Abstract
This study describes a novel, distinct phenotype of urinary symptoms named “myofascial urinary frequency syndrome” (MUFS) present in one-third of individuals presenting with urinary frequency. In addition to a characteristic symptom constellation suggestive of myofascial dysfunction, MUFS subjects exhibit “persistency”: a persistent feeling of needing to urinate regardless of urine volume. On examination, 97% of MUFS patients demonstrated pelvic floor hypertonicity with either global tenderness or myofascial trigger points, and 92% displayed evidence of impaired muscular relaxation, hallmarks of myofascial dysfunction. To confirm this symptom pattern was attributable to the pelvic floor musculature, we confirmed the presence of “persistency” in 68 patients with pelvic floor myofascial dysfunction established through comprehensive examination and electromyography and corroborated by improvement with pelvic floor myofascial release. These symptoms distinguish subjects with myofascial dysfunction from subjects with OAB, IC/BPS, and asymptomatic controls, confirming MUFS is a distinct LUTS symptom complex.
Subject terms: Health care, Signs and symptoms, Urological manifestations, Urology, Urological manifestations
Introduction
Storage lower urinary tract symptoms (LUTS), such as urinary frequency, urgency, and bladder discomfort, dramatically impact both urologic well-being and global quality of life. During their lifetime, the majority of women will experience at least one of these symptoms1, which are often chronic and degrade both physical and social functioning2. While many options exist for LUTS management, in reality, clinical care remains inadequate and most women do not seek out care for these symptoms3. For those who attempt to get treatment, many will not receive an accurate diagnosis4, with more than 90% abandoning medical therapies within a year of seeking care5.
Accurate diagnosis remains a challenge for defining appropriate management of LUTS. Patients with LUTS are commonly classified into one of several symptom clusters, such as overactive bladder (OAB) and interstitial cystitis/bladder pain syndrome (IC/BPS), with obscure relationships to the pathophysiology underlying those symptoms. OAB is typically distinguished by urinary urgency, a sudden, compelling desire to pass urine which is difficult to defer6, while interstitial cystitis/bladder pain syndrome (IC/BPS) is distinguished by bladder pain7,8. While laboratory and diagnostic testing can help to rule out other etiologies for lower urinary tract symptoms, such as urinary retention, bladder tumors or urinary stones, or urinary tract infection, these two syndromes are defined solely by a collection of patient-reported symptoms, making diagnosis subjective and clinician-dependent9,10. Even more advanced urologic evaluation (such as cystoscopy or urodynamic testing) is frequently normal in both of these conditions, and there are no laboratory markers to identify either condition. As there is substantial symptomatic overlap between OAB and IC/BPS11–13, we previously proposed a novel nomogram using patient-reported symptoms that has substantial power to distinguish IC/BPS from OAB based on widely used, validated surveys14.
Many patients, however, do not fall within the categories of OAB or IC/BPS due to their lack of key features associated with these diagnoses. Many patients present with a similar urinary frequency and desire to urinate (“urge”) as that expressed by OAB patients, but lack the sudden urge to urinate with little warning (“urgency”) that is central to an OAB diagnosis. These patients describe bladder pressure or discomfort, but in the absence of the distinct bladder pain that suggests a diagnosis of IC/BPS. Such patients have been allocated into various categories (e.g., frequency-urgency syndrome), but currently are misdiagnosed as “OAB-dry” due to their lack of incontinence (or fear of incontinence). While the name suggests a similar spectrum as OAB, the absence of true urinary urgency is inconsistent with a diagnosis of OAB and suggests a different pathophysiology. A recent study of the Lower Urinary Tract Dysfunction Research Network (LURN) cohort revealed that such “OAB-dry” individuals exhibit unique patterns of presenting features that differ from classic OAB subjects, including straining with urination, bladder discomfort, and a feeling of incomplete emptying15. The severity of symptomatic bother in these OAB-dry subjects was most closely related to the severity of pelvic floor tenderness on exam, implicating a possible myofascial dysfunction in this LUTS subgroup.
In the application of our previously described, diagnostic nomogram to a real-world population of subjects with storage LUTS, we hypothesized that the nomogram would successfully identify and classifying subjects with OAB and IC/BPS, but also identify patient subgroups outside the classical LUTS diagnostic paradigm. This analysis describes the features of that group of highly bothered patients who lacked the bladder pain or true urgency characteristic of IC/BPS or OAB, but were symptomatic with urinary frequency, an uncomfortable urge to void without incontinence and bladder pressure, similar to the OAB-dry subset described within the LURN cohort15. In further characterizing this inadequately described phenotype of LUTS, we sought to confirm previously suggested associations with pelvic floor myofascial dysfunction, defining a novel subtype of storage LUTS we termed myofascial urinary frequency syndrome (MUFS).
Results
A highly bothered subset of patients lacks clear bladder pain or urgency/urge incontinence
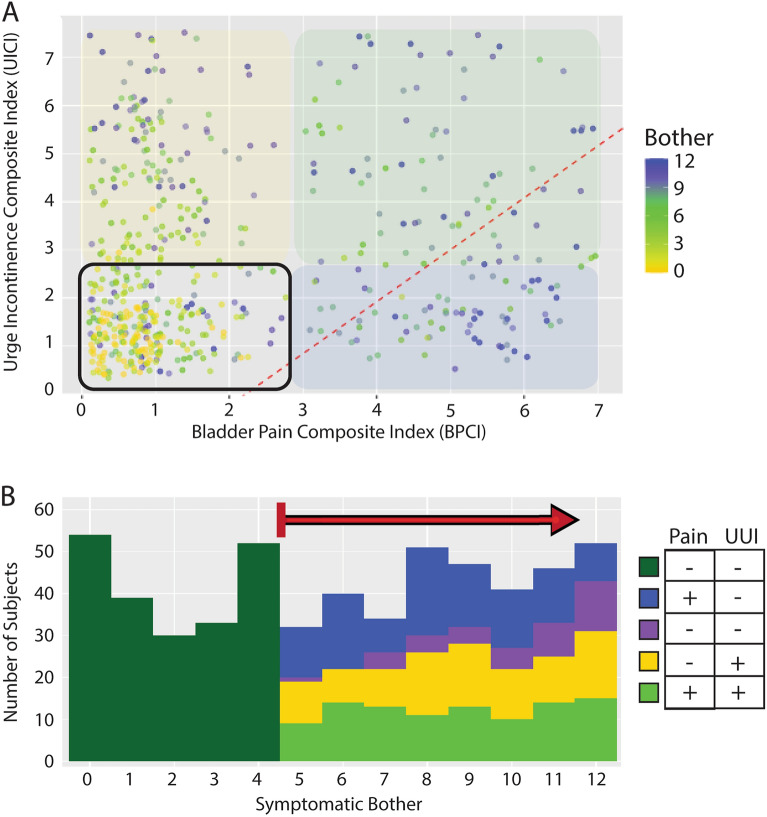
The Exploratory cohort included 551 female subjects sequentially presenting to a tertiary urology clinic in the 2017 calendar year (Fig. 1) were assessed by four validated symptom questionnaires: (1) the female Genitourinary Pain Index (fGUPI)16, (2) Overactive Bladder Questionnaire (OAB-q)17, (3) Pelvic Floor Distress Inventory (PFDI-20)18, and (4) the O’Leary–Sant Indices19. Of these, 208 subjects were not significantly bothered by genitourinary complaints [fGUPI Quality of Life(QoL) < 5), leaving 343 patients dissatisfied with their urinary symptoms. Application of the previously described storage LUTS nomogram (Fig. 2) classified 96 (28%) subjects as IC/BPS, defined by a bladder pain composite index (BPCI) score ≥ 3 in the absence of significant urinary urgency. This subgroup exhibited symptoms consistent with that diagnosis, demonstrating the highest levels of bladder pain (ICSI4, ICPI4) aggravated by filling (fGUPI 2c, 2d) (Table 1). An additional 137 subjects (40%) were categorized as OAB [urgency/incontinence composite index (UICI) scores ≥ 2.5], with characteristically high scores describing urgency often associated with a fear of incontinence (UDI-6 2, OABq8, ICSI1, ICPI3; Table 1). Within the OAB group, there were 38 (11%) subjects with urgency and urgency-related incontinence as their dominant symptoms, but who also had substantial bladder pain (BPCI ≥ 3) conveyed on questionnaires [double-positive (DP) for pain and urgency].
