Abstract
An extensive evaluation of disease occurrence after statin use based on a “hypothesis-free” approach remains scarce. To examine the effect of statin use on the potential risk of developing diseases, a propensity score–matched cohort study was executed using data from the National Sample Cohort in South Korea. A total of 7847 statin users and 39,235 nonstatin users were included in the final analysis. The period of statin use was defined as our main time-dependent exposure and was divided into three periods: current, recent, and past. The main outcomes were defined as new-onset diseases with ≥100 events based on the International Statistical Classification of Diseases, 10th Revision. We calculated the adjusted hazard ratios and 95% confidence intervals (CIs) using Cox regression. We found that statin use significantly increased the risk of developing iron deficiency anemia up to 5.04 times (95% CI, 2.11 to 12.03). Therefore, the iron levels of patients using statins should be monitored carefully.
Statin use might increase the risk of iron deficiency anemia under a hypothesis-free approach to evaluating multiple diseases.
INTRODUCTION
The number of adults diagnosed with dyslipidemia is on the rise in the Republic of Korea (hereafter, Korea). The age-standardized prevalence of hypercholesterolemia in individuals aged 20 years and above increased from 9.2% in 2008 to 18.0% in 2018 (1). Dyslipidemia contributes to the global burden of diseases, including ischemic heart disease and ischemic stroke, which are the leading causes of death (2). Statins—3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) inhibitors—have been popularly prescribed according to experts’ guidelines to manage dyslipidemia (3–5), accounting for approximately 90% of dyslipidemia treatment in Korea since 2006 (1). As a result of the trend toward earlier initiation of statin therapy, more adults are being exposed to it, which could inevitably lead to increased risks of adverse effects (AEs).
Several unintended positive effects of statins have been identified, including anti-inflammation, anti-oxidative activity, anti-atherogenic activity, and improvement of endothelial function (6). However, in randomized controlled trials (RCTs) or meta-analyses (4, 5, 7, 8), statin-associated AEs including muscle symptoms and liver toxicity are frequently reported. Moreover, numerous observational studies have associated statin use with an increase in the incidence of type 2 diabetes mellitus (T2DM) in many populations (9–14). Similarly, epidemiological studies (15–18) have also linked statin use with Parkinson’s disease (PD).
Furthermore, patients’ medication adherence is known to be influenced by potential AEs (7, 19). However, comprehensive evaluation of the potential AEs that require long-term follow-up or those that are uncommon is challenging within the RCT framework. Various observational studies have suggested navigating these limitations and investigating real-world risks (20). A previous study (21) attempted to evaluate the broad-spectrum effects of statins by focusing on diseases likely associated with them as other studies (9, 15, 22) have done. This hypothesis-based study design could potentially overlook underlying AEs. A hypothesis-free data-mining approach (23) detected rosuvastatin-specific AEs including iron deficiency anemia (IDA) without considering causation and confounders. However, these studies are vulnerable to biases such as measured or unmeasured confounders (24), necessitating careful design. Hence, it is crucial to conduct real-world pharmacovigilance studies based on a hypothesis-free design using various methods to navigate each study’s limitations.
Our study used a large-scale cohort database provided by the National Health Insurance Service (NHIS) in Korea, offering at least 10 years of follow-up data. The completeness of this database, collected independently of our study, ensured the representativeness of the real-world setting, minimized biases (24, 25), and facilitated the evaluation and comparison of the disease-wise time-dependent effect of statin use in Korea. Therefore, we investigated statin-related AEs within a “hypothesis-free” or “agnostic” framework.
RESULTS
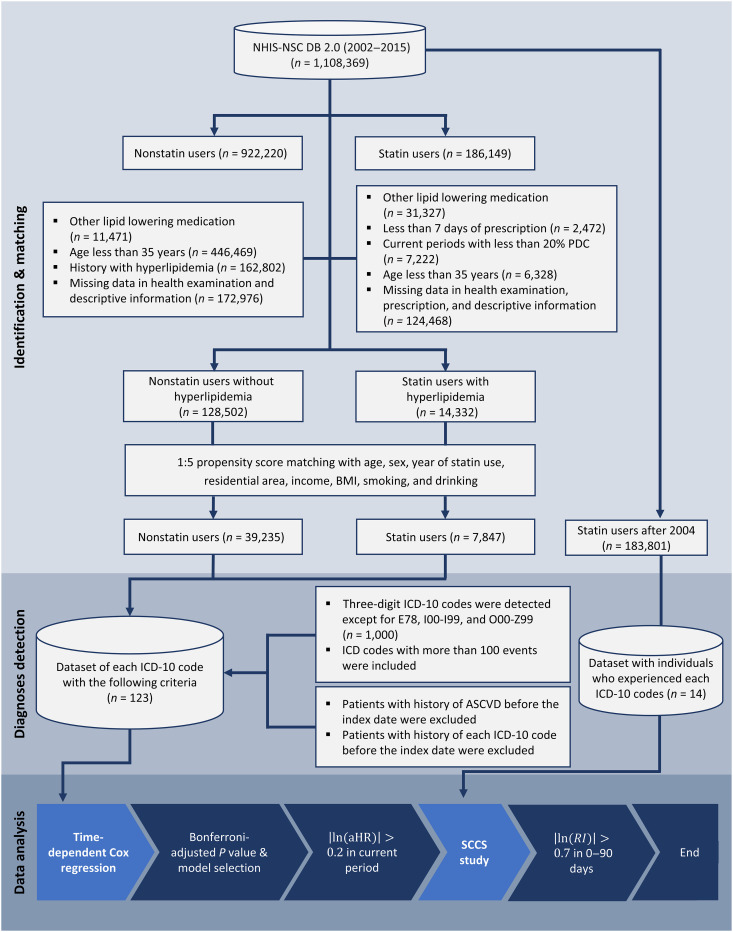
After applying the inclusion and exclusion criteria, 14,332 statin users with hyperlipidemia and 128,502 nonstatin users were eligible for the study (Fig. 1). We identified that the propensity score-based matched dataset consisted of 7847 statin users and 39,235 nonstatin users.
Fig. 1. Flowchart of the study process.
aHR, adjusted hazard ratio; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; ICD-10, International Statistical Classification of Diseases, 10th Revision; NHIS-NSC, National Health Insurance Service–National Sample Cohort; PDC, possession days covered; RI, relative incidence; SCCS, self-controlled case series.
