Abstract
Background
Ocimum americanum L. (O. americanum) and Ocimum basilicum L. (O. basilicum) are highly valued aromatic medicinal plants. Their leaves are widely used as spices in traditional cuisine. Their essential oils (EOs) are extensively used in food, cosmetic, and pharmaceutical industries. This study aimed to investigate the main chemical profiles of O. americanum and O. basilicum leaf EOs and assess their effects on antibacterial, antioxidant, and larvicidal properties.
Methods
EOs were extracted from the leaves of O. basilicum and O. americanum using steam distillation in a Clevenger-type apparatus. The chemical constituents of the EOs were analyzed using gas chromatography–mass spectrometry. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and metal-chelating techniques were used to assess the free-radical scavenging capability of the oils. The extracted oils were also tested for their antibacterial activities via a disk-diffusion test and the broth microdilution method. Furthermore, the mosquito larvicidal (Aedes aegypti) activity was tested using standard protocols.
Results
Camphor (33.869%), limonene (7.215%), longifolene (6.727%), caryophyllene (5.500%), and isoledene (5.472%) were the major compounds in O. americanum leaf EO. The EO yield was 0.4%, and citral (19.557%), estragole (18.582%) camphor (9.224%) and caryophyllene (3.009%) were the major compounds found among the 37 chemical constituents identified in O. basilicum oil. O. basilicum exhibited a more potent antioxidant activity in DPPH, FRAP, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid tests than O. americanum. The zones of inhibition and minimum inhibitory concentration of the oils in the microdilution and disk diffusion methods were 8.00 ± 0.19 mm to 26.43 ± 2.19 mm and 3.12–100 µg/mL, respectively. At 400 ppm, O. basilicum and O. americanum EOs demonstrated larvicidal activity, with mortality ratios of 73.60% ± 0.89% and 78.00% ± 1.00%, respectively. Furthermore, after 30 min of exposure to O. americanum and O. basilicum EOs, the larval death rates were 73.60% ± 0.89% and 78.00% ± 1.00%, respectively.
Conclusions
The findings revealed that the EOs extracted from the leaves of O. basilicum and O. americanum exhibited reasonable antioxidant, antibacterial, and mosquito larvicidal potentials, and can be used as alternative medicine for the treatment of human health and larvicidal mosquito control.
Keywords: Ocimum, Basil leaf, Antibacterial, Flavor, Aromatherapy
Background
Plants are essential for maintaining the ecological dynamics of ecosystems. They also offer numerous substances with potential healing properties [1, 2]. Medicinal plant extracts and essential oils (EOs) are factories of valuable natural bioactive compounds, which are widely used in the food, pharmaceutical, nutraceutical, and cosmetic industries for their fragrance, taste, and therapeutic capabilities [3–6]. Terpenes and their oxygenated derivatives, benzoids, and phenylpropanoids are volatile, complex mixtures of EOs [4, 7]. In general, EOs are often mixtures of large amounts of two or three compounds as the main components (> 20%–95%) these compounds and others that may be present in very small amounts.
Free radicals, such as reactive oxygen species (ROS), form in redox processes, and are the leading cause of degenerative diseases, such as stroke, rheumatoid arthritis, diabetes, inflammation, aging, cancer, and neurological disorders of the human body [2, 8]. ROS have been associated with lipid oxidation, peroxidation, and DNA mutations of proteins [9]. Antioxidants include any substance that can remove ROS. Given their resonance-stabilizing effect, phenolic compounds can effectively scavenge free radicals [10]. Interest in the search for new plant-based herbal medicines with antioxidant properties has grown in modern times.
Bacterial infections are increasing nowadays. As with the use of antibiotics for bacterial treatment, the rate of therapeutic drug failure is increasing due to the development of resistance of microbial strains. Despite the medical advances in treatment of infectious diseases, chemotherapy and immunization remain serious health problems [11]. The resistance of microorganisms to antimicrobial drugs necessitates the research on new medications with a potential antimicrobial activity [12]. Studies have focused on plant-derived EOs, which contain various volatile bioactive constituents that can act as therapeutics in humans.
Aedes aegypti L. is the vector of arboviruses that cause diseases, such as yellow fever, dengue fever, chikungunya, and zika in tropical regions [13–15]. Rapid urbanization, the lack of infrastructure, and poor sanitation favor the spread of female Aedes aegypti mosquito, which is resistant to pesticides and commercially available repellents [13, 15]. In addition, the disadvantages of chemical pesticides, such as pollutants, and harm to nontarget species, have limited their widespread use [16]. Therefore, alternative pesticides with high efficacy, minimal environmental impact, and low toxicity to humans must be developed to control mosquitoes. Currently, mosquito control programs focus on the eradication of mosquito larvae with herbal remedies [17]. In this context, plant-derived EOs have attracted considerable attention as potential sources of mosquito repellents and larvicides [18].
The genus Ocimum (basil) comprises 160 species and is the major species in Lamiaceae globally. Ocimum is called “the king of herbs” because of its enormous applications in traditional medicine, pharmaceuticals, and cosmetic industry [19, 20]. Several species of Ocimum, such as O. basilicum, O. gratissimum, O. tenuiflorum, O. americanum, O. kilimandscharicum, and O. micranthum, are cultivated for their high-value fragrance, flavor, and medicinal properties [21]. Ocimum has been traditionally used to treat a variety of ailments, including rheumatism, epilepsy, paralysis, diarrhea, sunstroke, influenza, high fever, gonorrhea, abdominal pains, mental illness, colds, and coughs; it also possesses antipyretic, antiemetic, stomatic, antihelmintic, and antimalarial activities [22]. Furthermore, Ocimum leaves have been investigated as a food and flavoring agent because of their aromatic properties [23]. The EOs and aromatic fragrance of Ocimum leaves have therapeutic potentials, especially antimicrobial, antioxidant, insecticidal, and nematocidal properties [24]. Interestingly, Ocimum EOs are rich in terpenoids and phenylpropanoids, such as methyl cinnamate, linalool, thymol, camphor, citrol, eugenol, and geraniol, which are important active constituents that are variable and influenced by environmental factors [23–25].
Ocimum basilicum L. is an aromatic herb and main commercial crop with numerous biological uses [26]. This plant is used as a supplement in the perfume, pharmaceutical, food, aromatherapy, and cosmetic industries [27]. EOs and aromatic leaves have been used in plant-based healthcare since ancient times in traditional systems of medicine [28]. Ocimum basilicum has an aromatic fragrance distinguished by its chemotypes, especially methyl chavicol-, linalool-, eugenol-, methyl eugenol-, and methyl cinnamate-rich chemotypes, which have been documented in India [23]. Earlier studies have reported that the EOs extracted from O. basilicum exhibited potential antimicrobial, fungicidal, antioxidant, antiviral, antiproliferative, anti-inflammatory, antispasmodic, and sedative properties [29].
Ocimum americanum L. (syn. O. canum Sims.) is another renowned species of Ocimum, and it is commonly spread through India and tropical Africa. It is also known as “hairy basil.” Preparations achieved from its aerial parts are repetitively used in folk remedies for the treatment of insomnia and anxiety [30]. In Nigeria, O. americanum leaf decoction or infusion is used to control fever, coughs, colds, piles, and diabetes [31]. In Yoruba tribals, O. americanum is used to prepare soup given its aroma and flavor [32]. O. americanum acetone extract inhibited neurotoxins that caused brain damage in rats [33]. With these regards, the current work was conducted to determine the chemical profiles of EOs extracted from O. basilicum and O. americanum leaves and investigate their antibacterial, antioxidant, and ant mosquito larvicidal properties.
Methods
Plant material and extraction of EOs
Fresh leaves of O. basilicum and O. americanum were collected from an agricultural field (permission was obtained from the landowner) in Coimbatore, Tamil Nadu, India. The identifications of the leaves were confirmed by Dr. Jagathes Kumar, Assistant Professor, and Botanist, Department of Botany, Sri Vijay Vidyalaya College of Arts & Science. The voucher specimens (LO005 and LO006) were deposited at the herbarium center. The collected leaves of O. basilicum and O. americanum were shade dried at room temperature. The EOs were then isolated from 500 g dried leaves through hydrodistillation for 3 h using a Clevenger apparatus. The extracted oils were dried with anhydrous sodium sulfate (1.0 g), separated, and stored at 4 °C for further use.
Chemical constituents of EOs
After the separation of EOs, their chemical composition was determined by gas chromatography–mass spectrometry (GC–MS) using a Clarus 680 GC–MS (PerkinElmer®) equipped with a capillary column (30 m × 0.25 mm) of diphenyl dimethyl polysiloxane model Elite-5 MS. The oven temperature was slowly increased from 60 C to 300 C at the rate of3 °C min–1. The temperatures of injection and mass were set at 220 C and 240 C, respectively. The EOs (1.0 µL) were injected and with a split ratio of 1:200. The flow rate of helium gas was 1 mL/min. GC–MS was performed using the same chromatography and a mass spectrometer. The GC conditions were also the same. The chemical constituents were identified using the retention time and mass spectra in the Wiley and NIST libraries of GC–MS and literature.
