Abstract
Low 25-Hydroxyvitamin D (25(OH)D) in preterm infants is a risk factor for bronchopulmonary dysplasia (BPD), but increased supplementation failed to demonstrate a beneficial effect on BPD. In neonatal animal models, deficiency and excessive vitamin D exposure have been associated with increased mortality and histological alterations in the lung evocative of BPD. Our hypothesis is that 25(OH)D levels ≥ 120 nmol/L are also a risk factor for BPD or death. This retrospective single-center cohort study included only infants born at <31 weeks gestational age without major malformations with at least a determination of 25(OH)D at <36 weeks corrected age and no determination <50 nmol/L. Routine 25(OH)D determination was performed at 1 month and monthly thereafter. A total of 175 infants were included. Infants with BPD or who died had a significantly lower term and weight, but a similar frequency of 25(OH)D ≥120 nmol/L (50.5% vs. 43.9%, p = 0.53). The logistic regression identified weight (OR 0.997, 95% CI [0.995–0.998]) and term (OR 0.737, 95% CI [0.551–0.975]) as significantly associated with BPD or death; the occurrence of excessive 25(OH)D was not significantly associated (OR 1.029, 95% CI [0.503–2.093]). The present study did not demonstrate any significant association between excessive 25(OH)D after one month of age and BPD or death.
Keywords: vitamin D, premature infants, bronchopulmonary dysplasia, low-birthweight infant, very-low-birthweight infant
1. Introduction
Bronchopulmonary dysplasia (BPD) is a frequent and sometimes severe complication of premature infants with long-term consequences [1]. Vitamin D is implicated in lung development, as demonstrated by multiple animal studies in rodents [2], and supplementation with low doses of native vitamin D in rodent pups exposed to hyperoxia is reported to attenuate the histological and some biochemical markers of BPD [3]. Low concentrations of 25-Hydroxyvitamin D (25(OH)D) at birth and at one month of age have been associated with increased risk of BPD as demonstrated by a meta-analysis [4] and a study adjusting for the factors known to be associated with BPD [5]. Furthermore, low 25(OH)D at one month of age has also been associated with increased risk of bronchopulmonary dysplasia [6]. However, studies investigating high-dose supplementation (compared with low-dose) failed to demonstrate any significant effect on the frequency of BPD [7,8] but have found high 25(OH)D concentrations in the groups exposed to high doses [8,9,10]. The data on the consequence of vitamin D excess in this population (except for the risk of nephrocalcinosis and/or hypercalcemia) are sparse [11]. In rodents receiving vitamin D in excess during gestation and lactation, pups had an abnormal lung histology; there was a greater mean linear intercept, a greater total respiratory system resistance, and a lower basal proliferation of their lung mesenchymal stem cells with a lower adipogenic and a greater myogenic potential [12,13]. Furthermore, in a model of bronchopulmonary dysplasia, neonatal pups exposed to oxygen receiving high doses of 1,25-di-Hydroxyvitamin D (1,25(OH)2D) from the first day of life exhibited higher mortality and an altered lung histology (increased mean linear intercept, a decreased angiogenesis, and increased proinflammatory factors) when compared with animals receiving low doses [14]. Recent studies have demonstrated a high frequency of excessive levels of 25(OH)D in preterm infants with supplementation recommended at that time [15,16,17]. Our hypothesis was that these excessive 25(OH)D levels in very and extremely preterm infants may be deleterious to pulmonary development and may therefore be implicated in the pathogenesis of BPD. The primary objective of this study was, therefore, to determine whether excessive 25(OH)D levels are an independent risk factor for BPD or death.
2. Materials and Methods
In this retrospective cohort study, all infants born at <31 weeks gestational age between January 2018 and December 2019 were eligible for inclusion if they were hospitalized before 3 days of life and for at least 10 days in the neonatal intensive care unit (NICU) in the Hospital Femme Mere Enfant, Bron, France, and presented no major congenital malformation. They were included if they had at least a 25(OH) D determination at <36 weeks corrected age. They were excluded if they presented at least a 25(OH)D determination <50 nmol/L.
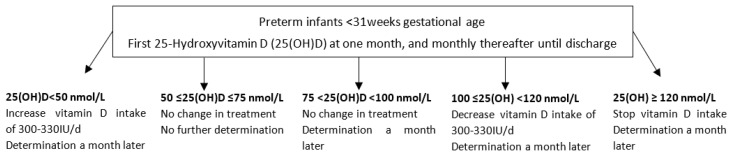
In this NICU, preterm infants receiving parenteral nutrition were supplemented with Cernevit (Baxter, Guyancourt, France) in an amount of ¼ vial daily (containing 55 IU cholecalciferol). When parenteral nutrition was stopped, infants with a weight below 1 kg received Sterogyl (DB pharma, La Varenne-St-Hilaire, France) in an amount of 3 drops daily (1200 IU ergocalciferol) while infants above 1 kg received Uvesterol ADEC (Crinex, Montrouge, France) in an amount of 0.3 mL daily (containing 1000 IU ergocalciferol). These supplementations were following or slightly above the European Society of Paediatric Gastroenterology, Hepatology and Nutrition’s recommendations at that time of 800–1000 IU daily during enteral nutrition and more than 30 IU during parenteral nutrition [18,19]. A routine determination of 25(OH)D was recommended in our unit at one month of age and monthly thereafter until discharge with a protocol for adaptation of the dose (Figure 1). The objective was to maintain 25(OH)D ≥ 50 and < 120 nmol/L.
Figure 1.
