Abstract
Purpose:
Synovial sarcoma (SS) is a rare, high-grade soft tissue tumor that requires multidisciplinary and multimodal care with surgery, radiotherapy, and chemotherapy. We examined the impact of sociodemographic and clinical factors on treatment patterns and survival in localized SS patients.
Methods:
Adolescents and young adults (AYAs, 15–39 years) and older adults (“adults,” ≥40 years) diagnosed with localized SS from 2000 to 2018 were identified in the California Cancer Registry. Multivariable logistic regression identified clinical and sociodemographic factors associated with receipt of chemotherapy and/or radiotherapy. Cox proportional hazards regression identified factors associated with overall survival (OS). Results are reported as odds ratios (ORs) and hazard ratios (HRs), respectively, with 95% confidence intervals (CIs).
Results:
More AYAs (n = 346) than adults (n = 272) received chemotherapy (47.7% vs. 36.4%) and radiotherapy (62.1% vs. 58.1%). Age at diagnosis, tumor size, treatment at National Cancer Institute-Children's Oncology Group (NCI-COG)-designated facilities, insurance status, and neighborhood socioeconomic status (SES) influenced treatment patterns. Among AYAs, treatment at NCI-COG-designated facilities was associated with receiving chemotherapy (OR 2.74, CI 1.48–5.07) and low SES was associated with worse OS (HR 2.28, 1.09–4.77). In adults, high SES was associated with receiving chemoradiotherapy (OR 3.20, CI 1.40–7.31), whereas public insurance was associated with decreased odds of chemoradiotherapy (OR 0.44, CI 0.20–0.95). With regard to treatment, absence of radiotherapy (HR 1.94, CI 1.18–3.20) was associated with worse OS in adults.
Conclusion:
In localized SS, both clinical and sociodemographic factors influenced treatment patterns. Further research should investigate how SES-related factors produce treatment disparities and identify interventions to improve treatment equity and outcomes.
Keywords: synovial sarcoma, adolescent and young adult, treatment patterns, sociodemographic factors, specialized cancer center, survival outcomes
Introduction
Synovial sarcomas (SS) are high-grade tumors of primitive mesenchymal origin, which account for up to 10% of all soft tissue sarcomas (STS).1,2 Although SS are the most common nonrhabdomyosarcoma STS in children, peak incidence is in the third decade of life.2,3 SS classically present as slow-growing soft tissue masses in distal extremities with symptoms relating to local invasion and compression of surrounding tissues.
The mainstay of treatment for localized SS is surgical resection, often in tandem with adjuvant or neoadjuvant radiotherapy for large (>5 cm) or incompletely resectable tumors.4 Although SS seem to be more chemosensitive than other STS, consensus regarding the use of chemotherapy in patients with localized disease remains unclear.5,6 A recent study by the Children's Oncology Group (COG; ARST0332, ages 0–30) evaluated an algorithm in which only patients with tumors >5 cm and of high histological grade based on Pediatric Oncology Group criteria or were unresectable at study entry received chemotherapy and documented 5-year overall survival (OS) of 85% or better for patients with localized disease.7,8 However, a standard of care in these patients remains controversial, especially in adults. Moreover, there is a paucity of data on treatment patterns and the use of chemotherapy and radiotherapy at the population level.
Overall, 5-year survival for SS of all stages across all ages is ∼60%–75%.6,9 The main predictors of outcome include age at diagnosis, tumor size, and the presence of metastases.3,5,8,10–13 Additional prognostic factors include extent of surgical resection, anatomic location (extremity vs. axial), and histological factors such as tumor grade, mitotic rate, and necrosis.10,11,14–16 However, studies of the impact of insurance coverage, socioeconomic status (SES), and location of treatment (Specialized Cancer Center [SCC] vs. community centers) on localized SS survival outcomes are lacking. We, therefore, sought to characterize the impact of sociodemographic and clinical factors on treatment patterns and survival among patients with localized SS.
Methods
Patients diagnosed with a first primary localized SS in California, United States from 2000 to 2018 were identified using the California Cancer Registry (CCR). All analyses were overseen by the Institutional Review Board of the University of California, Davis. After excluding patients with a diagnosis on death certificate only, autopsy only, or with no survival time (n = 4.06%), we extracted the following information from the CCR: tumor morphology based on the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3 codes: 9040–9044), tumor size, anatomic site, stage at diagnosis, treatment (surgery [surgical resection], chemotherapy, radiotherapy), and sociodemographic characteristics, including age at diagnosis, race/ethnicity (non-Hispanic [NH] White, NH Black, Hispanic, Asian/Pacific Islander, other), sex, health insurance (private, public, no insurance), and initial hospital type where patient was reported to have received care within the first year from diagnosis (SCC vs. other). We defined SCC as COG institutions and/or National Cancer Institute (NCI)-designated comprehensive cancer centers (NCICCC) for patients ≤21 years and NCICCC for patients >21 years.
We defined care at an SCC based on the first year after diagnosis, to capture the full length of potential SS treatment. Patients were considered to have received all care at an SCC if all admission files from the CCR pertaining to the patient's cancer diagnosis were reported from an SCC. Minimum distance from patient residence to the nearest SCC hospital was calculated using geodetic distance in miles between the patient's zip code (center of zip code) and hospital address.
