Abstract
Introduction
Psoriasis, an incurable chronic inflammatory disease, affects over 6 million people in China. Ixekizumab, a monoclonal antibody against interleukin-17A, has demonstrated efficacy and safety for the treatment of moderate-to-severe plaque psoriasis, although limited data are available regarding its use in routine clinical practice in China. We investigated the real-world application of ixekizumab in China.
Methods
Adults (≥ 18 years) with moderate-to-severe plaque psoriasis prescribed ixekizumab in routine clinical practice were enrolled in this prospective, observational, single-arm, multicenter, post-marketing surveillance study. The primary endpoint was the safety of ixekizumab at week 12. The effectiveness of ixekizumab, based on the Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI), was assessed as a secondary endpoint.
Results
In total, 666 patients were enrolled; 663 were included in the safety analysis, and 612 in the effectiveness analysis. At least one adverse event (AE) was reported by 42.7% (283/663) of patients, most of which were mild (242/283, 85.5%), and 32.7% (217/663) of patients reported AEs related to study treatment. The most frequently reported AEs were injection site reactions. AEs led to discontinuation in five patients (0.8%). Only three patients had a serious AE. Mean ± standard deviation (SD) change from baseline in PASI score was reduction in 10.79 ± 9.55 at week 2 and 16.80 ± 12.15 at week 12. At week 2, 63.7% of patients achieved PASI 50. At week 12, 93.2%, 77.4%, and 45.1% of patients achieved PASI 75, PASI 90, and PASI 100, respectively. Mean ± SD change from baseline in DLQI was reduction in 5.91 ± 6.27 at week 2 and 9.76 ± 7.16 at week 12. DLQI 0/1 was achieved by 19.8% and 59.9% of patients at week 2 and 12, respectively.
Conclusion
Ixekizumab was well tolerated and effective in real-world clinical practice in Chinese adults with moderate-to-severe plaque psoriasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02672-1.
Keywords: Efficacy, Ixekizumab, Psoriasis, Real world, Safety
Key Summary Points
| We investigated the real-word application of ixekizumab, a monoclonal antibody against interleukin-17A, in China. |
| Ixekizumab was well tolerated and effective in routine clinical practice in Chinese adults with moderate-to-severe plaque psoriasis. |
| No new safety signals were observed and the safety profile of ixekizumab was consistent with that of previous clinical trials. |
Introduction
Psoriasis is an incurable, immune-mediated, chronic, recurrent, systemic inflammatory disease characterized by the local or extensive distribution of scaly plaques [1]. Psoriasis is common in China, with a prevalence of 0.47% in 2009 [2]; in a population of ~ 1.33 billion people, this indicates that over 6 million people in China have psoriasis. The majority of people with psoriasis have plaque psoriasis, accounting for around 96.5% of Chinese people with psoriasis [3, 4].
Plaque psoriasis is associated with a considerable economic burden [5]. In addition, while psoriasis does not generally affect mortality, it significantly affects an individual’s quality of life (QoL). As moderate-to-severe psoriasis can affect physical functioning and mental health, which can ultimately negatively impact social functioning, employment, and finances [6]. These effects on patient QoL can be assessed using the patient-reported Dermatology Life Quality Index (DLQI) score (range 0–30; higher scores indicate greater impairment) [1].
Ixekizumab is a humanized immunoglobulin (Ig) G4 monoclonal antibody that was designed to selectively inhibit IL-17A [7]. It binds with high affinity (< 3 pM) and specificity to IL-17A, a proinflammatory cytokine, and does not bind to human IL-17B, IL-17C, IL-17D, IL-17E, or IL-17F [7]. Neutralizing IL-17A with ixekizumab reduces excess keratinocyte proliferation and activation, leading to skin normalization in patients with plaque psoriasis [8]. The efficacy of ixekizumab to treat moderate-to-severe plaque psoriasis and to improve QoL were demonstrated in three global, randomized, phase 3 clinical trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3, and also in a phase 3 study undertaken in China [9–11].
