Abstract
Background: Scarce data exist that analyze the outcomes of hematological patients with SARS-CoV-2 infection during the Omicron variant period who received treatment with remdesivir or nirmatrelvir/ritonavir. Methods: This study aims to address this issue by using a retrospective observational registry, created by the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group, spanning from 27 December 2021 to 30 April 2023. Results: This study included 466 patients, 243 (52%) who were treated with remdesivir and 223 (48%) with nirmatrelvir/ritonavir. Nirmatrelvir/ritonavir was primarily used for mild cases, resulting in a lower COVID-19-related mortality rate (1.3%), while remdesivir was preferred for moderate to severe cases (40%), exhibiting a higher mortality rate (9%). A multivariate analysis in the remdesivir cohort showed that male gender (odds ratio (OR) 0.35, p = 0.042) correlated with a lower mortality risk, while corticosteroid use (OR 9.4, p < 0.001) and co-infection (OR 2.8, p = 0.047) were linked to a higher mortality risk. Prolonged virus shedding was common, with 52% of patients shedding the virus for more than 25 days. In patients treated with remdesivir, factors associated with prolonged shedding included B-cell malignancy as well as underlying disease, severe disease, a later onset of and shorter duration of remdesivir treatment and a higher baseline viral load. Nirmatrelvir/ritonavir demonstrated a comparable safety profile to remdesivir, despite a higher risk of drug interactions. Conclusions: Nirmatrelvir/ritonavir proved to be a safe and effective option for treating mild cases in the outpatient setting, while remdesivir was preferred for severe cases, where corticosteroids and co-infection significantly predicted worse outcomes. Despite antiviral therapy, prolonged shedding remains a matter of concern.
Keywords: remdesivir, nirmatrelvir/ritonavir, molnupiravir SARS-CoV-2 vaccines, Omicron, respiratory virus, hematological malignancies, allogeneic stem cell transplantation, autologous stem cell transplantation, COVID-19, mRNA vaccine, immunocompromised patients
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has revealed the heightened vulnerability of immunocompromised patients, especially those with hematological disorders, leading to increased morbidity and mortality compared to healthier individuals or patients with solid tumors [1,2]. However, the advancements in medical interventions, including supportive care, vaccination and antiviral drugs, have significantly decreased COVID-19 mortality rates from the first waves (>25%) [2,3] to the Omicron variant of concern (VOC) period (<2%) [4,5,6]. Interestingly, this positive trend was observed even before the introduction of SARS-CoV-2 vaccines and antiviral drugs in hematopoietic stem cell transplant recipients [7], also supporting the contribution of less severe emergent SARS-CoV-2 VOCs.
Direct-acting antiviral drugs such as remdesivir [8], VV116 [9] azvudine [10], nirmatrelvir/ritonavir [11] and, to a lesser extent, molnupiravir [12], have proven to reduce hospitalization, severe disease and death in the general population. Unlike vaccines and monoclonal antibodies, the antiviral activity of these drugs remains unaffected by the mutational evolution of SARS-CoV-2 VOCs [13], making them valuable tools against COVID-19, especially for immunosuppressed individuals who respond poorly to vaccination.
Despite a lack of clinical trials specifically evaluating the safety and efficacy of these antiviral drugs in immunosuppressed patients, national health authorities have approved their use and scientific societies have provided treatment recommendations for this vulnerable population [14]. For instance, nirmatrelvir/ritonavir is suggested for outpatient cases with asymptomatic or mild–moderate COVID-19, while remdesivir is preferred in moderate to critical cases, particularly for patients with pneumonia and oxygen requirements [14].
Understanding the safety and effectiveness of antiviral drugs in treating COVID-19 in immunocompromised patients is critical for optimizing treatment strategies and improving patient outcomes. To date, very few data exist on the safety and effectiveness of these antiviral drugs in treating hematological patients with SARS-CoV-2 infection or the underlying mechanisms that may influence their efficacy and tolerability [15,16,17,18].
In this context, the current multicenter, nationwide study assesses the outcomes of a large series of hematological patients with SARS-CoV-2 infection during the Omicron VOC period, who received treatment with remdesivir or nirmatrelvir/ritonavir in accordance with existing guidelines [14]. This study also investigates factors associated with poor outcomes and prolonged SARS-CoV-2 shedding. This research is based on a retrospective observational registry created by the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC).
2. Patients and Methods
2.1. Study Population
In April 2023, the Infectious Complications Subcommittee (GRUCINI) of the GETH-TC launched a national retrospective multicenter registry that evaluated the outcome of Omicron VOC SARS-CoV-2 infection in immunocompromised patients with hematological diseases (HDs), including cell therapy recipients. The registry included consecutive HD patients with Omicron SARS-CoV-2 infection diagnosed either via PCR or rapid antigen tests. The status of all included patients was updated until 10 July 2023. The local Research Ethical Committee of the Hospital Clínico Universitario of Valencia approved the study protocol (reference code 35.21).
2.2. Inclusion Criteria, Variables of Interest and Clinical and Virological Monitoring
To assess the severity of epidemiologically defined Omicron VOC SARS-CoV-2 infections, the inclusion criteria were consecutive hematological patients with SARS-CoV-2 infection with clinical symptoms or asymptomatic cases who received antiviral therapy from 27 December 2021 to 30 May 2023. Variables of interest included demographics, base-line hematological disease characteristics, vaccination status before infection, clinical, virological and biological features of each SARS-CoV-2 infection episode (i.e., molecular or clinical resolution date); and data related to antiviral drugs such as schedule, duration, discontinuations, dose modifications and toxicities grade ≥3 according to the Common Toxicity Criteria (CTC). In this series, 53% of remdesivir-treated patients and 55% of those treated with nirmatrelvir/ritonavir were monitored on a weekly or biweekly basis until PCR negativity.
2.3. Definitions
Although we did not sequence SARS-CoV-2 strains in any case, the inference of the Omicron VOC was based on the Spanish sequencing epidemiological data, which started from 27 December 2021. The usage of corticosteroids was categorized into two main groups: individuals who were already receiving corticosteroid treatment at the time of SARS-CoV-2 infection and those who were administered corticosteroids as an anti-inflammatory therapy to manage severe COVID-19 cases. Complete vaccination schedules were defined as at least three vaccine doses (except COVID-19 Vaccine Janssen®, which requires at least 2 doses). Incomplete vaccination was considered when 2 or less doses were given before SARS-CoV-2 infection. SARS-CoV-2 infection was defined as molecular (PCR test) or antigenic evidence of SARS-CoV-2 infection. COVID-19 severity includes mild (no pneumonia nor oxygen support), moderate (pneumonia without oxygen support) and severe (pneumonia and oxygen support). Prolonged shedding was defined as persistent PCR positivity after 25 days from the first detection. The duration of COVID-19 was defined as the time from first detection until the first PCR negativity, or until symptom resolution in the case of no PCR monitoring. The length of SARS-CoV-2 shedding after treatment was calculated from the date of antiviral therapy until PCR negativity in those with available data. Co-infection was defined as a significant co-pathogen that was detected in concurrent nasopharyngeal or other body sites (including lungs, urine, blood or stools) that required antimicrobial intervention during SARS-CoV-2 infection and until its clinical and/or microbiological resolution. Co-infections also included the detection of community acquired respiratory viruses (CARVs) in the same sample as that of SARS-CoV-2. Regarding antiviral SARS-CoV-2 therapy, we considered a second antiviral course when a minimum break of at least 5 days was observed between courses to distinguish it from continuous and/or extended therapy. None of the patients in this current study received concomitant treatment with remdesivir plus nirmatrelvir/ritonavir.
