Abstract
Turicella otitidis belongs to the Corynebacteriaceae family and is a normal inhabitant of the ear and exists in a commensal relationship with its host. In children, T. otitidis is frequently associated with otitis media. The emergence of Turicella otitidis as a pathogen is concerning, particularly due to the limited availability of data on its pathogenic properties. The objective of this study is to conduct a systematic review of T. otitidis infections occurring in both the ear and other anatomical sites, and to summarize the differences in metabolism and genome sequences between isolates obtained from the ear and blood.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-023-08721-y.
Keywords: Turicella otitidis, Otitis media, Extra-otic, Corynebacterium
Introduction
Turicella otitidis is a Gram-positive Corynebacterium that grows aerobically [1]. It is commonly part of normal ear resident flora, although it is frequently linked to external ear canal infection and acute and chronic otitis media in children [2]. Recent reports showed that the organism can cause extra-otic infections such as microbial keratitis, bacteremia, and posterior auricular abscess [3] in patients with or without underlying conditions, indicating its pathogenicity. In a diagnostic microbiology laboratory, T. otitidis is often not distinguished from commensal skin coryneform isolates. Depending on the source of the specimen and the number of specimens sent for culture, such as blood culture, the organism is reported as “Corynebacterium species, not C. jeikeium or C. striatum”. Nevertheless, advanced methodologies such as Matrix-Assisted Laser Desorption/Ionization Mass Spectroscopy (MALDI-TOF MS) have facilitated the accurate and rapid speciation of Corynebacterium [4]. Despite this, the traditional API Coryne method remains fundamental to the identification process, and further biochemical reactions, such as catalase, CAMP, DNase positive reaction, and oxidase negative reaction, aid in the identification of Turicella otitidis. Corynebacterium species are commonly treated with penicillin, macrolides, rifampin, vancomycin, and fluoroquinolones. Notably, T. otitidis exhibits resistance towards sulfamethoxazole, cotrimoxazole, and macrolide [5]. Thus, accurate identification and speciation are imperative for optimal treatment.
Since T. otitidis has traditionally been deemed non-pathogenic [6], little attention has been paid to its clinical relevance. Recent reports of extra-otic infections warrant the need to study the factors associated with the organism’s pathogenicity and adaptability in various niches. As the genome code and metabolism are crucial for all biological processes, including virulence, it is imperative to examine the factors and mechanisms underlying the virulence of T. otitidis. We herein summarized the information on the metabolism and genome sequence variation in Turicella otitidis isolated from two distinct sources, namely the ear and blood, as well as cases of otic- and extra-otic infection resulted from T. otitidis.
Objective
Turicella otitidis is frequently involved in both acute and chronic otitis media. Its adaptability and isolation from extra-otic infections make it an emerging pathogen. This study aims to understand the niche-specific modifications in T. otitidis metabolism and genome sequences and its role in pathogenesis in extra-otic infections compared to ear infections.
Methods
We conducted a comprehensive search of the literature by utilizing multiple databases, including PubMed, Science Direct, Cochrane, EMBASE, CINAHL, and Google Scholar. Our search was focused on identifying pertinent cases of T. otitidis infection, both within the otic and extra-otic domains, spanning from 1994 to 2023. Two authors independently screened all abstracts resulting from the initial literature search, with duplicate and non-pertinent articles subsequently removed. Quality assessment was conducted to ensure that the included articles solely focused on the association or discussion of Turicella otitidis concerning infection in either the ear or other sources. To filter the articles, keywords such as “Turicella otitidis”, “T. otitidis”, “Turicella otitidis metabolism”, “Turicella otitidis genome sequences”, and “T. otitidis genome sequence and metabolism” were used (Electronic Supplementary file, S1). Articles that mentioned T. otitidis in the abstract or studies in which only ear specimens were analyzed for their microbiota and their association with ear infections were included. Exclusion criteria included duplicate and extraneous articles and papers other than case reports and retrospective studies. The search was not restricted to regions and languages, but solely to publication types. The relevance and accuracy of the articles were carefully assessed after inclusion and exclusion parameters were established. A total of seven articles were in non-English languages (NEL), specifically Spanish (5), Czech (1), and French (1). To determine the eligibility of the articles, Google Translate was initially utilized to analyze their abstracts. Eligible articles were subsequently translated by volunteers, including students, researchers, and healthcare professionals. The Stony Brook University Language Department and Clinical Pathology specifically Clinical Microbiology and Hematology departments were solicited for volunteers. The translators proofread and edited the text.
