Abstract
Over 30% of cases may present with acute airway obstruction due to anaplastic thyroid cancer (ATC). In such situations, performing an emergency tracheostomy may be mandatory to save the life. A retrospective, single-centre study at our centre was conducted between 1 January 2021 to 31 December 2022. We had included 17 patients with asphyxia due to ATC and subsequently underwent tracheostomy for stridor. The overall survival (OS) ranged from 2 days to 16 months (median = 11 months). The 30-day mortality was 17.6%. One-year overall survival was 36%. A statistically significant difference in the OS among patients with distant metastasis and Shin grade IV tracheal infiltration (p < 0.001, Log Rank (Mantel-Cox), CI:95%). The degree of tracheal deviation correlated with the patent age group (Pearson chi-square (pc), p = 0.031), type of anaesthesia used local versus general (pc, p < 0.001) and site of tracheostoma (pc, p = 0.028). The degree of tracheal infiltration correlated with the presence of distant metastasis (pc, p = 0.01) and OS (pc, p = 0.013). Tracheostomy in patients with ATC is performed in extreme circumstances to support an airway. Patients often require isthmectomy to obtain adequate access for a tracheostomy, highlighting the importance of having a highly experienced surgeon involved. An attempt to perform the tracheostomy in the ward or the emergency room under local anaesthesia should be avoided. Patients and relatives should be educated to communicate evolving issues and tracheostomy care in the patient’s best interests, given the unusual context of ATC.
Level of evidence, IV.
Keywords: Thyroid gland, Thyroid cancer, Anaplastic thyroid cancer, Tracheostomy, Head and neck cancer
Introduction
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive cancers in humans [1] with a 5-year overall survival (OS) of 2 to 10% with a median survival rate of 3 to 6 months [2, 3]. Anaplastic carcinomas are relatively rare, and they account for only 1 to 2% of thyroid cancers. Regrettably, they constitute up to 14 to 50% of all mortalities due to all thyroid cancers [4–6]. The median overall survival ranges between 3 and 6 months in locally advanced and metastatic ATC [3, 6, 7], and disease-specific mortality approaches 100% [4]. There is no such thing as an early-stage ATC due to its aggressive nature, which invariably leads to fatality. All anaplastic thyroid cancers are, by definition, stage IV cancers [8]. The group staging only considers gross extrathyroidal extension, node positivity and distant metastasis to upstage the disease further [9].
The recent data from the Surveillance, Epidemiology and End Results (SEER) database suggests that the incidence of ATC is rising. The overall incidence of ATC is 2 to 3 cases per 1 million persons [10, 11]. The ATCs are frequently found in patients with long-standing goitre and lower education background [12]. The American Thyroid Association (ATA), in their recent guidelines for ATC management, strongly emphasise obtaining a rapid and definitive diagnosis of ATC [13]. Unfortunately, patients with ATC seldom visit early, and they usually seek medical attention when they develop symptoms suggestive of locally advanced disease [13–15].
Over 30% of cases may present with acute airway obstruction due to ATC [16]. In such situations performing an emergency tracheostomy may be mandatory to save the life. Due to the progressive and infiltrative nature of the disease, performing such a tracheostomy during emergencies can often be challenging, and considerable expertise is necessary to perform such difficult tracheostomies [16]. Controversy exists in performing an elective/prophylactic tracheostomy in cases with locally advanced ATC without asphyxia. One school of thought suggests that early tracheostomy in a diagnosed ATC will prevent airway obstruction due to disease progression. The other rational school of thought suggests that prophylactic tracheostomy be avoided as it impedes the quality of life and is best avoided until there is an impending airway problem [13, 16, 17]. Due to the upward trend in incidence of anaplastic carcinoma of the thyroid, all surgeons encountering these cases must know the technical nuances in emergency management of anaplastic thyroid carcinoma as the expert Head and Neck or Endocrine surgeon may not be available at every setup. In this study, we describe the rationale, indications, challenges and survival following tracheostomy in locally advanced ATC based on our experience.
