Dear Editor:
The clinical implications of inactivating mutations of the THRB gene are well known with the development of the syndrome of resistance to thyroid hormone beta (RTHβ). Affected individuals exhibit a tissue-specific reduction in thyroid hormone (TH) signaling, with high circulating serum-free thyroxine (fT4) and free triiodothyronine (fT3) levels with unsuppressed thyrotropin (TSH).1 The clinical implications of having RTHβ may also include pregnancy outcomes2–4 and altered tissue TH responsiveness in healthy offspring of these pregnancies.5
We have previously reported data from an extended Azorean family harboring a missense mutation in the THRB gene, which leads to the substitution of arginine by glicine at position 243 of the TH receptor β (R243Q).2 We demonstrated that high serum TH levels of pregnant affected mothers (AfMo) were congruent to that of similarly affected fetuses (AfFe) and resulted in a normal pregnancy and fetal outcomes. In contrast, when carrying a normal fetus (NlFe), without a THRB gene mutation, the fetus's exposure to supraphysiological levels of TH increased the risk for miscarriage and resulted in lower birth weight and suppressed TSH at birth.2 The same study showed no abnormalities at birth in AfFe exposed during embryonic life to subphysiological levels of TH, which occurs when AfFe with RTHβ inherited from the father, was carried by a normal mother (NlMo).2 Notably, NlFe born to AfMo manifest reduced sensitivity to TH in adulthood.5 While much attention has been given to the effect of maternal TH on the fetus, it is unknown how the genotype of the fetus might influence the thyroid function of the mother.
We retrospectively reviewed data from the previously reported Azorean family, harboring the THRB mutation R243Q,2,5 and we examined the maternal thyroid function tests (TFTs) throughout pregnancies of AfMo and NlMo, carrying either AfFe or NlFe fetuses, respectively. At birth, we also measured TFTs in cord blood as surrogate markers for newborn TFTs.
In this retrospective case series, we studied the maternal TFT, including TSH, fT3, and fT4, throughout nine pregnancies in six AfMo; six carrying AfFe, and three NlFe. TFTs were also obtained from their cord blood. In addition, TFTs were obtained during eight pregnancies of seven NlMo carrying four AfFe who inherited the THRB mutation from the father and four NlFe. Data points throughout these pregnancies were less frequent, and cord blood samples were unavailable. TSH, fT3, and fT4 were measured using a chemiluminescent microparticle immunoassay (ARCHITECT; Abbott). For all mothers, serum thyroid peroxidase antibodies (TPOab), heart rate, and body weight measurements were obtained, and for the newborns, the sex, body weight, and gestational week at delivery. The parameters obtained from the mothers during all pregnancies are reported in the Supplementary Data. The Internal Review Board of Hospital Divino Espirito Santo approved the study (1017/CES/2020). Written informed consent was obtained from all women.
A locally weighted scatterplot smoothing model generated with R software was used to depict the pregnancy week-related changes in TFTs. For further comparison, TFT results were divided into six groups (pror to gestation and five periods each of eight gestational weeks). The first group contained the mean values before pregnancy (week 0), and the other five groups were mean values from five gestational intervals of 8 weeks each (from weeks 1 to 40). Data were analyzed using Prism software (GraphPad), and a two-tailed Student's t-test was used to perform comparisons.