2.4. Endpoints and Statistical Analysis
The primary objective of this study is to describe how and in what context antivirals have been prescribed, the clinical outcome of the treated patients and what factors are associated with these outcomes. Secondary end-points include the evaluation of prolonged shedding and to elucidate factors related with this condition, as well as to estimate the antiviral effect on viral burden through PCR cycle threshold (Ct) kinetics analysis. We also analyze the effect of other authorized therapies on SARS-CoV-2 infection outcomes, as well as safety issues related to antiviral drugs.
The main patient characteristics were reported using descriptive statistics on the total available information; medians and ranges were used for continuous variables, while absolute and percentage frequencies were used for categorical variables. For comparisons between antiviral cohorts, a Fisher exact test, Mann–Whitney’s U test or a median test were used when appropriate. Univariate and multivariate analyses of risk factors for SARS-CoV-2-related mortality were calculated using logistic regression models. A median test analysis to check the conditions that were potentially associated with prolonged shedding was carried out for each antiviral cohort in patients with available PCR monitoring data. A p-value < 0.05 was considered statistically significant. All p-values are two-sided. Analyses were performed using the statistical software SPSS v. 25(IBM SPSS Statistics, Armonk, New York, NY, USA).
3. Results
3.1. Patient Characteristics
The patient characteristics are summarized in Table 1. This study includes two cohorts: one treated with remdesivir (n = 243) and the other with nirmatrelvir/ritonavir (n = 223). Patients in both cohorts had a median age of 65 years (range 19–92), with a higher proportion of males in both groups. Baseline diseases varied among patients, with B-cell non-Hodgkin lymphoma (NHL) being the most common in both cohorts. The majority of patients in both cohorts experienced COVID-19 within 6 months of their last therapy. Anti-CD20 therapy was given in 31% and 40% of patients in the remdesivir and nirmatrelvir/ritonavir cohorts, respectively, whereas corticosteroids at the time of infection comprised over 20% of patients in both cohorts. The only significant differences in the baseline clinical characteristics between both cohorts were the vaccination status, with complete vaccination (at least three doses) being more common in the nirmatrelvir/ritonavir cohort (77% vs. 64%, p = 0.003) as well as a higher rate of previous pulmonary disease in the remdesivir cohort (14% vs. 7%, p = 0.05). The median follow-up for survivors after SARS-CoV-2 infection was 150 days (range 25–549).
Table 1.
Patients’ characteristics.
| Characteristics | Remdesivir Cohort (n = 243) |
Nirmatrelvir/Ritonavir Cohort (n = 223) |
p Value |
|---|---|---|---|
| Age (years), median (range) | 64 (19–92) | 65 (19–90) | 0.4 |
| • 0–40 years, n (%) | 18 (8) | 19 (9) | 0.28 |
| • 41–60 years, n (%) | 86 (35) | 66 (30) | |
| • 61–70 years, n (%) | 49 (20) | 60 (27) | |
| • >71 years, n (%) | 90 (37) | 78 (35) | |
| Male, n (%) | 150 (62) | 131 (59) | 0.63 |
| Baseline disease, n (%) | 0.34 | ||
| • AML | 41 (17) | 26 (12) | |
| • ALL | 9 (4) | 6 (3) | |
| • MDS | 21 (9) | 13 (6) | |
| • CMPD | 8 (3) | 7 (3) | |
| • B-cell NHL | 78 (32) | 88 (40) | |
| • T-cell NHL | 7 (3) | 2 (1) | |
| • CLL | 20 (8) | 12 (5) | |
| • Plasmatic cell disorder | 38 (16) | 57 (25.5) | |
| • HD | 12 (5) | 11 (5) | |
| • AA or others | 6 (2) | 1 (0.5) | |
| Time from last therapy to COVID-19 | 0.61 | ||
| • <6 months | 182 (75) | 158 (71) | |
| • 6–12 months | 19 (8) | 20 (9) | |
| • >12 months or not treated | 42 (17) | 45 (20) | |
| Anti-CD 20 and time from to COVID-19, n (%) | 74 (31) | 88 (40) | 0.13 |
| • <6 months | 56 (24) | 68 (31) | |
| • 6–12 months | 6 (3) | 3 (1.5) | |
| • >12 months or not treated | 12 (5) | 17 (8) | |
| Cell therapy, n (%) | 94 (39) | 81 (36) | 0.63 |
| - ASCT, n (%) | 36 (15) | 41 (18) | |
| - Allo-SCT donor, n (%) | 46 (19) | 25 (11) | |
| • HLA identical sibling | 21 (9) | 8 (3.6) | |
| • Unrelated Donor | 12 (5) | 9 (4) | |
| • Haplo-identical family donor | 12 (5) | 8 (3.6) | |
| • UCBT | 1 (0.5) | 0 | |
| - CAR-T type, n (%) | 12 (5) | 15 (7) | |
| • Axi-cell | 6 | 6 | |
| • Tisa-cell | 1 | 2 | |
| • Anti-BCMA | 1 | 1 | |
| • ARI-001 (anti-CD19) | 4 | 5 | |
| Corticosteroids at the time of COVID-19, n (%) | 68 (28) | 48 (22) | 0.1 |
| Number of vaccine doses, n (%) | 0.017 | ||
| • 0, n (%) | 22 (9) | 14 (6) | |
| • 1, n (%) | 8 (3) | 8 (4) | |
| • 2, n (%) | 56 (23) | 29 (13) | |
| • 3, n (%) | 87 (36) | 92 (41) | |
| • >3, n (%) | 70 (29) | 80 (36) | |
| Vaccination status ©, n (%) | 0.003 | ||
| • Incomplete | 86 (36) | 51 (23) | |
| • Complete | 157 (64) | 172 (77) | |
| Tixagevimab/cilgavimab pre-exposure prophylaxis n (%) | 4 (1.6) | 8 (3.6) | 0.25 |
| Pulmonary/cardiovascular RF, n (%) | |||
| • Active smoking | 21 (9) | 21 (9) | 0.87 |
| • Arterial hypertension | 105 (43) | 79 (35) | 0.2 |
| • Cardiomyopathy | 49 (20) | 30 (14) | 0.14 |
| • Pulmonary disease | 33 (14) | 15 (7) | 0.05 |
| Overall mortality, n (%) | 50 (21) | 20 (9) | <0.001 |
| Median F/U after COVID-19, days (range) | 119 (3–549) | 136 (5–539) | 0.4 |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; cMPD, chronic myeloproliferative disease; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; HD, Hodgkin lymphoma; AA, aplastic anemia; ASCT, autologous hematopoietic stem cell transplantation; allo-SCT, allogeneic hematopoietic stem cell transplantation; UCBT, umbilical cord blood transplantation; CAR-T, chimeric antigen receptor T-cell therapy; BCMA, B-cell maturation antigen; ARI-001, academic CART-T; F/U, follow-up. © Complete vaccination was defined as at least 3 vaccine doses, whereas incomplete include 2 or less.