Subjects and Methods
This is a retrospective, single-centre study at the Department of Otorhinolaryngology and Head and Neck Surgery at All India Institute of Medical Sciences in Raipur. This study has included treatment-naive patients presenting with airway emergency in a suspected case of locally advanced thyroid tumours, who were later diagnosed with anaplastic thyroid cancer. All the included subjects have undergone tracheostomy for stridor. Ours is a large tertiary care referral centre; we follow strict working guidelines for the management of trauma and emergencies. Patients presenting with such symptoms are triaged and shifted to OT (after consent) in less than 15 to 20 min. The instances with nonanaplastic carcinoma as the diagnosis in the cytology were excluded. The patients admitted and treated at our centre from 1 January 2021 to 31 December 2022 were included in the data analysis. Written and informed consent was taken from all the participating patients. A detailed analysis of demographic data, clinical presentation, investigations, intraoperative findings, postoperative care and follow-up charts was done. The data analysis was performed using IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. The Institutional ethical exemption was taken for the study with deidentified patient data vide letter no 2634/IEC-AIIMSRPR/2022 dated 2 December 2022. All procedures performed in the study were conducted by the ethical standards in the 1964 Declaration of Helsinki, as revised in 2013. The STROCSS 2021 Guidelines have been utilised for data reporting [18].
Results
We had included 17 patients with a thyroid mass who presented with airway emergency and subsequently underwent tracheostomy for asphyxia. All the cases were diagnosed as anaplastic thyroid cancer, confirmed by cytology or histopathology.
General
There were 7 (41.2%) male and 10 (58.8%) female cases. The age ranged from 51 to 75 years (mean = 64.12 years ± σ 7.45). The age group was divided into < 55 and > 55 years as per the AJCC staging. Three (17.6%) cases were < 55 years, and 14 (82.4%) cases were ≥ 55 years of age. The performance status—Eastern Cooperative Oncology Group (ECOG) score was > 2 for all the patients and > 3 for three patients.
Type of Anaesthesia
All the patients had undergone emergency tracheostomies. The tracheostomies were performed under general (GA) in 13 (76.5%) cases, and in 4 (23.5%) cases, the tracheostomy was done under local anaesthesia (LA), mainly due to a drop in oxygen saturation. In all four cases, a temporary needle cricothyrotomy was performed in the outpatient department to improve saturation before a formal tracheostomy in the operation theatre.
Type of Intubation
We were fortunate to intubate all the cases planned under GA. An awake fibreoptic intubation was performed in 7 (41.2%) cases, intubation with the video laryngoscope aid was performed in 3 (17.6%) cases, and conventional intubation was done in 3 (17.6%) patients. In four cases (23.5%), intubation was not done due to temporary needle cricothyrotomy.
Degree of Laryngotracheal Deviation
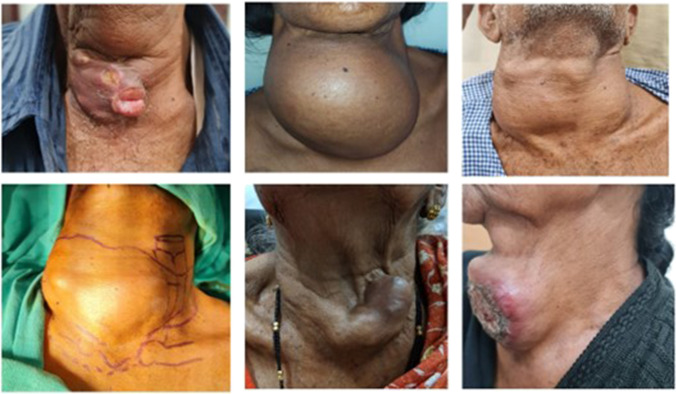
Ikekubo et al. have described four grades (mild, moderate, severe and arched) of tracheal deviation due enlarged thyroid gland [19]. The laryngotracheal complex was deviated in all the cases due to the large tumour (Fig. 1). Moderate deviation was seen in 4 (23.5% cases, severe deviation in 8 (47.1%) cases and arched in 5 (29.4%) cases. Due to the inability to palpate the laryngotracheal complex, in these 3 cases, the airway was marked by laryngotracheal transillumination method with flexible fibreoptic laryngoscope (Fig. 2). The trachea was palpable at the lower pole in 14 (82.4%) cases, and in 3 (17.6%) patients, the tracheal was not discernible at lower pole due to the tumour tracking into the mediastinum. The grade IV tumour infiltrating the trachea (per SHIN grading [20]) was seen in 5 (29.4%) cases.