Results
A total of 4809 articles were identified from PubMed, Science Direct, Cochrane, EMBASE, CINAHL, and Google Scholar databases. After excluding duplicates (n = 2134) and non-pertinent studies (n = 2633) from the thorough article evaluation, a full-length review was performed on the eligible articles (n = 42) and additional articles (n = 4) that did not meet the inclusion criteria were excluded. A comprehensive full-text assessment of the articles was thoroughly conducted according to the PRISMA flow chart [7] (Fig. 1). In total, thirty eight articles were included in the study and two of these studies compared the metabolism changes and genome variation in T. otitidis isolated from two sources (ear and blood). Out of 36 articles, 52.7% (19 articles) were case studies (Table 1) and 47.2% (17 articles) were retrospective studies (Table 2) [1–4, 8–39]. Of the total studies, 81.5% of the articles were in English, while 18.4% were in NEL. Based on the case description, thirteen cases (54.2%) of T. otitidis infection were in males while eleven cases (45.8%) were in females. Of the total 24 cases, ages ranged from 6 months to 75 years, 37.5% of the cases were reported in age < 5 years (male = 16.7%, female = 20.8%), while 29.2% were among individuals aged 5–15 years (male = 16.7%, female = 12.5%). Furthermore, 8.3% of cases were reported in the age group of 20–35 years (male = 4.17%, female = 4.17), and an equal percentage of 8.3% was seen in the age group 40–55 years of age (male = 8.33%, female = 0). Lastly, 16.7% of the cases were reported in individuals > 55 years of age (male = 8.33%, female = 8.33%). Of the total case reports, 16 cases (66.7%) were associated with ear infections, 4 cases (16.7%) were attributed to a bloodstream infection, and 2 cases (8.3%) were associated with ocular infection. Moreover, 4.2% of the cases were linked to skin and 4.2% were associated with abscesses in the cervical region. In light of the outcome of these cases, all (100%) of the patients survived with improvement in symptoms post-treatment. Of the total twenty four cases, 50% used a combination of various techniques including API Coryne, Vitek MS, biochemical analysis, microcopy, culture, Rapid CB Plus kit, High-performance liquid chromatography (HPLC), and MALDI MS for identification of T. otitidis. Meanwhile, the remaining 50% used a single method of identification such as Culture or MALDI-TOF MS or PCR. Of the total retrospective studies, 52.9% used multiple modalities, including VITEK MS, Microscan Panels (PC42 HIND), API Coryne, API Zym, API 50CH, as well as morphological and phenotypic tests such as CAMP test, DNASE test in the identification process. On the other hand, 41.2% relied solely on a single method such as 16S rRNA sequencing, DNA sequencing, Immunoblot DNAB II proteins, Western blot and 16S metagenomics, for the identification of T. otitidis. Additionally, in one study (5.9%), method of identification was not specified. The common treatment regime was amoxicillin-clavulanic acid (25%), and vancomycin (12.5%). In 20.8% of the cases, topical treatment was used. Additionally, the utilization of amoxicillin, fosfomycin, penicillin, rifampin, gentamycin, cefotaxime, and ciprofloxacin was reported in 41.7% of the cases. These antibiotics were administered either as monotherapy or given in combination [1–4, 8–39].
Fig. 1.
PRISMA flowchart of literature search and inclusion process of studies. Adapted from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021
Table 1.
Shows the summary of Turicella otitidis case reports. NS = not specified, Polymerase Chain Reaction (PCR)
| No. | Author & year | Age & sex | Condition | Source of isolation | Method of identification | Treatment |
|---|---|---|---|---|---|---|
| 1 | Mastroianni, A., et al., 2023 [40] | 11-year old, male | Acute lymphoblastic leukemia with acute or chronic otitis, mastoiditis, sinusitis | Central venous catheter (CVC) blood | Direct microscopy, Vitek2-Vitek MS | Intravenous rifampicin, vancomycin CVC lock therapy, and CVC removal |
| 2 | Priyadarshini, S.R., et al.,2021 [36] |
10-year-old, male |
Microbial keratitis | Corneal graft | VITEK® 2 automated system | Topical gatifloxacin 0.5% eye-drops |
| 3 | Koumaki, D., et al., 2020 [33] | 74-year-old, female | Palmoplantar eczema | Skin | VITEK® 2 automated system and biochemicals | Cefuroxime |
| 4 | Mammo, D.A., D. Watson, and K.R. Armbrust,2020 [26] | 71-year-old, male | Neovascular age-related macular degeneration | Endophthalmitis | PCR | NS |
| 5 | Li, D., et al., 2019 [11] | 69-year-old, female | Diffuse large B cell lymphoma, fever | Blood | MALDI-TOF MS | Vancomycin |