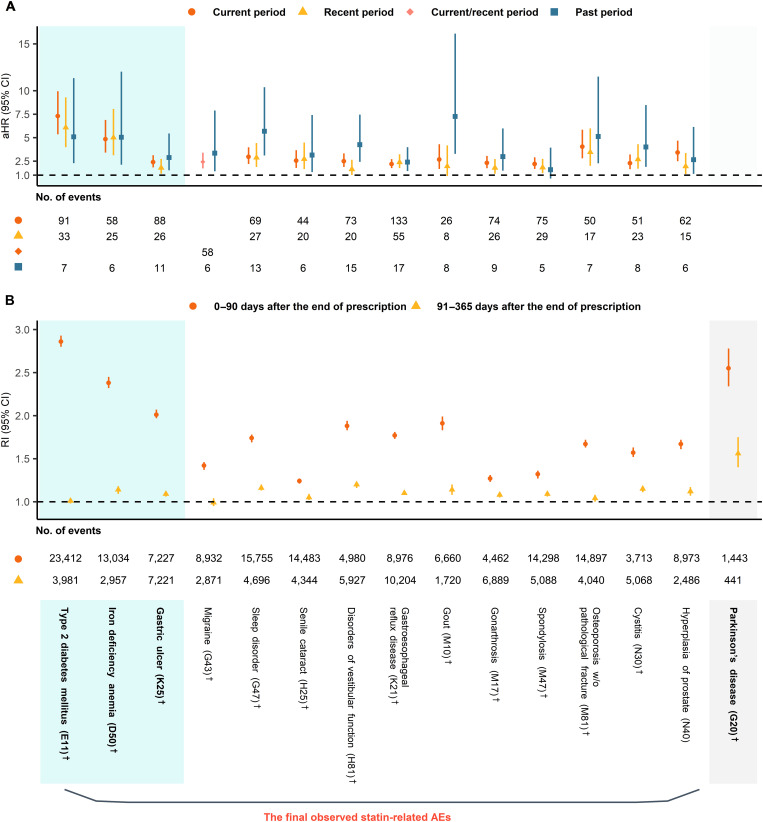
A total of 14 diagnoses with the International Statistical Classification of Diseases, 10th Revision (ICD-10) were identified from the cohort study (Fig. 2A): T2DM (ICD-10 code, E11), IDA (D50), gastric ulcer (GU; K25), migraine (G43), sleep disorder (G47), senile cataract (H25), disorders of vestibular function (H81), gastroesophageal reflux disease (GERD; K21) gout (M10), gonarthrosis (M17), spondylosis (M47), osteoporosis (M81), cystitis (N30), and hyperplasia of prostate (N40). Of these, only three, i.e., T2DM, IDA, and GU, satisfied the criteria for our self-controlled case series (SCCS) design (Fig. 2B).
Fig. 2. Adjusted hazard ratios and relative incidences of candidate statin-related adverse effects.
All aHRs were adjusted for total cholesterol and residential area as a random effect. PD was excluded in (A) because of the insufficient sample size. (A) Results of time-dependent Cox regression for candidate statin-related AEs with aHRs and 95% CIs for each period. (B) Results of SCCS design for candidate statin-related AEs, and PD with RIs and 95% CIs for each period. We detected 14 statin-related AEs using (A) and (B). †Diagnoses matched on the previously known AE. AE, adverse effect; aHR, adjusted hazard ratio; CI, confidence interval; PD, Parkinson's disease; RI, relative incidence; SCCS, self-controlled case series; w/o, without.
In the retrospective cohort analysis, the exposure level to each statin prescription was categorized into three periods: current (up to 3 months from the prescription end date), recent (up to 12 months from the prescription end date), and past (more than 12 months after the prescription end date). In the case-only design, each observation period was divided similarly to the cohort study, with the past period as a baseline.
In the cohort analysis, we confirmed the strongly time-dependent relationship of T2DM risks in each period [adjusted hazard ratio (aHR) 7.30, 95% confidence interval (CI) 5.36 to 9.95 (for the “current period”); aHR 6.08, 95% CI 3.98 to 9.30 (for the “recent period”); and aHR 5.09, 95% CI 2.28 to 11.34 (for the “past period”)]. The effect of statin use on IDA was also significant across the periods [aHR 4.84, 95% CI 3.40 to 6.89 (for the “current period”); aHR 5.01, 95% CI 3.12 to 8.06 (for the “recent period”); and aHR 5.04, 95% CI 2.11 to 12.03 (for the “past period”)]. A slight increase in the trend of IDA risk was observed between the recent and past periods, with overlapping 95% CIs. The observed trend in GU risk fluctuated regardless of the exposure level to statins [aHR 2.39, 95% CI 1.83 to 3.13 (for the “current period”); aHR 1.78, 95% CI 1.16 to 2.73 (for the “recent period”); and aHR 2.88, 95% CI 1.52 to 5.44 (for the “past period”)].
We found that the risk of T2DM was the highest in both designs [relative incidence (RI) 2.86, 95% CI 2.80 to 2.93 (for “0 to 90 days”); RI 1.01, 95% CI 0.98 to 1.04 (for “91 to 365 days”)] followed by IDA [RI 2.38, 95% CI 2.32 to 2.45 (for “0 to 90 days”); RI 1.14, 95% CI 1.09 to 1.18 (for “91 to 365 days”)] and GU [RI 2.01, 95% CI 1.97 to 2.07 (for “0 to 90 days”); RI 1.09, 95% CI 1.06 to 1.12 (for “91 to 365 days”)], for current statin users versus no statin users in this cohort study. Their matched RIs in the SCCS design were similarly high, while the RI of GU in 0 to 90 days was high in the SCCS design compared to the result of Cox regression (Fig. 2).
In addition, the RI of PD in 0 to 90 days after the end of statin prescription was 2.55 (95% CI 2.34 to 2.78) and that of the next interval was 1.56 (95% CI 1.40 to 1.75) even though PD was excluded in the Cox regression owing to insufficient sample size (Fig. 2B).