Fig. 1.
Various presentations of anaplastic thyroid carcinoma as a large thyroid mass displacing the laryngotracheal complex to varying degrees
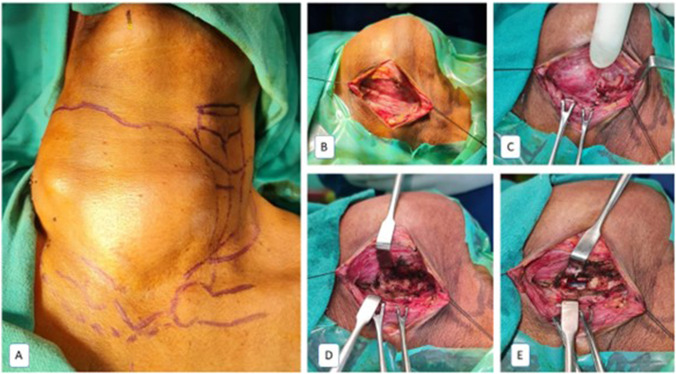
Fig. 2.
Tracheostomy technique. A Fibreoptic trailing with transillumination to mark the laryngotracheal complex (LTC). B Horizontal Kocher’s incision with raised flaps. C Tumour mass occluding the LTC. D Thyroid isthmectomy to expose the LTC. D Tracheotomy to visualise the endotracheal tube
Site of Tracheotomy
The tracheostomy was done below the isthmus if the trachea was readily palpable. In two cases, the laryngotracheal complex was deep-seated. It was located with the aid of intraoperative ultrasonography. The tracheal incision was defined as median (midline entry), paramedian (between midline and tracheoesophageal groove) and lateral tracheostomy (close to tracheoesophageal groove) (Fig. 3). Isthmectomy followed by tracheotomy (Fig. 2) was performed in 10 (58.8%) cases. The site of tracheal entry was dictated by the degree of tracheal deviation and tumour infiltrating into the trachea. Midline tracheotomy was done in 7 (41.2%), paramedian in 7 (41.2%) and lateral in 3 (17.6%) cases.
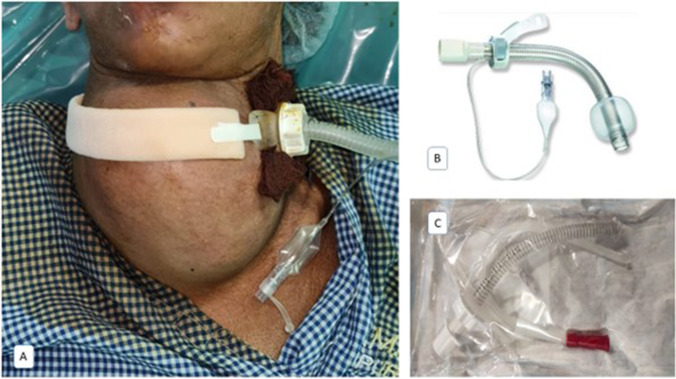
Fig. 3.
A Paramedian tracheostomy with an adjustable flange tracheostomy tube. B, C Adjustable flange tracheostomy tube is reinforced with metallic rings. This also acts as a stent to keep the airway patent
Type of Tracheostomy Tube
In large and bulky tumours or deep-seated trachea, conventional tracheostomy tubes may not be sufficient to secure the airway. An adjustable flange tracheostomy tube (Fig. 3) must be used in such situations. In 5 (29.4%) cases, adjustable flange tracheostomy tubes were utilised due to the large thyroid mass. Conventional cuffed double-lumen tracheostomy tubes must be used in other instances, as changing the tracheostomy tube in the postoperative period may be challenging. We used a conventional cuffed double lumen tracheostomy tube in 12 (70.6%) cases (Fig. 4).
