Abstract
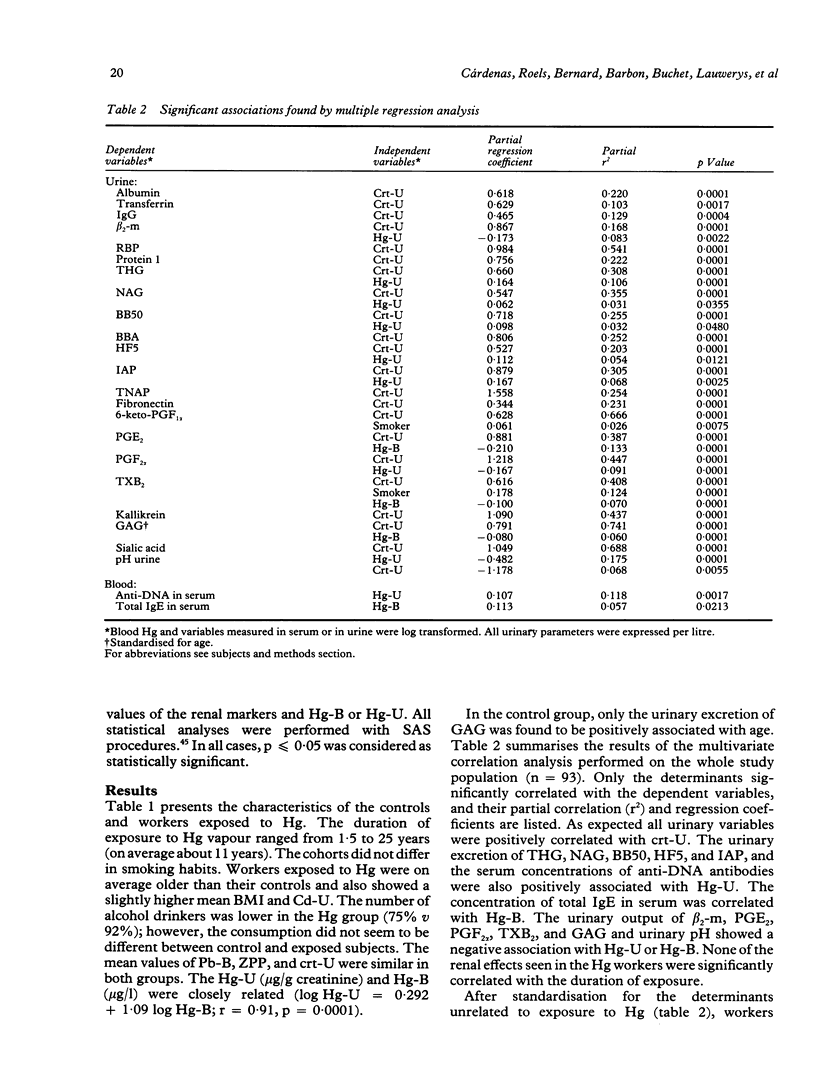
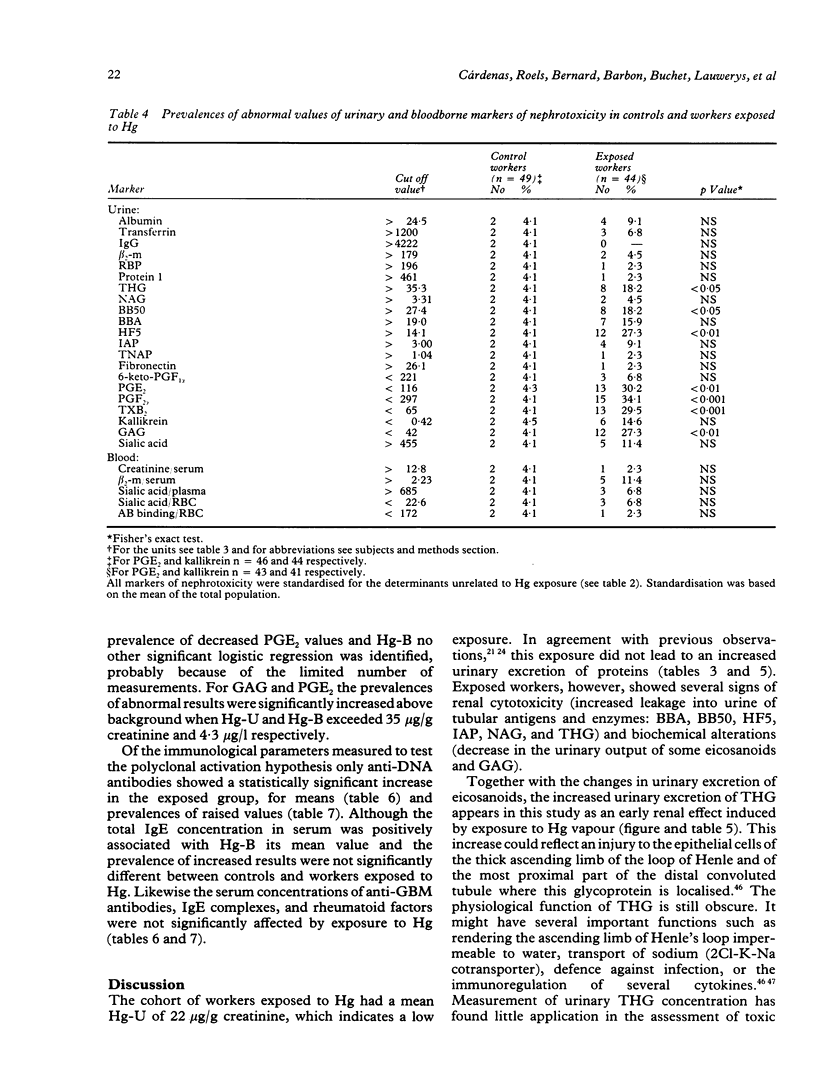
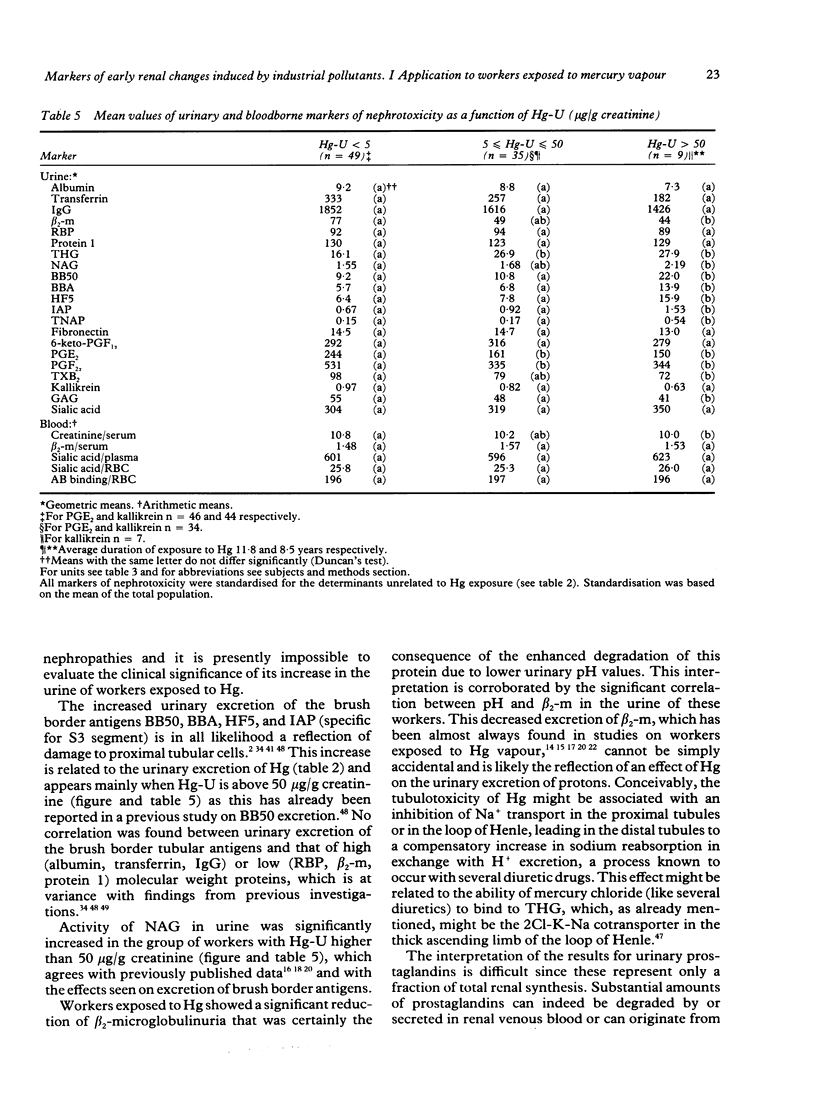
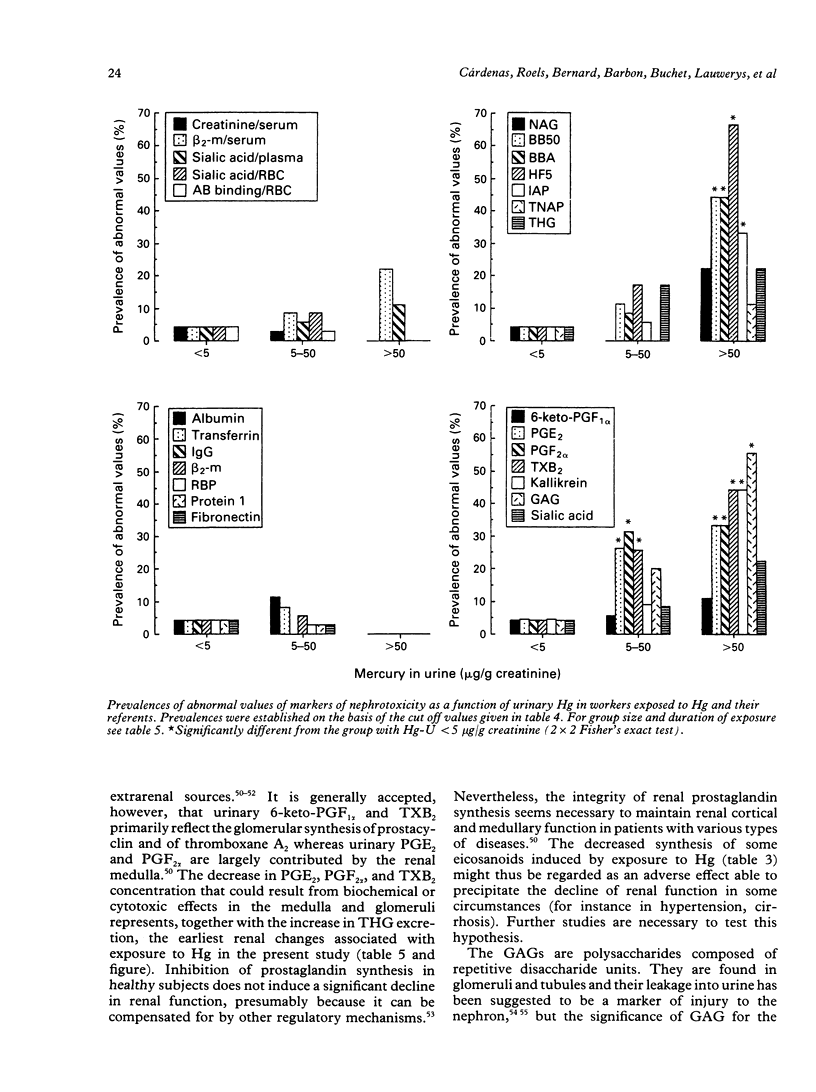
Several markers of renal changes have been measured in a cohort of 50 workers exposed to elemental mercury (Hg) and in 50 control workers. After application of selection criteria 44 exposed and 49 control workers were retained for the final statistical analysis. Exposed workers excreted on average 22 micrograms Hg/g creatinine and their mean duration of exposure was 11 years. Three types of renal markers were studied--namely, functional markers (creatinine and beta 2-microglobulin in serum, urinary proteins of low or high molecular weight); cytotoxicity markers (tubular antigens and enzymes in urine), and biochemical markers (eicosanoids, thromboxane, fibronectin, kallikrein, sialic acid, glycosaminoglycans in urine, red blood cell membrane negative charges). Several bloodborne indicators of polyclonal activation were also measured to test the hypothesis that an immune mechanism might be involved in the renal toxicity of elemental Hg. The main renal changes associated with exposure to Hg were indicative of tubular cytotoxicity (increased leakage of tubular antigens and enzymes in urine) and biochemical alterations (decreased urinary excretion of some eicosanoids and glycosaminoglycans and lowering of urinary pH). The concentrations of anti-DNA antibodies and total immunoglobulin E in serum were also positively associated with the concentration of Hg in urine and in blood respectively. The renal effects were mainly found in workers excreting more than 50 micrograms Hg/g creatinine, which corroborates our previous estimate of the biological threshold of Hg in urine. As these effects, however, were unrelated to the duration of exposure and not accompanied by functional changes (for example, microproteinuria), they may not necessarily represent clinically significant alterations of renal function.