3.2. Characteristics of SARS-CoV-2 Infection and Efficacy According to the Type of Antiviral Used
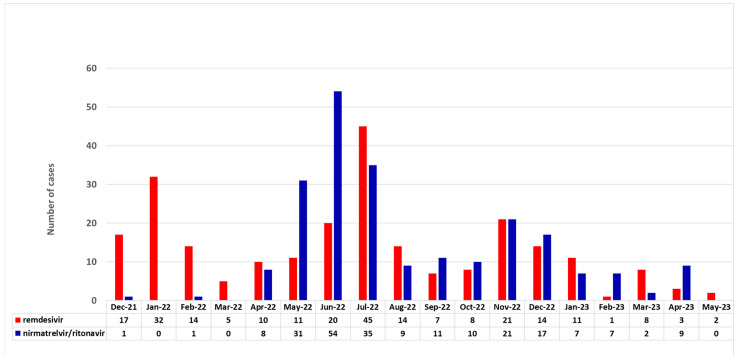
The clinical characteristics of SARS-CoV-2 infection are summarized in Table 2. The distribution of cases during the study period according to the antiviral drug are provided in Figure 1. The remdesivir cohort had a higher proportion of patients diagnosed using PCR compared to the nirmatrelvir/ritonavir cohort (90% vs. 60%, p < 0.001). The symptoms and severity of COVID-19 significantly varied between the cohorts, with the nirmatrelvir/ritonavir group showing a higher percentage of milder disease cases. Fever, respiratory symptoms, hospital admission, pneumonia and the need for oxygen support were significantly more prevalent in the remdesivir cohort (p < 0.001 for all comparisons). The duration of antiviral therapy also differed, with most nirmatrelvir/ritonavir patients (91%) receiving five days of treatment. However, 69 out of 466 (15%) (54 in the remdesivir group (22%) and 15 in the nirmatrelvir/ritonavir group, (7%)), received longer antiviral therapy. Co-infections were more common in the remdesivir cohort (p < 0.001). The nirmatrelvir/ritonavir group showed a higher rate of COVID-19 recovery at the last follow-up and a shorter median time to PCR negativity compared to the remdesivir cohort. Moreover, the nirmatrelvir/ritonavir cohort had a significantly lower COVID-19-related death rate (1.3% vs. 9%, p < 0.001) and fewer ICU admissions than the remdesivir group (0.5% vs. 6%, p < 0.001).
Table 2.
Clinical and laboratory characteristics of SARS-CoV-2 infection.
| Characteristics | Remdesivir Cohort (n = 243) |
Nirmatrelvir/Ritonavir Cohort (n = 223) |
p Value |
|---|---|---|---|
| Diagnostic test, n (%) | <0.001 | ||
| • PCR | 218 (90) | 135 (60) | |
| • Antigen-test-based | 25 (10) | 88 (40) | |
| Asymptomatic, n (%) | 2 (1) | 6 (3) | 0.1 |
| Fever, n (%) | 127 (52) | 71 (32) | <0.001 |
| Respiratory symptoms, n (%) | 172 (71) | 107 (48) | <0.001 |
| Pneumonia, n (%) | 97 (40) | 14 (6) | <0.001 |
| COVID-19-related hospital admission, n (%) | 118 (49) | 19 (9) | <0.001 |
| Oxygen support, n (%) | 69 (28) | 5 (2) | <0.001 |
| COVID-19 severity, n (%) | <0.001 | ||
| • Mild | 146 (60) | 209 (94) | |
| • Moderate | 28 (12) | 9 (4) | |
| • Severe | 69 (28) | 5 (2) | |
| Median time from dx to ATV therapy, days (range) | 1 (0–57) | 1 (0–140) | |
| • <5 days, n (%) | 190 (78) | 203 (91) | <0.001 |
| • ≥5 days, n (%) | 53 (22) | 20 (9) | <0.001 |
| Median duration of ATV therapy, days (range) | 5 (1–19) | 5 (2–15) | |
| • <5 days, n (%) | 89 (37) | 4 (2) | <0.001 |
| • 5 days, n (%) | 100 (41) | 204 (91) | <0.001 |
| • >5 days, n (%) | 54 (22) | 15 (7) | <0.001 |
| Co-infection, n (%) | 46 (19) | 9 (4) | <0.001 |
| • Bacterial | 35 (14) | 5 (2) | |
| • viral | 6 (3) | 3 (1.5) | |
| • Fungal | 5 (2) | 1 (0.5) | |
| Other COVID-19 therapies, n (%) | <0.001 | ||
| • Sotrovimab | 43 (19) | 7 (3) | |
| • Tixagevimab/cilgavimab | 4 (2) | 0 | |
| ➢ Median onset after COVID-19, days (range) | 6 (0–129) | 62 (0–104) | <0.001 |
| • Convalescent plasma | 30 (12) | 6 (3) | <0.001 |
| • Corticosteroids as COVID-19 therapy | 79 (32) | 6 (3) | <0.001 |
| ➢ Median onset after COVID-19, days (range) | 2 (-5–46) | 8 (0–79) | <0.001 |
| Laboratory characteristics at the time of SARS-CoV-2 detection | |||
| (A) ANC < 0.5× 109/L, n/evaluable (%) | 18/144 (13) | 10/96 (10) | 0.2 |
| (B) ALC < 0.5× 109/L, n/evaluable (%) | 50/144 (35) | 30/96 (31) | 0.35 |
| • CRP > 8 IU/mL, n/evaluable, %) | 76/142 (53) | 50/90 (55) | 0.6 |
| • Ct value at diagnosis available, n (%) | 142 (58) | 104 (47) | 0.2 |
| • Ct value at diagnostic, median (range) | 20 (0–40) | 21 (9–40) | 0.8 |
| Recovery from COVID-19 at last F/U (n/evaluable, %) | 163/234 (70) | 170/173 (98) | <0.001 |
| • PCR monitoring (n/evaluable, %) | 128/243 (53) | 122/223 (55) | 0.3 |
| • PCR negativity at last F/U, n (%) | 132 (63) | 125 (69) | 0.45 |
| • Median time from diagnosis to negativity, days (range) | 31 (2–209) | 22 (2–237) | <0.001 |
| • Prolonged shedding, n/evaluable (%) | 80/128 (63) | 49/122 (40) | <0.001 |
| • COVID-19-related death, n (%) | 21 (9) | 3 (1.3) | <0.001 |
| Median time from diagnosis to death (range) | 30 (4–111) | 24 (18–77) | 0.2 |
| ICU admission, n (%) | 14 (6) | 1 (0.5) | 0.005 |
Abbreviations:, PCR, real-time polymerase chain reaction; dx, diagnosis of COVID-19; ATV, antiviral drugs; ICU, intensive care unit admission; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; CRP, C-reactive protein; PCR, polymerase chain reaction; Ct, PCR cycle threshold; F/U, follow-up; ICU, intensive care unit.
Figure 1.
Distribution of antiviral use over the study period.
3.3. Univariate and Multivariate Analyses for Mortality
Table 3 presents the results of both the univariate and multivariate analyses that were conducted to identify risk factors for SARS-CoV-2-related mortality in the remdesivir cohort, consisting of 243 patients, out of which 21 patients experienced COVID-19 mortality. Such analyses were not performed in the nirmatrelvir/ritonavir group since such events were very uncommon (n = 3).
Table 3.
Univariate and multivariate analysis of risk factors for SARS-CoV-2-related mortality in the remdesivir cohort.