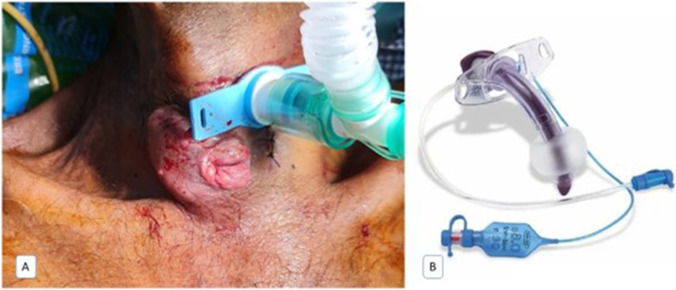
Fig. 4.
A Conventional tracheostomy tube. B Double lumen cuffed tracheostomy tube
Diagnosis of ATC
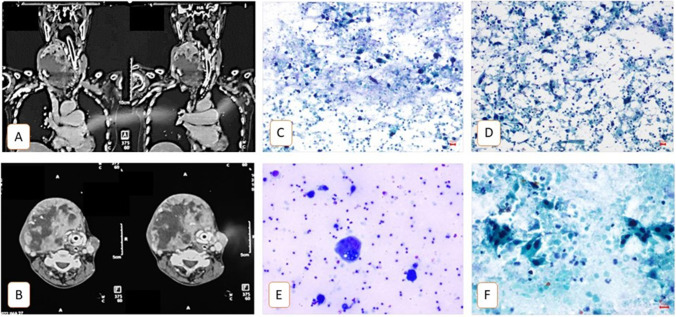
Intraoperative isthmectomy specimen led to the diagnosis of ATC in 7 cases, postoperative ultrasound-guided (USG) fine needle cytology (FNAC) or core needle biopsy led to the diagnosis of ATC in the remaining 10 cases (Fig. 5).
Fig. 5.
A, B Coronal and axial contrast-enhanced computerised tomography images suggesting a large thyroid mass grossly displacing the laryngotracheal complex. C–F Fine needle aspiration. Cytology images show scattered and tiny clusters of large polygonal with hyperchromatic nuclei and moderate to abundant orangophilic cytoplasm (atypical cells). Few tadpole-like cells and tumour giant cells were seen. The background shows dirty necrosis admixed with predominantly polymorphs, lymphocytes and foamy macrophages with phagocytosis
Postoperative Course
The tracheostomy tube was changed from a conventional double-lumen tube to a fenestrated double-lumen tracheostomy tube to facilitate the speech was done in 5 (29.4%) cases. Four (23.5%) cases had pulmonary metastasis, seen on chest radiographs and CT thorax. One patient died on postoperative day two due to the disease (Clavien–Dindo, grade 5). The cause of death was a type I respiratory failure due to pulmonary metastasis leading to myocardial infarction. One patient presented on the 35th postoperative day with fungating disease from the tracheostoma. The disease was diagnosed with biopsy and tracheal secretions, which were positive for viable anaplastic cells.
Cancer-Directed Therapy
Due to meagre performance status and general condition in 13 (76.5%) patients, they did not receive palliative radiotherapy following institute tumour board discussion, palliative metronomic chemotherapy was offered in those patients; Three patients received palliative radiotherapy followed by taxane/platinum-based chemotherapy, as they could not afford targeted therapy. The best supportive care was offered to the two patients.
