Abstract
Introduction
Patient-reported outcomes (PROs) provide an insightful method of assessing the subjective impact of therapies for those affected by multiple sclerosis (MS), a chronic neurologic disease notable for symptoms of fatigue and reduced physical function. The ongoing CLAWIR study aims to assess the effect of cladribine tablets (3.5 mg/kg cumulative dose over 2 years) in patients with highly active relapsing MS focusing on PROs of fatigue, physical function, treatment satisfaction, and work productivity. Here, we report on a pre-planned analysis at 12 months after treatment initiation with cladribine tablets.
Methods
CLAWIR is a 2-year, multicenter, prospective, observational study of patients with relapsing MS newly initiating cladribine tablets. The following PROs were analyzed: PRO Measurement Information System (PROMIS®) Fatigue MS (v1.0) and Physical Function MS (v2.1), Treatment Satisfaction Questionnaire for Medication (TSQM, v1.4), and Work Productivity and Activity Impairment MS Questionnaire (WPAI-MS). Data were analyzed descriptively.
Results
In total, 128 patients were eligible for analysis: 95 females (74.2%); median (range) age 34.5 (29, 44) years; 34 patients (26.6%) were treatment-naïve, and 89 (69.5%) were early switchers from platform therapies (the remaining 5 patients [3.9%] switched from a high-efficacy disease-modifying therapy). PROMIS® Fatigue MS mean (± standard deviation [SD]) T-scores decreased from 54.6 (± 9.59) at baseline to 51.8 (± 10.30) at 12 months, indicating an alleviation of fatigue, whereas PROMIS® Physical Function MS mean T-scores remained stable over time [baseline: 49.4 (± 10.69); 12 months: 50.3 (± 10.88)]. TSQM v1.4 mean scores indicated an improvement over time, increasing from 52.2 (± 27.79) at baseline to 81.4 (± 17.06) at 12 months for global satisfaction. WPAI-MS scores also showed an improvement across all four domains over 12 months.
Conclusion
This real-world study demonstrates the effect of cladribine tablets over 12 months on PROs of fatigue, physical function, treatment satisfaction, and work productivity.
Trial Registration
The CLAWIR study is registered at the German Federal Institute for Drugs and Medical Devices with the internal NIS number 7469.
Keywords: Cladribine tablets, Multiple sclerosis, Patient-reported outcomes
Key Summary Points
| Patient-reported outcomes (PROs) provide an insightful method of assessing the real-world benefit of disease-modifying therapies for patients with multiple sclerosis (MS), in particular, the effect on major contributors to the disabling symptoms of MS such as fatigue and reduced physical function |
| The ongoing 2-year CLAWIR study aims to assess the effect of cladribine tablets in patients with highly active relapsing MS, focusing on PROs describing fatigue, physical function, treatment satisfaction, and work productivity |
| At 12 months after initiating treatment with cladribine tablets, results of this real-world study show an improvement in the PROMIS® Fatigue MS score, stability in the PROMIS® Physical Function MS score, an increase in treatment satisfaction, and an improvement in work productivity |
Introduction
Within the field of multiple sclerosis (MS), a chronic disabling neurologic disease, the benefit of a disease-modifying therapy (DMT) from a patient’s perspective can be greatly advanced by describing patient-reported outcomes (PROs) [1, 2]. Assessing the impact of fatigue, a major contributor to the disabling symptoms experienced by people with MS (pwMS), and monitoring physical function provide further information on a patient’s well-being and satisfaction with their medication. Against this background, the PRO Measurement Information System (PROMIS®), a patient-centric measure to evaluate physical, mental, and social well-being of individuals living with chronic conditions [3], was established. The PROMIS® measure has been further developed and expanded to provide a valid and informative assessment of fatigue [4, 5] and physical function [6] in pwMS.
Data on PROs with one such oral DMT for the treatment of relapsing MS, cladribine tablets (3.5 mg/kg cumulative dose over 2 years administered as one treatment course of 1.75 mg/kg per year) are relatively scant; yet real-world data are accumulating [7–11] and have been recently reviewed [12, 13]. The ongoing CLAWIR (CLAdribine tablets in patients WIth Relapsing MS) study was therefore designed to further evaluate PROs over the 2-year treatment course with cladribine tablets in a real-world setting, using standardized questionnaires. As recent clinical studies have suggested, an early onset of action for cladribine tablets in terms of routine clinical/MRI outcomes [14] and monitoring and evaluation of PROs from the start of treatment would represent a valuable addition to the understanding of the effects of cladribine tablets from the patient’s perspective. In this report, we therefore describe the results of a pre-planned interim analysis focusing on PROs to evaluate fatigue, physical function, treatment satisfaction, and work productivity over 12 months from the start of treatment with cladribine tablets.