| Variables | Log. Regr. COVID-19 Mortality * (n = 21 Out of 243) |
|||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| OR (95% C.I.) % (95%C.I.) |
P | OR (95% C.I.) | P | |
| Age (years), | ||||
| • <41 years | 1 | |||
| • 41–60 years | 0.6 (0.06–6.2) | 0.68 | ||
| • 61–70 years | 1.5 (0.15–14.5) | 0.7 | ||
| • >71 years | 2.87 (0.35–23) | 0.3 | ||
| Male, n (%) | 0.41 (0.17–1.06) | 0.069 | 0.35 (0.13–0.96) | 0.042 |
| Baseline disease, n (%) | ns | |||
| • AML | 1 | |||
| • ALL | - | |||
| • MDS | 1.02 (0.12–3.5) | 0.8 | ||
| • CMPD | 1.7 (0.14–21) | 0.65 | ||
| • B-cell NHL | 3.9 (0.83–18) | 0.08 | ||
| • T-cell NHL | 3.2 (0.25–41) | 0.36 | ||
| • CLL | 3.44 (0.52–22) | 0.19 | ||
| • Plasmatic cell disorder | 0.52 (0.04–6.06) | 0.6 | ||
| • HD | - | |||
| • AA or others | - | |||
| Time from last therapy to COVID-19 | ||||
| • <6 months | 1 | |||
| • 6–12 months | 2.08 (0.54–7.9) | 0.28 | ||
| • >12 months or not treated | 0.85 (0.23–3.1) | 0.8 | ||
| Prior anti-CD20 therapy | 3.44 (1.38–8.5) | 0.008 | ns | |
| Time from anti-CD 20 to COVID-19, n (%) | ns | |||
| • No anti-CD20 | 1 | |||
| • <6 months | 2.47 (0.87–6.9) | 0.08 | ||
| • 6–12 months | 17.6 (3.12–100) | 0.001 | ||
| • >12 months or not treated | 3.5 (0.6–18.5) | 0.13 | ||
| Corticosteroids at the time of COVID-19 | 1.03 (0.38–2.7) | 0.9 | ||
| Procedure | ||||
| • Non-SCT | 0.88 (.27–3.2) | 0.8 | ||
| • Allo-SCT | 1 | |||
| • ASCT | 1.2 (0.06–5.6) | 0.8 | ||
| • CAR-T | 1.83 (0.25–13) | 0.54 | ||
| Vaccination status© | ||||
| • Complete | 1 | |||
| • Incomplete | 1.1 (0.45–2.85) | 0.78 | ||
| Pulmonary/cardiovascular risk factors, n (%) | ||||
| • Active smoking | 0.5 (0.06–3.9) | 0.5 | ||
| • Arterial hypertension | 2.8 (1.1–7.2) | 0.032 | ns | |
| • Cardiomyopathy | 3.3 (1.3–8.5) | 0.011 | ns | |
| • Pulmonary disease | 2.8 (1.01–7.8) | 0.05 | ns | |
| Other COVID-19 therapies | ||||
| • Convalescent plasma | 0.33 (0.04–2.5) | 0.29 | ||
| • Monoclonal antibodies | 0.99 (0.31–3.1) | 0.9 | ||
| • Corticosteroids | 11 (3.55–33.8) | <0.0001 | 9.4 (2.9–30.2) | <0.001 |
| Co-infection | 4.59 (1.8–11.6) | 0.001 | 2.8 (1.01–7.7) | 0.047 |
| ATV onset | ||||
| • <5 days | 1 | |||
| • ≥5 days | 1.48 (0.54–4.04) | 0.43 | ||
| Duration of remdesivir | ||||
| • <5 days | 1.13 (0.4–3.1) | 0.8 | ||
| • 5 days | 1 | |||
| • >5 days | 1.17 (0.36–3.78) | 0.78 | ||
| Ct value at diagnosis * | ||||
| • ≥20 | 1 | |||
| • <20 | 1.35 (0.41–4.3) | 0.61 | ||
| CRP > 8 UI/mL * | 1.8 (0.5–5.2) | 0.9 | ||
| ALC < 0.5 × 109/L * | 0.74 (0.13–3.9) | 0.7 | ||
| ANC < 0.5 × 109/L * | 1.1 (0.13–10.2) | 0.9 | ||
Abbreviations: SCT, stem cell transplantation; ASCT, autologous hematopoietic stem cell transplantation; allo-SCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; cMPD, chronic myeloproliferative disease; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; AA, aplastic anemia; Ct, PCR cycle threshold; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; CRP, C-reactive protein; ns, not significant. * These variables have not been tested since there is a lack of data in more than 40% of cases. © Complete vaccination was defined as at least 3 vaccine doses, whereas incomplete includes 2 or less.
In the univariate analysis, variables associated with COVID-19 mortality were male gender, prior anti-CD20 therapy and timing from anti-CD20 therapy to COVID-19, pulmonary/cardiovascular risk factors, such as arterial hypertension, cardiomyopathy and pulmonary disease, the use of corticosteroids as COVID-19 therapy and co-infections. In the multivariate analysis, male gender was associated with a lower risk of mortality (odds ratio (OR) of 0.35, 95% confidence interval (C.I.) of 0.13–0.96, p = 0.042) whereas corticosteroid use showed a significant association with an increased risk of mortality (OR of 9.4, 95% C.I. of 2.9–30.2, p < 0.001), and co-infection (OR of 2.8, 95% C.I. of 1.01–7.7, p = 0.047) remained independently associated with a higher mortality risk. We also compared the COVID-19-related mortality in mild cases according to the antiviral drug used: only one case out of 209 in the N/R group died, compared to none in the 146 cases with mild disease who were treated with remdesivir (p = 0.99).
3.4. Cause of Death
The overall mortality rate in the remdesivir cohort was 21% (n = 50), whereas it was significantly lower in the nirmatrelvir/ritonavir cohort, with a mortality rate of 9% (n = 20) (p < 0.001). The median time to death was 53 days (range: 4–406) in the remdesivir group and 72 days (range: 18–296) in the nirmatrelvir/ritonavir group. Among the deceased patients, 12.4% (29 patients) in the remdesivir group and 7.7% (17 patients) in the nirmatrelvir/ritonavir group died from causes that were not related to COVID-19 (p < 0.001). Overall, the most common causes of death were baseline disease progression, accounting for 12% (29 cases) in the remdesivir group, and 4.5% (10 cases) in the nirmatrelvir/ritonavir group. Additionally, the nirmatrelvir/ritonavir group experienced nine cases of death due to other infectious complications after COVID-19 recovery, including one case of pneumocystis jirovecii pneumonia, three cases of pneumonia with an unknown origin, one case of invasive pulmonary fungal infection, three cases of septic shock and one case attributed to neurological deterioration caused by the JC virus.
3.5. Prolonged Shedding
SARS-CoV-2 PCR monitoring was available in 128 (53%) and 122 (55%) patients in the remdesivir and nirmatrelvir/ritonavir cohorts, respectively. Overall, the median duration of SARS-CoV-2 detection from the day of antiviral onset was 28.5 days (range 1–208) in the remdesivir cohort and 20 days (range 3–220) in the nirmatrelvir/ritonavir cohort (p = 0.004). Table 4 presents the median days of SARS-CoV-2 shedding, calculated from the day of antiviral drug onset according to different baseline factors in both cohorts.
Table 4.
Median days of SARS-CoV-2 shedding, calculated from the day of antiviral drug onset according to different conditions.