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O., Nielsen J. B. Effects of diethyldithiocarbamate on the toxicokinetics of cadmium chloride in mice. Toxicology. 1989 Apr;55(1-2):1–14. doi: 10.1016/0300-483x(89)90170-4. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Baggio B., Briani G., Cicerello E., Gambaro G., Bruttomesso D., Tiengo A., Borsatti A., Crepaldi G. Urinary glycosaminoglycans, sialic acid and lysosomal enzymes increase in nonalbuminuric diabetic patients. Nephron. 1986;43(3):187–190. doi: 10.1159/000183827. [DOI] [PubMed] [Google Scholar]
- Barregård L., Hultberg B., Schütz A., Sällsten G. Enzymuria in workers exposed to inorganic mercury. Int Arch Occup Environ Health. 1988;61(1-2):65–69. doi: 10.1007/BF00381609. [DOI] [PubMed] [Google Scholar]
- Bernard A. M., Lauwerys R. R. Continuous-flow system for automation of latex immunoassay by particle counting. Clin Chem. 1983 Jun;29(6):1007–1011. [PubMed] [Google Scholar]
- Bernard A. M., Ouled A., Roels H., Lauwerys R., Vandeleene B., Lambert A. Lack of relationship between urinary glycosaminoglycans and indices of tubular or glomerular renal damage. Urinary GAG are an unreliable nephrotoxicity index. Nephron. 1988;48(1):82–83. doi: 10.1159/000184878. [DOI] [PubMed] [Google Scholar]
- Bernard A., Dieryckx J. P., Viau C., Bazin H., Lauwerys R. Determination of IgE complexes and of total IgE by latex immunoassay. J Clin Chem Clin Biochem. 1987 Apr;25(4):245–251. doi: 10.1515/cclm.1987.25.4.245. [DOI] [PubMed] [Google Scholar]
- Bernard A., Roels H. A., Buchet J. P., Lauwerys R. R. Comparison, by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, of urinary proteins excreted by workers exposed to cadmium, mercury or lead. Toxicol Lett. 1980 Mar;5(3-4):219–222. doi: 10.1016/0378-4274(80)90063-6. [DOI] [PubMed] [Google Scholar]
