The authors wish to make the following corrections to this published paper [1].
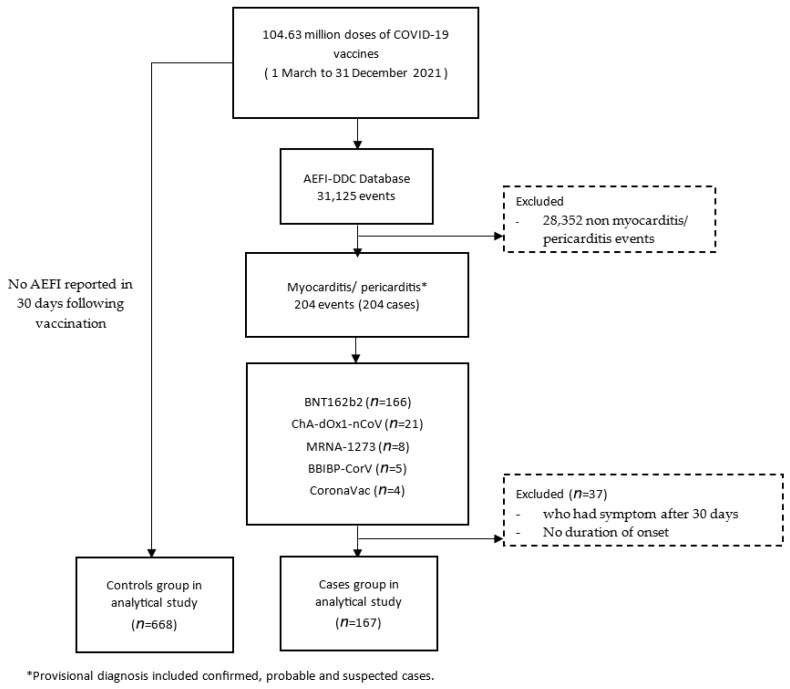
In Figure 1, the names of the vaccines have been replaced with their common names, i.e., Comirnaty has been replaced with BNT162B2, AstraZeneca has been replaced with ChA-dOx1-nCoV, Moderna has been replaced with MRNA-1273 and Sinopharm has been replaced with BBIBP-CorV. The corrected Figure 1 is shown below:
Figure 1.
Flow chart of the study.
In Table 1, we have changed the unit of the number of vaccination doses administered from per 1 million doses to per 100,000 vaccination doses administered and corrected the errors for the overall AEFI rate from 1.95 to be 0.195 per 100,000 doses. We have corrected the number of reported ages for ChAdOx1-nCoV and BNT162b from 21 and 166 to 20 and 165, respectively. The corrected Table 1 is shown below:
Table 1.
Characteristics of the myocarditis/pericarditis cases following immunization in Thailand.
| ChAdOx1-nCoV n = 21 |
BNT162b2 n = 166 |
BBIBP-CorV n = 5 |
CoronaVac n = 4 |
MRNA-1273 n = 8 |
Total n = 204 |
|
|---|---|---|---|---|---|---|
| No. of case reports to AEFI-DDC | 21 | 166 | 5 | 4 | 8 | 204 |
| No. of vaccination doses administered | 44,159,927 | 17,137,233 | 14,578,943 | 26,385,393 | 2,371,390 | 104,632,886 |
| Rate (cases/100,000 doses) | 0.048 | 0.970 | 0.034 | 0.015 | 0.337 | 0.195 |
| Reported age, No. | 20 | 165 | 5 | 4 | 8 | 202 |
| Age, median (IQR), y | 61 (41–66) | 14 (13–16) | 33 (13–47) | 23 (21.5–27.5) | 39 (32.5–52) | 15 (13–17) |
| Reported onset, No. | 20 | 163 | 3 | 3 | 8 | 197 |
| Time to symptom onset, median (IQR), d | 4 (1–23) | 2 (1–4) | 5 (0–44) | 3 (2–16) | 2 (0–6) | 2 (1–4) |
| Reported sex, No. | 21 | 166 | 5 | 4 | 8 | 204 |
| Male (%) | 13 (61.9) | 120 (72.29) | 2 (40) | 2 (50) | 3 (37.5) | 140 (68.63) |
| Female (%) | 8 (38.1) | 46 (27.71) | 3 (60) | 2 (50) | 5 (62.5) | 64 (31.37) |
| Reported occupation No. | 21 | 166 | 5 | 4 | 8 | 204 |
| Occupation (%) | ||||||
| Student | 3 (14.29) | 135 (81.33) | 2 (40) | 1 (25) | 0 (0) | 141 (85.45) |
| HCP | 0 (0) | 2 (1.2) | 0 (0) | 0 (0) | 0 (0) | 2 (1.21) |
| Monk | 0 (0) | 2 (1.2) | 0 (0) | 0 (0) | 0 (0) | 2 (1.21) |
| Government officer | 0 (0) | 1 (0.6) | 0 (0) | 1 (25) | 0 (0) | 2 (1.21) |
| Merchant | 1 (4.76) | 1 (0.6) | 0 (0) | 1 (25) | 1 (12.5) | 4 (2.42) |
| Unemployed | 3 (14.29) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1.82) |
| Housework | 2 (9.52) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.21) |
| Employee | 4 (19.05) | 0 (0) | 1 (20) | 0 (0) | 2 (25) | 7 (4.24) |
| Farmer | 1 (4.76) | 0 (0) | 0 (0) | 0 (0) | 1 (12.5) | 2 (1.21) |
| Unknown | 7 (33.33) | 25 (15.06) | 2 (40) | 1 (25) | 4 (50) | 39 (19.12) |
| Reported history of COVID-19 infection, No. | 21 | 166 | 5 | 4 | 8 | 204 |
| No | 20 (95.24) | 164 (98.8) | 5 (100) | 4 (100) | 7 (87.5) | 200 (98.04) |
| Yes | 1 (4.76) | 2 (1.2) | 0 (0) | 0 (0) | 1 (12.5) | 4 (1.96) |
| Reported dose, No. | 21 | 166 | 5 | 4 | 8 | 204 |
| Dose 1 | 5 (23.81) | 54 (32.53) | 3 (60) | 4 (100) | 2 (25) | 68 (33.33) |
| Dose 2 | 16 (76.19) | 111 (66.87) | 2 (40) | 0 (0) | 2 (25) | 129 (63.24) |
| Dose 3 | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) | 4 (50) | 7 (3.43) |
| Reported prognosis, No. | 21 | 166 | 5 | 4 | 8 | 204 |
| Status $ (%) | ||||||
| Full recovery | 1 (4.76) | 49 (29.52) | 1 (20) | 0 (0) | 1 (12.5) | 52 (25.49) |
| Improved | 6 (28.57) | 82 (49.4) | 0 (0) | 1 (25) | 4 (50) | 93 (45.59) |
| Death | 9 (42.86) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 10 (4.90) |
| Unknown | 5 (23.81) | 35 (21.08) | 4 (80) | 2 (50) | 3 (37.5) | 49 (24.02) |
| Reported hospitalization, No. | 21 | 166 | 5 | 4 | 8 | 204 |
