Abstract
The survival of Cryptosporidium parvum oocysts in soil and water microhabitats may be affected by the environmental production and release of free ammonia. The objective of this study was to determine the effects of increasing free ammonia concentrations and times of exposure on oocyst viability. Wild-type oocysts were obtained from naturally infected calf feces by chemical (continuous-flow) centrifugation and sucrose gradients. Ammonia (NH3) from a commercial solution was applied in concentrations ranging from 0.007 to 0.148 M. Exposure times ranged from 10 min to 24 h at a constant temperature of 24 ± 1°C. Viability of oocysts was determined with a dye permeability assay and an in vitro excystation assay (M. B. Jenkins, L. J. Anguish, D. D. Bowman, M. J. Walker, and W. C. Ghiorse, Appl. Environ. Microbiol. 63:3844–3850, 1997). Even the lowest concentration of ammonia decreased significantly the viability of oocysts after 24 h of exposure. Increasing concentrations of ammonia increased inactivation rates, which ranged from 0.014 to 0.066 h−1. At the highest concentration of ammonia, a small fraction of viable oocysts still remained. Exposure to pH levels corresponding to those associated with the ammonia concentrations showed minimal effects of alkaline pH alone on oocyst viability. This study shows that environmentally relevant concentrations of free ammonia may significantly increase the inactivation of oocysts in ammonia-containing environments.
Unlike other common disinfectants, ammonia is a natural product. It occurs in natural environments as a product of urea hydrolysis and of the microbial degradation of proteins and other nitrogen-containing compounds (referred to as ammonification). Significant concentrations of ammonia may be present in decomposing manure. Several studies have documented NH3 evolution from cow manure during storage (7, 11–13, 19, 21, 22), even when the pH of the manure was <6.8 (13). The pH of NH3 evolving in manure generally ranged from 7.5 (10) to 8.8 (7). Although Cryptosporidium parvum oocysts can survive many months in naturally infected manure under static conditions at 4°C (9), inactivation may be enhanced under anaerobic conditions in which ammonia persists. The objective of this study was to determine the effects on inactivation of freshly purified oocysts of environmentally relevant ammonia concentrations, ranging from 0.007 to 0.148 M, associated with manure slurries (22).
Oocyst purification.
Feces from 6- to 20-day-old Holstein calves with cryptosporidiosis were processed by a continuous-flow differential density flotation method, as described by Jenkins et al. (9). Purified oocysts were adjusted to a concentration of 107 ml−1 and stored in distilled water containing 100 U of penicillin G sodium ml−1, 100 μg of streptomycin sulfate ml−1, and 0.25 μg of amphotericin B ml−1 of oocyst suspension. The viability of each lot was estimated with a fluorochrome dye permeability assay, and, for some lots, in vitro excystation was performed as described below. Batches of purified oocysts for all experiments ranged from 3 weeks to 4 months old and ranged in viability from 90 to 95%.
Dye permeability assay.
The dye permeability assay, for determining viability and potential infectivity of oocysts from environmental water samples, has been described previously (1, 4, 9). Stock solutions of DAPI (4′-6-diamidino-2-phenylindole) (2 mg ml−1 in high-performance liquid chromatography-grade methanol) and propidium iodide (PI) (1 mg ml−1 in 0.1 M phosphate-buffered saline [PBS]) were added to aliquots of the sample in microcentrifuge tubes at a ratio of 1:10 (vol/vol); the sample was then mixed with a Vortex mixer and incubated in the dark at 37°C for 2 h. Each aliquot was stained with Hydrofluor antibody (Hydrofluor Combo; EnSys, Research Triangle Park, N.C.), washed twice with PBS, and resuspended to the original volume in 0.3 M DABCO (1,4-diazabicyclo[2.2.2]octane) in 0.1 M PBS (DABCO-PBS). The samples were stored in the dark at 4°C until being examined (within 72 h).
In vitro excystation assay.