Real-world data on the usage of ixekizumab support and supplement clinical trial findings and inform clinical decision-making. For example, patients with psoriasis from the USA and Canada included in the Corrona Psoriasis Registry were less likely to discontinue ixekizumab than TNF inhibitors (64% lower risk) and other IL-17 inhibitors (31% lower risk) [12]. Data from the same registry showed comparable incidence rates of adverse events (AEs) during ixekizumab treatment in a real-world setting compared with clinical trials [13]. In another US real-world study, adults using ixekizumab had a significantly higher persistence rate, lower discontinuation rate, and a higher likelihood of adherence than those receiving the IL-17 inhibitor secukinumab, with a similar risk of switching treatment [14]. However, despite the importance of real-world evidence for informing clinical practice, there are currently limited data available regarding the use of ixekizumab in Chinese patients in real-world settings. This real-world study was therefore undertaken to investigate the safety and effectiveness of ixekizumab for the treatment of moderate-to-severe plaque psoriasis in routine clinical practice in China.
Methods
Study Design and Patients
This prospective, observational, single-arm, multicenter, post-marketing surveillance study was undertaken in China to confirm the safety and efficacy of ixekizumab for the treatment of moderate-to-severe plaque psoriasis in routine clinical practice. Adults (≥ 18 years of age), who had been diagnosed with moderate-to-severe plaque psoriasis and prescribed ixekizumab, were recruited from the dermatology departments of hospitals across China. Patients who had previously received ixekizumab were excluded from the study, as were those who were participating in other clinical studies. As a real-world study, the usage of ixekizumab was at the investigator’s discretion in collaboration with the patient. However, the recommended ixekizumab regimen for the treatment of moderate-to-severe plaque psoriasis is an initial dose of 160 mg subcutaneously (two 80mg injections), followed by 80 mg subcutaneously every 2 weeks until week 12, and then maintenance therapy with 80 mg subcutaneously every 4 weeks.
All patients were prospectively followed for 12 weeks from baseline (visit 1, day 0 prior to the first dose of ixekizumab) or until their last dose of ixekizumab, whichever occurred first. Patients were encouraged to attend a routine follow-up visit at around 2 weeks (± 5 days) and 12 weeks (± 3 weeks) after the first dose of ixekizumab and were followed up by telephone 6 weeks (± 5 days) after the first dose.
The study was conducted in compliance with Good Clinical Practice and the Declaration of Helsinki. The study protocol and its amendments were approved by Independent Ethics Committees at each study center (Table S1: Supplementary Material). All patients provided written, informed consent prior to participating in the study.
Assessments
During the baseline visit, data were collected on patient demographics, psoriasis disease characteristics including treatment history, other medical history, and concomitant medications. The following data were collected at baseline, and 2 weeks (± 5 days) and 12 weeks (± 3 weeks) after the first dose of ixekizumab, if available: Psoriasis Area and Severity Index (PASI) score, DLQI, and body surface area (BSA) affected by psoriasis. Safety data were collected during the suggested follow-up visits at 2 weeks (± 5 days) and 12 weeks (± 3 weeks), and on the telephone 6 weeks (± 5 days) after the first dose of ixekizumab. Patients were instructed to inform the investigator about any adverse events (AEs) that occurred between the visits. All AEs were recorded, irrespective of any potentially causal relationship with ixekizumab. The severity and causality of AEs were based on the investigator’s opinion and AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA v.25.0). The outcome of AEs and any discontinuation due to AEs were also recorded.
Outcomes
The primary endpoint of the study was the safety of ixekizumab over 12 weeks in routine clinical practice, as assessed by AEs and serious AEs (SAEs). The efficacy of ixekizumab, in terms of the proportion of patients achieving ≥ 50% reduction from baseline in PASI (PASI 50) at 2 weeks and the proportion achieving a ≥ 75%, ≥ 90%, and 100% reductions from baseline in PASI (PASI 75, PASI 90, and PASI 100, respectively) at week 12, were evaluated as secondary endpoints. The effect of ixekizumab on health-related QoL, determined by the change from baseline DLQI at weeks 2 and 12, was also evaluated as a secondary endpoint. The minimal clinically important difference in PASI score was defined as a 75% improvement from baseline, and for DLQI was a 5-point change from baseline [15].