Pearson’s correlation coefficients between IDA and each ICD-10 code of diseases of the digestive system are shown in table S1. The absolute values of them were ranged from <0.01 to 0.19 and their mean (SD) was 0.04 (0.04). In particular, the magnitude of the correlation between IDA and GU was not strong (correlation coefficient = 0.12; table S1). After adjusting the history of GU in the analysis, a similar effect regarding IDA was observed (table S2). This confirms that statin use increased the risk of IDA regardless of GU.
In our sensitivity analyses, 9 of 14 ICD-10 codes, including T2DM and IDA, were detected when we accounted for total cholesterol, comorbidity, and comedications. Furthermore, when we limited our analysis to patients with a history of dyslipidemia, we found consistent results for T2DM, IDA, and GU (tables S3 and S4). For individuals of European ancestry, we also observed a considerable relationship between proxies of HMGCR inhibition and IDA or T2DM, respectively (see Supplementary Text, figs. S1 and S2, and tables S5 to S7).
DISCUSSION
We designed a hypothesis-free approach for identifying statin-related AEs by disease type based on time-dependent usage of statins by applying statistically appropriate methods on large-scale populations. It was successful in two aspects; most of the estimated risks were replicated on the basis of previously published literature and a prominent statin-related risk was found.
Validation with previous studies
To mitigate false-positive results, we concurrently adopted the retrospective cohort study and case-only design (SCCS). Findings of the former were in accordance with those of the previously published studies in 12 of the 14 statin-related AEs (9, 26–29). Evaluation of the overall results of the two designs, including the case-only design, identified T2DM and GU as AEs.
Several previous studies (9, 26, 30) have repeatedly confirmed the increased risk of new-onset T2DM with statin use, and recent meta-analyses (27, 28) have supported these results. We found that the risk of T2DM was higher than that of other observed AEs, and the extent of risk was reflected in the active status of therapy with statin use. This replicated result strengthens the validity of our study design.
An increased risk of GU was also observed in both designs. Previous observational and meta-analysis studies have not been in agreement (31–33) with some studies suggesting no effect (34–36); however, a nested case-control study (29) reported that statin use could increase the odds of peptic ulcer by 45% in Korea. We also identified 10 other known statin-related AEs in our study: migraine (37–39), sleep disorder (40–42), senile cataract (21, 22), disorders of vestibular function (43), GERD (44), gouts (45), gonarthrosis (46, 47), spondylosis (48), osteoporosis (49, 50), and cystitis (51). However, all their associations with statin use were weak in both designs, and the biological shreds of evidence for these findings are unidentified.
In addition, the validity of our case-only design was confirmed in that given only statin-prescribed patients diagnosed with PD, it was likely to increase the risk of this diagnosis in both durations since exposure. Unfortunately, the risk for PD could not be evaluated in this cohort study owing to events being less than 100. Although the relationship between statin use and PD was unclear, the results obtained in this study were similar to those of previous studies with statin use (15, 16) or low-density lipoprotein cholesterol (LDL-C) (52).
Potential association between statin use and IDA
A large population-based cohort study (21) in the United Kingdom reported no risk of anemia with statin use. Since our results indicated that the risks related to statin use increased consistently over time, the findings from the U.K. population may be partially attributed to the use of a time-independent risk model. The outcomes of our study were comparable to that of a data-mining approach study (23) on the signals of rosuvastatin for IDA compared to other statins in Korea. Meanwhile, our study had more achievements in connoting the causally related effects through our elaborate study designs. This was supported by our sensitivity analyses and two-sample Mendelian randomization (MR) results.
To the best of our knowledge, this is the first study that observed the risk of IDA as a possible AE related to statin use in real-world data. Our study suggested that statin could affect iron metabolism besides controlling LDL-C levels. In general, the effect of statins on atherosclerotic cardiovascular disease (ASCVD) is explained by lowering serum LDL-C levels. However, it has been proposed that statins have so-called pleiotropic effects, such as potential anti-inflammatory effects on the development of atherosclerotic plaque by reducing C-reactive protein concentrations (53, 54). Iron was suggested as another mediator for the effect of anti-inflammation (55). An excess of non–transferrin-bound iron was reported to accelerate redox cycling mainly causing the inflammatory process (56, 57). Therefore, iron deficiency leads to ameliorating oxidative stress (58). Statins have already been reported to inhibit hepcidin expression, the key hormonal regulator of iron distribution (59). Consequently, their use may have contributed to improved cardiovascular disease (CVD) risk through a reduction in iron levels. These inferences are in line with the finding that ferritin levels could result in better CVD outcomes without interacting with LDL-C levels (58).
There was a supportive result that there could be another pathway causing statin-related IDA. The most common class of anemia is IDA, in which iron is deficient, making hemoglobin carry oxygen in serum (60). Since iron is mainly absorbed in the duodenum, gastrointestinal diseases are one of the causes of iron deficiency (61). We also found a negligible correlation between IDA and any diseases of the digestive system, and IDA risk due to statin use is similar even after adjusting the history of GU (table S1 and S2). Therefore, statins could affect iron homeostasis leading to IDA regardless of the risk of GU.
Our study had some limitations. First, we focused on relatively common diseases with more than 100 events in the population that occurred during the observation period, and rare statin-related AEs, such as rhabdomyolysis (38), were not evaluated in our study. Second, we adopted the SCCS design to increase the statistical validity; nonetheless, our estimates may be biased because of potential confounders that were not considered. Third, we sought to compare the results of cohort and case-only studies with the recommendation of Farrington et al. (62) However, a dose-response relationship could not be detected. Fourth, our result should be carefully interpreted since we evaluated three-letter ICD-10 codes, not meaningful categories. Last, we considered only the Korean population, which limits the generalization of our results for pharmacokinetic and pharmacogenetic properties to other populations (63).
Our modeling strategy in pharmacovigilance with a population-based cohort was validated using the increased, time-dependent risk of T2DM with statin use. Here, we found an association between statin use and the risk of developing IDA in real-world data that indicated that iron levels in patients receiving statin therapy need to be monitored regularly. Further preclinical and clinical studies are necessary to validate our findings.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Review Board (no. E1910/001-001) of the Seoul National University, Korea. The need for informed consent was waived owing to the anonymized nature of the collected data.
Data collection
The National Sample Cohort (NSC) 2.0 from 1 January 2002 to 31 December 2015 was provided by NHIS in Korea and used in this study. NHIS, a single insurance institution, provides a universal healthcare coverage system to almost the entire population in Korea (64). This database randomly included approximately 2% of all citizens who qualified for this program for 1 year in 2006 or received medical aid (64). It contained electronic information regarding demographics, details of drug prescriptions, and medical records including diagnoses. Diagnoses were coded on the basis of the ICD-10.