Methods
CLAWIR is an ongoing multicenter, prospective, observational, non-interventional cohort study of adult patients newly initiating therapy with cladribine tablets for relapsing MS. The observation time is 2 years, including seven routine clinic visits (therapy start and at months 2, 6, 12, 14, 18, and 24). At therapy start, details on demographics (gender and age) and MS history—including type of MS, time since diagnosis, number of relapses in the previous 12 months, and treatment history—were collected. This pre-planned interim analysis (data cutoff January 2023) includes patients who completed the PRO assessments at month 12 after treatment initiation.
Outcomes
PROs were analyzed descriptively at baseline and months 6 and 12. The primary outcome was the change in fatigue and physical function. Fatigue was assessed by the PROMIS® Short Form version (v) 1.0—Fatigue-Multiple Sclerosis 8a [4, 5]. This measure consists of eight items that capture the severity of fatigue, conceptualized as the experience (i.e., intensity and frequency) and impact of fatigue, with minimal respondent burden. It is scored on a T-score metric, which is standardized for the US general population, and has a mean of 50 and a standard deviation (SD) of 10; higher T-scores indicate higher levels of fatigue. The minimal important difference (MID) is a decrease in T-score of 3.4–4.0 points, as described by Kamudoni et al. [5]. Physical function was assessed using PROMIS® Short Form v2.1—Physical Function-Multiple Sclerosis 15a [6]. A T-score is calculated based on the response from 15 items and standardized as described earlier; higher T-scores indicate improved physical function. Score changes of 2.3–2.7 points are proposed as the MID threshold for ‘minimal worsening’ in physical function [6]. In our study, we conservatively considered the MID for improvement as an increase in T-score of > 2.7 points, with changes in T-score in the range of 2.7 to − 2.7 points correlating with stability of physical function (P. Kamudoni, personal communication).
The secondary outcome was the comparison of treatment satisfaction and work productivity/activity impairment before and after treatment initiation. The Treatment Satisfaction Questionnaire for Medication (TSQM v1.4) was utilized, a scale designed to assess participants’ overall satisfaction with their medication [15, 16]. It consists of 14 items that assess the following four areas: effectiveness, side effects, convenience, and global satisfaction, with higher scores indicating greater treatment satisfaction. The Work Productivity and Activity Impairment MS Questionnaire (WPAI-MS) was selected as a patient-reported quantitative assessment of impairment attributable to general health or a specific health problem comprising of four types of scores [17, 18]: activity impairment—percent activity impairment due to health; presenteeism—percent impairment while working due to health/reduced on-the-job effectiveness; work productivity loss—percent overall work impairment due to health; and absenteeism—percentage of worktime missed due to health. The questions refer to the past 7 days, with higher scores indicating greater impairment of work productivity/activity.
Compliance with Ethics Guidelines
The study was performed in accordance with the 1964 Declaration of Helsinki, and its later amendments, and all applicable local rules and regulations. The CLAWIR study protocol and the informed consent form were approved by the local ethics review board (Landesärztekammer Baden-Württemberg, No. F-2020-147). All patients provided written informed consent to participate.
Analysis
All data are descriptive; corresponding 95% confidence intervals (CI) were calculated where appropriate. Data used for this report cover a maximum observation period of 12 months under therapy with cladribine tablets; only complete and valid data have been included in the analysis. Continuous variables are presented in terms of mean (SD) and median values (range), while categorical variables are summarized using frequencies and percentages. All analyses were performed using the statistical software SAS version 9.2 (or higher).
Results
Study Population
Patient demographics and clinical characteristics are shown in Table 1. A total of 128 patients were evaluable at the point of data cutoff (January 2023), including 95 females (74.2%). Median (range) age at enrollment was 34.5 (29, 44) years, and 125 patients (97.7%) were diagnosed with relapsing-remitting MS (the remaining patients were diagnosed with secondary progressive MS). A total of 89 patients had experienced one or more relapses in the previous year. Patients’ prior DMT history is shown in Table 1; 34 patients (26.6%) were treatment-naïve. Of the DMT-experienced patients, 69.5% (N = 89) were early switchers from platform therapies such as interferon, glatiramer acetate, teriflunomide, and dimethyl fumarate, while the remaining 3.9% (N = 5) had switched from the high-efficacy DMT natalizumab.
