Dear Editor,
Peripheral neuropathy is a group of devastating diseases affecting periphe-ral nerves and may cause symptoms such as extreme numbness, muscle weakness, and paralysis. Currently, there are no effective therapies for these diseases. Great progress has been made in identifying genetic causes for peripheral neuropathy owing to the advances in genetic testing in the last decade. For example, >100 genes have been identified to be associated with Charcot–Marie–Tooth (CMT) neuropathy, a group of disorders among the most common forms of inherited peripheral neuropathy. GARS, encoding glycyl-tRNA synthetase (GlyRS), was the first member identified in the family of aminoacyl-tRNA synthetases, the largest gene family implicated in CMT (Patzko and Shy, 2011). Although GARS is ubiquitously expressed in all cells, mutations in this gene cause a dominant axonal form of CMT, termed CMT type 2D (CMT2D), leading to the degeneration of peripheral axons and consequent deficits in distal motor and sensory functions (Antonellis et al., 2003). Our previous studies have demonstrated that CMT2D-associated mutations alter the conformation of GlyRS, enabling mutant GlyRS (GlyRSCMT2D) to bind to the Nrp1 receptor and disrupt the vascular endothelial growth factor (VEGF)/Nrp1 pathway (He et al., 2015). VEGF is best known for its essential role in blood vessel growth. However, many lines of evidence suggest that VEGF also protects neurons from a variety of damaging insults, including mechanical trauma, hypoxia, serum deprivation, chemical toxicity, and excitotoxicity (Mackenzie and Ruhrberg, 2012). Motor neurons express Nrp1 receptors and appear to be dependent on muscle-derived VEGF for survival (He et al., 2015). Mutations in the VEGF promoter reduced its expression levels and led to late-onset motor neuron degeneration (Oosthuyse et al., 2001). Altogether, these studies linked CMT2D pathology to the neomorphic binding activity of GlyRSCMT2D that antagonizes the neurotrophic VEGF/Nrp1 pathway.
Emerging evidence suggests that CMT neuropathy may also be influenced by environmental factors, such as vitamin deficiencies, heavy metals, biogenic metals, and pesticides (Staff and Windebank, 2014). For example, low serum vitamin D (VD) levels are associated with an increased risk of diabetic neuropathy (Skalli et al., 2012). The effects of VD are mainly mediated by the VD receptor (VDR), which is a member of the nuclear receptor family. Upon activation by VD, VDR binds to hormone response elements to regulate target gene expression (Plum and DeLuca, 2010). However, the role of VD/VDR signaling in CMT pathogenesis remains elusive. A better understanding of the underlying mechanisms may provide clues for developing effective therapies for CMT neuropathy.
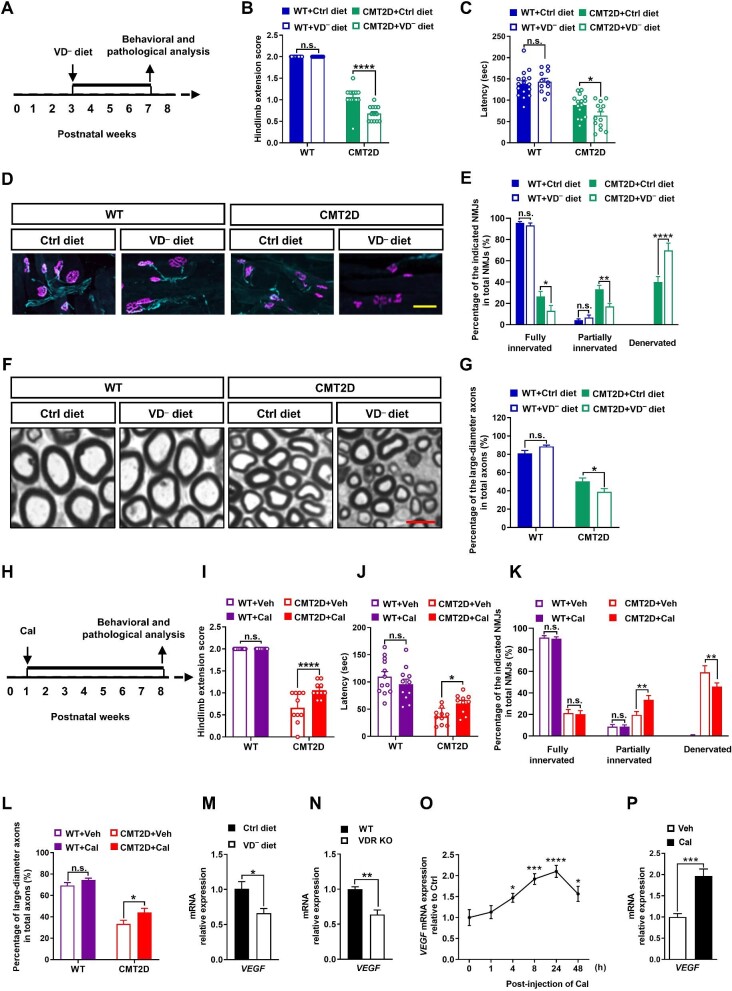
Considering that food is the major source of VD intake, we first explored the effects of VD deficiency on CMT pathogenesis by using a CMT2D mouse model (P234KY-GarsCMT2D mutant) and a VD-deficient diet. Calcium and phosphorus were enriched in the diet to compensate for potential metabolic derangements caused by VD deficiency. Previous studies have demonstrated that CMT-like phenotypes appeared at 3 weeks of age in CMT2D mice and gradually worsened throughout the remainder of their life (CMT2D mice began to die after 8 weeks) (Seburn et al., 2006). Therefore, we began to feed CMT2D or wild-type (WT) mice a VD-deficient diet at postnatal Week 3 to ensure their independent food intake and completed all the experiments at postnatal Week 7 before they died (Figure 1A). As shown in Supplementary