herman C., Orsini G.M., Hirsch A.H., Palma Avila M., Testo G., Jaramillo S.A., Bolyen E. Predicting neurodegenerative disease using Prepathology gut microbiota composition: A longitudinal study in mice modeling Alzheimer’s disease pathologies. Microbiol. Spectr. 2023;11:e03458-22. doi: 10.1128/spectrum.03458-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ling Z., Zhu M., Yan X., Cheng Y., Shao L., Liu X., Jiang R., Wu S. Structural and functional dysbiosis of fecal microbiota in Chinese patients with Alzheimer’s disease. Front. Cell Dev. Biol. 2021;8:634069. doi: 10.3389/fcell.2020.634069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lei W., Cheng Y., Gao J., Liu X., Shao L., Kong Q., Zheng N., Ling Z., Hu W. Akkermansia muciniphila in neuropsychiatric disorders: Friend or foe? Front. Cell. Infect. Microbiol. 2023;13:1224155. doi: 10.3389/fcimb.2023.1224155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 74.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 75.Minter M.R., Zhang C., Leone V., Ringus D.L., Zhang X., Oyler-Castrillo P., Musch M.W., Liao F., Ward J.F., Holtzman D.M. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laudani S., Torrisi S.A., Alboni S., Bastiaanssen T.F., Benatti C., Rivi V., Moloney R.D., Fuochi V., Furneri P.M., Drago F. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain Behav. Immun. 2023;107:385–396. doi: 10.1016/j.bbi.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Hamamah S., Aghazarian A., Nazaryan A., Hajnal A., Covasa M. Role of microbiota-gut-brain axis in regulating dopaminergic signaling. Biomedicines. 2022;10:436. doi: 10.3390/biomedicines10020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ojha S., Patil N., Jain M., Kole C., Kaushik P. Probiotics for Neurodegenerative Diseases: A Systemic Review. Microorganisms. 2023;11:1083. doi: 10.3390/microorganisms11041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bisaglia M. Mediterranean Diet and Parkinson’s Disease. Int. J. Mol. Sci. 2022;24:42. doi: 10.3390/ijms24010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baert F., Matthys C., Maselyne J., Van Poucke C., Van Coillie E., Bergmans B., Vlaemynck G. Parkinson’s disease patients’ short chain fatty acids production capacity after in vitro fecal fiber fermentation. NPJ Park. Dis. 2021;7:72. doi: 10.1038/s41531-021-00215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vervier K., Moss S., Kumar N., Adoum A., Barne M., Browne H., Kaser A., Kiely C.J., Neville B.A., Powell N. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut. 2022;71:1821–1830. doi: 10.1136/gutjnl-2021-325177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Minerbi A., Gonzalez E., Brereton N.J., Anjarkouchian A., Dewar K., Fitzcharles M.-A., Chevalier S., Shir Y. Altered microbiome composition in individuals with fibromyalgia. Pain. 2019;160:2589–2602. doi: 10.1097/j.pain.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 83.Shade A. Diversity is the question, not the answer. ISME J. 2017;11:1–6. doi: 10.1038/ismej.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirzayi C., Renson A., Genomic Standards Consortium. Massive Analysis and Quality Control Society. Cesare F., Susanna-Assunta S., Zohra F., Elsafoury S., Zohra F., Elsafoury S., et al. Reporting guidelines for human microbiome research: The STORMS checklist. Nat. Med. 2021;27:1885–1892. doi: 10.1038/s41591-021-01552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and Supplementary Materials.