Abstract
Introduction
Progression of fibrosis in interstitial lung diseases (ILD) has been associated with poor prognosis, lower quality of life for patients and caregivers, and higher healthcare costs. This study estimated the burden of disease and productivity loss of progressively fibrosing ILD, focusing on progressive pulmonary fibrosis other than idiopathic pulmonary fibrosis (non-IPF PPF) and systemic sclerosis-associated ILD (SSc-ILD) in the European Economic Area (EEA).
Methods
An economic model was built to estimate the clinical burden of SSc-ILD and non-IPF PPF. The model was based on published data on disease prevalence and disease burden (in terms of comorbidities, exacerbations, and deaths) as well as on productivity loss (in terms of sick days, early retirement, permanent disability, and job loss). Aggregate income loss was obtained by multiplying productivity loss by the median daily income in each country/area of investigation. A sensitivity analysis was performed to test the impact of the variability of the model assumptions.
Results
In the whole EEA, a total of 86,794 and 13,221 individuals were estimated to be affected by non-IPF PPF and SSc-ILD, respectively. Estimated annual sick days associated with the diseases were 3,952,604 and 672,172, early retirements were 23,174 and 5341, permanently disabled patients were 41,748 and 4037, and job losses were 19,789 and 2617 for non-IPF PPF and SSc-ILD, respectively. Annual exacerbations were estimated to be 22,401–31,181 and 1259–1753, while deaths were 5791–6171 and 572–638 in non-IPF PPF and SSc-ILD, respectively. The estimated annual aggregate income loss in EEA, accounting for losses due to annual sick days, early retirements, and permanently disabled patients, was €1433 million and €220 million in non-IPF PPF and SSc-ILD, respectively. The productivity loss due to job losses was €194 million and €26 million in non-IPF PPF and SSc-ILD, respectively. The main driver of aggregate income loss variability was the prevalence.
Conclusion
The impact of non-IPF PPF and SSc-ILD on society is definitely non-negligible. Actions to reduce the burden on our societies are highly needed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02701-z.
Keywords: Disease burden, Income loss, Indirect costs, Productivity loss, Progressive fibrosing interstitial lung disease, Systemic sclerosis
Key Summary Points
| Why carry out this study? |
| The clinical burden of ILD is becoming increasingly recognized. The progressive forms of ILDs have particularly been associated with worse prognosis, decreased quality of life, and increased healthcare resource use. |
| Adopting the hypothesis that measuring the impact of ILD on society would help in allocating the proper resources, we aimed to estimate the burden of disease and productivity loss of progressive pulmonary fibrosis other than idiopathic pulmonary fibrosis (non-IPF PPF) and systemic sclerosis-associated ILD (SSc-ILD) in the European Economic Area (EEA). |
| What was learned from the study? |
| Non-IPF PPF and SSc-ILD heavily affect society, as indirect costs in the EEA were estimated at approximately €1432 million and €220 million, respectively. Job losses were responsible for costs of approximately €194 million and €26 million in non-IPF PPF and SSc-ILD, respectively. |
| In conclusion, ILD is a significant economic burden for society. Beyond providing health and palliative care to patients, it is crucial to find solutions that help minimize the impact of the disease on productivity. |
Introduction
Interstitial lung diseases (ILDs) include more than 200 parenchymal pulmonary disorders, which are mostly rare [1]. They display varying degrees of inflammation, fibrosis, or both, and a wide range of clinical courses and prognoses [2]. The progressive fibrosis of lung parenchyma is responsible for the progressive deterioration in lung function and respiratory symptoms [1] and is associated with poor prognosis [1, 3], lower quality of life for patients and caregivers [4], and higher healthcare costs [4, 5]. The clinical objectives of managing progressive ILD are to prevent lung function deterioration, exacerbations, and quality of life deterioration and to increase survival [4]. In 2022, new practice guidelines defined progressive pulmonary fibrosis as an ILD of known or unknown etiology other than idiopathic pulmonary fibrosis (IPF) that has radiological evidence of pulmonary fibrosis, with at least two of the following three criteria occurring within the past year with no alternative explanation: (a) worsening respiratory symptoms; (b) physiological evidence of disease progression; and (c) radiological evidence of disease progression [6]. These guidelines also mention that nintedanib is the only drug recommended for treating progressive pulmonary fibrosis (PPF) as the phase III clinical trial showed a statistically significant reduction in disease progression and the adverse events are reversible with discontinuation of the medication.
According to Cottin and colleagues [1], ILDs may be categorized as idiopathic interstitial pneumonias (IIPs), autoimmune ILDs, hypersensitivity pneumonitis, sarcoidosis, and other ILDs. As part of autoimmune ILDs, systemic sclerosis (SSc) associated with ILD (SSc-ILD) is of particular interest as it is an autoreactive immune connective tissue disease characterized by inflammation, immune dysregulation, microvascular damage, and progressive fibrosis of the skin and internal organs. SSc mostly affects women, and its peak onset is at 30–70 years of age. Thus, this represents a population whose productivity can be affected by the disease [1]. ILD in SSc presents early in the disease course [7] and impacts both survival and quality of life [8, 9].
Within the group of fibrosing ILDs (F-ILD), around 30% of them can become progressive. Results of a multinational study showed that some subtypes such as hypersensitivity pneumonitis are more prone to progression while others like sarcoidosis are unlikely to be progressive. In that study, the rate of progression of SSc-ILD was 37% [2].
While scientific knowledge of the clinical impact of ILD on patient outcomes has increased a lot in recent years, only some studies have calculated the direct costs borne by the national healthcare systems in Europe due to progressive pulmonary fibrosis other than idiopathic pulmonary fibrosis (non-IPF PPF) [10, 11] and SSc-ILD [12]. Indirect costs (i.e., the productivity loss) should also be taken into consideration to give a more complete picture of the disease burden.
Our study aimed to estimate the productivity loss and burden of two types of ILD, namely non-IPF PPF and SSc-ILD.
Methods
A simple economic model was built to estimate the productivity loss of SSc-ILD and non-IPF PPF in the European Economic Area (EEA). The model was based on published data on disease prevalence and impact, and on the region-specific economic value of labor.