Study population
Individuals aged over 36 years were selected for this study. Lipoprotein metabolism disorders and lipidemias were coded as E78, per the ICD-10.
We defined the respective study population and applied two methodologies:
Retrospective cohort study
E78 patients who used at least one type of statins were considered statin users, and individuals without dyslipidemia who had never been prescribed statins were considered nonstatin users (Fig. 1). The index date of each statin user was assigned as the first observed date of statin prescription, and the same was used as the index date for their matched nonstatin user. Each statin user was matched with five nonstatin users. Patients with ASCVD were excluded because ASCVD can confound the effect of statins.
Case-only design
Follow-ups began after the index date that was set as 1 January 2004. Since this study design could cancel out time-invariant confounding variables, we included all patients with an E78 diagnosis and at least one exposure to any of the statins.
Main exposure
The main exposure in our analysis was statin use. Statins included simvastatin, lovastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, and pitavastatin. To isolate the effect of statins, other lipid-lowering medications and combinations, including fibrates, bile acid sequestrants, nicotinic acid and derivatives, other lipid-modifying agents, and statins in combinations with other agents, were excluded.
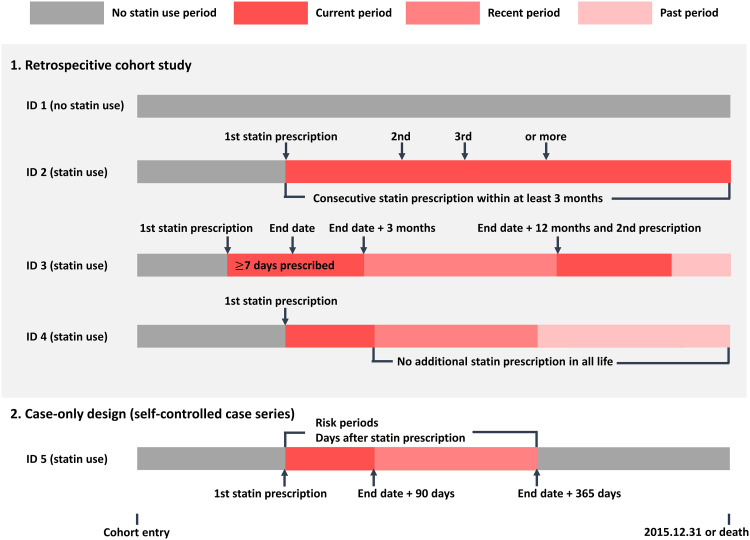
We separately specified the exposures (Fig. 3):
Fig. 3. Diagram for the definition of exposure.
For the retrospective cohort study, exposure level to statin was divided into three periods: current, recent, and past. For the case-only design, each observation period was split into two risk periods: a current risk period of 0 to 90 days after the end date of prescription followed by a recent risk period of 91 to 365 days after the end date of prescription.
Retrospective cohort study
We excluded statin users with prescriptions <7 days and nonadherent statin users who used statins for <20% of the days required for adherence (65). To differentiate between statin-related AEs based on the follow-up period of statin use in the cohort study, the exposure level was divided into three periods: current, recent, and past (66). The period with successive prescriptions of ≤3 months from the end date of the prescription was considered “current.” The period of up to 12 months from the end date of the current period was considered “recent,” and the period from the end date of the recent period to the next current period was defined as “past.”
Case-only design
Each period was split into two risk periods: a current risk period of 0 to 90 days after the end date of prescription, followed by a recent risk period of 91 to 365 days after the end date of prescription.
Outcome
ICD-10 codes have been developed since 1900 and are continuously updated to allow us to systematically record morbidity and mortality (67). For our clinical outcomes, different three-letter ICD-10 codes were considered separately. To achieve sufficiently large statistical power, codes with <100 events were excluded. E78 and diseases of the circulatory system, coded between I00 and I99, were excluded because of reverse causation. Codes O00 to Z99 were excluded because they were consequences of external causes (pregnancy and perinatal conditions, congenital anomalies, injuries, and poisoning). Consequently, a total of incident 123 diagnoses out of the 1000 ICD-10 codes were considered eligible. Death or when no outcome occurred was considered censored.
Statistical analyses
A multiple logistic regression model was used to calculate propensity scores, which considered confounding factors such as age, sex, year of statin use, income, residential area, body mass index, history of tobacco smoking, and alcohol consumption. We set a caliper at 0.15 and examined the absolute standardized mean differences according to statin use in table S8. We estimated the aHRs with their 95% CIs using the Cox proportional hazard model for time-dependent exposures after adjusting for total cholesterol and residential area. Residential area was considered as a random variable, with a gamma distribution to account for heterogeneity in dwelling locations. We assumed that there were five different scenarios of statin exposure under other fixed conditions: (i) current, recent, and past period; (ii) current/recent and past period; (iii) current and recent/past period; (iv) current, recent, and past as linear; and (v) statin use and no use. Model selection for each selected diagnosis was performed using the Bayesian information criterion. Scaled Schoenfeld residual plots with the frailties as an offset and cumulative raw Schoenfeld residuals by Brownian motion (68) were examined for proportional hazard assumption.
We prepared an SCCS as a case-only design for each diagnosis with |ln(aHR)| > 0.2 in the current period in the cohort study. Because of a violation of the SCCS assumption, as evidenced by event-dependent exposure shown in the centered event plot, we used the standard SCCS, incorporating a pre-exposure period of 20 days and checked the crude incidences of main outcomes because of rarity (62, 69). Last, statin-related AE was defined when |ln(RI)| > 0.7 for 0 to 90 days after the cessation of the prescribed use of statins.
Pearson’s correlation coefficients were calculated to evaluate the relationship between IDA and diseases of the digestive system (“K” based on ICD-10), using NHIS-NSC data, where the results were summarized. Furthermore, we performed two sensitivity analyses: (i) considering total cholesterol, comorbidities, anticoagulants, and antiplatelet in 1:1 propensity score matching (PSM) and (ii) selecting only patients with dyslipidemia using the previous 1:1 PSM. In these cases, we adjusted only the residential area as a random effect in the Cox model. Last, a two-sample MR was conducted to investigate the causal association using an independent dataset. Details are provided in the Supplementary Materials.
