Abstract
Understanding the factors associated with elevated risks and adverse consequences of traumatic brain injury (TBI) is an integral part of developing preventive measures for TBI. Brain injury outcomes differ based on one’s sex (biological characteristics) and gender (social characteristics reflecting norms and relationships), however, whether it is sex or gender that drives differences in early (30-day) mortality and discharge location post-TBI is not well understood. In the absence of a gender variable in existing data, we developed a method for “measuring gender” in 276,812 residents of Ontario, Canada who entered the emergency department and acute care hospitals with a TBI diagnostic code between April 1st, 2002, and March 31st, 2020. We applied logistic regression to analyse differences in diagnostic codes between the sexes and to derive a gender score that reflected social dimensions. We used the derived gender score along with a sex variable to demonstrate how it can be used to separate the relationship between sex, gender and TBI outcomes after severe TBI. Sex had a significant effect on early mortality after severe TBI with a rate ratio (95% confidence interval (CI)) of 1.54 (1.24–1.91). Gender had a more significant effect than sex on discharge location. A person expressing more “woman-like” characteristics had lower odds of being discharged to rehabilitation versus home with odds ratio (95% CI) of 0.54 (0.32–0.88). The method we propose offers an opportunity to measure a gender effect independently of sex on TBI outcomes.
Subject terms: Epidemiology, Trauma, Public health, Risk factors
Introduction
Traumatic brain injury (TBI) is defined as an alteration in the brain function or other evidence of brain pathology resulting from an external force1. It is one of the leading causes of death and disability around the world, affecting 104 million people annually, costing the international economy approximately US$400 billion each year1,2. Growing evidence suggests that TBI is an acute injury and a chronic disorder, where the clinical and functional outcomes are affected by both sex, which is a biological status, and gender, which represents an amalgamation of social, cultural, and behavioural elements3. The distinction between sex and gender effects in an individual’s injury trajectory and outcomes in clinical brain injury research is challenging, given the complex interplay between the magnitude of biological factors and social gradients that interact among themselves in a non-linear manner. However, sex and gender has been successfully integrated into rehabilitation sciences brain injury research to help explain differences in health and functional outcomes between and within male and female persons4–6. For example, sex affects the risk of developing certain diseases before and after TBI due to a range of genetic, hormonal, and metabolic factors that shape distinctive patterns of morbidity and mortality7,8. Meanwhile, gender is linked to a propensity to engage in risk-taking behaviors and exposure to violence9,10. For instance, men tend to take more risks to prove their masculinity, and consequently, they are more likely than women to be involved in serious car accidents or sports injuries, whereas women are more likely to be exposed to gender based violence4,5,11–13. Therefore, it is possible that there are gender-related characteristics apart from biological sex that are important to be aware of as they may affect TBI outcomes differently. Developing a method to measure gender as well as understanding the distribution of gender-related characteristics within the TBI population and its association with injury outcomes in population-based studies constitute significant gaps in the current literature and remains an area of a much-needed development.
In this study, we present a new method to operationalize gender in the context of TBI by constructing a gender score, following Lippa and Connelly’s concept14. We hypothesized that gender-related characteristics would reflect gender-based division of labour and gender-based violence, which might be associated with discharge location and that, in line with previous preclinical and clinical research4, early mortality will be largely affected by biological sex as opposed to gender. In order to show this, we: (1) constructed a gender score based on distinguished characteristics encoded in International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10-CA) diagnostic codes of male and female patients with TBI, and (2) showed that there is a difference in association of the resulting gender score and/or sex with early (i.e., 30 days) mortality after TBI and discharge location after TBI hospitalization.
Results
The full dataset used in the analysis, containing information about the first TBI events extracted from the National Ambulatory Care Reporting System (NACRS) and Discharge Abstract Database (DAD) datasets for 276,812 patients aged 16–64 years and their characteristics, are presented in Table 1. The majority of records (87%) came from NACRS, and the proportion of females (44.5%) was slightly lower than the proportion of males (55.5%).
Table 1.
Characteristics of datasets used in each analysis.
| Parameter | All patients | Mortality modela, test set | Discharge modelb, test set |
|---|---|---|---|
| N = 276,812 | N = 4389 | N = 7343 | |
| Records source (%) | |||
| Acute care | 12.7 | 63.2 | 100.0 |
| Emergency Dept. | 87.3 | 36.8 | – |
| Sex (%) | |||
| Female | 44.5 | 29.0 | 27.3 |
| Age (years) | |||
| Median | 31 | 45 | 42 |
| Q1 | 21 | 28 | 26 |
| Q3 | 47 | 56 | 54 |
| Rural (%) | |||
| Yes | 15.5 | 12.6 | 16.4 |
| Income quintile (%) | |||
| 1 (lowest) | 21.5 | 23.4 | 24.7 |
| 2 | 19.9 | 20.4 | 20.4 |
| 3 | 19.7 | 19.0 | 18.6 |
| 4 | 19.7 | 19.2 | 19.0 |
| 5 (highest) | 19.2 | 18.1 | 17.4 |
| Length of stayc (days) | |||
| Median | 2.9 | 5.8 | 5.0 |
| Q1 | 1.7 | 3.0 | 2.0 |
| Q3 | 4.8 | 14.0 | 12.0 |
| ADG scorec | |||
| Median | 2 | 2 | 2 |
| Q1 | 1 | 1 | 1 |
| Q3 | 3 | 4 | 4 |
| TBI severity (%) | |||
| Unknown | 48.6 | – | 19.6 |
| Mild | 41.5 | – | 39.0 |
| Moderate | 2.8 | – | 7.3 |
| Severe | 7.1 | 100.0 | 34.0 |
TBI: traumatic brain injury; Dept.: Department; ADG: Johns Hopkins’ Aggregated Diagnosis Groups; Q1 = 1st quartile; Q3 = 3rd quartile. Data given as median (Q1, Q3) for continuous variables or (%) for categorical variables.
aSubjects with severe TBI who had recorded survival status at day 30 after the first TBI event.
bSubjects who had a record of discharge location from acute care hospitals and non-missing ADG score.
cBased on non-missing records.