Antioxidant activities of EOs
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH free-radical scavenging potential of EOs was measured as described by Blois [34] but with minor changes. A total of 300 µL EO of varying concentrations (20–100 µg/mL) was mixed with the DPPH solution (3 mL 0.1 mM). The oil mixtures were kept in a darkroom for 30 min, and the color reduction by the EOs of the stable DPPH radical was measured at 517 nm on a spectrophotometer. Ascorbic acid, rutin, and butylated hydroxytoluene (BHT) were used as standards. DPPH was calculated using the equation below:
The concentration of oil that reduced the DPPH solution by 50% (half-maximal inhibitory concentration (IC50)) was calculated.
Measurement of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS•) radical cation assay
The ABTS• radical cation decolorization potential of EO from O. americanum and O. basilicum was measured in accordance with the work of Re et al. [35]. ABTS radical was produced by ABTS (7 mmol/L) with potassium persulfate (2.4 mM) in the dark for 12–16 h. Then, the ABTS solution was diluted in ethanol (1:89 v/v) to give an absorbance of 0.700 ± 0.02 at 734 nm. Triplicates of 10 μL essential oil (20 mg/mL in DMSO) and Trolox (concentration 0–15 μM) were added to 1 mL of ABTS solution. The reaction mixture was incubated at 30 °C for 30 min and the absorbance was measured at 734 nm. The total antioxidant activity was validated as Trolox equivalent µM/g oil. Ascorbic acid was used as a standard.
Ferric reducing antioxidant power (FRAP) assay
The FRAP-reducing antioxidant power potentials of O. basilicum and O. americanum EOs were esteemed following the work of Pulido et al. [36]. 900 µL of FRAP reagent was mixed with 90 µL of distilled water and 50 µL essential oil (20 mg/mL). The essential oil and blank were incubated at 37 °C for 30 min. After incubation, the optical density was taken at 593 nm using a spectrophotometer. Methanolic solutions with known Fe (II) (FeSO4·7H2O) concentrations, ranging from 100 to 2000 µmol/L were used for the preparation of the calibration curve. FRAP was indicated as mM Fe (II) equivalent/g oil. Ascorbic acid was used as a standard.
Metal-chelating activity
The chelation of ferrous ions by O. basilicum and O. americanum EOs was assessed in accordance with the method of Dinis et al. [37]. 100 µL of essential oil (20 mg/mL), 600 µL distilled water, and 100 µL ferrous chloride (2 mmol/L) were mixed, shaken vigorously, and incubated for 30 s. Then, 200 µL ferrozine (5 mmol/L) was added to the above mixture and incubated for 10 min at room temperature and the absorbance was recorded at 562 nm with a UV–vis spectrophotometer. EDTA (0–2 µg) was used as a standard for the preparation of the calibration curve. The metal chelating ability of antioxidants was expressed as mg Ethylenediaminetetraacetic acid (EDTA) equivalent/g oil.
Antibacterial activity
The antibacterial activity of O. basilicum and O. americanum EOs was assessed by two tests: (i) the c and (ii) broth microdilution (determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)) tests. The antibacterial efficacies of O. basilicum and O. americanum oils were tested against gram-positive bacteria, namely, Bacillus subtilis (MTCC-441), Streptococcus pyogenes (MTCC 1928), Enterococcus faecalis (MTCC 439), and Staphylococcus aureus (MTCC-96), and gram-negative bacteria, including Escherichia coli (MTCC-724), Klebsiella pneumoniae (MTCC-432), Proteus vulgaris (MTCC-426), Salmonella paratyphi (MTCC-735), Aeromonas hydrophila (MTCC-7646), Pseudomonas aeruginosa (MTCC 2453), and Enterobacter aerogenes (MTCC 39).
Diameter of the zone of inhibition (ZI) by disk diffusion
The antibacterial activities of O. basilicum and O. americanum EOs were evaluated using the paper-disk diffusion method, in accordance with the work of Mahendran and Ranjitha Kumari [38]. Muller–Hinton (Himedia, India) agar plates were prepared and stored at 20 °C ± 2 °C. A test bacterial suspension (100 µL) containing 108 CFU/mL of each bacterial suspension was dispensed and uniformly spread above the sterile plates using a glass spreader. Then, 20 µL (5, 10, and 20 mg/mL dissolved in DMSO) of each EO was loaded onto a sterile paper disk (6 mm). Oil-loaded paper disks were placed on the surface of the inoculated plates, and the plates were incubated at 37 °C ± 2 °C for 24 h. Streptomycin (20 µg/disk) and cefotaxime (20 µg/disk) disks were used as a reference (positive control) and DMSO (negative control). The experiment was carried out on three plates (in triplicate) for each organism. The ZI diameter was measured in millimeters (mm). The results were presented as mean ± standard deviation (SD).
Broth microdilution method for MIC and MBC
The MIC and MBC of O. basilicum and O. americanum oils were evaluated using the microdilution method, in accordance with the work of Duarte et al. [39]. Briefly, 100 µL twofold serially diluted (100 µg/mL to 0.781 µg/mL) oils of O. basilicum and O. americanum were deposed in each well in a 96-well plate. Then, 100 µL bacteria at 106 CFU were added to all wells. After incubation at 37 °C overnight, 40 µL (200 µg/mL) p-iodonitrotetrazolium violet (INT) was deposed to all wells for the evaluation of bacterial growth. Bacterial growth was shown by detecting the reduction of yellow to red formazan after 2 h of incubation at 37 °C [40]. To analyze the MBC, we sub-cultured 10 µL of each bacterial culture on Muller–Hinton plates for 24 h.
Mosquito larvicidal bioassay
The eggs of Aedes aegypti were acquired from the Center for Research in Medical Entomology, Madurai, India. The larvae were fed with yeast powder and dog biscuits at a 1:3 ratio. A total of 10% sucrose with chicks for blood meal was nourished for adults. Mosquitoes were maintained at 70%–85% relative humidity and 28 °C ± 2 °C temperatures with 12 h light.
The larval mortality was measured in accordance with the work of Panneer Selvam et al. [41] with slight modifications. A total of 25 larvae at the 3rd and 4th instar stages were separately kept in 200 mL paper cups containing 99 mL water added with 1 mL of essential oil at various concentrations (25, 50, 100, 200, and 400 ppm) dissolved in 1 mL of DMSO. The mortality was detected after 24 and 48 h. Toxicity (mortality) and larvicidal activities were described using 50% lethal concentration (LC50) and LC90 at 50% concentration of essential oil showed mortality. 95% confidence limit levels were calculated by probit analysis (SPSS ver. 26).
Statistical analysis
All the biological activities of both EOs were repeated in triplicate, and the results were given as mean ± SD and calculated using SPSS version 26. The IC50 value was investigated statistically with a one-way analysis of variance followed by Duncan’s multiple range test. Probit analysis was used to estimate the LC50 and LC90 lethal concentrations with a 95% confidence limit (CL) were all calculated.
Results
Chemical constituents of O. basilicum and O. americanum EOs
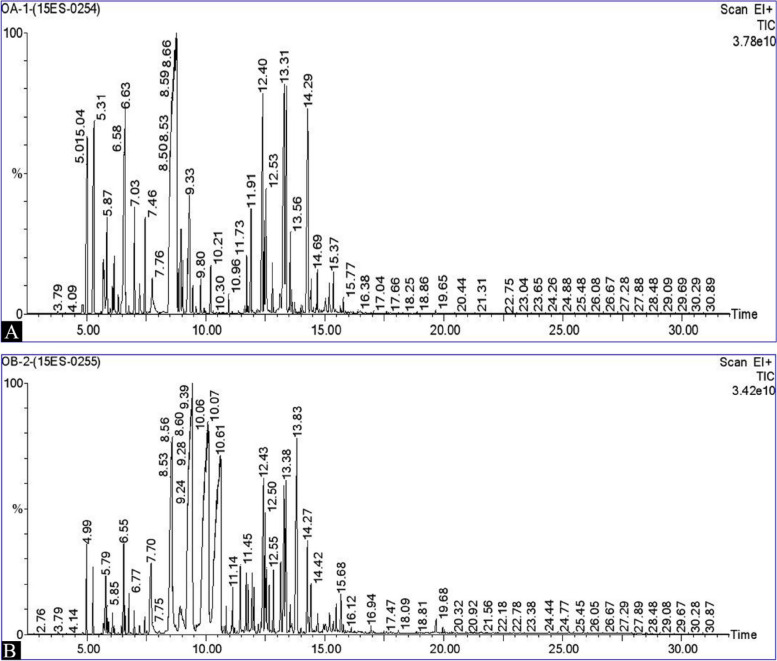
Hydrodistillation of O. basilicum and O. americanum yielded EOs about 0.6% (3.2 mL) and 0.4% (2.3 mL), respectively. The EOs of O. basilicum and O. americanum were investigated, and their chromatographic profiles are shown in Fig. 1A and B, respectively. Their chemical compositions from the experiment were compared with those of a previous study (Tables 1 and 2). The results exhibited that 37 and 34 compounds were identified, and they accounted for 81.141% and 100% of the oil, respectively, in O. basilicum and O. americanum. As shown in Table 1, the main components of O. basilicum were 3,7-dimethyl-,(Z)-(-citral) (19.557%), estragole (18.582%), camphor (9.224%) and caryophyllene (3.009%).
Fig. 2.