Figure S1A and B, both WT and CMT2D mice fed the VD-deficient diet showed a drop (>90%) in their blood VD level, without significant changes in body weight, compared to the mice fed the VD-adequate diet (Ctrl diet). Interestingly, VD deficiency only affected the motor functions in CMT2D mice. We found that CMT2D mice fed the VD-deficient diet exhibited significantly worse motor performance, compared with those fed the Ctrl diet, in both the hindlimb extension test (Figure 1B) and rotarod test (Figure 1C). In contrast, all the WT mice appeared normal, without any significant difference between Ctrl diet and VD-deficient diet groups (Figure 1B and C).
Figure 1.
CMT2D neuropathy is influenced by the VD pathway. (A) Experimental diagram showing the deprivation of VD from the diet. VD− diet, VD-deficient diet. (B and C) VD deficiency worsened motor defects of CMT2D mice in the hindlimb extension test and rotarod test (n ≥ 11 for each group). (D) Representative images showing NMJs in the gastrocnemius muscles with motor nerve terminals (labelled in cyan) and acetylcholine receptors on the muscle (labelled in magenta). Scale bar, 20 μm. (E) Quantification of NMJ denervation (n = 3 for each group). (F) Representative images of sciatic nerve sections. Scale bar, 5 μm. (G) The percentage of axons with a diameter >2 μm (n = 3 for each group). (H) Experimental diagram of Cal administration. (I and J) Cal treatment improved the motor performance of CMT2D mice in the hindlimb extension test and rotarod test (n ≥ 10 for each group). (K) Cal treatment significantly attenuated NMJ denervation in CMT2D mice (n = 3 for each group). (L) Cal treatment significantly increased the percentage of large-diameter axons (with a diameter >2 μm) in the sciatic nerves of CMT2D mice (n = 3 for each group). (M–P) RT-qPCR analysis of VEGF (VEGF-A164 isoform) mRNA expression levels. (M) VD deficiency significantly downregulate VEGF-A164 expression level in the gastrocnemius muscles of 7-week-old mice (n = 6 for each group). (N) VEGF-A164 expression level was significantly decreased in the gastrocnemius muscles of adult VDR KO mice (n ≥ 3 for each group). (O) VEGF-A164 expression level was upregulated in WT mouse gastrocnemius muscles after a single injection of 60 μg/kg Cal (n = 4 for each timepoint). (P) Cal (10 nM) treatment induced a rapid (<1 h) increase in VEGF-A164 expression level in C2C12 cells (n = 3 independent experiments). Data are presented as mean ± SEM. Statistical analysis was performed with two-way ANOVA (B, C, E, G, and I–L), Student's t-test (M, N, and P), or one-way ANOVA (O). n.s., not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The motor defects caused by VD deficiency were accompanied by significant pathological changes in the peripheral nerves and neuromuscular junctions (NMJs). We found that NMJs displayed a normal apposition of nerve fibers and postsynaptic acetylcholine receptors in the gastrocnemius muscles of WT mice fed either the Ctrl diet or VD-deficient diet (Figure 1D and E), suggesting that VD deficiency did not affect the NMJs of WT animals. However, the loss of nerve terminals at NMJs was observed in 7-week-old CMT2D mice, and the percentage of denervated NMJs was markedly increased by VD deficiency (Figure 1D and E). Likewise, the absence of large-diameter axons, another CMT2D pathological hallmark (Seburn et al., 2006), was also augmented in the sciatic nerves of CMT2D mice fed the VD-deficient diet (Figure 1F and G). These findings demonstrated that VD deficiency was an important environmental factor influencing CMT2D pathogenesis.