Gender score derivation
After filtering out diagnostic codes as described in the methods section, univariate logistic models were fit to 3815 codes in the training set and 2939 codes in the validation set; of these, 281 codes were statistically significantly associated with sex (50 of them were associated with higher odds of being female, and 231 with higher odds of being male) in both datasets after Bonferroni correction and were further included in the multiple logistic regression model to define gender score. The descriptions of the top 10 ICD-10-CA diagnostic codes associated with being male (Table 2) and with being female (Table 3) are presented.
Table 2.
ICD-10-CA codes with the highest effects (OR and 95% CI) in predicting male vs. female in the training set.
| ICD-10-CA code | Code description | OR (95% CI) for male vs. female | |
|---|---|---|---|
| Training N = 138,600 |
Validation N = 122,230 |
||
| W12 | Fall on and from scaffolding | 23.9 (9.8, 58.2) | 12.6 (4.6, 34.5) |
| V685 | Occupant of heavy transport vehicle injured in noncollision transport accident, driver, traffic accident | 22.0 (5.4, 90.3) | 26.8 (3.7, 195.1) |
| V8608 | Driver of other all-terrain or other off road motor vehicle injured in traffic accident | 8.6 (4.3, 16.9) | 7.1 (2.8, 18.0) |
| V274 | Motorcycle rider injured in collision with fixed or stationary object, driver, traffic accident | 8.4 (4.2, 16.6) | 3.9 (1.9, 7.9) |
| Z21 | Asymptomatic human immunodeficiency virus [HIV] infection status | 7.0 (3.0, 16.2) | 26.0 (3.6, 189.1) |
| V234 | Motorcycle rider injured in collision with car, pick-up truck or van, driver, traffic accident | 6.9 (5.1, 9.3) | 8.0 (5.0, 13.1) |
| V280 | Motorcycle rider injured in noncollision transport accident, driver, nontraffic accident | 6.7 (4.6, 9.8) | 6.9 (4.1, 11.8) |
| V293 | Motorcycle rider [any] injured in unspecified nontraffic accident | 6.7 (2.2, 22.0) | 5.7 (1.7, 19.0) |
| S02410 | Fracture of malar and maxillary bones, LeFort 2, closed | 6.7 (4.0, 11.2) | 9.9 (4.0, 24.7) |
| W27 | Contact with nonpowered hand tool | 5.9 (3.6, 9.8) | 2.9 (1.6, 5.2) |
ICD-10-CA: International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Canada, OR: odds ratio, CI: confidence interval. OR estimates are not used for further inference, hence, only point estimates are presented.
Table 3.
ICD-10-CA codes with the highest effects (OR and 95% CI) in predicting female vs. male in the training set.
| ICD-10-CA code | Code description | OR (95% CI) for female vs. male | |
|---|---|---|---|
| Training N = 138,600 |
Validation N = 122,230 |
||
| Y070 | (Assault) By spouse or partner | 41.5 (19.6, 88.0) | 18.1 (8.9, 37.0) |
| U99064 | Aesthetic sports | 27.5 (3.8, 198.9) | 9.9 (2.3, 42.6) |
| T741 | Physical abuse | 13.1 (4.7, 36.6) | 27.2 (3.8, 196.6) |
| U99037 | Horse riding sports | 10.3 (5.2, 20.6) | 6.2 (2.9, 13.2) |
| V800 | Animal-rider or occupant of animal-drawn vehicle injured by fall from or being thrown from animal or animal-drawn vehicle in noncollision accident | 9.1 (7.6, 10.9) | 8.8 (6.8, 11.3) |
| W04 | Fall while being carried or supported by other persons | 7.6 (4.7, 12.1) | 10.9 (5.0, 23.9) |
| U99068 | Other specified gymnastic and aesthetic sports and recreational activity | 7.5 (2.9, 19.3) | 7.4 (2.2, 25.1) |
| W54 | Bitten or struck by dog | 5.4 (3.8, 7.8) | 6.5 (3.6, 11.7) |
| V809 | Animal-rider or occupant of animal-drawn vehicle injured in other and unspecified transport accidents | 4.2 (2.4, 7.4) | 4.7 (1.9, 11.6) |
| Z630 | Problems in relationship with spouse or partner | 3.6 (1.9, 7.0) | 7.4 (2.2, 25.1) |
ICD-10-CA: International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Canada, OR: odds ratio, CI: confidence interval. OR estimates are not used for further inference, hence, only point estimates are presented.
The codes of the greatest separation of male from female persons with TBI expressed gender-based division of labour and gender-based violence, highlighting normative roles, relationships, and behaviours ascribed to male and female persons on the basis of biological sex. As such, we assigned “man-like’ and “woman-like’ titles on a gender score continuum, where 0 refers to strongest man-like and 1 to strongest woman-like characteristics. We derived these terms for convenience reflecting the methodology used.
The codes associated with a higher degree of being “man-like” than “woman-like” reflect distinct behavioral and social characteristics, such as occupations (fall from scaffolding for males), risk taking behaviours (motorcycle riding for males). Codes associated with a higher degree of being “woman-like” (Supplementary Table 5 for the full list of codes) included gender-related vulnerabilities (partner violence related codes for females).
The final logistic regression model that defined gender scores as a probability of being female included 281 ICD-10-CA codes. Figure 1 presents the distribution of gender scores in male and female sexes in the test dataset, which is heavier to the left tail (lower values) indicating that scores were skewed to defining “man-like” persons more than “woman-like”; this is possibly because approximately 80% of the included diagnostic codes showed higher odds for male. Additional analysis investigating the relationship between gender score and age showed that the overall pattern of distribution was similar among different age groups (Supplementary Fig. 5).
Figure 1.
