Abstract
Sediments from the sulfate-reduction zone of a petroleum-contaminated aquifer, in which benzene persisted, were inoculated with a benzene-oxidizing, sulfate-reducing enrichment from aquatic sediments. Benzene was degraded, with apparent growth of the benzene-degrading population over time. These results suggest that the lack of benzene degradation in the sulfate-reduction zones of some aquifers may result from the failure of the appropriate benzene-degrading sulfate reducers to colonize the aquifers rather than from environmental conditions that are adverse for anaerobic benzene degradation.
Persistence of benzene under sulfate-reducing conditions in petroleum-contaminated aquifers.
There are extensive anoxic zones in many petroleum-contaminated aquifers (1, 7). Although anaerobic microbial processes can remove alkylated monoaromatic hydrocarbons from petroleum-contaminated aquifers, highly toxic benzene often persists under in situ anaerobic conditions (7). For example, benzene appears to be degraded slowly, if at all, under sulfate-reducing conditions in petroleum-contaminated aquifers (2, 14, 16). This is despite the fact that the potential for benzene oxidation coupled to sulfate reduction in marine and estuarine sediments has been demonstrated (3, 4, 6, 9, 17). Furthermore, benzene degradation was observed under sulfate-reducing conditions in an enrichment culture initiated with aquifer sediments (5).
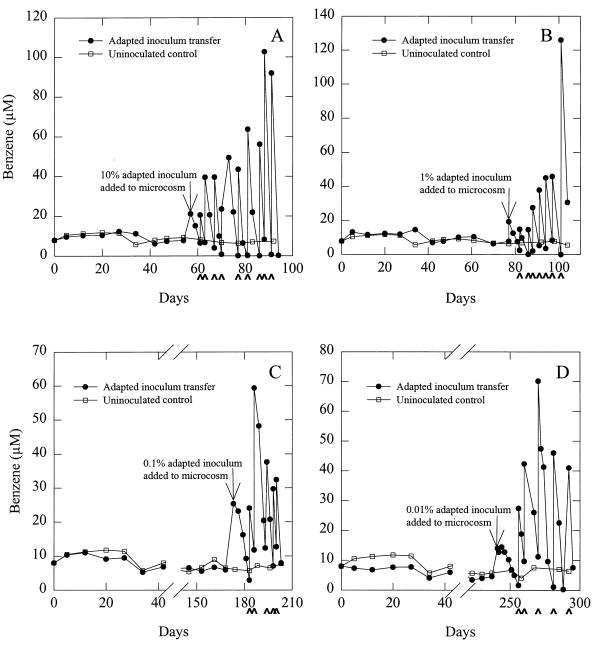
In order to further evaluate the potential for anaerobic benzene degradation coupled to sulfate reduction in petroleum-contaminated aquifers, aquifer sediments were collected from the sulfate-reducing zone of an aquifer contaminated with jet fuel (8, 18) as previously described (13). Strict anaerobic conditions were used in the incubation (12) of sediments (30 ml) under N2-CO2 (93:7) in 50-ml serum bottles sealed with thick butyl rubber stoppers. Sodium sulfate was added from an anaerobic stock solution (300 mM) in order to provide ca. 1 mM sulfate and ensure that the sediments did not become sulfate depleted. The sediment bottles were incubated inverted in the dark at 20°C. Benzene was added to these sediments from anaerobic aqueous stocks, and the loss of benzene was monitored by measuring benzene concentrations in the headspace with gas chromatography as previously described (9, 12). There was no degradation of benzene even after more than 250 days of incubation (Fig. 1).
FIG. 1.
Benzene uptake in inoculated and uninoculated aquifer sediments. Arrowheads along the x axes indicate readditions of benzene. Arrows in the graphs indicate the times of inoculation. The inoculation procedure required opening the bottles under a stream of N2-CO2, which flushed benzene from the system. Thus, benzene had to be added back to the sediments, which accounts for the slight increases in benzene concentrations at the times of inoculation. The data are from one bottle for each treatment.
Benzene oxidation coupled to sulfate reduction in freshwater aquatic sediments.