Figure 1.
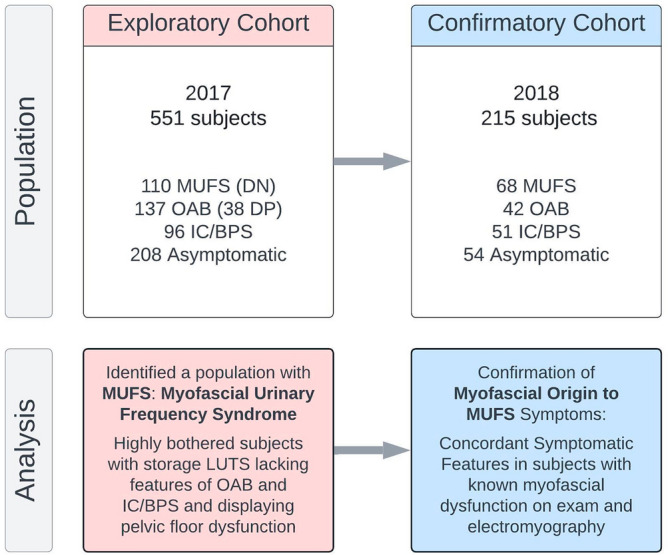
Study design. The exploratory cohort served initially to investigate what symptomatic complaints and exam findings were common to patients presenting with bothersome LUTS who did not exhibit the classic symptoms of overactive bladder (OAB) or interstitial cystitis/bladder pain syndrome (IC/BPS). After identifying that these patients displayed multiple features of myofascial dysfunction, a phenotype we dubbed myofascial urinary frequency syndrome (MUFS), we verified the most salient features of MUFS by comparison of a group of subjects with LUTS, abnormal pelvic floor findings on exam and EMG findings of a tonically contracted pelvic floor to asymptomatic subjects and patients with confirmed OAB and IC/BPS (Reassessment Cohort).
Figure 2.
A highly bothered subset of patients lacks clear bladder pain or urgency/urge incontinence. (A) In a test population of 551 subjects with a variety of LUTS, the Bother Index identified those patients dissatisfied with their urinary symptoms (yellow to blue heat map). (B) A dot plot of BPCI and UICI symptomatic scores for all subjects with high bother (green gradient indicating bother is as in (A) displays the degrees of bladder pain (BP) and urgency/urge incontinence (UI), respectively. OAB patients with UI (yellow overlay) occupy the upper left quadrant, while IC/BPS patients with BP (blue overlay) are in the lower left quadrant. A substantial population negative for the symptom domains originally described for this diagnostic LUTS nomogram is seen in the left lower quadrant, dubbed the Double Negative (DN) group (purple). These data also reveal a population with both UI and BP in the upper right quadrant (green; double Positive: DP). The division previously defined between OAB and IC/BPS patients from our prior nomogram is indicated by the red dotted line.
Table 1.
Symptomatic features of the exploratory cohort by nomogram quadrant.
| Question | Scale | DN | Cont | OAB | IC/BPS | Adjusted P value for DN | |||
|---|---|---|---|---|---|---|---|---|---|
| (n = 110) | (n = 208) | (n = 99) | (n = 96) | vs. Cont | vs. OAB | vs. IC | |||
| Age | 0–95 | 48.08 (17.98) | 54.55 (18.27) | 65.82 (16.05) | 46.60 (13.24) | 0.09 | < 0.001 | 0.91 | |
| OABq1 | Frequent urination during the daytime hours | 1–6 | 3.22 (1.56) | 1.70 (1.24) | 3.56 (1.64) | 3.24 (1.29) | < 0.001 | 0.20 | > 0.999 |
| OABq2 | An uncomfortable urge to urinate | 1–6 | 3.23 (1.35) | 1.73 (1.19) | 3.84 (1.52) | 3.85 (1.41) | < 0.001 | 0.004 | 0.004 |
| OABq3 | A sudden urge to urinate | 1–6 | 2.05 (1.29) | 1.56 (1.01) | 4.30 (1.19) | 2.51 (1.44) | < 0.001 | < 0.001 | 0.01 |
| OABq4 | Accidental loss of small amounts of urine | 1–6 | 1.93 (1.30) | 1.72 (1.01) | 4.26 (1.32) | 1.68 (0.90) | > 0.999 | < 0.001 | > 0.999 |
| OABq5 | Nighttime urination | 1–6 | 2.73 (1.65) | 2.04 (1.38) | 4.00 (1.67) | 3.07 (1.62) | < 0.001 | < 0.001 | 0.11 |
| OABq6 | Waking at night because you had to urinate | 1–6 | 2.92 (1.65) | 2.15 (1.46) | 4.25 (1.55) | 3.47 (1.55) | < 0.001 | < 0.001 | 0.02 |
| OABq7 | An uncontrollable urge to urinate | 1–6 | 2.94 (1.54) | 1.28 (0.78) | 4.06 (1.65) | 2.98 (1.58) | < 0.001 | < 0.001 | > 0.999 |
| OABq8 | Urine loss associated with a strong desire to urinate | 1–6 | 1.49 (0.85) | 1.45 (0.91) | 4.45 (1.34) | 1.71 (1.01) | 0.58 | < 0.001 | 0.29 |
| ICSI1 | Strong need to urinate with little or no warning | 0–5 | 1.21 (1.36) | 0.64 (0.95) | 2.74 (1.66) | 2.20 (1.61) | < 0.001 | < 0.001 | < 0.001 |
| ICSI2 | Urinate less than 2 h after you finished urinating | 0–5 | 2.63 (1.67) | 1.57 (1.45) | 3.50 (1.29) | 3.34 (1.42) | < 0.001 | < 0.001 | 0.007 |
| ICSI3 | How often typically up at night to urinate | 0–5 | 1.58 (1.29) | 1.33 (1.27) | 2.28 (1.41) | 1.95 (1.24) | 0.18 | 0.002 | 0.12 |
| ICSI4 | Experienced pain or burning in your bladder | 0–4 | 0.89 (1.53) | 0.40 (0.79) | 0.70 (1.05) | 2.55 (1.48) | < 0.001 | 0.81 | < 0.001 |
| ICPI1 | Frequent urination during the day | 0–4 | 1.90 (1.35) | 0.84 (1.16) | 2.78 (1.06) | 2.66 (1.19) | < 0.001 | < 0.001 | < 0.001 |
| ICPI2 | Getting up at night to urinate | 0–4 | 1.47 (1.39) | 1.02 (1.23) | 2.56 (1.33) | 2.17 (1.30) | 0.009 | < 0.001 | 0.001 |
| ICPI3 | Need to urinate with little warning | 0–4 | 0.92 (1.12) | 0.58 (0.95) | 2.72 (1.14) | 1.58 (1.29) | 0.01 | < 0.001 | < 0.001 |
| ICPI4 | Burning, pain, discomfort or pressure in your bladder | 0–4 | 1.35 (1.53) | 0.48 (0.93) | 1.22 (1.41) | 3.24 (0.96) | < 0.001 | 0.69 | < 0.001 |
| fGUPI1a† | Pain/discomfort at the entrance to the vagina | 0–1 | 0.37 | 0.12 | 0.20 | 0.39 | < 0.001 | 0.03 | 0.85 |
| fGUPI1b† | Pain/discomfort in the vagina | 0–1 | 0.37 | 0.08 | 0.22 | 0.42 | < 0.001 | 0.07 | 0.77 |
| fGUPI1c† | Pain/discomfort in the urethra | 0–1 | 0.38 | 0.07 | 0.16 | 0.53 | < 0.001 | 0.002 | 0.10 |
| fGUPI1d† | Pain/discomfort below the waist, in pubic or bladder area | 0–1 | 0.37 | 0.08 | 0.34 | 0.86 | < 0.001 | 0.71 | < 0.001 |
| fGUPI2a† | Pain or burning during urination | 0–1 | 0.34 | 0.05 | 0.23 | 0.54 | < 0.001 | 0.15 | 0.01 |
| fGUPI2b† | Pain or discomfort during or after sexual intercourse | 0–1 | 0.60 | 0.14 | 0.15 | 0.52 | < 0.001 | < 0.001 | 0.51 |
| fGUPI2c† | Pain or discomfort as your bladder fills | 0–1 | 0.05 | 0.02 | 0.05 | 1.00 | 0.67 | 0.87 | < 0.001 |
| fGUPI2d† | Pain or discomfort relieved by voiding | 0–1 | 0.20 | 0.07 | 0.17 | 0.67 | 0.002 | 0.59 | < 0.001 |