We further examined whether motor functions can be improved by activating the VD/VDR pathway in CMT2D mice. VD analogs, such as calcipotriol (Cal), are widely used to potently activate VD-related pathways (Plum and DeLuca, 2010). In this study, we treated WT and CMT2D mice by intraperitoneal (i.p.) injection of Cal starting from postnatal Week 1 and completed all the experiments at postnatal Week 8 (Figure 1H). Cal treatment did not impact the body weight of treated animals (Supplementary Figure S2). Compared with vehicle (Veh) treatment, Cal treatment did not affect motor functions in WT mice but restored the neuromuscular capacity of CMT2D mice to exhibit significantly higher strength and better motor performance in the hindlimb extension test and rotarod test (Figure 1I and J). Cal treatment also attenuated NMJ denervation in CMT2D mice (Figure 1K) and restored the percentage of large-diameter axons in the sciatic nerves of CMT2D mice (Figure 1L). These findings demonstrated that Cal treatment alleviated CMT-like symptoms in mice.
Next, we explored how VD signaling influenced GlyRSCMT2D-mediated patho-genic pathways in CMT2D mice. Since
VEGF/Nrp1 is a critical neurotrophic pathway dysregulated in CMT pathogenesis (He et al., 2015), we examined whether this pathway was influenced by VD/VDR signaling. RT-qPCR analysis revealed significantly decreased expression levels of VEGF in the gastrocnemius muscles of mice fed the VD-deficient diet and VDR knockout (VDR KO) mice, compared to their controls (Figure 1M and N). However, the expression levels of Nrp1 and GARS in mouse spinal cords were not changed (Supplementary Figure S3). A single i.p. injection of Cal significantly increased VEGF expression level in mouse gastrocnemius muscles, peaking at 24 h and lasting for at least 48 h after injection (Figure 1O; Supplementary Figure S4). Likewise, a rapid increase in VEGF expression level was observed within 1 h after Cal treatment in cultured mouse myoblast C2C12 cells, suggesting that VEGF may be a direct downstream target gene of VD/VDR signaling (Figure 1P).
It is an emerging concept that neurodegenerative diseases may arise from a combination of genetic factors and environmental influences. However, the identification of environmental risk factors is challenging. The underlying molecular link between genetic and environmental pathways also remains poorly understood. Here, we found that VD deficiency worsened CMT-like symptoms in CMT2D mice, highlighting the possibility that VD deficiency is an important environmental risk factor for CMT. Furthermore, we found that VD deficiency led to the downregulation of VEGF expression level in mice. Considering that VEGF is an important neurotrophic factor for motor neuron survival, this observation suggests that VEGF may act as a potential downstream effector of VD-mediated environmental pathways to influence CMT2D pathogenesis. It is also noteworthy that VEGF transcription may be directly regulated by VD/VDR signaling via binding to its promoter (Cardus et al., 2009). Together with our previous finding that the VEGF pathway is disrupted by GlyRSCMT2D through its aberrant binding to Nrp1 (He et al., 2015), these results suggest that VEGF may serve as a molecular link integrating VD-mediated environmental pathways and GlyRSCMT2D-mediated genetic pathways in CMT2D pathogenesis.
CMT is a devasting disease affecting 1 in 2500 people worldwide; however, there is no effective treatment available for this disease (Patzko and Shy, 2011). We previously reported that lentivirus-mediated overexpression of VEGF improved motor functions in CMT2D mice (He et al., 2015). However, strong VEGF overexpression may increase the risk of tumorigenesis due to its proangiogenic activity. Here, we found that Cal administration could moderately upregulate endogenous VEGF expression level, which was sufficient to alleviate CMT-like symptoms in mice without causing noticeable side effects. Considering that several VD analogs, including Cal, are currently used in the clinic (Plum and DeLuca, 2010), our findings highlight the potential of VD analogs to be repurposed for treating CMT2D neuropathy. Moreover, given the clinical similarities among different forms of CMT, our study may also shed light on treating other CMT subtypes.