In California, uninsured patients become eligible for public insurance (Medicaid) after receiving a cancer diagnosis, thus we analyzed patients with public insurance and no insurance together. However, patients 65 years or older with Medicare and a supplement were grouped with those with private insurance. To describe a patient's SES, we used a previously developed multicomponent index based on a patient's census block of residence at the time of diagnosis, as geocoded by the CCR.17 The index is based on U.S. Census and American Community Survey data on education, occupation, unemployment, household income, poverty, rent, and home values of census tracts and grouped into tertiles (low, medium, and high SES). Comorbidities were determined using the Charlson Comorbidity Index.18
Patients were divided into two age groups: adolescents and young adults (AYAs) ages 15–39 years and older individuals ≥40 years (adults). We used descriptive statistics (frequencies, percentages) to compare baseline characteristics by age group. We used multivariate logistic regression to assess associations between sociodemographic and clinical factors and receipt of chemotherapy, radiotherapy, or both by age group. Results are presented as adjusted odds ratios (ORs) and their associated 95% confidence intervals (CIs). We used multivariable Cox proportional hazards regression to evaluate sociodemographic and clinical factors associated with OS and cancer-specific survival (CSS). Survival time was calculated from date of diagnosis to the date of death from any cause for OS and to the date of death from cancer for CSS. Patients who died of other causes were censored at the time of death for CSS. Patients alive at the study end date (October 31, 2020) were censored at this time or at the date of last follow-up.
We assessed proportional hazards assumption with tests based on Schoenfeld residuals and inspection of the survival curves (survival function vs. survival time and log [−log] of the survival function vs. the log of time) for all variables in the model. Variables that violated proportional hazards assumption were included in the strata statement (primary tumor site, distance to SCC, Charlson Comorbidity Score). Results are presented as adjusted hazard ratios (HRs) and their associated 95% CIs. We used separate regression models for AYAs and adults. Analyses were conducted using SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Cohort characteristics
The study population consisted of 346 AYAs and 272 adults (Table 1). Male and female patients were evenly represented in both cohorts. Among AYA patients, 46.5% were Hispanic and 35.8% were NH white, with a similar pattern in adults. A large proportion of patients had a primary extremity tumor (74.9%) and presented with tumors ≤5 cm. Only 5.5% of AYAs, in contrast to 20.6% of adults had comorbidities. Approximately 70% of patients in both age groups were privately insured. A majority of patients lived within a 20-mile radius of an SCC (70%). Most patients (95.4% AYAs and 95.2% adults) underwent surgical resection. More AYAs than adults received chemotherapy (47.7% vs. 36.4%) and radiotherapy (62.1% vs. 58.1%).
Table 1.
Selected Demographic and Clinical Characteristics of Patients with Localized Synovial Sarcoma, 2000–2018
| Characteristics | AYA 15–39 years, N = 346, % (N) | Adult ≥40 years, N = 272, % (N) | p |
|---|---|---|---|
| Age | <0.001 | ||
| 15–18 | 18.5 (64) | ||
| 19–21 | 13.3 (46) | ||
| 22–30 | 31.5 (109) | ||
| 31–39 | 36.7 (127) | ||
| 40–65 | 86.0 (234) | ||
| >65 | 14.0 (38) | ||
| Sex | |||
| Female | 48.3 (167) | 50.4 (137) | 0.61 |
| Male | 51.7 (179) | 49.6 (135) | |
| Health insurance | |||
| Private | 69.9 (242) | 70.2 (191) | 0.99 |
| Public/uninsured | 26.3 (91) | 26.1 (71) | |
| Unknown | 3.8 (13) | 3.7 (10) | |
| Race/ethnicity | |||
| NH White | 35.8 (124) | 47.4 (129) | 0.03 |
| Black | 5.8 (20) | 5.9 (16) | |
| Hispanic | 46.5 (161) | 34.6 (94) | |
| Asian/Pacific Islander | 9.8 (34) | 10.3 (28) | |
| Other | 2.0 (7) | 1.8 (5) | |
| Neighborhood socioeconomic status | |||
| Low | 32.7 (113) | 30.9 (84) | 0.19 |
| Medium | 35.8 (124) | 30.9 (84) | |
| High | 31.5 (109) | 38.2 (104) | |
| Tumor size | |||
| 0–5 cm | 46.5 (161) | 40.1 (109) | 0.05 |
| 5.1–10 cm | 31.2 (108) | 31.6 (86) | |
| >10 cm | 11.6 (40) | 19.1 (52) | |
| Unknown | 10.7 (37) | 9.2 (25) | |
| Primary site | |||
| Body wall | 13.6 (47) | 15.1 (41) | 0.06 |
| Extremity | 77.7 (269) | 72.1 (196) | |
| Head/neck | 5.8 (20) | 5.5 (15) | |
| Visceral | 2.6 (9) | 7.4 (20) | |
| Other | 0.3 (1) | 0 (0) | |
| Radiotherapy | |||
| Yes | 62.1 (215) | 58.1 (158) | 0.29 |
| No | 37.6 (130) | 41.9 (114) | |
| Unknown | 0.3 (1) | 0 (0) | |
| Chemotherapy | |||
| Yes | 47.7 (165) | 36.4 (99) | 0.003 |
| No | 50.0 (173) | 62.9 (171) | |
| Unknown | 2.3 (8) | 0.7 (2) | |
| Surgery | |||
| Yes | 95.4 (330) | 95.2 (259) | 0.67 |
| No | 4.0 (14) | 4.8 (13) | |
| Unknown | 0.6 (2) | 0 (0) | |
| Comorbidities | |||
| No | 61.0 (211) | 57.0 (155) | <0.001 |
| Yes | 5.5 (19) | 20.6 (56) | |
| Unknown | 33.5 (116) | 22.4 (61) | |
| Distance to SCC | |||
| 0–10 miles | 53.5 (185) | 47.4 (129) | 0.29 |
| 11–20 miles | 17.3 (60) | 23.5 (64) | |
| 21–30 miles | 7.5 (26) | 9.2 (25) | |
| 31–40 miles | 4.3 (15) | 3.3 (9) | |
| >40 miles | 17.3 (60) | 16.5 (45) | |
| Initial cancer care at an SCC | |||
| All | 24.6 (85) | 13.2 (36) | <0.001 |
| Part | 37.0 (128) | 33.1 (90) | |
| None | 38.4 (133) | 53.7 (146) | |
| Vital status | |||
| Alive | 79.5 (275) | 62.9 (171) | <0.001 |
| Death | 20.5 (71) | 37.1 (101) | |
| From sarcomas | 66.2 (47) | 52.5 (53) | 0.07 |
| From other causes | 33.8 (24) | 47.5 (48) | |
AYAs, adolescents and young adults; NH, non-Hispanic; SCC, Specialized Cancer Center.
