Abstract
Saccharomyces cerevisiae plasma membrane H+-ATPase activity was stimulated during octanoic acid-induced latency, reaching maximal values at the early stages of exponential growth. The time-dependent pattern of ATPase activation correlated with the decrease of cytosolic pH (pHi). The cell population used as inoculum exhibited a significant heterogeneity of pHi, and the fall of pHi correlated with the loss of cell viability as determined by plate counts. When exponential growth started, only a fraction of the initial population was still viable, consistent with the role of the physiology and number of viable cells in the inoculum in the duration of latency under acid stress.
The biological target sites of octanoic acid in Saccharomyces cerevisiae may be related to processes of transport across membranes, particularly the plasma membrane (21). Like other weak acids at low pH, octanoic acid, a highly toxic by-product of yeast alcoholic fermentation (23) and an antimicrobial food additive (6), leads to the reduction of cytosolic pH (pHi) due to its dissociation in the approximately neutral cytoplasm following the entrance of the undissociated toxic form into the cell by passive diffusion (5, 20, 23). It is likely that this highly liposoluble weak acid significantly affects the spatial organization of the plasma membrane, affecting its function as a matrix for enzymes and as a selective barrier, thereby leading to the dissipation of the proton motive force across the plasma membrane and to intracellular acidification (16, 18). Significantly, the H+-ATPase in the plasma membrane in yeast, which creates the electrochemical proton gradient that drives the secondary transport of solutes and is implicated in the maintenance of pHi around neutrality, has been pointed out as a critical component of yeast adaptation to weak acids (8, 19, 24). Indeed, yeast plasma membrane H+-ATPase is activated during exponential growth with octanoic acid (19, 24), and the duration of lag phase before yeast cells enter exponential growth in the presence of sorbic acid is significantly extended in a mutant with reduced levels of plasma membrane ATPase activity (8). The activation of the H+-ATPase in the plasma membrane in yeast cells exposed to other stresses that also lead to the dissipation of the H+ gradient and intracellular acidification (such as subcritical inhibitory concentrations of ethanol [12, 14, 15], supraoptimal temperatures below 40°C [25], presence of other organic acids at low pH [1, 5, 8], and deprivation of nitrogen source [2]) have also been observed. Several lines of evidence indicate that ATPase activation is due to posttranslational modifications of the PMA1 ATPase (2, 12, 24, 25). Considerable information has been obtained on the variation of plasma membrane ATPase activity during exponential growth and early stationary phase of yeast cells cultivated in media, at low pH, supplemented or not with octanoic acid (24). However, this is not the case during the period of latency preceding exponential growth at concentrations of octanoic acid close to the maximal concentration allowing growth. The main objective of the present work was to obtain information about the pattern of ATPase activity and the changes in pHi and cell viability during the lag phase necessary for yeast adaptation to the physiological effects of octanoic acid before exponential growth.
Duration of yeast growth latency in octanoic acid-supplemented media.
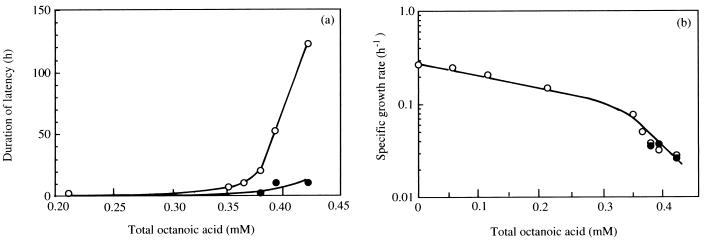
When cells of S. cerevisiae IGC3507III grown, at 30°C, in medium that had not been supplemented with octanoic acid were used to inoculate buffered YG media (30 g of glucose liter−1, 6.7 g of Yeast Nitrogen Base [Difco] liter−1) (pH 4.0) supplemented with increasing concentrations of this toxic acid up to around 0.35 mM total acid (19, 23), exponential growth was initiated without significant delay (Fig. 1a), although a dose-dependent decrease in specific growth rate was observed (Fig. 1b). However, for higher concentrations up to the maximal that allowed growth (0.42 mM), a lag phase was observed and its duration strongly increased with the severity of octanoic acid stress (Fig. 1a). The duration of latency was drastically reduced when exponential cells used as inoculum were grown in medium with an identical concentration of octanoic acid (Fig. 1a), but the specific growth rate was not modified (Fig. 1b). At a concentration of total octanoic acid of 0.39 mM, a lag phase of around 55 h was necessary for yeast cells, which had been cultivated under nonstressing conditions, to adapt to the deleterious effects of octanoic acid and to initiate inhibited exponential growth (Fig. 2).