Survival and Statistical Correlations
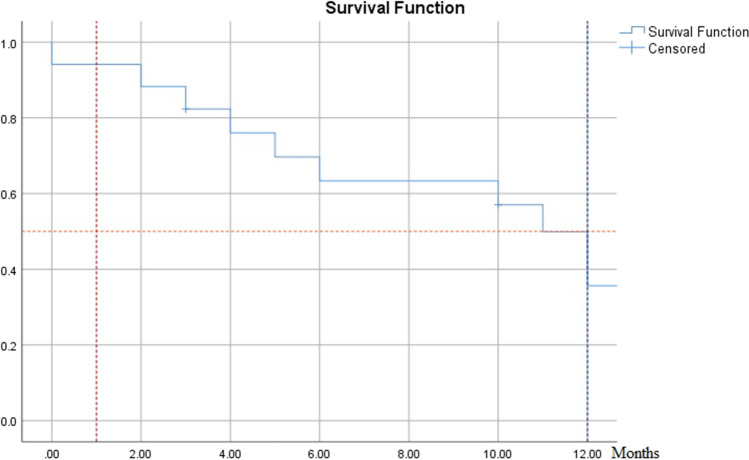
The patients’ overall survival ranged from 2 days to 16 months (median = 11 months). The 30-day mortality was 17.6%. One-year overall survival was 36% (Fig. 6). There was a statistically significant difference in the overall survival among patients with distant metastasis (median = 3 months versus 12 months; p < 0.001, Log Rank (Mantel-Cox), CI:95%) and among patients with Shin grade IV tracheal infiltration (median = 4 months versus 12 months; p < 0.001, Log Rank (Mantel-Cox), CI:95%). The age group did not affect the overall survival (p = 0.31). The degree of tracheal deviation correlated with the patient age group (Pearson chi-square (pc), p = 0.031), the type of anaesthesia used local versus general anaesthesia (pc, p < 0.001) and the site of tracheal entry for tracheostomy (pc, p = 0.028). The degree of tracheal infiltration correlated with the presence of distant metastasis (pc, p = 0.01) and overall survival (pc, p = 0.013) (Table 1).
Fig. 6.
The patients’ survival ranged from 2 days to 16 months (median = 11 months). The 30-day mortality was 17.6%. One-year overall survival was 36%
Table 1.
Patient outcome as a function of various independent factors
| Outcome | |||
|---|---|---|---|
| Alive with disease | Dead due to disease | ||
| Age group | < 55 | 0 | 3 |
| > 55 | 7 | 7 | |
| Gender | Male | 3 | 4 |
| Female | 4 | 6 | |
| SHIN IV | Yes | 0 | 5 |
| No | 7 | 5 | |
| Local vs general anaesthesia | LA | 3 | 1 |
| GA | 4 | 9 | |
| Site of tracheotomy | Median | 3 | 4 |
| Paramedian | 4 | 3 | |
| Lateral | 0 | 3 | |
| Degree of tracheal deviation | Moderate | 1 | 3 |
| Severe | 3 | 5 | |
| Arched | 3 | 2 | |
| Distant metastasis | Yes | 0 | 4 |
| No | 7 | 6 | |
Discussion
ATC is an aggressive tumour with a poor prognosis and rarely survives more than 2 years after diagnosis [1, 21]. The WHO defines ATC as “a highly aggressive thyroid malignancy composed of undifferentiated follicular thyroid cells” [22]. The American Thyroid Association has described eight critical steps in the management of ATC in 2021. Early action is crucial to preventing severe airway impairment. The first essential step described is quickly and definitively making the ATC diagnosis. Wendler and colleagues, in their study, nearly 1/3rd of ATC had extrathyroidal infiltration (stage IVB), and > 50% of cases had cervical lymph node metastases and 54% distant metastases (stage IVC) [23]. We can hypothesise the reason for the late presentation of ATC as they develop from neglected goitres among medically underserved communities, lower education background and areas with high tribal population; these individuals have a higher affinity to seek local and traditional therapies for their medical condition and neglect the medical care till they develop compressive symptoms [23–25]. With improving medical technologies, there is a paradigm shift in the management of the ATC. The best chance of cure is offered by surgery (if operable) followed by adjuvant therapy [1]. Cross-sectional imaging must be performed with positron emission computerised tomography (PET-CT) to evaluate the disease and metastasis. The ATC patients and their family members must receive counselling regarding the therapeutic options available, such as aggressive pain management and palliative care, as well as admissions to intensive care units. More importantly, end-of-life decisions must be made with the assistance of psychologists.
Tracheostomy was once used as a palliative measure in almost all cases of ATC, but modern treatment focuses on a suitable multidisciplinary care plan for tumour control. Tracheostomy is handled with more significant trepidation and concern due to the possibility of complications; while tracheostomy may prolong longevity by preventing acute airway compromise, it reduces the quality of life due to the accompanying morbidity. Furthermore, the tumour may erode the tracheostomy site or fungate around the tracheostomy site [26]. One patient had a similar issue on the 35th day following the tracheostomy.