Statistical Analysis
The sample size was calculated based on the incidence of SAEs in patients with plaque psoriasis during the first 12 weeks of treatment with ixekizumab in the phase 3 clinical trials [2.0% (46/2328)] [10]. A sample size of 600 patients would therefore provide a 2-sided 95% confidence interval (CI) of 2.2% for an incidence of SAEs of 2.0%, based on the large sample normal approximation. Assuming a drop-out rate of 10%, the target sample size was set at 667 patients.
Safety was evaluated in all patients who had received at least one dose of ixekizumab (safety population). Efficacy was evaluated in all patients included in the safety analysis who had at least one post-baseline observation (efficacy population). Efficacy was also evaluated in the following pre-defined subgroups: age (< 65, ≥ 65 years), sex (male, female), body weight (< 60 kg, 60– < 80 kg, ≥ 80 kg), number of ixekizumab 80-mg injections after baseline (0–1, 2–4, 5–7), and biologic treatment history (experienced, naïve). In addition, the changes in psoriatic BSA from baseline at weeks 2 and 12 were also evaluated post hoc.
Descriptive statistics were used to describe baseline characteristics, and safety and efficacy outcomes. Differences in the mean PASI score or DLQI among each subgroup at baseline were analyzed using analysis of variance, and mean differences in the change from baseline in PASI score or DLQI between/among each subgroup were analyzed using analysis of covariance with the baseline value as the covariate at weeks 2 and 12. Differences in the proportions of patients achieving PASI 50, PASI 75, PASI 90, PASI 100, and DLQI (0/1) among each subgroup were determined using a Pearson’s chi-square test or Fisher’s exact test.
Statistical analyses were performed using SAS v.9.4. A two-sided test was used for all statistical analyses with a significance level of 5%. There was no imputation for missing data unless otherwise specified, and all analyses were based on non-missing values.
Results
Patients
Of 666 patients enrolled in the study across 26 hospitals in China (Table S1) between August 31, 2020 and May 9, 2022, 663 were included in the safety population and 612 in the efficacy population.
Baseline demographics and disease characteristics are shown in Table 1. In the safety population, the mean duration of plaque psoriasis was 9.19 years and the vast majority of patients had received previous treatment (86.3%). Over half of all patients in the safety population had received prescription topical agents (58.5%), and 27.9% had received non-prescription topical agents. Systemic non-biologic agents had been received by 54.1% of patients and 10.6% had received biologic therapy, most often secukinumab or adalimumab.
Table 1.
Patient demographics and disease characteristics at baseline
| Characteristic | Safety population (n = 663) | Effectiveness population (n = 612) |
|---|---|---|
| Age, years | 40.40 ± 13.296 | 40.00 ± 13.047 |
| < 65 years | 627 (94.6) | 581 (94.9) |
| ≥ 65 years | 36 (5.4) | 31 (5.1) |
| Sex, male | 477 (71.9) | 440 (71.9) |
| Body weight, kg | 71.55 ± 14.761 | 71.71 ± 14.944 |
| < 60 kg | 144 (21.8) | 135 (22.1) |
| 60– < 80 kg | 320 (48.3) | 292 (47.7) |
| ≥ 80 kg | 198 (29.9) | 185 (30.2) |
| BMI, kg/m2 | 24.67 ± 4.048 | 24.71 ± 4.104 |
| < 18.5 kg/m2 | 27 (4.1) | 25 (4.1) |
| 18.5– < 24 kg/m2 | 278 (42.0) | 257 (42.0) |
| 24– < 28 kg/m2 | 241 (36.4) | 222 (36.3) |
| ≥ 28 kg/m2 | 116 (17.5) | 108 (17.6) |
| Duration of plaque psoriasis, years | 9.19 ± 9.55 | 9.16 ± 9.51 |