FIG. 1.
Effect of the addition to the growth medium of increasing concentrations of octanoic acid on the duration of lag phase (a) and the specific growth rate of S. cerevisiae IGC 3507III (b) for exponentially growing cells (used as inoculum) cultivated at 30°C at pH 4.0 in the absence (○) or presence (•) of concentrations of toxic lipophilic acid identical to those present in the growth medium. Results are representative of the many growth experiments carried out.
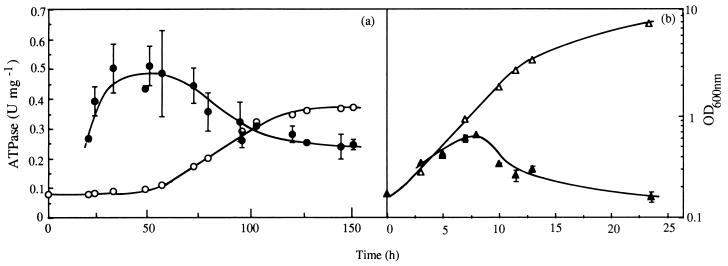
FIG. 2.
Specific activity of plasma membrane H+-ATPase (filled symbols) and growth curve (open symbols) of S. cerevisiae IGC 3507III during cultivation in the presence (a) or absence (b) of 0.39 mM total octanoic acid (at pH 4.0, 30°C). The data are averages with standard deviations for at least three enzyme assays using cells from at least two independent growth experiments. OD, optical density.
Activation of plasma membrane ATPase during octanoic acid-induced latency.
The specific activity of plasma membrane ATPase assayed in crude membrane suspensions prepared from nonadapted cells, as previously reported (19, 25), during cultivation in buffered medium (at pH 4.0) supplemented with 0.39 mM octanoic acid, increased during the 55 h of latency (Fig. 2a). A peak of activity was reached during the early stages of exponential growth and values of ATPase activity were consistently higher (twofold) in cells grown under octanoic acid stress (Fig. 2), as described by Viegas et al. (24). Yeast cells must adapt to the physiological effects of octanoic acid during an extended lag period, the length of which depended on the severity of acid stress (Fig. 1a), before eventually recovering and entering exponential growth; the activation of plasma membrane H+-ATPase observed during this period of latency reinforces the idea that this proton pump is an important component of this adaptative response (5, 8, 19, 24). In fact, the ability of yeast cells to grow in the presence of lipophilic acids at a low pH reflects their capacity to maintain control over their internal pH by excluding protons. This adaptative phenomenon, reported for the first time in the present work, complements the observation of Holyoak et al. (8) that a strain with reduced plasma membrane H+-ATPase activity displayed increased lag phase in the presence of the weak-acid preservative sorbic acid. Significantly, plasma membrane H+-ATPase activity was also pointed out to play a critical role in yeast tolerance of ethanol (15) or supraoptimal temperatures (13, 25). The mechanism underlying plasma membrane ATPase activation during octanoic acid-induced latency remains obscure at the present time, but it is likely that this is due to a posttranslational modification of ATPase, as proposed for ATPase activation during octanoic acid-stressed exponential growth (24). It is likely that during lag phase the amount of H+-ATPase in the plasma membrane slightly decreases, as found by Benito et al. (2) in yeast cells deprived of nitrogen source where ATPase activation also occurred (2), as the estimated half-life of the enzyme is about 11 h (2). ATPase activation during latency can hardly be attributed to the adaptative modification of the ATPase lipid environment in cells grown under lipophilic acid stress, as suggested by Alexandre et al. (1).
Changes in yeast pHi and viability during octanoic acid-stressed cultivation.