- Bompart G., Pécher C., Prévot D., Girolami J. P. Mild renal failure induced by subchronic exposure to molybdenum: urinary kallikrein excretion as a marker of distal tubular effect. Toxicol Lett. 1990 Aug;52(3):293–300. doi: 10.1016/0378-4274(90)90039-o. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Roels H., Bernard A., Lauwerys R. Assessment of renal function of workers exposed to inorganic lead, calcium or mercury vapor. J Occup Med. 1980 Nov;22(11):741–750. [PubMed] [Google Scholar]
- Druet P., Bernard A., Hirsch F., Weening J. J., Gengoux P., Mahieu P., Birkeland S. Immunologically mediated glomerulonephritis induced by heavy metals. Arch Toxicol. 1982 Sep;50(3-4):187–194. doi: 10.1007/BF00310850. [DOI] [PubMed] [Google Scholar]
- Dunn M. J. Nonsteroidal antiinflammatory drugs and renal function. Annu Rev Med. 1984;35:411–428. doi: 10.1146/annurev.me.35.020184.002211. [DOI] [PubMed] [Google Scholar]
- Ehrenberg R. L., Vogt R. L., Smith A. B., Brondum J., Brightwell W. S., Hudson P. J., McManus K. P., Hannon W. H., Phipps F. C. Effects of elemental mercury exposure at a thermometer plant. Am J Ind Med. 1991;19(4):495–507. doi: 10.1002/ajim.4700190407. [DOI] [PubMed] [Google Scholar]
- Fish A. J., Kleppel M., Jeraj K., Michael A. F. Enzyme immunoassay of anti-glomerular basement membrane antibodies. J Lab Clin Med. 1985 Jun;105(6):700–705. [PubMed] [Google Scholar]
- Gaultier M., Fournier E., Gervais P., Morel-Maroger L., Bismuth C., Rain J. D. Deux cas de syndrome néphrotique dans une fabrique de thermomètres. Bull Mem Soc Med Hop Paris. 1968;119(1):47–61. [PubMed] [Google Scholar]
- Gelpí E., Ramis I., Hotter G., Bioque G., Bulbena O., Roselló J. Modern high-performance liquid chromatographic-radioimmunoassay strategies for the study of eicosanoids in biological samples. J Chromatogr. 1989 Aug 11;492:223–250. doi: 10.1016/s0378-4347(00)84470-9. [DOI] [PubMed] [Google Scholar]