| Received previous treatment for plaque psoriasis | 572 (86.3) | 529 (86.4) |
| Topical prescription-only agent | 388 (58.5) | 354 (57.8) |
| Systemic non-biologic | 359 (54.1) | 331 (54.1) |
| Topical non-prescription agent | 185 (27.9) | 172 (28.1) |
| Phytotherapy | 125 (18.9) | 117 (19.1) |
| Biologic | 70 (10.6) | 61 (10.0) |
| TNF-II IgG (yisaipu) | 7 (1.1) | 6 (1.0) |
| Recombinant human IL-2 | 1 (0.2) | 1 (0.2) |
| Secukinumab | 37 (5.6) | 31 (5.1) |
| Adalimumab | 22 (3.3) | 20 (3.3) |
| Etanecept | 4 (0.6) | 4 (0.7) |
| Guselkumab | 4 (0.6) | 3 (0.5) |
| Ustekinumab | 3 (0.5) | 2 (0.3) |
| Infliximab | 2 (0.3) | 2 (0.3) |
| Other | 2 (0.3) | 2 (0.3) |
Data are mean ± standard deviation, n (%)
BMI body mass index, IL-2 interleukin-2, TNF-II IgG tumor necrosis factor-II immunoglobulin G
Safety
An overall summary of safety data and AEs reported in > 2% of patients by preferred term (PT) are shown in Table 2. At least one AE was reported by 283 (42.7%) patients. AEs related to study treatment were reported in 217 (32.7%) patients. The most frequently reported AEs by MedDRA system organ class (SOC) were general disorders and administration site conditions (151 patients, 22.8%), skin and subcutaneous tissue disorders (97 patients, 14.6%), and infections and infestations (80 patients, 12.1%). The most commonly reported AEs by MedDRA PT were all injection site reactions, including injection site erythema (64 patients, 9.7%), injection site swelling (62 patients, 9.4%), and injection site pain (31 patients, 4.7%).
Table 2.
Summary of safety (safety population)
| Patients (n = 663) | Events | |
|---|---|---|
| ≥ 1 AE | 283 (42.7) | 744 |
| AEs reported in ≥ 2% of patients | ||
| General disorders and administration site conditions (SOC) | 151 (22.8) | 380 |
| Injection site erythema | 64 (9.7) | 102 |
| Injection site swelling | 62 (9.4) | 96 |
| Injection site pain | 31 (4.7) | 35 |
| Injection site reaction | 24 (3.6) | 38 |
| Injection site pruritis | 20 (3.0) | 38 |
| Fatigue | 14 (2.1) | 14 |
| Skin and subcutaneous tissue disorders (SOC) | 97 (14.6) | 125 |
| Pruritis | 23 (3.5) | 23 |
| Urticaria | 22 (3.3) | 24 |
| Infections and infestations (SOC) | 80 (12.1) | 92 |
| Upper respiratory tract infection | 23 (3.5) | 23 |
| ≥ 1 drug-relateda AE | 217 (32.7) | 544 |
| ≥ 1 SAE | 3 (0.5) | 3 |
| ≥ 1 drug-relateda SAE | 2 (0.3) | 2 |
| ≥ 1 AE leading to discontinuation of study drug | 5 (0.8) | 8 |
Data are n (%) or n
AE adverse event, SAE serious adverse event, SOC system organ class
aConsidered drug-related by the treating physician
The vast majority of AEs were mild in severity (242 patients, 85.5%). Only 3 (0.5%) patients had an SAE (hypersensitivity, pustular psoriasis, and diabetes mellitus inadequate control), 2 of which were considered drug related (hypersensitivity and pustular psoriasis). No deaths were reported during the study.
AEs leading to discontinuation occurred in 5 (0.8%) patients (more than one AE could lead to discontinuation in a single patient): pustular psoriasis, dermatitis atopic, erythema, psoriasis, oedema, hypersensitivity, and hypoproteinemia.
Efficacy
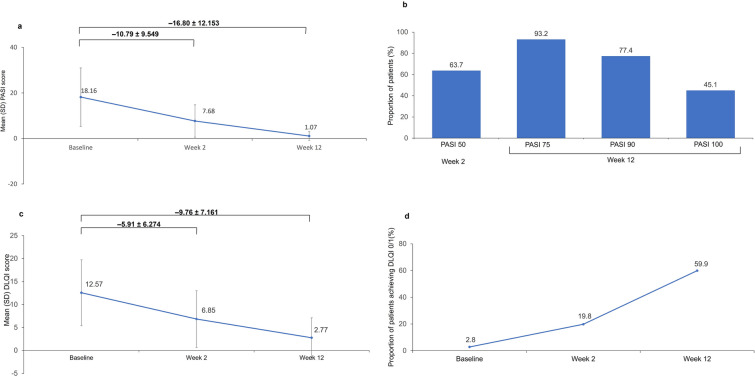
The mean PASI score at baseline was 18.16 dstandard deviation (SD) 12.84] (Fig. 1a; Table S2). The mean reduction from baseline in PASI score was 10.79 (SD 9.55) at week 2 and 16.80 (SD 12.15) at week 12. At week 2, 63.7% of patients achieved PASI 50 and at week 12, 93.2% of patients achieved PASI 75, 77.4% achieved PASI 90, and 45.1% achieved PASI 100 (Fig. 1b).