Previous studies that have reported benzene oxidation coupled to sulfate reduction were conducted with marine or estuarine sediments (3, 4, 6, 9, 17). In a study in which benzene oxidation coupled to sulfate reduction was simultaneously investigated in both marine and freshwater sediments, benzene degradation was only observed in the marine sediments (17). Therefore, one possible explanation for the lack of benzene degradation under sulfate-reducing conditions in freshwater aquifer sediments was that benzene oxidation coupled to sulfate reduction does not take place under freshwater conditions.
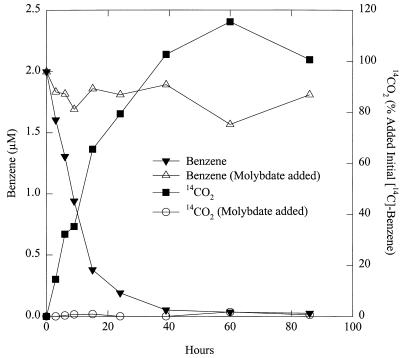
However, freshwater aquatic sediments from the previously described (10) Gunston Cove site in the Potomac River were adapted for benzene oxidation coupled to sulfate reduction within 120 days (data not shown). When 0.39 μCi of [14C]benzene (58.2 mCi/mmol, diluted in sterile, anoxic water to provide ca. 2 μCi/ml) was added to these benzene-adapted sediments and 14CO2 and 14CH4 were monitored with a gas proportional counter as previously described (12), there was a steady production of 14CO2 over time that corresponded with a loss of benzene that was monitored in parallel incubations without added [14C]benzene (Fig. 2). When molybdate, a specific inhibitor of sulfate reduction (15), was added from an anaerobic, concentrated stock of sodium molybdate (500 mM) to a final concentration of 10 mM 1 h prior to these incubations, loss of benzene and production of 14CO2 over time were inhibited (Fig. 2). Studies on the stoichiometry of benzene degradation and sulfate depletion in these sediments were conducted as previously described for benzene-adapted marine sediments (9). The amount of benzene-dependent sulfate reduction was 81% ± 13% (n = 3) of the sulfate reduction expected if the benzene metabolized was completely oxidized to carbon dioxide, with sulfate serving as the sole electron acceptor, according to the following reaction: 4C6H6 + 15SO42− + 12H2O→24HCO3− + 15HS− + 9H+. Similar percentages of benzene-dependent sulfate reduction have been observed in studies with benzene-adapted marine and estuarine sediments (9, 17).
FIG. 2.
Loss of benzene and production of 14CO2 from [14C]benzene over time in freshwater aquatic sediments adapted for benzene degradation coupled to sulfate reduction. The data are the means of duplicate incubations for each treatment.
The results with the freshwater aquatic sediments demonstrate that microorganisms that can oxidize benzene with the reduction of sulfate can flourish under freshwater conditions. Thus, a lack of appropriate salinity cannot account for the persistence of benzene in freshwater petroleum-contaminated aquifers. Another possibility for the persistence of benzene in the aquifer sediments was that even though environmental conditions might be suitable for benzene oxidation coupled to sulfate reduction, the sediments lack the appropriate benzene-degrading sulfate-reducing microorganisms. In order to evaluate this, aquifer sediments were inoculated (10% [wt/wt]) with the benzene-adapted freshwater aquatic sediments (Fig. 1A). Following inoculation, benzene degradation proceeded without a lag. With the depletion of the benzene initially present in the sediments, more benzene was added, resulting in continued degradation (Fig. 1A). After the inoculated aquifer sediments of Fig. 1A had been refed benzene five times, this sediment was used to provide a 10% inoculum for another bottle of unadapted aquifer sediments, which then also rapidly metabolized benzene (Fig. 1B). This procedure was repeated two more times, with continued rapid benzene degradation in all inoculated sediments (Fig. 1C and D). This was the case even in the final transfer (Fig. 1D) in which the amount of aquatic sediment from the initial benzene-degrading inoculation constituted less than 1 part per 10,000 of the sediment mass.
The finding that benzene continued to be rapidly degraded after the aquatic sediment had been effectively diluted out indicates that the addition of the aquatic sediments did not stimulate benzene degradation by changing the physical-chemical characteristics of the aquifer sediments. The fact that the capacity for benzene degradation was maintained with successive transfers of adapted sediments into unadapted aquifer sediments suggests that the factor responsible for benzene degradation was capable of replication, i.e., that it was a benzene-degrading microorganism that originated from the freshwater aquatic sediments.