- Gerosa A., Violante F. S., Trevisan A. Effetti renali dell'esposizione a mercurio in una industria di termometri. Med Lav. 1986 Nov-Dec;77(6):635–638. [PubMed] [Google Scholar]
- Goldman M., Baran D., Druet P. Polyclonal activation and experimental nephropathies. Kidney Int. 1988 Aug;34(2):141–150. doi: 10.1038/ki.1988.159. [DOI] [PubMed] [Google Scholar]
- Halligan S., Graham M. J., Gray T. J., Harpur E. S., Bonner F. W. A quantitative method for the measurement of urinary glycosaminoglycans--potential use in studies of xenobiotic nephrotoxicity. Toxicol Lett. 1990 Sep;53(1-2):183–185. doi: 10.1016/0378-4274(90)90121-2. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Tiderström G. Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta. 1973 Feb 12;43(3):305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Hursh J. B., Cherian M. G., Clarkson T. W., Vostal J. J., Mallie R. V. Clearance of mercury (HG-197, HG-203) vapor inhaled by human subjects. Arch Environ Health. 1976 Nov-Dec;31(6):302–309. doi: 10.1080/00039896.1976.10667240. [DOI] [PubMed] [Google Scholar]
- KAZANTZIS G., SCHILLER K. F., ASSCHER A. W., DREW R. G. Albuminuria and the nephrotic syndrome following exposure to mercury and its compounds. Q J Med. 1962 Oct;31:403–418. [PubMed] [Google Scholar]
- Koopmans P. P., Thomas C. M., van de Berg R. J., Thien T., Gribnau F. W. The urinary excretion of prostaglandins and thromboxane B2 in healthy volunteers: a study in males and females, and on the influence of seminal fluid contamination. Prostaglandins Leukot Essent Fatty Acids. 1988 Jun;32(3):107–111. [PubMed] [Google Scholar]
- Kumar S., Muchmore A. Tamm-Horsfall protein--uromodulin (1950-1990). Kidney Int. 1990 Jun;37(6):1395–1401. doi: 10.1038/ki.1990.128. [DOI] [PubMed] [Google Scholar]