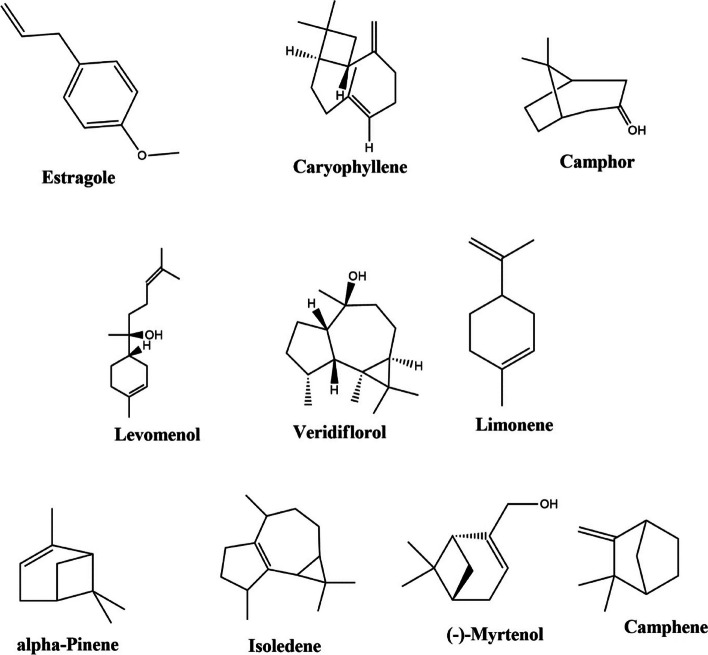
Predominant phytochemicals are present in the EOs of O. americanum and O. basilicum leaves
Fig. 1.
GC–MS chromatogram of leaf EOs from Ocimum species. A O. americanum B O. basilicum. The EOs obtained from O. americanum had 34 compounds and contained camphor (33.86%), limonene (7.21%), longifolene (6.72%), veridiflorol (5.85%), isoledene (5.472), and α-pinene (5.19%) as the main chemical components (Table 2 and Fig. 2)
Table 1.
Chemical composition of O. basilicum oil
| Peak | Retention time (min) | Mass | Compounds | Area (%) | [Ref]a |
|---|---|---|---|---|---|
| 1 | 4.994 | 136 | α-Pinene | 1.010 | 1, 3 |
| 2 | 5.264 | 136 | Camphene | 0.729 | 1, 3 |
| 3 | 5.795 | 128 | 1-Octen-3-ol | 0.771 | 4 |
| 4 | 5.850 | 136 | β-Pinene | 0.246 | 1, 3 |
| 5 | 6.550 | 136 | D-Limonene | 1.287 | 2 |
| 6 | 6.600 | 154 | Eucalyptol | 0.251 | 1, 2, 3 |
| 7 | 6.770 | 136 | α-Ocimene | 0.391 | |
| 8 | 6.990 | 136 | 3-Carene | 0.235 | 7 |
| 9 | 7.700 | 154 | Cis-β-Terpineol | 2.715 | |
| 10 | 8.596 | 152 | Camphor | 9.224 | 1, 2, 3 |
| 11 | 8.921 | 152 | (-)-Myrtenol | 0.781 | 1, 3 |
| 12 | 8.981 | 152 | ( +)- trans-Limonene oxide | 0.757 | 7 |
| 13 | 9.441 | 148 | Estragole | 18.582 | 5 |
| 14 | 10.071 | 152 | 2,6-Octadienal, 3,7-Dimethyl-, (Z)- (β – citral) | 19.557 | 5 |
| 15 | 10.862 | 204 | α- farnesene | 0.250 | 1, 3 |
| 16 | 11.137 | 166 | 4,8-dimethyl-nona-3,8-dien-2-one | 0.479 | |
| 17 | 11.452 | 154 | (. ± .)-Lavandulol, | 0.855 | 4 |
| 18 | 11.707 | 180 | Geranyl vinyl ether | 0.734 | |
| 19 | 11.952 | 204 | Cadrene | 0.766 | |
| 20 | 12.427 | 204 | Caryophyllene | 3.009 | 1 |
| 21 | 12.497 | 204 | trans- α-Bergamotene | 1.470 | 2 |
| 22 | 12.547 | 204 | isoledene | 0.685 | |
| 23 | 12.662 | 204 | α-Cubebene | 0.560 | 1, 3 |
| 24 | 12.847 | 204 | α-Caryophyllene | 0.793 | 3 |
| 25 | 13.153 | 204 | Aromadendrene | 1.311 | 6 |
| 26 | 13.298 | 204 | (-)-Iso aromadendrene -(V) | 2.268 | |
| 27 | 13.378 | 204 | Patchoulene | 2.300 | |
| 28 | 13.553 | 204 | Longifolene | 0.309 | |
| 29 | 13.623 | 204 | (-)-α-panasinsen | 0.199 | |
| 30 | 13.833 | 222 | Levomenol | 4.888 | |
| 31 | 14.273 | 222 | (-)-Globulol | 1.282 | |
| 32 | 14.418 | 220 | Caryophyllene oxide | 0.219 | 2 |
| 33 | 14.708 | 166 | Cyclohexane, Butylidene | 0.761 | |
| 34 | 15.193 | 222 | Epiglobulol | 0.443 | |
| 35 | 15.484 | 222 | α -Bisabolol | 0.320 | 2 |
| 36 | 15.684 | 222 | Veridiflorol | 0.495 | 2 |
| 37 | 19.680 | 222 | Ledol | 0.209 | |
| Total | 81.141% |
a1 (Abou El-Soud, Deabes, Abou El-Kassem, & Khalil 2015)
2 (Hussain, Anwar, Hussain Sherazi, & Przybylski 2008)
3 (Ismail 2006)
4 (Politeo, Jukic, & Milos 2007)
5 (Beatovic et al. 2015)
6 (Avetisyan et al. 2017), and
7 (Chalchat & Özcan 2008)
Table 2.
Chemical composition of O. americanum oil
| Peak | Retention time (min) | Mass | Compounds | Area (%) | [Ref]a |
|---|---|---|---|---|---|
| 1 | 5.039 | 136 | α-Pinene | 5.195 | 1, 2, 3 |
| 2 | 5.315 | 136 | Camphene | 4.674 | 2 |
| 3 | 5.710 | 204 | α -farnesene | 0.972 | |
| 4 | 5.755 | 128 | 1-Octen-3-ol | 0.398 | 5 |
| 5 | 5.870 | 136 | β-Pinene | 1.694 | 1,2,3 |
| 6 | 6.165 | 136 | α-Phellandrene | 0.773 | |
| 7 | 6.625 | 136 | Limonene | 7.215 | 1,3 |
| 8 | 7.025 | 136 | 3-Carene | 1.673 | |
| 9 | 7.235 | 154 | Cis-β-Terpineol | 0.535 | 3 |
| 10 | 7.460 | 136 | Cyclohexene | 1.433 | |
| 11 | 7.756 | 154 | Eucalyptol | 1.220 | 1,2,3 |
| 12 | 8.771 | 152 | Camphor | 33.869 | 1,2,3 |
| 13 | 8.856 | 154 | Iso Borneol | 0.388 | 2 |
| 14 | 8.971 | 154 | Borneol | 1.394 | 1 |
| 15 | 9.046 | 154 | α-terpinenol | 0.671 | 1,3 |
| 16 | 9.241 | 148 | Estragole | 0.937 | 5 |
| 17 | 9.326 | 152 | (-)-Myrtenol | 3.206 | 1 |
| 18 | 9.796 | 152 | Octadienal | 0.476 | |
| 19 | 10.212 | 204 | Naphthalene | 0.691 | 2 |
| 20 | 11.727 | 204 | Copaene | 0.696 | 1 |
| 21 | 11.907 | 204 | Cadrene | 1.508 | |
| 22 | 12.402 | 204 | Caryophyllene | 5.500 | |
| 23 | 12.462 | 204 | β-Caryophyllene | 1.060 | 4 |
| 24 | 12.532 | 204 | Aromadendrene | 1.696 | 3 |
| 25 | 12.803 | 204 | α-Caryophyllene | 0.661 | 5 |
| 26 | 13.118 | 204 | α-Cubebene | 0.450 | |
| 27 | 13.308 | 204 | Longifolene | 6.727 | |
| 28 | 13.403 | 204 | Isoledene | 5.472 | |
| 29 | 13.558 | 204 | Epizonarene | 0.992 | |
| 30 | 14.293 | 222 | Veridiflorol | 5.858 | |
| 31 | 14.693 | 222 | Humulane | 0.541 | 5 |
| 32 | 15.038 | 222 | α-cadinol | 0.341 | 1 |
| 33 | 15.189 | 222 | Ledol | 0.512 | |
| 34 | 15.369 | 222 | γ-Muurolene | 0.572 | |
| Total | 100% |
a1 (Singh, Tewari, Pande, & Singh 2013)
2 (Bhatt, Tewari, Pande, & Rana 2018)
3 (Matasyoh, Bendera, Ogendo, Omollo, & Deng 2006)
4 (Coulibaly, Hema, Sawadogo, Toe, Kiendrebeogo, & Nébié 2023), and
5 (Wungsintaweekul, Sitthithaworn, Putalun, Pfeifhoffer, & Brantner 2010)
Antioxidant activity
In the present research, the antioxidant activities of O. basilicum and O. americanum EOs were determined via four different investigations, including DPPH, FRAP, ABTS, and metal-chelating assays.