Table 1.
Patient characteristics/demographics
| Parameter | Study population, N = 128 (100%) |
|---|---|
| Median age, years (range) | 34.5 (29, 44) |
| Gender, n (%) | |
| Female | 95 (74.2) |
| Male | 33 (25.8) |
| Type of MS, n (%) | |
| Relapsing-remitting MS | 125 (97.7) |
| Secondary progressive MS | 3 (2.3) |
| Median time since MS diagnosis at enrollment, years (range) | 5.0 (1, 10) |
| Number of relapses/patient in the previous 12 months, n | |
| 0 | 39 |
| 1 | 57 |
| 2 | 22 |
| ≥ 3 | 6 |
| Unknown | 4 |
| Prior DMT history, n (%) | |
| No DMT | 34 (26.6) |
| One DMT | 42 (32.8) |
| Two DMTs | 33 (25.8) |
| ≥ Three DMTs | 19 (14.8) |
DMT disease-modifying therapy, MS multiple sclerosis, n number of patients
PROs
The PROMIS® Fatigue MS T-scores showed a numerical decrease over time from a mean score (± SD) of 54.6 (± 9.59) at baseline to 53.8 (± 9.12) after 6 months and 51.8 (± 10.30) after 12 months (Fig. 1). The 95% CI for change from baseline to 12 months was − 4.8 to − 1.6 points. Within this range, the number of patients that achieved the MID, suggesting an improvement of fatigue, was 56.7% (N = 59).
Fig. 1.
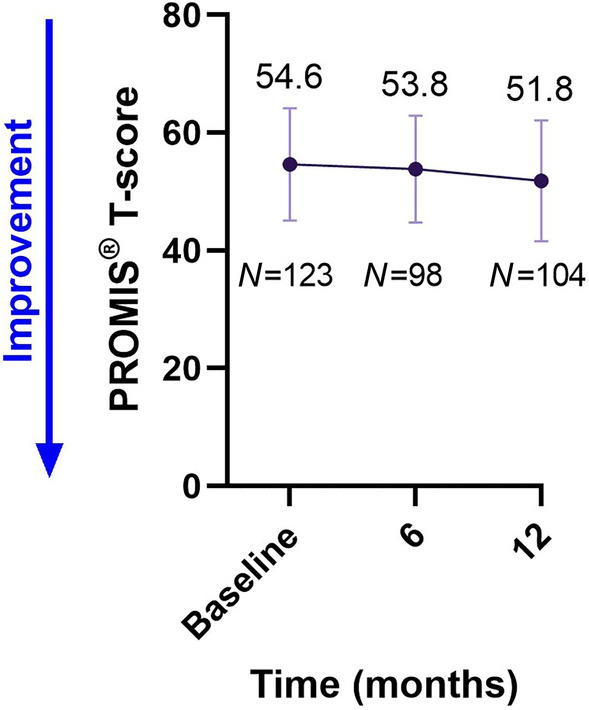
PROMIS® Fatigue MS scores over time. Values shown are mean ± SD. MS multiple sclerosis, N number of patients, PROMIS® Patient-Reported Outcome Measurement Information System
The PROMIS® Physical Function MS T-scores remained stable over time from a mean score (± SD) of 49.4 (± 10.69) at baseline to 49.0 (± 10.62) and 50.3 (± 10.88), respectively, after 6 and 12 months (Fig. 2). The 95% CI for change from baseline to 12 months was 0.2–2.4 points. Overall, 82.6% of patients (N = 86) experienced an improvement or stability of their physical function (range from − 2.7 to > 2.7 points).
Fig. 2.