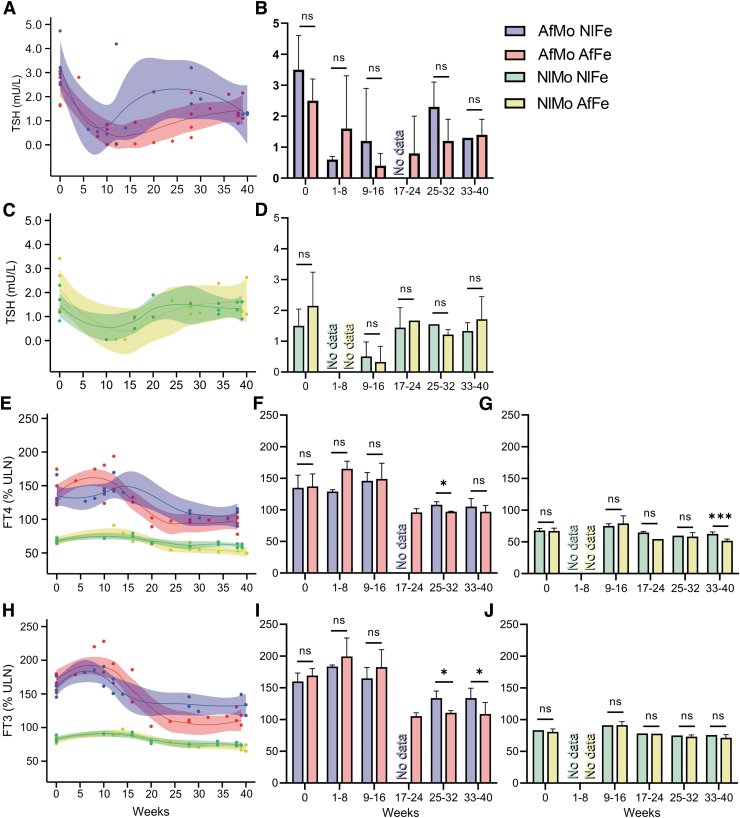
Maternal TSH values throughout pregnancies of RTHβ women ranged from 0.04 to 4.73 mIU/mL (Fig. 1A and C; normal range: 0.35–4.94 mIU/mL). The lowest TSH values were at the end of the first trimester, being undetectable in some cases. No significant differences were observed in the TSH values of RTHβ pregnancies carrying a NlFe compared to AfFe (Fig. 1B) nor of NlMo pregnancies carrying AfFe as compared to NlFe (Fig. 1C, D). fT4 measurements of AfMo during pregnancies were above the upper limit of normal (ULN) set at 100% (Fig. 1E). The fT4 values of AfMo peaked during the first 8–16 weeks of pregnancy, reaching 138% and 158% of the ULN when carrying a NlFe or an AfFe, respectively. From weeks 25 to 32, the AfMo carrying a NlFe exhibited significantly higher fT4 than when carrying a AfFe (108 ± 5.3 vs. 96 ± 2% ULN; p < 0.05; Fig. 1F). Similarly, NlMo, from weeks 33 to 40, exhibited significantly higher fT4 when carrying NlFe than AfFe (62.5 ± 3.2 vs. 51.8 ± 2.8% ULN; p < 0.001; Fig. 1G). fT3 values of AfMo were also above the ULN (Fig. 1H). Notably, during the first 8–16 weeks of AfMo pregnancies, fT3 values increased to 174% ULN and 191% ULN when carrying a NlFe and AfFe, respectively. From weeks 25 to 40, AfMo exhibited significantly higher fT3 values when carrying a NlFe than an AfFe (134 ± 13 vs. 110 ± 11% ULN; p < 0.05; Fig. 1I). Differently, NlMo carrying either AfFe or NlFe showed similar fT3 levels during pregnancies (Fig. 1J).
FIG. 1.
Maternal TFTs throughout pregnancy. Serum TSH levels for AfMo (A) and NlMo (C) as a function of gestational age. The best-fitted line and the CIs bandwidth are included. Histograms for mean TSH levels for AfMo (B) and NlMo (D). Values before pregnancy (week 0) and pools for 8-week intervals from weeks 1 to 40. (E) Serum fT4 and (H) serum fT3 levels as a function of gestational age for AFMo and NlMo. Histograms for the mean fT4 values for AFMo (F) and NLMo (G). Histograms for the mean fT3 values for AFMo (I) and NLMo (J). Values are mean ± SEM: *p < 0.05, ***p < 0.001. AfFe, affected fetus; AfMo, affected mother; CI, 95% confidence interval; fT3, free triiodothyronine; fT4, free thyroxine; NlFe, normal fetus; NlMo, normal mother; SEM, standard error of the mean; TFTs, thyroid function tests; TSH, thyrotropin; ULN, the upper limit of normal as 100%.
Among all the mothers, three AfMo were positive for TPOab (one carrying AfFe and two a NlFe); one NlMo carrying an AfFe had positive TPOab. Throughout the pregnancies, resting heart rate was significantly higher in AfMo than in NlMo. Differences were already significant before the onset of pregnancy (80.9 ± 3.5 vs. 72.3 ± 3.3 beat/minute; p < 0.01), increasing at delivery (96.4 ± 6.2 vs. 89.0 ± 2.6 beat/minute; p < 0.001). In contrast, AfMo and NlMo exhibited similar body weight at the beginning of the pregnancy (62.2 ± 9.4 vs. 65.5 ± 7.3 kg) and at delivery (72.3 ± 10.3 vs. 76.8 ± 7.2 kg).