| fGUPI3 | Frequency of pain or discomfort over the last week | 0–5 | 2.92 (1.24) | 0.52 (0.83) | 1.51 (1.57) | 3.45 (1.21) | < 0.001 | < 0.001 | 0.009 |
| fGUPI4 | Number that best describes average pain/discomfort | 0–10 | 3.43 (2.93) | 0.73 (1.43) | 2.66 (2.89) | 5.71 (2.13) | < 0.001 | 0.09 | < 0.001 |
| fGUPI5 | Frequency of sensation of incomplete emptying | 0–5 | 1.85 (1.80) | 0.85 (1.15) | 2.01 (1.59) | 2.52 (1.71) | < 0.001 | 0.29 | 0.02 |
| fGUPI6 | Need to urinate < 2 h after last urinating | 0–5 | 2.50 (1.61) | 1.25 (1.15) | 3.19 (1.36) | 3.35 (1.41) | < 0.001 | 0.005 | < 0.001 |
| fGUPI7 | Have your symptoms kept you from doing the kinds of things you would usually do | 0–3 | 1.27 (1.07) | 0.14 (0.35) | 1.49 (1.16) | 1.47 (1.08) | < 0.001 | 0.48 | 0.48 |
| fGUPI8 | How much did you think about your symptoms | 0–3 | 2.38 (0.81) | 0.45 (0.57) | 2.34 (0.83) | 2.45 (0.71) | < 0.001 | > 0.999 | > 0.999 |
| fGUPI9 | Satisfaction with current symptoms | 0–6 | 4.68 (1.10) | 1.36 (1.08) | 4.83 (0.98) | 4.92 (1.05) | < 0.001 | > 0.999 | 0.53 |
| POPDI-1 | Pressure in the lower abdomen | 0–4 | 1.36 (1.39) | 0.47 (0.92) | 1.11 (1.32) | 2.25 (1.23) | < 0.001 | 0.46 | < 0.001 |
| POPDI-2 | Heaviness or dullness in the lower abdomen | 0–4 | 1.28 (1.33) | 0.35 (0.79) | 0.87 (1.28) | 1.75 (1.31) | < 0.001 | 0.03 | 0.03 |
| POPDI-3 | A bulge or something falling out that can see or feel in the vaginal area | 0–4 | 0.39 (1.03) | 0.12 (0.52) | 0.35 (0.86) | 0.20 (0.65) | 0.07 | > 0.999 | > 0.999 |
| POPDI-4 | A need to push on the vagina or around the rectum to have a complete bowel movement | 0–4 | 0.53 (1.02) | 0.30 (0.85) | 0.50 (1.04) | 0.34 (0.91) | 0.05 | > 0.999 | 0.67 |
| POPDI-5 | A feeling of incomplete bladder emptying | 0–4 | 2.67 (0.97) | 0.72 (1.01) | 1.68 (1.27) | 1.81 (1.52) | < 0.001 | < 0.001 | < 0.001 |
| POPDI-6 | A need to push up in the vagina area to start or complete urination | 0–4 | 0.14 (0.52) | 0.14 (0.52) | 0.23 (0.68) | 0.12 (0.46) | > 0.999 | > 0.999 | > 0.999 |
| CRADI-8–1 | A need to strain too hard to have a bowel movement | 0–4 | 1.26 (1.41) | 0.28 (0.74) | 0.91 (1.28) | 0.88 (1.16) | < 0.001 | 0.22 | 0.02 |
| CRADI-8–2 | A feeling that you have not completely emptied your bowels after a bowel movement | 0–4 | 1.02 (1.20) | 0.31 (0.76) | 0.98 (1.33) | 0.82 (1.21) | < 0.001 | > 0.999 | 0.83 |
| CRADI-8–3 | Losing stool without control when stools are well formed | 0–4 | 0.12 (0.59) | 0.04 (0.34) | 0.27 (0.81) | 0.05 (0.34) | 0.27 | 0.63 | 0.98 |
| CRADI-8–4 | Losing stool without control when stool is loose or liquid | 0–4 | 0.23 (0.73) | 0.09 (0.50) | 0.64 (1.16) | 0.24 (0.72) | 0.03 | 0.01 | > 0.999 |
| CRADI-8–5 | Losing gas from the rectum without control | 0–4 | 0.64 (1.20) | 0.29 (0.68) | 0.80 (1.18) | 0.32 (0.84) | 0.26 | > 0.999 | 0.26 |
| CRADI-8–6 | Pain with passing stools | 0–4 | 0.29 (0.79) | 0.05 (0.33) | 0.31 (0.82) | 0.22 (0.72) | 0.001 | > 0.999 | > 0.999 |
| CRADI-8–7 | A strong sense of urgency and have to rush to the bathroom to have a bowel movement | 0–4 | 0.58 (1.10) | 0.25 (0.68) | 0.78 (1.18) | 0.37 (0.82) | 0.05 | 0.44 | 0.44 |
| CRADI-8–8 | Stool passes through the rectum and bulges outside during or after a bowel movement | 0–4 | 0.18 (0.71) | 0.08 (0.43) | 0.32 (0.88) | 0.06 (0.35) | 0.79 | 0.79 | 0.79 |
| UDI-6–1 | Bothered by frequent urination | 0–4 | 1.74 (1.43) | 0.77 (1.14) | 2.78 (1.37) | 2.15 (1.30) | < 0.001 | < 0.001 | 0.12 |
| UDI-6–2 | Bothered by leakage related to feeling of urgency | 0–4 | 0.64 (1.00) | 0.89 (1.00) | 3.21 (1.00) | 1.21 (1.23) | 0.02 | < 0.001 | 0.001 |
| UDI-6–3 | Bothered by leakage related to physical activity, coughing, or sneezing | 0–4 | 0.74 (1.19) | 0.58 (0.99) | 1.91 (1.44) | 0.76 (1.10) | > 0.999 | < 0.001 | > 0.999 |
| UDI-6–4 | Bothered by small amounts of leakage (drops) | 0–4 | 0.65 (1.15) | 0.41 (0.85) | 2.20 (1.47) | 0.45 (0.87) | 0.36 | < 0.001 | 0.61 |
| UDI-6–5 | Bothered by difficulty emptying bladder | 0–4 | 0.95 (1.29) | 0.22 (0.62) | 0.90 (1.31) | 1.19 (1.26) | < 0.001 | > 0.999 | 0.48 |
| UDI-6–6 | Bothered by pain or discomfort in the lower abdominal or genital area | 0–4 | 1.21 (1.53) | 0.20 (0.70) | 0.78 (1.30) | 2.43 (1.21) | < 0.001 | 0.10 | < 0.001 |
Means (standard deviation) are shown for all interval variables, except for the binary variables which document the population proportions scoring positive for each individual feature. Pairwise comparison of interval variables were compared using Welch’s t test, while binary variables (designated with†) were examined using the Chi-square test. DN, Double Negative Population; Cont, Asymptomatic Controls; OAB, Overactive Bladder; IC/BPS, Interstitial Cystitis/Bladder Pain Syndrome; OABq, Overactive Bladder Questionnaire; ICSI, Interstitial Cystitis Symptoms Index; ICPI, Interstitial Cystitis Problem Index; fGUPI, Female Genitourinary pain index; UDI-6, Urinary Distress Inventory Short Form; CRADI-8, Colorectal-Anal Distress Inventory; POPDI-6, Pelvic Organ Prolapse Distress Inventory; ns, Not significant.
The remaining 110 patients (32%) demonstrated significantly bothersome urinary symptoms but lacked either the bladder pain or urgency that characterized the IC/BPS and OAB groups, respectively (Supplemental Fig. 1). Despite lacking pain or urgency, this population [double-negative (DN) for pain and urgency] was bothered at similar levels to the other subject groups and was younger than the other populations.
