Abstract
One major problem with the overuse of antibiotics is that the microorganisms acquire resistance; thus the dose must be increased unsustainably. To overcome this problem, researchers from around the world are actively investigating new types of antimicrobials. Zinc oxide (ZnO) nanoparticles (NPs) have been proven to exhibit strong antimicrobial effects; moreover, the Food and Drugs Administration (FDA) considers ZnO as GRAS (generally recognized as safe). Many essential oils have antimicrobial activity and their components do not generate resistance over time. One of the drawbacks is the high volatility of some components, which diminishes the antimicrobial action as they are eliminated. The combination of ZnO NPs and essential oils can synergistically produce a stronger antimicrobial effect, and some of the volatile compounds can be retained on the nanoparticles’ surface, ensuring a better-lasting antimicrobial effect. The samples were characterized with X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), ultraviolet-visible spectroscopy (UV-Vis), and thermal analysis (TG-DSC) coupled with analysis of evolved gases using FTIR. The ZnO NPs, with a size of ~35 nm, exhibited a loading between 1.44% and 15.62%—the lower values were specific for limonene-containing oils (e.g., orange, grapefruit, bergamot, or limette), while high values were obtained from cinnamon, minzol, thyme, citronella, and lavender oils—highlighting differences among non-polar terpenes and alcohol or aldehyde derivatives. The antibacterial assay indicated the existence of a synergic action among components and a high dependency on the percentage of loaded oil. Loaded nanoparticles offer immense potential for the development of materials with specific applications, such as wound dressings or food packaging. These nanoparticles can be utilized in scenarios where burst delivery is desired or when prolonged antibacterial activity is sought.
Keywords: ZnO, citronella, orange, thyme, lavender, grapefruit, bergamot, cinnamon, rosemary, minzol, limette, antimicrobial
1. Introduction
One major problem with the overuse of antibiotics is that the microorganisms are acquiring resistance; thus the dose must be increased unsustainably [1]. To overcome this problem, new types of antimicrobials are being researched worldwide, from innovative synthetic organic compounds [2] to metallic and oxide nanoparticles [3], natural antimicrobials like plant extracts or essential oils [4], and synthetic peptides that can be designed to have antibacterial properties similar to antibiotics [5].
Zinc oxide (ZnO) nanoparticles (NPs) have been proven to exhibit strong antimicrobial effects, but at the same time U.S. Food and Drugs Administration (FDA) considers ZnO as GRAS (generally recognized as safe) [6]. The scientific literature is rich with numerous reports highlighting the antibacterial activity of ZnO nanoparticles against model pathogens, including both Gram-negative and Gram-positive strains. These nanoparticles have garnered significant interest due to their remarkable ability to inhibit the growth and proliferation of bacteria [7,8,9]. The antifungal activity is researched and reported much less, and usually in food-related applications. At the same time, the debate about the exact mechanism involved in the antimicrobial activity is still ongoing. The ZnO with its strong photocatalytic activity is expected to generate considerable levels of reactive oxygen species (ROS) under light irradiation [10], which are the main toxic species for microorganisms. However, ZnO NPs also exhibit antimicrobial activity under dark conditions, which supports alternative killing pathways. The most-often-mentioned ones are the mechanical damage to the cellular membrane and the internalization of NPs followed by the release of Zn2+ ions. All these factors promote ZnO NPs as an alternative microbicide agent in various domains, like food packaging, topical ointments, water treatment, and others [11,12,13].
Natural plant extracts and essential oils are also a major research topic, due to their multiple health benefits, which include antioxidant, antimutagenic, antiproliferative, or antimicrobial activities [14,15]; some of them—like citronella (lemongrass) essential oil—are classified as non-toxic biopesticides in the USA [16] while having a strong antifungal activity [17]. The antimicrobial activity of each essential oil derives from one or two main components. Additionally, minor components can also exhibit useful activities, and by synergism, the essential oil is usually more potent than the main components. The orange essential oil is composed mainly of D-limonene which is listed as GRAS by the FDA, but it is also considered a biopesticide and bug-repellant [18,19]. The antiseptic properties of thyme essential oil are generated by the main component, thymol [20]. The thyme essential oil was used as an antiparasitic or to treat minor wounds from ancient times due to its strong antimicrobial properties [21,22]. Lavender [23,24], grapefruit [25,26], bergamot [27,28], cinnamon [29,30], and rosemary [31,32] essential oils have strong antimicrobial activity and therefore are good candidates for innovative antibacterial and antifungal therapies and materials. Nevertheless, the essential oils must be loaded or encapsulated in a matrix, be it organic or inorganic, to ensure a longer release profile, and a sustained antimicrobial activity [33,34].
The nanoparticles can be loaded with various antimicrobials by multiple techniques—some being solvent-free and others involving a solvent. The solvent-free methods can be physical mixing, co-milling, melt method, or microwave irradiation. Moreover, solvent-mediated loading can be carried out using adsorption, incipient wetness impregnation, solvent evaporation, supercritical and liquid CO2 technology, one-pot drug loading and synthesis, chaperone assistance, etc. [35,36,37,38,39,40].
By combining the ZnO NPs with essential oils, the synergic activities result in either a stronger antimicrobial or a decrease in the amounts needed to combat microorganisms. Such loaded nanoparticles can be further used in topical ointments or other antimicrobial applications like food packaging [9]. By using essential oils loaded on ZnO NPs, some physical characteristics can also be improved, including retaining the volatile components into the mixture for a longer time, and slow release of compounds (due to interactions with nanoparticles surface) that can improve the availability of the essential oil components over time, providing a long-lasting antimicrobial activity.
In this study, we obtained polyhedral shape ZnO NPs with a diameter of ~35 nm, which were loaded with a series of the following essential oils: citronella, orange, thyme, lavender, grapefruit, bergamot, cinnamon, rosemary, minzol, and limette. Since these essential oils are provided as liquids, we chose a solvent-mediated loading mixed procedure, namely impregnation, followed by excess solvent evaporation. The ZnO NPs loaded with essential oils were characterized with thermogravimetry-differential scanning calorimetry (TG-DSC), Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), scanning electron microscopy (SEM), powder X-ray diffraction (XRD), ultraviolet-visible spectroscopy (UV-Vis) and fluorescence spectroscopy (PL).
The essential oil-loaded ZnO NPs were tested against a series of model pathogen bacterial strains—Gram-negative Salmonella typhimurium (ATCC 14028) and Escherichia coli (ATCC 25922), and Gram-positive Staphylococcus aureus (ATCC 25923) and Listeria monocytogenes (ATCC 19114)—which are one of the common causes of food poisoning [41,42,43,44,45]. The presence of both ZnO NPs and essential oils leads to an increase in antibacterial activity, indicating a synergic action.
2. Materials and Methods
Zinc acetate dihydrate (Zn(CH3COO)2·2H2O) with 99.9% purity was purchased from Merck (Merck Group, Darmstadt, Germany). The 1-butanol was obtained from Sigma (Redox Lab Supplies Com SRL, Bucharest, Romania). The nutrient growth medium used was TSA (Tryptone Soya Agar) type, produced by Oxoid Ltd. (Cheshire, UK).
Essential oils of citronella, orange, thyme, lavender, grapefruit, bergamot, cinnamon, rosemary, minzol, and limette were obtained from Carl-Roth (Karlsruhe, Germany), with major components being listed in Section 3.3.2.
All substances were used without further purification.
ZnO synthesis was performed as presented in [10]. Briefly, 5 g of zinc acetate dihydrate was poured in 50 mL of 1-butanol. The solution was refluxed for 24 h under continuous stirring. The flask was allowed to rest for 24 h at 25 °C. The precipitate was separated by decantation and subjected to repeated centrifugation. Between centrifugation, the powder was washed with absolute ethanol—the process being repeated thrice. Finally, the obtained white powder was dried in an electrical oven at 80 °C. An amount of 0.1 g of the obtained ZnO nanopowder was loaded with each essential oil (30 µL essential oil mixed with 100 µL ethanol). The mixture was ultrasonicated for 10 min and then evaporated at 40 °C in an electrical oven. The ZnO NPs loaded with essential oils were further used for microbiological assay. The sample labels are presented in Table 1.
Table 1.
Sample code label for each essential oil.
| Sample Label | Essential Oil Used |
|---|---|
| ZnO | - |
| ZnO_Cit | Citronella |
| ZnO_Ora | Orange |
| ZnO_Thy | Thyme |
| ZnO_Lav | Lavender |
| ZnO_Grap | Grapefruit |
| ZnO_Berg | Bergamot |
| ZnO_Cin | Cinnamon |
| ZnO_Ros | Rosemary |
| ZnO_Mint | Minzol |
| ZnO_Lime | Limette |
Information about the crystallinity of the obtained ZnO nanopowder was obtained with a PANalytical Empyrean equipment (from Malvern PANalytical, Bruno, The Netherlands) using the equation λCuKα = 1.54184 Å. The X-ray diffractograms (XRDs) were recorded in the 2θ range 10–70°, with a step of 0.02° and a time per step of 0.1 s.
A Tecnai G2F30 S-TWIN high-resolution transmission electron microscope (TEM) from FEI (FEI Company, Eindhoven, The Netherlands) was used to record the micrographs of the ZnO NPs.
The surface morphology and microstructure of ZnO nanopowder were investigated with a scanning electron microscope, QUANTA INSPECT F50 (FEI Company, Eindhoven, The Netherlands).
Ultraviolet-visible (UV-Vis) reflectance spectrum was recorded with a V560 spectrophotometer from JASCO (Easton, PA, USA), using a 60 mm integrating sphere (ISV-469), between 200 and 900 nm.
An LS55 fluorimeter from Perkin Elmer (Perkin Elmer, Waltham, MA, USA), was used to record the fluorescence spectrum. The 320 nm radiation from the Xe lamp was used as an excitation wavelength. The emission spectrum was recorded between 350 and 700 nm, employing a 350 nm cut-off filter, with a scan speed of 200 nm min−1.
Thermogravimetry–differential scanning calorimetry was performed with a STA 449C F3 equipment, from Netzsch (Selb, Germany) coupled with a Tensor 27 FTIR from Bruker (Bruker Optik GmbH, Ettlingen, Germany), equipped with an internal thermostatic gas cell. A typical sample, weighing ~10 mg, was placed in an alumina crucible and was heated up at a rate of 10 °C∙min−1, up to 900 °C, in air.
A Nicolet iS50R spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) device was used to record the Fourier transform infrared (FTIR) spectra, in attenuated total reflection (ATR). For every spectrum, an average of 32 scans was performed, from 400 to 4000 cm−1, with a resolution of 4 cm−1.
To study in-vitro antibacterial activity, pure cultures of four reference bacterial strains obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) were used. These pathogenic bacteria (Table 2) naturally contaminate food products obtained under improper manufacturing conditions, producing food poisoning and infections. The nutrient growth medium used was TSA (Tripton Soy Agar), produced by Oxoid (Cheshire, UK).
Table 2.
Bacteria strains used in this study.
| No | Bacteria Strains | Gram-Positive | Gram-Negative |
|---|---|---|---|
| 1 | Listeria monocytogenes, ATCC 19114 | Yes | |
| 2 | Staphylococcus aureus, ATCC 25923 | Yes | |
| 3 | Salmonella typhimurium, ATCC 14028 | Yes | |
| 4 | Escherichia coli, ATCC 25922 | Yes |
Quantification of the working inoculum was carried out using the Plate Count technique, which involved the enumeration of the cells that determine the formation of colonies by cultivation on the nutrient culture medium. The qualitative screening of the sensitivity of different pathogenic bacteria to the samples of interest was performed with disk diffusion techniques on agar, adapted according to the standard issued by the Clinical and Laboratory Standards Institute (Wayne, PA, USA). In order to determine the antimicrobial activity of ZnO NPs, an adapted diffusion assay that follows the CLSI 2020 guidelines was employed. All antimicrobial experiments were performed in triplicates.