- Lauwerys R. R., Buchet J. P. Occupational exposure to mercury vapors and biological action. Arch Environ Health. 1973 Aug;27(2):65–68. doi: 10.1080/00039896.1973.10666319. [DOI] [PubMed] [Google Scholar]
- Lauwerys R., Bernard A. Preclinical detection of nephrotoxicity: description of the tests and appraisal of their health significance. Toxicol Lett. 1989 Mar;46(1-3):13–29. doi: 10.1016/0378-4274(89)90113-6. [DOI] [PubMed] [Google Scholar]
- Lubec G., Kircher S. Noninvasive diagnosis of tubular damage by the use of urinary chondroitin-4-sulfate/heparan sulfate ratio. Nephron. 1986;42(4):340–340. doi: 10.1159/000183699. [DOI] [PubMed] [Google Scholar]
- Magos L., Clarkson T. W. Atomic absorption determination of total, inorganic, and organic mercury in blood. J Assoc Off Anal Chem. 1972 Sep;55(5):966–971. [PubMed] [Google Scholar]
- Meyer B. R., Fischbein A., Rosenman K., Lerman Y., Drayer D. E., Reidenberg M. M. Increased urinary enzyme excretion in workers exposed to nephrotoxic chemicals. Am J Med. 1984 Jun;76(6):989–998. doi: 10.1016/0002-9343(84)90847-7. [DOI] [PubMed] [Google Scholar]
- Mutti A. Detection of renal diseases in humans: developing markers and methods. Toxicol Lett. 1989 Mar;46(1-3):177–191. doi: 10.1016/0378-4274(89)90126-4. [DOI] [PubMed] [Google Scholar]
- Mutti A., Lucertini S., Valcavi P., Neri T. M., Fornari M., Alinovi R., Franchini I. Urinary excretion of brush-border antigen revealed by monoclonal antibody: early indicator of toxic nephropathy. Lancet. 1985 Oct 26;2(8461):914–917. doi: 10.1016/s0140-6736(85)90850-5. [DOI] [PubMed] [Google Scholar]
- Patrono C., Dunn M. J. The clinical significance of inhibition of renal prostaglandin synthesis. Kidney Int. 1987 Jul;32(1):1–12. doi: 10.1038/ki.1987.164. [DOI] [PubMed] [Google Scholar]