| 6 | De Frutos, M., et al., 2018 [39] | 46-year-old male | Right otalgia with suppuration | Right ear | Pure culture | Oral and topical ciprofloxacin |
| 7 | De Frutos, M., et al., 2018 [39] | 2-year-old, male | Left otitis media | Left ear | Otic smear, culture | Amoxicillin |
| 8 | De Frutos, M., et al., 2018 [39] | 28-year-old, female (21 weeks pregnant) | Episodes of otitis | Ear | Culture | Topical beclomethasone and clioquinol |
| 9 | De Frutos, M., et al., 2018 [39] | 9-year-old, female | Suppurative otitis media with left ear pain | Left ear | Smear and Culture |
Amoxicillin-clavulanic acid and topical ciprofloxacin |
| 10 | De Frutos, M., et al., 2018 [39] | 13-month-old, male | Erythema in the left retroauricular area and detachment of the auricular pavilion | Ear exudate | Culture | Meropenem and oral amoxicillin-clavulanic acid |
| 11 | Halle, T.R., N.W. Todd, and J. Fainberg, 2017 [18] | 10-month-old female | Recurrent acute otitis media | Right ear | Culture | Ciprodex-otic drops, bilateral cochlear implantation |
| 12 | Bîrluţiu, V et al., 2017 [12] | 75-years-old, male | Spastic paraplegia, and confusion, altered general condition | Blood culture | API® Coryne and culture | Fosfomycin |
| 13 | Gaona, C.E. and J.S. Castañón, 2017 [25] | 6 year old, female | Otitis externa | Ear exudate | Gram stain, media growth, API Coryne, Vitek MS system | Ciprofloxacin ear drops |
| 14 | Gaona, C.E. and J.S. Castañón, 2017 [25] | 53-year old, male | Right otorrhea with purulent discharge | Right ear | Gram stain, media growth, API Coryne, Vitek MS system | Tobramycin ear drops |
| 15 | Johnson, A.K. and B. Isaacson., 2016 [17] | 4-year-old, male | Progressive right post-auricular erythema, otalgia, fever, and vomiting | Purulent middle ear effusion | Culture | Intravenous antibiotics |
| 16 | MA, S.C. 2014 [41] | 14-month-old female |
Fever, retroauricular edema with detachment of the left pinna. |
Otic exudate | Culture |
Cefotaxime and Amoxicillin-clavulanic acid |
| 17 | Ježek, P., et al., 2011 [29] | 4 year old, male | Otitis media and spontaneous tympanic membrane perforation | Left ear | Rapid CB Plus kit, MALDI MS, culture, biochemicals | Beta-Lactam |
| 18 | Jeziorski, E., et al.2009 [21] | 3 years and 3 months old, female |
Acute perforated otitis media complicated by mastoiditis |
Spontaneous otorrhoea | Smear and culture | Cefotaxime, vancomycin, amoxicillin–clavulanic acid |
| 19 | Poulter, M.D. and C.J. Hinnebusch, 2005 [35] | 23-year-old, male | Right tympanic membrane retracted with a middle ear effusion | Middle ear effusion | Smear, culture, API Coryne system, and biochemicals | Augmentin |
| 20 | C. Loiez et al., 2002 [34] | 10-year-old, male | Acute lymphoblastic leukemia B | Blood culture and ear swab | API Coryne system, culture, CAMP test | Oral amoxicillin |
| 21 | Dana, A., R. Fader, and D. Sterken, 2001 [2] | 5-year-old, female | Bilateral ear pain | Right and left middle | Gram Stain, API Coryne, culture | Intravenous cefotaxime |
| 22 | Reynolds, S.J., M. Behr, and J. McDonald, 2001 [3] | 3-year-old, female | Pain and swelling behind her right ear | Ear | PCR, culture, CAMP test, High-performance liquid chromatography analysis | Intravenous cefuroxime and cloxacillin |
| 23 | Fernandez Perez, A., et al. 1996 [13] | 7-year-old, male | Upper right neck pain radiating to the mastoid region | Cervical abscess | API Coryne and culture | NS |
| 24 | Renaud, F.N., et al. 1996 [1] | 6-month-old, female | Bilateral maxillolabiopalatine cleft with mucopurulent discharge | Ear | API Coryne, culture and biochemicals | Amoxicillin-clavulanic acid |
Table 2.
Shows the summary of retrospective studies of patients with T. otitidis infection
| No. | Author and year | Age | Sample size | No. of Turicella otitidis cases or percentage | Condition | Source of isolation | Identification | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Gavrilovici, C., et al., 2022 [9] | 2 months-7 years | 147 | n = 1 | Acute otitis media | Pus | MICROSCAN panels (PC42, HIND) and MALDI-TOF MS | NA | |
| 2 | Mendez-Legaza, J.M., et al.,2021 [23] | 0–14 years and 2–81 years | 1089 samples | n = 22 | ear exudate | External auditory canal | Culture, API Coryne, MALDI-TOF MS | ||
| 3 | Chen, T.Y., et al. 2021 [20] | NS | 107 culturable isolates | n = 10 | Otitis media | Middle ear sample | NS | ||
| 4 | Álvarez, A.S. and M.G. Coca, 2021 [32] | NS | 273 ear exudates | n = 18 | Acute otitis media | Middle ear fluid samples | ANC card of the Vitek2® system | ||
| 5 | Barron, C.L., et al., 2020 [19] | 9 months- 19 years | 38 effusion | n = 1 | Chronic middle ear effusions | Middle ear fluid | Culture, Immunoblot (DNABII proteins) | ||
| 6 | Ari O., et al., 2019 [15] | NS | 25 children | 6% | Otitis media with effusion | Ear | 16 S rRNA metagenomics | ||