3. Results and Discussion
The obtained white ZnO nanopowder was investigated to determine the purity, composition, and morphology of the nanoparticles.
3.1. X-ray Diffraction Analysis (XRD)
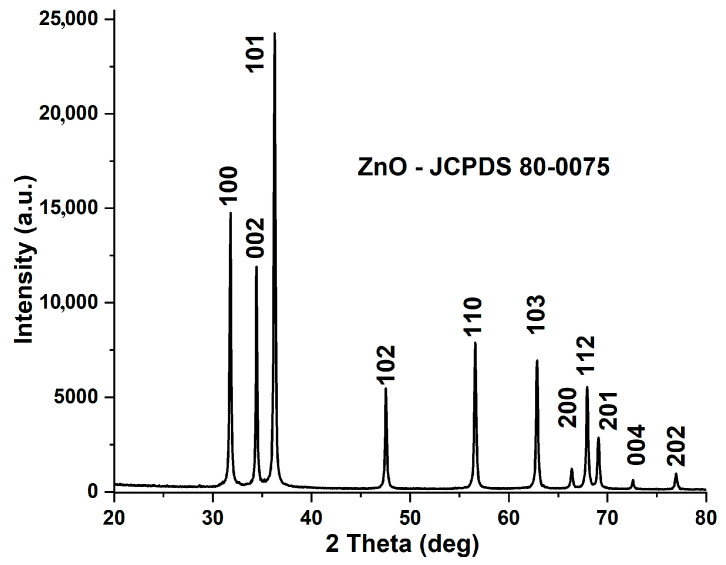
The XRD analysis has revealed that the crystalline phase composed of only ZnO, Figure 1.
Figure 1.
XRD pattern for the obtained ZnO nanopowder (JCPDS 80-0075).
The XRD peaks were found at 2θ values (with Miller indices inside brackets): 31.78° (100), 34.43° (002), 36.25° (101), 47.56° (102), 56.61° (110), 62.85° (103), 66.39° (200), 67.95° (112), 69.09° (201), 72.60° (004), and 76.97° (202) [46]. The pattern was indexed as single-phase hexagonal wurtzite (ZnO) corresponding to JCPDS card no. 80-0075. Average crystallite diameter (D), microstrain (ε), and lattice parameters were calculated using Rietveld refinement, and the values are presented in Table 3.
Table 3.
Lattice parameters for obtained ZnO nanopowder.
| Unit Cell | a = b [Å] | c [Å] | V [Å3] | c/a | Microstrain (%) | Average Crystallite Size (nm) | Dislocation Density (δ) ×10−4 |
|---|---|---|---|---|---|---|---|
| ZnO | 3.25081 | 5.20661 | 47.65065 | 1.60163 | 0.26 ± 0.10 | 34.45 ± 4.93 | 8.43 |
The number of surface defects of the nanoparticles can be calculated as the dislocation density (δ) and is given by the length of dislocation lines in a volume unit. The δ value is calculated as 1/D2 (where D is the average crystallite diameter) [47]. The active centers, in the photocatalytic and antimicrobial activities, are represented by the defects on the nanoparticle surface. Such defects act as reactive oxygen species (ROS) generators; therefore, a sample with a high δ value can exhibit strong antimicrobial activity.
3.2. Scanning and Transmission Electron Microscopy
3.2.1. Scanning Electron Microscopy (SEM)
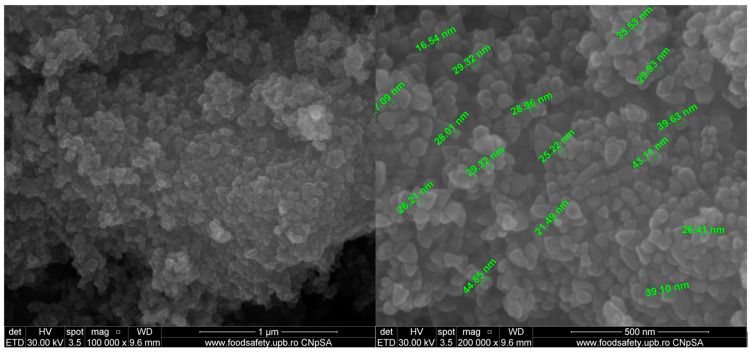
Morphology of the ZnO NPs was investigated with scanning electron microscopy (SEM). The SEM micrographs (Figure 2) indicate a uniform morphology, mostly polyhedral or triangular, with particle size averaging around ~30 nm, and a tendency to form soft agglomerates. The literature indicates that in the case of ZnO, smaller particles exhibit a higher antimicrobial activity [48]. Additionally, some microorganisms are more sensitive to the shape of nanoparticles, which can cause mechanical damage to the membrane and can lead to easier internalization [10].
Figure 2.
The SEM micrographs for the obtained ZnO nanopowder.
3.2.2. Transmission Electron Microscopy (TEM)
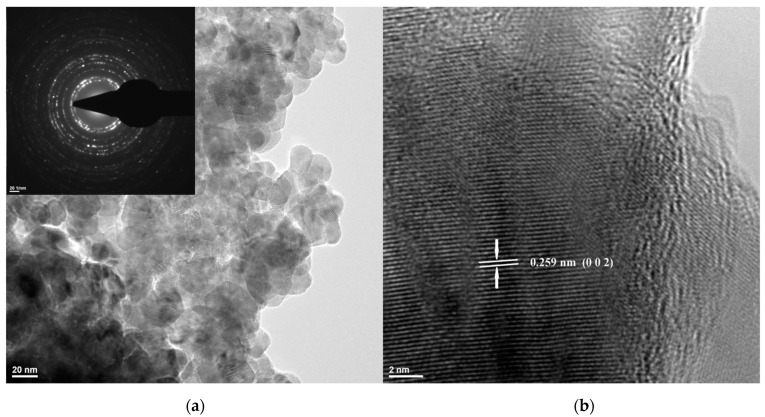
For a better image of ZnO NP size, shape, and morphology, TEM bright-field images were obtained (Figure 3), while the crystallinity of the sample was assessed with selected area electron diffraction (SAED). Images confirm that most ZnO NPs were polyhedral-shaped, with a diameter in the interval of 20–40 nm.
Figure 3.
The TEM, SAED (inset) (a) and HRTEM (b) micrographs for the obtained ZnO nanopowder.
The SAED pattern obtained on the ZnO sample confirms that the only identified crystalline phase was the wurtzite form of ZnO. The high-resolution transmission electron microscopy (HRTEM) image (Figure 3) allows clear identification of atomic planes corresponding to the (0 0 2) Miller indices of wurtzite. Moreover, the regular succession of the lattice fringes, at distances of d = 2.59 Å, indicates the uniform crystalline structure, without the presence of an amorphous phase.
3.3. Spectroscopic Studies
3.3.1. UV-Vis and Fluorescence (PL) Spectroscopy
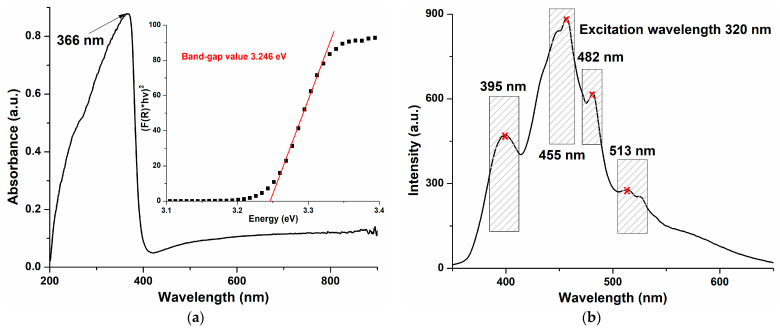
The UV-Vis spectrum for the ZnO nanopowders is presented in Figure 4a. The large and intense absorption band centered at 366 nm is typical for ZnO and similar to other literature reports [49]. Protected textiles or sunscreen cosmetics contain ZnO due to this strong absorption band in the UV domain [11].
Figure 4.
The UV-Vis spectrum and Tauc plot (inset) (a), and fluorescence spectrum (b) for the obtained ZnO nanopowder.
ZnO is a direct band-gap semiconductor, with a wide band-gap of ~3.37 eV between the conduction and the valence bands. Therefore, a relatively large absorption band located in the ultraviolet domain is typical for the UV-Vis spectrum. The electron jump from the valence band to the conduction band generates this absorption peak, and its numerical value can be used to calculate the band-gap value of ZnO [50]. Some literature reports wrongly assign this band to a surface plasmon resonance, which does not exist in semiconductors like ZnO [51].
The band-gap value was determined using a Tauc plot (inset of Figure 4a) by applying the Kubelka–Munk function F(R) = (1 − R)2/2R—where R is the sample diffuse reflectance—and graphical extrapolation to [F(R)∙hν]2 = 0 [52]. The obtained value, 3.246 eV, was smaller than the theoretical value, indicating that the presence of additional electronic levels inside the band gap was most probably induced by the existence of various crystal defects [53]. Such defects were also responsible for the visible emission bands from the ZnO fluorescence spectrum (Figure 4b), called deep-level emission (DLE). The electronic levels induced inside the band-gap—by the defects like zinc vacancies (VZn), oxygen anti-sites (OZn), oxygen vacancies (VO), zinc interstitials (Zni), or oxygen interstitials (Oi)—generated the emission bands from 455, 482 and 513 nm, as previously reported in the literature [52,54,55].
On the contrary, the UV emission band, located near-band-edge (NBE) at 395 nm, was assigned to the recombination of free excitons. The recombination of excited electrons and holes could be blocked by the presence of defects, that could trap the free electrons. Therefore, a high density of surface defects can cause a decreased intensity of the NBE emission [56]. The production of ROS was increased by the surface defects, especially by the oxygen vacancies, and therefore their density was correlated with a higher antimicrobial activity under light irradiation [57].
3.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
The FTIR spectrum displayed two characteristic zones for the ZnO NPs. The very strong absorption band around ~420–450 cm−1 was assigned to the stretching vibrations of the Zn–O bond [8,58], while the weak peaks from ~610/680 cm−1 were assigned to Zn–OH bending vibrations [58,59] (Figure S1a). As previously reported in the literature, the band in the domain 400–500 cm−1 becomes very strong for the single phases of wurtzite, surpassing all other vibrations. Furthermore, the peaks corresponding to the bending of Zn–OH bonds remain at low intensity in the interval of 600–700 cm−1 [58]. Additionally, the peaks between 800 and 1100 cm−1 could be assigned to the ν1 and ν2 stretches of carbonate [60], indicating the existence of minute impurities on the surface of nanoparticles generated by the acetate precursor [55]. Finally, the peak at 873 cm−1 could also be assigned to the Zn2+ presence in tetrahedral coordination [61,62].
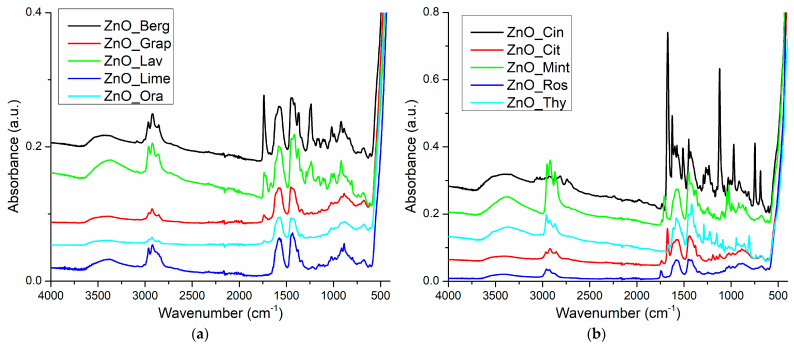
The loading process of ZnO NPs with essential oils was monitored with the help of FTIR spectroscopy (Figure S1b). Specific peaks for the essential oils components were identified in Table 3.