The present results revealed that O. basilicum oil had more noticeable activity than O. americanum oil because its IC50 value was lower (11.56 ± 0.89 µg/mL) than that of O. americanum (13.42 ± 1.03 µg/mL). However, the standards, which included ascorbic acid (3.72 ± 0.60 µg/mL), rutin (4.65 ± 0.52 µg/mL), and BHT (7.89 ± 0.49 µg/mL), exhibited higher activities.
The Trolox equivalent antioxidant capacity (TEAC) was also assessed by neutralizing the radical cation of ABTS. The ABTS activity of O. basilicum EO (2842.12 ± 10.39 µM TEAC/g oil) showed more potency than that of O. americanum (2085.07 ± 7.43 µM TEAC/g oil). Table 3 shows the results on FRAP and metal chelation activity of O. americanum and O. basilicum EOs. In ferric reducing and chelating metal ions, O. basilicum presented a higher FRAP (907.24 ± 18.29 mM Fe (II)/g oil) and metal-chelating (189.16 ± 09.04 mg EDTA Eq/g oil) activity.
Table 3.
ABTS•+-scavenging, FRAP, and metal-chelating activities of EOs obtained from O. basilicum and O. americanum leaves
| Sample | ABTS˙ + (μM TEAC/g oil) | FRAP (mM Fe (II) Eq/g oil) | Metal chelating activity (mg EDTA Eq/g oil) |
|---|---|---|---|
| O. americanum | 2085.07 ± 7.43c | 851.32 ± 13.71c | 106.01 ± 12.07c |
| O. basilicum | 2842.12 ± 10.39b | 907.24 ± 18.29b | 189.16 ± 09.04b |
| Ascorbic acid | 4174.87 ± 12.65a | 2365.65 ± 11.87a | 312.65 ± 10.21a |
Values are means of three independent analyses ± SD (n = 3). Mean values followed by different superscript letters (a,b,c) indicate significant statistical differences (p < 0.05)
Antibacterial activity of O. americanum and O. basilicum EOs
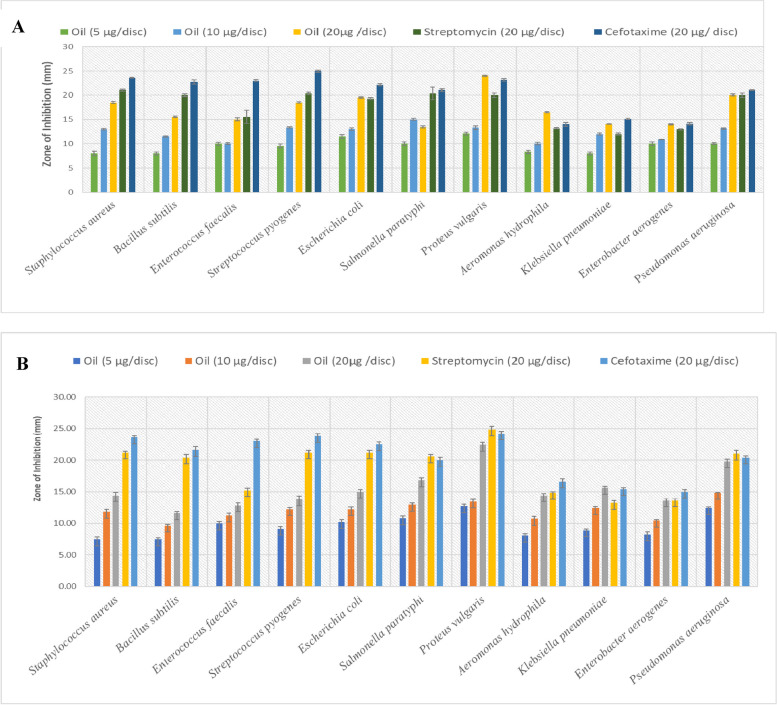
Figure 3 shows the ZI of O. basilicum and O. americanum oils evaluated via the disk-diffusion method.
Fig. 3.
Antibacterial activity of EOs of O. basilicum and O. americanum leaves. A Antibacterial activity of O. americanum leaf EOs. B Antibacterial activity of O. basilicum leaf EOs. Values are means of three independent analyses ± SD (n = 3)
The EOs obtained from O. basilicum and O. americanum displayed strong antibacterial activity against the tested pathogenic bacteria, with a ZI of 8.00–26.43 mm. Remarkably, the highest antibacterial activity was observed in P. vulgaris within the ZI of O. basilicum EO (26.43 mm) followed by that of O. americanum (24.00 mm), and such finding was comparable to that of streptomycin (20 µg/disk; 23.17 mm). Bactericidal activities against S. paratyphi, E. aerogenes, and E. faecalis ranged from 10.00 mm to 23.00 mm (Figs. 3A and 3B). In this case, the antibacterial activity of both plants exhibited dose dependence as it improved under a series of increased concentrations of the EO (5, 10, and 20 µg/disk).
The occurrence of live microorganisms in wells, which is an indication of colony formation, was confirmed by the decreases in yellow INT to triphenyl formazan (pink). This color was not detected in dead cells. The specific concentration of EO that can prevent the development of bacteria was investigated using this technique. The MIC and MBC of O. basilicum and O. americanum oils are displayed in Table 4 and differed from the range detected in the microdilution method (3.12 µg/mL to 100 µg/mL).
Table 4.
MIC and MBC values of O. americanum and O. basilicum Eos
| Microorganisms | O. americanum | O. basilicum | Cefotaxime | |||
|---|---|---|---|---|---|---|
| MIC value (μg/mL) | MBC value ( μg/mL) | MIC value (μg/mL) | MBC value (μg/mL) | MIC value (μg/mL) | MBC value (μg/mL) | |
| Staphylococcus aureus | 100.00 | 100.00 | 12.50 | 12.50 | 3.12 | 3.12 |
| Bacillus subtilis | 50.00 | 12.50 | 3.12 | 3.12 | 12.50 | 12.50 |
| Enterococcus faecalis | 100.00 | 100.00 | 12.50 | 12.50 | 4.88 | 3.12 |
| Streptococcus pyogenes | 50.00 | 50.00 | 6.25 | 6.25 | 3.12 | 2.40 |
| Escherichia coli | 9.76 | 12.50 | 3.12 | 3.12 | 3.12 | 3.12 |
| Salmonella paratyphi | 9.76 | 12.50 | 3.12 | 3.12 | 6.25 | 6.25 |
| Proteus vulgaris | 9.76 | 12.50 | 3.12 | 3.12 | 3.12 | 3.12 |
| Aeromonas hydrophila | 50.00 | 19.53 | 6.25 | 6.25 | 6.25 | 6.25 |
| Klebsiella pneumoniae | 50.00 | 50.00 | 6.25 | 6.25 | 3.12 | 3.12 |
| Enterobacter aerogenes | 100.00 | 100.00 | 12.50 | 12.50 | 6.25 | 6.25 |
| Pseudomonas aeruginosa | 50.00 | 12.50 | 6.25 | 6.25 | 12.50 | 12.50 |
Values are means of three independent analyses (n = 3)
Larvicidal activity
The larvicidal efficiency of O. basilicum and O. americanum EOs was studied. The EOs had a notable larvicidal action, and the results are presented in Table 5.
Table 5.