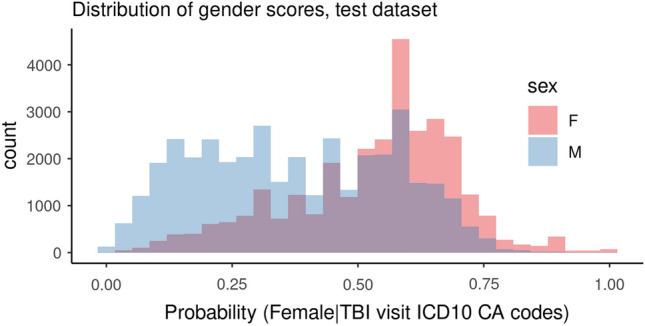
Gender score distribution in males and females, test dataset (N = 68,900).
Predicting TBI-related excess mortality
This analysis included 4389 patients in the test dataset with a severe TBI who had a survival status recorded at day 30 after their first TBI event, of which 402 (9.2%) of them died within 30 days from their injury event. Characteristics of the datasets are presented in Table 1 and Supplementary Table 4.
In TBI-related excess mortality prediction (Table 4), all models contained the following control variables: age, Aggregated Diagnosis Groups (ADG) score, rurality indicator, income quintile, and cause of injury as well as population mortality as an offset. Sex only model, i.e., Model 1, the rate ratio (RR) for sex was 1.54 (1.24–1.91). In Model 2, gender score only model, the RR for gender score was 2.02 (1.02–4.0).
Table 4.
Models predicting TBI-related mortality.
| Model predictor(s) | Rate ratio (95% CI) | Likelihood ratio (df, LRT p-value) |
|---|---|---|
| Model 1 | ||
| Sex (Female = 1) | 1.54 (1.24, 1.91) | 14.33 (1, 0.0002) |
| Model 2 | ||
| Gender score | 2.02 (1.02, 4.00) | 4.01 (1, 0.0453) |
| Model 3 | ||
| Sex (female = 1) | 1.49 (1.19, 1.86) | 11.50 (1, 0.0007) |
| Gender score | 1.48 (0.73, 3.01) | 1.18 (1, 0.28) |
CI: confidence interval; LRT: Likelihood-Ratio Test; df: degrees of freedom. Poisson survival models using binary sex and continuous gender score (1 is more woman-like vs. 0 is more man-like) for test set with N = 4389.
To compare the models, we first look at Model 3. Sex was a significant predictor with p-val = 0.0007 (likelihood ratio test (LRT) = 11.50, df = 1) while gender score was not significant with p-val = 0.28 (LRT= 1.18, df = 1). This is very good evidence that sex is a better predictor than gender (i.e., sex only model is preferred over gender score only model in predicting TBI-related excess mortality)15. To directly quantify the strength of this evidence, Bayes factor (BF) was calculated from the LRT statistics in Model 3 where the BF for Model 1 (sex only) over Model 2 (gender score only) was 174, which is considered "very strong” evidence on the Kass-Raftery scale16.
Predicting discharge location
This analysis included 7343 persons in the test set who had a record of discharge location from acute care hospitals and non-missing ADG scores. The cohort’s characteristics are presented in Table 1 and Supplementary Table 3.
In Table 5, all models were controlled for age, length of stay (LOS), ADG score, rurality, and income quintile. Table 4 shows that both sex and gender score were significant in their separate models with a p-val = 0.0224 (LRT = 13.10, df = 5) and p-val < 0.0001 (LRT = 38.92, df = 5), respectively. In sex only model (Model 1), the odds ratio (OR) for being discharged to LTC (versus home) for females versus males was 2.14 (1.09–4.22), making sex a significant predictor for this category. In gender score only model (Model 2), gender score was a significant predictor for two discharge locations, particularly, OR for “other” (versus home) for “woman-like” versus “man-like” was 0.34 (0.22–0.51) and rehab (versus home) was 0.54 (0.32–0.88). To further investigate the relationship between gender score and “other” discharge location, we fit a series of logistic regression models comparing chances of going to a particular location included in “other” as compared to “home” (Supplementary Table 6). It showed that the relationship observed in the main model could be driven by the largest subgroup within the “other” category, namely, those discharged to another hospital/acute care facility because more “woman-like” persons had a lower chance of being discharged to that location (versus home) as compared to more “man-like” persons with an OR of 0.22 (0.13–0.40).
Table 5.
Models predicting discharge location post-TBI hospitalization.
| Model predictor(s) | Home with support vs home OR (95% CI) |
LTC vs home OR (95% CI) |
CCC vs home OR (95% CI) |
Other vs home OR (95% CI) |
Rehab vs home OR (95% CI) |
Likelihood ratio (df, LRT p-value) |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Sex (female = 1) | 1.18 (0.98, 1.43) | 2.14 (1.09, 4.22) | 1.10 (0.72, 1.68) | 0.89 (0.76, 1.03) | 1.11 (0.93, 1.34) | 13.10 (5, 0.0224) |
| Model 2 | ||||||
| Gender score | 1.22 (0.74, 2.02) | 3.68 (0.57, 23.84) | 1.72 (0.58, 5.14) | 0.34 (0.22, 0.51) | 0.54 (0.32, 0.88) | 38.92 (5, < 0.0001) |
| Model 3 | ||||||
| Sex (female = 1) | 1.17 (0.96, 1.43) | 1.98 (0.98, 4.04) | 1.04 (0.67, 1.63) | 1.00 (0.85, 1.18) | 1.22 (1.01, 1.48) | 8.59 (5, 0.126) |
| Gender score | 1.07 (0.63, 1.81) | 2.14 (0.30, 15.46) | 1.63 (0.52, 5.14) | 0.34 (0.22, 0.52) | 0.45 (0.27, 0.77) | 34.41 (5, < 0.0001) |
OR: odds ratio, CI: confidence interval; LRT: Likelihood-Ratio Test; df: degrees of freedom; LTC: Long Term Care; CCC: Inpatient Complex Continuing Care. Baseline category logit model using binary sex and continuous gender score (1 is more woman-like vs. 0 is more man-like) for test set with N = 7343.