The DN population exhibits a symptomatic profile distinct from other LUTS symptom clusters
To identify the nature and severity of bothersome genitourinary symptoms present in the DN population, we examined patient-reported scores on 51 individual questions from validated instruments (Table 1). Individual questions were clustered into redundant groups to attempt to identify specific symptoms common to and shared by this DN population. Only two symptomatic features demonstrated higher values in this population than in the other groups: a sensation of incomplete bladder emptying (POPDI-6 5) and a need to strain with defecation (CRADI-8 1). While these sensations of incomplete bladder and bowel emptying were significant in comparison to controls, companion questions describing the actual “difficulty emptying the bladder” (UDI-6 5) or “difficulty emptying your bowels at the end of a bowel movement” (CRADI-8 2) were not significantly different from other LUTS populations.
In addition, the endorsement of urge differed based on the individual wording of the questionnaire (Table 1): DN subjects endorsed a similar magnitude of urge as classic OAB patients when expressed as a “strong” or “uncomfortable” need to urinate (OABq2). However, this population was minimally symptomatic and significantly different from OAB subjects when the urge was expressed as a “need to urinate with little warning” (ICSI1, ICPI2) or “sudden urge” (OABq3).
A similar pattern was seen for pain symptoms, with the questions divided into two categories. Scores for the DN population were similar to the IC/BPS population when the question wording included the terms “pressure” or “discomfort” alone or in combination with other pain terms (ICPI4) but were significantly less when the terms “burning” or “pain” were used in isolation (ICSI4). The patterns of pain localization also differed between the DN and IC/BPS populations. IC/BPS subjects were characterized primarily by pain with bladder filling relieved by bladder emptying (fGUPI 2c, 2d). In contrast, the DN population exhibited additional, variable manifestations of genitourinary pain, but only pain related to intercourse (fGUPI 2b) was present in more than half of DN subjects (Table 1).
Urinary frequency was a dominant aspect of this DN population (OABq1, ICSI2, ICPI3, UDI-6 1), although the objective severity and bother attributed to this symptom was lower than that seen for OAB or IC/BPS subjects (Table 1). Urinary incontinence (OABq4, OABq8, UDI-6 1–3), fecal urgency/incontinence (CRADI-8 3–5, CRADI-8 7), and nocturia (OABq5, ICSI3, ICPI2) were low in this population, clearly distinguishing this group from the classic OAB population. Thus, the dominant features of the DN population in validated questionnaires were a perception of incomplete emptying, urinary frequency, pelvic discomfort, pelvic pressure unrelated to bladder filling, and straining to void/defecate without sudden-onset urgency, nocturia, significant incontinence, or bladder-specific pain.
The double-negative population exhibits multiple aspects of myofascial dysfunction
As symptom scores on the Colorectal-Anal Distress Inventory (CRADI-8) were similar in pattern to patients with pelvic floor dyssynergia described in the colorectal literature20, we examined the diagnoses coded in association with the initial specialist consultation of the 110 subjects classified into the DN group. Ninety-seven (88%) of these subjects carried a coded diagnosis of high-tone pelvic floor dysfunction at initial evaluation. The remainder of the patient visits carried codes associated with symptomatic features, such as urinary frequency, dysuria, and pelvic pain.
Eighty-four (76%) of DN subjects had positive documentation of increased pelvic floor hypertonicity with either global tenderness or distinct, painful myofascial trigger points, despite pain not being a primary complaint of the patient without elicitation. Only 3 (3%) of the subjects in this group did not have any evidence of myofascial hypertonicity or tenderness documented on physical exam; most subjects with sufficient assessment (67/73, 92% of those assessed, 61% overall) also had evidence of impaired muscular relaxation after voluntary pelvic floor contraction, a hallmark of myofascial dysfunction. We therefore named the symptom complex experienced by the DN population “myofascial frequency syndrome” to reflect both LUTS and myofascial dysfunction.
Thematic analysis of DN patient histories links uncomfortable bladder pressure to urinary frequency
Qualitative review of individual histories of present illness using thematic analysis (Table 2) confirmed the pattern of symptomatology revealed by the questionnaires, identifying bothersome urinary frequency resulting from a perception of bladder pressure or discomfort and conveying the sensation of fullness with an uncomfortable need to urinate as common among these patients. This constellation of complaints was frequently attributable to a persistent feeling of bladder fullness or pressure with the accompanying need to urinate regardless of urine volume, a symptomatic constellation we termed “persistency”. Several additional symptomatic features that could not be captured by questionnaires were common among these subjects, such as a minimal or positive effect of fluid intake on symptom severity, minimal nocturia once asleep with increases in frequent urination experienced with initially lying down at night, worsening of symptoms with certain activities (e.g., travel, extended periods of sitting, or very intense exercise), a sensation of bladder fullness unrelieved by voiding, and occasional insensate, small volume incontinence. These highly bothered subjects often had no clear dominant symptom, but almost all complained of “persistency”. Their urinary frequency appeared assocated with the persistent feeling of needing to urinate regardless of how much urine was in the bladder; this sensation of an uncomfortable inability to defer voiding combined with a sensation of incomplete emptying and pelvic discomfort that was difficult to localize.
Table 2.
Thematic analysis of presenting features of patients with MFS.
| Global theme | Category | Subcategory | Code | Raw data (examples) |
|---|---|---|---|---|
| Urinary frequency | Persistent need to void | Constant frequency | Persistent feeling of needing to void | "She can never be comfortable for any period of time, even right after voiding" |
| Fullness out of proportion with volume | "She states that there is never very much there, but it feels like I'm going to burst, so I have to go." | |||
| Intrusive urge | "She notes the need to go is powerful, and very hard to ignore." | |||
| Prone frequency without nocturia | Nighttime Frequency | "She has to go to the bathroom 5–6 times before she can finally get to sleep" | ||
| Association with lying down | "Very little comes out each time, but 5 min after she gets back in bed, she feels like she has to go again to get empty." | |||
| No Nocturia | "Once she gets to sleep, she stays asleep for ~ 6 h, but has to go multiple times when she first lies down." | |||
| Activities exacerbate symptoms | Excessive physical activity | Exercise brings on urge | "She cannot exercise without feeling like she has to urinate every 20 min" | |
| Physical activity worsens symptoms | "The day after a lot of walking, like a trip to Disneyland, will be much worse" | |||
| Immobility for long periods of time | Worsened by immobility | "Travelling in the car for long trips is terrifying because it will make these symptoms so much worse." | ||
| Symptoms persist after immobility | "Always gets the feeling of needing to go constantly right after a long plane flight, almost like the beginning of a UTI but it never progresses." | |||
| Extended Periods of Sitting | Sitting worse than other positions | "She has to lie down in the back seat on long car trips, or she will have to urinate every 10 min." | ||
| Sitting increases urge | "She notes that she dreads long meetings, cause the urgency is so much worse sitting in one place for that long and she feels like she can't go or her coworkers will think something is wrong with her | |||
| Sensation of Bladder Fullness | Sensation of incomplete emptying | Constant sensation of fullness | Persistant urge | "it always feels like there is something there, even right after going" |
| Rapid return of fullness | "Within 15 min of going, she feels like her bladder is completely full again." | |||
| Perception of fullness | "She states she knows there's nothing there, but feels like she is still full." | |||
| Poor Toileting Behaviors | Straining to Void | "She sits on the toilet for at least a few minutes after trying to push any remaining urine out, even though little comes, just to make sure she is empty" | ||
| Double Voiding | "She will typically get up and then go right back to the toilet and only a little bit more will come out." | |||
| Persistant Voiding | "She complains that she is losing a lot of time to just sitting on the toilet in an attempt to finally feel empty" | |||
| Bladder discomfort and pressure | Pressure | Building pressure | "It is not a sudden-onset urgency, more of a building pressure that is hard to ignore." | |
| Pressure throughout pelvis | "there is a pressure that begins midline over the bladder and then spreads across the low pelvis if she tries to hold it." | |||
| Heaviness | "there is a heaviness in the pelvis that is always there, it just gets worse the longer she tried to wait before urinating." | |||
| Discomfort | Discomfort builds | "If she does not go when the desire comes, she will not be incontinent, but will be more and more uncomfortable until she just has to go" | ||
| Pain with deferring urination | "If she had to hold it past the early sensation of needing to go, it would just get painful to hold it, so she never does." | |||
| Uncomfortable urge | "it is not painful, but just a very uncomfortable pressure that interferes with her ability to do anything else." | |||
| Uncomfortable Urge, not Urgency | Minimal impact of fluids | Little dependence on fluid | No impact of fluid limitation | "if she drinks more, she goes more, but even when she limits fluids heavily, the feeling of constantly needing to void never goes away." |
| Low volume urge | "She states as soon as she takes a sip of any fluids, the urine hits her bladder and she has to go again" | |||
| Frequency independent of intake | "It doesn't matter how little she drinks, the feeling [of bladder fullness] never goes away." | |||
| Improved voiding with more volume | better voiding efficiency with more fluids | "she notes that if she drinks more, sometimes it will help her feel like she can empty better and be more comfortable" | ||
| no impairment in capacity | "She notes that the only time she feels she can really get empty is first thing in the morning when her bladder is really full." | |||
| Insensate incontinence | Activity-associated leakage | Worse leak with intense activity | "While exercising, never feels any leakage, but notices that she can be very wet afterwards" | |
| Minor activities bring on leakage | "Never feels a gush, but notes her liner is pretty wet when she gets home after a long walk." | |||
| No awareness of leaks | Feeling of wetness, not leak | "she never notices anything coming out, but when she gets undressed at the end of the day, she notices her underwear is wet." | ||
| Constant incontinence | "No particular events or activities bring it on, she just notices a little wetness in her underwear when she goes to the bathroom." | |||
| Not stress incontinence | No leakage with stress maneuvers | "denies any leakage with cough, laugh or sneeze, but feels a little bit wet all throughout the day" | ||
| No association with fullness | "She denies any period when she can be predictably dry for any length of time" | |||
| Very low volume | Bothered by smell | "never soaks through the inner cotton layer of her underwear, but definitely smells like urine, so she keeps a change of underwear in her purse." | ||
| Low volume | "she just feels a little bit wet, all throughout the day, but never enough to wear a pad." | |||
| No urgency incontinence | No urgency or urgency incontinence | No sudden-onset urgency | "She never leaks or has to run suddenly to the bathroom, but constantly needs to go." | |
| No incontinence with urge | "She states if she had to hold it and couldn't get to a bathroom, she could make it." | |||
| Volitional voiding to avoid discomfort | "She could wait to void, but doesn't want to." |
Descriptions of unique symptomatic features were extracted from the history of present illness (HPI) at each patient’s initial consultation.