| Remdesivir Cohort Ω SARS-CoV-2 Shedding |
Nirmatrelvir/Ritonavir Cohort Ω SARS-CoV-2 Shedding |
|||||
|---|---|---|---|---|---|---|
| Variable | Median Days (Range) |
n | p Value | Median Days (Range) |
n | p Value |
| Age | 0.25 | 0.87 | ||||
| • <41 years | 21 (2–32) | 7 | 17 (6–95) | 12 | ||
| • 41–60 years | 30 (2–162) | 55 | 20.5 (2–220) | 40 | ||
| • 61–70 years | 30 (1–208) | 32 | 17.5 (2–132) | 30 | ||
| • >71 years | 25 (2–108) | 34 | 21 (2–167) | 40 | ||
| SARS-CoV-2 infection severity | 0.013 | NT | ||||
| • Mild | 27 (2–162) | 91 | 20 (2–220) | 116 | ||
| • Moderate | 27.5 (3–108) | 16 | 7.5 (2–25) | 4 | ||
| • Severe | 39 (1–208) | 21 | 5 (2–8) | 2 | ||
| Fever | 0.035 | 0.83 | ||||
| • Yes | 32 (1–208) | 61 | 20 (2–122) | 42 | ||
| • No | 23 (2–162) | 67 | 19.5 (2–220) | 80 | ||
| Corticosteroids at infection | 0.82 | 0.13 | ||||
| • Yes | 31 (3–208) | 39 | 14.5 (2–75) | 32 | ||
| • No | 28 (1–162) | 89 | 21 (2–220) | 90 | ||
| Pneumonia | 0.022 | NT | ||||
| • Yes | 34 (1–208) | 37 | 7.5 (2–25) | 6 | ||
| • No | 27 (2–162) | 91 | 20.5 (2–220) | 116 | ||
| Hospital admission | 0.15 | 0.88 | ||||
| • Yes | 30 (1–117) | 69 | 25 (2–52) | 11 | ||
| • No | 24 (2–208) | 59 | 20 (2–220) | 111 | ||
| Oxygen requirement | 0.022 | 0.8 | ||||
| • Yes | 39 (1–208) | 21 | 5 (2–8) | 2 | ||
| • No | 26.5 (2–162) | 106 | 20 (2–220) | 120 | ||
| Monoclonal antibodies | 0.055 | 0.82 | ||||
| • Yes | 35 (7–162) | 26 | 20.5 (2–132) | 11 | ||
| • No | 25 (1–208) | 98 | 20 (2–220) | 111 | ||
| Convalescent plasma | 0.7 | 0.49 | ||||
| • Yes | 30 (4–106) | 24 | 25 (2–59) | 5 | ||
| • No | 28 (1–208) | 104 | 19 (2–220) | 117 | ||
| Corticosteroids for COVID-19 therapy | 0.004 | 0.11 | ||||
| • Yes | 37 (1–208) | 31 | 2 (2–25) | 3 | ||
| • No | 26 (2–117) | 97 | 20 (2–220) | 119 | ||
| Time from last therapy to COVID-19 | 0.49 | 0.87 | ||||
| • <6 months | 29 (1–162) | 104 | 19.5 (2–167) | 90 | ||
| • 6–12 months | 29 (11–62) | 9 | 18.5 (2–122) | 12 | ||
| • >12 months or not treated | 17 (3–208) | 15 | 22.5 (2–220) | 18 | ||
| Anti-CD20 | 0.015 | 0.34 | ||||
| • Yes | 33 (1–162) | 39 | 20 (2–220) | 59 | ||
| • No | 27 (2–208) | 89 | 20 (2–167) | 63 | ||
| Time from anti-CD 20 to COVID-19 | 0.027 | 0.69 | ||||
| • <6 months | 34 (1–162) | 32 | 20 (6–132) | 49 | ||
| • 6–12 months | 67.5 (62–73) | 2 | 32 (2–63) | 2 | ||
| • >12 months | 29 (7–63) | 5 | 16 (2–220) | 8 | ||
| • Not treated | 27 (2–208) | 89 | 20 (2–167) | 63 | ||
| B-cell NHL/CLL vs. others | 0.005 | 0.61 | ||||
| • Yes | 33 (1–162) | 49 | 19.5 (2–220) | 64 | ||
| • No | 25 (2–208) | 79 | 20.5 (2–167) | 58 | ||
| Ct value at diagnosis * | 0.001 | 0.82 | ||||
| • ≥20 | 19 (2–108) | 49 | 19 (2–90) | 28 | ||
| • <20 | 38 (1–208) | 47 | 21 (5–95) | 34 | ||
| ATV onset from the first positive PCR | 0.008 | 0.6 | ||||
| • <5 days | 27.5 (2–208) | 100 | 20 (2–167) | 110 | ||
| • ≥5 days | 31 (1–162) | 28 | 20.5 (2–220) | 12 | ||
| Duration of ATV | 0.001 | 0.83 | ||||
| • <5 days | 19 (2–89) | 42 | 21 (20–21) | 2 | ||
| • 5 days | 33 (1–208) | 52 | 19 (2–220) | 108 | ||
| • >5 days | 31.5 (4–108) | 34 | 24 (4–132) | 12 | ||
| Co-infections | 0.34 | 0.63 | ||||
| • Yes | 34 (2–208) | 17 | 38 (13–63) | 2 | ||
| • No | 27 (1–162) | 111 | 20 (2–220) | 120 | ||
| ANC < 0.5 × 109/L | 0.4 | 0.25 | ||||
| • Yes | 38 (2–108) | 11 | 14 (6–51) | 5 | ||
| • No | 30 (1–117) | 77 | 24 (2–122) | 49 | ||
| ALC < 0.5 × 109/L | 0.1 | 0.81 | ||||
| • Yes | 35 (2–117) | 36 | 20.5 (2–122) | 18 | ||
| • No | 27 (1–90) | 52 | 23 (2–95) | 36 | ||
| Vaccination status | 0.039 | 0.28 | ||||
| • Complete | 31 (1–208) | 82 | 20 (2–220) | 93 | ||
| • Incomplete | 24.5 (2–90) | 46 | 18 (2–167) | 29 | ||
Abbreviations: NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; Ct, PCR cycle threshold; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NT, not tested. Ω Median duration of SARS-CoV-2 detection estimated from the day of antiviral onset in the remdesivir cohort was 28.5 days (range 1–208), whereas in the nirmatrelvir/ritonavir cohort, it was 20 days (range 3–220) (p = 0.004). * These variables have not been tested since there is a lack of data in more than 40% of cases.
In the remdesivir cohort, severe COVID-19 shed the virus for a longer duration compared to those with mild or moderate cases. Fever, pneumonia and the need for oxygen support were also linked to longer shedding, as was a longer timing from SARS-CoV-2 detection to antiviral onset, the duration of antiviral therapy in cases of persistent SARS-CoV-2 shedding, anti-CD20 treatment, the time from anti-CD20 treatment to COVID-19, B-cell NHL or chronic lymphocytic leukemia (CLL) diagnosis and a PCR Ct value of <20. Patients receiving corticosteroids for COVID-19 therapy had a longer viral shedding period, whereas those with incomplete vaccination showed shorter shedding duration. In the nirmatrelvir/ritonavir cohort, we did not find any condition associated with prolonged shedding.
Among the total patient population (n = 466), 22 patients (4%) required a second course of antiviral therapy due to the persistence of SARS-CoV-2 and/or worsening symptoms. In the remdesivir group, 12 patients (5%) needed a second course of treatment. Among these 12 patients, 10 received a repeat of remdesivir course, while two transitioned to nirmatrelvir/ritonavir therapy. In the nirmatrelvir/ritonavir group, another 11 patients (5%) received a second course of antiviral therapy, of whom four repeated a nirmatrelvir/ritonavir course, while six patients switched to remdesivir and one to molnupiravir. When we looked at those who suffered from prolonged shedding (80 out of 128 (63%) in the remdesivir group and 49 out of 122 (40%) in the nirmatrelvir/ritonavir group), we observed that a second antiviral course was given to seven out of eighty (9%) in the remdesivir groups and to five out of forty-nine (10%) in the nirmatrelvir/ritonavir group. Patients who received a second course showed a non-significant higher mortality (three out of twenty-three, 13%) as compared to those who only received one course (21 out of 443, 5%) (p = 0.108).
3.6. Antiviral Effect According to the Antiviral Drug
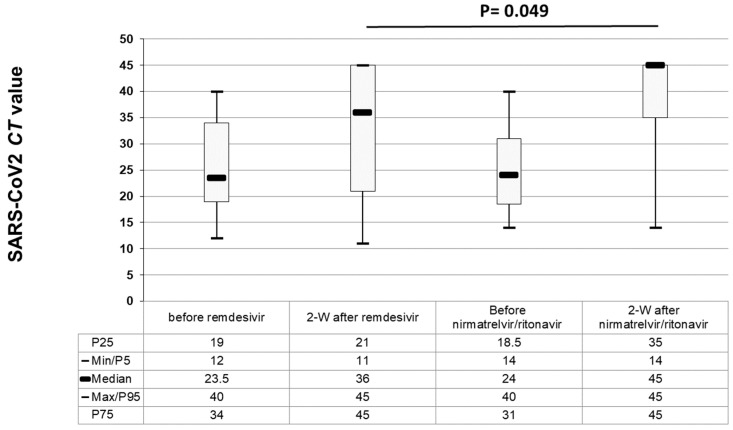
To assess the antiviral effect, we conducted a comparative analysis of the kinetics of the PCR Ct values in patients with mild COVID-19 severity. We specifically focused on individuals who had recorded Ct data at the time of initial SARS-CoV-2 detection and again after 2 weeks, if they had received antiviral drugs before the second PCR test. We categorized them based on the antiviral drug they received: remdesivir (n = 32 patients) or nirmatrelvir/ritonavir (n = 34 patients). For samples that tested negative at two weeks, we assigned a Ct value of 45 for representative purposes. In both groups, the median PCR Ct values increased after antiviral therapy (see Figure 2), with higher median Ct values in the nirmatrelvir/ritonavir group (p = 0.049). In the nirmatrelvir/ritonavir group, most patients achieved PCR negativity (29 out of 34 patients, 85%) compared to those in the remdesivir group (21 out of 32 patients, 65%) at the two-week mark, (p = 0.08). Eleven out of thirty-two patients (34%) from the remdesivir group had rebound infection and the Ct values remained the same (n = 3) or even decreased (n = 8) after 2 weeks. In the nirmatrelvir/ritonavir group, this phenomenon occurred in only five out of thirty four patients (15%) (two with the same Ct value and three with a lower Ct) (p = 0.09).