Larvicidal properties of leaf EOs from O. americanum and O. basilicum against the larvae of A. aegypti after 12 and 24 h of exposure
| Sample | Mosquito life stages | Time | Concentration (ppm) | % mortality ± SD | LC50 (LCL-UCL)a | LC90 (LCL-UCL)a | χ2 (df = 4)b |
|---|---|---|---|---|---|---|---|
| O. americanum | Second instar | After 12 h | 25 | 23.57 ± 0.42 | |||
| 50 | 33.88 ± 0.46 | 160.34 | 627.43 | ||||
| 100 | 45.00 ± 1.00 | (86.76–217.58) | (457.69–1202.18) | ||||
| 200 | 55.80 ± 0.83 | 5.406 | |||||
| 400 | 74.60 ± 1.14 | ||||||
| After 24 h | 25 | 23.56 ± 0.12 | |||||
| 50 | 47.00 ± 0.69 | 87.96 | 439.54 | 10.45* | |||
| 100 | 64.30 ± 1.02 | (12.70–163.11) | (292.16–716.18) | ||||
| 200 | 80.00 ± 1.50 | ||||||
| 400 | 91.54 ± 1.56 | ||||||
| Third instar | After 12 h | 25 | 11.80 ± 0.89 | ||||
| 50 | 31.91 ± 1.53 | 134.54 | 1119.07 | 2.34 | |||
| 100 | 45.00 ± 1.00 | (46.72–227.60) | (781.28–2828.96) | ||||
| 200 | 57.80 ± 1.30 | ||||||
| 400 | 73.60 ± 0.89 | ||||||
| After 24 h | 25 | 21.20 ± 1.30 | |||||
| 50 | 44.56 ± 1.28 | ||||||
| 100 | 58.20 ± 1.30 | 73.73(16.54–148.55) | 577.28(353.49–961.49) | 2.39* | |||
| 200 | 75.60 ± 0.54 | ||||||
| 400 | 82.60 ± 0.89 | ||||||
| O. basilicum | Second instar | After 12 h | 25 | 32.20 ± 0.44 | |||
| 50 | 44.60 ± 1.14 | 61.14 | 1114.68 | 2.18 | |||
| 100 | 53.20 ± 1.09 | (17.76–342.99) | (242.16–3126.01) | ||||
| 200 | 67.20 ± 0.83 | ||||||
| 400 | 76.40 ± 1.14 | ||||||
| After 24 h | 25 | 41.60 ± 1.14 | |||||
| 50 | 62.80 ± 1.92 | ||||||
| 100 | 71.40 ± 1.19 | 37.14 | 286.55 | 1.22* | |||
| 200 | 87.40 ± 1.14 | (0.08–137.77) | (30.43–548.49) | ||||
| 400 | 93.20 ± 0.83 | ||||||
| Third instar | After 12 h | 25 | 27.40 ± 1.24 | ||||
| 50 | 34.00 ± 0.87 | ||||||
| 100 | 56.76 ± 0.69 | 92.65 | 950.72 | 1.56 | |||
| 200 | 69.10 ± 1.88 | (0.03–259.13) | (495.34–3371.99) | ||||
| 400 | 78.00 ± 1.00 | ||||||
| After 24 h | 25 | 42.35 ± 1.20 | |||||
| 50 | 64.50 ± 1.57 | ||||||
| 100 | 79.25 ± 1.65 | 31.43 | 233.43 | 1.09* | |||
| 200 | 86.00 ± 1.72 | (0.41–111.35) | (37.22–459.36) | ||||
| 400 | 95.50 ± 1.89 |
LCL Lower confidence level, UCL Upper confidence level
a95% confidence interval
bDegrees of freedom; χ2 chi-square value
*Significance at P ≤ 0.05
After 12 and 24 h of exposure, larvicidal effects, and mortality were observed in second- and third-instar larvae. As shown in Table 5, the larval mortality of A. aegypti (second and third instars) increased after the treatment using O. basilicum and O. americanum EOs at different concentrations (25–400 µg/mL). The treatment with O. basilicum at 25 g/mL caused 41.60% and 42.35% mortalities in the second- and third-instar larvae, respectively, and the values increased to 93.20% and 95.50% after a 24 h treatment period (Table 5).
After a 24 h exposure period, the LC50 of O. basilicum was the most effective against Aedes aegypti (LC50: 37.14 and 31.43 ppm and LC90: 286.55 and 233.43 ppm for the second and third instars, respectively, followed by that of O. americanum (LC50 87.96 and 73.73 ppm LC90 439.54 and 577.28 ppm). From the Chi-squared test, O. basilicum result showed significance chi-level χ2 = 1.09 and χ2 = 1.22 at P ≤ 0.05 (Table 5).
Discussions
In Ocimum spp., EOs are generally rich in estragole and 2, 6-octadienal, as observed in our result for O. basilicum EO. The components vary from those reported by Mohamed Abdoul-Latif et al. [20], who reported linalool (41.2%) and estragole (30.1%) as the major compounds in O. basilicum from Djibouti in East Africa. The components also differed from those reported by Srivastava et al. [23], who observed methyl chavicol (51.2%–58.0%), methyl eugenol (10.0%–15.0%), meta-eugenol (4.5%–9.3%), and camphor (4.5%–5.7%) as the main chemotype II components in O. basilicum from India. The results of the EO analysis revealed methyl chavicol (88.6%) as the major compound of O. basilicum [42]. In contrast to our findings, some researchers reported that the main constituents of O. basilicum oil are 2,6-octadienal, 3,7-dimethyl-,(Z)-(20.01%), citral (20.11%), and eugenol (26.76) [43].
As shown in Table 2, the GC–MS analysis identified 34 compounds, which represent 100% of the total EOs in O. americanum. Interestingly, O. americanum oil was rich in camphor (33.86%) and limonene (7.21%). In contrast to our findings, Mohamed Abdoul-Latif et al. [20] reported that the main compounds in O. americanum EO include carvotanacetol (38.5%) and estragole (27.5%). Other investigators presented eugenol (45.2%), methyleugenol (14.8%), and (E)-caryophyllene (30.2%) as the major constituents of O. americanum from Rudrapur, India [44]. Similar to our result, camphor is a major chemical constituent of O. minimum, O. canum, and O. gratissimum but in different percentages [45–47]. These results suggest that the chemical composition of EOs in aromatic medicinal plants can vary depending on environmental factors and EO extraction methods [7, 48]. The different chemical profiles of the sources of plant materials affect the biological activity of EOs. Therefore, determining the chemical profiles of plant materials is essential for the quality control of their products.
EOs are capable natural scavengers that can diminish free-radical generation. They are offered as potential alternatives to artificial preservatives [48]. EOs react with the DPPH radical (deep-violet color) and alter it to hydrazine (DPPH-H discoloration). The extent of discoloration indicates the scavenging capability of samples [49]. This mechanism occurs due to the presence of antioxidants, which donate electrons/hydrogen atoms to stable radicals. Free-radical scavenging is generally indicated as the percentage of DPPH inhibition and the concentration essential for a 50% DPPH reduction (IC50). The present results indicated that O. basilicum oil had a more noticeable activity than O. americanum oil, with an IC50 value lower (11.56 ± 0.89 µg/mL) than that of O. americanum (13.42 ± 1.03 µg/mL). However, the standards, which included ascorbic acid (3.72 ± 0.60 µg/mL), rutin (4.65 ± 0.52 µg/mL), and BHT (7.89 ± 0.49 µg/mL), exhibited higher activities. These responses are consistent with those of Hazrati et al. [7] and Rezzoug et al. [27], who reported that EOs were less active than standard antioxidants.
Commonly, the antioxidant capability of oils is associated with their major bioactive constituents [50]. In a previous study, EOs, such as camphor, α-pinene, terpinene-4-ol borneol, eucalyptol, p-cymene, and β-pinene, were tested separately for their antioxidant activity, and the findings revealed their low free-radical scavenging capacities [51]. Zengin and Baysal [52] observed that linalool and α-terpineol have poor radical-scavenging capacity. Camphor, limonene, longifolene, caryophyllene, isoledene, β-citral, and estragole were the most abundant chemical constituents in O. americanum and O. basilicum in our study. This composition may clarify why oils display low antioxidant capability compared with synthetics. In numerous reports, the antioxidant capacity of EOs was determined by the occurrence of phenolic chemical constituents and synergistic effects of major chemical compounds, consistent with our results [7, 53–55]. EOs are a mixture of many chemical constituents derived from secondary metabolism. According to Tohidi et al. [56], oxygenated monoterpenes display high antioxidant, antifungal, and antibacterial activities. However, minor chemical constituents play an important role, and the constituents/combinations that are accountable for their antioxidant activities are not well known [57].
Usually, EOs and their chemical constituents are highly hydrophobic, which permits bacteria to lose their cell constituents and cause cell death [58]. The antibacterial activity of O. basilicum and O. americanum was studied in several bacteria. The agar-disk method is cheap, simple, and reproducible [59]. The EO of O. basilicum exhibited superior antibacterial activity. The MIC values of O. basilicum oil were lower than those of O. americanum oil. Similar observations were obtained for other EOs [60, 61]. In all tested organisms, the MBC was similar to the MIC values, which proved the antibacterial capability of these EOs. A similar observation was achieved in earlier research [59, 61].
The presence of oxygenated monoterpenes, such as geraniol formate, geraniol, citronellyl formate, citronellol, and linalool (64.93%–80.31%), as the main constituents is responsible for the EOs strong antibacterial activity against almost all susceptible microorganisms. According to specific studies, crude EOs have greater antibacterial activity than single compounds [62]. The antimicrobial activity of EOs has been linked to their hydrophobic nature, which allows them to penetrate the gram-positive bacterial cell membrane and inactivate molecular mechanisms that cause cell death [4, 7, 63].