Factors associated with receipt of chemotherapy
AYAs with tumor diameter >5 cm were more likely to receive chemotherapy (OR for tumors 5.1–10 cm 4.83, CI 2.66–8.79), whereas those with body wall tumors were less likely to receive this treatment (OR 0.37, CI 0.17–0.82) (Table 2). AYAs who obtained all or some treatment at an SCC were more likely to receive chemotherapy (All treatment: OR 2.26, CI 1.16–4.41; Some treatment: OR 2.74, CI 1.48–5.07). Similar to AYAs, adults with large tumors (tumors >10 cm: OR 5.21, CI 2.28–11.92) and who received some treatment at an SCC (OR 3.57, CI 1.83–6.98) were more likely to receive chemotherapy. In addition, adults, but not AYAs, living in high SES neighborhoods were more likely to receive chemotherapy (OR 3.20, CI 1.40–7.31). In contrast, adults with public/no insurance (vs. private insurance) were less likely to receive chemotherapy (OR 0.44, CI 0.20–0.95) and those older than 65 years (vs. 40–65 years) were significantly less likely to receive chemotherapy (OR 0.11, CI 0.03–0.43). Race/ethnicity and distance to SCC did not influence receipt of chemotherapy.
Table 2.
Factors Associated with Treatments Given (Chemotherapy, Radiotherapy, or Chemotherapy and Radiotherapy) in Patients with Localized Synovial Sarcoma, 2000–2018. Associations Reported as Odds Ratios with Respective 95% Confidence Intervals
| Characteristics |
Chemotherapy |
Radiotherapy |
Chemotherapy and radiotherapy |
|||
|---|---|---|---|---|---|---|
| AYA |
Adults |
AYA |
Adults |
AYA |
Adults |
|
| Total | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age | ||||||
| 15–18 | Reference | Reference | Reference | |||
| 19–21 | 0.68 (0.26–1.74) | 0.93 (0.37–2.34) | 0.85 (0.33–2.17) | |||
| 22–30 | 0.72 (0.34–1.51) | 1.17 (0.56–2.44) | 0.94 (0.44–2.00) | |||
| 31–39 | 0.67 (0.32–1.38) | 0.92 (0.45–1.88) | 0.77 (0.36–1.63) | |||
| 40–65 | Reference | Reference | Reference | |||
| >65 | 0.11 (0.03–0.43) | 0.79 (0.34–1.85) | 0.07 (0.01–0.55) | |||
| Sex | ||||||
| Female | Reference | Reference | Reference | Reference | Reference | Reference |
| Male | 0.71 (0.42–1.21) | 0.64 (0.34–1.18) | 0.9 (0.54–1.51) | 1.01 (0.57–1.77) | 0.79 (0.46–1.35) | 0.67 (0.34–1.31) |
| Health insurance | ||||||
| Private | Reference | Reference | Reference | Reference | Reference | Reference |
| Public/uninsured | 1.24 (0.64–2.43) | 0.44 (0.20–0.95) | 1.05 (0.54–2.06) | 0.43 (0.22–0.86) | 1.08 (0.55–2.11) | 0.32 (0.13–0.79) |
| Unknown | 0.81 (0.19–3.39) | 0.73 (0.14–3.88) | 0.42 (0.11–1.62) | 0.36 (0.09–1.44) | 0.58 (0.14–2.50) | 0.18 (0.02–1.71) |
| Race/ethnicity | ||||||
| NH White | Reference | Reference | Reference | Reference | Reference | Reference |
| Black | 2.21 (0.69–7.10) | 1.64 (0.41–6.62) | 0.5 (0.16–1.55) | 1.97 (0.56–6.98) | 1.26 (0.37–4.32) | 2.36 (0.51–10.81) |
| Hispanic | 1.36 (0.70–2.64) | 1.73 (0.83–3.61) | 1.21 (0.63–2.32) | 1.52 (0.76–3.05) | 1.52 (0.78–2.97) | 2.04 (0.91–4.57) |
| Asian/PI | 1.11 (0.44–2.75) | 1.01 (0.37–2.77) | 0.95 (0.38–2.34) | 1.81 (0.67–4.93) | 0.65 (0.24–1.76) | 1.74 (0.59–5.14) |
| Neighborhood socioeconomic status | ||||||
| Low | Reference | Reference | Reference | Reference | Reference | Reference |
| Medium | 0.61 (0.31–1.18) | 1.55 (0.68–3.56) | 1.83 (0.96–3.49) | 1.21 (0.56–2.64) | 1.2 (0.62–2.33) | 2.18 (0.88–5.40) |
| High | 0.83 (0.39–1.77) | 3.20 (1.40–7.31) | 1.71 (0.79–3.68) | 1.36 (0.65–2.87) | 1.31 (0.60–2.89) | 3.03 (1.24–7.41) |
| Tumor size | ||||||
| 0–5 cm | Reference | Reference | Reference | Reference | Reference | Reference |
| 5.1–10 cm | 4.83 (2.66–8.79) | 2.52 (1.22–5.22) | 2.08 (1.14–3.80) | 1.4 (0.71–2.77) | 5.11 (2.76–9.45) | 3.06 (1.39–6.72) |
| >10 cm | 6.95 (2.64–18.25) | 5.21 (2.28–11.92) | 1.13 (0.47–2.72) | 1.5 (0.69–3.28) | 2.29 (0.94–5.55) | 5.98 (2.45–14.56) |
| Unknown | 1.54 (0.66–3.61) | 1.16 (0.33–4.04) | 0.47 (0.21–1.08) | 0.22 (0.07–0.67) | 1.72 (0.67–4.43) | 0.54 (0.10–2.96) |
| Primary site | ||||||