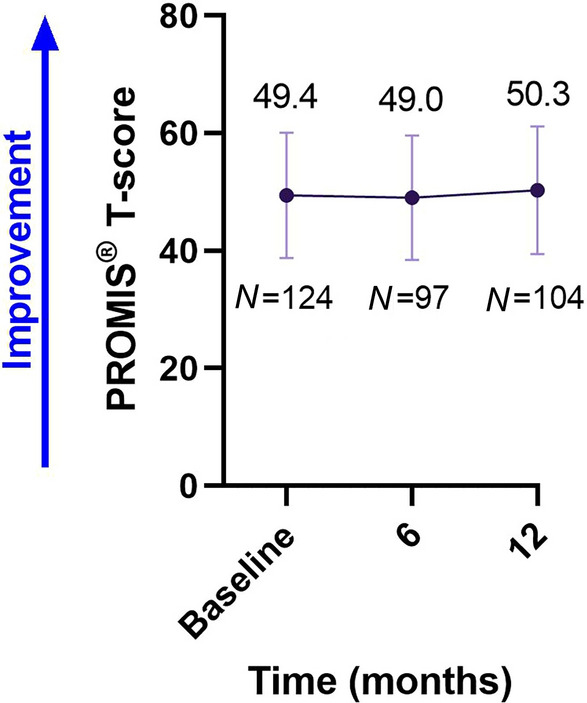
PROMIS® Physical Function MS scores over time. Values shown are mean ± SD. MS multiple sclerosis, N number of patients, PROMIS® Patient-reported Outcome Measurement Information System
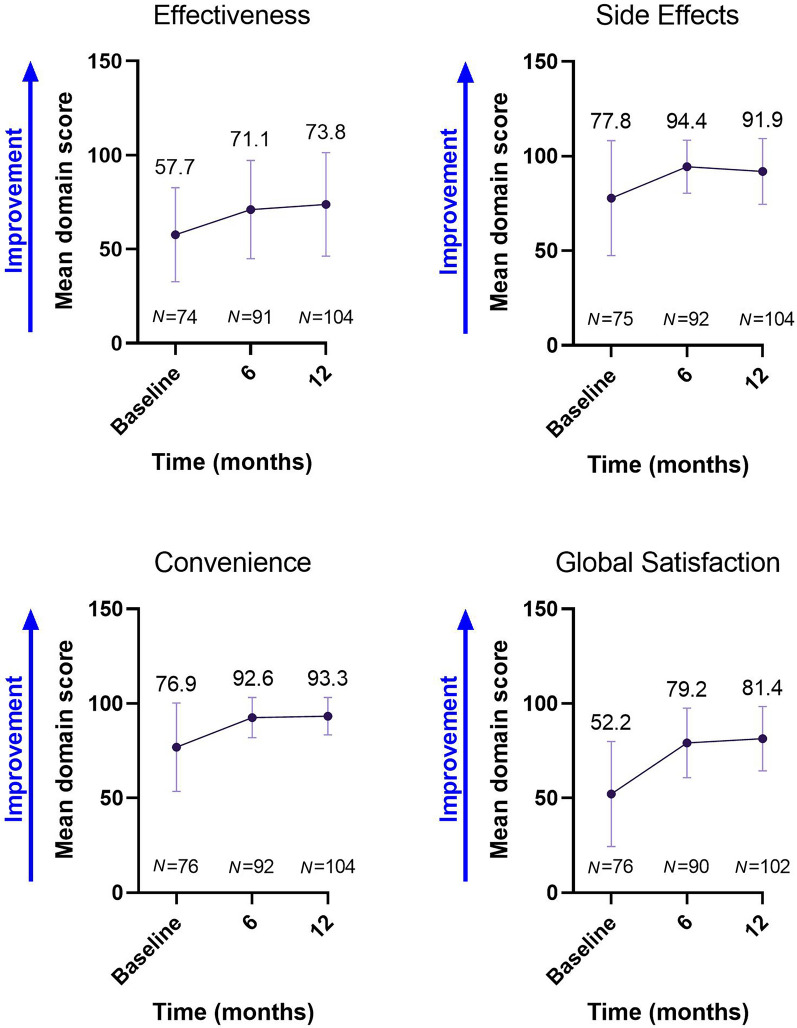
TSQM v1.4 domain scores showed a numerical increase over time. Regarding global satisfaction, the mean score (± SD) increased from 52.2 (± 27.79) at baseline to 79.2 (± 18.38) and 81.4 (± 17.06), respectively, after 6 and 12 months (Fig. 3).
Fig. 3.