Table 5 demonstrates that the larval mortality of A. aegypti (second and third instars) increased after the treatment with O. basilicum and O. americanum EOs at different concentrations (25–400 ppm). The treatment with O. basilicum EO at 25 ppm caused 41.60% and 42.35% mortalities in second- and third-instar larvae, respectively, and the values increased to 93.20% and 95.50% after a 24 h treatment period (Table 5). A similar tendency has been observed for both instars of A. aegypti at different concentrations of O. americanum EOs at 12 and 24 h. Mortality was dose-dependent in our study. Earlier reports showed agreement with our statement [64–66]. According to our findings, the chi-square value in O. basilicum for second-instar and third-instar larvicidal activity was significant at p ≤ 0. 05. This result indicates that O. basilicum essential oil was potent against A. aegypti (dengue fever vector). Likewise, Eos from Artemisia vulgaris showed the chi-square value (χ2 = 3.14) for third-stage A. aegypti [67]. The results for larvicidal capability revealed that the ratio of deaths was directly proportional to the oil concentration which demonstrated that concentration plays a vital role in larvicidal activity. In all the analyses, the mortality was found to increase when the concentration increased. The higher the concentration of toxic substances, the greater the potential of their toxic effect was observed. EOs isolated from plants have been documented as botanical pesticides [68]. The EO of Ipomoea cairica exhibited 100% mortality against Anopheles subpictus, Culex tritaeniorhynchus, and Aedes albopictus [69]. Tiwary et al. [70] perceived the larvicidal effects of linalool against A. stephensi (LC50 = 58 ppm), C. quinquefasciatus (LC50 = 49 ppm), and A. aegypti (LC50 = 54 ppm). Cheng et al. [71] observed that 36.0–86.8 µg/mL EOs were required to kill A. aegypti. Cavalcanti et al. [72] determined that the larvicidal efficacy of Brazil oils against Aedes aegypti ranged from 60 µg/mL to 69 µg/mL. Rahuman et al. [73] also perceived that Feronia limonia dried leaves were associated with high activity against C. quinquefasciatus, A. stephensi, and A. aegypti. The larvicidal efficacy of Thymus vulgaris, Mentha arvensis, Cymbopogan citratus, P. graveolens, and O. basilicum EOs were investigated against C. quinquefasciatus. Cheng et al. [71] reported that 1,8-cineole and eugenol from O. gratissimum had LC50 of 60 ppm against A. aegypti larvae. A similar activity was observed on the oils of O. basilicum and O. americanum. Early researchers identified the efficiency of EOs against mosquito larvae and adults [74]. Moreover, previous studies noted the larvicidal activity of EOs isolated from different plants, such as the Rutaceae plants [75] Eucalyptus globulus [76] and Syzygium [77]. Other works reported that the EOs extracted from different plants show characteristic chemical composition and affect various biological properties [77–81]. For example, the four main constituents of Rutaceae (Citrus aurantiumare) oil, namely, diethyl o-phthalate, limonene, methyl dihydrojasmonatem, and limonene, exhibit the highest larvicidal activity against Aedes albopictus [75]. The five major components identified in Eucalyptus globulus oil are 1,8-cineole (or eucalyptol), α-pinene, trans-pinocarveol, aromadendrene, and globulol. These compounds showed a potential larvicidal activity against Anopheles stephensi [76]. Compared with O. americanum and O. basilicum oils, some major chemical components, such as camphor, estragole, levomenol, and veridiflorol, differ from those in other works [75–77]. In this study, several factors were responsible for the good larvicidal activity against Aedes aegypti. Mainly, the activity may be related to their major chemical components in both oils, and the minor components can also contribute by acting in a synergetic manner [77]. In addition, the age and distribution of plants can affect larvicidal activity [81].
Conclusions
The results demonstrated that camphor, limonene, longifolene, caryophyllene and estragole are the most abundant components in O. basilicum and O. americanum. This study discovered that the oils of both plants have strong antibacterial and larvicidal activities but a low antioxidant activity. These EOs should be further evaluated to develop safe agents for larvicidal therapy. Further studies should be conducted to reveal the mode of action of these oils to understand their possible roles in human wealth.
Acknowledgements
We would like to express our gratitude to the Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand, and GM thankful to the Second Century Fund (C2F), Chulalongkorn University, for C2F Postdoctoral Fellowship. We gratefully acknowledge Dr. Pannipa Janta who is a postdoctoral researcher from the Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand for her help in revision throughout the manuscript.
Abbreviations
- EO
Essential oil
- GC–MS
Gas chromatography–mass spectrometry
- O. basilicum
Ocimum basilicum
- O. americanum
Ocimum americanum
- DPPH
2,2-Diphenylpicrylhydrazyl
- FRAP
Ferric reducing antioxidant power
- ABTS
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid
- MIC
Minimal inhibitory concentration
- MBC
Minimum bactericidal concentration
- LC50 and LC90
50% And 90% lethal concentration, respectively
- IC50
Half-maximal inhibitory concentration
- ppm
Parts per million
- BHT
Butylated hydroxytoluene
- ZI
Zone of inhibition
- EDTA
Ethylenediaminetetraacetic acid
Authors’ contributions
G.M. designed, and conceptualized the study, analyzed the data, drafted the report, and prepared the final manuscript. S.V. reviewed, revised, and edited the draft and the final manuscript. The author(s) read and approved the final manuscript.
Authors’ information
Not applicable.
Funding
Not applicable.
Availability of data and materials
All relevant data are included within the manuscript and are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All methods complied with relevant institutional, national, and international guidelines, and legislation. We furthermore declare that there is no ethical issue in our experiments.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ganesan Mahendran, Email: saidhana30@gmail.com.
Sornkanok Vimolmangkang, Email: sornkanok.v@pharm.chula.ac.th.
References
- 1.Sobrinho AC, de Souza EB, Rocha MF, Albuquerque MR, Bandeira PN, dos Santos HS, de Paula Cavalcante CS, Oliveira SS, Aragão PR, de Morais SM, dos Santos Fontenelle RO. Chemical composition, antioxidant, antifungal and hemolytic activities of essential oil from Baccharis trinervis (Lam.) Pers. (Asteraceae) Ind Crops Prod. 2016;84:108–15. doi: 10.1016/j.indcrop.2016.01.051. [DOI] [Google Scholar]
- 2.Nazir N, Zahoor M, Uddin F, Nisar M. Chemical composition, in vitro antioxidant, anticholinesterase, and antidiabetic potential of essential oil of Elaeagnus umbellata Thunb. BMC Complement Altern Med Ther. 2021;21(1):1–3. doi: 10.1186/s12906-021-03228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardaweel SK, Bakchiche B, ALSalamat HA, Rezzoug M, Gherib A, Flamini G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L (Lamiaceae) from Algerian Saharan atlas. BMC Complement Altern Med Ther. 2018;18(1):1–7. doi: 10.1186/s12906-018-2274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancarz GF, Laba LC, Silva TA, de Santi Pazzim M, de Souza D, Prado MR, de Souza LM, Nakashima T, Mello RG. Chemical composition and biological activity of Liquidambar styraciflua L. Leaf essential oil. Ind Crops Prod. 2019;138:111446. doi: 10.1016/j.indcrop.2019.06.009. [DOI] [Google Scholar]
- 5.Amirzadeh M, Soltanian S, Mohamadi N. Chemical composition, anticancer and antibacterial activity of Nepeta mahanensis essential oil. BMC Complement Altern Med Ther. 2022;22(1):1–2. doi: 10.1186/s12906-022-03642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He F, Wang W, Wu M, Fang Y, Wang S, Yang Y, Ye C, Xiang F. Antioxidant and antibacterial activities of essential oil from Atractylodes lancea rhizomes. Ind Crops Prod. 2020;153:112552. doi: 10.1016/j.indcrop.2020.112552. [DOI] [Google Scholar]
- 7.Hazrati S, Govahi M, Sedaghat M, Kashkooli AB. A comparative study of essential oil profile, antibacterial and antioxidant activities of two cultivated Ziziphora species (Z. clinopodioides and Z. tenuior) Ind Crops Prod. 2020;157:112942. doi: 10.1016/j.indcrop.2020.112942. [DOI] [Google Scholar]
- 8.Matkowski A, Kus P, Goralska E, Wozniak D. Mangiferin–a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev Med Chem. 2013;13(3):439–455. doi: 10.2174/138955713804999838. [DOI] [PubMed] [Google Scholar]
- 9.Wu CR, Lin WH, Hseu YC, Lien JC, Lin YT, Kuo TP, Ching H. Evaluation of the antioxidant activity of five endemic Ligustrum species leaves from Taiwan flora in vitro. Food Chem. 2011;127(2):564–571. doi: 10.1016/j.foodchem.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Atif M, Ilavenil S, Devanesan S, AlSalhi MS, Choi KC, Vijayaraghavan P, Alfuraydi AA, Alanazi NF. Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind Crops Prod. 2020;147:112239. doi: 10.1016/j.indcrop.2020.112239. [DOI] [Google Scholar]
- 11.Noumedem JA, Mihasan M, Kuiate JR, Stefan M, Cojocaru D, Dzoyem JP, Kuete V. In vitro antibacterial and antibiotic-potentiation activities of four edible plants against multidrug-resistant gram-negative species. BMC Complement Altern Med. 2013;13(1):1. doi: 10.1186/1472-6882-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bautista-Baños S, Hernández-López M, Bosquez-Molina E, Wilson CL. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, Anthracnose levels and quality of papaya fruit. Crop Prot. 2003;22(9):1087–1092. doi: 10.1016/S0261-2194(03)00117-0. [DOI] [Google Scholar]