Local protocol of adaptation of vitamins in extremely and very preterm infants.
The main outcome was BPD or death at 36 weeks corrected age. BPD was defined as the need for supplemental oxygen or respiratory support to maintain a saturation equal to or above 90% at 36 weeks corrected age with radiological evidence of parenchymal lung disease [20]. In the description of the population, it was classified into three grades according to Jobe et al. [21].
The main early predictive factors of BPD reported in recent studies were collected [22,23,24,25]: multiple gestations, antenatal corticosteroids, spontaneous delivery, gestational age at birth, birthweight, Apgar at 5 min (in categories 8 to 10, 4 to 7, 0 to 3), sex, respiratory support during the first 24 h (classified in 3 groups—mild FiO2 < 30% and noninvasive ventilation, moderate FiO2 < 30% and mechanical ventilation, severe FiO2 ≥ 30% and mechanical ventilation—as proposed by Baud et al. [22]), and breastfeeding defined as receiving any mother’s milk. Ethnic origin was not available; however, Baud et al. excluded it from their final predictive model in a French population [22].
“Small for gestational age” was defined as a weight below the tenth percentile according to the Fenton curves [26]. Enterocolitis was considered present if a grade of 2 or above was observed.
Data were extracted from electronic medical charts (IntelliSpace Critical Care and Anesthesia prescription software, Philips, Suresne, France) and completed with the discharge letter when infants were transferred to another hospital or another unit.
The number of subjects was calculated based on the unpublished results of a pilot study [27]. Based on the results of the multiple logistic regression simulation taking into account confounding parameters (term, spontaneous birth, and sex) and excessive 25(OH)D concentration, the number of infants necessary to find an OR of 2.8 for BPD and a 25(OH)D concentration association was 176, with a power of 80% and an alpha risk of 0.05.
The quantitative variables were described using the mean and standard deviation (SD), and qualitative variables using the number of patients and frequency (%) of each modality.
Patients were stratified according to the maximal 25(OH)D concentration between 1 month of life and 36 weeks corrected age (excessive [any determination ≥ 120 nmol/L] [28] or normal [all determinations ≥50 to <120 nmol/L]). Patients with BPD or who died at 36 weeks corrected age were compared with other patients using the Wilcoxon or chi-squared tests, as appropriate. The analysis of the association between BPD and excessive 25(OH)D concentration was investigated using a logistic regression model constructed using backward stepwise selection.
25(OH)D concentration was measured using a chemiluminescent microparticle immunoassay with an Isys analyzer (Immunodiagnostic Systems, Pouilly-en-Auxois, France).
This study was approved by the institutional review board (Comité Scientifique et Éthique) of the Hospices Civils de Lyon on 18 January 2023 (number 23_076). It also received the approval of the national data protection commission (Commission Nationale de l’Informatique et des Libertés; number 23_5076). According to French law, parental informed consent was not necessary, but all parents were informed and could refuse the participation of their infant.
This study is registered in ClinicalTrials.gov/study/NCT05944055 (accessed on 17 July 2023).
3. Results
3.1. Population
3.1.1. Study Flow-Chart
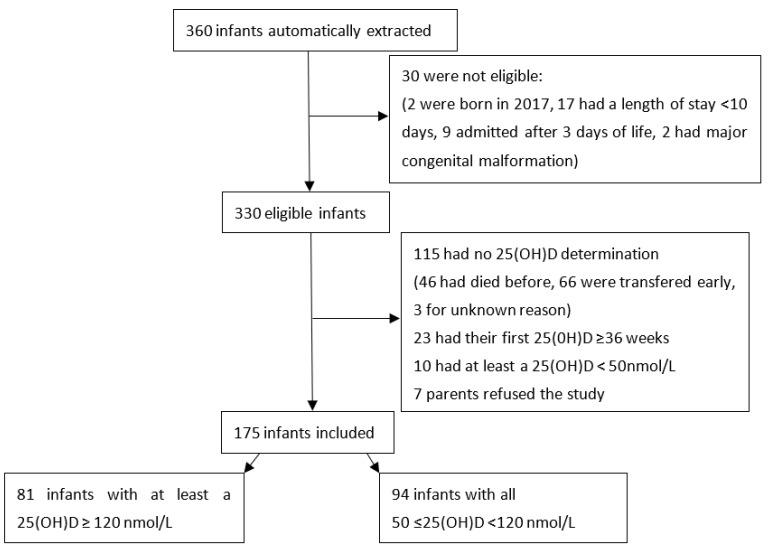
The study flow-chart is presented in Figure 2.
Figure 2.
Study flow-chart.
3.1.2. Description of the Population
A total of 175 infants were included, of which 81 (46.3%) had at least one 25(OH)D ≥ 120 nmol/L, and for the remaining 94 (53.7%) 25(OH)D was always between 50 and 120 nmol/L. The obstetrical characteristics of the included population and according to 25(OH)D concentration are described in Table 1.
Table 1.