TSQM v1.4 scores over time. Values shown are mean ± SD. N number of patients, TSQM Treatment Satisfaction Questionnaire for Medication
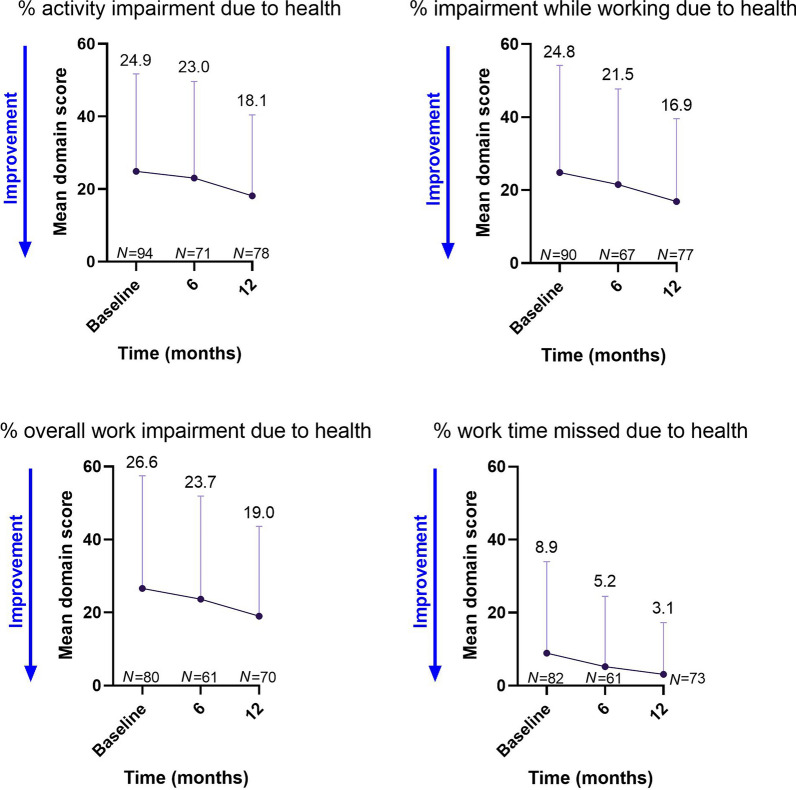
WPAI-MS mean scores also showed a numerical decrease over time, consistent with reduced impairment across all four domains (Fig. 4).
Fig. 4.
WPAI-MS scores over time. Values shown are mean ± SD. MS multiple sclerosis, N number of patients, WPAI-MS Work Productivity and Activity Impairment Multiple Sclerosis Questionnaire
Discussion
The findings of this pre-planned 12-month analysis build on our earlier results at 6 months after initiation of treatment with cladribine tablets [19], and show a numerical improvement in the PROMIS® Fatigue MS score, stability in the PROMIS® Physical Function MS score, an increase in treatment satisfaction, and an improvement in work productivity with reduced activity impairment. Our findings therefore provide an indication of the validity of the PROMIS® instruments in a real-world setting.
The PROMIS® Fatigue MS and Physical Function MS measurements represent valid, sensitive, patient-centric measures of fatigue and mobility in pwMS, and thus are of high value in both routine care and in clinical research as new methods to better capture these facets of patient symptoms [3, 5, 6]. The numerical decrease in scores from baseline to month 12 in the PROMIS® Fatigue MS score is therefore an important finding, as it indicates an alleviation of fatigue from the patient perspective. Indeed, 56.7% (N = 59) patients achieved the MID, confirming the validity of the result. Moreover, lessening the impact of fatigue, a common disabling symptom experienced by pwMS, would represent a substantial easing of the economic burden of MS [20].
The PROMIS® Physical Function MS scores remained stable over the 12-month time period, which is believed to represent an important outcome of treatment for those with MS. This analysis is consistent with the Expanded Disability Status Scale (EDSS) findings of the CLARITY and CLARITY Extension studies with cladribine tablets, in which EDSS scores remained stable for up to 5 years after treatment initiation [21–23].
Treatment satisfaction, as determined by TSQM v1.4, showed a sustained increase over 12 months in all four domains, correlating with improved treatment satisfaction regarding effectiveness, side effects, convenience, and global satisfaction. Indeed, the improvement in each domain score exceeded the recently reported bounds for clinically meaningful improvement [24]. Such findings are also consistent with those of the CLARIFY-MS study, which reported on the high treatment satisfaction using the same (TSQM) analytical method at 6 months and was the first reported use of TSQM v1.4 as a measure of treatment satisfaction with cladribine tablets [25]. In addition, the mean score for global satisfaction was consistent with that recently reported in the CLUE study, which also examined treatment satisfaction with cladribine tablets at 6 months using TSQM v1.4 [26].