| 7 | Man, W.H., et al., 2019 [28] | > 5 years | 94 children | n = 5 | Acute otitis media | Tympanostomy tube otorrhea | 16 S ribosomal RNA-based sequencing | ||
| 8 | Kolbe, A.R., et al., 2019 [10] | 3-176 months | 50 children | n = 26 | Chronic otitis media | Middle ear fluid | DNA sequencing | ||
| 9 | Kalcioglu, et al., 2018 [24] | NS | 102 samples | n = 5/26 (cholesteatomy), n = 4/18 (typanosclerotic plaque | Chronic otitis media | Middle ear | PCR, 16 ribosomal RNA | ||
| 10 | Sillanpää, S., et al., 2017 [42] | 5–42 months | 79 | n = 5 | Acute otitis media | Middle ear fluid samples | Nested-PCR amplification of the 16 S rRNA gene (V4 region), mass sequencing | ||
| 11 | Vila, P.M., et al., 2017 [22] | 2–6 years | 54 | n = 4 | Otitis media (cochlear implant) | Middle ear | Culture | ||
| 12 | Krueger, A., et al., 2017 [27] | 0–24 months (n = 25), > 24 month (n = 30) | 55 | 7.84% | Middle ear effusion | Middle ear | Western blot and DNA and molecular analysis | ||
| 13 | Quesnel, S., et al., 2010 [8] | 3 months-15 years | 188 | n = 4 | Acute mastoiditis | Drainage of retro auricular abscess, paracentesis | Culture | ||
| 14 | Jeziorski, E., et al.2009 [21] | NS | 12 | n = 12 | Acute otitis media, acute pyelonephritis, seromucous otitis, acute myeloid leukemia, acute lymphoid leukemia | Middle ear | Stain, culture, DNAse, CAMP test | ||
| 15 | Gomez-Garces, J.L., et al., 2004 [43] | 6 months-7 years | 153 ear exudates (112 patients) | n = 7 | Acute exudative otitis media or exacerbation of chronic otitis media | Middle ear | API Coryne, API Zym, API 50 CH, morphological and phenotypic tests | ||
| 16 | Holzmann, D., et al., 2002 [30] | NS | 60 children | n = 14 | Exudative otitis media | Middle ear effusion | Culture, biochemical and chemotaxonomic data | ||
| 17 | Funke, G., et al., 1994 [39] | 1–5 years | NS | NS | Otitis media | Middle ear fluid | Biochemical, API Coryne, and 16 S rRNA sequencing | ||
NS Not specified, PCR Polymerase chain reaction
We herein provide a summary of the genome and cellular fatty acid analysis of T. otitidis draft strain (TD1) isolated from a central line catheter tip culture in a patient with a history of bowel obstruction and ATCC 51,513 strain from the ear [35, 44]. Comparative studies were conducted to investigate the changes in the genome and metabolic pathways of both strains. Upon comparing the identity of TD1 to ATCC 51,523 (with a similarity of 98.75%), Greninger et al. performed genome analysis which showed that TD1 has a size of 2,150,112 bp with an N50 of 24,176 bp and a GC content of 71.2%. On the other hand, ATCC 51,513 has a size of 2,077,086 bp with an average G + C content of 71.35%. Direct mapping of TD1 reads to ATCC 51,513 revealed 20,176 variants between the two strains. Furthermore, the TD1 strain contains 85.4 kb unique sequences when compared to ATCC51513. Notably, this includes the locus of 50 kb that contains over 60 hypothetical protein-coding sequences. In TD1 strain, various genetic variants in the genome exist, which encompass exons coding for an arylsulfatase, a cadmium-cobalt antiporter, a phosphate/phosphonate transporter operon, and a unique ATP-binding cassette (ABC) transporter. These findings imply a potential role in the survival and adaptation of T. otitidis to diverse niches. Also, TD1 strain has antibiotic resistance genes, including a cfrA 50 S methyltransferase that shares 99% amino acid similarity with T. otitidis ATCC 51,513, as well as two major facilitator superfamily–type drug-resistance transporters that exhibit 99% amino acid similarity to T. otitidis ATCC 51,513 and 57–59% amino acid similarity to Corynebacterium species [35, 44].
Discussion
Turicella otitidis is a constituent of the normal ear microbiota [40] and has been linked to both acute and chronic otitis media [30]. T. otitidis is a non-branching, long, irregular, Gram-positive bacillus that forms non-hemolytic, creamy/whitish colonies after 24 h of incubation (Fig. 2A and B). The organism is negative for catalase, alkaline phosphatase, and nitrate reduction. However, it is positive for the CAMP test, leucine arylamidase, and pyrazinamidase. Additionally, it is non-motile and urease negative. The ultimate differentiation of Corynebacterium species is based on conventional diagnostics [1] together with MALDI-TOF MS. Recently, the identification of T. otitidis from extra-otic sources suggests the adaptability of the microorganism and its potential to cause significant extra-otic infections in susceptible individuals. Thus necessitating the appropriate speciation of Corynebacterium when recovered from sterile sites to ensure that patients receive optimal and timely treatment [41].
Fig. 2.