Main obstetrical characteristics of the studied population.
| Pregnancy Characteristics * | Excessive 25(OH)D N = 81 |
Normal 25(OH)D N = 94 |
Total N = 175 |
|
|---|---|---|---|---|
| Parity | 1 | 29 (35.8%) | 40 (42.6%) | 69 (39.4%) |
| 2 | 25 (30.9%) | 31 (33.0%) | 56 (32.0%) | |
| 3 | 19 (23.5%) | 14 (14.9%) | 33 (18.9%) | |
| ≥4 | 8 (9.9%) | 8 (8.5%) | 16 (9.1%) | |
| Unknown | 0 (0.0%) | 1 (1.1%) | 1 (0.6%) | |
| Multiple pregnancy | 30 (37.5%) | 25 (26.6%) | 56 (32.0%) | |
| Any hypertension during pregnancy | 19 (23.5%) | 21 (22.3%) | 40 (22.9%) | |
| Preterm premature rupture of membranes | 26 (32.1%) | 27 (28.7%) | 53 (30.3%) | |
| Any diabetes during pregnancy | 8 (9.9%) | 13 (13.8%) | 21 (12.0%) | |
| Histological chorioamnionitis | 21 (25.9%) | 13 (13.8%) | 34 (19.4%) | |
| Unavailable | 0 (0%) | 3 (3.2%) | 3 (1.7%) | |
| Clinical chorioamnionitis | 11 (13.6%) | 7 (7.4%) | 18 (10.3%) | |
| Unknown | 1/1.2%) | 3 (3.2%) | 4 (2.3%) | |
| Any antenatal corticosteroids | 76 (93.8%) | 83 (88.3%) | 159 (90.9%) |
* Data were available for all infants except if specified.
The main neonatal characteristics of the included population and according to 25(OH)D concentration are presented in Table 2.
Table 2.
Main neonatal characteristics of the studied population.
| Neonatal Characteristics * | N (%), Except if Specified | Excessive 25(OH)D N = 81 | Normal 25(OH)D N = 94 |
Total N = 175 |
|
|---|---|---|---|---|---|
| Birth season | Summer | 19 (23.5%) | 32 (34.0%) | 51 (29.1%) | |
| Fall | 20 (24.7%) | 26 (27.7%) | 46 (26.3%) | ||
| Winter | 21 (25.9%) | 18 (19.1%) | 39 (22.3%) | ||
| Spring | 21 (25.9%) | 18 (19.1%) | 39 (22.3%) | ||
| Sex | Male | 39 (48.1%) | 51 (54.3%) | 90 (51.4%) | |
| Term (weeks) | Mean (SD) | 27.58 (1.84) | 27.88 (1.56) | 27.74 (1.70) | |
| Weight (g) | Mean (SD) | 938 (272) | 998 (305) | 970 (291) | |
| Height (cm) | Mean (SD) | 35.05 (3.11) | 35.15 (3.98) | 35.11 (3.60) | |
| Head circumference (cm) | Mean (SD) | 24.83 (2.19) | 25.44 (2.72) | 25.16 (2,50) | |
| Small for gestational age ** | 14 (17.3%) | 17 (18.1%) | 31 (17.7%) | ||
| Apgar at 5 min | 8–10 | 55 (67.9%) | 52 (55.3%) | 107 (61.1%) | |
| 4–7 | 21 (25.9%) | 37 (39.4%) | 58 (33.1%) | ||
| 0–3 | 4 (4.9%) | 4 (4.3%) | 8 (4.6%) | ||
| Not available | 1 (1.2%) | 1 (1.1%) | 2 (1.1%) | ||
| Maximum ventilation during the first 24 h | FiO2 < 30% and noninvasive ventilation | 7 (8.6%) | 6 (6.4%) | 13 (7.4%) | |
| Assisted ventilation and FiO2 < 30% | 0 (0.0%) | 1 (1.1%) | 1 (0.6%) | ||
| Assisted ventilation or FiO2≥ 30% | 74 (91.4%) | 87 (92.6%) | 161 (92.0%) | ||
| Parenteral nutrition (days) | Median | 14 | 13 | 14 | |
| Interquartile range | 7–23 | 7–20 | 7–21 | ||
| Enteral feeding | Maternal or donor milk | 17 (21.0%) | 19 (20.2%) | 36 (20.6%) | |
| Mixed | 50 (61.7%) | 49 (52.1%) | 99 (56.6%) | ||
| Formula or donor milk | 14 (17.3%) | 26 (27.7%) | 40 (22.9%) | ||
| Any mother’s milk given | 66 (81.5%) | 68 (72.3%) | 134 (76.6%) | ||
| First determination of 25(OH)D (nmol/L) | Mean (SD) | 139.4 (43.1) | 86.1 (19.6) | 110.8 (42.1) | |
| Corrected age at first 25(OH)D determination | Mean (SD) | 32.4 (1.9) | 32.5 (1.5) | 32.4 (1.7) | |
| Second determination of 25(OH)D (nmol/L) | 25 (30.9%) | 20 (21.3%) | 45 (25.7%) | ||
| Mean (SD) | 144.40 (29.58) | 85.20 (17.76) | 118.1 (38.7) | ||
| Corrected age at second determination of 25(OH)D | Mean (SD) | 34.2 (1.2) | 34.2 (1.5) | 34.2 (1.3) | |
* Data were available for all infants except if specified. ** Weight below the tenth percentile according to Fenton curves [26]. SD: standard deviation.
3.2. Outcomes
The main outcomes of the cohort according to 25(OH)D concentration are presented in Table 3.
Table 3.