The decrease in WPAI scores from baseline to 12 months in each of the four categories showed a lessening of activity impairment after initiation of treatment with cladribine tablets. Notably, an improvement was observed across all domains, including absenteeism (worktime missed), presenteeism (impairment at work), work productivity loss (overall work impairment), and activity impairment (worktime missed) due to health. These improvements correspond with a reduction in the mean number of work days missed, and a mean increase in self-reported productivity, in patients treated with cladribine tablets in the CLARITY study [27].
PROs on the effectiveness and safety of cladribine tablets, including fatigue (through the 5-item version of Modified Fatigue Impact Scale), physical functioning (36-Item Short Form Health Survey), TSQM v1.4, and WPAI-MS, are also being evaluated as part of the CLICK-MS and MASTER-2 trials [28] and will provide a useful comparison with the results described in our study.
Concerning study limitations, the number of patients completing WPAI-MS was lower than the response rate of PROMIS® and TSQM, indicating that patients may engage better if the complexities of the questionnaires are reduced. We must also consider that the results from this pre-planned analysis at 12 months reflect only half the planned 2-year treatment course of cladribine tablets being received. At the time of this analysis, 76.3% of patients had received their second treatment course, and further analyses will determine if additional benefits may be observed for PROs at 24 months.
Conclusions
This real-world study builds on the data derived from the 6-month analysis, showing an alleviation of fatigue, no worsening of physical function, an increase in treatment satisfaction, and improved work productivity with cladribine tablets over 12 months from treatment initiation from the patient’s perspective. The study data also provide additional evidence of the use of validated PRO instruments, including PROMIS® Fatigue MS and Physical Function MS, TSQM v1.4, and WPAI-MS, in a real-world setting of treatment for MS.
Acknowledgements
The authors thank participating patients and their families, investigators, co-investigators, and study teams at each of the participating centers and at the healthcare business of Merck KGaA, Darmstadt, Germany.
Medical Writing and Editorial Assistance
Medical writing assistance for the development of this manuscript, under the direction of the authors, was provided by Claire Snaith and Steve Winter of inScience Communications, Springer Healthcare Ltd., UK, and was supported by the healthcare business of Merck KGaA, Darmstadt, Germany.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and take responsibility for the integrity of the work as a whole.
Author Contributions
Conceptualization, methodology/study design, validation, resources, data curation, writing—original draft, writing—review and editing: Daniela Rau, Beate Müller, Susanne Übler; software, formal analysis, visualization, project administration: Susanne Übler; investigation: Daniela Rau; supervision, funding acquisition: Beate Müller. All authors contributed to writing of the manuscript and have given their approval for this version to be published.
Funding
The healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945) provided funding for this study and payment of Rapid Service and Open Access Fees.
Data Availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data sharing portal for the healthcare business of Merck KGaA, Darmstadt, Germany https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When the healthcare business of Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA will endeavor to gain agreement to share data in response to requests.
Declarations
Conflict of Interest
Daniela Rau has received honoraria for speaking at scientific meetings, serving at scientific advisory boards and consulting activities from Bayer, Biogen, Celgene (BMS), Eli Lilly and Company, the healthcare business of Merck KGaA, Darmstadt, Germany, Novartis, Roche, Sanofi, and Teva. She has received research support from the healthcare business of Merck KGaA, Darmstadt, Germany. Beate Müller and Susanne Übler are employees of Merck Healthcare Germany GmbH, Weiterstadt, Germany, an affiliate of Merck KGaA, Darmstadt, Germany.
Ethical Approval
The study was performed in accordance with the 1964 Declaration of Helsinki, and its later amendments, and all applicable local rules and regulations. The CLAWIR study protocol and the informed consent form were approved by the local ethical review board (Landesärztekammer Baden-Württemberg, no. F-2020-147). All patients provided written informed consent to participate.