The acid pretreatment protocol was described previously (9, 16). Each 100-μl aliquot of oocyst suspension in microcentrifuge tubes was washed once with 1 ml of acidified Hanks’ balanced salt solution (HBSS) (10 μl of 1 M HCl in 10 ml of HBSS [pH 2.5]), resuspended in 1 ml of acidified HBSS, and incubated at 37°C for 1 h in the dark. Suspensions were then sedimented by centrifugation at 11,300 × g for 30 s; the pellets were washed three times with warm (37°C) HBSS to remove any residual acid and then resuspended to 100 μl in warm HBSS. Ten microliters of 2.2% sodium bicarbonate in HBSS and 10 μl of 1% sodium deoxycholate in Hanks’ minimal essential medium (Sigma Chemical Co., St. Louis, Mo.) were added to each sample. The suspensions were then mixed on a Vortex mixer and incubated for 3.5 h at 37°C in the dark. Ten microliters of primary Hydrofluor antibody was then added to each sample, and they were mixed on a Vortex mixer and returned to the incubator for 0.5 h, for a total excystation incubation time of 4 h. The samples were then washed in 1 ml of PBS, resuspended in 100 μl of PBS, and stained with 10 μl of Hydrofluor labeling reagent. After incubating at room temperature in the dark for 0.5 h, oocysts were washed with 1 ml of DABCO-PBS, suspended in 100 μl of DABCO-PBS, and stored at 4°C until being examined (within 72 h). The percentage of excysted oocysts was determined by subtracting the number of empty oocysts observed before excystation from the number of oocysts observed after excystation (9).
Microscopy.
All samples were examined with a Zeiss LSM-210 microscope (1) in conventional DIC and epifluorescence mode with a triple excitation-emission filter set (catalog no. 61001; Chroma Technology Corp., Brattleboro, Vt.) with excitations at 390 to 410, 485 to 510, and 555 to 585 nm and emissions at 450 to 475, 510 to 550, and 595 to 660 nm and a separate UV filter combination (excitation wavelength, 310 to 395 nm). Except for enumeration studies, a Zeiss 100×/1.3 plan-neofluor objective with 10× eyepieces was used for all other microscopy.
Experimental design.
The source of ammonia for experimentation was a commercial ammonia solution containing distilled water and ammonia. The NH3 concentration was proprietary and therefore was not given. All treatments were in triplicate. Freshly purified oocysts were suspended in distilled water at a concentration to 107 ml−1. A 0.1-ml aliquot of the oocyst suspension was pipetted into 1.5-ml microcentrifuge tubes. Additional quantities of distilled water were added to the tubes and corresponded to the ammonia dilutions used as treatments: 90, 50, 25, 10, 5, 1, and 0% (vol/vol). The total volume of oocyst suspension per tube was 1 ml. The pH of each dilution was measured with a standard, calibrated calomel electrode, and the total NH4 concentration was determined by the automated phenate method at the Soil Testing Laboratory of Cornell University. The concentrations of NH3 were calculated for each dilution with the equation pOH = pKb + log ([NH4]/[NH3]) (6), where Kb is the base dissociation constant. In descending order, final concentrations were 0.148 M (2,516 mg/liter), 0.103 M (1,751 mg/liter), 0.060 M (1,020 mg/liter), 0.039 M (663 mg/liter), 0.026 M (443 mg/liter), and 0.007 M (119 mg/liter). The tubes were incubated at room temperature (24 ± 1°C) in the dark. The tubes were labeled so that analysis would be performed in a blinded fashion. Tubes were sampled after 10 min, 1 h, and 24 h of incubation. The suspensions were washed four times in PBS and resuspended in 0.1 ml of PBS. For each sampling time, one set of tubes was assayed for viability with the dye permeability assay, and one set underwent in vitro excystation. Two 10-μl subsamples per replicate sample were observed microscopically, and 100 oocysts per subsample were characterized.