| Hospitalization (%) | ||||||
| IPD | 11 (52.38) | 139 (83.73) | 2 (40) | 1 (25) | 7 (87.5) | 160 (78.43) |
| OPD | 6 (28.57) | 18 (10.84) | 1 (20) | 2 (50) | 1 (12.5) | 28 (13.73) |
| ER | 2 (9.52) | 7 (4.22) | 0 (0) | 1 (25) | 0 (0) | 10 (4.90) |
| Unknown | 2 (9.52) | 2 (1.2) | 2 (40) | 0 (0) | 0 (0) | 6 (2.94) |
| Reported hospital stay, No. | 13 | 93 | 2 | 2 | 4 | 114 |
| Duration of hospital stay, median (IQR), in days | 3 (1–12) | 3 (1–4) | 22.5 (10–35) | 21 (7–35) | 2.5 (1–4.5) | 3 (1–5) |
IQR: interquartile range; HCP: healthcare provider; $: status was evaluated at date reported. IPD: inpatient department, OPD: outpatient department, ER: emergency room.
In Table 2, we have added a remark about the incidence of myocarditis in ChAdOx1-nCoV under Table 2 and changed the incidence of myocarditis in ChAdOx1-nCoV in children aged 12 to 17 to “NA”. The reason we decided to modify this is to avoid confusion, since ChAdOx1-nCoV was not recommended for people under 18 years old in Thailand, but there were some errors that occurred during the campaign as reported. Therefore, we think it would be clearer for readers if we remove the rate of ChAdOx1-nCoV among vaccinees age 12–17 from the table to the footnote. The corrected Table 2 is shown below:
Table 2.
Rate of suspected myocarditis/pericarditis (per 100,000 doses administered) by vaccine, age, and sex (n = 202).
| Age Group | Vaccine and Sex | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1-nCoV | BNT162b2 | BBIBP-CorV | CoronaVac | MRNA-1273 | Total | Background Incidence $ (Cases/100,000 Population) |
||||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| 05–11 y | NA | NA | 0.00 | 0.00 | 0.00 | 6.21 | NA | NA | 0.00 | 0.00 | 0.00 | 4.87 | 0.24 | 0.29 |
| 12–17 y | NA * | NA * | 2.87 | 1.03 | 2.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.89 | 1.02 | 0.45 | 0.37 |
| 18–20 y | 0.14 | 0.14 | 1.03 | 0.47 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.32 | 0.18 | 0.87 | 0.44 |
| 21–40 y | 0.01 | 0.01 | 0.13 | 0.05 | 0.00 | 0.03 | 0.04 | 0.04 | 0.20 | 0.61 | 0.03 | 0.05 | 0.83 | 0.51 |
| 41–60 y | 0.03 | 0.02 | 0.07 | 0.00 | 0.04 | 0.04 | 0.00 | 0.00 | 0.57 | 0.00 | 0.04 | 0.02 | 1.58 | 1.08 |
| 61–80 y | 0.19 | 0.04 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.61 | 0.13 | 0.03 | 3.88 | 3.25 |
| >80 y | 0.00 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 4.77 | 4.94 |
* ChA-dOx1-nCoV was not recommended for use in people less than 18 years old in Thailand; however, some unintentional misuse occurred, and one case of suspected myocarditis was reported among 7826 doses administered to males aged 12–17 years. The only case of suspected myocarditis after the ChAdOx1-nCoV vaccine was a male aged 17 years and 8 months who was reported as 18 years old on the vaccination day and intended to receive the ChAdOx1-nCoV vaccine. His medical record was not available to confirm the diagnosis of myocarditis. $: The background incidence is the 3-year median incidence of myocarditis/pericarditis in 2018–2020 prior to implementation of the COVID-19 vaccine.
The authors apologize for any inconvenience this may have caused and affirm that the scientific conclusions remain unaffected. The original publication has also been updated.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Reference
- 1.Mahasing C., Doungngern P., Jaipong R., Nonmuti P., Chimmanee J., Wongsawat J., Boonyasirinant T., Wanlapakorn C., Leelapatana P., Yingchoncharoen T., et al. Myocarditis and Pericarditis following COVID-19 Vaccination in Thailand. Vaccines. 2023;11:749. doi: 10.3390/vaccines11040749. [DOI] [PMC free article] [PubMed] [Google Scholar]