Abstract
Objectives - To determine whether dexamethasone `matures' the phosphatidylcholine (PC) composition of broncheoalveolar fluid in infants at high risk of neonatal chronic lung disease (CLD), either by increasing the proportion of dipalmitoylphosphatidylcholine (DPPC), expressed as a percentage of total PC (%DPPC), or by increasing the ratio of DPPC to palmitoyloleoylphosphatidylcholine (DPPC:POPC ratio).
Design - Double blind, placebo controlled.
Setting and patients - Sixteen infants <32 weeks' gestation, <1250 g birth weight who were dependent on mechanical ventilation and requiring a fractional inspired oxygen of >0·30 at 12 days of chronological age.
Intervention - Randomisation to receive a two week reducing course of dexamethasone base at an initial dose of 0·2 mg/kg three times a day, or equivalent volumes of normal saline, starting at 14 days. Eight infants were randomised into each group. Broncheoalveolar lavage was performed serially throughout the study period or until extubation. PC composition of the fluid was analysed by high performance liquid chromatography.
Outcome measures - The %DPPC and the DPPC:POPC ratios were calculated for individual infants for days −1 and 0 combined, days 1 and 3 combined, and days 5 and 7 combined. Analysis of covariance was used to analyse the results.
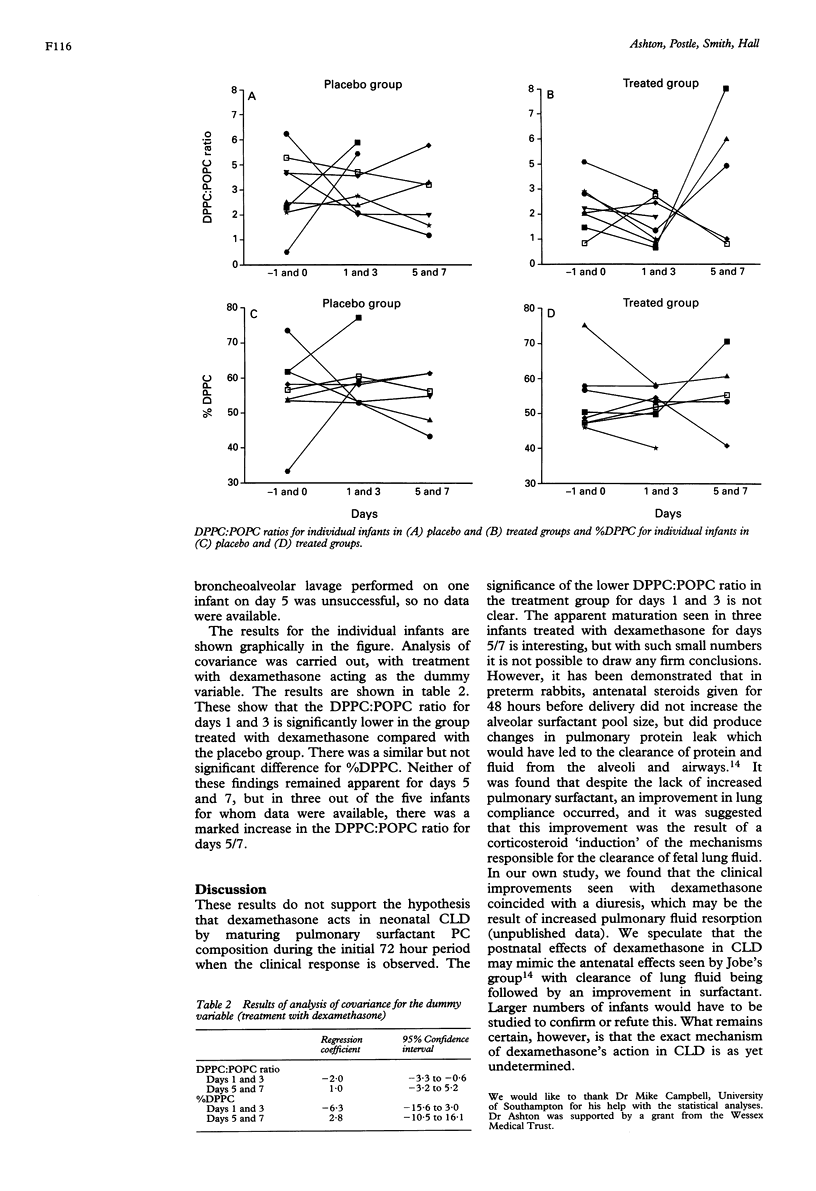
Results - The DPPC:POPC ratio was significantly less in the treated group than the placebo group on days 1 and 3, and not greater as the hypothesis stated. Three out of five infants treated with dexamethasone and for whom data were available showed a substantial rise in DPPC:POPC ratio on days 5/7, compared with the placebo group, but overall these changes were not statistically significant.