To compare sex only model versus gender only model, we looked at Model 3 which contains both sex and gender effects. Gender score was significant with p < 0.0001 (LRT = 34.41, df = 5) while sex was not significant with p = 0.126 (LRT = 8.59, df = 5). This is very good evidence that gender score is the stronger predictor, i.e., gender score only model is preferred over sex only model, of acute care hospital discharge location15. To directly quantify the strength of this evidence, BF was calculated from the LRT statistics in Model 3 where BF for the gender score only model over the sex only model was 4 × 105 which is considered as a “very strong” evidence on the Kass-Raftery scale16.
Discussion
We utilized ICD-10-CA diagnostic codes billed for patients with TBI during their hospital or emergency department (ED) visits to derive gender-related characteristics of male and female persons with TBI. We applied Lippa and Connelly’s “gender diagnosticity” concept which refers to a “probability that an individual is predicted to be a male or a female based on some set of gender-related diagnostic indicators”14 and showed how this score can help in distinguishing between sex and gender in study of the TBI outcomes. Prior research used the concept of gender diagnosticity to construct a gender score based on information derived from psychosocial variables and showed that gender score was associated with cardiovascular disease risk factors, independently of biological sex17.
To the best of our knowledge, this is the first large-scale population-based study using health administrative data that investigated sex and gender effects in TBI outcomes simultaneously. To achieve this goal, we constructed a gender score metric based on information from ICD-10-CA diagnostic codes recorded during TBI-related ED or acute care hospital visits, and then used this score along with biological sex to predict early mortality and discharge location. Biological sex and gender score characterized persons with TBI differently and had distinctive predictive effect for early mortality and acute care discharge location. There is evidence of very strong effect of sex and gender score in predicting early mortality and discharge location, respectively, based on the Kass-Raftery score criteria18. Therefore, it can be used to alert clinicians and policymakers to these distinctive effects, and to develop preventive and rehabilitation strategies. This study also provides researchers who have access to large administrative healthcare databases with a method to a derive gender score in their population of interest and use it in their analysis to predict clinically and functionally meaningful outcomes.
As expected, the gender score metric we created was able to separate man-like from woman-like patients based on gender-based division of labour and gender-based violence indicators, which clearly differs from biological sex, contributing important explanatory power in understanding TBI outcomes. The distribution of the score towards woman-like characteristics in our study was opposite to results reported earlier in a cohort of younger persons with myocardial infraction19, where researchers found a more asymmetrical distribution with a stronger clustering of male persons in the man-like characteristics and a broader distribution of female persons over the whole gender score continuum. Our results, across adulthood ages suggest that that female patients might possess their woman-like characteristics more strongly in the fifth and sixth decades of life whereas male patients acquire a wider range of characteristics on the gender score continuum, although their man-like characteristics were more profoundly seen in younger ages. Future studies should consider derivation of gender scores in population based TBI research by the decades of life.
The significance of studying biological sex as a separate entity from gender-related characteristics in early mortality after TBI has been increasingly emphasized in preclinical20 and clinical21 research. It has been suggested that female hormones oestrogen is neuroprotective, acting on the steroidogenic central nervous system to attenuate neural damage post-injury, particularly in females, given the occurrence of the hormone at higher levels in females relative to males4. Several mechanisms22,23 of action have been suggested for its neuroprotective capacity, including post-injury levels of brain-derived neurotrophic factor, given its role in the survival, differentiation, and outgrowth of neurons, and its purported regulation by oestrogen. As level of oestrogen changes over the lifetime of the female persons, with low points at the beginning and end of life, if either of these hormones is to afford protection following TBI24, it is conceivable that its influences would be most potent in adulthood ages we studied as opposed to early or later life, which remain to be explored in future research.
Different gender‐related characteristics, including societal norms, roles, and responsibilities (i.e., gender-based division of labour), gender-based violence, and gender inequity in access to and control over resources have been reported as being important to the socially driven outcomes after TBI3. Prior research has shown that female patients are more likely than male patients to be discharged to care facilities versus home after TBI25,26, possibly due to differences in the existing familiar and social support. In our cohort of adults with TBI, we observed, in line with prior research and our hypotheses, that woman-like gender characteristics were a predictor of lower probability to be discharged to “rehabilitation” after acute care hospital stay, even after controlling for relevant variables. The gender score was shown to contain gender related characteristics such as “assault by spouse or partner” and “physical abuse,” among others turned to be stronger predictors of discharge location than biological sex. Considering an evolving society with closing gender inequity gaps in the household27 and global efforts to eradicate gender-based violence28, further research is imperative to evaluate whether the effect of gender score on discharge locations would diminish with time. Furthermore, gender related characteristics among older persons who are women may be more impactful on discharge location than among younger persons. Future investigation into children, adolescents, and older persons’ groups is needed, which may show different influence of gender-related characteristics on discharge location after TBI.
There are several limitations to this analysis. We used the same information related to TBI from ICD-10-CA codes to create a gender score metric and investigated its relationship with TBI outcomes. Gender is a multi-dimensional notion, and the metric we built only incorporates limited dimensions of gender, such as risk-taking behaviors, gender-based violence, and employment/occupations. However, we believe that defining gender score based on characteristics that predict a person to be more likely a male or a female is in keeping with the existing methods to measure gender. Also, the resulting gender score reflect degree of “man-like” more than of “woman-like”, which is an important finding22. Further, our “sex” variable was binary. The relatively small number of persons in the dataset that did not identify as a male or a female did not make matched analyses feasible. We also recognize the limitations of using administrative data overall.
In conclusion, this study, to the best of our knowledge, is the first example of applying the concept of gender diagnosticity to the ICD-10-CA diagnostic codes data in a province-wide Canadian cohort of patients with TBI. When creating potentially time-dependent gender score and testing its association with outcomes of interest (i.e., excess mortality and discharge locations), we defined relatively restricted (from historical perspective) time windows for analysis. The derived gender score metric allowed us to gain additional insights into relationship between sex, gender, and TBI outcomes when no explicit measure of gender is available in a data source comprised of predominantly ICD-10-CA codes. Our results highlight that sex and gender effects expressed differently in TBI outcomes that are driven to a greater extent by physiological responses to injury in the context of genetics, endocrine, metabolic, and immune systems (i.e., sex) or by interpersonal family and community relationships, and socioeconomic factors within the person’s living environment. More research is needed to further test and validate this approach in different age cohorts and across different clinical conditions, as well as gender metric variability over time.