Defining the constellation of urinary symptoms associated with pelvic floor myofascial dysfunction
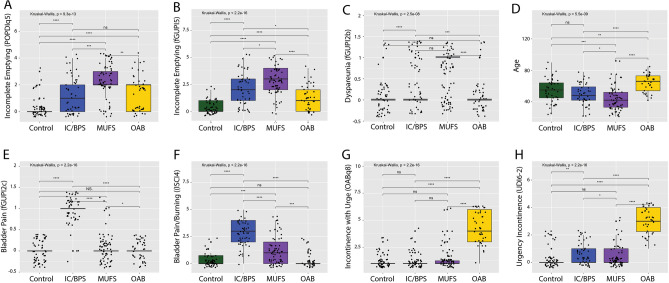
We next sought to confirm the presence of “persistency” in the Reassessment cohort, an independent cohort containing patients with a known myofascial origin to their urinary symptoms. From all patients with primary assessment in 2018, we identified 68 patients established through comprehensive diagnostic evaluation to have pelvic floor myofascial dysfunction as the source of their urinary symptoms. Urodynamic testing with pelvic floor electromyography demonstrated a tonically contracted, non-relaxing pelvic floor, even during voiding. Subjective symptomatic improvement in LUTS following pelvic floor physical therapy or biofeedback served as secondary confirmation of myofascial dysfunction as the source of their symptoms. Consistent with the clinical profiles of the MUFS group, these individuals had sought care for urinary complaints, typically urinary frequency and a persistent urge to void, in the absence of significant bladder or pelvic pain (Fig. 3). These subjects were then combined with 42 subjects with OAB, 51 subjects with IC/BPS, and 54 asymptomatic subjects seen during the same interval to create a Reassessment cohort.
Figure 3.
MUFS subjects exhibit symptomatic features distinct from OAB and IC/BPS subjects. While multiple symptomatic features, such as urinary frequency, were increased in all LUTS groups, the MUFS demonstrated significantly increased severity of a sensation (A) and frequency (B) of incomplete emptying and dyspareunia (C). Subjects with MDFS were also significant younger than the other populations (D). In contrast, subjects with IC/BPS exhibited higher levels of pain with bladder filling (E) and bladder pain and burning (F), while OAB subjects demonstrated consistent elevations in the sudden incontinence associated with urgency (G) and a strong desire to urinate (H). Brackets indicate pairwise comparisons by Wilcoxon rank sum tests. *p < 0.05, **p < 0.005, ***P < 0.005, ns: not significant.
Bivariate logistic regression models were used to identify individual patient-reported symptomatic features associated with myofascial dysfunction from the OABq symptom assessment, PFDI-20, fGUPI, and ICSI/ICPI (51 items in total). Nineteen symptom features found to have significant associations were included in multivariable models predicting myofascial dysfunction (Supplemental Table 1), one including and one excluding the asymptomatic controls, to ensure that the identified features could distinguish myofascial dysfunction subjects from both controls and other subjects with genitourinary symptoms. Only two variables remained positively associated with myofascial dysfunction in both models (fGUPI 5, POPDI-6 5), both of which describe a sensation of incomplete bladder emptying. The sensation of lower abdominal pressure (POPDI-6 1) as well as pain with sexual intercourse (fGUPI 2b) were both positively associated with myofascial dysfunction, although the association was not statistically significant in the LUTS-only model (p = 0.057). Both urine loss associated with a strong desire to urinate (OABq8) and pain with bladder filling (fGUPI 2c) demonstrated significant negative associations with myofascial dysfunction, clearly distinguishing this group from the OAB and IC/BPS groups, respectively. Thus, this independent cohort of patients with known myofascial dysfunction demonstrated the symptoms of “persistency”, the persistent feeling of needing to void occurring in conjunction with a sensation of incomplete emptying, bladder pressure, and dyspareunia, but without urgency, urge incontinence, or isolated pain with bladder filling.
The clinical presentation of DN subjects is associated with significant diagnostic confusion
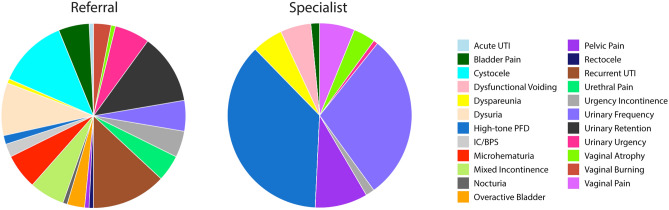
Pre-referral diagnoses for the myofascial dysfunction patients were highly varied, including cystocele, urinary retention, dysuria, recurrent UTI, hematuria, urethral pain, urinary frequency, and urgency, without a single dominant diagnosis. After initial specialist evaluation, more than 75% of MUFS subjects were given a primary diagnosis of either high-tone pelvic floor dysfunction (37%), urinary frequency (29%) or pelvic pain (9%) (Fig. 4). Other less common diagnoses included dysfunctional voiding (5%), dyspareunia (5%), and vaginal pain (6%), all of which encompass features common to the MUFS symptom cluster.
Figure 4.
Referral and Specialist Diagnosis for Myofascial Urinary Frequency Syndrome Subjects in the Validation Dataset. At referral, subjects with myofascial urinary frequency syndrome were frequently diagnosed with a wide range of presumptive diagnoses. As there is no specific code for LUTS derived from a myofascial dysfunction, the coded diagnoses reflected the typical symptoms of this disorder (e.g., urinary frequency, pelvic pain, vaginal pain, dyspareunia) and pelvic floor muscle dysfunction (high-tone pelvic floor dysfunction, dysfunctional voiding).