Main outcomes of the studied population.
| Outcomes * | N (%), Except if Specified | Excessive 25(OH)D N = 81 | Normal 25(OH)D N = 94 |
Total N = 175 |
|---|---|---|---|---|
| Intraventricular hemorrhage | 1 | 6 (7.4%) | 11 (11.7%) | 17 (9.7%) |
| 2 | 2 (2.5%) | 4 (4.3%) | 6 (3.4%) | |
| 3 | 1 (1.2%) | 1 (1.1%) 4 (4.3%) |
2 (1.1%) | |
| 4 | 3 (3.7%) | 4 (4.3%) | 7 (4.0%) | |
| Cystic periventricular leukomalacia | 0 (0.0%) | 5 (5.3%) | 5 (2.9%) | |
| Retinopathy of prematurity | 1 | 9 (11.1%) | 15 (16.0%) | 24 (13.7%) |
| 2 | 18 (22.2%) | 14 (14.9%) | 32 (18.3%) | |
| 3 | 7 (8.6%) | 5 (5.3%) | 12 (6.9%) | |
| Unknown | 9 (11.1%) | 13 (13.8%) | 22 (12.6%) | |
| PDA requiring treatment | 22 (27.2%) | 30 (31.9%) | 52 (29.7%) | |
| Enterocolitis grade ≥2 | 2 | 0 (0.0%) | 1 (1.1%) | 1 (0.6%) |
| 3 | 0 (0.0%) | 2 (2.1%) | 2 (1.1%) | |
| Number of sepsis events | 1 | 23 (28.4%) | 19 (20.2%) | 42 (24.0%) |
| 2 | 4 (4.9%) | 5 (5.3%) | 9 (5.1%) | |
| 3 | 1 (1.2%) | 1 (1.1%) | 2 (1.1%) | |
| BPD grade | No BPD | 18 (22.8%) | 22 (23.9%) | 40 (23.4%) |
| Mild | 29 (36.7%) | 38 (41.3%) | 67 (39.2%) | |
| Moderate | 24 (30.4%) | 31 (33.7%) | 55 (32.2%) | |
| Severe | 8 (10.1%) | 1 (1.1%) | 9 (5.3%) | |
| Moderate or severe BPD or death | 34 (42.0%) | 34 (36.2%) | 68 (38.9%) | |
| Moderate or severe BPD | 32 (39.5%) | 32 (34.0%) | 64 (36.6%) | |
| Death before 36 weeks corrected age | 2 (2.5%) | 2 (2.1%) | 4 (2.3%) |
* Data were available for all infants except if specified. BPD: bronchopulmonary dysplasia; PDA: persistent ductus arteriosus.
3.3. Analysis
Univariate analysis found that term (BPD or death: median 26.50, interquartile range (25.57–27.79) vs. no BPD or death: median 28.29 interquartile range (27.36–29.43), p < 0.001) and weight (BPD or death: median 775 g, interquartile range (635–892) vs. no BPD or death: median 1050 g, interquartile range (900–1232), p < 0.001) were significantly different between infants with BPD or death and those without. The occurrence of 25(OH)D ≥ 120 nmol/l (50.0% vs. 43.9%, p = 0.53) was not significantly different between the two groups (with BPD or death and without). Multiple pregnancy, Apgar score, sex, any mother’s milk given, spontaneous birth, and maximum ventilation during the first 24 h of life were not significantly different between groups.
The results of the multivariable analysis are presented in Table 4 with the full saturated model and the final model. In the final model, term (OR 0.737, 95% CI [0.551–0.975], p = 0.035) and weight (OR 0.997, 95% CI [0.995–0.998], p = 0.001) were significantly associated with BPD or death; there was no significant association with any 25(OH)D determination ≥120 nmol/L (OR 1.029, 95% CI [0.503–2.093], p = 0.936).
Table 4.
Multivariable analysis to evaluate the risk of bronchopulmonary dysplasia or death taking into account the classically described factors and the occurrence of a 25(OH)D ≥120 nmol/L.
| Variables | OR | 95%CI * | p-Value | |
|---|---|---|---|---|
| Full model | ||||
| Any 25(OH)D determination ≥ 120 nmol/L | 1.011 | 0.475–2.145 | 0.977 | |
| Weight (g) | 0.997 | 0.994–0.999 | 0.019 | |
| Term (weeks) | 0.661 | 0.436–0.989 | 0.046 | |
| Multiple pregnancy | 1.881 | 0.855–4.248 | 0.120 | |
| Sex, female | 0.530 | 0.242–1.134 | 0.106 | |
| Maximal ventilation during the first 24 h | 0.838 | 0.210–3.743 | 0.806 | |
| Any mother’s milk administrated | 1.237 | 0.520–3.011 | 0.634 | |
| Apgar at 5 min | 8–10 | 1.000 | ||
| 4–7 | 0.912 | 0.402–2.041 | 0.823 | |
| 0–3 | 0.795 | 0.147–4.108 | 0.782 | |
| Spontaneous birth | 0.631 | 0.223–1.770 | 0.381 | |
| Final model | ||||
| Any 25(OH)D determination ≥ 120 nmol/L | 1.029 | 0.503–2.093 | 0.936 | |
| Weight (g) | 0.997 | 0.995–0.998 | 0.001 | |
| Term (weeks) | 0.737 | 0.551–0.975 | 0.035 | |
* 95% CI: 95% confidence interval.
A post hoc analysis was performed to evaluate whether the occurrence of a 25(OH)D above 150 nmol/L was associated with the occurrence of BPD or death. Again, only term (OR 0.736, 95% CI [0.551–0.971], p = 0.033) and weight (OR 0.997, 95% CI [0.995–0.999], p = 0.001) were significantly associated; there was no significant effect of 25(OH)D >150 nmol/L (OR 1.291, 95% CI [0.558–2.982], p = 0.548).
4. Discussion
As reported herein, a high frequency of excessive 25(OH)D levels with high enteral intakes of vitamin D has been reported [15,16,17] and the recommendations from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition have been updated with a decreased recommended intake during enteral nutrition (400–700 IU daily) and an increased recommended intake during parenteral nutrition [29,30].