Methods
Study design and data sources
For this retrospective cohort study, we accessed the population-wide health administrative data for all publicly funded services provided to the residents of Ontario, Canada from ICES (formerly the Institute for Clinical Evaluative Sciences)18 data repository. We combined the records for ED visits with acute care visits, gathered from National Ambulatory Care Reporting System (NACRS) and Discharge Abstract Database (DAD), respectively. These datasets contained primary and secondary diagnoses recorded using ICD-10-CA codes (up to 10 codes per record in NACRS, and up to 25 codes in DAD) as well as clinical, demographic, and socio-economic information about each person. We only included the first incidence of a TBI-related visit during the study period, defined as the “first TBI event”, for patients who were discharged from the ED or acute care hospitals with a TBI diagnostic code (S020, S021, S023, S027, S028, S029, S040, S071, S06) between April 1st, 2002, and March 31st, 202029. We restricted the cohort to patients who were aged 16–64 years in order ensure homogeneity of gender attributes within the adult group (versus pediatric or senior population). Data on age, sex, and calendar year specific death rates in the general population were extracted from Statistics Canada life tables30. The Abbreviated Injury Severity Score generated according to previously published severity classifications, was used as a measure of TBI severity and was measured on a 6-point scale based on ICD-10-CA codes and categorized as mild (1–2), moderate (3), or severe (> 4)31,32.
Data on discharge locations were derived from DAD.
The combined dataset was randomly split into 50% for training, 25% for validation, and 25% for testing to prevent overfitting and to ensure model validation. Training and validation sets were used for model building and internal validation, whereas the reported results were based on the test set performance.
Ethical approval and informed consent
Approval: The study protocol was approved by the Research Ethics Boards of the University of Toronto (20-5823) and the University Health Network (#20-5823) All methods were carried out in accordance with the relevant guidelines and regulations.
Informed consent: This research utilised encrypted administrative health data authorised under Section 45 of Ontario's Personal Health Information Protection Act. The data are housed at Institute for Clinical Evaluative Sciences (ICES), an independent, non-profit research institute, whose legal status under Ontario's health information privacy law allowed it to collect and analyse healthcare and patient characteristics data, without individual patient consent, for health system evaluation and improvement.
Statistical approaches
Gender score derivation
We used logistic regression approach to derive gender score reflecting a probability of each person being male or female based on a set of indicator variables of diagnostic codes that reflect biological (associated with binary sex, such as diseases) and social (associated with behavioral and other socially defined characteristics considered as man-like or woman-like) attributes of people.
Each person’s sex was compiled from the Registered Persons Database33. The ICD-10-CA diagnostic codes recorded in each TBI visit were converted into a matrix of indicator variables for each distinct diagnostic code using natural language processing tools (creating document-term matrix using R package “tm”34). Diagnostic codes that were not common, i.e., present in a single person in training and/or validation datasets, as well as codes that occurred only in males or only in females, were removed from both sets; the latter was done to ensure derived gender characteristics were relevant to both sexes. To select the subset of diagnostic codes to include in the gender score model, we assessed the significance of each unique diagnostic code in predicting the sex (Female = 1) of persons who were diagnosed with that code by fitting univariate logistic regression models. All diagnostic code indicators that were significant at 5% level after Benjamini–Hochberg correction35,36 in both training and validation sets were subsequently included into the gender score model predicting the probability of sex reported as female in the training set. Consequently, model coefficients obtained from the training dataset were used to calculate the final gender scores in the test set. Therefore, the final gender score was a continuous variable ranging from 0 (man-like) to 1 (woman-like), estimating the probability of a person being male or female.
Predicting TBI-related excess mortality
Following our previous research8, we defined the acute phase of mortality due to injury sustained during a TBI event (in some studies it was called TBI-related mortality8,37) as death within a 30-day window. Exploratory analysis showed that 64% of the people who died within 30 days had a severe TBI diagnosis (Supplementary Table 1), therefore, the analysis was restricted to this subpopulation. In addition, people with unknown survival status 30 days following their first TBI event, or with unknown injury severity were excluded from this analysis.
The outcome was therefore defined as time-to-death within 30 days of the first TBI event and patients who were alive on the 30th day after the first TBI event were censored. Covariates in the model were selected based on previous research8, which included age as a continuous variable, mechanism of injury (determined using major external cause of injury group codes38: falls, struck by/against object, motor vehicle collisions, cyclist collisions, other), rurality indicator, income quintile (linear predictor), and Johns Hopkins Aggregated Diagnosis Groups (ADG) score, which is a weighted score representing the presence or absence of 32 ADG diagnosis groups as an indicator of comorbidities39 (Supplementary Table 2). To control for population death rates, we extracted the age, sex, and calendar year specific death rates for each person from the Statistics Canada life tables18 and used it as an offset term in the model. Excess mortality rate was modelled using a Poisson regression model8,40–42 by treating survival status of each subject as observations from Poisson distribution with rate parameter specific for each time interval (day); the model is equivalent to the piecewise exponential survival model42. Since mortality status is recorded daily or in discrete time intervals, Poisson distribution naturally fits the data structure and model allows for population-wide death rate to be accounted for in the model.
As part of data pre-processing, the mortality dataset was transformed into a person/period format, with 1 record per day until death or censoring occurred at day 30 with an event indicator equal to 0 for each day the person was alive and 1 for the day of death. To control for the underlying population death rate, daily death rate (dividing yearly death rate by 365) was calculated from Statistics Canada life tables30 for each patient, matched by sex, age, and year of the first traumatic brain injury (TBI)-related healthcare visit during our study period and was added to the model as an offset term. The resulting model was defined as following (Eq. 1):
| 1 |
where is the death rate for day from the first TBI event, is the average daily death rate derived from Statistics Canada30 mortality tables, matched by patient age, sex and year of the first TBI event, and X is the matrix of predictors. For patients who died on day 0, an interval of length 0.5 was assigned, and population rate included into the model was adjusted accordingly.