Discussion
In this study, we describe a previously underrecognized phenotype of storage LUTS that we named myofascial frequency syndrome (MUFS), which is distinct from other common forms of storage LUTS such as OAB and IC/BPS. Affected patients typically seek care for nonspecific symptoms of urinary frequency, an urge to urinate, pelvic pressure, or a sensation of incomplete bladder emptying, embodied in the symptomatic concept we identified as “persistency”. Demonstrating objective findings of pelvic floor hypertonicity and trigger points on physical exam, MUFS subjects demonstrate symptomatic improvements in these LUTS with myofascial-directed therapies, such as PFPT21. MUFS was common in this study, identified in 20% of all new urologic consultations and 32% of those presenting with urinary frequency. While MUFS shares many features with myofascial pelvic pain/pelvic floor myalgia (MPP/PFM), the presentation of these patients is distinct in that they do not report pain. Although pain can sometimes be elicited or trigger points identified on pelvic exam, patients’ reasons for seeking care are primarily urinary, which can lead to difficulties suspecting the pelvic floor as an etiology of their symptoms if a detailed pelvic floor exam is not performed. While the co-existence of urinary complaints in patients with MPP/PFM is well-documented22, few reports have detailed the urinary manifestations associated with myofascial dysfunction in the absence of baseline bladder/pelvic pain23–25.
In the colorectal literature, however, myofascial dysfunction has been well documented to contribute substantially to defecatory symptoms in the absence of pain26. Multiple phenotypes of myofascial dysfunction, including failed, incomplete, or paradoxical relaxation of the levator muscle complex, contribute to distinct patterns of defecatory symptoms, ranging from obstructive defecation to insensate fecal incontinence to painful defecation. Our data suggest that there is a similar breadth of urinary symptomatology attributable to myofascial dysfunction that extends beyond myofascial pain, of which MUFS is one such phenotype.
Our analysis reveals that MUFS patients commonly present with a combination of symptoms that overlap substantially with other categories of storage LUTS. The clinical behavior of MUFS as a “great pretender”, mimicking many of the features of better characterized conditions, may explain why this syndrome is frequently unrecognized and remains poorly documented in the literature. MUFS subjects describe urinary frequency often due to an uncomfortable feeling of needing to urinate; many will use the word “urgency” to describe this sensation, making MUFS easily confused with OAB, even though this sensation is distinct from true urgency. Subjects with MUFS often complain of pelvic pressure and heaviness and straining to defecate, similar to subjects with pelvic organ prolapse, although typically patients will deny a vaginal bulge or a need to splint to void/defecate. In addition, MUFS patients will often complain of a bladder/pelvic discomfort that is not described as overt pain, but, in combination with frequency and urge, could easily be conflated with IC/BPS. Some patients even exhibit a fluctuating urethral/vaginal discomfort or burning and a terminal dysuria that can be mistaken for recurrent urinary tract infections, although the baseline symptoms rarely resolve completely between aggravating episodes. The most discriminatory feature, however, was the feature of “persistency”: a persistent desire to urinate regardless of bladder volume, typically accompanied by urinary frequency and a sensation of incomplete bladder emptying that was unexpectedly distinct from the difficulty emptying the bladder experienced in patients with prolapse.
While the bladder pressure described by patients is similar to the bladder pressure and discomfort often described by many patients diagnosed with IC/BPS, these patients infrequently describe pain or discomfort when asked about their symptoms, often only using these terms when prompted or during examination. In addition, the persistent desire to urinate is a common complaint solicited from patients with IC/BPS as well. Patients with IC/BPS are often noted to have increased pelvic floor hypertonicity in addition to bladder-specific pain, which similarly benefits from pelvic floor physical therapy27. Before ruling out bladder-centric IC/BPS, it may be reasonable to consider a bladder anesthetic challenge to help differentiate whether the patients symptoms represent a reactionary pelvic floor dysfunction or pure myofascial dysfunction alone. While it might be reasonable to place MUFS patients under the umbrella of IC/BPS given this symptomatic overlap, MUFS patients responded with complete remission of their symptoms with physical therapy alone, which establishes MUFS as a distinct condition with a specific presentation and etiology that benefits from physical therapy as the first line of care. Improved recognition of this condition independent of IC/BPS will improve the care of affected individuals and help to avoid unnecessary invasive procedures or medications with significant side effects.
Given the wide range of referral diagnoses for the population with MUFS, it is reasonable to conclude that this mixture of symptoms elicits significant confusion for providers. While a standardized screening examination has been developed for identifying and quantifying internal hip and pelvic floor myofascial pain on palpation28, a diagnostic examination requires formal evaluation by a specially trained pelvic floor physical therapist or rehabilitation medicine specialist. Even for skilled urologists and urogynecologists, making a diagnosis of MUFS requires both a careful, discriminant pelvic exam and a detailed symptomatic history to identify potential myofascial dysfunction. This can be challenging in an era in which telemedicine is increasingly an expectation by patients and when the amount of time allowed for visits may limit the ability to perform this assessment adequately. Future derivation of an objective symptomatic measure associated with this phenotype could greatly assist providers in suspecting a myofascial origin to a patient’s urinary symptoms and focusing the physical assessment and possible treatments to be considered21.
An additional limitation to improving the diagnosis of MUFS is the absence of consensus terminology for myofascial pelvic floor dysfunction. For example, numerous terms have been used to describe a pelvic floor with abnormal pelvic floor contractile activity, including: nonfunctioning pelvic floor muscles, short pelvic floor syndrome, atonic or acontractile pelvic floor, hypertonic pelvic floor, high-tone pelvic floor dysfunction, and pelvic floor/levator muscle spasm29. The International Continence Society30 proposed classification of pelvic floor muscle dysfunction into overactive, underactive and non-functioning categories, but these general descriptions fail to capture the range of possible pathologies, noting only their association with genitourinary pain as well as voiding, defecatory, and sexual dysfunction. There is also a lack of diagnostic ICD-10 codes describing this specific pelvic floor condition, further confounding epidemiologic study of myofascial dysfunction. As we learn more about the role of the pelvic floor in urinary symptomatology, it is imperative we develop both consistent terminology and diagnostic codes to reflect these conditions. Common terminology is one necessary and surmountable step to improving the awareness and care for these patients moving forward; its deficiency limits the reproducibility of this analysis as no gold standard for the diagnosis of MUFS has yet been defined.
Diagnosis of MUFS could be achieved with objective, reproducible, and quantifiable assessments of pelvic floor muscle fitness. Such measures may help differentiate between forms of myofascial dysfunction and correlate those patterns with different symptomatologies, as has been done with defecatory disorders. Unfortunately, there are currently no accepted measures to quantify muscular fitness or dynamic myofascial function for the pelvic floor. While pelvic floor electromyography can identify paradoxical contraction during voiding that is characteristic of dysfunctional voiding, it is not reliable in assessing pelvic floor muscle strength or fitness/functionality31. Novel pelvic floor assessment methods that identify and objectively measure the types of myofascial dysfunction are greatly needed. Urinary symptoms may be caused by a shortened, acontractile pelvic floor, a hypertonic, fixed pelvic floor, or a dyssynergic pelvic floor. The causes, manifestations, and treatments for each of these pelvic floor dysfunctions may be unique; a better understanding of how to prevent and manage each etiology requires more advanced pelvic floor assessment.
Further, the underlying causes of each of these forms of myofascial dysfunction remain elusive. New tools to assess dynamic muscle function and fitness will be needed to establish these relationships more definitively. Many of the patients noted chronic constipation and defecatory dysfunction, volitional holding behaviors, musculoskeletal injuries or trauma, joint dysfunction (e.g., arthritis, sacroiliac joint dysfunction, etc.), and even anxiety disorders. While many of these described comorbidities could impact pelvic floor myofascial function, a large number of affected individuals did not have these features. In addition, coexistance of MUFS with myofascial hypertonicity tells us little about the direction of causality. Myofascial dysfunction in these individuals could be a result of urinary symptoms, with a guarding-type reflex incited by abnormal bladder sensations, or the direct cause of those symptoms, in which poor pelvic floor muscle fitness directly impairs bladder function to induce urinary frequency and a sensation of bladder fullness. The resolution of urinary symptoms seen in the Reassessment cohort after physical therapy with documented improvements in myofascial hypertonicity, however, implicates the pelvic floor musculature as a key player in the development of these urinary symptoms, although future studies will need to examine this connection propspectively. Interestingly, these noted associations are similar to co-occurrence patterns and symptoms seen in children with Bladder Bowel Dysfunction (BBD)32,33. Despite a high prevalence, BBD is underdiagnosed, raising the possibility that MUFS in adults could represent a later manifestation of unrecognized childhood BBD.