Figure 2.
Cycle threshold (Ct) kinetics before and two weeks after antiviral treatment, according to the antiviral drug in 66 patients with mild COVID-19 (32 treated with remdesivir and 34 with nirmatrelvir/ritonavir).
3.7. Tolerability of Antiviral Drugs
In Table 5, the tolerability and safety data of two antiviral treatments, remdesivir and nirmatrelvir/ritonavir, are compared. The data show that the risk of drug–drug interactions was significantly higher in the nirmatrelvir/ritonavir group compared to the remdesivir group (p = 0.001). Specifically, there were more cases of baseline treatment modifications (interruption or dose modification of baseline drugs), antiviral dose modifications and early antiviral discontinuations in the nirmatrelvir/ritonavir group. However, when it came to adverse events of grade ≥3, there was no significant difference between the two groups, with only one patient (0.5%) in the remdesivir group and three patients (1.3%) in the nirmatrelvir/ritonavir group experiencing such events (p = 0.3).
Table 5.
Tolerability and safety data.
| Characteristics | Remdesivir (n = 194) |
Nirmatrelvir/Ritonavir (n = 223) | p Value |
|---|---|---|---|
| Risk of drug–drug interactions, n (%) | 0.001 | ||
| • Baseline treatment modification | 1 (0.5) | 5 (2.2) | |
| • ATV dose modification | 1 (0.5) | 9 (4) | |
| • Early ATV interruption | 27 (14) | 38 (17) | |
| • Treatment completed | 165 (68) | 120 (54) | |
| Adverse events ≥ grade 3, n (%) | 1 (0.5) | 3 (1.3) | 0.3 |
Abbreviations: ATV, antiviral therapy.
4. Discussion
We present herein a large cohort of hematological patients treated with remdesivir or nirmatrelvir/ritonavir during the Omicron SARS-CoV-2 period. Nirmatrelvir/ritonavir was primarily administered to mild cases (94%), resulting in a lower SARS-CoV-2 mortality rate (1.3%), while remdesivir was more frequently used for moderate to severe cases (40%) with a higher mortality rate (9%). Multivariate analysis in the remdesivir cohort revealed that male gender correlated with a lower mortality risk, while corticosteroid use as COVID-19 therapy and co-infection were linked to a higher mortality risk. Remdesivir-treated patients (with more severe cases) showed significantly longer SARS-CoV-2 shedding from the antiviral onset day compared to the nirmatrelvir/ritonavir group (28.5 days vs. 20 days). Additionally, in the remdesivir cohort, longer viral shedding was associated with the severity of COVID-19, baseline immunosuppression factors and the timing from illness onset to antiviral therapy. Conversely, the nirmatrelvir/ritonavir group displayed higher PCR Ct values at two weeks after treatment compared to the remdesivir group in cases with mild COVID-19, albeit with a higher risk of drug–drug interactions. Importantly, no significant difference in the incidence of grade ≥3 adverse events was observed between the two groups, indicating a similar safety profile for both antiviral drugs in this patient population.
This real-life retrospective study yielded several noteworthy observations. Firstly, the use of each antiviral closely adhered to the current recommendations [14]. Specifically, nirmatrelvir/ritonavir was mainly employed for mild infections in the outpatient setting, while remdesivir was of choice for severe cases during hospital admission. This adherence to guidelines potentially explains the significantly higher COVID-19-related mortality in the remdesivir group. Secondly, the study revealed a higher non-COVID-19 mortality within the remdesivir group, primarily attributed to a relapse or progression of baseline HDs. This observation suggests that this cohort may comprise frailer patients with aggressive and/or uncontrolled HDs at the time of contracting COVID-19. Furthermore, it is likely that this group had a higher prevalence of comorbidities, particularly pulmonary conditions, and the risk of drug–drug interactions with their baseline medication may have favored the use of remdesivir in this group. Such circumstances create challenges when attempting to compare the outcomes between antiviral treatment groups with different severity-based recommendations, making it potentially unfeasible [19].
Similar to prior retrospective reports from the Omicron period [15], we reported an overall COVID-19-related mortality of 5%. However, it should be noted that the current series mainly focused on symptomatic treated patients, disregarding non-treated and asymptomatic cases, which likely represent more than 50% of SARS-CoV-2 infection cases in prospective surveys [4,5,6]. Thus, it is plausible that the true COVID-19-related mortality could currently be lower than 2% in this vulnerable population in the current period.
In our analysis, we found three factors that were associated with mortality in the remdesivir group. Surprisingly, male gender had a protective effect against mortality, contrary to prior reports on the pre-Omicron VOC, suggesting a more severe disease course for males [20,21,22,23]. While we did not observe significant differences in the distribution of comorbidities between genders, as previously observed, it is possible that comorbidities may impact COVID-19 death more in women [24]. Remarkably, mortality was primarily influenced by the use of corticosteroids and co-infections during COVID-19. These factors were closely linked to each other and associated with higher mortality rates in critically ill COVID-19 patients, particularly with the use of dexamethasone [25]. In our series, the use of corticosteroids to manage COVID-19 was also closely linked to co-infections, which have been also associated with higher mortality in COVID-19 patients [26,27]. Still, the use of corticosteroids during infections in immunosuppressed patients is a matter of concern. Prior experience with the use of corticosteroids at the time of CARV infection in stem cell transplant recipients has been consistently associated with a higher risk of lower respiratory tract progression and mortality [28,29,30,31,32,33]. In contrast, based on the RECOVERY study in non-immunosuppressed individuals [34], many scientific societies have made high-grade recommendations on the use of dexamethasone in hematological malignancy patients with severe COVID-19, as happened with SARS-CoV-2 vaccine recommendations [14]. However, we already know from real-life experiences that caution is needed when extrapolating results from non-immunosuppressed individuals to immunosuppressed patients, as vaccine responses were significantly lower in the latter group, and a three-dose program of mRNA vaccines is now recommended [14]. Although one might argue that corticosteroids in our study were administered in severe cases, conceivably explaining the higher mortality, recent experience from the EPICOVIDEHA registry also supports an increased risk of mortality in hematological patients receiving corticosteroids, as shown in a propensity-score-matched analysis [35]. The limited inflammatory response in hematological patients during COVID-19, due to their profound cellular and humoral immunosuppression, raises serious concerns regarding corticosteroid use, facilitating co-infections in immunocompromised patients whilst managing COVID-19. Thus, it appears to be suitable to perform prospective randomized clinical trials before recommending their use in this setting.