| Extremity | Reference | Reference | Reference | Reference | Reference | Reference |
| Body wall | 0.37 (0.17–0.82) | 1.06 (0.47–2.39) | 0.48 (0.24–0.99) | 0.58 (0.27–1.25) | 0.26 (0.11–0.62) | 0.7 (0.28–1.73) |
| Head/neck | 0.99 (0.35–2.79) | 0.32 (0.06–1.71) | 4.85 (1.25–18.80) | 3.63 (0.90–14.58) | 1.66 (0.56–4.89) | 0.62 (0.11–3.53) |
| Visceral | 1.76 (0.33–9.35) | 0.48 (0.13–1.71) | 0.08 (0.01–0.69) | 0.08 (0.02–0.31) | 0.17 (0.02–1.52) | 0.12 (0.02–0.74) |
| Comorbidities | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.84 (0.27–2.56) | 1.46 (0.66–3.21) | 5.89 (1.18–29.32) | 1.08 (0.51–2.27) | 1.17 (0.40–3.44) | 2.32 (0.98–5.51) |
| Unknown | 0.4 (0.22–0.72) | 0.75 (0.34–1.63) | 0.85 (0.49–1.48) | 0.86 (0.42–1.75) | 0.53 (0.29–0.99) | 0.87 (0.36–2.10) |
| Distance to SCC | ||||||
| 0–10 miles | Reference | Reference | Reference | Reference | Reference | Reference |
| 11–20 miles | 0.8 (0.39–1.62) | 1.2 (0.55–2.59) | 1.43 (0.70–2.89) | 0.8 (0.39–1.62) | 1.12 (0.55–2.27) | 1.13 (0.47–2.71) |
| 21–30 miles | 0.94 (0.37–2.43) | 0.96 (0.33–2.83) | 0.72 (0.27–1.90) | 1.88 (0.63–5.57) | 0.9 (0.32–2.51) | 1.46 (0.45–4.73) |
| 31–40 miles | 0.84 (0.20–3.57) | 1.03 (0.22–4.84) | 2.74 (0.64–11.76) | 7.87 (0.79–78.81) | 0.84 (0.24–2.99) | 2.3 (0.44–11.93) |
| >40 miles | 0.87 (0.41–1.86) | 0.8 (0.32–1.97) | 0.58 (0.28–1.16) | 1.13 (0.49–2.60) | 0.59 (0.27–1.28) | 1.32 (0.51–3.45) |
| Initial cancer care at an SCC | ||||||
| None | Reference | Reference | Reference | Reference | Reference | Reference |
| All | 2.26 (1.16–4.41) | 0.97 (0.38–2.48) | 0.88 (0.47–1.66) | 0.78 (0.34–1.80) | 1.31 (0.64–2.68) | 1.29 (0.46–3.65) |
| Some | 2.74 (1.48–5.07) | 3.57 (1.83–6.98) | 1.99 (1.07–3.70) | 1.48 (0.78–2.81) | 3.38 (1.77–6.46) | 3.09 (1.48–6.41) |
Bold values are statistically significant.
AYA, adolescents and young adults; CI, confidence interval; NH, non-Hispanic; OR, odds ratio; PI, Pacific Islander; SCC, Specialized Cancer Center.
Factors associated with receipt of radiotherapy
AYAs with tumor size 5.1–10 cm (vs. ≤5 cm) and head and neck (vs. extremity) tumors were more likely to be given radiotherapy (OR 2.08, CI 1.14–3.80; OR 4.85, CI 1.25–18.80, respectively). On the other hand, AYAs with visceral (vs. extremity) tumors were much less likely to receive radiotherapy (OR 0.08, CI 0.01–0.69), similar to what was found in adults (OR 0.08, CI 0.02–0.31). Among AYAs, but not adults, the presence of comorbidities was associated with a higher likelihood of radiotherapy (OR 5.89, CI 1.18–29.32). AYAs who received some treatment at an SCC (vs. none) were more likely to receive radiotherapy (OR 1.99, CI 1.07–3.70), but no association was found among adults. In contrast to AYAs, adults with public or no insurance (vs. private insurance) were less likely to receive radiotherapy (OR 0.43, CI 0.22–0.86). There was no significant association between age, sex, race/ethnicity, and receipt of radiotherapy in AYAs or adults.
Factors associated with receipt of chemoradiotherapy
In AYAs, having a tumor 5.1–10 cm in size (vs. ≤5 cm) or partial treatment at an SCC (vs. none) were associated with a higher likelihood of receiving chemoradiotherapy (OR 5.11, CI 2.76–9.45; OR 3.38, CI 1.77–6.46, respectively). AYAs with body wall tumors (vs. extremity) were less likely to be treated with chemoradiotherapy (OR 0.26, CI 0.11–0.62). Adults >65 years (vs. 40–65 years) were much less likely to receive chemoradiotherapy (OR 0.07, CI 0.01–0.55). In adults, large tumors (5.1–10 cm: OR 3.06, CI 1.39–6.72; >10 cm: OR 5.98, CI 2.45–14.56) and living in high SES neighborhoods (OR 3.03, CI 1.24–7.41) were associated with higher likelihood of chemoradiotherapy. Visceral tumor location (OR 0.12, CI 0.02–0.74) and having public/no insurance (OR 0.32, CI 0.13–0.79) were associated with lower likelihood of chemoradiotherapy in adult patients. Race/ethnicity and distance to SCC were not associated with receipt of chemoradiotherapy.