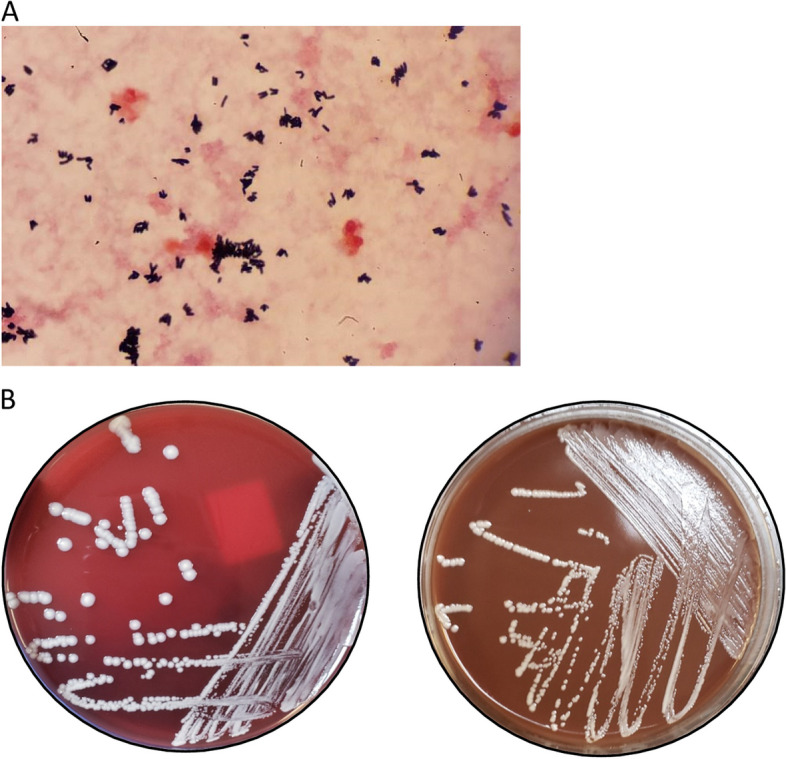
A shows pleomorphic Gram-positive rods, a characteristic morphology of Corynebacterium, “Chinese letters”. B shows the growth of non-pigmented white colonies of T. otitidis on blood and chocolate agar inoculated with a positive blood culture incubated for 24 hours at 35 OC
Human immune system is a multi-faceted confluence of intricate cellular and biochemical responses. Microorganisms react to the dynamic immune milieu by disrupting host defense mechanisms and adapting to the environment to thrive and propagate. To evade eradication by the host immune response and subsist in the new environment, bacteria employ various strategies such as genome alteration and changes to their metabolic profile [45]. In this study, we summarized genome and cellular fatty acid analysis of T. otitidis draft strain (TD1) isolated from a central line catheter tip and ATCC 51,513 strain isolated from the ear.
Unlike ATC5153, TD1 strain possesses genes for various transporters such as arylsulfatase. Arylsulfatases are enzymes that break down aryl sulfonate ester bonds to release sulfonate, a source of sulfur synthesis required in the biosynthesis of cysteine and methionine. Arylsulfatases play a role in redox reactions and are linked to bacterial pathogenesis [46]. TD1 strain also contains a cadmium-cobalt antiporter. This anti-porter helps to regulate bio-metal levels, which are important for metabolic processes and are inserted into specific metalloproteins during biosynthesis. Microorganisms adapt to environmental pressure by importing essential metals while preventing excessive accumulation and intoxication through transporters and anti-transporters [43], suggesting a similar role of these transporters in T. otitidis. In addition, TD1 possesses phosphate/phosphonate transporter operon and a unique ABC transporter. Phosphate is a crucial nutrient found as phosphorus in nature. Bacteria have diverse transport systems, including a high-affinity phosphate-specific unique ABC transporter, to ensure phosphorus availability. They also have a system for alternative phosphorus sources [47–49]. However, the role of these transporters in T. otitidis pathogenesis is not fully understood.
Microorganism’s distinctive metabolic characteristics are linked to its natural habitat. Like T. otitidis, which encodes for a four-step histidine utilization pathway that facilitates the conversion of L-histidine to L-glutamate [50]. The low concentration of histidine in the middle ear serves as a limiting factor for pathogenic bacteria that cause otitis media. The metabolism of glutamate plays a crucial role in providing resistance to bacterial stress responses. Both TD1 and ATC5153 possess the gene responsible for converting histidine to glutamate, enabling pathogenic T. otitidis to overcome the limiting factor as well as a stress response. This supports the colonization of Turicella otitidis in the middle ear, leading to otitis media, whereas other bacteria are unable to colonize and cause infection [44].
Turicella otitidis harbors genes for selenocysteine synthesis, which is incorporated into selenoproteins, in addition presence of nucleophilic amino acids and modulation of redox potential, suggests that T. otitidis is highly adaptable to environmental conditions, which enables it to cause extra-otic infections [41, 51]. Moreover, T. otitidis is capable of catabolizing taurine, a sulfur-containing β-amino acid that plays significant roles in antioxidative and anti-inflammatory reactions and promotes the immune defense against microbial infections by enhancing the metabolism and functions of immune cells such as monocytes and macrophages [51, 52]. T. otitidis lacks essential genes, namely mabA, inhA, kasA, and hadB (Fatty Acid Synthesis (FAS-II) pathway), which are involved in the biosynthesis of fatty and mycolic acid [52]. In addition, the fatty acid profile shows that T. otitidis possesses unsaturated menaquinones (MK-10 and MK-11) as opposed to dihydrogenated menaquinones (MK-8(H2) and MK-9(H2)). Menaquinone (Vitamin K2) is an essential, electron carrier intricately involved in anaerobic redox reactions leading to ATP generation. Furthermore, T. otitidis lacks mycolic acid, and this absence plays a role in preventing cell wall permeability, thereby imparting resistance to antibiotics and phagocytosis, unlike other forms of Corynebacterium species [37]. As a result of niche-specific changes in the genome and metabolism of a bacterium, the mechanism of adaptability and pathogenicity is suggested to be linked to the immune status of the host. Several retrospective studies have demonstrated that Turicella otitidis is a prevalent cause of ear infections in the pediatric population [22, 53].