Desktop Research
In July 2022, a medical literature search was conducted using PubMed for data about epidemiology and work productivity losses associated with non-IPF PPF (and SSc-ILD in particular). The keywords used for epidemiology data were “epidemiology”, “ILD”, “incidence”, “interstitial lung disease”, “prevalence”, and “pulmonary fibrosis”. The keywords used for productivity losses data were “burden of disease”, “disability”, “early retirement”, “economic impact”, “interstitial lung disease”, “job loss”, “productivity loss”, and “sick days”. Only articles reporting on European data were selected. Only articles in English with abstract available were considered. Data coming from institutional documents (gray literature) were used to determine the monetary value of working days in the EEA.
Ethical Considerations
This study is based on previously published data. No ethics committee approval was required for this study.
Input Data
Population
A prevalence of 27.9 per 105 for non-IPF PPF and of 4.25 per 105 for SSc-ILD was used as base case in the model. Furthermore, an incidence of 6.50 per 105 and 0.75 per 105 was used for non-IPF PPF and SSc-ILD, respectively. The data come from a retrospective study conducted in six European countries as the average of the minimum and maximum values for the overall countries’ value (mean of the six countries included) [2].
Affected population was calculated by applying the aforementioned prevalence and incidence rates to the working-age population (assumed from 20 to 64 years) of each country/area from European and international statistics institutions [13]. It was assumed that the population below 20 years old and above 64 years old do not work, thereby productivity loss due to the disease in these population groups has been considered negligible in this paper. For comorbidities, exacerbations, and deaths, the whole adult population (≥ 18 years) was considered.
Burden of Disease
Burden of disease measures the impact of living in a condition of illness. Here, we measure it by presenting data on comorbidities, exacerbations, and deaths due to both non-IPF PPF and SSc-ILD.
Comorbidities
Percentages of comorbidities were extracted from DELPHI studies conducted in a selection of European countries [4, 14] and are presented in Supplementary Materials (Table s1). They were multiplied by the relative affected adult population to calculate the estimated number of patients for each specific comorbidity.
Exacerbations
We assumed that only patients with a progressing form of ILD could experience exacerbations. We used the rate of disease progression from the PERSEIDS study for SSc-ILD to calculate the number of patients with progressive SSc-ILD [2]. Given the rate of exacerbation depends on whether patients with non-IPF PPF are treated, and nintedanib is the only treatment that has been granted marketing authorization for non-IPF PPF, we considered placebo and nintedanib-treated data from the INBUILD phase 3 trial [15]. Table 1 shows the methodology to calculate the yearly exacerbation rate in the EEA, which was then multiplied by the affected adult population (ranging between the two extreme assumptions of all patients/no patient treated with nintedanib) to afford an estimated number of patients with exacerbations.
Table 1.
Methodology to calculate the yearly exacerbation rate in the European Economic Area [15]
| Nintedanib | Placebo | |
|---|---|---|
| (A) Total exacerbations | 98 | 136 |
| (B) Median follow-up time in months | 19 | 19 |
| (C) Median follow-up time in years (B/12) | 1.58 | 1.58 |
| (D) Patients at beginning of trial | 332 | 331 |
| (E) Total person-year (C × D ) | 526 | 524 |
| Exacerbation rate (A/E) | 0.19 | 0.26 |
Deaths
The same as for exacerbations, death rate depends on whether patients are treated. Percentages were multiplied by the relative affected adult population (assuming that first all patients were treated with nintedanib and then all with placebo) to get an estimated number of deaths caused by non-IPF PPF and SSc-ILD in the INBUILD [15] and SENSCIS trials [16]. Annual death rate in untreated patients is 5.1% for patients with non-IPF PPF and 3.1% for patients with SSc-ILD, while it is 4.8% and 3.5% in treated patients for non-IPF PPF and SSc-ILD, respectively.
Income
Daily median income was calculated by dividing annual income [17] by the number of working days specific for EU27, UK, and EFTA (assumed to have the same working days as the EU [18]). Income was expressed in terms of euros (€).
Annual and daily median net incomes for each of the countries analyzed are reported in Table 2.
Table 2.
Annual and daily median net incomes in EEA
| Country/area | Annual median income (€) | Daily median income (€) |
|---|---|---|
| EU27 | 18,296 | 72 |
| Austria | 27,428 | 109 |
| Belgium | 25,739 | 102 |
| Bulgaria | 5157 | 20 |
| Croatia | 8061 | 32 |
| Cyprus | 16,686 | 66 |
| Czechia | 10,625 | 42 |
| Denmark | 32,088 | 127 |
| Estonia | 12,623 | 50 |
| Finland | 25,456 | 101 |
| France | 22,680 | 90 |
| Germany | 25,015 | 99 |
| Greece | 8752 | 35 |
| Hungary | 6614 | 26 |
| Ireland | 28,130 | 111 |
| Italy | 17,532 | 69 |
| Latvia | 9437 | 37 |
| Lithuania | 9669 | 38 |
| Luxembourg | 37,844 | 150 |
| Malta | 16,240 | 64 |
| Netherlands | 28,441 | 113 |
| Poland | 8295 | 33 |
| Portugal | 10,800 | 43 |
| Romania | 4832 | 19 |
| Slovakia | 8703 | 34 |
| Slovenia | 15,415 | 61 |
| Spain | 15,892 | 63 |
| Sweden | 25,498 | 101 |
| EFTA | 41,070a | 163 |
| Iceland | 39,918 | 158 |
| Liechtenstein | NA | NA |
| Norway | 40,241 | 159 |
| Switzerland | 43,051 | 170 |
| UK | 21,464 | 85 |
| EU27 + EFTA | 19,562a | 77 |
| EEA (EU27 + EFTA + UK) | 19,623a | 78 |
Data obtained from Eurostat 2021 (exceptions: Luxembourg, Malta, Portugal, Slovakia, Norway, Switzerland, and EU27 2020; Iceland and UK: 2018) [17] and National Central Banks, 2021 [18]
EEA European Economic Area, EFTA European Free Trade Association, EU27 European Union including 27 countries (from 2020), NA not available, Non-IPF PPF non-idiopathic pulmonary fibrosis progressive pulmonary fibrosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, UK United Kingdom
aMean of median incomes
The daily median income was about €78 in the whole EEA, but with huge variations, ranging from €19 in Romania to €170 in Switzerland.
Productivity Loss
The economic impact in terms of sick days, early retirement, permanent disability, and job loss was estimated as described in Supplementary Materials (Table s2).