Another differential aspect of COVID-19 in hematological patients as compared to the general population is the well-characterized prolonged virus shedding. While healthy individuals often shed non-viable virus fragments for an extended period, hematological patients may continue to shed viable SARS-CoV-2 for several months after the initial infection [36]. In our series, 52% of patients (149 out of 250) who were monitored through PCR until negativity showed a virus shedding for more than 25 days from the first detection. Prolonged viral shedding is the result of both a baseline compromised B- or T-cell function and the COVID-19 severity, along with a high viral burden. In this sense, we confirmed in the remdesivir group that conditions related to impaired B- and T-cell function (i.e., anti-CD20 treatment, the time from anti-CD20 treatment to COVID-19, B-cell NHL or CLL diagnosis and corticosteroid use for COVID-19) were significantly associated with prolonged virus shedding, in line with a prior report [37]. Importantly, the COVID-19 severity was also an important cause of prolonged shedding in our series, supported not only by the severe category but also by the development of symptoms such as fever, pneumonia and oxygen requirements. The fact that we only identified conditions that were associated with prolonged viral shedding in the remdesivir group supports this relation, since most severe cases were included in this group. In fact, the remdesivir cohort showed significantly longer SARS-CoV-2 shedding compared to the nirmatrelvir/ritonavir group (28.5 days vs. 20 days). A third condition associated with this phenomenon, and obviously linked to the disease severity, is the viral load, indirectly measured using the PCR Ct values, with a cut-off of 20 in our series. Prior studies have shown that severe cases exhibit an average viral load approximately 60 times greater than that of non-severe cases, indicating a potential correlation between higher viral loads and severe clinical outcomes [38]. However, early antiviral treatment can substantially reduce the duration of viral replication and accelerate the clearance of the virus [39]. In this sense, we also confirm in the remdesivir cohort that a period of less than 5 days from illness onset to antiviral therapy was significantly correlated with a shorter duration of SARS-CoV-2 shedding, supporting the idea that early therapy may contribute to improved outcomes [38,39,40,41]. Lastly, monitoring the dynamic change in the PCR Ct value can partially evaluate antiviral effectiveness [42]. Given that severe COVID-19 was over-represented in the remdesivir group, we restricted this analysis to mild COVID-19 cases in both groups, and we observed a higher antiviral effect in the nirmatrelvir/ritonavir cohort and a trend towards a shorter time to negativity. In vitro models have shown that both molecules have equipotent antiviral activity against the ancestral virus and the VOCs Alpha, Beta, Gamma, Delta and Omicron [13]. However, future research comparing the antiviral potency of each compound in vivo in this setting is warranted.
Finally, this study provides valuable insights into the safety of antiviral drugs in treating COVID-19 in immunocompromised patients with hematological diseases. As expected, nirmatrelvir/ritonavir was significantly associated with higher drug–drug interactions, although severe adverse events did not differ between the groups.
This study relies on historical data and observational analysis, potentially introducing limitations and biases. To substantiate these findings and establish stronger evidence for the efficacy of particular treatments in hematological patients during the Omicron variant period, further research and prospective studies are warranted.
5. Conclusions
In line with the current guidelines, remdesivir was the treatment of choice for severe cases, and mortality in this group was mainly associated with the use of corticosteroids and co-infections. Nirmatrelvir/ritonavir appears to be safe and effective in reducing SARS-CoV-2 shedding, having been a preferable option in the outpatient setting. Prolonged shedding was very common and closely linked to immunosuppression status, COVID-19 severity, viral burden and the timing from symptoms to antiviral onset.
Acknowledgments
REDCap was developed and supported by the Vanderbilt Institute for Clinical and Translational Research. We offer our sincere thanks to all hematology units from participating centers for their commitment to the current study. Finally, we also want to thank the patients, nurses and study coordinators for their foremost contributions to this study.
Author Contributions
The authors responsible for the conception and the design of this study: J.L.P., R.M., I.G.-C. and Á.C. The author who performed the data analysis and generated the tables and figures: J.L.P. The authors responsible for patient recruitment: J.L.P., I.H., T.F.A., I.G.-C., L.V., J.L.-J., P.C., C.A., C.G.-V., I.A., E.S.-E., L.L.-C., A.A.-P., A.A., V.G.-G., E.A., L.H.-M., C.G.-S., J.M., J.Á.H.-R., P.R.-G., M.M.-C., M.G. and D.C. The authors responsible for writing and supervising the writing of the manuscript: J.L.P., I.G.-C., D.N., C.S. and R.M. All co-authors were responsible for reviewing the analysis interpretation, suggesting modifications to the text, critically reviewing the manuscript and for the final approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the local research ethics committee of the Hospital Clínico Universitario of Valencia (protocol code 35.21).
Informed Consent Statement
All patients included in this registry gave their signed informed consent, in accordance with the declaration of Helsinki.
Data Availability Statement
Data are available upon formal request by email to the Spanish hematopoietic transplant and cell therapy group (GETH-TC).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Langerbeins P., Hallek M. COVID-19 in patients with hematologic malignancy. Blood. 2022;140:236–252. doi: 10.1182/blood.2021012251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungman P., de la Camara R., Mikulska M., Tridello G., Aguado B., Zahrani M.A., Apperley J., Berceanu A., Bofarull R.M., Calbacho M., et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35:2885–2894. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piñana J.L., Martino R., García-García I., Parody R., Morales M.D., Benzo G., Gómez-Catalan I., Coll R., De La Fuente I., Luna A., et al. Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH). Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp. Hematol. Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piñana J.L., López-Corral L., Martino R., Vazquez L., Pérez A., Martin-Martin G., Gago B., Sanz-Linares G., Sanchez-Salinas A., Villalon L., et al. Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC). SARS-CoV-2 vaccine response and rate of infection in patients with hematological disorders. J. Hematol. Oncol. 2022;15:54. doi: 10.1186/s13045-022-01275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piñana J.L., Martino R., Vazquez L., López-Corral L., Pérez A., Chorão P., Avendaño-Pita A., Pascual M.-J., Sánchez-Salinas A., Sanz-Linares G., et al. Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC). SARS-CoV-2-reactive antibody waning, booster effect and SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination. Bone Marrow Transpl. 2023;58:567–580. doi: 10.1038/s41409-023-01946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piñana J.L., Vazquez L., Calabuig M., López-Corral L., Martin-Martin G., Villalon L., Sanz-Linares G., Conesa-Garcia V., Sanchez-Salinas A., Gago B., et al. Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC). One-year SARS-CoV-2 infection and correlates of protection in fully vaccinated hematological patients. Blood Cancer J. 2023;13:8. doi: 10.1038/s41408-022-00778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljungman P., Tridello G., Piñana J.L., Ciceri F., Sengeloev H., Kulagin A., Mielke S., Yegin Z.A., Collin M., Einardottir S., et al. Improved outcomes over time and higher mortality in CMV seropositive allogeneic stem cell transplantation patients with COVID-19; An infectious disease working party study from the European Society for Blood and Marrow Transplantation registry. Front. Immunol. 2023;14:1125824. doi: 10.3389/fimmu.2023.1125824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., et al. Remdesivir for the treatment of Covid-19–final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L., Wang Z. Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur. J. Med. Chem. 2023;257:115503. doi: 10.1016/j.ejmech.2023.115503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J.L., Li Y.H., Wang L.L., Liu H.Q., Lu S.Y., Liu Y., Li K., Liu B., Li S.Y., Shao F.M., et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal. Transduct. Target Ther. 2021;6:414. doi: 10.1038/s41392-021-00835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simón-Campos A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer W.A., 2nd, Eron J.J., Jr., Holman W., Cohen M.S., Fang L., Szewczyk L.J., Sheahan T.P., Baric R., Mollan K.R., Wolfe C.R., et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 2022;14:eabl7430. doi: 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vangeel L., Chiu W., De Jonghe S., Maes P., Slechten B., Raymenants J., André E., Leyssen P., Neyts J., Jochmans D. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 2022;198:105252. doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesaro S., Mikulska M., Hirsch H.H., Styczynski J., Meylan S., Cordonnier C., Navarro D., von Lilienfeld-Toal M., Mehra V., Marchesi F., et al. Update of recommendations for the management of COVID-19 in patients with haematological malignancies, haematopoietic cell transplantation and CAR T therapy, from the 2022 European Conference on Infections in Leukaemia (ECIL 9) Leukemia. 2023;37:1933–1938. doi: 10.1038/s41375-023-01938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmanton-García J., Marchesi F., Gomes da Silva M., Farina F., Dávila-Valls J., Bilgin Y.M., Glenthøj A., Falces-Romero I., Van Doesum J., Labrador J., et al. EPICOVIDEHA registry. Nirmatrelvir/ritonavir in COVID-19 patients with haematological malignancies: A report from the EPICOVIDEHA registry. EClinicalMedicine. 2023;58:101939. doi: 10.1016/j.eclinm.2023.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun F., Lin Y., Wang X., Gao Y., Ye S. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis. 2022;22:1279. doi: 10.1016/S1473-3099(22)00430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magyari F., Pinczés L.I., Páyer E., Farkas K., Ujfalusi S., Diószegi Á., Sik M., Simon Z., Nagy G., Hevessy Z., et al. Early administration of remdesivir plus convalescent plasma therapy is effective to treat COVID-19 pneumonia in B-cell depleted patients with hematological malignancies. Ann. Hematol. 2022;101:2337–2345. doi: 10.1007/s00277-022-04924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aiello T.F., Puerta-Alcalde P., Chumbita M., Lopera C., Monzó P., Cortes A., Fernández-Avilés F., Suárez-Lledó M., Correa J., Ortiz-Maldonado V., et al. Current outcomes of SARS-CoV-2 Omicron variant infection in high-risk haematological patients treated early with antivirals. J. Antimicrob. Chemother. 2023;78:1454–1459. doi: 10.1093/jac/dkad105. [DOI] [PubMed] [Google Scholar]
- 19.Høeg T.B., Duriseti R., Prasad V. Potential “Healthy Vaccinee Bias” in a Study of BNT162b2 Vaccine against Covid-19. N. Engl. J. Med. 2023;389:284–285. doi: 10.1056/NEJMc2306683. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen N.T., Chinn J., De Ferrante M., Kirby K.A., Hohmann S.F., Amin A. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. PLoS ONE. 2021;16:e0254066. doi: 10.1371/journal.pone.0254066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bischof E., Wolfe J., Klein S.L. Clinical trials for COVID-19 should include sex as a variable. J. Clin. Investig. 2020;130:3350–3352. doi: 10.1172/JCI139306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bienvenu L.A., Noonan J., Wang X., Peter K. Higher mortality of COVID-19 in males: Sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116:2197–2206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., Rosser E.C., Webb K., Deakin C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida Y., Wang J., Zu Y. Sex differences in comorbidities and COVID-19 mortality-Report from the real-world data. Front. Public Health. 2022;10:881660. doi: 10.3389/fpubh.2022.881660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Søvik S., Barrat-Due A., Kåsine T., Olasveengen T., Strand M.W., Tveita A.A., Berdal J.E., Lehre M.A., Lorentsen T., Heggelund L., et al. Corticosteroids and superinfections in COVID-19 patients on invasive mechanical ventilation. J. Infect. 2022;85:57–63. doi: 10.1016/j.jinf.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton M.J., Orihuela C.J., Harrod K.S., Bhuiyan M.A.N., Dominic P., Kevil C.G., Fort D., Liu V.X., Farhat M., Koff J.L., et al. COVID-19 bacteremic co-infection is a major risk factor for mortality, ICU admission, and mechanical ventilation. Crit. Care. 2023;27:34. doi: 10.1186/s13054-023-04312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fehér Á., Szarvas Z., Lehoczki A., Fekete M., Fazekas-Pongor V. Co-infections in COVID-19 patients and correlation with mortality rate. Minireview Physiol. Int. 2022;109:1–8. doi: 10.1556/2060.2022.00015. [DOI] [PubMed] [Google Scholar]
- 28.Piñana J.L., Xhaard A., Tridello G., Passweg J., Kozijn A., Polverelli N., Heras I., Perez A., Sanz J., Berghuis D., et al. Seasonal Human Coronavirus Respiratory Tract Infection in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. J. Infect. Dis. 2021;223:1564–1575. doi: 10.1093/infdis/jiaa553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piñana J.L., Tridelo G., Xhaard A., Wendel L., Montoro J., Vazquez L., Heras I., Ljungman P., Mikulska M., Salmenniemi U., et al. Upper and/or lower respiratory tract infection caused by human metapneumovirus after allogeneic hematopoietic stem cell transplantation. J. Infect. Dis. 2023:jiad268. doi: 10.1093/infdis/jiad268. [DOI] [PubMed] [Google Scholar]
- 30.Chartrand C., Tremblay N., Renaud C., Papenburg J. Diagnostic Accuracy of Rapid Antigen Detection Tests for Respiratory Syncytial Virus Infection: Systematic Review and Meta-analysis. J. Clin. Microbiol. 2015;53:3738–3749. doi: 10.1128/JCM.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo S., Waghmare A., Scott E.M., Xie H., Kuypers J.M., Hackman R.C., Campbell A.P., Choi S.-M., Leisenring W.M., Jerome K.R., et al. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: Association with mortality. Haematologica. 2017;102:1120–1130. doi: 10.3324/haematol.2016.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo S., Xie H., Campbell A.P., Kuypers J.M., Leisenring W.M., Englund J.A., Boeckh M. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: Viral detection in the lung predicts outcome. Clin. Infect Dis. 2014;58:1357–1368. doi: 10.1093/cid/ciu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chemaly R.F., Hanmod S.S., Rathod D.B., Ghantoji S.S., Jiang Y., Doshi A., Vigil K., Adachi J.A., Khoury A.M., Tarrand J., et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood. 2012;119:2738–2745. doi: 10.1182/blood-2011-08-371112. [DOI] [PubMed] [Google Scholar]
- 34.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aiello T.F., Salmanton-Garcia J., Marchesi F., Weinbergerova B., Glenthoj A., Van Praet J., Farina F., Davila-Valls J., Martín-Pérez S., El-Ashwah S., et al. Dexamethasone Treatment for COVID-19 is Related with Increased Mortality in Haematological Malignancy Patients: Results from the EPICOVIDEHA Registry. SSRN. [(accessed on 8 September 2023)]. Available online: https://ssrn.com/abstract=4473151. [DOI] [PMC free article] [PubMed]
- 36.Aydillo T., Gonzalez-Reiche A.S., Aslam S., van de Guchte A., Khan Z., Obla A., Dutta J., van Bakel H., Aberg J., García-Sastre A., et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N. Engl. J. Med. 2020;383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., Barbian K., Judson S.D., Fischer E.R., Martens C., et al. Case study: Prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912.e9. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M., Peiris M., Poon L.L.M., Zhang W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J., Wang D., Liu C., Meng Y., Cui L., et al. Early antiviral treatment con¬tributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J. Intern. Med. 2020;288:128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 40.McCullough P.A., Kelly R.J., Ruocco G., Lerma E., Tumlin J., Wheelan K.R., Katz N., Lepor N.E., Vijay K., Carter H., et al. Pathophysiological basis and rationale for early outpatient treatment of SARS-CoV-2 (COVID-19) infection. Am. J. Med. 2021;134:16–22. doi: 10.1016/j.amjmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vassilopoulos A., Mylonakis E. In patients with COVID-19 at risk for severe disease, nirmatrelvir + ritonavir reduced hospitalization or death. Ann. Intern. Med. 2022;175:JC63. doi: 10.7326/J22-0038. [DOI] [PubMed] [Google Scholar]
- 42.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon formal request by email to the Spanish hematopoietic transplant and cell therapy group (GETH-TC).