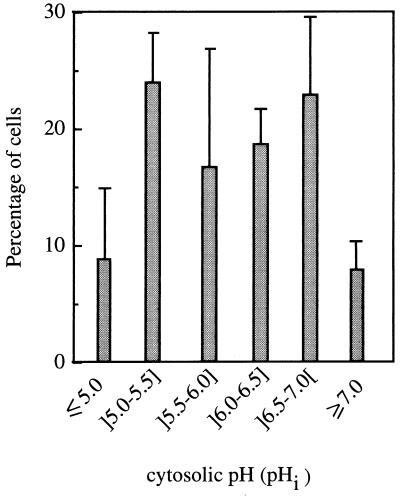
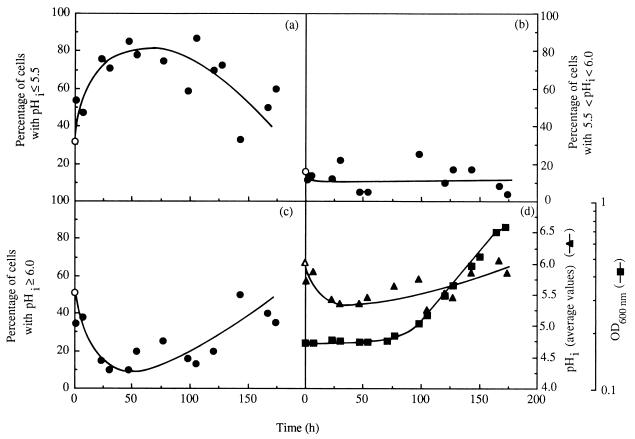
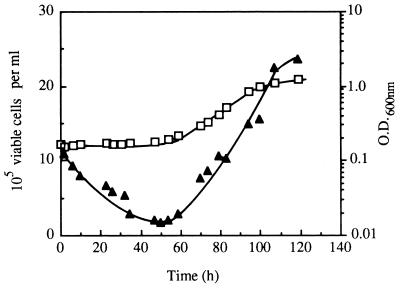
The change in pHi during cultivation of nonadapted cells with 0.39 mM octanoic acid was monitored by using an adaptation of the fluorescence microscopic image processing technique developed by Imai and Ohno (9); 5- (and 6)-carboxyfluorescein (cF) was used as the internal pH-dependent fluoroprobe. Cells washed and resuspended in cold CF buffer (citrate-phosphate buffer [at pH 4.0] with 50 mM glycine [Sigma], 110 mM NaCl, 5 mM KCl, and 1 mM MgCl2) to a cellular density of 2 × 108 ml−1 were loaded with cF by adding 20 μM of 5 (and 6)-carboxyfluorescein-diacetate (Sigma) and vortexing in two bursts of 1 min each, interspersed with 15 min on ice (9). After being washed twice with cold CF buffer, cF-loaded cells were immediately examined with a Zeiss Axioplan microscope equipped with adequate epifluorescence interference filters (Zeiss BP450-490 and Zeiss LP520) and connected to a video camera and to a computer with an image- analysis program (gel documentation system SW2000; UVP, San Gabriel, Calif.). Following a cell-by-cell analysis, the value of fluorescence intensity (fI) emitted by each cell, measured by direct densitometry, corresponded to the arithmetical mean value of fI measured in two or three different regions in the cytoplasm of the same cell, with the less fluorescent vacuole excluded. To estimate average pHi, an in vivo calibration curve was prepared (Fig. 3) by using cell suspensions grown in the absence of toxics which were loaded with cF as described above and incubated, at 30°C, with 0.5 mM carbonyl cyanide m-chlorophenylhydrazone (CCCP) to dissipate the plasma membrane pH gradient (4), before adjustment of external pH (in the range 3.5 to 7.5) by the addition of HCl or NaOH at 2 M. Fluorescence images were fixed 15 s after the occurrence of the excitation radiation in order to minimize interferences due to leakage of cF as well as fluorescence quenching (3, 7). Cells were kept on ice throughout the procedure, and CF buffer lacked glucose; therefore, the active efflux of cF (3) was minimized as confirmed by measuring the fluorescence in the medium surrounding the cells, which was negligible. Under the experimental conditions used and for the purpose of the study, this technique proved to be highly useful and suitable despite the limitations that might be raised (3, 7). It allowed a clear-cut picture of the pHi of individual cells, giving information about the distribution of pHi values of a yeast population (Fig. 4 and 5a to c), instead of solely an estimation of the average value of the whole population, as is the case with techniques based on the distribution of radioactive propionic acid (20) or on the in vivo 31P nuclear magnetic resonance (5). Moreover, values calculated for the average pHi of the whole yeast population during latency and exponential growth in medium with octanoic acid (Fig. 5d) were close to, although slightly lower than, the values previously obtained based on the distribution of [14C]propionic acid (20, 22). Results revealed that the cell population used to inoculate octanoic acid-supplemented medium exhibited a significant heterogeneity (Fig. 4); around 31% showed a pHi in the optimal range (above 6.5) (Fig. 4), with the average pHi value of the whole population estimated to