The significance level was set at 0.05, and the problem of multiple testing was adjusted with Bonferroni correction. Statistical analyses were performed using the SAS enterprise guide (version 7.13; SAS Institute, Cary, NC, USA), R (version 3.3.3; The R Development Core Team, Vienna, Austria), and Rex (70) (version 3.5.3; Rexsoft, Seoul, Korea).
Acknowledgments
The National Sample Cohort DB 2.0 (Research Administration No. NHIS-2020-2-035) was provided by the National Health Insurance Sharing Service and the results of the study are not related to the National Health Insurance Corporation. Our supplemental analysis related to Mendelian randomization was supported by the National Supercomputing Center with supercomputing resources including technical support (KSC-2022-CRE-0319) in the peer review process.
Funding: This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT grant NRF-2021R1A5A1033157 (to S.W.) and Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare grant HI22C0154 (to S.W.).
Author contributions: Conceptualization: S.L. and S.W. Methodology: J.A., S.L., and S.W. Investigation: J.A. and S.L. Visualization: J.A. Supervision: S.W. Writing—original draft: J.A. Writing—review and editing: J.A., S.L., and S.W. Software: J.A. Formal analysis: J.A. Data curation: J.A. Project administration: S.W. Funding acquisition: S.W.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The National Sample Cohort used in this study is provided by the National Health Insurance Sharing Service (NHISS; https://nhiss.nhis.or.kr/) with approval (Research Administration No. NHIS-2020-2-035). Any researchers who promise to follow the research ethics can personally request this database to NHISS by submitting IRB approval with the study protocol and the review committee will decide on the approval of the data provision. Raw data can be accessed by remote control desktop and releasing of the data by the researcher is not allowed legally because of the personal privacy policy of the NHISS. Genetic summary-level data were from the Global Lipids Genetics Consortium (https://csg.sph.umich.edu/willer/public/lipids2013/) and the “TwoSampleMR” R software package (https://mrcieu.github.io/TwoSampleMR/articles/introduction.html). All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 and S2
Tables S1 to S8
Legend for data file S1
Legend for code file S1
References
Other Supplementary Material for this manuscript includes the following:
Data file S1
Code file S1
REFERENCES AND NOTES
- 1.S. M. J. Cho, H. Lee, H. H. Lee, J. Baek, J. E. Heo, H. J. Joo, S. J. Hong, H. C. Kim, Dyslipidemia fact sheets in Korea 2020: An analysis of nationwide population-based data. J. Lipid Atheroscler. 10, 202–209 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.H. Du, Q. Shi, P. Song, X. F. Pan, X. Yang, L. Chen, Y. He, G. Zong, Y. Zhu, B. Su, Global burden attributable to high low-density lipoprotein-cholesterol from 1990 to 2019. Front. Cardiovasc. Med. 9, 903126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.K. B. Son, S. J. Bae, Patterns of statin utilisation for new users and market dynamics in South Korea: A 13-year retrospective cohort study. BMJ Open 9, e026603 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.S. M. Grundy, N. J. Stone, A. L. Bailey, C. Beam, K. K. Birtcher, R. S. Blumenthal, L. T. Braun, S. de Ferranti, J. Faiella-Tommasino, D. E. Forman, R. Goldberg, P. A. Heidenreich, M. A. Hlatky, D. W. Jones, D. Lloyd-Jones, N. Lopez-Pajares, C. E. Ndumele, C. E. Orringer, C. A. Peralta, J. J. Saseen, S. C. Smith, L. Sperling, S. S. Virani, J. Yeboah, 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 139, e1082–e1143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.E. J. Rhee, H. C. Kim, J. H. Kim, E. Y. Lee, B. J. Kim, E. M. Kim, Y. Song, J. H. Lim, H. J. Kim, S. Choi, M. K. Moon, J. O. Na, K. Y. Park, M. S. Oh, S. Y. Han, J. Noh, K. H. Yi, S. H. Lee, S. C. Hong, I. K. Jeong, 2018 guidelines for the management of dyslipidemia. Korean J. Intern. Med. 34, 723–771 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.J. K. Liao, U. Laufs, Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 45, 89–118 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.B. A. Golomb, M. A. Evans, Statin adverse effects. Am. J. Cardiovasc. Drugs 8, 373–418 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Y. He, X. Li, D. Gasevic, E. Brunt, F. McLachlan, M. Millenson, M. Timofeeva, J. Ioannidis, H. Campbell, E. Theodoratou, Statins and multiple noncardiovascular outcomes: Umbrella review of meta-analyses of observational studies and randomized controlled trials. Ann. Intern. Med. 169, 543–553 (2018). [DOI] [PubMed] [Google Scholar]
- 9.E. Na, S. Cho, D. J. Kim, J. Choi, E. Han, Time-varying and dose-dependent effect of long-term statin use on risk of type 2 diabetes: A retrospective cohort study. Cardiovasc. Diabetol. 19, 67 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.R. Bhattacharya, M. Ajmera, S. Bhattacharjee, U. Sambamoorthi, Use of antidepressants and statins and short-term risk of new-onset diabetes among high risk adults. Diabetes Res. Clin. Pract. 105, 251–260 (2014). [DOI] [PubMed] [Google Scholar]