References
- 1.D'Amico E, Haase R, Ziemssen T. Review: patient-reported outcomes in multiple sclerosis care. Mult Scler Relat Disord. 2019;33:61–66. doi: 10.1016/j.msard.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Nowinski CJ, Miller DM, Cella D. Evolution of patient-reported outcomes and their role in multiple sclerosis clinical trials. Neurotherapeutics. 2017;14(4):934–944. doi: 10.1007/s13311-017-0571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MK, Schalet BD, Cella D, Yost KJ, Dueck AC, Novotny PJ, et al. Establishing a common metric for patient-reported outcomes in cancer patients: Linking patient reported outcomes measurement information system (PROMIS), numerical rating scale, and patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Patient Rep Outcomes. 2020;4(1):106. doi: 10.1186/s41687-020-00271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamudoni P, Johns J, Cook KF, Salem R, Salek S, Raab J, et al. A comparison of the measurement properties of the PROMIS Fatigue (MS) 8a against legacy fatigue questionnaires. Mult Scler Relat Disord. 2022;66:104048. 10.1016/j.msard.2022.104048. [DOI] [PubMed]
- 5.Kamudoni P, Johns J, Cook KF, Salem R, Salek S, Raab J, et al. Standardizing fatigue measurement in multiple sclerosis: The validity, responsiveness and score interpretation of the PROMIS SF v1.0—Fatigue (MS) 8a. Mult Scler Relat Disord. 2021;54:103117. 10.1016/j.msard.2021.103117. [DOI] [PubMed]
- 6.Kamudoni P, Amtmann D, Johns J, Cook KF, Salem R, Salek S, et al. The validity, responsiveness, and score interpretation of the PROMIS Physical Function—Multiple Sclerosis 15a short form in multiple sclerosis. Mult Scler Relat Disord. 2022;62:103753. 10.1016/j.msard.2022.103753. [DOI] [PubMed]
- 7.Petracca M, Ruggieri S, Barbuti E, Ianniello A, Fantozzi R, Maniscalco GT, et al. Predictors of cladribine effectiveness and safety in multiple sclerosis: A real-world, multicenter, 2-year follow-up study. Neurol Ther. 2022;11(3):1193–1208. doi: 10.1007/s40120-022-00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeuffer S, Rolfes L, Hackert J, Kleinschnitz K, Ruck T, Wiendl H, et al. Effectiveness and safety of cladribine in MS: Real-world experience from two tertiary centres. Mult Scler. 2022;28(2):257–268. doi: 10.1177/13524585211012227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauma I, Viitala M, Kuusisto H, Atula S, Sipilä JOT, Ryytty M, et al. Finnish multiple sclerosis patients treated with cladribine tablets: A nationwide registry study. Mult Scler Relat Disord. 2022;61:103755. 10.1016/j.msard.2022.103755. [DOI] [PubMed]
- 10.Sorensen PS, Pontieri L, Joensen H, Heick A, Rasmussen PV, Schäfer J, et al. Real-world experience of cladribine treatment in relapsing-remitting multiple sclerosis: A Danish nationwide study. Mult Scler Relat Disord. 2023;70:104491. 10.1016/j.msard.2022.104491. [DOI] [PubMed]
- 11.Aerts S, Khan H, Severijns D, Popescu V, Peeters LM, Van Wijmeersch B. Safety and effectiveness of cladribine tablets for multiple sclerosis: Results from a single-center real-world cohort. Mult Scler Relat Disord. 2023;75:104735. 10.1016/j.msard.2023.104735. [DOI] [PubMed]
- 12.Moser T, Ziemssen T, Sellner J. Real-world evidence for cladribine tablets in multiple sclerosis: Further insights into efficacy and safety. Wien Med Wochenschr. 2022;172(15–16):365–372. doi: 10.1007/s10354-022-00931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oreja-Guevara C, Brownlee W, Celius EG, Centonze D, Giovannoni G, Hodgkinson S, et al. Expert opinion on the long-term use of cladribine tablets for multiple sclerosis: Systematic literature review of real-world evidence. Mult Scler Relat Disord. 2023;69:104459. 10.1016/j.msard.2022.104459. [DOI] [PubMed]
- 14.de Stefano N, Barkhof F, Montalban X, Achiron A, Derfuss T, Chan A, et al. Early reduction of MRI activity during 6 months of treatment with cladribine tablets for highly active relapsing multiple sclerosis: MAGNIFY-MS. Neurol Neuroimmunol Neuroinflamm. 2022;9(4):e1187. 10.1212/nxi.0000000000001187. [DOI] [PMC free article] [PubMed]
- 15.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermersch P, Hobart J, Dive-Pouletty C, Bozzi S, Hass S, Coyle PK. Measuring treatment satisfaction in MS: Is the treatment satisfaction questionnaire for medication fit for purpose? Mult Scler. 2017;23(4):604–613. doi: 10.1177/1352458516657441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 18.Glanz BI, Dégano IR, Rintell DJ, Chitnis T, Weiner HL, Healy BC. Work productivity in relapsing multiple sclerosis: Associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health. 2012;15(8):1029–1035. doi: 10.1016/j.jval.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Rau D, Richter J, Wagner T, Müller B, Übler S. Evaluation of patient-reported outcomes: CLAWIR study 6-month interim analysis. Abstract and poster presentation at the 30th Annual Meeting of the European Charcot Foundation, 17–19 November 2022. Baveno, Italy.