be approximately 6.0. This low pHi value results from cell cultivation in a rich medium with high production of organic acids (11) (external pH, 3.6), followed by washing of the cells with YG medium buffered at pH 4.0 (17). The introduction of the inoculum in octanoic acid-supplemented medium led to the very rapid (5-min) increase in the percentage of the cell population with pHi below 5.5, consistent with the rapid kinetics of cytosol acidification when yeast cells are exposed to weak acids (5). During extended incubation with octanoic acid and until the end of latency, the percentage of the population with a very low pHi (below 5.5) continued to increase, reaching 80% of the cell population, while the percentage of cell population with a pHi above 6.0 suffered a corresponding decrease (Fig. 5). During exponential growth, the opposite pHi modification was observed, consistent with a recovery of pHi to physiological levels (Fig. 5). The time-dependent pattern of internal acidification during lag phase correlated with plasma membrane ATPase activation (Fig. 2a and 5), suggesting that this activation was triggered by intracellular acidification, as proposed for acetic acid (5)- or nitrogen starvation (2)-induced activation. Immediately before yeast cells entered exponential growth, 80% of the initial viable population had lost viability, as assessed by the number of CFU (21) (Fig. 6), suggesting that octanoic acid-induced death during latency is related to internal acidification down to critical values (Fig. 5 and 6), in agreement with the relationship established by Imai and Ohno (10) between yeast viability and intracellular pH. Only about 20% of the initial population was able to start cell division in octanoic acid-supplemented medium, presumably those cells that in the inoculum exhibited pHi values around neutrality (Fig. 5 and 6). These results suggest that despite plasma membrane H+- ATPase activation, this system of pH homeostasis may not be able to fully counteract the physiological effects of increasing octanoic acid concentrations and eventually fails at very severe acid stress.
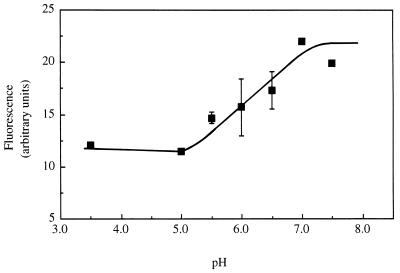
FIG. 3.
In vivo calibration curve, showing the pH dependence of the fI of cF-loaded-cells of S. cerevisiae IGC 3507III. Intracellular and extracellular pHs were equilibrated by incubation of cF-loaded cells, for 10 min at 30°C, with 0.5 mM CCCP. At each pH, values of fI correspond to the average fI of about 20 cells. The data are averages with standard deviations for three independent experiments.
FIG. 4.
Distribution of cells with different pHi values present in the inoculum of S. cerevisiae IGC 3507III prepared in growth medium without octanoic acid supplementation.
FIG. 5.
Percentage of yeast cells with pHi below 5.5 (a), between 5.5 and 6.0 (b), or above 6.0 (c); average pHi of the whole cell population (▴) during S. cerevisiae IGC3507III cultivation in medium supplemented with 0.39 mM total octanoic acid (pH 4.0, 30°C); and the optical density (OD) of the culture at 600 nm (▪). The average pHi values estimated for the whole cell population are the arithmetical mean values of the various average pHi values calculated for individual cells. The percentage of cells present in the inoculum with pHi values within the three ranges (○) and the average pHi of the inoculum cell population (▵) are indicated.
FIG. 6.