Antibiotics function by utilizing the virulence factors and anatomical features of a pathogen to disable its reproductive ability (bacteriostatic) or eradicate it (bactericidal). The resistance of Turicella otitidis to macrolides and lincosamides can be attributed to the presence of 23 S rRNA mutations [54]. The genome sequence of Turicella otitidis both strains, TD1 and ATCC 5153 possess a cfrA 50 S methyltransferase and two major facilitator superfamily-type drug resistance transporters. Given that T. otitidis is regarded as an emerging pathogen, it is of utmost importance to closely monitor the potential development of resistance in the future. Recent research has demonstrated that Turicella otitidis, in conjunction with other microorganisms, demonstrates resistance to high concentrations of ototopical antibiotics, including ciprofloxacin [5]. However, there is currently insufficient data available on Turicella otitidis resistance to the minimum inhibitory concentration (MIC) breakpoint, except in the presence of clindamycin and macrolides [55]. Although studies on otitis media have demonstrated the recurrence of infection when T. otitidis is not treated with appropriate antibiotics, there have been no reported occurrences of bacteremia or other invasive infections by Turicella otitidis. The organism has been reported to exhibit high susceptibility to beta-lactam antibiotics such as penicillin, cephalosporins, and carbapenems, as well as to chloramphenicol, linezolid, vancomycin, and teicoplanin. In all summarized cases, administration of broad-spectrum antibiotics showed successful recovery of patients infected with Turicella otitidis. However, the virulence factors of Turicella otitidis and its potential to cause extra-otic infections remain to be fully understood. One limitation of our study is the sparse number of cases available on Turicella otitidis. Though the genomic and metabolic analysis from two accessible studies has been summarized here, additional information is required to make conclusions about the observed variability in the genome and metabolic profile. Moreover, it is necessary to ascertain whether this information can be extrapolated to Turicella otitidis isolates from otitis media and extra otic infections in patients with or without underlying conditions, furthermore, the role of the host immune response in the adaptability and pathogenicity of the organism remains to be characterized in detail.
Summary
Turicella otitidis is a coryneform bacteria commonly associated with both acute and chronic otitis media. However, recent reports of extra-otic infections caused by Turicella otitidis and the emergence of antimicrobial resistance are concerning as the organism can cause infection in vulnerable populations. Despite this, our current understanding of the pathogenicity of Turicella otitidis remains limited. This study summarizes the metabolic and genomic characteristics of two isolates of T. otitidis strains from two different sources, suggesting that the bacterium is capable of shaping its environment and undergoes alterations in metabolic pathways, gene transcription, and proteome composition. These findings offer valuable insights into the metabolic pathophysiology of the bacterium, enabling it to adapt and survive in diverse niches.
Supplementary Information
Additional file 1: Electronic Supplementary file S1. Literature search summary (1994-2023).
Acknowledgements
We would like to thank the volunteers for reviewing the NEL articles and translating and editing the text where applicable. Also, we would like to thank Michael Huang for his guidance and feedback on the supplementary file.
Authors’ contributions
AA conceived the idea and wrote the original draft. CY, CC, BB, and AT conducted the research. All authors edited and agreed with the final version. CY and CC, and BB and AT contributed equally.
Funding
Stony Brook University.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cuishan Yuan and Casey Chung contributed equally to this work.
Briana Blair and Amy Tran contributed equally to this work.
References
- 1.Renaud FN, et al. Identification of Turicella otitidis isolated from a patient with otorrhea associated with surgery: differentiation from Corynebacterium afermentans and Corynebacterium auris. J Clin Microbiol. 1996;34(10):2625–2627. doi: 10.1128/jcm.34.10.2625-2627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dana A, Fader R, Sterken D. Turicella otitidis mastoiditis in a healthy child. Pediatr Infect Dis J. 2001;20(1):84–85. doi: 10.1097/00006454-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds SJ, Behr M, McDonald J. Turicella otitidis as an unusual agent causing a posterior auricular abscess. J Clin Microbiol. 