Citronellal (32.2%), geraniol (19.6%), geranylacetate (15.1%), citronellol (6.4%), and citronellylacetate (5.5%) were predominant in citronella essential oil, while the main ingredients of thyme essential oil were thymol (47.9%), γ-terpinene (31.3%) and p-cymene (8.5%). D-limonene was dominant in orange (94.9%), grapefruit (93.2%), and limette (42.5%) essential oils, with linalool (3%), β-myrcene (2.5%), or γ-terpinene (15.4%) and α/β-pinene (15.7%) as minor components in these oils. Limonene (59%) was also the major component of bergamot essential oil, along linalyl acetate (17.1%), linalool (9.5%), and β-pinene (5%).
Lavender essential oil contained an important amount of linalool (44.2%), linalyl acetate (30%), and terpinen-4-ol (11%). Whilst, the main component of cinnamon essential oil was (E)-cinnamaldehyde (72%) mixed with smaller quantities of linalool (7%), β-caryophyllene (6.5%), eucalyptol (5.5%), and eugenol (4.5%). A more equilibrated composition was found in rosemary essential oil—containing borneol (23.7%), α-pinene (9.9%), α-caryophyllene (8.1%), ledol (6.5%), eucalyptol (4.9%), camphor (4.9%), and γ-terpinene (4.5%)—and minzol essential oil that comprised menthol (45.4%), menthone (16.1%), menthofuran (8.9%), cis-carane (8.7%), 1,8-cineole (4.5%), and neo-menthol (4.2%).
The FTIR spectra showed mostly the typical signals of the terpene and associated functional groups.
The peaks from 3340 to 3430 cm−1 were assigned to the –OH stretching vibration from the alcohol, phenol, and carboxylic moieties. The peaks around 3000 cm−1 were from C–H stretching vibrations. Those from 3000 to 3030 cm−1 indicated the presence of unsaturated carbons Csp2–H like in thymol or γ-terpinene. Peaks in the region of 2800–3000 cm−1 were assigned to the methyl and methylene C–H symmetric and asymmetric vibrations and originated from all organic compounds (Figure 5a,b).
Figure 5.
The FTIR spectra for the ZnO NPs loaded with essential oils (a) and (b).
A distinct peak also was observed at ~1706/1743 cm−1, which could be attributed to the acetate or aldo (C=O) stretching vibration. These groups were present in geranylacetate/ citronellylacetate and citronellal, active ingredients of the citronella oil.
The peaks from 1620 to 1680 cm−1 were originating from C=C bonds, isolated or conjugated like in linalool or cinnamaldehyde from cinnamon essential oil. The signal of C=C–C stretching was responsible for the maximum from 1570 to 1585 cm−1 [63,64] (Figure S1c,d, Table 4).
Table 4.
Principal FTIR peaks of ZnO-essential oil samples and their assignment.
| Sample/ Wavenumber (cm−1) |
ZnO | ZnO_ Cit |
ZnO_ Ora |
ZnO_ Thy |
ZnO_ Lav |
ZnO_ Grap |
ZnO_ Berg |
ZnO_ Cin |
ZnO_ Ros |
ZnO_ Mint |
ZnO_ Lime |
|---|---|---|---|---|---|---|---|---|---|---|---|
| –OH | 3401 | 3340 | 3414 | 3374 | 3392 | 3423 | 3406 | 3379 | 3423 | 3379 | 3388 |
| –Csp2–H | - | - | - | 3020 | 3006 | 3010 | 3011 | 3027 | - | - | 3009 |
| –Csp3–H CH3 asym CH2 asym CH3 sym CH2 sym |
- - - - - |
2965 2920 2854 2833 |
2959 2926 2868 2854 |
2958 2923 2868 -tail |
2962 2925 2868 2855 |
2961 2927 2871 2855 |
2965 2921 2872 2856 |
2975 2930 2842 2813 |
2959 2927 2873 2854 |
2952 2918 2868 2847 |
2962 2923 2855 2832 |
| C=O | - | 1735 | 1740 | 1706 | 1735 | 1735 | 1735 | 1733 | 1743 | 1706/38 | - |
| C=C–C stretching | - | 1674 | 1620 | 1620 | 1622/74 | 1620 | 1622 | 1622/73 | 1620 | 1620 | 1620 |
| - | 1572 | 1584 | 1584 | 1575 | 1572 | 1572 | 1572 | 1584 | 1584 | 1584 | |
| C–O–str C–OHdef |
- | 1121 | - | - | 1117 | 1121 | 1115 | 1121 | 1045 | 1045 | 1045 |
| 1020 | 1024 | 1024 | 1020 | 1020 | 1020 | 1025 | 1024 | 1024 | 1024 | ||
| −bending | 615/673 | 612/677 | 614/680 | 612/677 | 613/682 | 613/680 | 612/678 | 606/688 | 613/677 | 612/678 | 614/678 |
| Zn–Ostr | 458 | 456 | 420 | 418 | 424 | 420 | 422 | 455 | 456 | 455 | 417 |
C–H symmetrical and asymmetrical bending was the cause for the strong signal in the range of 1417–1450 cm−1.
Finally, the –C–O– stretching and –C–OH deformation vibration of alcohols generated the signals from 1021–1121 cm−1, including menthol in minzol essential oil or linalool and eugenol from cinnamon essential oil [65].
Due to the loading process, interactions appeared between essential oil components and the surface of nanoparticles, on which Zn2+ represented positive centers with electron deficit and O2− as high electron density centers. Therefore, small modifications in the position of Zn–O and Zn–OH bands were noticeable in FTIR spectra of the essential-oils-loaded samples when compared with simple ZnO NPs.
3.4. Thermal Analysis (TG-DSC)
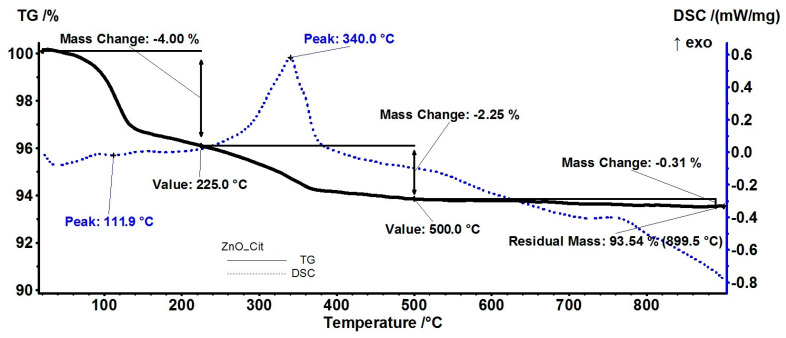
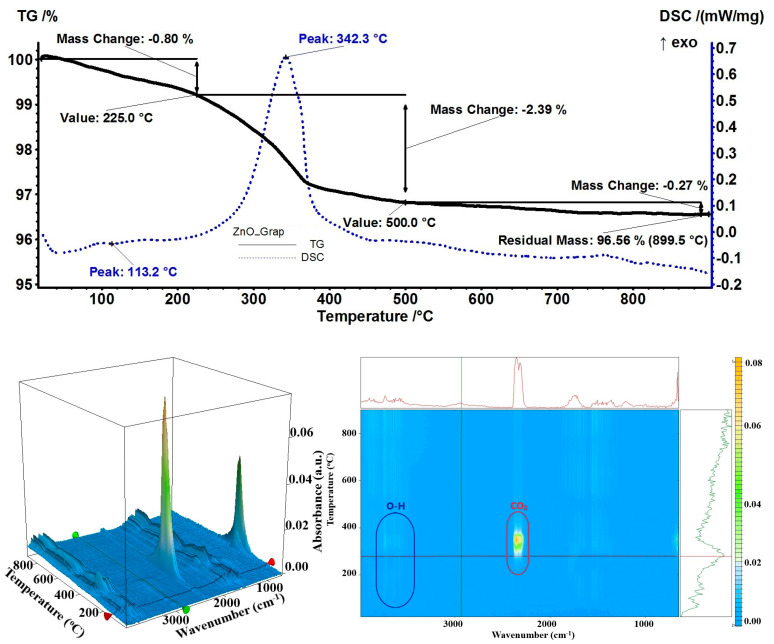
The thermal analysis (Figure S2) confirmed the synthesis of ZnO NPs using the solvothermal method. The small mass loss recorded up to 900 °C (1.54%) was caused by the elimination of solvent molecules adsorbed on the surface and some acetate impurities [10]. Evaluation of the quantity of essential oil loaded on ZnO NPs, as obtained from the synthesis, was carried out with thermal analysis (TG-DSC), Figure S3. By implementing the FTIR evaluation of the evolved gases from TG-DSC, monitoring of the desorption process of active components can be achieved.
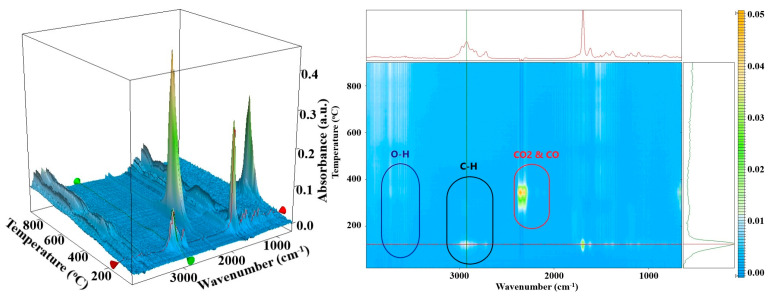
The thermal analysis of the ZnO_Cit sample presented a mass loss of 4.00% up to 225 °C accompanied by an endothermic effect with a minimum of 111.9 °C, indicating a desorption process (Figure 6). The upper part of the FTIR 2D projection presents the FTIR spectrum of the evolved gases at 115 °C, which could be assigned to the citronellal component [66]. The other less volatile components—geraniol, geranylacetate, and elemol—were eliminated in the second temperature interval of 225–500 °C, by oxidation and degradation, with a recorded mass loss of 2.25% [67]. An exothermic effect was associated with this process, with a maximum peak at 340.0 °C, indicating the presence of oxidative reactions. The FTIR 2D and 3D diagrams of evolved gases indicated the presence of carbon dioxide and water vapors in this temperature interval. On the right side of the FTIR 2D projection, there is a trace for hydrocarbon fragments (wavenumber 2925 cm−1) which presents a small peak at 350 °C indicating that fragmentation reaction also took place in this temperature interval. The estimated load of citronella essential oil was ~5%.
Figure 6.
The TG-DSC curves for the ZnO_Cit sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
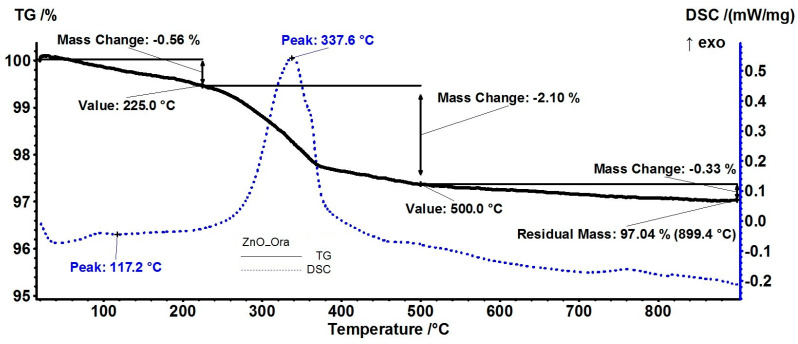
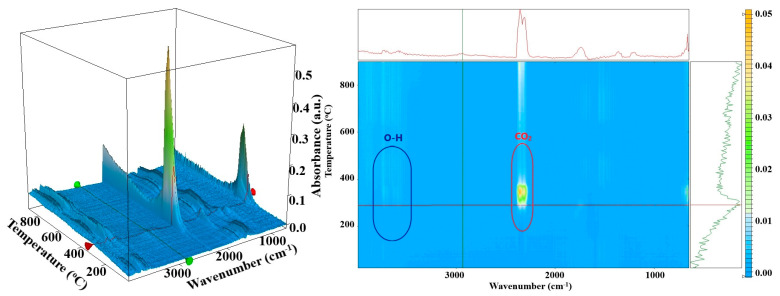
The samples obtained from orange and grapefruit essential oils exhibited the lowest total mass loss due to the low persistence of the loaded essential oils (estimated load of 1.44% and 1.93%, respectively). As the major component of these two essential oils was limonene (over 90%), it likely did not create strong enough bonds with ZnO NPs to avoid its rapid release by evaporation [68]. Nevertheless, the other minor components of essential oils, like linalool or β-myrcene were still present in the sample [69,70], and their degradation was followed by the elimination of water vapors, carbon dioxide, and some traces of hydrocarbon fragments at over 300 °C, as presented in Figure 7 and Figure 8.