Tracheostomy must be considered only if the patient develops acute symptoms of airway obstruction. The emergency tracheostomy must be performed in a formal operation theatre with the help of an experienced anaesthesiologist. Tracheostomy in such cases should be avoided at the bedside or in the outpatient department or emergency room because of the potential difficulty securing the airway secondary to overlying disease. The tracheostomy should be planned ahead of time and carried out by a qualified surgical team with the required tools. In the operating room, a planned tracheostomy should involve fibreoptic intubation whenever possible. The team must be ready to perform intubation as quickly as possible with utmost precision; the surgeons must be prepared to perform the surgical access to the airway if the intubation fails. The surgeons must always anticipate the nightmare of failed intubation and be trained to perform the procedure under LA; the possibility of this has to be conveyed to the patient party along with all the possible risks, including the risk of death on the table be thoroughly explained. When the tumour completely encases the laryngotracheal complex, we can mark the airway by trailing and transillumination method with the fibreoptic laryngoscope. This was a case report by Mehta and colleagues [27]. In three of our cases, we have used a similar technique to locate the laryngotracheal complex. Cricothyrotomy may be a simpler surgical operation and less likely to dislodge due to the bulk of the disease, so it should be carefully considered as an alternative to tracheostomy. In four patients with severe stridor and a drop in oxygen saturation, an emergency 14G needle cricothyrotomy was done; 100% high flow oxygen was delivered to maintain the oxygen saturation, and a formal tracheostomy was done by shifting the patient to the operation theatre.
Kocher’s horizontal collar incision with further extension to the swelling side was used in all of our cases. This incision will provide liberty to the surgeons so that the incision can be extended laterally on either side if the trachea is not palpable or the nodule/tumour is too large to negotiate. The initial steps of thyroidectomy till the identification of the thyroid gland must be followed for better exposure. One must always aim to identify the isthmus, as the possibility of encountering the larynx (above) and trachea (below) is higher. The operating surgeon must be cognizant of the median anastomosis between either side anterior glandular division of the superior thyroid artery and its accompanying vein at the superior border of the isthmus and pyramidal lobe; plexus thyroideus impar—a venous plexus at the lower border isthmus and rarely thyroidea ima artery. When encountered, the vessels must be ligated instead of using cautery, as these vessels may open up in the post-operative period due to cough and mechanical friction from the adjacent tracheostomy tube. This may lead to haemorrhage or lower airway obstruction if the tracheostomy tube is uncuffed.
Isthmectomy followed by tracheotomy may be considered in bulky tumours masking the trachea. The laryngotracheal complex usually would be deviated in many cases; paramedian or lateral tracheal entry tracheostomy can be safely considered in such cases. The precarious ATC airway prompts diligence in optimising the tracheostomy tube placement. The surgeon must have a sound knowledge of the intricate anatomy. If cross-sectional imaging is available, it will help with the surgery. In some instances, a high tracheostomy or a cricothyrotomy may be considered. In cases with large and bulky tumours or deep-seated trachea, conventional tracheostomy tubes may not be sufficient to secure the airway. In such situations, an adjustable flange tracheostomy tube must be used. Conventional cuffed double-lumen tracheostomy tubes must be used in other cases, as changing the tracheostomy tube in the post-operative period may be challenging. We recommend monitoring the airway closely after tracheostomy. An experienced surgical team and critical care staff should be available if further intervention is required. Meticulous regular gentle intermittent suctioning must be performed to prevent tube obstruction, primarily if a single lumen tracheostomy tube or adjustable flange tracheostomy tubes are used.