2001;39(4):1672–1673. doi: 10.1128/JCM.39.4.1672-1673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papp Z, Elgabsi H, Tóth L. MALDI-TOF mass spectrometry reveals a highly complex bacterial profile of otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2016;86:189–192. doi: 10.1016/j.ijporl.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Boumghar-Bourtchai L, et al. Resistance to macrolides by ribosomal mutation in clinical isolates of Turicella otitidis. Int J Antimicrob Agents. 2009;34(3):274–277. doi: 10.1016/j.ijantimicag.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Lappan R, Jamieson SE, Peacock CS. Reviewing the pathogenic potential of the otitis-associated bacteria Alloiococcus Otitidis and Turicella otitidis. Front Cell Infect Microbiol. 2020;10:51. doi: 10.3389/fcimb.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bîrluţiu V, et al. Bacteremia with Turicella otitidis in an institutionalized elderly patient with multiple hospital admissions: a case report. Biomedical Res (India) 2017;28(5):2196–2198. [Google Scholar]
- 9.Correa Martínez L, et al. External otitis due to Turicella otitidis: two case reports. Revista Esp De Quimioterapia. 2017;30(6):474–475. [PubMed] [Google Scholar]
- 10.Fernandez Perez A, et al. Cervical abscess due to Turicella otitidis. Acta Otorrinolaringologica Espanola. 1999;50(4):333–335. [PubMed] [Google Scholar]
- 11.Gaona CE, Castañón JS. Otitis externa por Turicella otitidis: a propósito de dos casos. Rev Esp Quimioter. 2017;30(6):474–475. [PubMed] [Google Scholar]
- 12.Jeziorski E, et al. Infections à Turicella otitidis: à propos D’un cas d’otite moyenne compliquée de mastoïdite. Arch Pediatr. 2009;16(3):243–247. doi: 10.1016/j.arcped.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AK, Isaacson B. Extra-luminal sigmoid sinus granulation tissue resulting in otitic hydrocephalus. Int J Pediatr Otorhinolaryngol Extra. 2016;12:5–6. doi: 10.1016/j.pedex.2016.01.001. [DOI] [Google Scholar]
- 14.Koumaki D, Koumaki V, Boumpoucheropoulos S, Katoulis A, Bitados P, Stefanidou M, et al. Turicella otitidis as an unusual agent causing palmoplantar eczema: an emerging pathogen. Eur J Case Rep Intern Med. 2020;7(2):001458. 10.12890/2020_001458. [DOI] [PMC free article] [PubMed]
- 15.Li D, et al. Bacteremia caused by Turicella otitidis in a patient with diffuse large B-cell lymphoma. Clin Lab. 2020;66(3). [DOI] [PubMed]
- 16.Loïez C, et al. Turicella otitidis in a bacteremic child with acute lymphoblastic leukemia. Clin Microbiol Infect. 2002;8(11):758–759. doi: 10.1046/j.1469-0691.2002.00474.x. [DOI] [PubMed] [Google Scholar]
- 17.Mastroianni A, Vangeli V, Mauro MV, Manfredi R, Greco S. Turicella otitidis central venous-related bacteremia during pediatric acute lymphoblastic leukemia. Rev Esp Quimioter. 2023;36(5):531–532. doi: 10.37201/req/126.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez-Legaza JM, et al. Isolation of Turicella otitidis in ear infection. Revista Esp De Quimioterapia. 2021;34(3):264–266. doi: 10.37201/req/144.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poulter MD, Hinnebusch CJ. Turicella otitidis in a young adult with otitis externa. Infect Dis Clin Pract. 2005;13(1):31–32. doi: 10.1097/01.idc.0000152473.70296.8b. [DOI] [Google Scholar]
- 20.Priyadarshini SR, et al. Turicella otitidis: a rare agent causing microbial keratitis. BMJ Case Rep. 2021;14(7):e241371. doi: 10.1136/bcr-2020-241371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smruti RP, et al. Turicella otitidis: a rare agent causing microbial keratitis. BMJ Case Rep. 2021;14(7):e241371. doi: 10.1136/bcr-2020-241371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funke G, et al. Corynebacterium confusum sp. nov., isolated from human clinical specimens. Int J Syst Evol MicroBiol. 1998;48(4):1291–1296. doi: 10.1099/00207713-48-4-1291. [DOI] [PubMed] [Google Scholar]
- 23.De Frutos M, et al. Turicella otitidis, contributions to its role in the etiology of ear Infections. Rev Esp Quimioter. 2018;31(3):278–281. [PMC free article] [PubMed] [Google Scholar]
- 24.Halle TR, Todd NW, Fainberg J. Iatrogenic trichloroacetic acid injury causing necrotizing otitis media and deafness. Int J Pediatr Otorhinolaryngol. 2017;97:139–142. doi: 10.1016/j.ijporl.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Ježek P, et al. Turicella otitidis - neobvyklý původce otitis media. Zprávy Epidemiologie a Mikrobiologie. 2011;20(2):56–58. [Google Scholar]
- 26.Mastroianni A, et al. Turicella otitidis central venous-related bacteremia during pediatric acute lymphoblastic leukemia. Rev Esp Quimioter. 2023:mastroianni18jul2023-mastroianni18jul2023. [DOI] [PMC free article] [PubMed]
- 27.MA SC. Alloiococcus otitidis and Turicella otitidis: emerging germs as a cause of mastoiditis. Pediatric Society of Western Andalusia and Extremadura. 2014;21. 2014(1):69–70. https://spaoyex.es/articulo/alloiococcus-otitidis-y-turicella-otitidis-g%C3%A9rmenes-emergentes-como-causa-demastoiditis.