- 13.Botelho ADS, Ferreira OO, de Oliveira MS, Cruz JN, Chaves SHDR, do Prado AF, Nascimento LDD, da Silva GA, Amarante CBD, Andrade EHDA. Studies on the phytochemical profile of Ocimum basilicum var. minimum (L.) Alef. essential oil, its larvicidal activity and in silico interaction with acetylcholinesterase against Aedes aegypti (Diptera: Culicidae) Int J Mol Sci. 2022;23(19):11172. doi: 10.3390/ijms231911172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Rocha GomesVoris D, dos Santos Diaz L, Alencar Lima J, dos Santos Cople Lima K, Pereira Lima JB, dos Santos Lima AL. Evaluation of larvicidal, adulticidal, and anticholinesterase activities of essential oils of Illicium verum Hook. f., Pimenta dioica (L.) Merr., and Myristica fragrans Houtt. against Zika virus vectors. Environ Sci Pollut Res. 2018;25(23):22541–51. doi: 10.1007/s11356-018-2362-y. [DOI] [PubMed] [Google Scholar]
- 15.Pereira Filho AA, Pessoa GC, Yamaguchi LF, Stanton MA, Serravite AM, Pereira RH, Neves WS, Kato MJ. Larvicidal activity of essential oils from Piper species against strains of Aedes aegypti (Diptera: Culicidae) resistant to pyrethroids. Front Plant Sci. 2021;12:685864. doi: 10.3389/fpls.2021.685864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luz TR, Leite JA, de Mesquita LS, Bezerra SA, Silveira DP, de Mesquita JW, Gomes RE, Vilanova CM, de Sousa Ribeiro MN, do Amaral FM, Coutinho DF. Seasonal variation in the chemical composition and biological activity of the essential oil of Mesosphaerum suaveolens (L.) Kuntze. Ind Crops Prod. 2020;153:112600. doi: 10.1016/j.indcrop.2020.112600. [DOI] [Google Scholar]
- 17.Akrami AR, Vatandoost H, Ghoodarzi S, Abai MR, Toofighi Z. Study on components and larvicidal activities of essential oils of different parts of Prangos ferulacea plant against larvae of Cx. quinquefasciatus and Anopheles stephensi. Int J Trop Insect Sci. 2022;42(4):3151–9. doi: 10.1007/s42690-022-00865-9. [DOI] [Google Scholar]
- 18.Li J, Tang X, Chen B, Zheng W, Yan Z, Zhang Z, Li J, Su K, Ang S, Wu R, Zhang K. Chemical compositions and anti-mosquito activity of essential oils from Pericarpium Citri Reticulataes of different aging years. Ind Crops Prod. 2022;188:115701. doi: 10.1016/j.indcrop.2022.115701. [DOI] [Google Scholar]
- 19.Chowdhury T, Mandal A, Roy SC, De Sarker D. Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC–MS) analysis. J Genet Eng Biotechnol. 2017;15(1):275–286. doi: 10.1016/j.jgeb.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed Abdoul-Latif F, Elmi A, Merito A, Nour M, Risler A, Ainane A, Bignon J, Ainane T. Essential oils of Ocimum basilicum L. and Ocimum americanum L. from Djibouti: chemical composition, antimicrobial and cytotoxicity evaluations. Processes. 2022;10(9):1785. doi: 10.3390/pr10091785. [DOI] [Google Scholar]
- 21.Padalia RC, Verma RS, Upadhyay RK, Chauhan A, Singh VR. Productivity and essential oil quality assessment of promising accessions of Ocimum basilicum L. from north India. Ind Crops Prod. 2017;97:79–86. doi: 10.1016/j.indcrop.2016.12.008.. [DOI] [Google Scholar]
- 22.Saha S, Dhar TD, Sengupta C, Ghosh P. Biological activities of essential oils and methanol extracts of five Ocimum species against pathogenic bacteria. Czech J Food Sci. 2013;31(2):195–202. doi: 10.17221/234/2012-CJFS. [DOI] [Google Scholar]
- 23.Srivastava S, Lal RK, Yadav K, Pant Y, Bawitlung L, Kumar P, Mishra A, Gupta P, Pal A, Rout PK, Chanotiya CS. Chemical composition of phenylpropanoid rich chemotypes of Ocimum basilicum L. and their antimicrobial activities. Ind Crops Prod. 2022;183:114978. doi: 10.1016/j.indcrop.2022.114978. [DOI] [Google Scholar]
- 24.Pandey AK, Singh P, Tripathi NN. Chemistry and bioactivities of essential oils of some Ocimum species: an overview. Asian Pac J Trop Biom. 2014;4(9):682–94. doi: 10.12980/APJTB.4.2014C77. [DOI] [Google Scholar]
- 25.Chou ST, Lai CC, Lai CP, Chao WW. Chemical composition, antioxidant, anti-melanogenic and anti-inflammatory activities of Glechoma hederacea (Lamiaceae) essential oil. Ind Crops Prod. 2018;122:675–685. doi: 10.1016/j.indcrop.2018.06.032. [DOI] [Google Scholar]
- 26.Pirmoradi MR, Moghaddam M, Farhadi N. Chemotaxonomic analysis of the aroma compounds in essential oils of two different Ocimum basilicum L. varieties from Iran. Chem Biodivers. 2013;10(7):1361–71. doi: 10.1002/cbdv.201200413. [DOI] [PubMed] [Google Scholar]
- 27.Rezzoug M, Bakchiche B, Gherib A, Roberta A, Kilinçarslan Ö, Mammadov R, Bardaweel SK. Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement Altern Med. 2019;19(1):1. doi: 10.1186/s12906-019-2556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sajjadi SE. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. Daru J Pharm Sci. 2006;14(3):128–30. [Google Scholar]
- 29.Telci I, Bayram E, Yılmaz G, Avcı B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.) Biochem Syst Ecol. 2006;34(6):489–97. doi: 10.1016/j.bse.2006.01.009. [DOI] [Google Scholar]
- 30.Souza Filho AP, Bayma JC, Guilhon GM, Zoghbi MG. Potentially allelophatic activity of the essential oil of Ocimum americanum. Planta Daninha. 2009;27:499–505. doi: 10.1590/S0100-83582009000300010. [DOI] [Google Scholar]
- 31.Oyedemi SO, Oyedemi BO, Coopoosamy RM, Prieto JM, Stapleton P, Gibbons S. Antibacterial and norfloxacin potentiation activities of Ocimum americanum L. against methicillin resistant Staphylococcus aureus. S Afr J Bot. 2017;109:308–14. doi: 10.1016/j.sajb.2016.12.025. [DOI] [Google Scholar]
- 32.Bassole IH, Nebie R, Savadogo A, Ouattara CT, Barro N, Traore SA. Composition and antimicrobial activities of the leaf and flower essential oils of Lippia chevalieri and Ocimum canum from Burkina Faso. Afr J Biotechnol. 2005;4(10):1156–1160. [Google Scholar]
- 33.Aluko BT, Oloyede OI, Afolayan AJ. Hepatoprotective activity of Ocimum americanum L leaves against paracetamol–induced liver damage in rats. Am J Life Sci. 2013;1(2):37–42. [Google Scholar]
- 34.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 35.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 36.Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48(8):3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 37.Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315(1):161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 38.Mahendran G, Kumari BR. Biological activities of silver nanoparticles from Nothapodytes nimmoniana (Graham) Mabb. fruit extracts. Food Sci Hum Wellness. 2016;5(4):207–18. doi: 10.1016/j.fshw.2016.10.001. [DOI] [Google Scholar]
- 39.Duarte A, Ferreira S, Silva F, Domingues FC. Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomed. 2012;19(3–4):236–238. doi: 10.1016/j.phymed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Shai LJ, McGaw LJ, Aderogba MA, Mdee LK, Eloff JN. Four pentacyclic triterpenoids with antifungal and antibacterial activity from Curtisia dentata (Burm f) CA Sm. leaves. J Ethnopharmacol. 2008;119(2):238–44. doi: 10.1016/j.jep.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 41.Panneerselvam C, Murugan K, Kovendan K, Mahesh KP. Mosquito larvicidal, pupicidal, adulticidal, and repellent activity of Artemisia nilagirica (Family: Compositae) against Anopheles stephensi and Aedes aegypti. Parasitol Res. 2012;111(6):2241–2251. doi: 10.1007/s00436-012-3073-9. [DOI] [PubMed] [Google Scholar]
- 42.Kačániová M, Galovičová L, Borotová P, Vukovic NL, Vukic M, Kunová S, Hanus P, Bakay L, Zagrobelna E, Kluz M, Kowalczewski PŁ. Assessment of Ocimum basilicum wssential oil anti-insect activity and antimicrobial protection in fruit and vegetable quality. Plants. 2022;11(8):1030. doi: 10.3390/plants11081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mokat DN, Kharat TD. Essential oil composition in leaves of Ocimum species found in Western Maharashtra. India J Essent Oil-Bear Plants. 2022;25(1):1–8. doi: 10.1080/0972060X.2022.2043190. [DOI] [Google Scholar]
- 44.Singh S, Tewari G, Pande C, Singh C. Variation in essential oil composition of Ocimum americanum L. from north-western Himalayan region. J Essent Oil Res. 2013;25(4):278–90. doi: 10.1080/10412905.2013.775079. [DOI] [Google Scholar]
- 45.Martín T, Rubio B, Villaescusa L, Fernández L, Díaz AM. Polyphenolic compounds from pericarps of Myrtus communis. Pharm Biol. 1999;37(1):28–31. doi: 10.1076/phbi.37.1.28.6327. [DOI] [Google Scholar]
- 46.Chagonda LS, Makanda CD, Chalchat JC. The essential oils of Ocimum canum Sims (basilic camphor) and Ocimum urticifolia Roth from Zimbabwe. Flavour Fragr J. 2000;15(1):23–26. doi: 10.1002/(SICI)1099-1026(200001/02)15:1<23::AID-FFJ866>3.0.CO;2-W. [DOI] [Google Scholar]
- 47.Selvi MT, Thirugnanasampandan R, Sundarammal S. Antioxidant and cytotoxic activities of essential oil of Ocimum canum Sims from India. J Saudi Chem Soc. 2015;19(1):97–100. doi: 10.1016/j.jscs.2011.12.026. [DOI] [Google Scholar]
- 48.Mohafrash SM, Fallatah SA, Farag SM, Mossa AT. Mentha spicata essential oil nanoformulation and its larvicidal application against Culex pipiens and Musca domestica. Ind Crops Prod. 2020;157:112944. doi: 10.1016/j.indcrop.2020.112944. [DOI] [Google Scholar]
- 49.Horvathova E, Navarova J, Galova E, Sevcovicova A, Chodakova L, Snahnicanova Z, Melusova M, Kozics K, Slamenova D. Assessment of antioxidative, chelating, and DNA-protective effects of selected essential oil components (eugenol, carvacrol, thymol, borneol, eucalyptol) of plants and intact Rosmarinus officinalis oil. J Agric Food Chem. 2014;62(28):6632–9. doi: 10.1021/jf501006y. [DOI] [PubMed] [Google Scholar]
- 50.Sarikurkcu C, Arisoy K, Tepe B, Cakir A, Abali G, Mete E. Studies on the antioxidant activity of essential oil and different solvent extracts of Vitex agnus-castus L. fruits from Turkey. Food Chem Toxicol. 2009;47(10):2479–83. 10.1021/jf501006y. [DOI] [PubMed]
- 51.Riahi L, Elferchichi M, Ghazghazi H, Jebali J, Ziadi S, Aouadhi C, Chograni H, Zaouali Y, Zoghlami N, Mliki A. Phytochemistry, antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L. in Tunisia. Ind Crops Prod. 2013;49:883–9.10.1016/j.indcrop.2013.06.032.