- 11.O. Currie, D. Mangin, J. Williman, B. McKinnon-Gee, P. Bridgford, The comparative risk of new-onset diabetes after prescription of drugs for cardiovascular risk prevention in primary care: A national cohort study. BMJ Open 3, e003475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.N. L. Zaharan, D. Williams, K. Bennett, Statins and risk of treated incident diabetes in a primary care population. Br. J. Clin. Pharmacol. 75, 1118–1124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.S. W. Rha, B. G. Choi, H. S. Seo, S. H. Park, J. Y. Park, K. Y. Chen, Y. Park, S. Y. Choi, M. S. Shim, J. B. Kim, T. Park, J. Park, J. J. Lee, E. J. Park, S. H. Park, J. Y. Choi, S. Lee, J. O. Na, C. U. Choi, H. E. Lim, J. W. Kim, E. J. Kim, C. G. Park, D. J. Oh, Impact of statin use on development of new-onset diabetes mellitus in Asian population. Am. J. Cardiol. 117, 382–387 (2016). [DOI] [PubMed] [Google Scholar]
- 14.K. L. Wang, C. J. Liu, T. F. Chao, C. M. Huang, C. H. Wu, S. J. Chen, T. J. Chen, S. J. Lin, C. E. Chiang, Statins, risk of diabetes, and implications on outcomes in the general population. J. Am. Coll. Cardiol. 60, 1231–1238 (2012). [DOI] [PubMed] [Google Scholar]
- 15.S. M. Jeong, W. Jang, D. W. Shin, Association of statin use with Parkinson's disease: Dose-response relationship. Mov. Disord. 34, 1014–1021 (2019). [DOI] [PubMed] [Google Scholar]
- 16.C. Becker, S. S. Jick, C. R. Meier, Use of statins and the risk of Parkinson’s disease: A retrospective case-control study in the UK. Drug Saf. 31, 399–407 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Y. C. Lee, C. H. Lin, R. M. Wu, M. S. Lin, J. W. Lin, C. H. Chang, M. S. Lai, Discontinuation of statin therapy associates with Parkinson disease: A population-based study. Neurology 81, 410–416 (2013). [DOI] [PubMed] [Google Scholar]
- 18.G. Liu, N. W. Sterling, L. Kong, M. M. Lewis, R. B. Mailman, H. Chen, D. Leslie, X. Huang, Statins may facilitate Parkinson's disease: Insight gained from a large, national claims database. Mov. Disord. 32, 913–917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.J. L. Johnson, I. B. Loomis, A case of simvastatin-associated pancreatitis and review of statin-associated pancreatitis. Pharmacotherapy 26, 414–422 (2006). [DOI] [PubMed] [Google Scholar]
- 20.I. Mansi, C. R. Frei, C. P. Wang, E. M. Mortensen, Statins and new-onset diabetes mellitus and diabetic complications: A retrospective cohort study of US healthy adults. J. Gen. Intern. Med. 30, 1599–1610 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.L. Smeeth, I. Douglas, A. J. Hall, R. Hubbard, S. Evans, Effect of statins on a wide range of health outcomes: A cohort study validated by comparison with randomized trials. Br. J. Clin. Pharmacol. 67, 99–109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.M. Casula, D. Soranna, G. Corrao, L. Merlino, A. L. Catapano, E. Tragni, Statin use and risk of cataract: A nested case-control study within a healthcare database. Atherosclerosis 251, 153–158 (2016). [DOI] [PubMed] [Google Scholar]
- 23.N. K. Choi, Y. Chang, Y. K. Choi, S. Hahn, B. J. Park, Signal detection of rosuvastatin compared to other statins: Data-mining study using national health insurance claims database. Pharmacoepidemiol. Drug Saf. 19, 238–246 (2010). [DOI] [PubMed] [Google Scholar]
- 24.L. C. Thygesen, A. K. Ersbøll, When the entire population is the sample: Strengths and limitations in register-based epidemiology. Eur. J. Epidemiol. 29, 551–558 (2014). [DOI] [PubMed] [Google Scholar]
- 25.J. Olsen, Register-based research: Some methodological considerations. Scand. J. Public Health 39, 225–229 (2011). [DOI] [PubMed] [Google Scholar]
- 26.M. J. Ko, A. J. Jo, Y. J. Kim, S. H. Kang, S. Cho, S. H. Jo, C. Y. Park, S. C. Yun, W. J. Lee, D. W. Park, Time- and dose-dependent association of statin use with risk of clinically relevant new-onset diabetes mellitus in primary prevention: A nationwide observational cohort study. J. Am. Heart Assoc. 8, e011320 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.M. Casula, F. Mozzanica, L. Scotti, E. Tragni, A. Pirillo, G. Corrao, A. L. Catapano, Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 27, 396–406 (2017). [DOI] [PubMed] [Google Scholar]
- 28.N. Sattar, D. Preiss, H. M. Murray, P. Welsh, B. M. Buckley, A. J. M. de Craen, S. R. K. Seshasai, J. J. McMurray, D. J. Freeman, J. W. Jukema, P. W. Macfarlane, C. J. Packard, D. J. Stott, R. G. Westendorp, J. Shepherd, B. R. Davis, S. L. Pressel, R. Marchioli, R. M. Marfisi, A. P. Maggioni, L. Tavazzi, G. Tognoni, J. Kjekshus, T. R. Pedersen, T. J. Cook, A. M. Gotto, M. B. Clearfield, J. R. Downs, H. Nakamura, Y. Ohashi, K. Mizuno, K. K. Ray, I. Ford, Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 375, 735–742 (2010). [DOI] [PubMed] [Google Scholar]
- 29.K. J. Kim, M. J. Jung, S. H. Lee, Association of statin therapy with risk of peptic ulcer disease: A nested case-control study using National Insurance Health Service Database. Korean Soc. Pharmacoepidemiol. Risk Manag. 12, 20–28 (2020). [Google Scholar]
- 30.J. Y. Park, S. W. Rha, B. G. Choi, J. W. Choi, S. K. Ryu, S. Kim, Y. K. Noh, S. Y. Choi, R. G. Akkala, H. Li, J. Ali, S. Xu, H. A. Ngow, J. J. Lee, G. N. Lee, J. B. Kim, S. Lee, J. O. Na, C. U. Choi, H. E. Lim, J. W. Kim, E. J. Kim, C. G. Park, S. S. Hong, D. J. Oh, Impact of low dose atorvastatin on development of new-onset diabetes mellitus in Asian population: Three-year clinical outcomes. Int. J. Cardiol. 184, 502–506 (2015). [DOI] [PubMed] [Google Scholar]
- 31.A. Feng, E. Chuang, S. H. Wu, J. C. Wang, S. N. Chang, C. L. Lin, C. H. Kao, The effect of statins on the occurrence of peptic ulcer. Eur. J. Intern. Med. 26, 731–735 (2015). [DOI] [PubMed] [Google Scholar]