Fig. 1.
a Mean (SD) PASI score. b Proportion of patients achieving at least a 50% improvement in PASI at week 2 (PASI 50), or at least a 75% or 90%, or a 100% improvement (PASI 75, PASI 90, PASI 100, respectively) at week 12. c Mean (SD) DLQI score. d Proportion of patients achieving a DLQI 0/1. Effectiveness population; n = 612. DLQI Dermatology Life Quality Index, PASI Psoriasis Area and Severity Index, SD standard deviation
The mean psoriatic BSA at baseline was 27.6% (SD 22.097), which decreased by 10.1% (SD 14.22) at week 2. (Table S2) At week 12, the mean psoriatic BSA was 2.4% (SD 5.88), a decrease of 24.3% (SD 21.04) from baseline (Table S2).
The mean DLQI at baseline was 12.57 (SD 7.17), at which time only 2.8% of patients had a DLQI 0/1 (Fig. 1c, d; Table S2). At week 2, the mean reduction from baseline in DLQI was 5.91 (SD 6.27), and 19.8% of patients achieved DLQI 0/1. At week 12, the mean reduction from baseline DLQI was 9.76 (SD 7.16), and 59.9% of patients achieved DLQI 0/1.
Subgroup Analysis
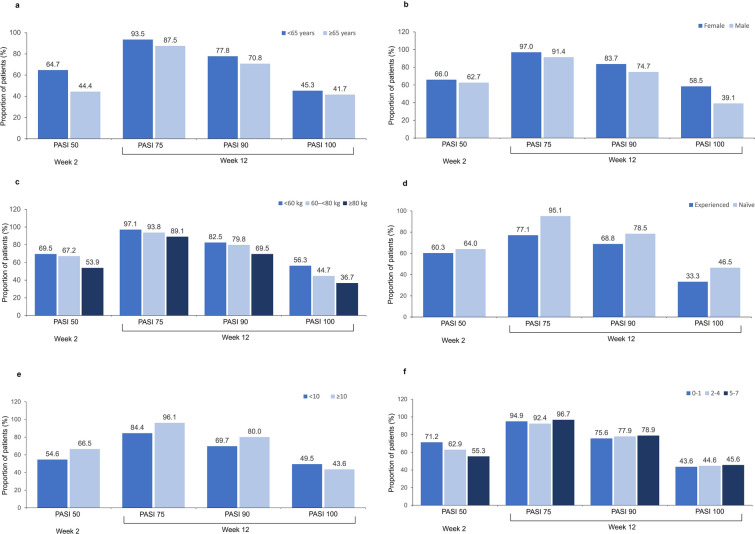
Differences within subgroups were analyzed for baseline values and change from baseline at weeks 2 and 12 for PASI score and DLQI, the proportion of patients achieving PASI 50 or DLQI 0/1 at week 2, and the proportion of patients achieving PASI 75, PASI 90, and PASI 100, or DLQI 0/1 at week 12 (see Fig. 2; Tables S3 and S4).
Fig. 2.