Concentration of viable cells (▴) and culture optical density (O.D.) at 600 nm (□) during lag and exponential phases of S. cerevisiae IGC 3507III growth in medium supplemented with 0.39 mM octanoic acid, at pH 4.0 and 30°C.
Adaptative response to octanoic acid.
The adaptation of yeast cells to octanoic acid at a low pH appears to depend on their H+-exporting ability, but this requires not only a highly active H+-ATPase in the plasma membrane but the provision of sufficient ATP to drive this energy-demanding process as indicated by the results of Holyoak et al. (8). It is likely that increased ATPase activity under octanoic acid stress may reduce cellular ATP levels and that ATP depletion contributes to the failure of the maintenance of pHi homeostasis, particularly among the subpopulation that in the inoculum exhibited the lowest pHi values. The loss of viability might occur for those cells where pHi decreased down to nonphysiological values. The eventual recovery of growth therefore depends on the remaining viable population, in agreement with the well-known critical role played by the physiology and number of viable cells in the inoculum in the duration of latency under acid stress. The observation that octanoic acid-adapted cells reinoculated into the same fresh medium can resume growth after a much shorter latency (Fig. 1a) is a good example of the importance of the physiology of the inoculum cells. Besides the increased plasma membrane H+-ATPase activity of octanoic acid-adapted cells, other mechanisms may underlie the adaptation to acid stress, such as the increased cellular buffering capacity of octanoic acid-grown cells due to their lower intracellular volume (20), the more favorable plasma membrane lipid composition (1), and the possible induction of the active efflux of the anion (26).
Acknowledgments
This work was supported by JNICT, FEDER, and the PRAXIS XXI Programme (grants PRAXIS2/2.1/BIO/20/94, PRAXIS2/2.1/BIO/37/94, and PBIC/C/BIO/2031/95).
REFERENCES
- 1.Alexandre H, Mathieu B, Charpentier C. Alteration in membrane fluidity and lipid composition, and modulation of H+-ATPase activity in Saccharomyces cerevisiae caused by decanoic acid. Microbiology. 1996;142:469–475. doi: 10.1099/13500872-142-3-469. [DOI] [PubMed] [Google Scholar]
- 2.Benito B, Portillo F, Lagunas R. In vivo activation of the yeast plasma membrane ATPase during nitrogen starvation: identification of the regulatory domain that controls activation. FEBS Lett. 1992;300:271–274. doi: 10.1016/0014-5793(92)80861-a. [DOI] [PubMed] [Google Scholar]
- 3.Breeuwer P. Assessment of viability of microorganisms employing fluorescence techniques. Ph.D. thesis. Wageningen, The Netherlands: Wageningen Agricultural University; 1996. [Google Scholar]
- 4.Calahorra M, Opekarová M, Ramirez J, Peña A. Leucine transport in plasma membrane vesicles of Saccharomyces cerevisiae. FEBS Lett. 1989;247:235–238. doi: 10.1016/0014-5793(89)81342-0. [DOI] [PubMed] [Google Scholar]
- 5.Carmelo V, Santos H, Correia I S. Effect of extracellular acidification on the activity of plasma membrane ATPase and on the cytosolic and vacuolar pH of Saccharomyces cerevisiae. Biochim Biophys Acta. 1997;1325:63–70. doi: 10.1016/s0005-2736(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 6.Freese E, Sheu C W, Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature (London) 1973;241:321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- 7.Graber M L, Dilillo D C, Friedman B L, Pastoriza-Munoz E. Characteristics of fluoroprobes for measuring intracellular pH. Anal Biochem. 1986;156:202–212. doi: 10.1016/0003-2697(86)90174-0. [DOI] [PubMed] [Google Scholar]
- 8.Holyoak C D, Stratford M, McMullin Z, Cole M B, Crimmins K, Brown A J P, Coote P J. Activity of the plasma membrane H+-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl Environ Microbiol. 1996;62:3158–3164. doi: 10.1128/aem.62.9.3158-3164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai T, Ohno T. Measurement of yeast intracellular pH by image processing and the change it undergoes during growth phase. J Biotechnol. 1995;38:165–172. doi: 10.1016/0168-1656(94)00130-5. [DOI] [PubMed] [Google Scholar]