Factors associated with OS
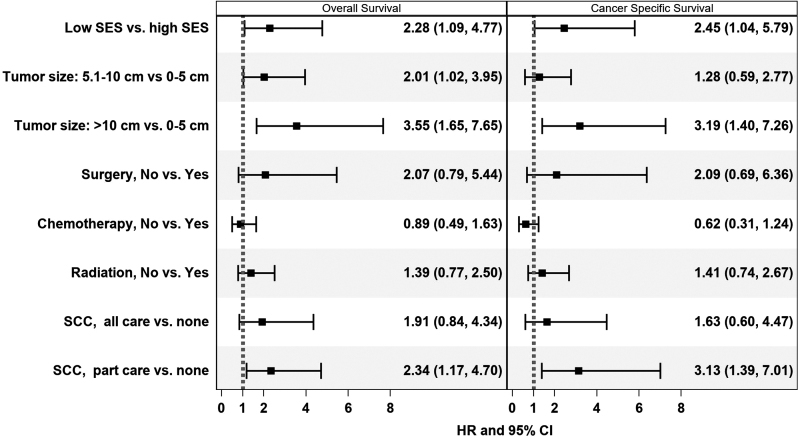
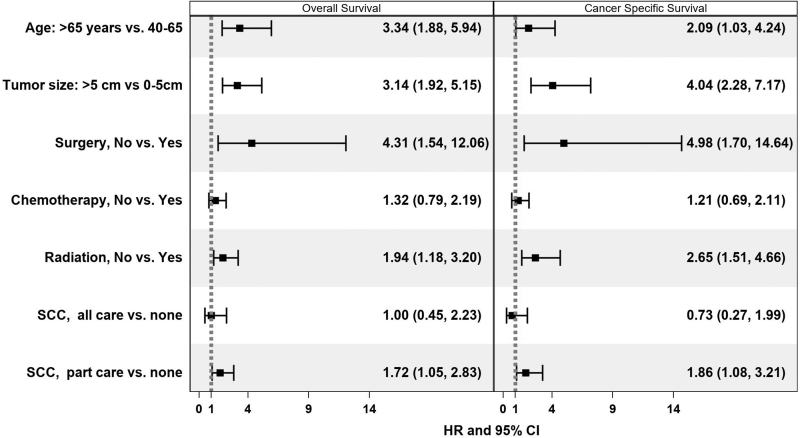
Age >65 (vs. 40–65 years) was associated with worse OS (HR 3.34, CI 1.88–5.93), but there was no association between age and survival in AYAs (Tables 3 and 4 and Figs. 1 and 2). Large tumors were associated with poor OS in both AYAs (>10 cm: HR 3.55, CI 1.65–7.65) and adults (>5 cm: 3.14, CI 1.92–5.15). In adults, lack of radiotherapy (HR 1.94, CI 1.18–3.20) and surgical resection (HR 4.31, CI 1.54–12.06) were associated with worse OS; no association was found for AYAs. Receiving some at an SCC in adults was associated with worse OS (HR 1.72, CI 1.05–2.83), a finding that was also seen in AYAs (HR 2.34, CI 1.17–4.70). In AYA patients, residing in a low SES neighborhood was associated with worse OS (HR 2.28, CI 1.09–4.77), a finding that was not seen in adults. Sex, race/ethnicity, and health insurance were not significantly associated with OS in AYAs or adults.
Table 3.
Multivariable-Adjusted Hazard Ratios and 95% Confidence Intervals of Associations with Overall and Cancer-Specific Survival in Adolescents and Young Adults 15–39 Years with Localized Synovial Sarcoma, 2000–2018
| |
Overall survival |
Cancer-specific survival |
||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | p | HR (95% CI) | p |
| Age | ||||
| 15–18 | Reference | Reference | ||
| 19–30 | 1.45 (0.65–3.24) | 0.364 | 1.59 (0.67–3.82) | 0.296 |
| 31–39 | 1.76 (0.78–3.98) | 0.175 | 1.40 (0.56–3.50) | 0.472 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.61 (0.36–1.03) | 0.065 | 0.61 (0.34–1.09) | 0.097 |
| Health insurance | ||||
| Private | Reference | Reference | ||
| Public/uninsured | 1.49 (0.80–2.78) | 0.209 | 1.44 (0.72–2.88) | 0.307 |
| Unknown | 1.30 (0.27–6.25) | 0.747 | 1.36 (0.26–6.97) | 0.716 |
| Race/ethnicity | ||||
| NH White | Reference | Reference | ||
| Not White | 0.98 (0.52–1.85) | 0.943 | 1.01 (0.49–2.05) | 0.986 |
| Neighborhood socioeconomic status | ||||
| Low | 2.28 (1.09–4.77) | 0.028 | 2.45 (1.04–5.79) | 0.042 |
| Medium | 1.50 (0.71–3.20) | 0.288 | 1.66 (0.70–3.93) | 0.253 |
| High | Reference | Reference | ||
| Tumor size | ||||
| 0–5 cm | Reference | Reference | ||
| 5.1–10 cm | 2.01 (1.02–3.95) | 0.043 | 1.28 (0.59–2.77) | 0.535 |
| >10 cm | 3.55 (1.65–7.65) | 0.001 | 3.19 (1.40–7.26) | 0.006 |
| Radiotherapy treatment | ||||
| Yes | Reference | Reference | ||
| No | 1.39 (0.77–2.50) | 0.272 | 1.41 (0.74–2.67) | 0.298 |
| Chemotherapy treatment | ||||
| Yes | Reference | Reference | ||
| No | 0.89 (0.49–1.63) | 0.707 | 0.62 (0.31–1.24) | 0.174 |
| Surgery | ||||
| Yes | Reference | Reference | ||
| No | 2.07 (0.79–5.44) | 0.138 | 2.09 (0.69–6.36) | 0.194 |
| Initial cancer care at an SCC | ||||
| None | Reference | Reference | ||
| All | 1.91 (0.84–4.34) | 0.124 | 1.63 (0.60–4.47) | 0.338 |
| Some | 2.34 (1.17–4.70) | 0.017 | 3.13 (1.39–7.01) | 0.006 |
Bold values are statistically significant.