Unlike the results obtained in an animal model [12,13,14], the present study did not find that excessive 25(OH)D concentration was a risk factor for BPD or death. This result may be related to the temporality of the excessive concentration, as in animal studies native vitamin D was administrated throughout the gestation period [12,13] or 1,25(OH)2D was administrated immediately at birth [14]. With such early administration, the lungs are more immature and their development may be severely impaired. We chose to study 25(OH)D at the first month because the frequency of excessive concentrations at birth in preterm infants is very low [31,32,33]. In France, Courbebaisse et al. reported that in the general population of newborns, 93% of cord blood concentrations were below 75 nmol/L [34], and Papalia et al. reported that in infants born below 29 weeks gestational age, 74% had a cord blood concentration ≤75 nmol/L [35]. In France, the current recommendation for vitamin D supplementation during pregnancy is to administer 100,000 IU once during the seventh month of pregnancy. This recommendation was followed in 88% of the pregnant patients in a recent large cohort study [34] and may explain these results. Furthermore, the vitamin D intake during parenteral nutrition was low herein (55 IU daily), and the median duration of parenteral nutrition was 14 days. Taking into account these aspects and the results of the study reported by Fort et al. (who described the increase in 25(OH)D in preterm infants receiving 200, 400, and 1000 IU daily) [9], we estimated that the risk of early excessive concentration was low in the study population.
The upper limit of normal 25(OH)D was established in accordance with the current recommendation of the European Society for Paediatric Nephrology for infants with chronic kidney disease [28,36] and recent French and European recommendations for preterm infants [29,37]. It was justified by an increase in mortality with higher concentrations in the general population [38,39]. This threshold is reinforced by the results of a case series study that identified 16 preterm infants referred to nephrology clinics for symptomatic hypervitaminosis D with 25(OH)D concentrations between 119 and 350 nmol/L [11]. In two previous studies, higher concentrations were associated with a high frequency of hypercalciuria [15,16]. However, it remains possible that the effect of excessive 25(OH)D concentration on lung development necessitates concentrations above 120 nmol/L, although the results of the post hoc analysis with concentrations above 150 nmol/L do not support this hypothesis.
The next factor that could explain the discrepancy between the results observed in the animal model reported by Chen et al. [14] and the present study is that in this animal study the active form of vitamin D, namely 1,25(OH)2D, was used, whereas native vitamin D was used herein, according to clinical practice. Using 1,25(OH)2D, the physiological regulation of the production of 1,25(OH)2D is circumvented, even though the limiting factor of this synthesis as classically described is the availability of 25(OH)D in preterm infants [40], but the regulation in extremely and very preterm infants and in particular the function of the C3 epimers are still not fully elucidated [41,42]. The results of the studies reported by Wang et al. and Mandell et al. in rodent pups exposed to hyperoxia receiving native vitamin D demonstrated improved lung histology but 25(OH)D was measured at low and normal levels, not allowing conclusions on supraphysiological doses [3,43]. However, the results reported by Yurt et al. and Sakurai et al. using supraphysiological doses of native vitamin D during rat gestation demonstrated deleterious consequences on the lung with high doses even without oxygen exposure [12,13].
The main limitation of this study is the absence of determination of 25(OH)D at birth; some infants from both groups may have experienced an early deficiency in 25(OH)D, which is a recognized risk factor for BPD [4,6], and they may not be equally distributed between groups, decreasing the difference between groups for the primary outcome. Despite this limitation, these results are important because they show that in the absence of an early determination of 25(OHD), a 25(OH)D concentration above 120 nmol/L before 36 weeks corrected age is not a significant risk factor for BPD or death. In addition, there does not seem to be a great difference in terms of morbidity according to 25(OH)D concentration herein, although this was not formally tested to avoid multiplicity of comparisons. Another limitation is the retrospective nature of this study. Some variables such as ethnicity which are known to interfere with vitamin D metabolism [8] and BPD frequency [44] were not available, and the risk of bias was increased.
Further studies are necessary to determine the appropriate modalities of administration of native vitamin D in extremely and very preterm infants as it is a modifiable factor that could impact the risk of BPD [4,6] and the risk of sepsis [45,46,47,48], two essential factors for the future of premature infants, but also nephrological and bone-related outcomes. The prevention of vitamin D deficiency at birth and the effectiveness of treatment with native vitamin D in infants with depleted and normal levels at birth should be evaluated with a careful monitoring of respiratory and infectious outcomes. Retrospective studies may give us clues (indication and dosage regimen) for further randomized control trials.
Acknowledgments
We acknowledge Frank Plaisant for his help in extracting data from electronic medical charts. We thank Philip Robinson (DRS, Hospices Civils de Lyon) for helping with manuscript preparation.
Author Contributions
Conceptualization, S.L. and J.B.; methodology, M.B. (Maxime Bonjour). and S.L.; validation, S.L. and M.B. (Maxime Bonjour); formal analysis, M.B. (Maxime Bonjour); investigation, M.M. and S.L.; data curation, M.M. and S.L.; writing—original draft preparation, S.L.; writing—review and editing, S.L., M.B. (Maxime Bonjour), M.M., J.B. and M.B. (Marine Butin); visualization, S.L. and M.B. (Maxime Bonjour); supervision, M.B. (Marine Butin) and J.B.; project administration, M.B. (Marine Butin). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Hospices civils de Lyon (protocol code 23_076 and date of approval: 18 January 2023).