Main sources of data were from DELPHI studies conducted in a selection of European countries [4, 14]. Other retrieved publications provided information on some aspects of productivity loss, and were used in the sensitivity analyses.
Aggregate Income Loss
Aggregate income loss is an economic measure of the potential loss, in terms of economic productivity, that non-IPF PPF and SSc-ILD create in society, in particular the working class. The estimation is based on the appropriate combination of different variables affecting the working-age population on a yearly basis, considering the value loss due to sick days, early retirement, and permanent disability.
Total sick days were obtained by multiplying the affected population by mean sick days per patient, which in turn was the result of the number of sick leaves per year multiplied by duration of the sick leave in days. To obtain the economic value to society, total sick days were then multiplied by the median daily income in each country/area of investigation, computed by dividing total annual income by working days [18] in a year.
Productivity loss due to early retirement was obtained by subtracting the affected incident population from the affected prevalent one and multiplying the result by the median annual income; the affected incident population is multiplied by half median annual income, thus assuming that the retirement happened on average during the middle of the year. Finally, productivity loss due to early retirement was computed as the sum of the two aforementioned figures.
Analogously, productivity loss due to permanent disability was obtained by first subtracting the affected incident population from the affected prevalent one and multiplying the result by the median annual income. To obtain total productivity loss due permanent disability, this figure was then added to the product of the affected incident population and half median annual income, again assuming that the event happened on average during the middle of the year.
Finally, aggregate annual income loss was computed by summing all the items obtained above. This cost is an estimation of the productivity loss that the disease inflicts on society in a year, in particular by lost added value for the aggregate economy.
Job Loss
Productivity loss due to job loss was left out of the aggregate result computation to prevent the likely risk of overlapping calculations which may lead to an overestimation of the total economic loss.
As above, productivity loss due to job loss was obtained by first subtracting the affected incident population from the affected prevalent one and multiplying the result by the median annual income. To obtain total productivity loss due to job loss, this figure was then added to the product of the affected incident population and half median annual income, again assuming that the event (i.e., being unemployed) happened on average during the middle of the year.
Sensitivity Analyses
A one-way sensitivity analysis was performed to evaluate the sensitivity of the aggregate income lost to each parameter. A specific variable was varied one at a time while maintaining all others fixed to test its impact on aggregate income lost. Parameters of prevalence and incidence were varied following their minimum and maximum value retrieved in the literature. All minimum and maximum values were taken from Hilberg et al. [2], except for the minimum value of non-IPF PPF prevalence, which was taken from Nasser et al. [19] (Supplementary Materials, Table s3). Cost for annual sick days, early retirement, and permanent disability varied by ± 20%.
While Cottin et al. [20] found that 20.7% of patients with SSc-ILD had sick leave daily allowances, it was not possible to input this information into our sensitivity analysis to calculate productivity loss.
Results
Base Case
Affected Population
In the whole EEA, a total of 86,794 individuals were estimated to be affected by non-IPF PPF, and 13,221 by SSc-ILD. Germany, being the country with the greatest number of adult patients, accounts for the most elevated number of patients, i.e., an estimated 13,824 and 2106 for non-IPF PPF and SSc-ILD, respectively.
It was calculated that every year, non-IPF PPF determined an estimated 3,952,604 sick days in the whole EEA. Prevalent retired, permanently disabled, and unemployed patients add up to 23,174, 41,748, and 19,789 individuals, respectively. With regards to SSc-ILD, 672,172 sick days were estimated in the whole EEA and prevalent annual retired patients, permanent disabled patients, and unemployed patients added up to 5341, 4037, and 2617 individuals, respectively. Overall results are presented in Table 3 and details per country can be found in Supplementary Materials, Tables s4 and s5.
Table 3.
Estimation of the total number of individuals affected by non-IPF PPF and SSc-ILD and number of annual events of sick days, affected prevalent individuals regarding early retirements, permanent disability, and job loss
| Country/area | Affected population | Sick days (annual) | Patients retired early (annual) | Patients permanently disabled (annual) | Patients who lost their job (annual) |
|---|---|---|---|---|---|
| Non-IPF PPF | |||||
| EU27 | 73,535 | 3,348,800 | 19,634 | 35,371 | 16,766 |
| EFTA | 2441 | 111,167 | 652 | 1174 | 557 |
| EEA (EU27 + EFTA + UK) | 86,794 | 3,952,604 | 23,174 | 41,748 | 19,789 |
| SSc-ILD | |||||
| EU27 | 11,202 | 569,490 | 4525 | 3420 | 2217 |
| EFTA | 372 | 18,905 | 150 | 114 | 74 |
| EEA (EU27 + EFTA + UK) | 13,221 | 672,172 | 5341 | 4037 | 2617 |
Working-age population figures, on which calculations were based, are from Eurostat, 2021 [21] (except for UK: last available data 2019)
EEA European Economic Area, EFTA European Free Trade Association, EU27 European Union including 27 countries (from 2020), Non-IPF PPF non-idiopathic pulmonary fibrosis progressive pulmonary fibrosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, UK United Kingdom
Burden of Disease
Exacerbations and Deaths
The estimated annual number of patients who directly experienced an exacerbation or death due to non-IPF PPF is shown in Table 4. Between 22,401 and 31,181 patients with non-IPF PPF are estimated to have had an exacerbation and 5791–6171 patients with non-IPF PPF are estimated to have died across Europe. Between 1259 and 1753 patients with SSc-ILD are estimated to have had an exacerbation and 572–638 patients with SSc-ILD are estimated to have died across Europe. Details per country can be found in Supplementary Materials, Tables s6 and s7.
Table 4.