In all RTHβ pregnancies with an AfMo, the gestational age at delivery was similar, and the birth body weight was lower in the NlFe than the AfFe (2.3 ± 0.1 vs. 3.1 ± 0.2 kg; p < 0.01; Table 1). Cord blood TSH from of NlFe was undetectable (<0.1), but in AfFe was 9.51 mU/L (2.2–10 mU/L, range in normal pregnancies). fT4 levels were similar in NlFe and AfFe (98.4 ± 16.4 vs. 89.5 ± 9.4% ULN; Table 1). Differently, fT3 levels were higher in NlFe than in AfFe (90.7 ± 13.0 vs. 51.9 ± 10.5% ULN; p < 0.05; Table 1). In pregnant NlMo, the gestational age at delivery (on average 39.0 ± 0.5 weeks) and the birth body weight (on average 3.1 ± 0.2 kg) were similar regardless of the THRβ genotype of the fetus.
Table 1.
Thyroid Function Tests in the Fetal Cord Blood
| NlFe | AfFe | p | |
|---|---|---|---|
| RTHβ with AfMo | |||
| Number of subjects | 3 | 3 | |
| Delivery week | 39.7 ± 0.6 | 38.8 ± 1.0a | ns |
| Birth weight (Kg) | 2.3 ± 0.11 | 3.0 ± 0.2 | <0.01 |
| TSH (mU/L) | <0.1 | 9.5 ± 0.6 | <0.001 |
| fT4 (%ULN) | 98.4 ± 16.4 | 89.47 ± 9.4 | ns |
| fT3 (%ULN) | 90.7 ± 13.0 | 51.93 ± 10.5 | <0.05 |
| RTHβ with NlMo | |||
| Number of subjects | 4 | 4 | |
| Delivery week | 38.75 ± 0.5 | 39.25 ± 1.0 | ns |
| Birth weight (Kg) | 3.31 ± 0.1 | 2.91 ± 0.3 | ns |
Six subjects; values are mean ± SEM.
AfFe, affected fetus; AfMo, affected mother; fT3, free triiodothyronine; fT4, free thyroxine; NlFe, normal fetus; NlMo, normal mother; ns, not significant; RTHβ, resistance to thyroid hormone beta; SEM, standard error of the mean; TSH, thyrotropin; ULN, the upper limit of normal as 100%.
The high maternal TH of pregnant women with RTHβ negatively impact the outcome of the fetus.1,2,6 Notwithstanding, the changes in maternal thyroid function during pregnancies in women carrying fetuses with RTHβ have not been extensively studied. Our observations agree with previous reports7,8 and show that TSH values in women with RTHβ were within the normal range and exhibited a human chorionic gonadotropin-mediated nadir at the end of the first trimester.7 In parallel, the fT4 and fT3 levels increased during the first trimester and declined afterward though remaining above the ULN.
The supraphysiological levels of fT4 and fT3 of AfMo may have adverse effects only when carrying a NlFe2,6 and must be closely monitored. Some treating physicians believe that in women with RTHβ carrying a NlFe, the fT4 level during pregnancy should be maintained below 50% the ULN to prevent fetal complications.6,9 Notably, we observed that AfMo carrying NlFe exhibited a more prolonged period of high serum fT3 than fT4 levels. This observation might be clinically relevant because the current approach for managing AfMo carrying NlFe is based only on maternal fT4 levels.6 fT3 levels are rarely followed in pregnancy, yet an increase in fT3 levels may result in increased TH-signaling in most tissues,10 contributing to the unfavorable outcome of RTHβ pregnancies carrying a NlFe.2 Therefore, refocusing attention on maternal fT3 levels may help clinicians manage RTHβ pregnancies. Future studies should aim at correlating RTHβ pregnancy outcomes with maternal serum fT3 levels to clarify the clinical relevance of the observed elevated fT3 levels in AfMo carrying a NlFe. However, the changes observed in the maternal TFTs are insufficient to predict the fetal genotype.
This retrospective study was limited by the fact that the blood samples were collected at variable times during gestation and also because data were missing at some temporal points, which could affect the statistical comparison between groups.