Informed Consent Statement
Parental consent was waived due to the French law regarding retrospective studies. However, according to the law, the parents were informed by a letter or an email and could refuse to participate in the study, which was taken into account.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cheong J.L.Y., Doyle L.W. An Update on Pulmonary and Neurodevelopmental Outcomes of Bronchopulmonary Dysplasia. Semin. Perinatol. 2018;42:478–484. doi: 10.1053/j.semperi.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Lykkedegn S., Sorensen G.L., Beck-Nielsen S.S., Christesen H.T. The Impact of Vitamin D on Fetal and Neonatal Lung Maturation. A Systematic Review. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015;308:L587–L602. doi: 10.1152/ajplung.00117.2014. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Jiang L. Role of Vitamin D–Vitamin D Receptor Signaling on Hyperoxia-induced Bronchopulmonary Dysplasia in Neonatal Rats. Pediatr. Pulmonol. 2021;56:2335–2344. doi: 10.1002/ppul.25418. [DOI] [PubMed] [Google Scholar]
- 4.Park H.W., Lim G., Park Y.-M., Chang M., Son J.S., Lee R. Association between Vitamin D Level and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. PLoS ONE. 2020;15:e0235332. doi: 10.1371/journal.pone.0235332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byun S.Y., Bae M.H., Lee N.R., Han Y.M., Park K.H. Association between Vitamin D Deficiency at One Month of Age and Bronchopulmonary Dysplasia. Medicine. 2021;100:e27966. doi: 10.1097/MD.0000000000027966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H., Fu J., Feng Y. Utility of Umbilical Cord Blood 25-Hydroxyvitamin D Levels for Predicting Bronchopulmonary Dysplasia in Preterm Infants with Very Low and Extremely Low Birth Weight. Front. Pediatr. 2022;10:956952. doi: 10.3389/fped.2022.956952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y., Li Z., Yan G., Jie Q., Rui C. Effect of Different Doses of Vitamin D Supplementation on Preterm Infants—An Updated Meta-Analysis. J. Matern. Fetal Neonatal Med. 2018;31:3065–3074. doi: 10.1080/14767058.2017.1363731. [DOI] [PubMed] [Google Scholar]
- 8.Aristizabal N., Holder M.P., Durham L., Ashraf A.P., Taylor S., Salas A.A. Safety and Efficacy of Early Vitamin D Supplementation in Critically Ill Extremely Preterm Infants: An Ancillary Study of a Randomized Trial. J. Acad. Nutr. Diet. 2023;123:87–94. doi: 10.1016/j.jand.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Fort P., Salas A.A., Nicola T., Craig C.M., Carlo W.A., Ambalavanan N. A Comparison of 3 Vitamin D Dosing Regimens in Extremely Preterm Infants: A Randomized Controlled Trial. J. Pediatr. 2016;174:132–138.e1. doi: 10.1016/j.jpeds.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natarajan C.K., Sankar M.J., Agarwal R., Pratap O.T., Jain V., Gupta N., Gupta A.K., Deorari A.K., Paul V.K., Sreenivas V. Trial of Daily Vitamin D Supplementation in Preterm Infants. Pediatrics. 2014;133:e628–e634. doi: 10.1542/peds.2012-3395. [DOI] [PubMed] [Google Scholar]
- 11.Vierge M., Laborie S., Bertholet-Thomas A., Carlier M.-C., Picaud J.-C., Claris O., Bacchetta J. Intoxication néonatale à la vitamine D chez des anciens prématurés: Une série de 16 cas. Arch. Pédiatrie. 2017;24:817–824. doi: 10.1016/j.arcped.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai R., Singh H., Wang Y., Harb A., Gornes C., Liu J., Rehan V.K. Effect of Perinatal Vitamin D Deficiency on Lung Mesenchymal Stem Cell Differentiation and Injury Repair Potential. Am. J. Respir. Cell Mol. Biol. 2021;65:521–531. doi: 10.1165/rcmb.2020-0183OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yurt M., Liu J., Sakurai R., Gong M., Husain S.M., Siddiqui M.A., Husain M., Villarreal P., Akcay F., Torday J.S., et al. Vitamin D Supplementation Blocks Pulmonary Structural and Functional Changes in a Rat Model of Perinatal Vitamin D Deficiency. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014;307:L859–L867. doi: 10.1152/ajplung.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C., Weng H., Zhang X., Wang S., Lu C., Jin H., Chen S., Liu Y., Sheng A., Sun Y. Low-Dose Vitamin D Protects Hyperoxia-Induced Bronchopulmonary Dysplasia by Inhibiting Neutrophil Extracellular Traps. Front. Pediatr. 2020;8:335. doi: 10.3389/fped.2020.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laborie S., Denis A., Raverot V., Claris O., Bacchetta J., Butin M. A Third of Premature Neonates Displayed Inadequate 25-hydroxyvitamin D Levels before Being Discharged from a French Neonatal Intensive Care Unit. Acta Paediatr. 2022;111:104–106. doi: 10.1111/apa.16126. [DOI] [PubMed] [Google Scholar]