Predicting discharge location
Discharge location prediction was restricted to acute care visits. Patients who were alive when discharged, with a recorded discharge location and non-missing baseline ADG score were included in this analysis (Table 1). The outcome variable was discharge location from acute care, categorized into six groups: discharged home, discharged home with support, inpatient complex continuing care (CCC), long term care (LTC), rehab, and other. The category “other” was composed of smaller subgroups including transfer to another inpatient care/hospital/acute care facility, long term/continued care, other ambulatory care/palliative care/hospice/addiction treatment centers/jails, died in facility, left against medical advice, and signed out against medical advice25,43. Covariates identified from previous study included age, length of stay (LOS), ADG score, rurality indicator, and income quintiles25. The most common discharge location (Supplementary Table 3) was “discharged home”, which was used as the reference level in the baseline category logistic regression models44.
Measuring effects of sex versus gender score
We compared the predictive performances of gender score versus biological sex in predicting TBI-related outcomes (early mortality and discharge location) using the test set. To achieve this, we considered the following three models for each outcome: Model 1 with binary sex and control variables as covariates, Model 2 with gender score and control variables, and Model 3 with both sex and gender score in addition to the control variables. We used two metrics/statistics to assess the effects of sex and gender score in predicting TBI-related outcomes: (1) profile likelihood based confidence intervals (CI) and (2) likelihood-ratio tests (LRT) while controlling for any relevant variables. We reported the p-value (p-val) and the degrees of freedom (df) for LRT, p-val < 0.05 was considered statistically significant. All model-derived estimates are reported with 95% CI, and if the CI contains 1, it is not significant at 5% level. Effect estimates were reported to compare the unit difference in gender score and sex. Sex is a binary variable coded as Female = 1 and Male = 0, and gender score is a continuous variable ranging from 0 to 1 (towards 0 is more man-like and towards 1 is more woman-like).
To assess whether sex or gender score is more informative in predicting TBI-related outcomes, we can use Model 3 to assess whether Model 1 or Model 2 is preferred using an indirect and a direct approach for comparison. An indirect approach is to test the significance of the sex effect and the gender effect, if one effect is significant and the other effect is not significant, then that is an evidence that the model with just the significant effect (and the control variables) is the preferred model15 for that outcome. We can also use Bayes factors (BF)16 to directly measure the strength of evidence that the data supports one model versus the other, therefore, prefers one effect over the other. More detailed description of the two approaches is presented below45.
Statistical methods: comparing different models
An important question in this paper is to decide if a TBI-related outcome is more likely due to sex or gender. From a statistical testing point of view, this translates to a question of whether the evidence from the data better supports a model with sex effect only or a model with gender effect only. Normally, we would test this using nested models where one model contains a subset of predictors of the other model and test if there is a significant difference between the fitness of the two models. However, in our case we have two models that are not nested, invalidating the direct procedure for comparison. Instead, we can assess the effects of sex and gender by looking at a full model which contains both variables and check whether one effect is statistically significant while the other effect is not significant. Cox discussed such testing method to compare a model with only effect 1 versus a model with only effect 2, one can do this by putting them both in a full model which contains both effects15. According to Cox, "If [effect 1] is very highly significant whereas [effect 2] is not [in the full model], a clear conclusion can be reached that the data agree better with [the model with effect 1] than the [model with effect 2]"15. This provides a basic procedure, but it does not directly measure the weight of evidence that one model is preferred over the other.
Theoretically, a way to directly compare the two model is to calculate the Bayes Factor (BF)16. This provides us with an estimate of the weight of evidence from the data that it supports one model over the other. We can approximate BF using the Bayesian Information Criterion (BIC)45. As mentioned in the main text we have the following three models: Model 1 contains sex effect only, Model 2 contains gender effect only, and Model 3 contains both sex and gender effects, in addition to the same control variables for all three models. Let be the loglikelihood () for the th model. Then, the difference in BIC () comparing the sex only model (Model 1) to the gender only model (Model 2) is equal to (Eq. 2):
| 2 |
where is the degrees of freedom for model and is the sample size. Finally, BF12 comparing the weight of evidence that supports Model 1 over Model 2 can be approximated by (Eq. 3):
| 3 |
Note, if one wants to know the weight of evidence for Model 2 over Model 1, then can be calculated as . Kass and Raftery suggest the following criteria to interpret BF16:
| BF12 | Evidence for Model 1 over Model 2 |
|---|---|
| 1 to 3 | Not worth more than a mention |
| 3 to 20 | Positive |
| 20 to 150 | Strong |
| > 150 | Very strong |
In this paper, we are comparing the sex effect only model versus the gender effect only model. (That is, where both models include the same set of control variables.) Since we are only comparing two models and both models have the same degrees of freedom, then turns out to be the difference in the likelihood ratio test (LRT) statistics used to test for the significance of the sex effect and the gender effect in the full model. To see this, note that the LRTS to test for the sex effect in the full model compares the of the model WITHOUT sex to the of the full model. The model without sex is the model with gender only which is Model 2. So, the LRTS is equal to the following (Eq. 4):
| 4 |
The LRT for the gender effect is similar except it uses LL1 instead of LL2. Since both Model 1 and Model 2 have the same degrees of freedom, it can be shown that (Eq. 5):
| 5 |
which implies that (Eq. 6):
| 6 |
So, since there are only two models being compared and the two models have the same degrees of freedom, then the BF can be approximated from the LRT statistics in the full model.
Missing data
All analyses were based on complete records with all variables relevant to a particular analysis recorded. Three variables used in the analysis had missing values: ADG score (10.1% missing), LOS (10.6% missing), and TBI severity (48.6% had “unknown” injury severity). The last variable was only used in TBI-related excess mortality analysis and exploratory analysis showed that the death rate of persons with “unknown” injury severity was similar to that of persons who sustained a mild TBI (Supplementary Table 4). Considering that excess mortality analysis was restricted to persons with a severe TBI and much higher mortality rate, excluding persons with unknown severity status should not result in biased estimates.