Previous studies have demonstrated that benzene also persists in anaerobic sediments from the Fe(III) reduction zone of this aquifer unless the availability of Fe(III) is artificially enhanced with the addition of Fe(III) chelators or humic substances (12, 13). Sulfate reduction is generally inhibited in the presence of Fe(III) because Fe(III) reducers outcompete sulfate reducers for electron donors (11). However, it seemed possible that benzene degradation in the Fe(III)-reducing aquifer sediments could also be stimulated with the benzene-oxidizing, sulfate-reducing inoculum since previous studies (12, 13) had indicated that there should be no Fe(III) reducers that would be able to compete with the sulfate reducers for benzene.
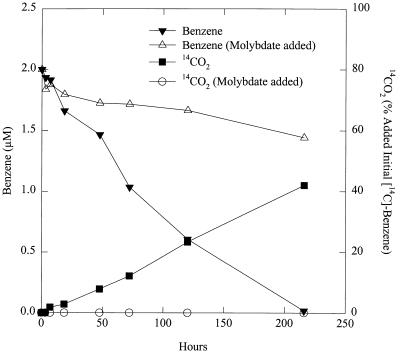
In order to evaluate this, aquifer sediments in which Fe(III) reduction was the terminal electron accepting process (TEAP) were amended with 20 mM ferrous sulfate and then inoculated with benzene-adapted aquatic sediments as described above. Inoculation of the Fe(III)-reducing sediments stimulated benzene degradation just as it had in the sediments in which sulfate reduction was the TEAP. Once the inoculated Fe(III)-reducing sediments were adapted for rapid benzene degradation, they could be used as inocula to stimulate benzene degradation in unadapted aquifer sediments. The involvement of sulfate reduction in this benzene degradation was evaluated with molybdate after the third such 10% transfer, when the volume of the original aquatic sediment inoculum was no more than 1 part per 1,000 of the sediment mass. Molybdate inhibited both the loss of benzene over time and the production of 14CO2 from [14C]benzene (Fig. 3).
FIG. 3.
Effect of molybdate on benzene uptake and production of 14CO2 from [14C]benzene in aquifer sediments from the Fe(III) reduction zone that were amended with the benzene-oxidizing, sulfate-reducing inoculum. As outlined in the text, the original inocula accounted for no more than 0.1% if the sediment mass. The data are the means of duplicate determinations.
These results demonstrate that the inoculated benzene-degrading sulfate reducers were effective in stimulating benzene oxidation coupled to sulfate reduction in Fe(III)-containing sediments. This finding is significant because Fe(III) is prevalent in large sections of many petroleum-contaminated aquifers (1, 7). Thus, if Fe(III) inhibited the activity of the benzene degraders, the number of sites at which benzene degradation could be stimulated with a benzene-degrading, sulfate-reducing inoculum would be limited.
Conclusions.
These studies indicate that it is the lack of benzene-oxidizing sulfate reducers in the aquifer sediments rather than the inability of benzene-oxidizing sulfate-reducers to grow and metabolize under freshwater conditions that is responsible for the persistence of benzene under sulfate-reducing conditions in this petroleum-contaminated aquifer. Although it is generally regarded that a population of the appropriate degradative organisms will develop if the proper conditions are present in an environment, the studies reported here demonstrate that this is not always true because an active benzene-degrading population was not established in the aquifer sediments even though conditions were suitable for benzene oxidation coupled to sulfate reduction. Attempts to identify the organisms responsible for benzene degradation in the aquifer sediments upon inoculation are under way.
The finding that supplementing aquifer sediments with benzene-oxidizing sulfate reducers can greatly accelerate anaerobic benzene degradation not only provides insight into the factors limiting the intrinsic anaerobic bioremediation of benzene but also suggests a novel strategy for anaerobic bioremediation of petroleum-contaminated aquifers. This and other (12, 13) anaerobic approaches may be more economically feasible and less technically difficult than commonly employed aerobic strategies for enhancing benzene degradation in heavily contaminated anaerobic aquifers (7).