Figure 7.
The TG-DSC curves, for the ZnO_Ora sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
Figure 8.
The TG-DSC curves, for the ZnO_Grap sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
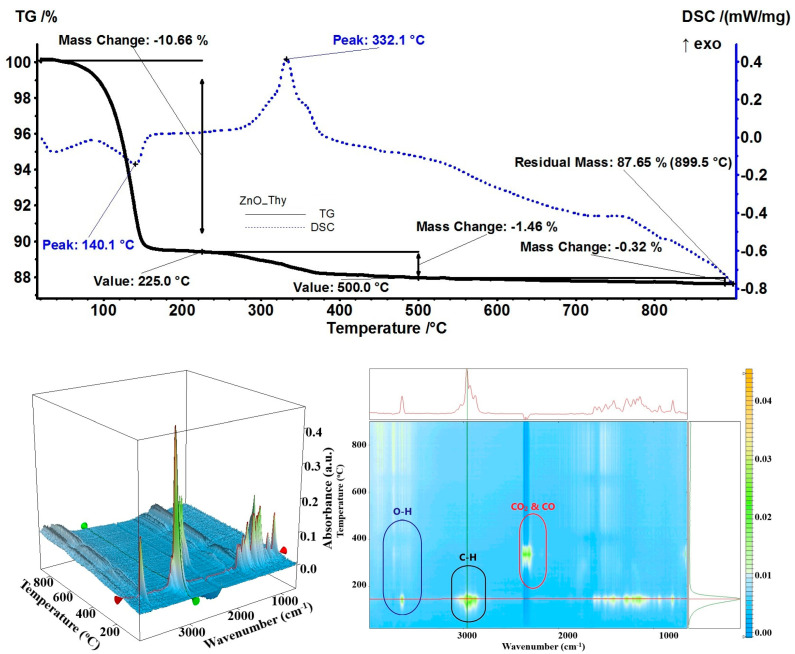
The sample ZnO_Thy exhibited a mass loss of 10.66% between RT-225 °C; the process being correlated with an endothermic effect on the DSC curve at 140.1 °C (Figure 9). The FTIR 2D and 3D diagrams of evolved gases indicated the presence of organic compounds in this temperature interval. On top of the 2D projection, the FTIR spectrum at 150 °C indicated mainly the presence of thymol in the evolved gases (both γ-terpinene and p-cymene have FTIR spectra that are masked by the one of thymol) [63,71]. Between 225 and 500 °C, the residual organics from the ZnO NPs were burned away—the recorded mass loss was 1.46% with an associated exothermic effect at 332.1 °C—and the only products identified in the evolved gases spectra were carbon dioxide and water vapors. The estimated load of thyme essential oil was 10.98%.
Figure 9.
The TG-DSC curves, for the ZnO_Thy sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
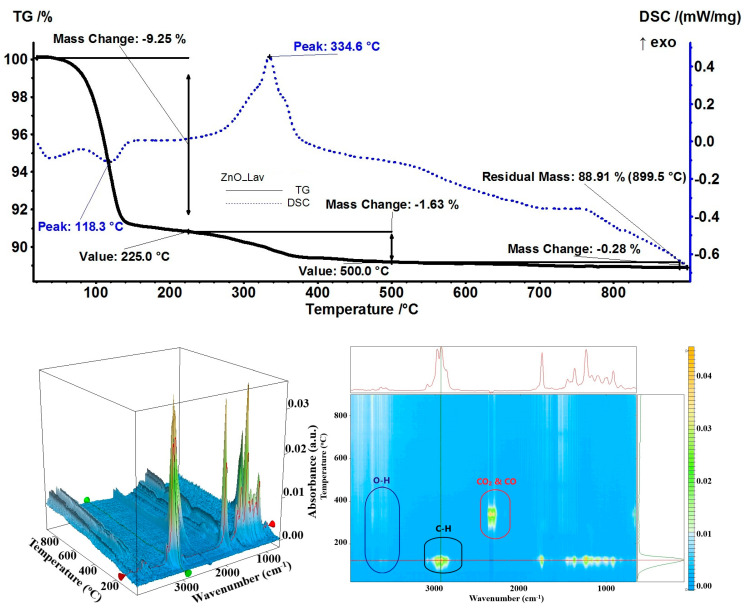
Another sample with a high load of essential oil (9.70%) was ZnO_Lav. At a temperature up to 225 °C, the sample presented a mass loss of 9.25% with an endothermic effect at 118.3 °C. On top of the 2D projection from Figure 10, the FTIR of evolved gases at 125 °C showed a mixture of linalool and linalyl acetate, as indicated by the presence of –OH, C–H, and C=O vibrations at 3566, ~3000, and 1753 cm−1, respectively [72]. The sample lost an additional 1.63% in the temperature interval of 225–500 °C by oxidation of residual organics, as indicated by the presence of specific peaks of carbon dioxide and water vapors from the FTIR diagram of the evolved gases around 350 °C. The residual mass at 900 °C represented 88.91% which permitted evaluation of loaded lavender essential oil at 9.25%.
Figure 10.
The TG-DSC curves, for the ZnO_Lav sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
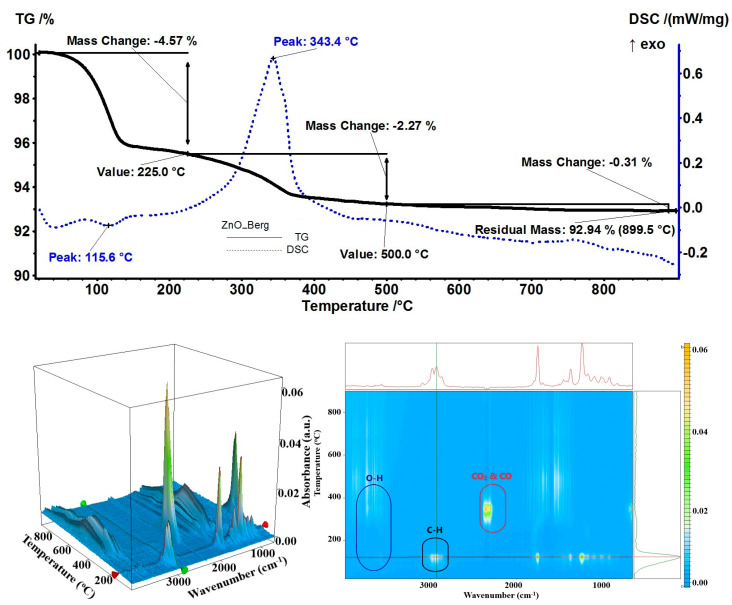
Although the bergamot essential oil’s major component is limonene, and it has a low affinity for ZnO NPs as shown by samples with orange and grapefruit essential oils, the lower percentage (59%) in the essential oil composition indicates that the other components (linalyl acetate, linalool, or β-pinene) can still be observed [73]. Therefore, a 4.57% mass loss was recorded at the temperature up to 225 °C, as an endothermic process with a minimum at 115.6 °C on the DSC curve (Figure 11). The FTIR of evolved gases indicated the presence of linalyl acetate as C–H and C=O specific vibrations were identified (around 2864–3088 cm−1 and 1753 cm−1, respectively). In the interval of 225–500 °C, the rest of the organic compounds were burned away from the NP surface as indicated by the FTIR of the evolved gases (carbon monoxide, carbon dioxide, and water-specific vibrations were identified at 2177, 2355, 3566 cm−1, respectively). The residual mass of 92.94% was used to evaluate the loading of bergamot essential oil at 5.61%.
Figure 11.
The TG-DSC curves, for the ZnO_Berg sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
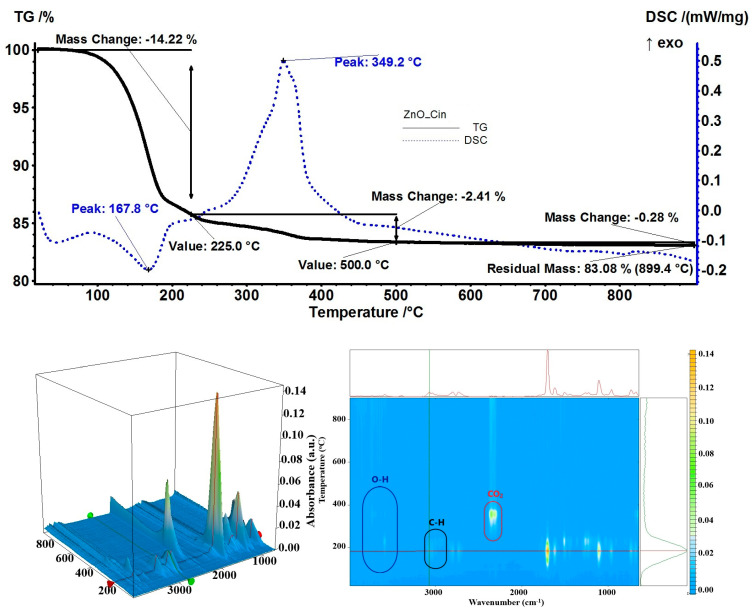
The highest loading capacity, at 15.62%, calculated from the residual mass of 83.08%, was obtained from the sample with cinnamon essential oil. In the temperature interval of 20–225 °C, the sample exhibited a mass loss of 14.22% accompanied by an endothermic effect at 167.8 °C (Figure 12). The FTIR of evolved gases assessed the presence of O–H, C–H, and C=O bonds, which were consistent with cinnamaldehyde from the essential oil, with a maximum at 183 °C, while the maximum for eugenol was observed at 230 °C [74]. At the temperature of up to 500 °C, the sample presented a mass loss of 2.41% due to the oxidation of remaining organic compounds as indicated by the strong exothermic effect from 349.2 °C; at this temperature, the evolved gases were composed of carbon dioxide and water vapors, confirming the complete burning process.
Figure 12.