Conclusions - The data do not support the hypothesis that dexamethasone's action in producing a clinical improvement within the first 72 hours of treatment for neonatal CLD is by the `maturation' of pulmonary surfactant PC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton M. R., Postle A. D., Hall M. A., Smith S. L., Kelly F. J., Normand I. C. Phosphatidylcholine composition of endotracheal tube aspirates of neonates and subsequent respiratory disease. Arch Dis Child. 1992 Apr;67(4 Spec No):378–382. doi: 10.1136/adc.67.4_spec_no.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery G. B., Fletcher A. B., Kaplan M., Brudno D. S. Controlled trial of dexamethasone in respirator-dependent infants with bronchopulmonary dysplasia. Pediatrics. 1985 Jan;75(1):106–111. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Crowley P., Chalmers I., Keirse M. J. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990 Jan;97(1):11–25. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Cummings J. J., D'Eugenio D. B., Gross S. J. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med. 1989 Jun 8;320(23):1505–1510. doi: 10.1056/NEJM198906083202301. [DOI] [PubMed] [Google Scholar]
- Gladstone I. M., Ehrenkranz R. A., Jacobs H. C. Pulmonary function tests and fluid balance in neonates with chronic lung disease during dexamethasone treatment. Pediatrics. 1989 Dec;84(6):1072–1076. [PubMed] [Google Scholar]
- Ikegami M., Berry D., elKady T., Pettenazzo A., Seidner S., Jobe A. Corticosteroids and surfactant change lung function and protein leaks in the lungs of ventilated premature rabbits. J Clin Invest. 1987 May;79(5):1371–1378. doi: 10.1172/JCI112964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazzi N. J., Brans Y. W., Poland R. L. Dexamethasone effects on the hospital course of infants with bronchopulmonary dysplasia who are dependent on artificial ventilation. Pediatrics. 1990 Nov;86(5):722–727. [PubMed] [Google Scholar]
- Mammel M. C., Green T. P., Johnson D. E., Thompson T. R. Controlled trial of dexamethasone therapy in infants with bronchopulmonary dysplasia. Lancet. 1983 Jun 18;1(8338):1356–1358. doi: 10.1016/s0140-6736(83)92139-6. [DOI] [PubMed] [Google Scholar]
- Ng P. C. The effectiveness and side effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Arch Dis Child. 1993 Mar;68(3 Spec No):330–336. doi: 10.1136/adc.68.3_spec_no.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle A. D. Method for the sensitive analysis of individual molecular species of phosphatidylcholine by high-performance liquid chromatography using post-column fluorescence detection. J Chromatogr. 1987 Apr 10;415(2):241–251. doi: 10.1016/s0378-4347(00)83216-8. [DOI] [PubMed] [Google Scholar]
- Yeh T. F., Torre J. A., Rastogi A., Anyebuno M. A., Pildes R. S. Early postnatal dexamethasone therapy in premature infants with severe respiratory distress syndrome: a double-blind, controlled study. J Pediatr. 1990 Aug;117(2 Pt 1):273–282. doi: 10.1016/s0022-3476(05)80547-5. [DOI] [PubMed] [Google Scholar]