Estimated annual number of patients who directly experienced an exacerbation or death due to non-IPF PPF and SSc-ILD
| Country | Exacerbations all treated | Exacerbations all placebo | Deaths all treated | Deaths all placebo |
|---|---|---|---|---|
| Non-IPF PPF | ||||
| EU27 | 19,038 | 26,500 | 4921 | 5245 |
| EFTA | 609 | 848 | 157 | 168 |
| EEA (EU27 + EFTA + UK) | 22,401 | 31,181 | 5791 | 6171 |
| SSc-ILD | ||||
| EU27 | 1070 | 1490 | 542 | 486 |
| EFTA | 34 | 48 | 17 | 16 |
| EEA (EU27 + EFTA + UK) | 1259 | 1753 | 638 | 572 |
EEA European Economic Area, EFTA European Free Trade Association, EU27 European Union including 27 countries (from 2020), Non-IPF PPF non-idiopathic pulmonary fibrosis progressive pulmonary fibrosis, UK United Kingdom
Comorbidities
Table 5 shows the estimated total number of patients affected by each specific comorbidity in the EEA both for SSc-ILD and non-IPF PPF. More than 30,000 patients with non-IPF PPF were estimated to suffer from fatigue, gastroesophageal reflux disease, pulmonary infection and depression and more than 4,000 patients with SSc-ILD to suffer from gastroesophageal reflux disease, fatigue, pulmonary hypertension and depression across Europe.
Table 5.
Estimated total number of patients affected by each specific comorbidity in the EEA both for SSc-ILD and non-IPF PPF
| Non-IPF PPF | SSc-ILD | |
|---|---|---|
| Respiratory function | ||
| Pulmonary infection | 35,206 | 3807 |
| Chronic bronchitis | 9372 | 897 |
| Asthma | 2764 | 366 |
| Obstructive sleep apnea | 17,062 | 494 |
| Lung cancer | 5647 | 458 |
| Pulmonary embolism | 4686 | 421 |
| Pulmonary hypertension | 23,791 | 4338 |
| Chronic obstructive pulmonary disease | 9132 | 604 |
| Emphysema | 11,775 | 604 |
| Cardiovascular function | ||
| Coronary artery disease (excluding MI) | 14,900 | 1080 |
| Heart failure | 16,582 | 1354 |
| Atrial fibrillation | 14,179 | 1043 |
| Myocardial infarction | 4566 | 183 |
| Deep vein thrombosis | 3364 | 458 |
| Cerebrovascular disease | 3845 | 238 |
| Metabolic disease | ||
| Diabetes | 19,586 | 1592 |
| Obesity | 17,303 | 1062 |
| Gastroesophageal reflux disease | 35,086 | 9848 |
| Other diseases | ||
| Depression | 32,683 | 4137 |
| Osteoporosis | 24,392 | 3478 |
| Cataracts | 12,256 | 1354 |
| Fatigue | 54,792 | 7377 |
EEA European Economic Area, EFTA European Free Trade Association, EU27 European Union including 27 countries (from 2020), MI myocardial infarction, Non-IPF PPF non-idiopathic pulmonary fibrosis progressive pulmonary fibrosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, UK United Kingdom
Productivity Loss
Aggregate Income Loss
Table 6 presents productivity loss figures for non-IPF PPF and SSc-ILD. In the EEA, the annual aggregate income loss estimated for non-IPF PPF was €1,432,657,033, with the loss due to permanently disabled patients being the most burdensome among the factors considered (€723,809,823). Regarding SSc-ILD, the annual aggregate income loss was estimated to be €220,009,339, with the loss due to early retired patients being the most burdensome among the factors considered (€95,556,241). Tables s8 and s9 in Supplementary Materials provide data per country.
Table 6.
Estimated productivity loss and aggregate income loss due to non-IPF PPF
| Country | Annual sick days loss (€) | Annual early retired patients’ loss (€) | Annual permanent disabled patients’ loss (€) | Annual aggregate income loss (€) |
|---|---|---|---|---|
| Non-IPF PPF | ||||
| EU27 | 242,561,148 | 317,380,712 | 571,768,296 | 1,131,710,156 |
| EFTA | 18,074,923 | 23,656,320 | 42,589,590 | 84,320,833 |
| EEA (EU27 + EFTA + UK) | 307,067,510 | 401,779,700 | 723,809,823 | 1,432,657,033 |
| SSc-ILD | ||||
| EU 27 | 41,249,447 | 75,480,148 | 57,046,928 | 173,776,523 |
| EFTA | 3,073,812 | 5,606,055 | 4,271,280 | 12,951,147 |
| EEA (EU27 + EFTA + UK) | 52,219,292 | 95,556,241 | 72,233,807 | 220,009,339 |
EEA European Economic Area, EFTA European Free Trade Association, EU27 European Union including 27 countries (from 2020), Non-IPF PPF non-idiopathic pulmonary fibrosis progressive pulmonary fibrosis, UK United Kingdom
Job Loss
Table 7 shows an estimation of the productivity loss due to people losing their job as a consequence of both non-IPF PPF and SSc-ILD. These results are not part of the aggregate income loss above so that any possible double counting is ruled out. The estimated productivity loss due to job losses for both diseases is €219,841,167 (88.3% of which non-IPF PPF).
Table 7.