[Supplementary material is available at Journal of Molecular Cell Biology online. This work was supported by grants from the STI2030-Major Projects (2021ZD0202501), the National Natural Science Foundation of China (NSFC) (82150003, 91949104, and 31871022), Zhejiang Province NSFC (LY19H090135), the National Institutes of Health (NIH) (R35 GM139627), and Open Project from the State Key Laboratory of Genetic Resources and Evolution of China (GREKF20-08). G.B., L.Z., and X.-L.Y. conceived and supervised the project; H.L., M.T., H.Y., and T. L. performed the experiments; Z.-Y.W. and N.S. contributed to the experimental design and/or scientific advice. H.L., M.T., Q.C., L.G., and G.B. wrote the primary draft of the manuscript.]
Supplementary Material
Contributor Information
Huaqing Liu, Key Laboratory of Novel Targets and Drug Study for Neural Repair of Zhejiang Province, School of Medicine, Hangzhou City University, Hangzhou 310015, China.
Mingmin Tang, Key Laboratory of Novel Targets and Drug Study for Neural Repair of Zhejiang Province, School of Medicine, Hangzhou City University, Hangzhou 310015, China; Department of Neurobiology and Department of Neurology of Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310058, China; Liangzhu Laboratory, MOE Frontier Science Center for Brain Science and Brain–Machine Integration, State Key Laboratory of Brain–Machine Intelligence, Zhejiang University, Hangzhou 311121, China.
Hualin Yu, Key Laboratory of Novel Targets and Drug Study for Neural Repair of Zhejiang Province, School of Medicine, Hangzhou City University, Hangzhou 310015, China.
Tongfei Liu, The Institute for Brain Science and Disease, Chongqing Medical University, Chongqing 400016, China; Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA 92037, USA.
Qinqin Cui, Liangzhu Laboratory, MOE Frontier Science Center for Brain Science and Brain–Machine Integration, State Key Laboratory of Brain–Machine Intelligence, Zhejiang University, Hangzhou 311121, China.
Linfan Gu, Department of Neurobiology and Department of Neurology of Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310058, China.
Zhi-Ying Wu, Department of Neurobiology and Department of Neurology of Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310058, China; Key Laboratory of Medical Neurobiology of Zhejiang Province, Zhejiang University School of Medicine, Hangzhou 310009, China.
Nengyin Sheng, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China.
Xiang-Lei Yang, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA 92037, USA.
Linghui Zeng, Key Laboratory of Novel Targets and Drug Study for Neural Repair of Zhejiang Province, School of Medicine, Hangzhou City University, Hangzhou 310015, China.
Ge Bai, Department of Neurobiology and Department of Neurology of Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310058, China; Liangzhu Laboratory, MOE Frontier Science Center for Brain Science and Brain–Machine Integration, State Key Laboratory of Brain–Machine Intelligence, Zhejiang University, Hangzhou 311121, China.
References
- Antonellis A., Ellsworth R.E., Sambuughin N.et al. (2003). Glycyl tRNA synthetase mutations in Charcot–Marie–Tooth disease type 2D and distal spinal muscular atrophy type V. Am. J. Hum. Genet. 72, 1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardus A., Panizo S., Encinas M.et al. (2009). 1,25-dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis 204, 85–89. [DOI] [PubMed] [Google Scholar]
- He W., Bai G., Zhou H.et al. (2015). CMT2D neuropathy is linked to the neomorphic binding activity of glycyl-tRNA synthetase. Nature 526, 710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie F., Ruhrberg C. (2012). Diverse roles for VEGF-A in the nervous system. Development 139, 1371–1380. [DOI] [PubMed] [Google Scholar]
- Oosthuyse B., Moons L., Storkebaum E.et al. (2001). Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 28, 131–138. [DOI] [PubMed] [Google Scholar]
- Patzko A., Shy M.E. (2011). Update on Charcot–Marie–Tooth disease. Curr. Neurol. Neurosci. Rep. 11, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L.A., DeLuca H.F. (2010). Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 9, 941–955. [DOI] [PubMed] [Google Scholar]
- Seburn K.L., Nangle L.A., Cox G.A.et al. (2006). An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a Charcot–Marie–Tooth 2D mouse model. Neuron 51, 715–726. [DOI] [PubMed] [Google Scholar]
- Skalli S., Muller M., Pradines S.et al. (2012). Vitamin D deficiency and peripheral diabetic neuropathy. Eur. J. Intern. Med. 23, E67–E68. [DOI] [PubMed] [Google Scholar]
- Staff N.P., Windebank A.J. (2014). Peripheral neuropathy due to vitamin deficiency, toxins, and medications. Continuum 20, 1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.