- 52.Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sökmen A, Akpulat HA. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol. 2003;87(2-3):215–20. 10.1016/S0378-8741(03)00149-1. [DOI] [PubMed]
- 53.Zengin H, Baysal AH. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19(11):17773–98. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Masry MH, Khalil AI, Hassouna MS, Ibrahim HA. In situ and in vitro suppressive effect of agricultural composts and their water extracts on some phytopathogenic fungi. World J Microbiol Biotechnol. 2002;18(6):551–8. doi: 10.1023/A:1016302729218. [DOI] [Google Scholar]
- 55.López V, Akerreta S, Casanova E, García-Mina JM, Cavero RY, Calvo MI. In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Foods Hum. Nutr. 2007;62(4):151–5. doi: 10.1007/s11130-007-0056-6. [DOI] [PubMed] [Google Scholar]
- 56.Fallah S, Mouguee S, Rostaei M, Adavi Z, Lorigooini Z. Chemical compositions and antioxidant activity of essential oil of wild and cultivated Dracocephalum kotschyi grown in different ecosystems: a comparative study. Ind Crops Prod. 2020;143:111885. doi: 10.1016/j.indcrop.2019.111885. [DOI] [Google Scholar]
- 57.Tohidi B, Rahimmalek M, Arzani A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017;220:153–61. doi: 10.1016/j.foodchem.2016.09.203. [DOI] [PubMed] [Google Scholar]
- 58. Wang W, Wu N, Zu YG, Fu YJ. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008;108(3):1019–22. 10.1016/j.foodchem.2007.11.046. [DOI] [PubMed]
- 59.Luís Â, Duarte A, Gominho J, Domingues F, Duarte AP. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind Crops Prod. 2016;79:274–82. doi: 10.1016/j.indcrop.2015.10.055. [DOI] [Google Scholar]
- 60.Bachir RG, Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac J Trop Biom. 2012;2(9):739–42. doi: 10.1016/S2221-1691(12)60220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Silva F, Ferreira S, Queiroz JA, Domingues FC. Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry. J Med Microbiol. 2011;60(10):1479–86. [DOI] [PubMed]
- 62.Radaelli M, Silva BP, Weidlich L, Hoehne L, Flach A, Costa LA, Ethur EM. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz J Microbiol. 2016;47:424–30. doi: 10.1016/j.bjm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorman HD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308–16. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 64. Sayout A, Ouarhach A, Dilagui I, Soraa N, Romane A. Antibacterial activity and chemical composition of essential oil from Lavandula tenuisecta Coss. ex Ball. an endemic species from Morocco. Eur J Integr Med. 2020;33:101017. 10.1016/j.eujim.2019.101017.
- 65.Pavela R. Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crops Prod. 2015;76:174–87. doi: 10.1016/j.indcrop.2015.06.050. [DOI] [Google Scholar]
- 66.Murugan K, Benelli G, Panneerselvam C, Subramaniam J, Jeyalalitha T, Dinesh D, Nicoletti M, Hwang JS, Suresh U, Madhiyazhagan P. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp Parasitol. 2015;153:129–38. doi: 10.1016/j.exppara.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Govindarajan M, Rajeswary M, Veerakumar K, Muthukumaran U, Hoti SL, Mehlhorn H, Barnard DR, Benelli G. RETRACTED ARTICLE: Novel synthesis of silver nanoparticles using Bauhinia variegata: a recent eco-friendly approach for mosquito control. Parasitol Res. 2016;115(2):723–33. doi: 10.1007/s00436-021-07295-5. [DOI] [PubMed] [Google Scholar]
- 68.Balasubramani S, Sabapathi G, Moola AK, Solomon RV, Venuvanalingam P, Bollipo Diana RK. Evaluation of the leaf essential oil from Artemisia vulgaris and its larvicidal and repellent activity against dengue fever vector Aedes aegypti—an experimental and molecular docking investigation. ACS Omega. 2018;3(11):15657–65. doi: 10.1021/acsomega.8b01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gbolade AA, Oyedele AO, Sosan MB, Adewoyin FB, Soyelu OL. Mosquito repellent activities of essential oils from two Nigerian Ocimum species. J Trop Med Plants. 2000;1(1/2):146–8. [Google Scholar]
- 70.Tiwary M, Naik SN, Tewary DK, Mittal PK, Yadav S. Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. J Vector Borne Dis. 2007;44(3):198. [PubMed] [Google Scholar]
- 71.Cheng SS, Liu JY, Huang CG, Hsui YR, Chen WJ, Chang ST. Insecticidal activities of leaf essential oils from Cinnamomum osmophloeum against three mosquito species. Bioresour Technol. 2009;100(1):457–464. doi: 10.1016/j.biortech.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 72. Cheng SS, Liu JY, Huang CG, Hsui YR, Chen WJ, Chang ST. Insecticidal activities of leaf essential oils from Cinnamomum osmophloeum against three mosquito species. Bioresour Technol. 2009;100(1):457–64. 10.1016/j.biortech.2008.02.030. [DOI] [PubMed]
- 73.Rahuman AA, Gopalakrishnan G, Ghouse BS, Arumugam S, Himalayan B. Effect of Feronia limonia on mosquito larvae. Fitoterapia. 2000;71(5):553–555. doi: 10.1016/S0367-326X(00)00164-7. [DOI] [PubMed] [Google Scholar]
- 74.Dua VK, Pandey AC, Raghavendra K, Gupta A, Sharma T, Dash AP. Larvicidal activity of neem oil ( Azadirachta indica) formulation against mosquitoes. Malar J. 2009;8(1):1–6. doi: 10.1186/1475-2875-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jian R, Lin Y, Li Y, Wu W, Ren X, Liang Z, Kong L, Cai J, Lao C, Wu M, Chen W, Chen J, Hong WD, Sheng Z. Larvicidal activity of two Rutaceae plant essential oils and their constituents against Aedes albopictus (Diptera: Culicidae) in multiple formulations. J Med Entomol. 2022;59:1669–1677. doi: 10.1093/jme/tjac083. [DOI] [PubMed] [Google Scholar]
- 76.Alipanah H, Abdollahi A, Firooziyan S, Zarenezhad E, Jafari M, Osanloo M. Nanoemulsion and nanogel containing Eucalyptus globulus essential oil; larvicidal activity and antibacterial properties. Interdiscip Perspect Infect Dis. 2022;2022:1–9. doi: 10.1155/2022/1616149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huong LT, Thinh BB, Hung NH, Phu HV, Hieu NC, Dai DN. Chemical composition, antimicrobial and larvicidal activities of essential oils of two Syzygium species from Vietnam. Braz J Biol. 2023;84:e270967. doi: 10.1590/1519-6984.270967. [DOI] [PubMed] [Google Scholar]
- 78.Bassolé IHN, Guelbeogo WM, Nebie R, Costantini C, Sagnon N, Kabore ZI, Traore SA. Ovicidal and larvicidal activity against Aedes aegypti and Anopheles gambiae complex mosquitoes of essential oils extracted from three spontaneous plants of Burkina Faso. Parassitologia. 2003;45(1):23–26. [PubMed] [Google Scholar]
- 79.Chau DTM, Chung NT, Huong LT, Hung NH, Ogunwande IS, Dai DN, Setzer WN. Chemical compositions, mosquito larvicidal and antimicrobial activities of leaf essential oils of eleven species of Lauraceae from Vietnam. Plants. 2020;9(5):606. doi: 10.3390/plants9050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Senthilkumar A, Venkatesalu V. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi: a malarial vector mosquito. Parasitol Res. 2010;107(5):1275–1278. doi: 10.1007/s00436-010-1996-6. [DOI] [PubMed] [Google Scholar]
- 81.Senthilkumar A, Venkatesalu V. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi: a malarial vector mosquito. Parasitol Res. 2010;107(5):1275–8. 10.1007/s00436-010-1996-6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included within the manuscript and are available from the corresponding author upon reasonable request.