The TG-DSC curves, for the ZnO_Cin sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
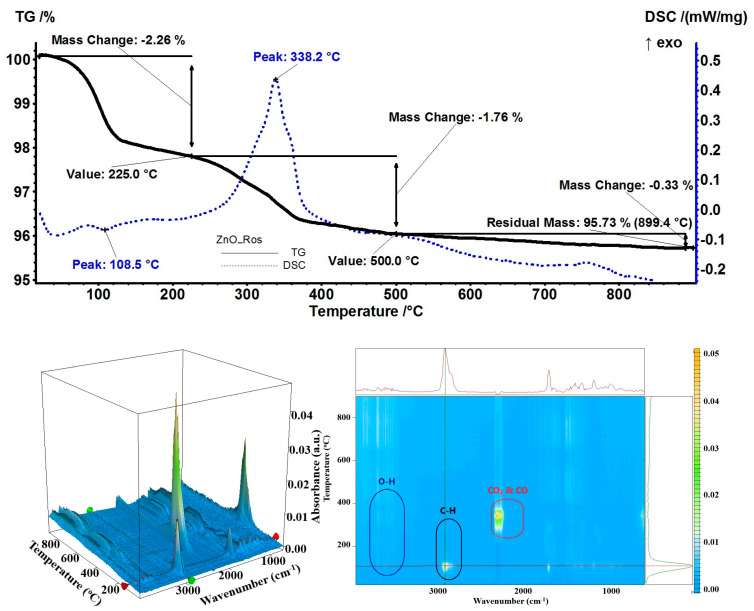
For the sample with rosemary essential oil, a mass loss of 2.26% was recorded up to 225 °C. The desorption process was endothermic as indicated by the minimum of 108.5 °C on the DSC curve (Figure 13). The FTIR for evolved gases indicated the presence of borneol at 107 °C, quickly followed by α-caryophyllene at 112 °C [75]. The complete oxidation of remaining organic molecules occurred between 225 and 500 °C when a mass loss of 1.76% was recorded, accompanied by a strong exothermic effect at 338.2 °C. The residual mass of 95.73% indicated that only a fraction of rosemary essential oil had been loaded on ZnO NPs, estimated at 2.77%.
Figure 13.
The TG-DSC curves, for the ZnO_Ros sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
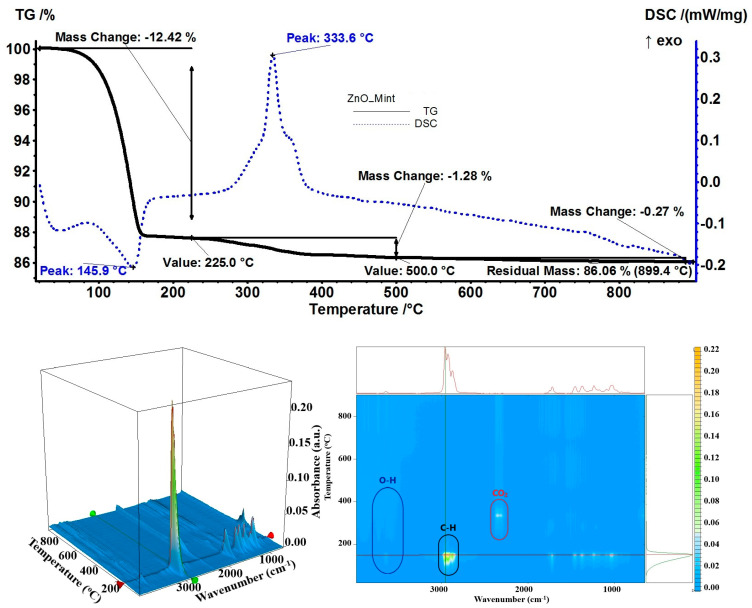
The sample ZnO_Mint exhibited the second-highest estimated load of 12.60%—most probably due to menthol’s high affinity for ZnO. The mass loss at the temperature up to 225 °C was 12.42%, with the desorption endothermic effect presenting the minimum at 145.9 °C (Figure 14). Both menthone and menthol were eliminated rapidly one after the other—menthone first—and the evolved gases FTIR indicated maximum peaks at 140 °C and 150 °C [76]. The elimination of residual organics took place after 225 °C when a mass loss of 1.28% was recorded together with a sharp and strong exothermic effect at 333.6 °C on the DSC curve. For this sample, the residual mass was 86.06%.
Figure 14.
The TG-DSC curves, for the ZnO_Mint sample, FTIR 3D diagram of the evolved gases and its 2D projection in the wavenumber/temperature plan.
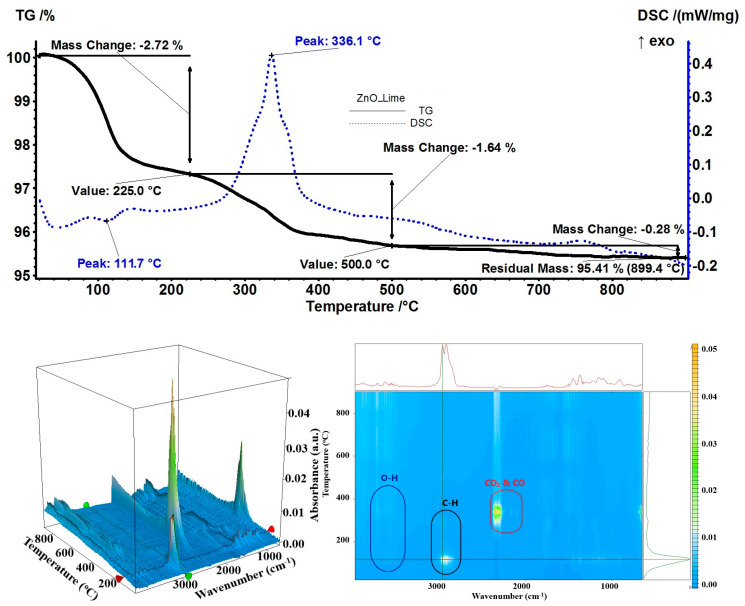
As limette essential oil contains limonene and has a low affinity for the NPs, the estimated load was only 3.10%, based on the residual mass of 95.41%. The recorded mass loss at the temperature up to 225 °C was 2.72%, with an additional 1.64% up to 500 °C (Figure 15). In the first step, volatile organics were desorbed from the ZnO NP surfaces, as indicated by the endothermic effect from 111.7 °C, while in the second step, residual organic compounds were oxidized as indicated by the exothermic effect from 336.1 °C. The FTIR of evolved gases indicated the presence of γ-terpinene and β-pinene (C–H stretching vibrations around 3000 cm−1, for both sp3 and sp2 carbons; C=C stretching vibration at 1652 cm−1; C–H bending vibration at 1442 cm−1; and C=C bending vibration at 912 cm−1) [77,78]. The temperature at which these compounds were identified with the FTIR of evolved gases was 116 °C.
Figure 15.
The TG-DSC curves, for the ZnO_Lime sample, FTIR 3D diagram of the evolved gases, and its 2D projection in the wavenumber/temperature plan.
The principal numeric data from thermal analysis are presented in Table 5.
Table 5.
Temperature intervals for the principal mass loss processes, and associated thermal effects.
| Sample | Mass Loss (%) RT-225 °C |
Endothermic Peak (°C) |
Mass Loss (%) 225–500 °C |
Exothermic Peak (°C) | Residual Mass (%) | Estimated Load (%) |
|---|---|---|---|---|---|---|
| ZnO | 0.11% | 70.1 | 1.03% | 328.2 | 98.46% | - |
| ZnO_Cit | 4.00% | 111.9 | 2.25% | 340.0 | 93.54% | 5.00% |
| ZnO_Ora | 0.56% | 117.2 | 2.10% | 337.6 | 97.04% | 1.44% |
| ZnO_Thy | 10.66% | 140.1 | 1.46% | 332.1 | 87.65% | 10.98% |
| ZnO_Lav | 9.25% | 118.3 | 1.63% | 334.6 | 88.91% | 9.70% |
| ZnO_Grap | 0.80% | 113.2 | 2.39% | 342.3 | 96.56% | 1.93% |
| ZnO_Berg | 4.57% | 115.6 | 2.27% | 343.4 | 92.94% | 5.61% |
| ZnO_Cin | 14.22% | 167.8 | 2.41% | 349.2 | 83.08% | 15.62% |
| ZnO_Ros | 2.26% | 108.5 | 1.76% | 338.2 | 95.73% | 2.77% |
| ZnO_Mint | 12.42% | 145.9 | 1.28% | 333.6 | 86.06% | 12.60% |
| ZnO_Lime | 2.72% | 111.7 | 1.64 | 336.1 | 95.41% | 3.10% |
To conclude, the limonene had a low affinity for ZnO NPs and a high volatility leading to a low loading of samples with essential oils containing limonene, such as orange or grapefruit—both with under 2% load. The limonene represents around half of the mass of the bergamot or limette essential oil; therefore, the overall loading was higher due to the remaining compounds like linalyl acetate and linalool or γ-terpinene and α/β-pinene, respectively. The rosemary oil also exhibited a low loading capacity of only 2.77% due to the high content of non-polar hydrocarbons and low content of bulky alcohols.
For the citronella essential oil, a medium loading capacity of 5% was found as compounds like citronellal, geraniol or geranylacetate are more polar due to functional oxygen-based moieties, and therefore exhibited a higher affinity towards ZnO NPs.
The high-affinity phenols and alcohols for ZnO NPs led to high loading of essential oils that contain such compounds as major components: thymol in thyme oil, linalool in lavender oil, and menthol in minzol oil. The presence of additional menthone in minzol oil led to a higher load of 12.60%. However, the highest load, 15.62%, was obtained from cinnamon oil due to the presence of (E)-cinnamaldehyde in a high quantity (72%) with the additional presence of linalool and eugenol.
The essential oil used to load ZnO NPs represents ~20% of each sample mass. However, none of the essential oils reached the theoretical value—the maximum load achieved being 15.62%. These results confirmed that some of the essential oil components (especially the non-polar ones) did not interact strongly enough with the ZnO NP surface to be retained.
3.5. Antibacterial Activity Assay
The disk-in-agar diffusion method was employed to evaluate the antibacterial activity of the essential oils-loaded ZnO NP samples. This diffusion-based method is considered suitable for identifying the most potent antibacterial agents. The zone of inhibition determined by the diffusion method is proportional to the bacterial susceptibility to the essential oil present in the paper disc. The zone of inhibition around the impregnated disc defines the extent of antimicrobial activity and is determined as zone diameter in mm (which includes the size of the paper disc). The factors that affect the diameter of this zone are bacterial cell growth and the rate of diffusion.
The results employing the diffusion method are shown in Table 6. The evaluation consisted of assessing the size of the zones of inhibition induced by ZnO NPs loaded with essential oils, and areas where microbial growth was absent. The tests were performed in triplicates and the results were reported as the average diameter. These are directly proportional to the sensitivity of the germ used; the more active the substance in the structure of the tested sample, the wider the zone of inhibition of microbial development. The results were read after 24 h of incubation at 37 °C.
Table 6.
Diameters of zones of inhibition (mm *).