To determine if the pHs of the ammonia solutions were the principle factor in oocyst inactivation, freshly purified oocysts were also exposed to three pH levels, 9, 10, and 11, which bridged the pHs of the ammonia dilutions. A 0.01 M solution of CAPS [3-(cyclohexylamino)-1-propanesulfonic acid] (Sigma) was adjusted with either HCl or NaOH to obtain each pH level. Like the ammonia experiments above, 107 oocysts suspended in 0.1 ml of distilled water were pipetted into 1.5-ml microfuge tubes. One milliliter of each CAPS solution was added. The tubes were mixed on a Vortex mixer and incubated at 24 ± 1°C (room temperature). Duplicate tubes were sampled at 10 min, 1 h, and 24 h. Both the dye permeability assay and in vitro excystation were used to assess viability, as described above. Oocysts suspended in PBS, pH 7.2, were used as controls.
To validate the rates of oocyst inactivation as determined by the results of the dye permeability assay (see Table 1), purified oocysts were exposed (as described above) to an NH3 concentration of 0.060 M for 8.2 days, the projected time to reach 99.999% inactivation (see Table 1). This concentration of NH3 was chosen in order to compare our results with a claim made by Ruxton (18). To determine if temperature is a factor in oocyst inactivation by NH3, one set of replicate samples (including controls) was incubated at 24 ± 1°C, and another set was incubated at 4°C.
TABLE 1.
Inactivation rates of C. parvum oocysts exposed to measured concentrations of ammoniaa
| [NH3] (mol/liter) | K ± 95% CI/hb | Days to reach 99.999% inactivationc |
|---|---|---|
| 0.007 | 0.014 ± 0.004 | 27 |
| 0.026 | 0.027 ± 0.007 | 14.5 |
| 0.039 | 0.050 ± 0.005 | 7.6 |
| 0.060d | 0.047 ± 0.014 | 8.2 |
| 0.104 | 0.059 ± 0.034 | 6.5 |
| 0.148 | 0.066 ± 0.030 | 5.8 |
| 3.9e | 0.384 | 1 |
Based on data from the dye permeability assay after a 24-h exposure.
It was assumed that oocyst inactivation was a first-order process. The coefficient of inactivation was determined by regressing ln (P0/Pt) against time (derived from the equation Pt = P0 · e−Kt, where P0 is the initial percentage of viable oocysts, Pt is the percentage of viable oocysts at time t, in hours, and K is the coefficient of inactivation). The 95% confidence intervals (CI) were determined by calculating the Student t value at the appropriate degree of freedom at an α level (two-sided) of 0.025.
Calculated by the equation t = ln (P0/Pt)/K.
This concentration of NH3 and exposure time were used in the validation experiment shown in Table 2.
A power function, y = 2.037x−0.522 (r2 = 0.998), that fit the regression of [NH3] against days to reach 99.999% inactivation was used to determine the concentration of ammonia that would reduce the viability of oocysts by 99.999% in 1 day. The K value for this concentration of ammonia was then derived.
Statistical analysis.
Except where otherwise stated, all statistical analyses of data were performed with Minitab Statistical Software (Minitab Inc., State College, Pa.).
Effect of ammonia.