Estimation of the productivity loss due to people losing their job as a consequence of both non-IPF PPF and SSc-ILD
| Country | Job loss due to non-IPF PPF (€) | Job loss due to SSc-ILD (€) |
|---|---|---|
| EU27 | 153,375,368 | 20,281,116 |
| Austria | 4,799,900 | 630,844 |
| Belgium | 5,521,016 | 733,562 |
| Bulgaria | 672,989 | 87,669 |
| Croatia | 616,667 | 80,610 |
| Cyprus | 292,005 | 41,715 |
| Czechia | 2,140,938 | 281,563 |
| Denmark | 3,449,460 | 449,232 |
| Estonia | 309,264 | 44,181 |
| Finland | 2,520,144 | 330,928 |
| France | 27,057,240 | 3,572,100 |
| Germany | 39,423,640 | 5,215,628 |
| Greece | 1,728,520 | 227,552 |
| Hungary | 1,230,204 | 162,043 |
| Ireland | 2,630,155 | 351,625 |
| Italy | 19,407,924 | 2,568,438 |
| Latvia | 330,295 | 42,467 |
| Lithuania | 517,292 | 67,683 |
| Luxembourg | 491,972 | 56,766 |
| Malta | 170,520 | 24,360 |
| Netherlands | 9,300,207 | 1,222,963 |
| Poland | 6,100,973 | 804,615 |
| Portugal | 2,084,400 | 275,400 |
| Romania | 1,758,848 | 231,936 |
| Slovakia | 939,924 | 126,194 |
| Slovenia | 616,600 | 84,783 |
| Spain | 14,572,964 | 1,922,932 |
| Sweden | 4,768,126 | 624,701 |
| EFTA | 11,437,995 | 1,519,590 |
| Iceland | 279,426 | 39,918 |
| Liechtenstein | NA | NA |
| Norway | 4,084,462 | 543,254 |
| Switzerland | 7,275,619 | 968,648 |
| UK | 26,465,112 | 3,498,632 |
| EU27 + EFTA | 169,436,840 | 22,408,347 |
| EEA (EU27 + EFTA + UK) | 194,163,923 | 25,677,244 |
EEA European Economic Area, EFTA European Free Trade Association, EU27 European Union including 27 countries (from 2020), NA not available, Non-IPF PPF non-idiopathic pulmonary fibrosis progressive pulmonary fibrosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, UK United Kingdom
Sensitivity Analyses
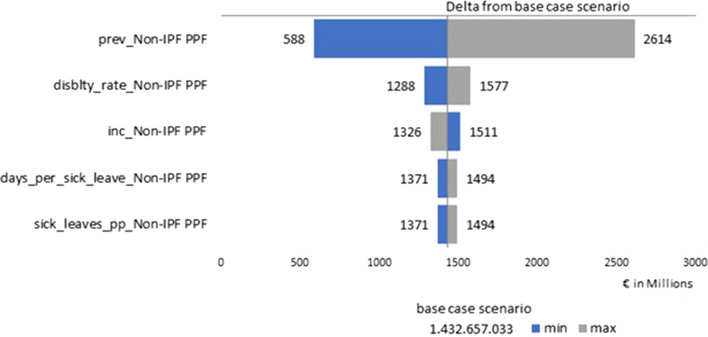
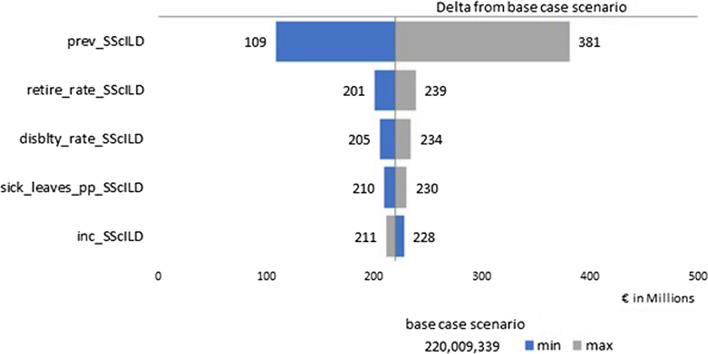
Results of the deterministic sensitivity analyses are summarized as a tornado diagram to highlight the variables that had the most impact on aggregate income lost. The input providing the highest variability with respect to the base case for both non-IPF PPF (Fig. 1) and SSc-ILD (Fig. 2) was the prevalence.
Fig. 1.
Tornado diagram about the deterministic sensitivity analysis for non-IPF PPF. Non-IPF PPF non-idiopathic pulmonary fibrosis progressive pulmonary fibrosis
Fig. 2.
Tornado diagram about the deterministic sensitivity analysis for SSc-ILD. SSc-ILD systemic sclerosis-associated interstitial lung disease
Discussion
Our results show that the burden of non-IPF PPF and SSc-ILD on society is significant in Europe. It was estimated that 86,794 patients with non-IPF PPF and 13,221 patients with SSc-ILD were living in Europe. While the prognosis of patients with non-IPF PPF and patients with SSc-ILD is poor, comorbidities, sick leaves, permanent disability, and early retirement contribute to the disease burden, not only for the healthcare systems but also on society overall. We estimated the aggregated income loss to be €1433 million for non-IPF PPF and €220 million for SSc-ILD. Permanent disability and early retirement were the most important source of aggregate income loss, representing around half and 43% of costs for non-IPF PPF and SSc-ILD, respectively. Additionally, job losses resulting from the diseases were estimated to cost €194 million for non-IPF PPF and €26 million for SSc-ILD.
There are very few economic studies around the burden of PPF and SSc-ILD. A cost-effectiveness analysis about non-IPF PPF was conducted in the Netherlands by Westerink and colleagues [22], whose model was based on data coming from the INBUILD trial [23]. It showed that the societal costs per patient, including round trip to hospital, general practitioner, and specialist, healthcare worker/domestic support at home, and sick days, were in the range €1627–2403, with the lowest cost associated with patients treated with nintedanib. These figures are much lower than those calculated in our study, as we found that the estimated annual cost per patient was €16,506. However, it is difficult to compare such costs since the methodologies used are different, as were the nature of costs. In the Westerink study, costs related to early retirements, permanent disability, and job losses were not included.
Another study conducted in France assessed the disease burden associated with progressive fibrosing. In this study, total median annual costs per patient were €18,362, including all hospitalizations, specific laboratory tests, imagery and treatment, and specific medical and paramedical costs but excluding sick leave, daily allowances, and transport costs [19]. While these costs are similar to those in Westerink et al.’s study, as our study focuses on productivity losses, comparison with the two aforementioned studies is irrelevant.
SSc is generally diagnosed during working age (mean 46 years) [24] and associated ILD arises within the first 5 years after diagnosis [25]. Therefore, in addition to increased direct costs, a huge economic burden is expected due to the loss of productivity. A small US claims data analysis performed by Zhou et al. [26] evaluated sick days and the relevant costs of 52 insured and employed patients with SSc-ILD compared with healthy controls. In the 6 months immediately following the diagnosis, they found that the sick days per patient were 23.2. Hypothesizing a comparison with our results by doubling the aforementioned figures to convert to 12-month values, we obtain that 46.4 annual sick days per patient. These data are very similar to the assumptions of our study (50.8 annual sick days). Nonetheless, comparing US to EEA productivity loss costs is irrelevant, as the median income in the USA [27] is 3-fold than that found in the EEA [17, 18].
The first follow-up European study to evaluate indirect costs associated with SSc-ILD was the study by Knarborg and colleagues [12] based on a Danish national patients registry. They evaluated the excess direct healthcare costs and indirect costs associated with SSc in patients with and without ILD 5 years before and 4 years after the diagnosis, finding reduced mean annual income per case of €9763 in SSc-ILD cohort compared with €6604 in the non-ILD SSc cohort. Foregone earnings were calculated not only for working cases but also for the entire Danish SSc population. Disability pension was the key driver of excess public transfer income in both cohorts after diagnosis. In our SSc-ILD study cohort, around one-third of annual income loss was due to permanent disabled patients’ loss, which is a lower proportion than that found in our non-IPF PPF cohort, maybe as a result of the slow progression rate of fibrosis in SSc-ILD [1].