The mechanism by which the fetal THRB genotype may influence the maternal thyroid function remains unknown. An AfFe but not NlFe has the potential of influencing the mother's thyroid function via a TH-mediated response because the TSH levels of NlFe, measured in cord blood, were undetectable while in AfFe were normal, indicating that the thyroid function in NlFe could be restricted during pregnancy but not in AfFe. The placental TH regulatory mechanisms, mainly TH transporters and deiodinases, could also be in play since the THRB genotype of the fetus influences them.3 The TH-derived metabolite T2S of fetal origin could also play a role as it is considered a fetal mechanism responsible for reducing maternal serum T3 concentrations.
This study should be expanded in the future. Prospective studies including a greater number of pregnant women with RTHβ caused by different THRB mutations are needed. Such studies should collect detailed clinical data as well as TFTs throughout pregnancy, from the cord blood as well as from the fetal and maternal components of the placenta.
Supplementary Material
Authors' Contributions
F.S.-L.: methodology (equal); visualization (equal); formal analysis (equal); writing—original draft (lead); writing—review and editing (equal). X.-H.L.: methodology (equal); formal analysis (equal); writing—review and editing (equal). H.J.: formal analysis (equal); writing—review and editing (equal); visualization (lead). A.M.D.: conceptualization (equal); formal analysis (equal); writing—review and editing (equal). S.R.: conceptualization (lead); formal analysis (equal); writing—review and editing (lead). J.A.: conceptualization (equal); resources (lead); writing—review and editing (equal).
Author Disclosure Statement
No competing financial interests exist.
Funding Information
F.S.-L.: The National Institutes of Health, DK15070. X.-H.L.: The National Institutes of Health, DK15070. H.J.: no funding information to declare. A.M.D.: The National Institutes of Health, DK15070. S.R.: The National Institutes of Health, DK15070. J.A.: no funding information to declare.
Supplementary Material
References
- 1. Pappa T, Refetoff S. Resistance to thyroid hormone beta: A focused review. Front Endocrinol (Lausanne) 2021;12:656551; doi: 10.3389/fendo.2021.656551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anselmo J, Cao D, Karrison T, et al. Fetal loss associated with excess thyroid hormone exposure. JAMA 2004;292(6):691–695; doi: 10.1001/jama.292.6.691 [DOI] [PubMed] [Google Scholar]
- 3. Salas-Lucia F, Stan MN, James H, et al. Effect of the fetal THRB genotype on the placenta. J Clin Endocrinol Metab 2023; doi: 10.1210/clinem/dgad243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moran C, Chatterjee K. Maternal resistance to thyroid hormone β and pregnancy outcomes. J Clin Endocrinol Metab 2023; doi: 10.1210/clinem/dgad350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Srichomkwun P, Anselmo J, Liao XH, et al. Fetal exposure to high maternal thyroid hormone levels causes central resistance to thyroid hormone in adult humans and mice. J Clin Endocrinol Metab 2017;102(9):3234–3240; doi: 10.1210/jc.2017-00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pappa T, Anselmo J, Mamanasiri S, et al. Prenatal diagnosis of resistance to thyroid hormone and its clinical implications. J Clin Endocrinol Metab 2017;102(10):3775–3782; doi: 10.1210/jc.2017-01251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anselmo J, Kay T, Dennis K, et al. Resistance to thyroid hormone does not abrogate the transient thyrotoxicosis associated with gestation: Report of a case. J Clin Endocrinol Metab 2001;86(9):4273–4275; doi: 10.1210/jcem.86.9.7858 [DOI] [PubMed] [Google Scholar]
- 8. Dhingra S, Owen PJ, Lazarus JH, et al. Resistance to thyroid hormone in pregnancy. Obstet Gynecol 2008;112(2 Pt 2):501–503; doi: 10.1097/AOG.0b013e3181809e3a [DOI] [PubMed] [Google Scholar]
- 9. Weiss RE, Dumitrescu A, Refetoff S. Approach to the patient with resistance to thyroid hormone and pregnancy. J Clin Endocrinol Metab 2010;95(7):3094–3102; doi: 10.1210/jc.2010-0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salas-Lucia F, Bianco AC. T3 levels and thyroid hormone signaling. Front Endocrinol (Lausanne) 2022;13:1044691; doi: 10.3389/fendo.2022.1044691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.