Included in strata statement: primary site, distance to NCI-COG facility, Charlson score.
HR, hazard ratio; NCI-COG, National Cancer Institute-Children's Oncology Group; NH, non-Hispanic; SCC, Specialized Cancer Center (Children's Oncology Group and/or National Cancer Institute-designated Comprehensive Cancer Center).
Table 4.
Multivariable-Adjusted Hazard Ratios and 95% Confidence Intervals of Associations with Overall and Cancer-Specific Survival in Adults 40 Years and Older with Localized Synovial Sarcoma, 2000–2018
| |
Overall survival |
Cancer-specific survival |
||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | p | HR (95% CI) | p |
| Age | ||||
| 40–65 | Reference | Reference | ||
| >65 | 3.34 (1.88–5.94) | <0.001 | 2.09 (1.03–4.24) | 0.042 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.89 (0.58–1.37) | 0.594 | 0.86 (0.53–1.40) | 0.549 |
| Health insurance | ||||
| Private | Reference | Reference | ||
| Public/uninsured | 1.18 (0.72–1.92) | 0.512 | 1.00 (0.58–1.73) | 0.991 |
| Race/ethnicity | ||||
| NH White | Reference | Reference | ||
| Not White | 1.28 (0.78–2.09) | 0.329 | 1.31 (0.74–2.32) | 0.346 |
| Neighborhood socioeconomic status | ||||
| Low | 1.20 (0.68–2.11) | 0.540 | 1.51 (0.79–2.87) | 0.210 |
| Medium | 0.86 (0.49–1.52) | 0.611 | 0.77 (0.40–1.49) | 0.445 |
| High | Reference | Reference | ||
| Tumor size | ||||
| 0–5 cm | Reference | Reference | ||
| >5 cm | 3.14 (1.92–5.15) | <0.001 | 4.04 (2.28–7.17) | <0.001 |
| Radiotherapy treatment | ||||
| Yes | Reference | Reference | ||
| No | 1.94 (1.18–3.20) | 0.009 | 2.65 (1.51–4.66) | 0.001 |
| Chemotherapy treatment | ||||
| Yes | Reference | Reference | ||
| No | 1.32 (0.79–2.19) | 0.286 | 1.21 (0.69–2.11) | 0.511 |
| Surgery | ||||
| Yes | Reference | Reference | ||
| No | 4.31 (1.54–12.06) | 0.005 | 4.98 (1.70–14.64) | 0.004 |
| Comorbidities | ||||
| No | Reference | Reference | ||
| Yes | 1.53 (0.91–2.57) | 0.111 | 1.50 (0.83–2.71) | 0.181 |
| Unknown | 0.62 (0.33–1.18) | 0.147 | 0.64 (0.32–1.29) | 0.214 |
| Initial cancer care at an SCC | ||||
| None | Reference | Reference | ||
| All | 1.00 (0.45–2.23) | 0.996 | 0.73 (0.27–1.99) | 0.542 |
| Some | 1.72 (1.05–2.83) | 0.031 | 1.86 (1.08–3.21) | 0.025 |
Bold values are statistically significant.
Included in strata statement: primary site, distance to NCI-COG facility.
NH, non-Hispanic; HR, hazard ratio; NCI-COG, National Cancer Institute-Children's Oncology Group; SCC, Specialized Cancer Center (Children's Oncology Group and/or National Cancer Institute-designated Comprehensive Cancer Center).
FIG. 1.
Associations of overall and cancer-specific survival in adolescents and young adults with localized synovial sarcoma, 2000–2018.
FIG. 2.
Associations of overall and cancer-specific survival in adults with localized synovial sarcoma, 2000–2018.
Discussion
In this population-based study of AYAs and adults with localized SS, we found that age at diagnosis and tumor size, as well as treatment at an SCC, insurance status, and neighborhood SES influenced treatment patterns. Although age, tumor size, and lack of surgical resection are well studied in SS, our study, to our knowledge, is the first to additionally consider the impact of sociodemographic factors on treatment patterns in AYAs and adults with localized SS outside of a clinical trial.7,10,13,16 As there is currently no completely agreed-upon standard of care for treatment of localized SS beyond surgical resection, increased understanding of the factors that influence treatment patterns could inform potential areas for intervention to improve care for patients with localized SS.
Our analysis demonstrates that age at diagnosis and tumor size impact the receipt of adjunct chemotherapy and radiotherapy and survival. This finding is consistent with prior studies of patients with localized SS.6,10,13 In older adults, decisions to not pursue chemotherapy and/or radiotherapy can be influenced by concerns for toxicity and morbidity in patients with comorbidities that are often more prevalent in older adults, such as the higher prevalence of renal disease in older patients.19 However, our study did not reveal any associations between comorbidities and treatment patterns or survival outcomes in adults. In our study, only the absence of radiotherapy was associated with worse survival in adults. We observed no other associations between treatment patterns and survival in either age group. While several prospective studies have shown radiotherapy to be beneficial for local control, but not survival, our findings suggest that radiotherapy could potentially provide some benefit in older patients with localized SS, however, we were unable to control for other factors that may impact survival in this cohort of older patients who received radiotherapy.9,20,21
While SS is generally described as more chemosensitive than most other STS, our findings do not demonstrate a survival advantage with chemotherapy.22,23 However, given the observational nature of our data, and confounding factors, we are unable to account for, it is possible that higher risk patients may have been more likely to get chemotherapy and that, in fact, receipt of chemotherapy did improve outcomes. In addition, we cannot control for the selection bias inherent in treating patients across multiple center types without knowledge of the risk assessment process for each individual patient.