Antifibrotics were already used in IPF when the INBUILD trial [23], which reported its efficacy also in non-IPF PPF, was published, thus resulting in a paradigm shift toward an en bloc approach to antifibrotic therapy [6]. Pirfenidone is also an antifibrotic agent, with anti-inflammatory, antioxidative, and antiproliferative properties. It was recommended for treatment of IPF in prior guidelines. However, the official ATS/ERS/JRS/ALAT Clinical Practice Guideline [6] found that data about the use of pirfenidone were insufficient to give a recommendation for its use in non-IPF PPF, as two randomized clinical trials with small sample sizes were analyzed [28, 29], one of which was terminated early as a result of futility triggered by slow recruitment [28].
Dyspnea, cough, and fatigue are the most common symptoms of non-IPF PPF and heavily affect health-related quality of life (HRQoL) [30]. Nintedanib proved to be able to significantly reduce the worsening of cough and dyspnea in patients with non-IPF PPF [31]. In fact, nintedanib had a statistically [32] and clinically [33] meaningful impact on HRQoL on the cough domain score of Living with Pulmonary Fibrosis (L-PF) questionnaire administered to patients with non-IPF PPF recruited in the INBUILD trial [15].
In addition to the disease-modifying drugs, a supportive care program should also be offered to patients in order to meet their needs and relieve the disease burden [34, 35]. The early diagnosis and management of the progressive behavior of the disease is particularly important as it may be able to prevent the worsening of the quality of life, thus reducing the burden of the disease [34].
These measures are underused in Europe and generally accessed only at the final stages of the disease, despite carrying the potential to reduce symptom burden [36]. One of the reasons is that they are too often called “palliative care” and such terms often make patients perceive themselves as “doomed” [35]. Therefore, a comprehensive support strategy, generally indicated as “holistic care”, able not only to slow down the progression of the disease but also to offer supportive measures, symptom relief, and end-of-life care [34], has emerged in the last decade as an unmet need for patients affected by non-IPF PPF.
In particular, supportive measures include support groups, both nurse-led and among peers, pulmonary rehabilitation, and supplemental oxygen. Also education programs, where attended, have received the appreciation of patients [34]. In the BUILDup study [4], most panelists agreed that progressive fibrosing ILDs affect caregivers’ quality of life in terms of sleep and health, daily activities, emotional well-being, social life, and finances. As a result of substantial psychological burden carried by caregivers, the need for emotional and educational support is apparent [35]. However, both for emotional and educational support groups, the impact in the long-term on well-being is still to be demonstrated. Pulmonary rehabilitation proved to be able to significantly increase exercise capacity, quality of life, and dyspnea in the short term, but again data about the long term are lacking, even though the gains in quality of life seem to be preserved [34]. Supplemental oxygen is recommended by the official ATS/ERS/JRS/ALAT Clinical Practice Guideline [6] in patients with hypoxia, but few data support these recommendations [34].
Finally, end-of-life care means facing the conversation about end-of life decisions, in accordance with patient’s preference and religious beliefs. As a result of the frequent avoidance of such a conversation, even though the majority of patients prefer to die at home, most of them actually die in the hospital [34].
In a review by van Manen and colleagues about IPF [37], a comprehensive program was summarized as the “ABCDE of IPF care”, and then adapted by Kreuter et al. [35] to ILD care in general, i.e., “Assessing patients’ needs; Backing patients by giving information and support; delivering Comfort care by focusing on treating symptoms and taking into account Comorbidities; striving to prolong life by Disease modification; helping and preparing patients and their caregivers for the eventual End-of-life events that are likely to occur”. Besides, our study shows a significant proportion of patients with SSc-ILD and patients with non-IPF PPF were estimated to have fatigue or depression. A Greek study reported that depression was indirectly associated with disease severity, symptom burden, and quality of life in patients with IPF, reinforcing the need for mental support for patients with ILD [38].
In Europe, surveys conducted in European countries among healthcare professionals (HCPs) and patients with IPF and non-IPF PPF showed that less than 50% of patients had access to pulmonary rehabilitation, maybe as a result of incomplete reimbursements, unawareness, and physical distance issues [36]. In addition, as already reported in the literature, patients and their caregivers feel the need for better emotional and psychological support [36], but reimbursement and access to psychologists for this type of patients are still restricted. Therefore, the support groups mentioned above are the best alternative and have been shown to be appreciated by patients. In addition, the need for educational activities and active involvement of patients in the development of treatment plans emerged from these surveys [36].
Even though the course of non-IPF PPF is unpredictable, supportive care should be offered early, as it is able to partly relieve the disease burden [35]. Brereton and colleagues, who analyzed several models of palliative care life-limiting illnesses, found that models of palliative care show several benefits for patients and caregivers and just few disadvantages, but the final conclusion about the cost-effectiveness of palliative care cannot be drawn because of the heterogeneity of diseases and strategies of palliative care considered [39]. We speculate that an improved state of health may reduce sick days and the relevant productivity loss.
In the future, novel approaches should be taken to diagnose ILDs early on or to prevent or limit their progression, ultimately limiting the burden on healthcare systems and societies. In the field of precision medicine, biomarkers at an acceptable cost could provide actionable information to clinicians for instance regarding disease activity or the need for treatment modification [40].
Our study has some limitations. First of all, as evidenced by Hilberg and colleagues [2], prevalence and incidence estimates about ILDs are highly variable, both according to the type of ILD considered and among European countries. Besides, in our study we used the mean of several countries and therefore did not reflect country variations or any potential fluctuation in prevalence with age. The uncertainty about the economic impact is the direct consequence of the uncertainty about epidemiological data. In our sensitivity analysis, disease prevalence is the factor that impacts aggregate income loss estimates the most. Given the large range of prevalence used in the sensitivity analysis based on a multicountry epidemiology study, it is likely that the actual aggregate income loss falls within that range.