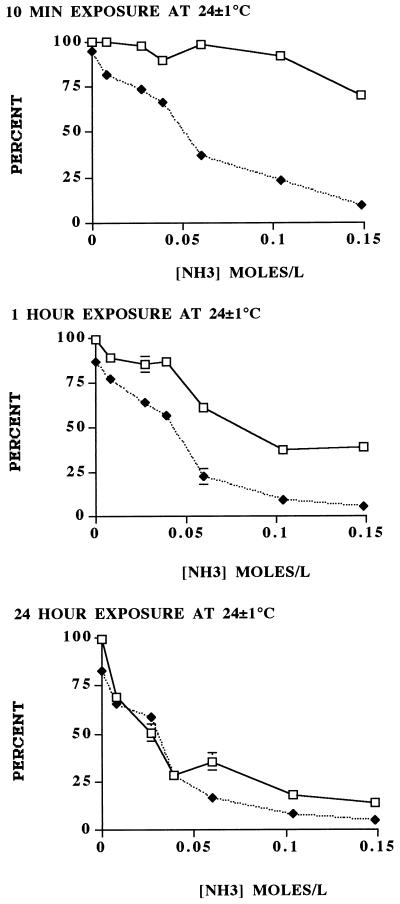
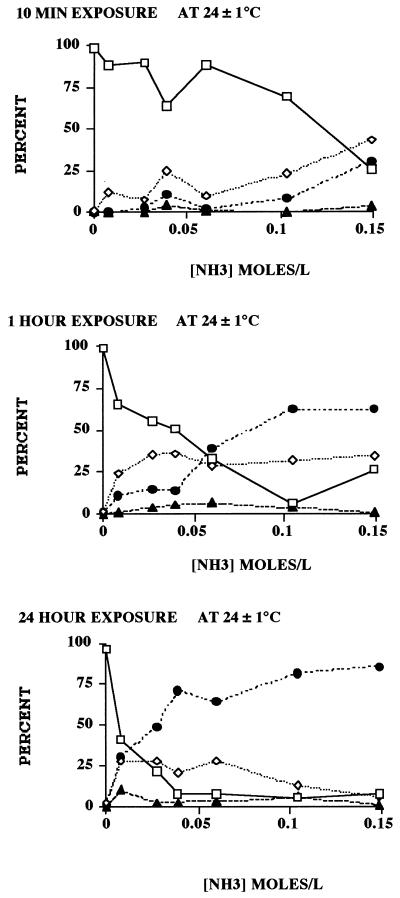
Based on the dye permeability assay, both increased time of exposure and the concentration of ammonia increased the inactivation rate of wild-type oocysts. Compared to the 1-h and 24-h exposure times, the 10-min exposure to the various concentrations showed the greatest discrepancies between the dye permeability assay and in vitro excystation, the results of which appeared not to be as time dependent as those of the dye permeability assay (Fig. 1). One-hour exposure showed better agreement, and 24-h exposure showed the best agreement, between the two assays. As previously observed (9), the dye permeability assay generally indicated a slightly higher survival rate than in vitro excystation. Results of the dye permeability assay indicated that exposure to all ammonia concentrations increased oocyst wall permeability (Fig. 2). The 10-min exposure showed generally increased frequencies of DAPI-positive, PI-negative (DAPI+ PI−) oocysts with increased ammonia concentrations. Increased time of exposure resulted in increased frequencies of both DAPI+ PI− and DAPI+ PI+ oocysts. After 1-h exposure, the frequency of DAPI+ PI− oocysts appeared to reach a maximum between 24 and 36%, after which the frequency of DAPI+ PI+ oocysts increased. A 24-h exposure to 0.007 M ammonia at room temperature affected the permeability of the oocyst wall in more than 50% of the purified population; the proportion of DAPI+ PI+ (nonviable) oocysts was slightly less than the proportion of DAPI+ PI− (potentially viable and/or infective) oocysts (Fig. 2). The progression of increased permeability of the oocyst wall from impermeable (DAPI− PI−) to partially permeable (DAPI+ PI−) to lethally permeable (DAPI+ PI+) has been reported previously in relationship to increasing temperatures (9). The data indicated that increased times of exposure to the lowest concentrations of ammonia led to increases in the frequency of inactive (DAPI+ PI+) oocysts. Exposure to greater concentrations of ammonia also increased the percentage of inactivated oocysts. With the increased frequency of these inactivated oocysts, the correspondence between viability, as determined by the dye permeability assay (9), and the frequency of excysted oocysts, as determined by the in vitro excystation assay, increased. The principle difference between the two viability assays at the 1-h exposure was attributable to the number of partially permeable (DAPI+ PI−) oocysts, which are considered viable and potentially infective (1, 4, 9). Untreated oocyst controls that paralleled these experiments showed no significant increase in oocyst wall permeability under the same conditions of time and temperature.
FIG. 1.