| Sample | Gram-Positive Bacteria | Gram-Negative Bacteria | ||
|---|---|---|---|---|
| L. monocytogenes | S. aureus | S. typhimurium | E. coli | |
| ZnO_Cit | 12.3 ± 0.6 b | 12.0 ± 1.0 b | 16.3 ± 0.6 b | 14.7 ± 0.6 d |
| ZnO_Ora | 11.0 ± 0.0 a | 8.7 ± 0.6 a | 15.0 ± 0.0 a | 10.7 ± 0.6 a |
| ZnO_Thy | 13.7 ± 0.6 c | 14.3 ± 0.6 c | 18.3 ± 0.6 c | 15.7 ± 0.6 d |
| ZnO_Lav | 10.7 ± 0.0 a | 12.7 ± 0.6 b | 14.7 ± 0.0 a | 11.7 ± 0.6 b |
| ZnO_Grap | 10.7 ± 0.6 a | 9.0 ± 0.0 a | 14.3 ± 0.0 a | 10.3 ± 0.6 a |
| ZnO_Berg | 13.3 ± 0.6 b,c | 12.7 ± 0.6 b | 13.0 ± 1.0 d | 12.0 ± 0.0 b |
| ZnO_Cin | 13.7 ± 0.6 c | 12.0 ± 0.6 b | 16.0 ± 0.0 b | 13.3 ± 0.6 c |
| ZnO_Ros | 11.0 ± 0.0 a | 12.3 ± 0.6 b | 11.7 ± 0.6 d | 13.0 ± 1.0 b,c |
| ZnO_Mint | 11.3 ± 0.6 a | 12.0 ± 0.0 b | 12.0 ± 1.0 d | 11.7 ± 0.6 b |
| ZnO_Lime | 10.3 ± 0.6 a | 9.3 ± 0.6 a | 12.3 ± 0.6 d | 10.3 ± 0.6 a |
| ZnO | 10.7 ± 0.6 a | 8.7 ± 0.6 a | 14.7 ± 0.6 a | 10.3 ± 0.6 a |
| Citronella | 22.0 ± 1.0 | 16.3 ± 0.6 | 9.7 ± 0.6 | 37.7 ± 1.2 |
| Orange | 13.0 ± 1.0 | 9.7 ± 0.6 | 10.0 ± 0.0 | 9.7 ± 0.6 |
| Thyme | 24.3 ± 0.6 | 57.7 ± 0.6 | 55.3 ± 0.6 | 59.0 ± 1.0 |
| Lavender | 12.0 ± 0.0 | 20.3 ± 0.6 | 15.0 ± 1.0 | 17.0 ± 1.0 |
| Grapefruit | 12.3 ± 0.6 | 12.7 ± 0.6 | 11.7 ± 0.6 | 12.3 ± 0.6 |
| Bergamot | 8.0 ± 0.0 | 7.3 ± 0.6 | 8.7 ± 0.6 | 6.7 ± 0.6 |
| Cinnamon | 30.7 ± 0.6 | 31.7 ± 0.6 | 22.0 ± 1.0 | 15.3 ± 0.6 |
| Rosemary | 9.0 ± 0.0 | 9.0 ± 0.0 | 8.3 ± 0.6 | 11.7 ± 0.6 |
| Minzol | 15.0 ± 1.0 | 15.0 ± 1.0 | 13.0 ± 1.0 | 19.3 ± 0.6 |
| Limette | 7.3 ± 0.6 | 11.0 ± 1.0 | 9.0 ± 0.0 | 10.0 ± 1.0 |
* The diameter of the paper disc is included; mean value of three replicates ± standard deviation; a, b, c, d different small letters indicate statistically significant differences between samples on same column (p < 0.05).
ZnO NPs showed a moderate inhibitory effect on the tested bacteria, but the inhibitory activity was more pronounced in combination with the essential oils, thus demonstrating their synergistic effect. The synergistic activity between ZnO NPs and the studied essential oils was most visible, especially for citronella, thyme, and cinnamon essential oils against all the bacterial strains. The antibacterial activity of ZnO was very evident in the case of S. typhimurium, followed by E. coli and L. monocytogenes, demonstrating that ZnO has significant antibacterial potential.
All combinations of ZnO with essential oils showed antimicrobial activity on the test microorganism strains by direct contact with the environment. Samples proved less active against Gram-positive bacteria than Gram-negative ones (S.aureus being the most resistant strain), but the differences in sensitivity were not distinct.
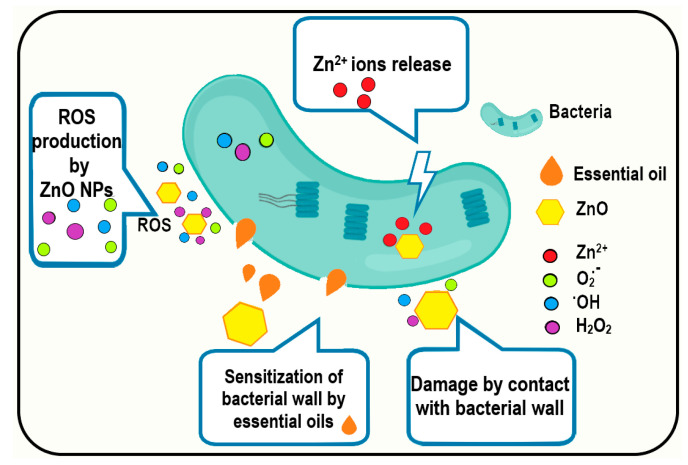
The inhibitory effect of ZnO on S. aureus was relatively weak (8.7 mm of inhibition diameter), but seven samples loaded with essential oils presented a significant increase in the inhibition zone. For S. aureus strain, only the ZnO NPs loaded with orange, grapefruit, and limette essential oils presented a negligible increase in the diameter of the inhibition zone—these being also the samples with the smallest EO load. For all the other samples, the presence of essential oils along ZnO NPs led to increases in inhibition zones, including samples containing bergamot and rosemary essential oils, which exhibited only 7–9 mm diameter of inhibition zones as stand-alone substances; this was likely because the essential oils were sensitizing the bacterial cell to become less resistant to ZnO NPs (Figure 16). The mechanism could be related to the presence of essential oils, which changed the concentration of adenosine triphosphate and/or hyperpolarization of the cell wall, leading to a decrease in the cytoplasmic pH [79], which in turn made it easier for the ZnO NPs to damage the membrane, and zinc ions to bind the proteins and enzymes, disrupting vital processes inside the cell [80]. The combined synergistic antibacterial activity of ZnO and essential oils was demonstrated in this study against both Gram-positive and Gram-negative bacteria.
Figure 16.
The proposed mechanism for the antibacterial activity of ZnO-EO NPs.
Overall, the ZnO NP samples loaded with thyme, citronella, and cinnamon oils were the most effective, showing significant areas of inhibition of bacterial growth. We have previously reported strong antimicrobial activity of chitosan or alginate-based films with ZnO and citronella essential oil [13,67]. The literature also reported some antibacterial nanocomposites with thyme essential oil used in specific applications [81,82]. In addition, some packaging films containing ZnO and cinnamon essential oils have been reported lately [83,84]. The antimicrobial activity of ZnO NPs loaded with bergamot [85], orange [86], grapefruit, limette, or minzol essential oils is not well studied. Nevertheless, some studies directly used limonene instead of oils to obtain antimicrobial packaging [87]. Similarly, there are some reports on ZnO loaded directly with menthol for wound dressing applications [88].
The loaded nanoparticles can be further used with a polymer matrix to obtain innovative composite materials with specific applications like wound dressing or food packaging [33,89,90,91], tailoring them for prolonged release of the loaded essential oils or for rapid, burst desorption.
4. Conclusions
The obtained ZnO NPs presented good antibacterial activity on all four bacterial strains (better on Gram-negative than on Gram-positive bacteria), supporting further use in medical applications.
In this study, we loaded ZnO NPs with ten different essential oils, and the antibacterial activity of the samples was determined. Due to composition and high volatility, some essential oils exhibited low loading capacity, especially those containing non-substituted terpenes like limonene (e.g., 1.44% estimated load for orange essential oil). At the same time, the essential oils containing alcohol, aldehyde, and ketone derivatives presented a higher loading capacity (e.g., 15.62% for cinnamon essential oil). Synergic antibacterial activity between ZnO NPs and essential oils was observed for most of the samples, related to the effective load on the surface of ZnO NPs. Therefore, samples with a higher content of strong antibacterial oils like thyme, cinnamon, or citronella essential oils present a higher potential for antibacterial applications (diameter of inhibition zone higher than 15 mm) like wound dressing or food packaging.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15102470/s1, Figure S1: The FTIR spectra for the ZnO NPs (a) and ZnO NPs loaded with essential oils (b); detail of the 1000–1800 cm−1 zone (c) and (d); Figure S2. The TG-DSC curves for the ZnO NPs; Figure S3. The TG curves for the ZnO NPs and ZnO NPs loaded with essential oils
Author Contributions
Conceptualization, O.-C.O., G.M. and E.A.; methodology, O.-C.O. and A.F.; investigation, L.M., D.F., A.-V.S., E.L.U., A.A.D. and B.-S.V.; writing—original draft preparation, O.-C.O., L.M., D.F., A.-V.S., G.M., E.L.U., A.A.D. and B.-S.V.; writing—review and editing, A.F. and E.A.; supervision, O.-C.O. and A.F. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was supported by a grant from the Ministry of Research, Innovation and Digitization, CCCDI-UEFISCDI, project number PN-III-P2-2.1-PED-2021-3414, within PNCDI III; 573PED/2022 “Ambalaje inovative cu activitate antimicrobiana pentru siguranta alimentara”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Deaconu M., Nicu I., Tincu R., Brezoiu A.M., Mitran R.A., Vasile E., Matei C., Berger D. Tailored doxycycline delivery from mcm-41-type silica carriers. Chem. Pap. 2018;72:1869–1880. doi: 10.1007/s11696-018-0457-z. [DOI] [Google Scholar]
- 2.Holban A.M., Gestal M.C., Grumezescu A.M. Control of biofilm-associated infections by signaling molecules and nanoparticles. Int. J. Pharm. 2016;510:409–418. doi: 10.1016/j.ijpharm.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 3.Gherasim O., Grumezescu A.M., Grumezescu V., Iordache F., Vasile B.S., Holban A.M. Bioactive surfaces of polylactide and silver nanoparticles for the prevention of microbial contamination. Materials. 2020;13:768. doi: 10.3390/ma13030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogosanu G.D., Grumezescu A.M., Huang K.S., Bejenaru L.E., Bejenaru C. Prevention of microbial communities: Novel approaches based natural products. Curr. Pharm. Biotechnol. 2015;16:94–111. doi: 10.2174/138920101602150112145916. [DOI] [PubMed] [Google Scholar]
- 5.Feger G., Angelov B., Angelova A. Prediction of amphiphilic cell-penetrating peptide building blocks from protein-derived amino acid sequences for engineering of drug delivery nanoassemblies. J. Phys. Chem. B. 2020;124:4069–4078. doi: 10.1021/acs.jpcb.0c01618. [DOI] [PubMed] [Google Scholar]
- 6.Oprea O., Andronescu E., Ficai D., Ficai A., Oktar F.N., Yetmez M. Zno applications and challenges. Curr. Org. Chem. 2014;18:192–203. doi: 10.2174/13852728113176660143. [DOI] [Google Scholar]
- 7.Motelica L., Oprea O.C., Vasile B.S., Ficai A., Ficai D., Andronescu E., Holban A.M. Antibacterial activity of solvothermal obtained zno nanoparticles with different morphology and photocatalytic activity against a dye mixture: Methylene blue, rhodamine b and methyl orange. Int. J. Mol. Sci. 2023;24:5677. doi: 10.3390/ijms24065677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spoiala A., Ilie C.I., Trusca R.D., Oprea O.C., Surdu V.A., Vasile B.S., Ficai A., Ficai D., Andronescu E., Ditu L.M. Zinc oxide nanoparticles for water purification. Materials. 2021;14:4747. doi: 10.3390/ma14164747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ficai D., Oprea O., Ficai A., Holban A.M. Metal oxide nanoparticles: Potential uses in biomedical applications. Curr. Proteomics. 2014;11:139–149. doi: 10.2174/157016461102140917122838. [DOI] [Google Scholar]
- 10.Motelica L., Vasile B.S., Ficai A., Surdu A.V., Ficai D., Oprea O.C., Andronescu E., Jinga D.C., Holban A.M. Influence of the alcohols on the zno synthesis and its properties: The photocatalytic and antimicrobial activities. Pharmaceutics. 2022;14:2842. doi: 10.3390/pharmaceutics14122842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preda M.D., Popa M.L., Neacsu I.A., Grumezescu A.M., Ginghina O. Antimicrobial clothing based on electrospun fibers with zno nanoparticles. Int. J. Mol. Sci. 2023;24:1629. doi: 10.3390/ijms24021629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lungu I.I., Holban A.M., Ficai A., Grumezescu A.M. Nanostructures for Antimicrobial Therapy. Springer; Berlin/Heidelberg, Germany: 2017. Zinc oxide nanostrucures: New trends in antimicrobial therapy; pp. 503–514. [Google Scholar]