When considering missingness in variables included in models as covariates (ADG score and LOS), based on simulation study done by Lee and Carlin46, it can be concluded that given the strength of evidence (associations) shown in our models for main predictors (sex and gender) and relatively low proportion of missing values in these covariates, performing imputations would not change our main conclusions, so we present the complete case analysis.
Statistical software
All analyses were performed in R (version 3.6.3, R Foundation for Statistical Computing; http://www.R-project.org).
Supplementary Information
Acknowledgements
The research reported in this presentation was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS117921. The content is solely the authors' responsibility and does not necessarily represent the official views of the National Institutes of Health. During the work under the study researchers T.M. and A.C. were supported by Canada Research Chair programs (CRC-2021-00074 and CRC-2019-00019). T.M. was also supported by the Global Brain Health Institute (GBHI), Alzheimer’s Association, and the Alzheimer’s Society UK Pilot Awards (GBHI ALZ UK-23-971123).
This study contracted ICES Data & Analytic Services (DAS) and used de-identified data from the ICES Data Repository, which is managed by ICES with support from its funders and partners: Canada's Strategy for Patient-Oriented Research (SPOR), the Ontario SPOR Support Unit, the Canadian Institutes of Health Research, and the Government of Ontario. The opinions, results and conclusions reported are those of the authors. No endorsement by ICES or any of its funders or partners is intended or should be inferred. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed herein are those of the author, and not necessarily those of CIHI.
Author contributions
A.C., T.M., V.C. and M.E. conceived the original concept and initiated the work. M.E. designed and optimized statistical analyses for this work. A.T. carried out the analyses with M.E.’s support. A.T. and S.Z. wrote the manuscript and optimized reporting. A.C., T.M., M.E., contributed to the writing of the manuscript. All authors discussed the results biweekly and further steps for analyses and interpretation. All authors read the final version of the manuscript and commented on the text.
Data availability
ICES is an independent, non-profit research institute funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). As a prescribed entity under Ontario's privacy legislation, ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation, and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario. The dataset from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at http://www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-45683-2.
References
- 1.Menon DK, Schwab K, Wright DW, Maas AI. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.James SL, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mollayeva T, Mollayeva S, Pacheco N, Colantonio A. Systematic review of sex and gender effects in traumatic brain injury: Equity in clinical and functional outcomes. Front. Neurol. 2021;12:678971. doi: 10.3389/fneur.2021.678971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollayeva T, Mollayeva S, Colantonio A. Traumatic brain injury: Sex, gender and intersecting vulnerabilities. Nat. Rev. Neurol. 2018;14:711–722. doi: 10.1038/s41582-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 5.Colantonio A, Valera EM. Brain injury and intimate partner violence. J. Head Trauma Rehabil. 2022;37:2–4. doi: 10.1097/HTR.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabricius AM, D’Souza A, Amodio V, Colantonio A, Mollayeva T. Women’s gendered experiences of traumatic brain injury. Qual. Health Res. 2020;30:1033–1044. doi: 10.1177/1049732319900163. [DOI] [PubMed] [Google Scholar]
- 7.Mollayeva T, et al. Decoding health status transitions of over 200 000 patients with traumatic brain injury from preceding injury to the injury event. Sci. Rep. 2022;12:5584. doi: 10.1038/s41598-022-08782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollayeva T, et al. Pre-injury health status and excess mortality in persons with traumatic brain injury: A decade-long historical cohort study. Prev. Med. 2020;139:106213. doi: 10.1016/j.ypmed.2020.106213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyal L. Sex, gender, and health: The need for a new approach. BMJ. 2001;323:1061–1063. doi: 10.1136/bmj.323.7320.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miani C, Wandschneider L, Niemann J, Batram-Zantvoort S, Razum O. Measurement of gender as a social determinant of health in epidemiology—A scoping review. PLoS ONE. 2021;16:e0259223. doi: 10.1371/journal.pone.0259223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrigan JD, Wolfe M, Mysiw WJ, Jackson RD, Bogner JA. Early identification of mild traumatic brain injury in female victims of domestic violence. Am. J. Obstet. Gynecol. 2003;188:S71–S76. doi: 10.1067/mob.2003.404. [DOI] [PubMed] [Google Scholar]
- 12.Kwako LE, et al. Traumatic brain injury in intimate partner violence: A critical review of outcomes and mechanisms. Trauma Violence Abuse. 2011;12:115–126. doi: 10.1177/1524838011404251. [DOI] [PubMed] [Google Scholar]
- 13.Colantonio A. Sex, gender, and traumatic brain injury: A commentary. Arch. Phys. Med. Rehabil. 2016;97:S1–S4. doi: 10.1016/j.apmr.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Lippa R, Connelly S. Gender diagnosticity: A new Bayesian approach to gender-related individual differences. J. Pers. Soc. Psychol. 1990;59:1051–1065. doi: 10.1037/0022-3514.59.5.1051. [DOI] [Google Scholar]
- 15.Cox, D. R. Tests of separate families of hypotheses. In Berkeley Symposium on Mathematical Statistics and Probability, Vol. 4.1, 105–123 (1961).
- 16.Kass RE, Raftery AE. Bayes factors. J. Am. Stat. Assoc. 1995;90:773–795. doi: 10.1080/01621459.1995.10476572. [DOI] [Google Scholar]
- 17.Pelletier R, Ditto B, Pilote L. A composite measure of gender and its association with risk factors in patients with premature acute coronary syndrome. Psychosom. Med. 2015;77:517–526. doi: 10.1097/PSY.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 18.Iron, K. & Manuel, D. G. Quality Assessment of Administrative Data (QuAAD): An Opportunity for Enhancing Ontario’s Health Data. (2007).