Viability of C. parvum oocysts exposed for 10 min, 1 h, and 24 h at 24 ± 1°C to various concentrations of ammonia (0.007, 0.026, 0.039, 0.060, 0.104, and 0.148 M), based on the dye permeability assay and in vitro excystation. The sum of the impermeable (DAPI− PI−) and semipermeable (DAPI+ PI−) oocysts was the number of viable oocysts (9). Each datum point represents the mean ± standard error (SE) of at least six replicates of 100 oocysts observed per replicate. SE values not indicated are smaller than the symbols. □, viable; ⧫, excysted.
FIG. 2.
Dynamics of dye permeability of oocysts as determined by the dye permeability assay. Each point corresponds to, and is from the same experiments as, the viability determinations of oocysts exposed to various concentrations of ammonia for 10 min, 1 h, and 24 h at 24 ± 1°C. Each datum point represents the mean ± standard error (SE) of at least six replicates of 100 oocysts observed per replicate. SE values not indicated are smaller than the symbols. □, DAPI− PI−; ◊, DAPI+ PI−; •, DAPI+ PI+; ▴, empty.
Based on the results of the dye permeability assay for each ammonia concentration, rates of oocyst inactivation were determined by using a first-order kinetic model (Table 1). With the determination of these inactivation rates (K values) for each ammonia concentration, the time it took to reach 99.999% inactivation was calculated (Table 1). Regression analysis of the ammonia concentration against the number of days required to reach 99.999% inactivation led to a power function that best fit (r2 = 0.998) the regressed data. This power function (Table 1) indicated that it would take an ammonia concentration of 3.9 M to inactivate 99.999% of the freshly purified oocysts in 24 h.
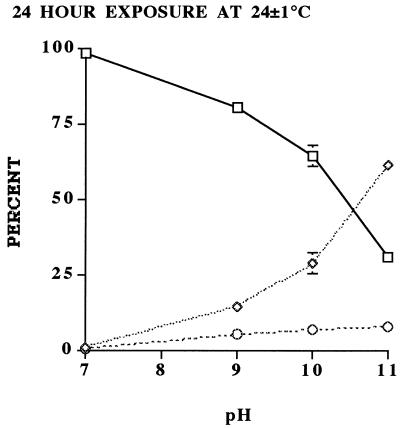
Effect of high pH on oocyst viability.
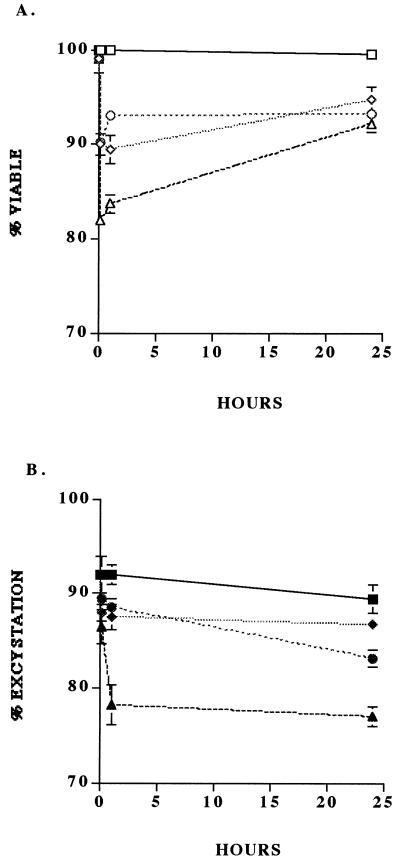
As indicated by the increased frequency of DAPI+ PI− oocysts, there was a change in the permeability status of the oocyst wall with increasing pH (Fig. 3). However, there was only minor change in viability status, as indicated by the minimal change in frequency of DAPI+ PI+ oocysts. The in vitro excystation assay also showed little significant change in percent excystation with increasing pH (Fig. 4). These data strongly suggest that the high pH levels associated with ammonia solutions may have primarily time-dependent effects on oocyst wall permeability, whereas free ammonia, which has previously been reported to be lipid soluble and able to penetrate biological membranes (17), is able to permeate immediately the oocyst wall and sporozoite membranes and inactivate the sporozoites and/or enzymes involved in excystation. Based on the model proposed by Robin (17), the equilibrium equation NH3 − NH4+ (6), and the nonpolar nature of NH3, we hypothesize that an increased interoocyst NH3 concentration raises the internal pH of the oocyst to deleterious levels. Because the components of pH, viz. H+ and OH−, are charged, they remain external to the oocyst wall, thus explaining the inability of pH alone to inactive oocysts. The differential action of the elevated pH and free ammonia concentration on the oocyst may account for the initial differences (i.e., at 10-min exposure) between the dye permeability assay and in vitro excystation (Fig. 1). Increased oocyst wall permeability at elevated pHs associated with ammonia production would also lead to increased susceptibility to other forms of inactivation or disinfecting agents (15).