Acknowledgments
This work was supported by National Science Foundation grant DEB9523932.
We thank Frank Chapelle for help in collecting the aquifer sediments and Joan Woodward for technical assistance.
REFERENCES
- 1.Anderson R T, Lovley D R. Ecology and biogeochemistry of in situ groundwater bioremediation. Adv Microbial Ecol. 1997;15:289–350. [Google Scholar]
- 2.Chapelle F H, Bradley P M, Vroblesky D A, Lovley D R. Measuring rates of biodegradation in a petroleum hydrocarbon-contaminated aquifer. Groundwater. 1996;34:691–698. [Google Scholar]
- 3.Coates J D, Anderson R T, Lovley D R. Oxidation of polycyclic aromatic hydrocarbons under sulfate-reducing conditions. Appl Environ Microbiol. 1996;62:1099–1101. doi: 10.1128/aem.62.3.1099-1101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coates J D, Anderson R T, Woodward J C, Phillips E J P, Lovley D R. Anaerobic hydrocarbon degradation in petroleum-contaminated harbor sediments under sulfate-reducing and artificially imposed iron-reducing conditions. Environ Sci Technol. 1996;30:2784–2789. [Google Scholar]
- 5.Edwards E A, Grbic-Galic D. Complete mineralization of benzene by aquifer microorganisms under strictly anaerobic conditions. Appl Environ Microbiol. 1992;58:2663–2666. doi: 10.1128/aem.58.8.2663-2666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazumi J, Caldwell M E, Suflita J M, Lovley D R, Young L Y. Anaerobic degradation of benzene in diverse environments. Environ Sci Technol. 1997;31:813–818. [Google Scholar]
- 7.Lovley D R. Potential for anaerobic bioremediation of BTEX in petroleum-contaminated aquifers. J Ind Microbiol. 1997;18:75–81. [Google Scholar]
- 8.Lovley D R, Chapelle F H, Woodward J C. Use of dissolved H2 concentrations to determine the distribution of microbially catalyzed redox reactions in anoxic ground water. Environ Sci Technol. 1994;28:1205–1210. doi: 10.1021/es00056a005. [DOI] [PubMed] [Google Scholar]
- 9.Lovley D R, Coates J D, Woodward J C, Phillips E J P. Benzene oxidation coupled to sulfate reduction. Appl Environ Microbiol. 1995;61:953–958. doi: 10.1128/aem.61.3.953-958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovley D R, Phillips E J P. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol. 1987;53:2636–2641. doi: 10.1128/aem.53.11.2636-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovley D R, Woodward J C, Chapelle F H. Stimulated anoxic biodegradation of aromatic hydrocarbons using Fe(III) ligands. Nature. 1994;370:128–131. doi: 10.1038/370128a0. [DOI] [PubMed] [Google Scholar]
- 13.Lovley D R, Woodward J C, Chapelle F H. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl Environ Microbiol. 1996;62:288–291. doi: 10.1128/aem.62.1.288-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyngkilde J, Christensen T H. Fate of organic contaminants in the redox zones of a landfill leachate pollution plume (Vejen, Denmark) J Contam Hydrol. 1992;10:291–307. [Google Scholar]
- 15.Oremland R S, Capone D G. Use of “specific” inhibitors in biogeochemistry and microbial ecology. Adv Microb Ecol. 1988;10:285–383. [Google Scholar]
- 16.Patterson B M, Pribac F, Barber C, Davis G B, Gibbs R. Biodegradation and retardation of PCE and BTEX compounds in aquifer material from Western Australia using large-scale columns. J Contam Hydrol. 1993;14:261–278. [Google Scholar]
- 17.Phelps C D, Kazumi J, Young L Y. Anaerobic degradation of benzene in BTX mixtures dependent on sulfate reduction. FEMS Microbiol Lett. 1996;145:433–437. doi: 10.1111/j.1574-6968.1996.tb08612.x. [DOI] [PubMed] [Google Scholar]
- 18.Vroblesky D A, Chapelle F H. Temporal and spatial changes of terminal electron accepting processes in a petroleum hydrocarbon contaminated aquifer and the significance for contaminant biodegradation. Water Resour Res. 1994;30:1561–1570. [Google Scholar]