- 16.Mathilde M., Butin M., Pascal R., Plaisant F., Laborie S., Bacchetta J. Local Protocol Helped to Deliver Vitamin D Levels More Accurately in Preterm Infants. Acta Paediatr. 2022;111:76–85. doi: 10.1111/apa.16088. [DOI] [PubMed] [Google Scholar]
- 17.Kołodziejczyk-Nowotarska A., Bokiniec R., Seliga-Siwecka J. Monitored Supplementation of Vitamin D in Preterm Infants: A Randomized Controlled Trial. Nutrients. 2021;13:3442. doi: 10.3390/nu13103442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agostoni C., Buonocore G., Carnielli V., De Curtis M., Darmaun D., Decsi T., Domellöf M., Embleton N., Fusch C., Genzel-Boroviczeny O., et al. Enteral Nutrient Supply for Preterm Infants: Commentary From the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010;50:85–91. doi: 10.1097/MPG.0b013e3181adaee0. [DOI] [PubMed] [Google Scholar]
- 19.Koletzko B., Goulet O., Hunt J., Krohn K., Shamir R., for the Parenteral Nutrition Guidelines Working Group 8 Vitamins. J. Pediatr. Gastroenterol. Nutr. 2005;41:S47–S53. doi: 10.1097/01.mpg.0000181848.39370.b3. [DOI] [PubMed] [Google Scholar]
- 20.Higgins R.D., Jobe A.H., Koso-Thomas M., Bancalari E., Viscardi R.M., Hartert T.V., Ryan R.M., Kallapur S.G., Steinhorn R.H., Konduri G.G., et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018;197:300–308. doi: 10.1016/j.jpeds.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobe A.H., Bancalari E. Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 22.Baud O., Laughon M., Lehert P. Survival without Bronchopulmonary Dysplasia of Extremely Preterm Infants: A Predictive Model at Birth. Neonatology. 2021;118:385–393. doi: 10.1159/000515898. [DOI] [PubMed] [Google Scholar]
- 23.Laughon M.M., Langer J.C., Bose C.L., Smith P.B., Ambalavanan N., Kennedy K.A., Stoll B.J., Buchter S., Laptook A.R., Ehrenkranz R.A., et al. Prediction of Bronchopulmonary Dysplasia by Postnatal Age in Extremely Premature Infants. Am. J. Respir. Crit. Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapcharoensap W., Gage S.C., Kan P., Profit J., Shaw G.M., Gould J.B., Stevenson D.K., O’Brodovich H., Lee H.C. Hospital Variation and Risk Factors for Bronchopulmonary Dysplasia in a Population-Based Cohort. JAMA Pediatr. 2015;169:e143676. doi: 10.1001/jamapediatrics.2014.3676. [DOI] [PubMed] [Google Scholar]
- 25.Spiegler J., Preuß M., Gebauer C., Bendiks M., Herting E., Göpel W., Bendiks M., Berghäuser M.A., Böckenholt K., Bohnhorst B., et al. Does Breastmilk Influence the Development of Bronchopulmonary Dysplasia? J. Pediatr. 2016;169:76–80.e4. doi: 10.1016/j.jpeds.2015.10.080. [DOI] [PubMed] [Google Scholar]
- 26.Fenton T.R., Kim J.H. A Systematic Review and Meta-Analysis to Revise the Fenton Growth Chart for Preterm Infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laborie S. Les Surdosages en 25 OHD Pendant les Premiers Mois de vie Sont-Ils un Facteur de Risque de Dysplasie Bronchopulmonaire? JFRN; Paris, France: 2022. [Google Scholar]
- 28.Shroff R., Wan M., Nagler E.V., Bakkaloğlu S., Fischer D.-C., Bishop N., Cozzolino M., Bacchetta J., Edefonti A., Stefanidis C.J., et al. Clinical Practice Recommendations for Native Vitamin D Therapy in Children with Chronic Kidney Disease Stages 2–5 and on Dialysis. Nephrol. Dial. Transplant. 2017;32:1098–1113. doi: 10.1093/ndt/gfx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Embleton N.D., Moltu S.J., Lapillonne A., van den Akker C.H.P., Carnielli V., Fusch C., Gerasimidis K., van Goudoever J.B., Haiden N., Iacobelli S., et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2022 doi: 10.1097/MPG.0000000000003642. ahead of print . [DOI] [PubMed] [Google Scholar]
- 30.Bronsky J., Campoy C., Braegger C., Braegger C., Bronsky J., Cai W., Campoy C., Carnielli V., Darmaun D., Decsi T., et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Vitamins. Clin. Nutr. 2018;37:2366–2378. doi: 10.1016/j.clnu.2018.06.951. [DOI] [PubMed] [Google Scholar]
- 31.Burris H.H., Van Marter L.J., McElrath T.F., Tabatabai P., Litonjua A.A., Weiss S.T., Christou H. Vitamin D Status among Preterm and Full-Term Infants at Birth. Pediatr. Res. 2014;75:75–80. doi: 10.1038/pr.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassai M.S., Cafeo F.R., Affonso-Kaufman F.A., Suano-Souza F.I., Sarni R.O.S. Vitamin D Plasma Concentrations in Pregnant Women and Their Preterm Newborns. BMC Pregnancy Childbirth. 2018;18:412. doi: 10.1186/s12884-018-2045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onwuneme C., Martin F., McCarthy R., Carroll A., Segurado R., Murphy J., Twomey A., Murphy N., Kilbane M., McKenna M., et al. The Association of Vitamin D Status with Acute Respiratory Morbidity in Preterm Infants. J. Pediatr. 2015;166:1175–1180.e1. doi: 10.1016/j.jpeds.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 34.Courbebaisse M., Souberbielle J.