The limitations of this analysis bear mention. As this cohort included only women, utilizing multiple symptomatic measures specifics to women, it is not clear how these symptoms present in men and how myofascial dysfunction manifests as a cause for LUTS. In addition, our thematic analysis utilized the provider documentation of patient symptoms. While significant detail in these records was sufficient to gain substantial insight into the experiences of patients, future studies should attempt to capture these experiences directly from the patient perspective.
While MUFS can occur in subjects across the age spectrum, it comprised a larger proportion of younger patients presenting with LUTS. One explanation for this pattern is that insults to the pelvic floor that accompany aging (e.g., pelvic floor obstetrical trauma, increased muscular laxity) may be protective against MUFS. However, OAB incidence also increases dramatically at the menopausal transition and continues to increase thereafter. Subjects with concurrent urgency and urgency incontinence characteristic of OAB would not have been classified as MUFS, as this group was defined as lacking this symptom. Therefore, it is unclear but likely that MUFS co-exists with other storage LUTS, particularly in older patients. Many patients with IC/BPS demonstrate pelvic floor hypertonicity on exam and benefit significantly from PFPT27,34,35, suggesting that multiple forms of storage LUTS can coexist in a single patient. Hopefully, as we gain better recognition and tools for the evaluation of MUFS, we will be able to begin to dissect out the relative contributions of neurologic, inflammatory, detrusor, and myofascial dysfunction in these more complex clinical presentations.
Regardless of these limitations, this study takes the first step of defining and differentiating MUFS, a myofascial-driven, non-painful urologic symptom cluster, as a distinct storage LUTS subtype characterized by “persistency”: an uncomfortable, persistent desire to urinate associated with urinary frequency and a sensation of incomplete bladder emptying.
Online methods
Study inclusion
This study was approved by the Cedars-Sinai Office of Research Compliance and Quality Improvement Institutional Review Board (IRB#00040261), which waived the requirement for subject informed consent, and all research was performed in accordance with relevant guidelines/regulations. Female subjects seeking care from urologists board-certified in female pelvic medicine and reconstructive surgery (FPMRS) were administered four validated, written questionnaires at their initial clinical evaluation: (1) the female Genitourinary Pain Index (fGUPI)16, (2) Overactive Bladder Questionnaire (OAB-q)17, (3) Pelvic Floor Distress Inventory (PFDI-20)18, and (4) the O’Leary–Sant Indices19 as part of standard of care treatment. The fGUPI measures the nature and severity of genitourinary pain, and contains subscales assessing pain, urinary symptoms, and quality of life16. The OAB-q measures both continent and incontinent OAB symptoms and contains subscales for symptom bother, coping behaviors, concern/worry, social interaction, sleep, and health-related quality of life17. Only the symptom questions (1–8) were utilized in this analysis; the 25 health-related quality of life questions on the OABq (OABq 9–33), which address the impact of symptoms on activities and quality of life, were not examined for symptomatic discrimination. The PFDI-20 measures pelvic floor symptoms and includes domains that assess urinary [Urinary Distress Inventory (UDI-6)], defecatory [Colorectal-Anal Distress Inventory (CRADI-8)], and prolapse symptoms [Pelvic Organ Prolapse Distress Inventory (POPDI-6)]18. The O’Leary–Sant Indices, which include the Interstitial Cystitis Symptom and Problem Indices (ICSI/ICPI), are commonly used together to measure the severity of and bother associated with urinary frequency, urgency, nocturia, and bladder pain19.
Study cohorts
Two distinct groups of subjects were analyzed, an Exploratory cohort and a Reassessment cohort (Fig. 1). Patients who were not significantly bothered by their symptoms (i.e., Bother Index < 5) were excluded from analysis. Retrospective assessment of the diagnoses coded for these excluded subjects revealed that most sought care for conditions such as asymptomatic microscopic hematuria, nephrolithiasis, or pyuria. Patients with active or recurrent symptomatic urinary tract infections (UTI), prior invasive urinary tract therapies (such as neuromodulation, onabotulinumtoxinA injection, transurethral resection of the bladder, etc.), active smoking, current pregnancy, diabetes, or neurogenic lower urinary tract dysfunction (such as from spinal cord injury, multiple sclerosis, or spinal dysraphism) were excluded. Patients with cyclic pain at menses with concern for endometriosis were also excluded; however, patients with other comorbid functional pain syndromes, such as irritable bowel syndrome or fibromyalgia, were allowed to participate.
The Exploratory cohort consisted of 551 consecutive female subjects with a range of irritative storage LUTS evaluated at a tertiary urology practice between January and December 2017. The previously described diagnostic nomogram was applied to this population to classify subjects with characteristic urgency incontinence (UI) as OAB (UICI > 2.5, BPCI < 3), subjects reporting bladder pain (BP) as IC/BPS (BPCI > 3, UICI < 2.5), and subjects with low bother scores (Bother Index ≤ 5) as controls14. A mixed group of 110 patients were unclassified (UICI < 2.5, BPCI < 3, Bother > 5), and represented a range of symptomatic diagnoses such as urinary frequency, urgency, and bladder pressure. Validated questionnaire responses were used to characterize the symptomatic patterns distinguishing this group from the other classifications.
A Reassessment cohort of 215 subjects evaluated between January and December 2018 was employed to examine the correlation of distinct symptomatic LUTS patterns. This population contained a myofascial dysfunction group of 68 subjects with urinary symptoms and demonstrable myofascial discomfort or trigger points on physical exam confirmed by non-relaxing pelvic floor hypertonicity on electromyography. Initial evaluation ruled out other obvious sources of their symptoms with a negative urine culture, postvoid residual under 50 ml, and normal cystoscopy. Patients with cyclic symptoms related only to menstrual cycle were excluded from this group. Myofascial dysfunction was further implicated as the source of their symptomatology by demonstrable improvements in their urinary symptoms after myofascial release-based, pelvic floor physical therapy (PFPT) or biofeedback. This cohort contained two additional groups with LUTS: (1) 42 subjects diagnosed with OAB who endorsed significant urgency incontinence, lacked any bladder pain on exam or questionnaire assessment, and exhibited detrusor overactivity on urodynamic assessment, and (2) 51 subjects with a clinical diagnosis of IC/BPS with significant bladder pain on exam and questionnaires but who lacked incontinence. An additional 54 subjects with asymptomatic scores on all questionnaires served as controls.
Qualitative assessment of bothered subjects with LUTS lacking pain or urge
For subjects from the Exploratory cohort lacking both urgency incontinence and bladder pain [double-negative (DN) for pain and urgency], we captured findings on discriminant pelvic exams from initial consultations, including prolapse grade by compartment, the presence or absence of stress incontinence on cough stress test, pelvic floor hypertonicity, myofascial trigger points, Oxford grade for pelvic floor muscle strength, and the presence or absence of appropriate relaxation after attempted Kegel.
To determine the prevalent symptomatic features common to this population, we additionally performed a modified thematic analysis36. Descriptions of patient complaints and features were captured from the history of present illness and assessment in the initial consultation within the electronic medical record. Primary urinary complaints, additional bothersome symptoms, aggravating and relieving factors, compensatory behaviors, and terminology used to describe their urinary symptoms were catalogued by two reviewers into primary codes. These primary codes (263 in total) were compiled into common codes capturing similar concepts by each reviewer. A consensus was reached between reviewers regarding conceptual accuracy, resulting in 40 common codes across the population. These codes were clustered into 16 subcategories. The subcategories clustered into 7 categories, which were combined into 3 major themes. In addition, the diagnostic codes associated with both the specialist referral and consultation encounter were recorded.
Statistical analysis
Bivariate differences between phenotypic groups were examined in the Exploratory Cohort using Welch’s t test and chi-square tests. In the Reassessment cohort, bivariate logistic regression models predicting MUFS were used to examine the magnitude of the odds ratios for individual symptomatic features. Measures with significant, positive associations with MUFS were included in a multivariable model predicting MUFS. All analyses were conducted in Stata version 16.1, Stata Corp (College Station, Texas, USA).