- 10.Imai T, Ohno T. The relationship between viability and intracellular pH in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol. 1995;61:3604–3608. doi: 10.1128/aem.61.10.3604-3608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiorella B, Blanch H, Wilke C. By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng. 1983;25:103–121. doi: 10.1002/bit.260250109. [DOI] [PubMed] [Google Scholar]
- 12.Monteiro G A, Supply P, Goffeau A, Correia I S. The in vivo activation of Saccharomyces cerevisiae plasma membrane H+-ATPase by ethanol depends on the expression of the PMA1 gene, but not of the PMA2 gene. Yeast. 1994;10:1439–1446. doi: 10.1002/yea.320101107. [DOI] [PubMed] [Google Scholar]
- 13.Piper P W. Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993;11:339–356. doi: 10.1111/j.1574-6976.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 14.Rosa M F, Correia I S. In vivo activation by ethanol of plasma membrane ATPase of Saccharomyces cerevisiae. Appl Environ Microbiol. 1991;57:830–835. doi: 10.1128/aem.57.3.830-835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosa M F, Correia I S. Ethanol tolerance and activity of plasma membrane ATPase in Kluyveromyces marxianus and Saccharomyces cerevisiae. Enzyme Microb Technol. 1992;14:23–27. [Google Scholar]
- 16.Sá-Correia, I., S. P. Salgueiro, C. A. Viegas, and J. M. Novais. 1989. Leakage induced by ethanol and octanoic and decanoic acids in Saccharomyces cerevisiae. Yeast 5(Suppl.):S123–S127.
- 17.Slavik J, Kotyk A. Intracellular pH distribution and transmembrane pH profile of yeast cells. Biochim Biophys Acta. 1984;766:679–684. doi: 10.1016/0005-2728(84)90129-4. [DOI] [PubMed] [Google Scholar]
- 18.Stevens S, Hofemyer J-H S. Effects of ethanol, octanoic and decanoic acids on fermentation and the passive influx of protons through the plasma membrane of Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1993;38:656–663. [Google Scholar]
- 19.Viegas C A, Correia I S. Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J Gen Microbiol. 1991;137:645–651. doi: 10.1099/00221287-137-3-645. [DOI] [PubMed] [Google Scholar]
- 20.Viegas C A, Correia I S. Toxicity of octanoic acid in Saccharomyces cerevisiae at temperatures between 8.5 and 30°C. Enzyme Microb Technol. 1995;17:826–831. [Google Scholar]
- 21.Viegas C A, Correia I S. Effects of low temperatures (9–33°C) and pH (3.3–5.7) in the loss of Saccharomyces cerevisiae viability by combining lethal concentrations of ethanol with octanoic or decanoic acids. Int J Food Microbiol. 1997;34:267–277. doi: 10.1016/s0168-1605(96)01200-7. [DOI] [PubMed] [Google Scholar]
- 22.Viegas, C. A., and I. Sá-Correia. Unpublished results.
- 23.Viegas C A, Rosa M F, Correia I S, Novais J M. Inhibition of yeast growth by octanoic and decanoic acids produced during ethanolic fermentation. Appl Environ Microbiol. 1989;55:21–28. doi: 10.1128/aem.55.1.21-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viegas C A, Supply P, Capieaux E, van Dyck L, Goffeau A, Correia I S. Regulation of the expression of the H+-ATPase genes PMA1 and PMA2 during growth and effects of octanoic acid in Saccharomyces cerevisiae. Biochim Biophys Acta. 1994;1217:74–80. [PubMed] [Google Scholar]
- 25.Viegas C A, Sebastião P B, Nunes A G, Correia I S. Activation of plasma membrane H+-ATPase and expression of PMA1 and PMA2 genes in Saccharomyces cerevisiae cells grown at supraoptimal temperatures. Appl Environ Microbiol. 1995;61:1904–1909. doi: 10.1128/aem.61.5.1904-1909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warth A D. Effect of benzoic acid on glycolytic metabolite levels and intracellular pH in Saccharomyces cerevisiae. Appl Environ Microbiol. 1991;57:3415–3417. doi: 10.1128/aem.57.12.3415-3417.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]