- 32.C. J. Lin, W. C. Liao, Y. A. Chen, H. J. Lin, C. L. Feng, C. L. Lin, Y. J. Lin, M. C. Kao, M. Z. Huang, C. H. Lai, C. H. Kao, Statin therapy is associated with reduced risk of peptic ulcer disease in the Taiwanese population. Front. Pharmacol. 8, 210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.B. Boonpheng, L. Al Momani, S. Arikapudi, A. Bokhari, S. Rupal, Statins and risk of peptic ulcer disease and upper gastrointestinal bleeding: A systematic review and meta-analysis. Am. J. Gastroenterol. 113, S1551 (2018). [Google Scholar]
- 34.R. Badillo, R. Schmidt, E. M. Mortensen, C. R. Frei, I. Mansi, Statin therapy and gastrointestinal hemorrhage: A retrospective cohort study with propensity score-matching. Pharmacoepidemiol. Drug Saf. 24, 849–857 (2015). [DOI] [PubMed] [Google Scholar]
- 35.S. E. Gulmez, A. T. Lassen, C. Aalykke, M. Dall, A. Andries, B. S. Andersen, J. M. Hansen, M. Andersen, J. Hallas, Do statins protect against upper gastrointestinal bleeding? Br. J. Clin. Pharmacol. 67, 460–465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.K. Wijarnpreecha, P. Panjawatanan, P. Leelasinjaroen, P. Ungprasert, Statins and risk of peptic ulcer disease: A systematic review and meta-analysis. Arab J. Gastroenterol. 21, 135–138 (2020). [DOI] [PubMed] [Google Scholar]
- 37.C. S. Ramsey, Q. C. Snyder, Altitude-induced migraine headache secondary to pravastatin: Case report. Aviat. Space Environ. Med. 69, 603–606 (1998). [PubMed] [Google Scholar]
- 38.A. Gluba-Brzozka, B. Franczyk, P. P. Toth, J. Rysz, M. Banach, Molecular mechanisms of statin intolerance. Arch. Med. Sci. 12, 645–658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.M. Sayyah, K. Shirbandi, F. Rahim, R. Ganji, Statin for migraine headache: Is it worthwhile? Neurol. India 68, 1003–1007 (2020). [DOI] [PubMed] [Google Scholar]
- 40.G. B. J. Mancini, S. Baker, J. Bergeron, D. Fitchett, J. Frohlich, J. Genest, M. Gupta, R. A. Hegele, D. Ng, J. Pope, Diagnosis, prevention, and management of statin adverse effects and intolerance: Proceedings of a Canadian working group consensus conference. Can. J. Cardiol. 27, 635–662 (2011). [DOI] [PubMed] [Google Scholar]
- 41.M. Broncel, P. Gorzelak-pabiś, A. Sahebkar, K. Serejko, S. Ursoniu, J. Rysz, M. C. Serban, M. Mozdzan, M. Banach, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group , Sleep changes following statin therapy: A systematic review and meta-analysis of randomized placebo-controlled polysomnographic trials. Arch. Med. Sci. 11, 915–926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.M. Takada, M. Fujimoto, K. Yamazaki, M. Takamoto, K. Hosomi, Association of statin use with sleep disturbances: Data mining of a spontaneous reporting database and a prescription database. Drug Saf. 37, 421–431 (2014). [DOI] [PubMed] [Google Scholar]
- 43.A. Langeard, K. Saillant, E. Cloutier, M. Gayda, F. Lesage, A. Nigam, L. Bherer, S. Fraser, Association between statin use and balance in older adults. Int. J. Environ. Res. Public Health 17, 4662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.I. Smith, R. Schmidt, E. A. Halm, I. A. Mansi, Do statins increase the risk of esophageal conditions? Findings from four propensity score-matched analyses. Clin. Drug Investig. 38, 135–146 (2018). [DOI] [PubMed] [Google Scholar]
- 45.H. G. Choi, B. C. Kwon, M. J. Kwon, J. H. Kim, J. H. Kim, B. Park, J. W. Lee, Association between gout and dyslipidemia: A nested case–control study using a national health screening cohort. J. Pers. Med. 12, 605 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U. E. Makris, C. A. Alvarez, E. M. Mortensen, I. A. Mansi, Association of statin use with increased risk of musculoskeletal conditions: A retrospective cohort study. Drug Saf. 41, 939–950 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.J. Wang, J. Dong, J. Yang, Y. Wang, J. Liu, Association between statin use and incidence or progression of osteoarthritis: Meta-analysis of observational studies. Osteoarthr. Cartil. 28, 1170–1179 (2020). [DOI] [PubMed] [Google Scholar]
- 48.U. E. Makris, C. A. Alvarez, W. Wei, E. M. Mortensen, I. A. Mansi, Association of statin use with risk of back disorder diagnoses. JAMA Intern. Med. 177, 1044–1046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.M. Leutner, C. Matzhold, L. Bellach, C. Deischinger, J. Harreiter, S. Thurner, P. Klimek, A. Kautzky-Willer, Diagnosis of osteoporosis in statin-treated patients is dose-dependent. Ann. Rheum. Dis. 78, 1706–1711 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.S. M. Lin, J. H. Wang, C. C. Liang, H. K. Huang, Statin use is associated with decreased osteoporosis and fracture risks in stroke patients. J. Clin. Endocrinol. Metab. 103, 3439–3448 (2018). [DOI] [PubMed] [Google Scholar]
- 51.C. Y. Huang, S. D. Chung, L. T. Kao, H. C. Lin, L. H. Wang, Statin use is associated with bladder pain syndrome/interstitial cystitis: A population-based case-control study. Urol. Int. 95, 227–232 (2015). [DOI] [PubMed] [Google Scholar]
- 52.X. Huang, H. Chen, W. C. Miller, R. B. Mailman, J. L. Woodard, P. C. Chen, D. Xiang, R. W. Murrow, Y. Z. Wang, C. Poole, Lower low-density lipoprotein cholesterol levels are associated with Parkinson's disease. Mov. Disord. 22, 377–381 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.T. Jamialahmadi, M. Abbasifard, Ž. Reiner, M. Rizzo, A. H. Eid, A. Sahebkar, The effects of statin treatment on serum ferritin levels: A systematic review and meta-analysis. J. Clin. Med. 11, 5251 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.F. Montecucco, F. Burger, G. Pelli, N. K. Poku, C. Berlier, S. Steffens, F. Mach, Statins inhibit C-reactive protein-induced chemokine secretion, ICAM-1 upregulation and chemotaxis in adherent human monocytes. Rheumatology 48, 233–242 (2009). [DOI] [PubMed] [Google Scholar]