Proportion of patients achieving at least a 50% improvement in PASI at week 2 (PASI 50), or at least a 75% or 90%, or a 100% improvement (PASI 75, PASI 90, PASI 100, respectively) at week 12 by: a age; < 65 years n = 581, ≥ 65 years n = 31; b sex; female n = 172, male n = 440; c body weight; < 60 kg n = 135, 60– < 80 kg n = 292, ≥ 80 kg n = 185; d biologic treatment history; experienced n = 61, naïve n = 551; e baseline PASI score; < 10 n = 149, ≥ 10 n = 454. f Number of ixekizumab injections (80 mg) after baseline; 1, n = 151; 2–4, n = 327; 5–7 n = 105. PASI Psoriasis Area and Severity Index
Age
More patients < 65 years of age achieved PASI 50 at week 2 than those ≥ 65 years of age (64.7% vs. 44.4%, p = 0.033). Age did not significantly affect any other PASI parameters analyzed, changes from baseline in DLQI or the proportions of patients achieving a DLQI of 0/1 at week 2 and 12.
Sex
Improvements in PASI from baseline were greater in males than females at week 12 (mean 17.61 vs. 14.99; p = 0.002); however, males had higher baseline PASI scores than females (p = 0.004). Despite greater improvements from baseline in males, more females than males achieved PASI 75 (97.0% vs. 91.4%; p = 0.032), PASI 90 (83.7% vs. 74.7%; p = 0.037), and PASI 100 (58.5% vs. 39.1%; p < 0.001) at week 12. More females than males also achieved DLQI 0/1 at week 2 (25.3% vs. 17.6%; p = 0.043), and at week 12 (68.4% vs. 56.1%; p = 0.015).
Weight
There were differences in the change in PASI and DLQI from baseline to week 2 based on body weight category (p = 0.003 and p = 0.010, respectively), but with no clear patterns.
Ixekizumab Dose Pattern
No clear association was observed between the number of ixekizumab injections received by patients and change from baseline in PASI. However, receiving a higher number of injections was associated with a higher rate of achievement of PASI 50 at week 2 (p = 0.042). There was no association between number of ixekizumab injections received and change in DLQI from baseline.
Biologic treatment history
Patients naive to biologic treatment for plaque psoriasis had greater changes from baseline in PASI at week 12 than those who were treatment-experienced (p = 0.001); however, they also had higher baseline PASI scores (p = 0.0125). A higher proportion of patients naive to biologic therapy achieved PASI 75 at week 12 (p < 0.001). No difference in DLQI parameters was observed between patients native to biologic treatment and those who were treatment experienced.
Discussion
The results of this observational study show that ixekizumab is well tolerated in real-world clinical practice for the treatment of Chinese adults with moderate-to-severe plaque psoriasis. No new safety signals were observed and the safety profile of ixekizumab was consistent with that reported in a randomized phase 3 trial conducted in Chinese patients, and with the predominantly Caucasian patients included in the global UNCOVER-1 to -3 trials [10, 11, 16]. Furthermore, ixekizumab was highly effective when used in real clinical practice in China, with reductions in mean PASI score from baseline to week 12 of 16.80 (SD 12.153) and mean change from baseline DLQI of 9.76 (SD 7.161) with 59.9% of patients achieving DLQI 0/1. These efficacy findings are also consistent with the Chinese phase 3 trial and the global phase 3 trials [10, 11, 16].
At least one AE was reported by 42.7% of patients in this study, compared with 72.2% of patients who received ixekizumab 80 mg twice weekly (Q2W) and 70.7% of those who received ixekizumab 80 mg every 4 weeks (Q4W) for 12 weeks in the Chinese phase 3 trial [11], and 58.4% and 58.8% who received ixekizumab Q2W and Q4W, respectively, in UNCOVER-1 to -3 [10]. The lower proportion of patients reporting an AE in the present study may be due to underreporting of AEs due to the observational nature of the study. The rate of SAEs and discontinuations due to AEs in the present study were very low and comparable to those reported in patients who received ixekizumab for 12 weeks in previous randomized trials [10, 11, 16]. In addition, the profile of AEs observed in this real-world study was similar to that reported in the Chinese and global phase 3 trials of ixekizumab, with the most common AEs including injection site reactions [10, 11, 16]. Interestingly, upper respiratory tract infection was the most common AE reported among patients in the Chinese phase 3 trial (15.9%), but was reported by a lower proportion of patients in the present study (3.5%) and in the global UNCOVER-1 to -3 trials (4.4%) [10, 11, 16]. Injection site reactions were the most commonly reported AEs in this real-world study, while infections were the most reported AEs in the phase 3 trials (nasopharyngitis in UNCOVER-1 to -3[10] and upper respiratory tract infection in the Chinese phase 3 trial[11]). Given the broad inclusion criteria of the present study, these real-world findings support the safety profile of ixekizumab reported in the randomized phase 3 trials, and no new safety signals were observed.