-C., Baptiste A., Taieb J., Tsatsaris V., Guibourdenche J., Senat M.-V., Haidar H., Jani J., Guizani M., et al. Vitamin D Status during Pregnancy and in Cord Blood in a Large Prospective French Cohort. Clin. Nutr. 2019;38:2136–2144. doi: 10.1016/j.clnu.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 35.Papalia H., Samonini A., Buffat C., Gras E., des Robert C., Landrier J.-F., Pauly V., Boubred F. Low Vitamin D Levels at Birth and Early Respiratory Outcome in Infants With Gestational Age Less Than 29 Weeks. Front. Pediatr. 2022;9:790839. doi: 10.3389/fped.2021.790839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacchetta J., Schmitt C.P., Bakkaloglu S.A., Cleghorn S., Leifheit-Nestler M., Prytula A., Ranchin B., Schön A., Stabouli S., Van de Walle J., et al. Diagnosis and Management of Mineral and Bone Disorders in Infants with CKD: Clinical Practice Points from the ESPN CKD-MBD and Dialysis Working Groups and the Pediatric Renal Nutrition Taskforce. Pediatr. Nephrol. 2023;38:3163–3181. doi: 10.1007/s00467-022-05825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bacchetta J., Edouard T., Laverny G., Bernardor J., Bertholet-Thomas A., Castanet M., Garnier C., Gennero I., Harambat J., Lapillonne A., et al. Vitamin D and Calcium Intakes in General Pediatric Populations: A French Expert Consensus Paper. Arch. Pédiatrie. 2022;29:312–325. doi: 10.1016/j.arcped.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Durup D., Jørgensen H.L., Christensen J., Schwarz P., Heegaard A.M., Lind B. A Reverse J-Shaped Association of All-Cause Mortality with Serum 25-Hydroxyvitamin D in General Practice: The CopD Study. J. Clin. Endocrinol. Metab. 2012;97:2644–2652. doi: 10.1210/jc.2012-1176. [DOI] [PubMed] [Google Scholar]
- 39.Sempos C.T., Durazo-Arvizu R.A., Dawson-Hughes B., Yetley E.A., Looker A.C., Schleicher R.L., Cao G., Burt V., Kramer H., Bailey R.L., et al. Is There a Reverse J-Shaped Association Between 25-Hydroxyvitamin D and All-Cause Mortality? Results from the U.S. Nationally Representative NHANES. J. Clin. Endocrinol. Metab. 2013;98:3001–3009. doi: 10.1210/jc.2013-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salle B.L., Delvin E.E., Lapillonne A., Bishop N.J., Glorieux F.H. Perinatal Metabolism of Vitamin D. Am. J. Clin. Nutr. 2000;71:1317S–1324S. doi: 10.1093/ajcn/71.5.1317s. [DOI] [PubMed] [Google Scholar]
- 41.Matejek T., Zapletalova B., Stepan M., Malakova J., Palicka V. Dynamics of the Vitamin D C3-Epimer Levels in Preterm Infants. Clin. Chem. Lab. Med. CCLM. 2023;61:1084–1094. doi: 10.1515/cclm-2022-1128. [DOI] [PubMed] [Google Scholar]
- 42.Bailey D., Veljkovic K., Yazdanpanah M., Adeli K. Analytical Measurement and Clinical Relevance of Vitamin D3 C3-Epimer. Clin. Biochem. 2013;46:190–196. doi: 10.1016/j.clinbiochem.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 43.Mandell E.W., Ryan S., Seedorf G.J., Gonzalez T., Smith B.J., Fleet J.C., Abman S.H. Maternal Vitamin D Deficiency Causes Sustained Impairment of Lung Structure and Function and Increases Susceptibility to Hyperoxia-Induced Lung Injury in Infant Rats. Am. J. Respir. Cell Mol. Biol. 2020;63:79–91. doi: 10.1165/rcmb.2019-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan R.M., Feng R., Bazacliu C., Ferkol T.W., Ren C.L., Mariani T.J., Poindexter B.B., Wang F., Moore P.E., Chougnet C., et al. Black Race Is Associated with a Lower Risk of Bronchopulmonary Dysplasia. J. Pediatr. 2019;207:130–135.e2. doi: 10.1016/j.jpeds.2018.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhandai R., Jajoo M., Singh A., Mandal A., Jain R. Association of Vitamin D Deficiency with an Increased Risk of Late-Onset Neonatal Sepsis. Paediatr. Int. Child Health. 2018;38:193–197. doi: 10.1080/20469047.2018.1477388. [DOI] [PubMed] [Google Scholar]
- 46.Cizmeci M.N., Kanburoglu M.K., Akelma A.Z., Ayyildiz A., Kutukoglu I., Malli D.D., Tatli M.M. Cord-Blood 25-Hydroxyvitamin D Levels and Risk of Early-Onset Neonatal Sepsis: A Case–Control Study from a Tertiary Care Center in Turkey. Eur. J. Pediatr. 2015;174:809–815. doi: 10.1007/s00431-014-2469-1. [DOI] [PubMed] [Google Scholar]
- 47.Say B., Uras N., Sahin S., Degirmencioglu H., Oguz S.S., Canpolat F.E. Effects of Cord Blood Vitamin D Levels on the Risk of Neonatal Sepsis in Premature Infants. Korean J. Pediatr. 2017;60:248. doi: 10.3345/kjp.2017.60.8.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cetinkaya M., Cekmez F., Buyukkale G., Erener-Ercan T., Demir F., Tunc T., Aydın F.N., Aydemir G. Lower Vitamin D Levels Are Associated with Increased Risk of Early-Onset Neonatal Sepsis in Term Infants. J. Perinatol. 2015;35:39–45. doi: 10.1038/jp.2014.146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.