- 13.Motelica L., Ficai D., Oprea O., Ficai A., Trusca R.D., Andronescu E., Holban A.M. Biodegradable alginate films with zno nanoparticles and citronella essential oil-a novel antimicrobial structure. Pharmaceutics. 2021;13:1020. doi: 10.3390/pharmaceutics13071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavaloiu R.D., Sha’at F., Bubueanu C., Deaconu M., Neagu G., Sha’at M., Anastasescu M., Mihailescu M., Matei C., Nechifor G., et al. Polyphenolic extract from sambucus ebulus l. Leaves free and loaded into lipid vesicles. Nanomaterials. 2020;10:56. doi: 10.3390/nano10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niculescu A.G., Grumezescu A.M. Natural compounds for preventing ear, nose, and throat-related oral infections. Plants. 2021;10:1847. doi: 10.3390/plants10091847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francikowski J., Baran B., Cup M., Janiec J., Krzyzowski M. Commercially available essential oil formulas as repellents against the stored-product pest alphitobius diaperinus. Insects. 2019;10:96. doi: 10.3390/insects10040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go E.J., Song K.B. Effect of java citronella essential oil addition on the physicochemical properties of gelidium corneum-chitosan composite films. Food Sci. Biotechnol. 2020;29:909–915. doi: 10.1007/s10068-020-00740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Fajardo J.A., Flores-Mendez D.A., Suarez-Jacobo A., Torres-Martinez L.G., Granados-Vallejo M., Corona-Gonzalez R.I., Guatemala-Morales G.M., Arriola-Guevara E. Separation of d-limonene and other oxygenated compounds from orange essential oil by molecular distillation and fractional distillation with a wiped film evaporator. Processes. 2023;11:991. doi: 10.3390/pr11040991. [DOI] [Google Scholar]
- 19.Hollingsworth R.G. Limonene, a citrus extract, for control of mealybugs and scale insects. J. Econ. Entomol. 2005;98:772–779. doi: 10.1603/0022-0493-98.3.772. [DOI] [PubMed] [Google Scholar]
- 20.Vlaicu P.A., Untea A.E., Panaite T.D., Saracila M., Turcu R.P., Dumitru M. Effect of basil, thyme and sage essential oils as phytogenic feed additives on production performances, meat quality and intestinal microbiota in broiler chickens. Agriculture. 2023;13:874. doi: 10.3390/agriculture13040874. [DOI] [Google Scholar]
- 21.Ramos M., Beltran A., Fortunati E., Peltzer M., Cristofaro F., Visai L., Valente A.J.M., Jimenez A., Kenny J.M., Garrigos M.C. Controlled release of thymol from poly(lactic acid)-based silver nanocomposite films with antibacterial and antioxidant activity. Antioxidants. 2020;9:395. doi: 10.3390/antiox9050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailen M., Illescas C., Quijada M., Martinez-Diaz R.A., Ochoa E., Gomez-Munoz M.T., Navarro-Rocha J., Gonzalez-Coloma A. Anti-trypanosomatidae activity of essential oils and their main components from selected medicinal plants. Molecules. 2023;28:1467. doi: 10.3390/molecules28031467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diass K., Merzouki M., Elfazazi K., Azzouzi H., Challioui A., Azzaoui K., Hammouti B., Touzani R., Depeint F., Gotor A.A., et al. Essential oil of lavandula officinalis: Chemical composition and antibacterial activities. Plants. 2023;12:1571. doi: 10.3390/plants12071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todorova D., Yavorov N., Lasheva V., Damyanova S., Kostova I. Lavender essential oil as antibacterial treatment for packaging paper. Coatings. 2023;13:32. doi: 10.3390/coatings13010032. [DOI] [Google Scholar]
- 25.Rochin-Medina J.J., Mendoza-Lopez I.A., Campo N.C.D., Bastidas-Bastidas P.J., Ramirez K. Activity of plant essential oils against clinically and environmentally isolated salmonella enterica serotypes: In vitro assays and molecular docking. Lett. Appl. Microbiol. 2023;76:ovad045. doi: 10.1093/lambio/ovad045. [DOI] [PubMed] [Google Scholar]
- 26.Puscaselu R.G., Lobiuc A., Sirbu I.O., Covasa M. The use of biopolymers as a natural matrix for incorporation of essential oils of medicinal plants. Gels-Basel. 2022;8:756. doi: 10.3390/gels8110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brah A.S., Armah F.A., Obuah C., Akwetey S.A., Adokoh C.K. Toxicity and therapeutic applications of citrus essential oils (ceos): A review. Int. J. Food Prop. 2023;26:301–326. doi: 10.1080/10942912.2022.2158864. [DOI] [Google Scholar]
- 28.Zambito Y., Piras A.M., Fabiano A. Bergamot essential oil: A method for introducing it in solid dosage forms. Foods. 2022;11:3860. doi: 10.3390/foods11233860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S.K., Wang Y.F. Antimicrobial and antioxidant effects of kappa-carrageenan coatings enriched with cinnamon essential oil in pork meat. Foods. 2022;11:2885. doi: 10.3390/foods11182885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogwaro B.A., O’Gara E.A., Hill D.J., Gibson H. A study of the antimicrobial activity of combined black pepper and cinnamon essential oils against escherichia fergusonii in traditional african yoghurt. Foods. 2021;10:2847. doi: 10.3390/foods10112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaur R., Kaur L., Gupta T.B., Bronlund J. Manuka oil vs. Rosemary oil: Antimicrobial efficacies in wagyu and commercial beef against selected pathogenic microbes. Foods. 2023;12:1333. doi: 10.3390/foods12061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivas-Mendez P., Chavez-Martinez A., Santellano-Estrada E., Asorey L.G., Sanchez-Vega R., Renteria-Monterrubio A.L., Chavez-Flores D., Tirado-Gallegos J.M., Mendez-Zamora G. Antioxidant and antimicrobial activity of rosemary (Rosmarinus officinalis) and garlic (Allium sativum) essential oils and chipotle pepper oleoresin (Capsicum annum) on beef hamburgers. Foods. 2022;11:2018. doi: 10.3390/foods11142018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiriac A.P., Rusu A.G., Nita L.E., Chiriac V.M., Neamtu I., Sandu A. Polymeric carriers designed for encapsulation of essential oils with biological activity. Pharmaceutics. 2021;13:631. doi: 10.3390/pharmaceutics13050631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimino C., Maurel O.M., Musumeci T., Bonaccorso A., Drago F., Souto E.M.B., Pignatello R., Carbone C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics. 2021;13:327. doi: 10.3390/pharmaceutics13030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seljak K.B., Kocbek P., Gasperlin M. Mesoporous silica nanoparticles as delivery carriers: An overview of drug loading techniques. J. Drug Deliv. Sci. Technol. 2020;59:101906. doi: 10.1016/j.jddst.2020.101906. [DOI] [Google Scholar]
- 36.Sima S., Secuianu C. The effect of functional groups on the phase behavior of carbon dioxide binaries and their role in ccs. Molecules. 2021;26:3733. doi: 10.3390/molecules26123733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciuca M.D., Racovita R.C. Curcumin: Overview of extraction methods, health benefits, and encapsulation and delivery using microemulsions and nanoemulsions. Int. J. Mol. Sci. 2023;24:8874. doi: 10.3390/ijms24108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racovita R.C., Ciuca M.D., Catana D., Comanescu C., Ciocirlan O. Microemulsions of nonionic surfactant with water and various homologous esters: Preparation, phase transitions, physical property measurements, and application for extraction of tricyclic antidepressant drugs from aqueous media. Nanomaterials. 2023;13:2311. doi: 10.3390/nano13162311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrisor G., Motelica L., Ficai D., Ilie C.I., Trusca R.D., Surdu V.A., Oprea O.C., Mirt A.L., Vasilievici G., Semenescu A., et al. Increasing bioavailability of trans-ferulic acid by encapsulation in functionalized mesoporous silica. Pharmaceutics. 2023;15:660. doi: 10.3390/pharmaceutics15020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voicu G., Oprea O., Vasile B.S., Andronescu E. Antibacterial activity of zinc oxide—Gentamicin hybrid material. Dig. J. Nanomater. Biostructures. 2013;8:1191–1203. [Google Scholar]
- 41.Alandiyjany M.N., Abdelaziz A.S., Abdelfattah-Hassan A., Hegazy W.A.H., Hassan A.A., Elazab S.T., Mohamed E.A.A., El-Shetry E.S., Saleh A.A., ElSawy N.A., et al. Novel in vivo assessment of antimicrobial efficacy of ciprofloxacin loaded mesoporous silica nanoparticles against salmonella typhimurium infection. Pharmaceuticals. 2022;15:357. doi: 10.3390/ph15030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ardean C., Davidescu C.M., Nemes N.S., Negrea A., Ciopec M., Duteanu N., Negrea P., Duda-Seiman D., Muntean D. Antimicrobial activities of chitosan derivatives. Pharmaceutics. 2021;13:1639. doi: 10.3390/pharmaceutics13101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naskar A., Kim K.S. Potential novel food-related and biomedical applications of nanomaterials combined with bacteriocins. Pharmaceutics. 2021;13:86. doi: 10.3390/pharmaceutics13010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schito A.M., Piatti G., Caviglia D., Zuccari G., Zorzoli A., Marimpietri D., Alfei S. Bactericidal activity of non-cytotoxic cationic nanoparticles against clinically and environmentally relevant pseudomonas spp. Isolates. Pharmaceutics. 2021;13:1411. doi: 10.3390/pharmaceutics13091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceresa C., Fracchia L., Fedeli E., Porta C., Banat I.M. Recent advances in biomedical, therapeutic and pharmaceutical applications of microbial surfactants. Pharmaceutics. 2021;13:466. doi: 10.3390/pharmaceutics13040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purcar V., Somoghi R., Nitu S.G., Nicolae C.A., Alexandrescu E., Gifu I.C., Gabor A.R., Stroescu H., Ianchis R., Caprarescu S., et al. The effect of different coupling agents on nano-zno materials obtained via the sol-gel process. Nanomaterials. 2017;7:439. doi: 10.3390/nano7120439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumbrava A., Berger D., Prodan G., Matei C., Moscalu F., Diacon A. Influence of synthesis route on the structure and properties of zinc oxide nanoparticles functionalized with anthocyanins from raw vegetable extracts. ECS J. Solid State Sci. Technol. 2017;6:P870–P878. doi: 10.1149/2.0311712jss. [DOI] [Google Scholar]
- 48.Dumbrava A., Berger D., Matei C., Prodan G., Aonofriesei F., Radu M.D., Moscalu F. New composite nanomaterials with antimicrobial and photocatalytic properties based on silver and zinc oxide. J. Inorg. Organomet. Polym. 2019;29:2072–2082. doi: 10.1007/s10904-019-01166-4. [DOI] [Google Scholar]
- 49.Rogozea E.A., Olteanu N.L., Petcu A.R., Lazar C.A., Meghea A., Mihaly M. Extension of optical properties of ZnO/SiO2 materials induced by incorporation of au or nio nanoparticles. Opt. Mater. 2016;56:45–48. doi: 10.1016/j.optmat.2015.12.020. [DOI] [Google Scholar]
- 50.Popa M.L., Preda M.D., Neacsu I.A., Grumezescu A.M., Ginghina O. Traditional vs. Microfluidic synthesis of zno nanoparticles. Int. J. Mol. Sci. 2023;24:1875. doi: 10.3390/ijms24031875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imran H.J., Hubeatir K.A., Aadim K.A. Synthesis and characterization of zno nanoparticles by pulsed laser ablation in liquid using different wavelengths for antibacterial application. Int. J. Nanoelectron. Mater. 2023;16:345–358. [Google Scholar]
- 52.Oprea O., Andronescu E., Vasile B.S., Voicu G., Covaliu C. Synthesis and characterization of zno nanopowder by non-basic route. Dig. J. Nanomater. Biostructures. 2011;6:1393–1401. [Google Scholar]