Ixekizumab demonstrated high efficacy for the treatment of moderate-to-severe plaque psoriasis in real-world clinical practice in China. A similar proportion of patients in the present study achieved PASI 75 (93.2%), 90 (77.5%) and 100 (45.1%) at week 12 compared with patients receiving ixekizumab Q2W for 12 weeks in the Chinese phase 3 (93.8%, 82.4%, and 33.0%, respectively) and UNCOVER-1 (89.1%, 70.9%, and 35.3%, respectively) studies [10]. Similarly, the mean reduction from baseline DLQI in the present study (9.76) was comparable with that reported in the Chinese phase 3 trial (8.86). The efficacy findings of our study were also broadly similar to the results reported from a real-word study of secukinumab in Chinese patients with moderate-to-severe plaque psoriasis, in which rates of PASI 75, 90, and 100 after 16 weeks of treatment were 91.1%, 73.0%, and 38.3%, respectively [17]. Interestingly, a recent meta-analysis concluded that 93.0% of patients receiving ixekizumab 80 mg Q2W achieved PASI 75 at 12 weeks, which is very similar to the findings of the present study [18]. The meta-analysis also ranked ixekizumab 80 mg Q2W as having the highest efficacy in terms of short-term (week 12 or 16) achievement of PASI 75 compared with brodalumab, secukinumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab [18].
One strength of the present study is the broad inclusion criteria (and very narrow exclusion criteria), designed to avoid any potential recruitment bias. For example, the present study did not include any restrictions on previous treatment, whereas the UNCOVER-2 and -3 trials excluded patients previously treated with etanercept. The present study also had several limitations including those inherent in all single arm observational studies, such as a lack of a comparator arm and the potential for missing data to lead to bias. Furthermore, patients were recruited mainly at a large tertiary hospital, and the data may not be generalizable to the entire population of Chinese patients with psoriasis. In addition, we only report results for short-term treatment, up to week 12, and longer-term evaluation of ixekizumab during maintenance treatment requires further study.
Conclusions
The results of this observational study show that ixekizumab is well tolerated and effective in routine clinical practice in Chinese adults with moderate-to-severe plaque psoriasis, with no new safety signals observed. These real-world findings from a broad Chinese patient population align with and support the results of the Chinese phase 3 trial of ixekizumab and the global UNCOVER-1 to -3 trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study
Medical Writing/Editorial Assistance
Editorial support for this manuscript was provided by Jake Burrell PhD (Rude Health Consulting) and paid for by Eli Lilly and Company.
Author Contributions
Li Ying contributed to conception of the work, acquisition of data for the work, interpretation of data for the work and critical revision of the work for important intellectual content. Ji Suyun, Liang Yanhua and Yuling Shi contributed to design of the work, acquisition of data for the work, interpretation of data for the work and critical revision of the work for important intellectual content. Liang Yunsheng, Deng Li, Dang Lin, Lv Chengzhi, Lin Bingjiang and Zhang Furen contributed to acquisition of data for the work, and critical revision of the work for important intellectual content. Shi Wendi, Li Jinnan, Dong Yu and Dou Guanshen contributed to design of the work, interpretation of data for the work and critical revision of the work for important intellectual content.
Funding
Eli Lilly and Company funded this study as well as the Rapid Service Fee and Open Access fee. This work was also sponsored by grants from the National Natural Science Foundation of China (No. 82073429, 82273510, and 82203913), the Innovation Program of Shanghai Municipal Education Commission (No.2019-01-07-00-07-E00046), the Clinical Research Plan of SHDC (No. SHDC2020CR1014B) and the Program of Shanghai Academic Research Leader (No. 20XD1403300).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Shi Wendi, Li Jinnan, Dong Yu and Dou Guanshen are employees from Eli Lilly China. Li Ying, Ji Suyun, Liang Yanhua, Liang Yunsheng, Deng Li, Dang Lin, Lv Chengzhi, Lin Bingjiang, Zhang Furen, and Yuling Shi have no conflict of interest to declare.