Similarly, we applied data from BUILDup studies, which were based on opinions of European experts gathered and elaborated thanks to the DELPHI methods, by using averages [4, 14]. However, it is very likely that differences among countries exist, as reported about another respiratory disease, i.e., chronic obstructive pulmonary disease (COPD). In fact, in a systematic review conducted in some European countries [41], indirect costs varied greatly, as costs of work productivity loss ranged from €5735 (Germany) to €998 (Greece) and early retirement cost varied €19,031 (Germany) to €3695 (Sweden). Besides, as BUILDup was based on expert opinions and not from patients themselves, productivity loss data should be taken with caution, and more research on this topic should be done.
We used the comorbidity data from BUILDup studies and not an average of different real-world evidence studies, which presents limitations. In the BUILDup study on PPF, the most prevalent comorbidities were fatigue (45.6%), pulmonary infection (29.3%), gastroesophageal reflux disease (29.2%), depression (27.2%), osteoporosis (20.3%), and pulmonary hypertension (19.8%) [4]. In Nasser et al.’s study in France, the most prevalent diseases were arterial hypertension (63.8%), gastroesophageal reflux disease (55.4%), cardiac arrythmias (21.9%), depression (20.5%), and congestive heart failure (20.0%) [19]. While there is variability between studies, it seems our assumptions do not overestimate the high level of comorbidities in patients with ILD.
Our study excluded direct costs. Direct costs typically include hospitalizations such as for exacerbations, visits to health professionals, or medication. This type of information is not available in a several European countries and costs do not always follow the level of citizens income, making estimations difficult. Besides, some country healthcare systems can be composed of multiple financing systems and calculations of direct costs can differ across countries, some including value-added tax and others not.
We assumed in our model that patients with non-progressive SSc-ILD do not present exacerbations. As exacerbations are characterized by acute, clinically significant respiratory deteriorations, our assumption is valid for modeling disease burden and at worse underestimate the burden of disease. Besides, we assumed that 37% of patients with SSc-ILD are progressive, which was based on patient file review [2]. This is comparable to Wijsenbeek et al.’s study that shows that 31% of patients with SSc-ILD have progressive disease [3].
Other limitations include the fact that exacerbation rates and mortality with or without treatment are based on clinical trial data and not based on European real-world evidence over a longer follow-up period. While this might affect our results overall, we assumed this approach to be appropriate enough for modelling purposes.
Finally, our study relies on few articles assessing the epidemiology and the societal burden of ILD. Although we conducted a sensitivity analysis to reflect potential variations in assumptions, more investigation is needed on productivity loss for patients with ILD.
Conclusion
Despite the limitations and possible biases about indirect costs of non-IPF PPF and SSc-ILD in the EEA, the results of our present study point towards a unique direction, i.e., that the impact of non-IPF PPF and SSc-ILD on society is definitely non-negligible, as in the EEA it is estimated at €1,432,657,033 and €220,009,339, respectively. Job losses are responsible for estimated costs of €194,163,923 and €25,677,244 in non-IPF PPF and SSc-ILD, respectively. It seems reasonable to undertake actions to reduce the burden on our societies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing, Editorial, and Other Assistance
We acknowledge Laura Fascio Pecetto from SEEd Medical Publishers, who provided medical writing, publishing support, and journal styling services. These services were funded by Boehringer Ingelheim.
Authorship
All authors whose names appear on the submission have: (1) Made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work. (2) Drafted the work or revised it critically for important intellectual content. (3) Approved the version to be published; and (4) Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Author Contributions
All authors contributed to the study conception and design. Luca Castello compiled publicly available data and performed the analysis. Stephane Soulard provided methodological support. Anders Lokke, Pedro Pinheiro Martins, and Ole Hilberg provided scientific content to the study for the design and the interpretation of results. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Boehringer Ingelheim bv. (Amsterdam, the Netherlands), which also funded medical writing, publishing support, and journal styling services. The authors did not receive payment related to the development of the manuscript. The study sponsor is also funding the journal’s Rapid Service and Open Access Fees.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
This manuscript is supported and funded by Boehringer Ingelheim. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. Stephane Soulard and Pedro Pinheiro Martins are employees of Boehringer Ingelheim. Boehringer Ingelheim compensated AdRes HE&OR to do the analysis. Anders Lokke, Luca Castello, and Ole Hilberg have nothing to disclose.
Ethical Approval
This study is based on previously published data. No ethics committee approval was required for this study.
References
- 1.Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27:180076. doi: 10.1183/16000617.0076-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilberg O, Hoffmann-Vold A-M, Smith V, et al. Epidemiology of interstitial lung diseases and their progressive-fibrosing behaviour in six European countries. ERJ Open Res. 2022;8:00597–2021. doi: 10.1183/23120541.00597-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijsenbeek M, Kreuter M, Olson A, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin. 2019;35:2015–2024. doi: 10.1080/03007995.2019.1647040. [DOI] [PubMed] [Google Scholar]
- 4.Wuyts WA, Papiris S, Manali E, et al. The burden of progressive fibrosing interstitial lung disease: a DELPHI approach. Adv Ther. 2020;37:3246–3264. doi: 10.1007/s12325-020-01384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson AL, Maher TM, Acciai V, et al. Healthcare resources utilization and costs of patients with non-IPF progressive fibrosing interstitial lung disease based on insurance claims in the USA. Adv Ther. 2020;37:3292–3298. doi: 10.1007/s12325-020-01380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205:e18–47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeger VK, Wirz EG, Allanore Y, et al. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: a longitudinal EUSTAR study. PLoS ONE. 2016;11:e0163894. doi: 10.1371/journal.pone.0163894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann-Vold A-M, Bendstrup E, Dimitroulas T, et al. Identifying unmet needs in SSc-ILD by semi-qualitative in-depth interviews. Rheumatology (Oxford) 2021;60:5601–5609. doi: 10.1093/rheumatology/keab154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann-Vold A-M, Fretheim H, Halse A-K, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med. 2019;200:1258–1266. doi: 10.1164/rccm.201903-0486OC. [DOI] [PubMed] [Google Scholar]
- 10.Cottin V, Teague R, Nicholson L, Langham S, Baldwin M. The burden of progressive-fibrosing interstitial lung diseases. Front Med (Lausanne) 2022;9:799912. doi: 10.3389/fmed.2022.799912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maqhuzu PN, Kreuter M, Bahmer T, et al. Cost drivers in the pharmacological treatment of interstitial lung disease. Respir Res. 2021;22:218. doi: 10.1186/s12931-021-01807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knarborg M, Løkke A, Hilberg O, Ibsen R, Sikjaer MG. Direct and indirect costs of systemic sclerosis and associated interstitial lung disease: a nationwide population-based cohort study. Respirology. 2022;27:341–349. doi: 10.1111/resp.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eurostat. Glossary: retirement age (legal, standard, reference). https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Retirement_age_(legal,_standard,_reference). Accessed 08 Nov 2022.