- 55.R. G. Ruddell, D. Hoang Le, J. M. Barwood, P. S. Rutherford, T. J. Piva, D. J. Watters, P. Santambrogio, P. Arosio, G. A. Ramm, Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB–regulated signaling in rat hepatic stellate cells. Hepatology 49, 887–900 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.R. G. DePalma, V. W. Hayes, B. K. Chow, G. Shamayeva, P. E. May, L. R. Zacharski, Ferritin levels, inflammatory biomarkers, and mortality in peripheral arterial disease: A substudy of the Iron (Fe) and Atherosclerosis Study (FeAST) Trial. J. Vasc. Surg. 51, 1498–1503 (2010). [DOI] [PubMed] [Google Scholar]
- 57.E. Gammella, S. Recalcati, G. Cairo, Dual role of ROS as signal and stress agents: Iron tips the balance in favor of toxic effects. Oxid. Med. Cell. Longev. 2016, 8629024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.L. R. Zacharski, R. G. DePalma, G. Shamayeva, B. K. Chow, The statin-iron nexus: Anti-inflammatory intervention for arterial disease prevention. Am. J. Public Health 103, e105–e112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.L. Mascitelli, M. Goldstein, Might the beneficial effects of statin drugs be related to their action on iron metabolism? QJM 105, 1225–1229 (2012). [DOI] [PubMed] [Google Scholar]
- 60.B. Elstrott, L. Khan, S. Olson, V. Raghunathan, T. DeLoughery, J. J. Shatzel, The role of iron repletion in adult iron deficiency anemia and other diseases. Eur. J. Haematol. 104, 153–161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.F. Bermejo, S. García-López, A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J. Gastroenterol. 15, 4638–4643 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.P. Farrington, H. Whitaker, Y. Ghebremichael-Weldeselassie, Self-Controlled Case Series Studies: A Modelling Guide With R (Chapman and Hall/CRC, 2018). [Google Scholar]
- 63.B. K. Birmingham, S. R. Bujac, R. Elsby, C. T. Azumaya, J. Zalikowski, Y. Chen, K. Kim, H. J. Ambrose, Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur. J. Clin. Pharmacol. 71, 329–340 (2015). [DOI] [PubMed] [Google Scholar]
- 64.J. Lee, J. S. Lee, S. H. Park, S. A. Shin, K. W. Kim, Cohort profile: The National Health Insurance Service–National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidmiol. 46, e15 (2017). [DOI] [PubMed] [Google Scholar]
- 65.G. Chodick, V. Shalev, Y. Gerber, A. D. Heymann, H. Silber, V. Simah, E. Kokia, Long-term persistence with statin treatment in a not-for-profit health maintenance organization: a population-based retrospective cohort study in Israel. Clin. Ther. 30, 2167–2179 (2008). [DOI] [PubMed] [Google Scholar]
- 66.H. J. De Jong, T. P. van Staa, A. Lalmohamed, F. de Vries, R. J. Vandebriel, H. Van Loveren, O. H. Klungel, J. W. C. Tervaert, Pattern of risks of systemic lupus erythematosus among statin users: A population-based cohort study. Ann. Rheum. Dis. 76, 1723–1730 (2017). [DOI] [PubMed] [Google Scholar]
- 67.K. W. Fung, J. Xu, O. Bodenreider, The new international classification of diseases 11th edition: A comparative analysis with ICD-10 and ICD-10-CM. J. Am. Med. Inform. Assoc. 27, 738–746 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.R. Xu, A. Gamst, On proportional hazards assumption under the random effects models. Lifetime Data Anal. 13, 317–332 (2007). [DOI] [PubMed] [Google Scholar]
- 69.I. Petersen, I. Douglas, H. Whitaker, Self controlled case series methods: An alternative to standard epidemiological study designs. BMJ 354, i4515 (2016). [DOI] [PubMed] [Google Scholar]
- 70.RexSoft, Rex: Excel-Based Statistical Software, Version 3.5.3 (RexSoft, 2018); https://rexsoft.org/.
- 71.S. J. Yun, S. J. Byun, H.-J. Kim, B.-M. Oh, D. Y. Lee, S. J. Park, H. G. Seo, Statin use and pneumonia risk in Parkinson's disease. Parkinsonism Relat. Disord. 91, 124–127 (2021). [DOI] [PubMed] [Google Scholar]
- 72.C. J. Willer, E. M. Schmidt, S. Sengupta, G. M. Peloso, S. Gustafsson, S. Kanoni, A. Ganna, J. Chen, M. L. Buchkovich, S. Mora, J. S. Beckmann, J. L. Bragg-Gresham, H. Y. Chang, A. Demirkan, H. M. Den Hertog, R. Do, L. A. Donnelly, G. B. Ehret, T. Esko, M. F. Feitosa, T. Ferreira, K. Fischer, P. Fontanillas, R. M. Fraser, D. F. Freitag, D. Gurdasani, K. Heikkilä, E. Hyppönen, A. Isaacs, A. U. Jackson, Å. Johansson, T. Johnson, M. Kaakinen, J. Kettunen, M. E. Kleber, X. Li, J. Luan, L. P. Lyytikäinen, P. K. E. Magnusson, M. Mangino, E. Mihailov, M. E. Montasser, M. Müller-Nurasyid, I. M. Nolte, J. R. O'Connell, C. D. Palmer, M. Perola, A. K. Petersen, S. Sanna, R. Saxena, Global Lipids Genetics Consortium , Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.G. Hemani, J. Zheng, B. Elsworth, K. H. Wade, V. Haberland, D. Baird, C. Laurin, S. Burgess, J. Bowden, R. Langdon, V. Y. Tan, J. Yarmolinsky, H. A. Shihab, N. J. Timpson, D. M. Evans, C. Relton, R. M. Martin, G. D. Smith, T. R. Gaunt, P. C. Haycock, The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, e34408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.L. Sun, H. Ding, Y. Jia, M. Shi, D. Guo, P. Yang, Y. Wang, F. Liu, Y. Zhang, Z. Zhu, Associations of genetically proxied inhibition of HMG-CoA reductase, NPC1L1, and PCSK9 with breast cancer and prostate cancer. Breast Cancer Res. 24, 12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 and S2
Tables S1 to S8
Legend for data file S1
Legend for code file S1
References
Data file S1
Code file S1