In our study, the patient’s survival ranged from 2 days to 16 months (median = 11 months). The 30-day mortality was 17.6%. One-year overall survival was 36%. In a recent systematic review, patients were distributed according to the stage: IVA 10%, IVB 48% and IVC 36%. The average period surviving was 4.6 months. With adjuvant therapy, the median overall survival for patients who underwent surgery rose to 9.6 months from 6.6 months. A median of 2.1 months was the survival rate for patients treated nonsurgically. According to this data, the overall survival rate was 47.8% at six months and dropped to 6.6% at 2 years. Survival by clinical stage showed statistically significant variations [28]. We recommend molecular studies on the pathology samples and targeted therapy whenever feasible. Before offering different cancer-directed treatments, the tumour board must always consider the patient’s general condition, disease status, overall benefit, financial, social, cultural and economic domains. An active dialogue between the patient, caregivers, and surgeon is essential to chart the comprehensive care plan to manage the disease and complications.
We may never be able to compare the outcomes following emergency tracheostomy in patients with ATC and those without tracheostomy in a study, mainly due to the rarity and progressive nature of the disease. Hence, the data on functional and survival outcomes following the tracheostomy in ATC may remain elusive for a foreseeable time.
Emergency tracheostomies may increase survival by overcoming acute airway distress and impending mortality. The incidence of advanced local disease in ATC patients who need tracheostomies is increased, and their odds of long-term survival are slim. Increased secretions, coughing, altered speech and swallowing, a need for frequent suctioning and general discomfort are common side effects of tracheostomies.
Conclusion
In patients with ATC, tracheostomy is only performed in dire situations to support an airway causing life-threatening hypoxia since it results in significant post-operative morbidity. The 30-day mortality was 17.6%. One-year overall survival was 36%. In ATC patients, prophylactic tracheostomy should be avoided. The ideal approach to conduct a tracheostomy in an ATC patient is under general anaesthesia with endotracheal intubation, and thus, if the patient is experiencing acute airway distress, they should be taken to the operating room. Before beginning the procedure, the operating team must have a variety of endotracheal and tracheostomy tubes available in different sizes and types. A tracheostomy is not a proposed solution; it may only temporarily secure the airway. Additional aids such as fibreoptic optic laryngoscope and intraoperative sonography for localising the laryngotracheal complex must be considered in cases with bulky disease. Patients and relatives should be educated to communicate evolving issues and tracheostomy care in the patient’s best interests, given the unusual context of ATC.
Data Availability
Data is available with the corresponding author.
Declarations
Ethics Approval
Ethical review — No. 2634/IEC-AIIMSRPR/2022 dated 2 December 2022.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coca-Pelaz A, Rodrigo JP, Lopez F, Shah JP, Silver CE, Al Ghuzlan A et al. Evaluating new treatments for anaplastic thyroid cancer. Expert Rev Anticancer Ther. 2022;22(11):1239–47. [DOI] [PubMed]
- 2.Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011;2011:542358. doi: 10.1155/2011/542358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno F, Reyes C, Pineda CA, Castellanos G, Cálix F, Calderón J, et al. Anaplastic thyroid carcinoma with unusual long-term survival: a case report. J Med Case Rep. 2022;16(1):39. doi: 10.1186/s13256-021-03249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000–2019. JAMA Oncol. 2020;6(9):1397–1404. doi: 10.1001/jamaoncol.2020.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslan ZAT, Granados-García M, Luna-Ortiz K, Guerrero-Huerta FJ, Gómez-Pedraza A, Ñamendys-Silva SA et al (2014) Anaplastic thyroid cancer: multimodal treatment results [Internet]. [cited 2022 7th November]. Available from: http://ecancer.org/es/journal/article/449-anaplastic-thyroid-cancer-multimodal-treatment-results [DOI] [PMC free article] [PubMed]