- 53.Zhang C.J., Tu Q.M., Francis L.F., Kortshagen U.R. Band gap tuning of films of undoped zno nanocrystals by removal of surface groups. Nanomaterials. 2022;12:565. doi: 10.3390/nano12030565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dumbrava A., Matei C., Diacon A., Moscalu F., Berger D. Novel zno-biochar nanocomposites obtained by hydrothermal method in extracts of ulva lactuca collected from black sea. Ceram. Int. 2023;49:10003–10013. doi: 10.1016/j.ceramint.2022.11.178. [DOI] [Google Scholar]
- 55.Motelica L., Popescu A., Razvan A.G., Oprea O., Trusca R.D., Vasile B.S., Dumitru F., Holban A.M. Facile use of zno nanopowders to protect old manual paper documents. Materials. 2020;13:5452. doi: 10.3390/ma13235452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babayevska N., Przysiecka L., Nowaczyk G., Jarek M., Jarvekulg M., Kangur T., Janiszewska E., Jurga S., Iatsunskyi I. Fabrication of gelatin-zno nanofibers for antibacterial applications. Materials. 2021;14:103. doi: 10.3390/ma14010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shang C.Y., Bu J.Y., Song C. Preparation, antimicrobial properties under different light sources, mechanisms and applications of TiO2: A review. Materials. 2022;15:5820. doi: 10.3390/ma15175820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaningini A.G., Azizi S., Sintwa N., Mokalane K., Mohale K.C., Mudau F.N., Maaza M. Effect of optimized precursor concentration, temperature, and doping on optical properties of zno nanoparticles synthesized via a green route using bush tea (Athrixia phylicoides dc.) leaf extracts. ACS Omega. 2022;7:31658–31666. doi: 10.1021/acsomega.2c00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahalakshmi S., Hema N., Vijaya P.P. In vitro biocompatibility and antimicrobial activities of zinc oxide nanoparticles (zno nps) prepared by chemical and green synthetic route- a comparative study. Bionanoscience. 2020;10:112–121. [Google Scholar]
- 60.Pholnak C., Sirisathitkul C., Suwanboon S., Harding D.J. Effects of precursor concentration and reaction time on sonochemically synthesized zno nanoparticles. Mater. Res-Ibero-Am. J. 2014;17:405–411. doi: 10.1590/S1516-14392013005000192. [DOI] [Google Scholar]
- 61.Jayarambabu N., Siva Kumari B., Venkateswara Rao K., Prabhu Y.T. Beneficial role of zinc oxide nanoparticles on green crop production. Int. J. Multidiscip. Adv. Res. Trends. 2015;2:273–282. [Google Scholar]
- 62.Bazari M., Najmoddin N. The effect of cationic, anionic and nonionic surfactants on morphology and antibacterial properties of zinc oxide. Materia. 2022;27:e13172. doi: 10.1590/s1517-707620220002.1372. [DOI] [Google Scholar]
- 63.Catauro M., Bollino F., Tranquillo E., Sapio L., Illiano M., Caiafa I., Naviglio S. Chemical analysis and anti-proliferative activity of campania thymus vulgaris essential oil. J. Essent. Oil Res. 2017;29:461–470. doi: 10.1080/10412905.2017.1351405. [DOI] [Google Scholar]
- 64.Yang Y., Li Y.Q., Amoroso V., Acma F., Guiang M.M., Wu H. Comparison of production of bioactive components in zanthoxylum nitidum taproots from different regions in southern china. Biomed. Chromatogr. 2023;37:e5602. doi: 10.1002/bmc.5602. [DOI] [PubMed] [Google Scholar]
- 65.Li Y.Q., Kong D.X., Wu H. Analysis and evaluation of essential oil components of cinnamon barks using gc-ms and ftir spectroscopy. Ind. Crops Prod. 2013;41:269–278. doi: 10.1016/j.indcrop.2012.04.056. [DOI] [Google Scholar]
- 66.Wany A., Kumar A., Nallapeta S., Jha S., Nigam V.K., Pandey D.M. Extraction and characterization of essential oil components based on geraniol and citronellol from java citronella (Cymbopogon winterianus jowitt) Plant Growth Regul. 2014;73:133–145. doi: 10.1007/s10725-013-9875-7. [DOI] [Google Scholar]
- 67.Motelica L., Ficai D., Ficai A., Trusca R.D., Ilie C.I., Oprea O.C., Andronescu E. Innovative antimicrobial chitosan/zno/ag nps/citronella essential oil nanocomposite—Potential coating for grapes. Foods. 2020;9:1801. doi: 10.3390/foods9121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewczyk D.C., Cohan J.W., Goetz M.L., Trafford B.L., Fuller R.L., Sparks J.R. Kinetic treatment of evaporation via thermogravimetric analysis: The case of d-limonene. Ind. Eng. Chem. Res. 2020;59:15069–15074. doi: 10.1021/acs.iecr.0c02864. [DOI] [Google Scholar]
- 69.Lin X.C., Cao S., Sun J.Y., Lu D.L., Zhong B.L., Chun J. The chemical compositions, and antibacterial and antioxidant activities of four types of citrus essential oils. Molecules. 2021;26:3412. doi: 10.3390/molecules26113412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miya G., Nyalambisa M., Oyedeji O., Gondwe M., Oyedeji A. Chemical profiling, toxicity and anti-inflammatory activities of essential oils from three grapefruit cultivars from kwazulu-natal in south africa. Molecules. 2021;26:3387. doi: 10.3390/molecules26113387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Porte A., Godoy R.L.O. Chemical composition of thymus vulgaris l. (thyme) essential oil from the rio de janeiro state (brazil) J. Serbian Chem. Soc. 2008;73:307–310. doi: 10.2298/JSC0803307P. [DOI] [Google Scholar]
- 72.Pokajewicz K., Bialon M., Svydenko L., Fedin R., Hudz N. Chemical composition of the essential oil of the new cultivars of lavandula angustifolia mill. Bred in ukraine. Molecules. 2021;26:5681. doi: 10.3390/molecules26185681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cebi N., Erarslan A. Determination of the antifungal, antibacterial activity and volatile compound composition of citrus bergamia peel essential oil. Foods. 2023;12:203. doi: 10.3390/foods12010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Behbahani B.A., Falah F., Arab F.L., Vasiee M., Yazdi F.T. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of cinnamomum zeylanicum bark essential oil. Evid.-Based Complement. Altern. Med. 2020;2020:5190603. doi: 10.1155/2020/5190603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamel D.G., Mansour A.I.A., El-Diin M.A.H.N., Hammam A.R.A., Mehta D., Abdel-Rahman A.M. Using rosemary essential oil as a potential natural preservative during stirred-like yogurt making. Foods. 2022;11:1993. doi: 10.3390/foods11141993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taherpour A.A., Khaef S., Yari A., Nikeafshar S., Fathi M., Ghambari S. Chemical composition analysis of the essential oil of mentha piperita l. From kermanshah, iran by hydrodistillation and hs/spme methods. J. Anal. Sci. Technol. 2017;8:11. doi: 10.1186/s40543-017-0122-0. [DOI] [Google Scholar]
- 77.Indriyani N.N., Al Anshori J., Permadi N., Nurjanah S., Julaeha E. Bioactive components and their activities from different parts of citrus aurantifolia (christm.) swingle for food development. Foods. 2023;12:2036. doi: 10.3390/foods12102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin L.Y., Chuang C.H., Chen H.C., Yang K.M. Lime (Citrus aurantifolia (christm.) swingle) essential oils: Volatile compounds, antioxidant capacity, and hypolipidemic effect. Foods. 2019;8:398. doi: 10.3390/foods8090398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao S.J., Liu G.Z., Li J.G., Chen J., Li L.N., Li Z., Zhang X.L., Zhang S.M., Thorne R.F., Zhang S.Z. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of staphylococcus aureus and candida species. Front. Cell. Infect. Microbiol. 2020;10:603858. doi: 10.3389/fcimb.2020.603858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Motelica L., Ficai D., Oprea O.C., Ficai A., Ene V.L., Vasile B.S., Andronescu E., Holban A.M. Antibacterial biodegradable films based on alginate with silver nanoparticles and lemongrass essential oil-innovative packaging for cheese. Nanomaterials. 2021;11:2377. doi: 10.3390/nano11092377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zehra A., Wani S.M., Jan N., Bhat T.A., Rather S.A., Malik A.R., Hussain S.Z. Development of chitosan-based biodegradable films enriched with thyme essential oil and additives for potential applications in packaging of fresh collard greens. Sci. Rep. 2022;12:16923. doi: 10.1038/s41598-022-20751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin J., Naskar A., Ko D., Kim S., Kim K.S. Bioconjugated thymol-zinc oxide nanocomposite as a selective and biocompatible antibacterial agent against staphylococcus species. Int. J. Mol. Sci. 2022;23:6770. doi: 10.3390/ijms23126770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osaili T.M., Albiss B.A., Al-Nabulsi A.A., Alromi R.F., Olaimat A., Al-Holy M., Savvaidis I., Holley R. Effects of metal oxide nanoparticles with plant extract on viability of foodborne pathogens. J. Food Saf. 2019;39:e12681. doi: 10.1111/jfs.12681. [DOI] [Google Scholar]
- 84.Abdollahzadeh E., Hosseini H.M., Fooladi A.A.I. Antibacterial activity of agar-based films containing nisin, cinnamon eo, and zno nanoparticles. J. Food Saf. 2018;38:e12440. doi: 10.1111/jfs.12440. [DOI] [Google Scholar]
- 85.Sami R. Effects of chitosan-zinc oxide nano coating supplemented with bergamot essential oil on postharvest shelf life of table grapes (Vitisvinifera L., red globe) Mater. Express. 2023;13:89–97. doi: 10.1166/mex.2023.2325. [DOI] [Google Scholar]
- 86.Radulescu M., Andronescu E., Cirja A., Holban A.M., Mogoanta L., Balseanu T.A., Catalin B., Neagu T.P., Lascar I., Florea D.A., et al. Antimicrobial coatings based on zinc oxide and orange oil for improved bioactive wound dressings and other applications. Rom. J. Morphol. Embryol. 2016;57:107–114. [PubMed] [Google Scholar]
- 87.Sepulveda F.A., Rivera F., Loyo C., Canales D., Moreno-Serna V., Benavente R., Rivas L.M., Ulloa M.T., Gil-Castell O., Ribes-Greus A., et al. Poly (lactic acid)/d-limonene/zno bio-nanocomposites with antimicrobial properties. J. Appl. Polym. Sci. 2022;139:51542. doi: 10.1002/app.51542. [DOI] [Google Scholar]
- 88.Jiang L.H., Han Y.H., Xu J., Wang T. Preparation and study of cellulose-based zno nps@hec/c-?-cd/menthol hydrogel as wound dressing. Biochem. Eng. J. 2022;184:108488. doi: 10.1016/j.bej.2022.108488. [DOI] [Google Scholar]
- 89.Chiriac A.P., Stoleru E., Rosca I., Serban A., Nita L.E., Rusu A.G., Ghilan A., Macsim A.M., Mititelu-Tartau L. Development of a new polymer network system carrier of essential oils. Biomed. Pharmacother. 2022;149:112919. doi: 10.1016/j.biopha.2022.112919. [DOI] [PubMed] [Google Scholar]
- 90.Rapa M., Gaidau C., Mititelu-Tartau L., Berechet M.D., Berbecaru A.C., Rosca I., Chiriac A.P., Matei E., Predescu A.M., Predescu C. Bioactive collagen hydrolysate-chitosan/essential oil electrospun nanofibers designed for medical wound dressings. Pharmaceutics. 2021;13:1939. doi: 10.3390/pharmaceutics13111939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiriac A.P., Rusu A.G., Nita L.E., Macsim A.M., Tudorachi N., Rosca I., Stoica I., Tampu D., Aflori M., Doroftei F. Synthesis of poly(ethylene brassylate-co-squaric acid) as potential essential oil carrier. Pharmaceutics. 2021;13:477. doi: 10.3390/pharmaceutics13040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.