Importantly, our study demonstrates that sociodemographic factors impacted treatment patterns and survival. In adults, insurance and neighborhood SES influenced treatment patterns. While treatment did not impact survival directly in our study, our findings suggest that health insurance type and neighborhood SES impact the treatment a patient receives. This in turn could have a negative impact on patient outcomes, especially if care is dictated by sociodemographic factors rather than physician recommendations. In addition, for both AYAs and adults, receiving all or some treatment at an SCC was associated with a higher likelihood of chemotherapy or chemoradiation. The reason behind this association is unclear but may be indicative of greater access to specialized oncologists and ancillary resources at SCCs that result in more aggressive management of localized SS. This association could also have been observed if patients with more complex diseases were transferred to an SCC from a community hospital.
In adults, higher SES was also associated with an increased likelihood of chemotherapy, which might be due to increased access to specialized care in resource-rich communities. In contrast, unlike adults, many AYAs (up to age 21) in California are covered by California Children's Services, which mandates their evaluation at SCCs, possibly blunting an association between SES and treatment in the AYA group.
We did not observe a survival benefit for AYAs or adults who were treated at SCCs, which is in contrast to what has been described previously by our group and others.24,25 However, this may reflect the referral of higher-risk cases or those with other medical complexity with a less favorable overall prognosis to SCCs. Further investigation is needed to characterize differences in treatment patterns between SCCs and non-SCCs and identify the subset of SS patients that would benefit from specialized treatment. Furthermore, any patient and physician barriers to obtaining treatment at SCCs would need to be identified and addressed to ensure that these services are being maximally utilized by those requiring higher level of care.
As described above, various clinical and nonclinical factors influenced treatment patterns in this cohort of patients with localized SS. While residing in a low SES neighborhood was associated with worse survival among AYAs, no relationship was seen between neighborhood SES and treatment received. This finding may suggest that neighborhood SES may influence survival outcomes independent of clinical management in AYA patients with localized SS, who are known to otherwise have a better prognosis. However, it is also possible that patients with low SES experience treatment delays or face barriers in completed prescribed treatment. Additional research is needed to uncover SES-related factors, such as family support, transportation, distance to clinic, and medical literacy that could also affect clinical outcomes among these young patients with localized SS.
As with most population-based studies, there are inherent limitations to our study. While we have information on whether patients received chemotherapy or radiotherapy, we are unable to account for the specific chemotherapy regimens administered, variations in surgery and chemotherapy protocols across different institutions, the quality of surgery and radiotherapy, delays in treatment delivery or failure to complete planned therapy, or differences in management of treatment-related complications. Additionally, we do not have information on surgical margins or status of disease progression, which could impact prognosis. Since we derived our data from the CCR, which uses a comorbidity index score in lieu of specific comorbidity details, we are unable to account for specific comorbidities that may impact choice and delivery of treatment. Although we have information on where patients were treated overall, we do not have information on whether they were referred to a different institution than the reporting institution for different parts of their therapy such as chemotherapy, radiotherapy, or surgery.
Furthermore, our cohort of adults older than 65 years is relatively small compared with other age groups and it is possible that some associations were not detected due to sample size. Despite the above limitations, our study is the first to characterize the AYA and adult population with SS in this detail, and the first to describe sociodemographic factors that may affect treatment patterns and survival.
Conclusion
Among AYAs and adults with localized SS, both clinical and sociodemographic factors influence the type of treatment given to patients. Importantly, even in a young population of patients with localized disease, living in a low SES neighborhood was associated with worse survival among AYAs, underscoring the potential for SES-related factors to impact survival outcomes. Further research is needed to elucidate how sociodemographic factors lead to disparities in patterns of care and to identify interventions that could improve survival outcomes for these patients.
Data Availability Statement
The data that support the findings of this study are available from the CCR. Access to the data is granted through an application process by the management or data custodians.
Authors' Contributions
The authors confirm their contribution as follows: study conception and design: A.S., S.T., and E.A.; data collection and analysis: R.A. and F.M.; draft article preparation: A.S., E.A., R.A., F.M., and T.K. All authors reviewed and approved the final version of the article.
Disclaimer
The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Institutes of Health, the National Cancer Institute (NCI), and the Centers for Disease Control and Prevention (CDC) or their Contractors and Subcontractors.
Author Disclosure Statement
All authors have no relevant financial or nonfinancial interests to disclose.
Funding Information
E.A.: Hyundai Hope on Wheels Young Investigator Award. R.A.: Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number T32HP30037 for Research in Primary Care. T.K.: UC Davis Comprehensive Cancer Center award number P30CA093373. J.C-A.: Supported in part by the UC Davis Paul Calabresi Career Development Award for Clinical Oncology as funded by the NCI/National Institutes of Health through grant number 5K12-CA138464. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885;CDC's National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the NCI's Surveillance, Epidemiology, and End Results program (SEER) under contract HHSN261201800032I awarded to the University of California, San Francisco; contract HHSN261201800015I awarded to the University of Southern California; and contract HHSN261201800009I awarded to the Public Health Institute.