Supplementary Information
Abbreviations
- AUC
Area under curve
- BP
Bladder pain
- BPCI
Bladder pain composite index
- BMI
Body mass index
- CRADI-8
Colorectal-anal distress inventory
- DN
Double negative
- DP
Double positive
- fGUPI
Female genitourinary pain index
- FPMRS
Female pelvic medicine and reconstructive surgery
- IC/BPS
Interstitial cystitis/bladder pain syndrome
- ICSI
Interstitial cystitis symptom index
- ICPI
Interstitial cystitis problem index
- LASSO
Least angle shrinkage and selection operator
- LUTS
Lower urinary tract symptoms
- MUFS
Myofascial urinary frequency syndrome
- MPP/PFM
Myofascial pelvic pain/pelvic floor myalgia
- OAB
Overactive bladder
- OABq
Overactive bladder questionnaire
- PFDI
Pelvic floor distress index-20
- PFPT
Pelvic floor physical therapy
- POPDI-6
Pelvic organ prolapse distress inventory 6
- p-CLUS
Phenotyping of comprehensive lower urinary symptoms
- PA
Predictive accuracy
- UI
Urgency incontinence
- UICI
Urge incontinence composite index
- UDI-6
Urinary distress inventory 6
- UTI
Urinary tract infection
Author contributions
LA, MRK and JLL conceived and designed the study. All authors participated in data analysis and reviewed the manuscript. ALA and ATC drafted the manuscript.
Funding
This work was supported by the AUGS/Duke UrogynCREST (Urogynecology Clinical Research Educational Scientist Training) Program (R25HD094667 (NICHD)). Dr. Ackerman is supported by NIH/K08DK118176, NIH/R03AG067993, DOD W81XWH2110644.
Data availability
Data for this study are available in the supplemental figures and tables as well as upon request from the Corresponding Author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44862-5.
References
- 1.Coyne KS, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: Results from the epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104:352–360. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: Results from EpiLUTS. Urology. 2012;80:90–96. doi: 10.1016/j.urology.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Pakgohar M, Sabetghadam S, Vasegh Rahimparvar SF, Kazemnejad A. Quality of life (QoL) and help-seeking in postmenopausal women with urinary incontinence (UI): A population based study. Arch. Gerontol. Geriatr. 2014;59:403–407. doi: 10.1016/j.archger.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Berry SH, et al. Development, validation and testing of an epidemiological case definition of interstitial cystitis/painful bladder syndrome. J. Urol. 2010;183:1848–1852. doi: 10.1016/j.juro.2009.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benner JS, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105:1276–1282. doi: 10.1111/j.1464-410X.2009.09036.x. [DOI] [PubMed] [Google Scholar]
- 6.D'Ancona C, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol. Urodyn. 2019;38:433–477. doi: 10.1002/nau.23897. [DOI] [PubMed] [Google Scholar]
- 7.Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol. Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 8.Clemens JQ, Erickson DR, Varela NP, Lai HH. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J. Urol. 2022;208:34–42. doi: 10.1097/JU.0000000000002756. [DOI] [PubMed] [Google Scholar]
- 9.Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological A. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J. Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 10.Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J. Urol. 2015;193:1572–1580. doi: 10.1016/j.juro.2015.01.087. [DOI] [PubMed] [Google Scholar]
- 11.Hanno PM, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J. Urol. 2011;185:2162–2170. doi: 10.1016/j.juro.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai HH, Vetter J, Jain S, Gereau RWT, Andriole GL. The overlap and distinction of self-reported symptoms between interstitial cystitis/bladder pain syndrome and overactive bladder: A questionnaire based analysis. J. Urol. 2014;192:1679–1685. doi: 10.1016/j.juro.2014.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tincello DG, Walker AC. Interstitial cystitis in the UK: Results of a questionnaire survey of members of the Interstitial Cystitis Support Group. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005;118:91–95. doi: 10.1016/j.ejogrb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Ackerman AL, Lai HH, Parameshwar PS, Eilber KS, Anger JT. Symptomatic overlap in overactive bladder and interstitial cystitis/painful bladder syndrome—Development of a new algorithm. BJU Int. 2018 doi: 10.1111/bju.14568. [DOI] [PubMed] [Google Scholar]
- 15.Torosis M, Jackson N, Nitti V, Ackerman AL. Overactive bladder patients with and without urgency incontinence: A spectrum of one condition or different phenotypes? Urogynecology (Hagerstown) 2023;29:33–40. doi: 10.1097/SPV.0000000000001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens J, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74:983–987.e983. doi: 10.1016/j.urology.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyne K, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: The OAB-q. Qual. Life Res. 2002;11:563–574. doi: 10.1023/A:1016370925601. [DOI] [PubMed] [Google Scholar]
- 18.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am. J. Obstet. Gynecol. 2005;193:103–113. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 20.Rao SS, Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation. J. Neurogastroenterol. Motil. 2016;22:423–435. doi: 10.5056/jnm16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meister MR, et al. A pilot trial of movement-based pelvic floor physical therapy to address pelvic floor myofascial pain and lower urinary tract symptoms. Int. Urogynecol. J. 2022 doi: 10.1007/s00192-022-05353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastore EA, Katzman WB. Recognizing myofascial pelvic pain in the female patient with chronic pelvic pain. J. Obstet. Gynecol. Neonatal. Nurs. 2012;41:680–691. doi: 10.1111/j.1552-6909.2012.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erbes NA, Foster SN, Harris-Hayes M, Spitznagle TM. Movement Impairments in Women with and without Urinary Urgency/Frequency. J. Womens Health Phys. Therap. 2021;45:164–173. doi: 10.1097/jwh.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FitzGerald MP, Kotarinos R. Rehabilitation of the short pelvic floor. I: Background and patient evaluation. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2003;14:261–268. doi: 10.1007/s00192-003-1049-0. [DOI] [PubMed] [Google Scholar]
- 25.Foster SN, et al. Hip and pelvic floor muscle strength in women with and without urgency and frequency predominant lower urinary tract symptoms. J. Womens Health Phys. Therap. 2021;45:126–134. doi: 10.1097/jwh.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brusciano L, et al. Pelvic floor dyssynergia: The new iceberg syndrome. Tech. Coloproctol. 2020;24:393–394. doi: 10.1007/s10151-020-02164-2. [DOI] [PubMed] [Google Scholar]
- 27.FitzGerald MP, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J. Urol. 2012;187:2113–2118. doi: 10.1016/j.juro.2012.01.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meister MR, et al. Development of a standardized, reproducible screening examination for assessment of pelvic floor myofascial pain. Am. J. Obstet. Gynecol. 2019;220:255e251–255e259. doi: 10.1016/j.ajog.2018.11.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bo K, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol. Urodyn. 2017;36:221–244. doi: 10.1002/nau.23107. [DOI] [PubMed] [Google Scholar]
- 30.Messelink B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: Report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol. Urodyn. 2005;24:374–380. doi: 10.1002/nau.20144. [DOI] [PubMed] [Google Scholar]
- 31.Navarro Brazalez B, et al. The evaluation of pelvic floor muscle strength in women with pelvic floor dysfunction: A reliability and correlation study. Neurourol. Urodyn. 2018;37:269–277. doi: 10.1002/nau.23287. [DOI] [PubMed] [Google Scholar]
- 32.Aguiar LM, Franco I. Bladder bowel dysfunction. Urol. Clin. N. Am. 2018;45:633–640. doi: 10.1016/j.ucl.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Santos JD, Lopes RI, Koyle MA. Bladder and bowel dysfunction in children: An update on the diagnosis and treatment of a common, but underdiagnosed pediatric problem. Canadian Urological Association journal Journal de l'Association des urologues du Canada. 2017;11:S64–S72. doi: 10.5489/cuaj.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FitzGerald MP, et al. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J. Urol. 2009;182:570–580. doi: 10.1016/j.juro.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss JM. Pelvic floor myofascial trigger points: Manual therapy for interstitial cystitis and the urgency-frequency syndrome. J. Urol. 2001;166:2226–2231. doi: 10.1016/s0022-5347(05)65539-5. [DOI] [PubMed] [Google Scholar]
- 36.Braun V, Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study are available in the supplemental figures and tables as well as upon request from the Corresponding Author.