- 14.Davidsen JR, Miedema J, Wuyts W, et al. Economic burden and management of systemic sclerosis-associated interstitial lung disease in 8 European countries: the BUILDup Delphi consensus study. Adv Ther. 2021;38:521–540. doi: 10.1007/s12325-020-01541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive interstitial lung diseases: data from the whole INBUILD trial. Eur Respir J. 2022;59:2004538. doi: 10.1183/13993003.04538-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 17.Eurostat. Statistics. 2022. https://ec.europa.eu/eurostat/databrowser/view/ILC_DI03__custom_1275633/default/table?lang=en. Accessed 28 Oct 2022.
- 18.Euro area and EU working days to build Calendar Adjustment Regressor. CROS - European Commission. 2016. https://ec.europa.eu/eurostat/cros/content/euro-area-and-eu-working-days-build-calendar-adjustment-regressor_en. Accessed 28 Oct 2022.
- 19.Nasser M, Larrieu S, Boussel L, et al. Estimates of epidemiology, mortality and disease burden associated with progressive fibrosing interstitial lung disease in France (the PROGRESS study) Respir Res. 2021;22:162. doi: 10.1186/s12931-021-01749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottin V, Larrieu S, Boussel L, et al. Epidemiology, mortality and healthcare resource utilization associated with systemic sclerosis-associated interstitial lung disease in France. Front Med (Lausanne) 2021;8:699532. doi: 10.3389/fmed.2021.699532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eurostat. Population on 1 January by age group and sex. 2021. https://ec.europa.eu/eurostat/databrowser/view/demo_pjangroup/default/table?lang=en. Accessed 28 Oct 2022.
- 22.Westerink L, Nicolai JLJ, Postma MJ, van Boven JFM, Boersma C. Cost-effectiveness of nintedanib for patients with progressive fibrosing interstitial lung disease (PF-ILD) Pharmacoecon Open. 2022;6:647–656. doi: 10.1007/s41669-022-00354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 24.Mayes MD, Lacey JV, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 25.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Z, Fan Y, Thomason D, et al. Economic burden of illness among commercially insured patients with systemic sclerosis with interstitial lung disease in the USA: a claims data analysis. Adv Ther. 2019;36:1100–1113. doi: 10.1007/s12325-019-00929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Statistica. Median household income in the United States from 1990 to 2022. 2023. https://www.statista.com/statistics/200838/median-household-income-in-the-united-states/. Accessed 05 Jun 2023.
- 28.Behr J, Prasse A, Kreuter M, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021;9:476–486. doi: 10.1016/S2213-2600(20)30554-3. [DOI] [PubMed] [Google Scholar]
- 29.Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8:147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 30.Swigris JJ, Brown KK, Abdulqawi R, et al. Patients’ perceptions and patient-reported outcomes in progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27:180075. doi: 10.1183/16000617.0075-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency. OFEV Summary of Product Characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/ofev-epar-product-information_en.pdf. Accessed 30 Apr 2023.
- 32.Swigris JJ, Richeldi L, Wijsenbeek M, et al. Effects of nintedanib on dyspnea, cough and quality of life in patients with progressive fibrosing interstitial lung diseases: findings from the INBUILD Trial | B16 ILD THERAPY II. ATS 2020 Int Conf Am Thorac Soc Int Conf Meet Abstr. 2020 doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2754?role=tab. [DOI] [Google Scholar]
- 33.Swigris JJ, Bushnell DM, Rohr K, Mueller H, Baldwin M, Inoue Y. Responsiveness and meaningful change thresholds of the living with pulmonary fibrosis (L-PF) questionnaire dyspnoea and cough scores in patients with progressive fibrosing interstitial lung diseases. BMJ Open Respir Res. 2022;9:e001167. doi: 10.1136/bmjresp-2021-001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijsenbeek MS, Holland AE, Swigris JJ, Renzoni EA. Comprehensive supportive care for patients with fibrosing interstitial lung disease. Am J Respir Crit Care Med. 2019;200:152–159. doi: 10.1164/rccm.201903-0614PP. [DOI] [PubMed] [Google Scholar]
- 35.Kreuter M, Bendstrup E, Russell A-M, et al. Palliative care in interstitial lung disease: living well. Lancet Respir Med. 2017;5:968–980. doi: 10.1016/S2213-2600(17)30383-1. [DOI] [PubMed] [Google Scholar]
- 36.Moor CC, Wijsenbeek MS, Balestro E, et al. Gaps in care of patients living with pulmonary fibrosis: a joint patient and expert statement on the results of a Europe-wide survey. ERJ Open Res. 2019;5:00124–2019. doi: 10.1183/23120541.00124-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Manen MJG, Geelhoed JJM, Tak NC, Wijsenbeek MS. Optimizing quality of life in patients with idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2017;11:157–169. doi: 10.1177/1753465816686743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzouvelekis A, Karampitsakos T, Kourtidou S. Impact of depression on patients with idiopathic pulmonary fibrosis. Front Med (Lausanne) 2020;7(7):29. doi: 10.3389/fmed.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brereton L, Clark J, Ingleton C, et al. What do we know about different models of providing palliative care? Findings from a systematic review of reviews. Palliat Med. 2017;31:781–797. doi: 10.1177/0269216317701890. [DOI] [PubMed] [Google Scholar]
- 40.Karampitsakos T, Juan-Guardela BM, Tzouvelekis A, Herazo-Maya JD. Precision medicine advances in idiopathic pulmonary fibrosis. EBioMedicine. 2023;95:104766. doi: 10.1016/j.ebiom.2023.104766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehman AU, Hassali MAA, Muhammad SA, Harun SN, Shah S, Abbas S. The economic burden of chronic obstructive pulmonary disease (COPD) in Europe: results from a systematic review of the literature. Eur J Health Econ. 2020;21:181–194. doi: 10.1007/s10198-019-01119-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.