References
- 1. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer 2006;119(12):2922–2930; doi: 10.1002/ijc.22239 [DOI] [PubMed] [Google Scholar]
- 2. Joseph N, Laurent SS, Nelson JJ, et al. Incidence and prevalence of synovial sarcoma in the US: An analysis using SEER*Stat. J Clin Oncol 2019;37(15 Suppl):e22535; doi: 10.1200/JCO.2019.37.15_suppl.e22535 [DOI] [Google Scholar]
- 3. Aytekin MN, Öztürk R, Amer K, et al. Epidemiology, incidence, and survival of synovial sarcoma subtypes: SEER database analysis. J Orthop Surg (Hong Kong) 2020;28(2):2309499020936009; doi: 10.1177/2309499020936009 [DOI] [PubMed] [Google Scholar]
- 4. Gazendam AM, Popovic S, Munir S, et al. Synovial sarcoma: A clinical review. Curr Oncol 2021;28(3):1909–1920; doi: 10.3390/curroncol28030177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: A retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 2004;101(3):627–634; doi: 10.1002/cncr.20386 [DOI] [PubMed] [Google Scholar]
- 6. Shi W, Indelicato DJ, Morris CG, et al. Long-term treatment outcomes for patients with synovial sarcoma: A 40-year experience at the University of Florida. Am J Clin Oncol 2013;36(1):83–88; doi: 10.1097/COC.0b013e31823fe450 [DOI] [PubMed] [Google Scholar]
- 7. Spunt SL, Million L, Chi YY, et al. A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): A Children's Oncology Group prospective study. Lancet Oncol 2020;21(1):145–161; doi: 10.1016/s1470-2045(19)30672-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venkatramani R, Xue W, Randall RL, et al. Synovial sarcoma in children, adolescents, and young adults: A report from the Children's Oncology Group ARST0332 study. J Clin Oncol 2021;39(35):3927–3937; doi: 10.1200/jco.21.01628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guadagnolo BA, Zagars GK, Ballo MT, et al. Long-term outcomes for synovial sarcoma treated with conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys 2007;69(4):1173–1180; doi: 10.1016/j.ijrobp.2007.04.056 [DOI] [PubMed] [Google Scholar]
- 10. Spillane AJ, A'Hern R, Judson IR, et al. Synovial sarcoma: A clinicopathologic, staging, and prognostic assessment. J Clin Oncol 2000;18(22):3794–3803; doi: 10.1200/jco.2000.18.22.3794 [DOI] [PubMed] [Google Scholar]
- 11. Spurrell EL, Fisher C, Thomas JM, et al. Prognostic factors in advanced synovial sarcoma: An analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol 2005;16(3):437–444; doi: 10.1093/annonc/mdi082 [DOI] [PubMed] [Google Scholar]
- 12. Sultan I, Rodriguez-Galindo C, Saab R, et al. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: An analysis of 1268 patients. Cancer 2009;115(15):3537–3547; doi: 10.1002/cncr.24424 [DOI] [PubMed] [Google Scholar]
- 13. Vlenterie M, Ho VK, Kaal SE, et al. Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br J Cancer 2015;113(11):1602–1606; doi: 10.1038/bjc.2015.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bergh P, Meis-Kindblom JM, Gherlinzoni F, et al. Synovial sarcoma: Identification of low and high risk groups. Cancer 1999;85(12):2596–2607; doi: [DOI] [PubMed] [Google Scholar]
- 15. Guillou L, Benhattar J, Bonichon F, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: A multicenter, retrospective analysis. J Clin Oncol 2004;22(20):4040–4050; doi: 10.1200/jco.2004.11.093 [DOI] [PubMed] [Google Scholar]
- 16. Lewis JJ, Antonescu CR, Leung DHY, et al. Synovial sarcoma: A multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol 2000;18(10):2087–2094; doi: 10.1200/jco.2000.18.10.2087 [DOI] [PubMed] [Google Scholar]
- 17. Yost K, Perkins C, Cohen R, et al. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;12(8):703–711; doi: 10.1023/a:1011240019516 [DOI] [PubMed] [Google Scholar]
- 18. Lichtensztajn DY, Giddings BM, Morris CR, et al. Comorbidity index in central cancer registries: The value of hospital discharge data. Clin Epidemiol 2017;9:601–609; doi: 10.2147/clep.S146395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Younger E, Litière S, Le Cesne A, et al. Outcomes of elderly patients with advanced soft tissue sarcoma treated with first-line chemotherapy: A pooled analysis of 12 EORTC soft tissue and bone sarcoma group trials. Oncologist 2018;23(10):1250–1259; doi: 10.1634/theoncologist.2017-0598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gingrich AA, Marrufo AS, Liu Y, et al. Radiotherapy is associated with improved survival in patients with synovial sarcoma undergoing surgery: A National Cancer Database analysis. J Surg Res 2020;255:378–387; doi: 10.1016/j.jss.2020.05.075 [DOI] [PubMed] [Google Scholar]
- 21. Naing KW, Monjazeb AM, Li CS, et al. Perioperative radiotherapy is associated with improved survival among patients with synovial sarcoma: A SEER analysis. J Surg Oncol 2015;111(2):158–164; doi: 10.1002/jso.23780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gronchi A, Casali PG. Adjuvant therapy for high-risk soft tissue sarcoma in the adult. Curr Treat Options Oncol 2013;14(3):415–424; doi: 10.1007/s11864-013-0243-7 [DOI] [PubMed] [Google Scholar]
- 23. Canter RJ, Qin LX, Maki RG, et al. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res 2008;14(24):8191–8197; doi: 10.1158/1078-0432.Ccr-08-0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolfson JA, Sun C-L, Wyatt LP, et al. Impact of care at comprehensive cancer centers on outcome: Results from a population-based study. Cancer 2015;121(21):3885–3893; doi: 10.1002/cncr.29576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alvarez E, Spunt SL, Malogolowkin M, et al. Treatment at specialized cancer centers is associated with improved survival in adolescent and young adults with soft tissue sarcoma. J Adolesc Young Adult Oncol 2022;11(4):370–378; doi: 10.1089/jayao.2021.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the CCR. Access to the data is granted through an application process by the management or data custodians.