- 19.Leung Yinko SSL, et al. Health-related quality of life in premature acute coronary syndrome: Does patient sex or gender really matter? J. Am. Heart Assoc. 2014;3:e000901. doi: 10.1161/JAHA.114.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakholia MV, et al. Systemic estrone production and injury-induced sex hormone steroidogenesis after severe traumatic brain injury: A prognostic indicator of traumatic brain injury-related mortality. J. Neurotrauma. 2019;36:1156–1167. doi: 10.1089/neu.2018.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar RG, et al. Temporal acute serum estradiol and tumor necrosis factor-α associations and risk of death after severe traumatic brain injury. J. Neurotrauma. 2020;37:2198–2210. doi: 10.1089/neu.2019.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma C, et al. Sex differences in traumatic brain injury: A multi-dimensional exploration in genes, hormones, cells, individuals, and society. Chin. Neurosurg. J. 2019;5:24. doi: 10.1186/s41016-019-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner AK, et al. Acute serum hormone levels: Characterization and prognosis after severe traumatic brain injury. J. Neurotrauma. 2011;28:871–888. doi: 10.1089/neu.2010.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaya MO, Raval AP, Bramlett HM. Traumatic brain injury in women across lifespan. Neurobiol. Dis. 2022;164:105613. doi: 10.1016/j.nbd.2022.105613. [DOI] [PubMed] [Google Scholar]
- 25.Chen AY, et al. Factors associated with discharge destination from acute care after acquired brain injury in Ontario, Canada. BMC Neurol. 2012;12:16. doi: 10.1186/1471-2377-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown SB, Colantonio A, Kim H. Gender differences in discharge destination among older adults following traumatic brain injury. Health Care Women Int. 2012;33:896–904. doi: 10.1080/07399332.2012.673654. [DOI] [PubMed] [Google Scholar]
- 27.Waisath, W. et al. Closing the Gender Gap: a Summary of Findings and Policy Recommendations. https://www.worldpolicycenter.org/sites/default/files/WORLD_Policy_Brief_Summary_of_Findings_and_Policy_Recommendations_for_Closing_the_Gender_Gap_2015.pdf (2014).
- 28.Nowrojee, S. Gender equality in 2022: The best, the worst, the most surprising, and most ridiculous. United Nations Foundationhttps://unfoundation.org/blog/post/gender-equality-in-2022-the-best-the-worst-the-most-surprising-and-most-ridiculous/?gclid=EAIaIQobChMI-_mAj8rV_QIVTnZvBB1HAQ6yEAAYAiAAEgI7qfD_BwE (2022).
- 29.Chen AY, Colantonio A. Defining neurotrauma in administrative data using the International Classification of Diseases Tenth Revision. Emerg. Themes Epidemiol. 2011;8:4. doi: 10.1186/1742-7622-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Statistics Canada. Life expectancy and other elements of the complete life table, single-year estimates, Canada, all provinces except Prince Edward Island. (2022) 10.25318/1310083701-ENG.
- 31.Gedeborg R, Warner M, Chen LH, Gulliver P, Cryer C, Robitaille Y, Bauer R, Ubeda C, Lauritsen J, Harrison J, Henley G, Langley J. Internationally comparable diagnosis-specific survival probabilities for calculation of the ICD-10-based Injury Severity Score. J. Trauma Acute Care Surg. 2014;76(2):358–365. doi: 10.1097/TA.0b013e3182a9cd31. [DOI] [PubMed] [Google Scholar]
- 32.Association for the Advancement of Automotive Medicine. Abbreviated Injury Scale Association for the Advancement of Automotive Medicine 2019 (Available from https://www.aaam.org/abrreviated-injury-scale-ais/).
- 33.Registered Persons Database (RPDB). https://data.ontario.ca/dataset/registered-persons-database-rpdb.
- 34.Feinerer, I. & Hornik, K. tm: Text Mining Package.
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- 36.Jana S, et al. Application of multiple testing procedures for identifying relevant comorbidities, from a large set, in traumatic brain injury for research applications utilizing big health-administrative data. Front. Big Data. 2022;5:793606. doi: 10.3389/fdata.2022.793606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Z, et al. Predictors of 30-day mortality in traumatic brain-injured patients after primary decompressive craniectomy. World Neurosurg. 2020;134:e298–e305. doi: 10.1016/j.wneu.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 38.Fegan, S. Recommended ICD-10-CA Codes for Injury Core Indicators. Association of Public Health Epidemiologists in Ontariohttp://core.apheo.ca/index.php?pid=306 (2013).
- 39.Austin PC, van Walraven C. The Mortality Risk Score and the ADG Score: Two points-based scoring systems for the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med. Care. 2011;49:940–947. doi: 10.1097/MLR.0b013e318229360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colantonio A, et al. Predictors of postacute mortality following traumatic brain injury in a seriously injured population. J. Trauma. 2008;64:876–882. doi: 10.1097/TA.0b013e31804d493e. [DOI] [PubMed] [Google Scholar]
- 41.Ratcliff G, Colantonio A, Escobar M, Chase S, Vernich L. Long-term survival following traumatic brain injury. Disabil. Rehabil. 2005;27:305–314. doi: 10.1080/09638280400018338. [DOI] [PubMed] [Google Scholar]
- 42.Friedman M. Piecewise exponential models for survival data with covariates. Ann. Stat. 1982 doi: 10.1214/aos/1176345693. [DOI] [Google Scholar]
- 43.Jacob B, Chan V, Stock D, Colantonio A, Cullen N. Determinants of discharge disposition from acute care for survivors of hypoxic-ischemic brain injury: Results from a large population-based cohort data set. Arch. Phys. Med. Rehabil. 2021;102:1514–1523. doi: 10.1016/j.apmr.2021.01.083. [DOI] [PubMed] [Google Scholar]
- 44.Agresti A. Categorical Data Analysis. Wiley; 2013. [Google Scholar]
- 45.Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6:461–464. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- 46.Lee KJ, Carlin JB. Recovery of information from multiple imputation: A simulation study. Emerg. Themes Epidemiol. 2012;9:3. doi: 10.1186/1742-7622-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ICES is an independent, non-profit research institute funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). As a prescribed entity under Ontario's privacy legislation, ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation, and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario. The dataset from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at http://www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.