- 28.Quesnel S, et al. Acute mastoiditis in children: a retrospective study of 188 patients. Int J Pediatr Otorhinolaryngol. 2010;74(12):1388–1392. doi: 10.1016/j.ijporl.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Gavrilovici C, et al. Acute otitis media in children-challenges of antibiotic resistance in the post-vaccination era. Microorganisms. 2022;10(8):1598. doi: 10.3390/microorganisms10081598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Garces J, et al. Acute and chronic otitis media and Turicella otitidis: a controversial association. Clin Microbiol Infect. 2004;10(9):854–857. doi: 10.1111/j.1198-743X.2004.00965.x. [DOI] [PubMed] [Google Scholar]
- 31.Kalcioglu MT, et al. Metagenomics analysis of bacterial population of tympanosclerotic plaques and Cholesteatomas. Otolaryngol Head Neck Surg. 2018;159(4):724–732. doi: 10.1177/0194599818772039. [DOI] [PubMed] [Google Scholar]
- 32.Krueger A, et al. Relationship of the middle ear effusion microbiome to secretory mucin production in pediatric patients with chronic Otitis media. Pediatr Infect Dis J. 2017;36(7):635–640. doi: 10.1097/INF.0000000000001493. [DOI] [PubMed] [Google Scholar]
- 33.Vila PM, et al. Infectious complications of pediatric cochlear implants are highly influenced by otitis media. Int J Pediatr Otorhinolaryngol. 2017;97:76–82. doi: 10.1016/j.ijporl.2017.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen TY, et al. In vitro inhibition of clinical isolates of Otitis media pathogens by the probiotic Streptococcus salivarius BLIS K12. Probiotics and Antimicrobial Proteins. 2021;13(3):734–738. doi: 10.1007/s12602-020-09719-7. [DOI] [PubMed] [Google Scholar]
- 35.Brinkrolf K, et al. Draft genome sequence of Turicella otitidis ATCC 51513, isolated from middle ear fluid from a child with otitis media. J Bacteriol. 2012;194(21):5968–5969. doi: 10.1128/JB.01412-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barron CL, et al. Identification of essential biofilm proteins in middle ear fluids of otitis media with effusion patients. Laryngoscope. 2020;130(3):806–811. doi: 10.1002/lary.28011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baek I, et al. Phylogeny trumps chemotaxonomy: a case study involving Turicella otitidis. Front Microbiol. 2018;9:834. doi: 10.3389/fmicb.2018.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arı O, et al. Does the mcrobiata in Otitis media with effusion originate from the adenoids? Turkish Archives of Otolaryngology/Türk Otolarengoloji Arsivi. 2019;57:S10–0. [Google Scholar]
- 39.Álvarez AS, Coca MG. Turicella otitidis isolates in otic exudates during 2020. Revista Esp De Quimioterapia. 2021;34(4):390–392. doi: 10.37201/req/011.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holzmann D, et al. Turicella otitidis and corynebacterium auris do not cause otitis media with effusion in children. Pediatr Infect Dis J. 2002;21(12):1124–1126. doi: 10.1097/00006454-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Couloigner V, et al. Pathogens implicated in acute otitis media failures after 7-valent pneumococcal conjugate vaccine implementation in France: distribution, serotypes, and resistance levels. Pediatr Infect Dis J. 2012;31(2):154–8. doi: 10.1097/INF.0b013e3182357c8d. [DOI] [PubMed] [Google Scholar]
- 42.Mammo DA, Watson D, Armbrust KR. Post-intravitreal injection endophthalmitis secondary to Turicella otitidis: a case report. BMC Ophthalmol. 2020;20(1):1–4. doi: 10.1186/s12886-020-01412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dulay H, et al. Cobalt resistance via detoxification and mineralization in the iron-reducing bacterium geobacter sulfurreducens. Front Microbiol. 2020;11:600463. doi: 10.3389/fmicb.2020.600463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greninger AL, et al. Draft genome sequence of Turicella otitidis TD1, isolated from a patient with bacteremia. Genome Announc. 2015;3(5):01060–01015. doi: 10.1128/genomea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer A, et al. Diversity of immune strategies explained by adaptation to pathogen statistics. Proc Natl Acad Sci. 2016;113(31):8630–8635. doi: 10.1073/pnas.1600663113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stressler T, et al. Detection, production, and application of microbial arylsulfatases. Appl Microbiol Biotechnol. 2016;100(21):9053–9067. doi: 10.1007/s00253-016-7838-4. [DOI] [PubMed] [Google Scholar]
- 47.Metcalf WW, Wanner BL. Involvement of the Escherichia coli phn (psiD) gene cluster in assimilation of phosphorus in the form of phosphonates, phosphite, pi esters, and pi. J Bacteriol. 1991;173(2):587–600. doi: 10.1128/jb.173.2.587-600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stasi R, Neves HI, Spira B. Phosphate uptake by the phosphonate transport system PhnCDE. BMC Microbiol. 2019;19(1):79. doi: 10.1186/s12866-019-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Veen HW. Phosphate transport in prokaryotes: molecules, mediators and mechanisms. Antonie Van Leeuwenhoek. 1997;72(4):299–315. doi: 10.1023/A:1000530927928. [DOI] [PubMed] [Google Scholar]
- 50.Juliao PC, et al. Histidine auxotrophy in commensal and disease-causing nontypeable haemophilus influenzae. J Bacteriol. 2007;189(14):4994–5001. doi: 10.1128/JB.00146-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wessjohann LA, et al. Selenium in chemistry and biochemistry in comparison to sulfur. Biol Chem. 2007;388(10):997–1006. doi: 10.1515/BC.2007.138. [DOI] [PubMed] [Google Scholar]
- 52.Schaffer S, Kim HW. Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther (Seoul) 2018;26(3):225–241. doi: 10.4062/biomolther.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sillanpää S, et al. Next-generation sequencing combined with specific PCR assays to determine the bacterial 16S rRNA gene profiles of middle ear fluid collected from children with acute otitis media. mSphere. 2017;2(2):10. doi: 10.1128/mSphere.00006-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolk DM. Microbiology of middle ear infections: do you hear what I hear? Clin Microbiol Newsl. 2016;38(11):87–93. doi: 10.1016/j.clinmicnews.2016.05.002. [DOI] [Google Scholar]
- 55.Trinh KV, et al. Characterization of ciprofloxacin resistance levels: implications for ototopical therapy. Otol Neurotol. 2021;42(7):e887–893. doi: 10.1097/MAO.0000000000003113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Electronic Supplementary file S1. Literature search summary (1994-2023).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.