- 20.Le HH, Ken-Opurum J, LaPrade A, Maculaitis MC, Sheehan JJ. Assessment of economic burden of fatigue in adults with multiple sclerosis: An analysis of US National Health and Wellness Survey data. Mult Scler Relat Disord. 2022;65:103971. 10.1016/j.msard.2022.103971. [DOI] [PubMed]
- 21.Giovannoni G, Boyko A, Correale J, Edan G, Freedman MS, Montalban X, et al. Long-term follow-up of patients with relapsing multiple sclerosis from the CLARITY/CLARITY Extension cohort of CLASSIC-MS: An ambispective study. Mult Scler. 2023;29(6):719–730. doi: 10.1177/13524585231161494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giovannoni G, Comi G, Rammohan K, Rieckmann P, Dangond F, Keller B, et al. Long-term disease stability assessed by the Expanded Disability Status Scale in patients treated with cladribine tablets 3.5 mg/kg for relapsing multiple sclerosis: An exploratory post hoc analysis of the CLARITY and CLARITY Extension studies. Adv Ther. 2021;38(9):4975–85. 10.1007/s12325-021-01865-w. [DOI] [PMC free article] [PubMed]
- 23.Signori A, Ponzano M, Alexandri N, Giovannoni G, Sormani MP. Prevalence of disability improvement in relapsing-remitting multiple sclerosis patients treated with cladribine tablets. Eur J Neurol. 2022;29(7):2144–2147. doi: 10.1111/ene.15316. [DOI] [PubMed] [Google Scholar]
- 24.Greene N, Quere S, Bury DP, Mazerolle F, M'Hari M, Loubert A, et al. Establishing clinically meaningful within-individual improvement thresholds for eight patient-reported outcome measures in people with relapsing-remitting multiple sclerosis. J Patient Rep Outcomes. 2023;7(1):61. doi: 10.1186/s41687-023-00594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brochet B, Hupperts R, Langdon D, Solari A, Piehl F, Lechner-Scott J, et al. Treatment satisfaction, safety, and tolerability of cladribine tablets in patients with highly active relapsing multiple sclerosis: CLARIFY-MS study 6-month interim analysis. Mult Scler Relat Disord. 2022;57:103385. 10.1016/j.msard.2021.103385. [DOI] [PubMed]
- 26.Inshasi J, Farouk S, Shatila A, Hassan A, Szolics M, Thakre M, et al. Multicentre observational study of treatment satisfaction with cladribine tablets in the management of relapsing multiple sclerosis in the Arabian Gulf: The CLUE study. Neuro Ther. 2023;12(4):1309–18. 10.1007/s40120-023-00497-2. [DOI] [PMC free article] [PubMed]
- 27.Ali S, Paracha N, Cook S, Giovannoni G, Comi G, Rammohan K, et al. Reduction in healthcare and societal resource utilization associated with cladribine tablets in patients with relapsing-remitting multiple sclerosis: analysis of economic data from the CLARITY Study. Clin Drug Investig. 2012;32(1):15–27. doi: 10.2165/11593310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Miravalle AA, Katz J, Robertson D, Hayward B, Harlow DE, Lebson LA, et al. CLICK-MS and MASTER-2 Phase IV trial design: Cladribine tablets in suboptimally controlled relapsing multiple sclerosis. Neurodegener Dis Manag. 2021;11(2):99–111. doi: 10.2217/nmt-2020-0059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data sharing portal for the healthcare business of Merck KGaA, Darmstadt, Germany https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When the healthcare business of Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA will endeavor to gain agreement to share data in response to requests.