- 6.Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008;37(2):525–38. doi: 10.1016/j.ecl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid. 2017;27(6):751. doi: 10.1089/thy.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 10.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 11.Burke JP, Hay ID, Dignan F, Goellner JR, Achenbach SJ, Oberg AL, et al. Long-term trends in thyroid carcinoma: a population-based study in Olmsted County, Minnesota, 1935–1999. Mayo Clin Proc. 2005;80(6):753–758. doi: 10.1016/S0025-6196(11)61529-2. [DOI] [PubMed] [Google Scholar]
- 12.Zivaljevic VR, Vlajinac HD, Marinkovic JM, Kalezic NK, Paunovic IR, Diklic AD. Case-control study of anaplastic thyroid cancer: goiter patients as controls. Eur J Cancer Prev. 2008;17(2):111–115. doi: 10.1097/CEJ.0b013e3281108036. [DOI] [PubMed] [Google Scholar]
- 13.Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Jr TJC et al (2021) American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid [Internet]. 2021 12th March [cited 2022 7th November]; Available from: https://www.liebertpub.com/doi/10.1089/thy.2020.0944 [DOI] [PMC free article] [PubMed]
- 14.Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg. 2015;4(1):44–51. doi: 10.3978/j.issn.2227-684X.2014.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, et al. Anaplastic Thyroid Carcinoma: An Update. Cancers. 2022;14(4):1061. doi: 10.3390/cancers14041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaha AR, Ferlito A, Owen RP, Silver CE, Rodrigo JP, Haigentz M, et al. Airway issues in anaplastic thyroid carcinoma. Eur Arch Otorhinolaryngol. 2013;270(10):2579–2583. doi: 10.1007/s00405-013-2556-3. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Liao Z, Li JJ, Wu XF, Zhuang SM. The role of tracheostomy in anaplastic thyroid carcinoma. World J Oncol. 2015;6(1):262–264. doi: 10.14740/wjon899w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew G, Agha R, Albrecht J, Goel P, Mukherjee I, Pai P, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2021;96:106165. doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 19.Ikekubo K, Hashimoto S, Ika K, Kurahashi Y, Funazuka R, Kamino T, et al. A study on tracheal deviation due to thyroid disease. Health Eval Promot. 2016;1(43):567–575. doi: 10.7143/jhep.43.567. [DOI] [Google Scholar]
- 20.Shin DH, Mark EJ, Suen HC, Grillo HC. Pathologic staging of papillary carcinoma of the thyroid with airway invasion based on the anatomic manner of extension to the trachea: a clinicopathologic study based on 22 patients who underwent thyroidectomy and airway resection. Hum Pathol. 1993;24(8):866–870. doi: 10.1016/0046-8177(93)90136-5. [DOI] [PubMed] [Google Scholar]
- 21.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol R Coll Radiol G B. 2010;22(6):486–497. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhlin CC, Mete O, Baloch ZW. The 2022 WHO classification of thyroid tumors: novel concepts in nomenclature and grading. Endocr Relat Cancer. 2023;30(2):e220293. 10.1530/ERC-22-0293. [DOI] [PubMed]
- 23.Wendler J, Kroiss M, Gast K, Kreissl MC, Allelein S, Lichtenauer U, et al. Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: results of a multicenter study in Germany. Eur J Endocrinol. 2016;175(6):521–529. doi: 10.1530/EJE-16-0574. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri KG. Anaplastic cancer of the thyroid: the viper in the pit. Indian J Endocrinol Metab. 2019;23(1):1–2. doi: 10.4103/ijem.IJEM_91_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musa ASA, Musa MT, Baba I. Cultural beliefs and attitudes: the psychosocial and economic problems associated with goiter and thyroidectomy in an African population. Thyroid Res Pract. 2014;11(1):22. doi: 10.4103/0973-0354.124191. [DOI] [Google Scholar]
- 26.Mani N, McNamara K, Lowe N, Loughran S, Yap BK. Management of the compromised airway and role of tracheotomy in anaplastic thyroid carcinoma. Head Neck. 2016;38(1):85–88. doi: 10.1002/hed.23857. [DOI] [PubMed] [Google Scholar]
- 27.Mehta KA, Ambulkar RP, Sharma KS, Chaukar DA. Innovative use of the fiberoptic bronchoscope. Indian J Med Paediatr Oncol Off J Indian Soc Med Paediatr Oncol. 2012;33(4):233–235. doi: 10.4103/0971-5851.107090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu S, Helman SN, Hanly E, Likhterov I. The role of surgery in anaplastic thyroid cancer: A systematic review. Am J Otolaryngol. 2017;38(3):337–350. doi: 10.1016/j.amjoto.2017.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available with the corresponding author.