Ethical Approval
The study was conducted in compliance with Good Clinical Practice and the Declaration of Helsinki. The study protocol and its amendments were approved by Independent Ethics Committees at each study center (Table S1). All patients provided written, informed consent prior to participating in the study.
References
- 1.Committee of Psoriasis DB, Chinese Medical Association Guidelines for the Diagnosis and Treatment of Psoriasis in China: 2019 Concise Edition#. Int J Dermatol Venereol. 2020;3(1):14–26. doi: 10.1097/JD9.0000000000000074. [DOI] [Google Scholar]
- 2.Ding X, Wang T, Shen Y, Wang X, Zhou C, Tian S, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. 2012;22(5):663–667. doi: 10.1684/ejd.2012.1802. [DOI] [PubMed] [Google Scholar]
- 3.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Wang G, Jin H, Xu J, Zhu X, Zheng M, et al. Clinic characteristics of psoriasis in China: a nationwide survey in over 12000 patients. Oncotarget. 2017;8(28):46381–46389. doi: 10.18632/oncotarget.18453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawro T, Zalewska A, Hawro M, Kaszuba A, Krolikowska M, Maurer M. Impact of psoriasis severity on family income and quality of life. J Eur Acad Dermatol Venereol. 2015;29(3):438–443. doi: 10.1111/jdv.12572. [DOI] [PubMed] [Google Scholar]
- 6.Zhong H, Yang H, Mao Z, Chai X, Li S. Impact of moderate-to-severe psoriasis on quality of life in China: a qualitative study. Health Qual Life Outcomes. 2021;19(1):271. doi: 10.1186/s12955-021-01902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Lu J, Allan BW, Tang Y, Tetreault J, Chow CK, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016;9:39–50. doi: 10.2147/JIR.S100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130(1):145–54e9. doi: 10.1016/j.jaci.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- 10.Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Zheng J, Pan W, Zheng M, Lu Y, Li F, et al. Efficacy and safety of ixekizumab in Chinese patients with moderate-to-severe plaque psoriasis: 60-week results from a phase 3 study. Int J Dermatol Venereol. 2022 doi: 10.1097/JD9.0000000000000244. [DOI] [Google Scholar]
- 12.Lockshin B, Cronin A, Harrison RW, McLean RR, Anatale-Tardiff L, Burge R, et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: the Corrona Psoriasis Registry. Dermatol Ther. 2021;34(2):e14808. doi: 10.1111/dth.14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grace E, Zhu B, Malley W, O'Brien J, Mangner R, Agada N, et al., editors. Safety events occurring among patients exposed to ixekizumab in the Corrona Psoriasis Registry. In: 6th Congress of the Skin Inflammation and Psoriasis International Network; Paris, France; 25–27 April 2019.
- 14.Blauvelt A, Shi N, Burge R, Malatestinic WN, Lin CY, Lew CR, et al. Comparison of real-world treatment patterns among patients with psoriasis prescribed ixekizumab or secukinumab. J Am Acad Dermatol. 2020;82(4):927–935. doi: 10.1016/j.jaad.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664. doi: 10.1111/j.0022-202X.2005.23621.x. [DOI] [PubMed] [Google Scholar]
- 16.Leonardi C, Reich K, Foley P, Torii H, Gerdes S, Guenther L, et al. Efficacy and safety of ixekizumab through 5 years in moderate-to-severe psoriasis: long-term results from the UNCOVER-1 and UNCOVER-2 PHASE-3 RANDOMIZED CONTROLLED TRIALS. Dermatol Ther (Heidelb) 2020;10(3):431–447. doi: 10.1007/s13555-020-00367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, Cai ML, Hong XJ, Zheng LJ, Hu ZL, Yuan T, et al. Real-world data on the use of secukinumab as treatment for moderate-to-severe psoriasis in Chinese patients. Eur J Dermatol. 2020;30(5):554–560. doi: 10.1684/ejd.2020.3878. [DOI] [PubMed] [Google Scholar]
- 18.Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short-Term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161. doi: 10.1155/2019/2546161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.