FIG. 3.
Results of the dye permeability assay on oocysts exposed to various pH levels (7, 9, 10, and 11) corresponding to the various concentrations of ammonia and incubated at 24 ± 1°C for 24 h. The sampling scheme and statistical analysis were the same as described in the legend to Fig. 1. □, DAPI− PI−; ◊, DAPI+ PI−; ○, DAPI+ PI+.
FIG. 4.
Results of the dye permeability (A) and in vitro excystation (B) assays on oocysts exposed to various pH levels (7, 9, 10, and 11, corresponding to the various concentrations of ammonia) and incubated at 24 ± 1°C for 10 min, 1 h, and 24 h. The sampling scheme and statistical analysis were the same as described in the legend to Fig. 1. Squares, pH 7; diamonds, pH 9; circles, pH 10; triangles, pH 11.
Our results demonstrate that exposure to low concentrations of free ammonia in solution can have a deleterious effect on the survival of C. parvum oocysts. Fayer et al. (8) reported that oocysts suspended in water that were exposed to an atmosphere of pure ammonia at room temperature (21 to 23°C) for 24 h were totally inactivated, based on animal infectivity assays. At room temperature, the solubility of gaseous ammonia is 32% (wt/wt) (10) or ∼17.8 M. Two previous studies demonstrated the lethal effects of a 5% solution of Parsons’ Ammonia (Armour-Dial) (∼0.04 M) on oocyst viability (5, 20). Ransome et al. (14) reported that a 1-h exposure at 10°C to 0.008 M ammonia resulted in an 85.6% reduction in in vitro excystation compared to a control (excystation rate not stated), and Blewett (2) reported that a 30-min exposure at 22°C to a 0.009 M solution of ammonia reduced excystation by 55%; both of these reductions in excystation were greater than the reductions in excystation that we observed for 0.007 M and 0.026 M NH3 at 24°C for 24 h (Fig. 1). Ruxton (18) reported unpublished data suggesting that a 24-h exposure to a 0.1% solution (0.06 M) would completely inactivate oocysts. Our results showed that a 24-h exposure to 0.06 M ammonia inactivated between 64.5% (based on the dye permeability assay) and 83.7% (based on in vitro excystation) of the oocysts. Based on our kinetic analysis, exposure to 0.060 M ammonia would inactivate 99.999% of the freshly purified oocysts in 8.2 days (Table 1).
The data in Table 2 validated the inactivation rate coefficient (K) at an NH3 concentration of 0.060 M and at a constant temperature of 24 ± 1°C; the calculated K value based on these data is 0.043 h−1, which is within the 95% confidence interval as indicated in Table 1. The rate of inactivation for oocysts exposed to 0.060 M NH3 at 4°C was significantly less, however, at 0.0087 h−1, a K value that indicates a hypothetical 26.5 days to reach 99.999% inactivation. These data indicate that temperature is a significant factor in the inactivation of oocysts by NH3, confirming the broad generalization that temperature is an important environmental factor affecting oocyst permeability (9). It further supports previous observations that temperature affects the permeability of the oocyst wall; at temperatures near freezing (4°C), oocysts are more impermeable to solutes like nonpolar NH3 than at higher temperatures. The viability of the controls was not significantly different from one another or from the viability of the batch at time zero (90.8).
TABLE 2.
Validation experiment showing the results of the dye permeability assay on purified C. parvum oocysts exposed to 0.060 M NH3 for 8.2 days
| Temp (°C) | Treatment | % of oocystsa
|
||||
|---|---|---|---|---|---|---|
| DAPI− PI− | DAPI+ PI− | DAPI+ PI+ | Empty | Viable | ||
| 24 | NH3 | 1.3 ± 0.7 | 90.3 ± 1.0 | 8.3 ± 0 | 1.3 ± 1.1 | |
| None (control) | 86.5 ± 1.6 | 1.7 ± 0.6 | 11.3 ± 1.2 | 0.5 ± 0.5 | 88.2 ± 1.1 | |
| 4 | NH3 | 7.2 ± 0.5 | 31.7 ± 1.7 | 58.7 ± 1.8 | 2.3 ± 0.7 | 38.8 ± 1.7 |
| None (control) | 86.2 ± 1.0 | 2.8 ± 0.5 | 10.5 ± 1.3 | 0.5 ± 0.3 | 89.0 ± 1.2 | |
Data are means ± standard errors of replicate samples and duplicate subsamples (100 oocysts counted and characterized per subsample) per replicate per treatment. The total number of oocysts characterized was 600 per treatment.
Previous studies did not report on temperature affecting the effectiveness of ammonia to inactivate Cryptosporidium oocysts (2, 3, 8, 14, 20). The data in Table 2 indicate explicitly that oocysts exposed to NH3 at temperatures around 25°C will be inactivated more quickly than at temperatures close to freezing. Manure piles, for example, can warm up to 30°C and then, as in winter, cool to an ambient atmospheric temperature of 5°C (9a), thus changing the effectiveness of NH3 on the inactivation of oocysts.
Many municipal watersheds contain non-point sources of C. parvum oocysts, such as dairies. Neonatal calves are very commonly infected with C. parvum, and a single infected calf can shed a few billion oocysts in the few days of its infection. Several studies have indicated that the nitrogen content of cow manure is lost to the atmosphere as gaseous ammonia (3, 7, 11, 12, 19). Although loss of nitrogen as gaseous ammonia has been considered a loss of an important plant nutrient, the evolution of ammonia in barnyard manure may be used to inactivate C. parvum oocysts, as suggested by Ruxton (18). Whitehead and Raistrick (22) reported an increase of ammonia in a slurry of cattle manure over a 3-week period, from an initial concentration of 0.05 M to >0.2 M. Based on our results, exposure to such concentrations of ammonia would significantly reduce the number of viable oocysts even at cool temperatures, given longer times of exposure.
Although the concentration of ammonia is pH dependent, our study shows that the pH associated with various concentrations of ammonia was not a factor in oocyst inactivation but rather tended to affect the permeability of oocyst walls, thus suggesting an indirect effect of pH on oocyst wall permeability. It appears to be the chemical activity of free ammonia, and its ability to penetrate the oocyst wall and sporozoite membranes, that leads directly to oocyst inactivation.
Acknowledgments
This research was supported in part by the Center for Advanced Technology, sponsored by the New York State Science and Technology Foundation.
We are grateful to M. Frongillo and K. Wallace for purifying oocysts and to L. Anthony for laboratory assistance. The expert secretarial assistance of Patti Durfey is gratefully acknowledged.
REFERENCES
- 1.Anguish L J, Ghiorse W C. Computer-assisted laser scanning and video microscopy for analysis of Cryptosporidium parvum oocysts in soil, sediment, and feces. Appl Environ Microbiol. 1997;63:724–733. doi: 10.1128/aem.63.2.724-733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blewett D A. Disinfection of oocysts. In: Angus K W, Blewett D A, editors. Cryptosporidiosis. Edinburgh, United Kingdom: Animal Disease Research Association; 1988. pp. 107–116. [Google Scholar]
- 3.Buijsman E, Maas H F M, Asman W A H. Anthropogenic NH3 emissions in Europe. Atmos Environ. 1987;21:1009–1022. [Google Scholar]
- 4.Campbell A T, Robertson L J, Smith H V. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl Environ Microbiol. 1992;58:3488–3493. doi: 10.1128/aem.58.11.3488-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell I, Tzipori S, Hutchison G, Angus K W. Effect of disinfectants on survival of Cryptosporidium oocysts. Vet Rec. 1982;111:414–415. doi: 10.1136/vr.111.18.414. [DOI] [PubMed] [Google Scholar]
- 6.Christian G D. Analytical chemistry. New York, N.Y: John Wiley & Sons; 1980. [Google Scholar]
- 7.Dewes T, Schmitt L, Valentin U, Ahrens E. Nitrogen losses during the storage of liquid livestock manures. Biol Wastes. 1990;31:241–250. [Google Scholar]
- 8.Fayer R, Graczyk T K, Cranfield M R, Trout J M. Gaseous disinfection of Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1996;62:3908–3909. doi: 10.1128/aem.62.10.3908-3909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins M B, Anguish L J, Bowman D D, Walker M J, Ghiorse W C. Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1997;63:3844–3850. doi: 10.1128/aem.63.10.3844-3850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Jenkins, M. B., et al. Unpublished data.
- 10.Merck & Co., Inc. The Merck index. Whitehouse Station, N.J: Merck & Co., Inc.; 1996. [Google Scholar]
- 11.Muck R E, Richards B K. Losses of manurial nitrogen in free-stall barns. Agric Wastes. 1983;7:65–79. [Google Scholar]
- 12.Muck R E, Steenhuis T S. Nitrogen loss from manure storages. Agric Wastes. 1982;4:41–54. [Google Scholar]
- 13.Patni N K, Jui P Y. Nitrogen concentration variability in dairy-cattle slurry stored in farm tanks. Trans Am Soc Agric Eng. 1991;34:609–615. [Google Scholar]
- 14.Ransome M E, Whitmore T N, Carrington E G. Effect of disinfectants on the viability of Cryptosporidium parvum oocysts. Water Supply. 1993;11:103–117. [Google Scholar]
- 15.Robertson L J, Campbell A T, Smith H V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl Environ Microbiol. 1992;58:3494–3500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson L J, Campbell A T, Smith H V. In vitro excystation of Cryptosporidium parvum. Parasitology. 1993;106:13–19. doi: 10.1017/s003118200007476x. [DOI] [PubMed] [Google Scholar]
- 17.Robin E D. Of men and mitochondria—intracellular and subcellular acid-base relations. N Engl J Med. 1961;265:780–785. doi: 10.1056/NEJM196110192651605. [DOI] [PubMed] [Google Scholar]
- 18.Ruxton G D. Mathematical modelling of ammonia volatilization from slurry stores and its effects on Cryptosporidium oocyst viability. J Agric Sci. 1995;124:55–60. [Google Scholar]
- 19.Sommer S G, Christensen B T, Nielsen N E, Schjorring J K. Ammonia volatilization during storage of cattle and pig slurry: effect of surface cover. J Agric Sci. 1993;121:63–71. [Google Scholar]
- 20.Sunderman C A, Lindsay D S, Blagburn B L. Evaluation of disinfectants for ability to kill avian Cryptosporidium oocysts. Companion Anim Pract. 1987;1:36–39. [Google Scholar]
- 21.Termeer W C, Warman P R. Use of mineral amendments to reduce ammonia losses from dairy cattle and chicken-manure slurries. Bioresour Technol. 1993;44:217–222. [Google Scholar]
- 22.Whitehead D C, Raistrick N. Nitrogen in the excreta of dairy cattle: changes during short-term storage. J Agric Sci. 1993;121:73–81. [Google Scholar]