- Piikivi L., Ruokonen A. Renal function and long-term low mercury vapor exposure. Arch Environ Health. 1989 May-Jun;44(3):146–149. doi: 10.1080/00039896.1989.9935878. [DOI] [PubMed] [Google Scholar]
- Pusey C. D., Bowman C., Morgan A., Weetman A. P., Hartley B., Lockwood C. M. Kinetics and pathogenicity of autoantibodies induced by mercuric chloride in the brown Norway rat. Clin Exp Immunol. 1990 Jul;81(1):76–82. doi: 10.1111/j.1365-2249.1990.tb05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A. A., Klahr S. Bladder contributes to eicosanoids excreted in urine. Am J Physiol. 1990 Nov;259(5 Pt 2):F859–F861. doi: 10.1152/ajprenal.1990.259.5.F859. [DOI] [PubMed] [Google Scholar]
- Roels H. A., Lauwerys R. R., Bernard A. M., Buchet J. P., Vos A., Oversteyns M. Assessment of the filtration reserve capacity of the kidney in workers exposed to cadmium. Br J Ind Med. 1991 Jun;48(6):365–374. doi: 10.1136/oem.48.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H. A., Lauwerys R. R., Buchet J. P., Bernard A. M., Lijnen P., Van Houte G. Urinary kallikrein activity in workers exposed to cadmium, lead, or mercury vapour. Br J Ind Med. 1990 May;47(5):331–337. doi: 10.1136/oem.47.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H., Gennart J. P., Lauwerys R., Buchet J. P., Malchaire J., Bernard A. Surveillance of workers exposed to mercury vapour:validation of a previously proposed biological threshold limit value for mercury concentration in urine. Am J Ind Med. 1985;7(1):45–71. doi: 10.1002/ajim.4700070106. [DOI] [PubMed] [Google Scholar]
- Ronco P., Brunisholz M., Geniteau-Legendre M., Chatelet F., Verroust P., Richet G. Physiopathologic aspects of Tamm-Horsfall protein: a phylogenetically conserved marker of the thick ascending limb of Henle's loop. Adv Nephrol Necker Hosp. 1987;16:231–249. [PubMed] [Google Scholar]
- Rosenman K. D., Valciukas J. A., Glickman L., Meyers B. R., Cinotti A. Sensitive indicators of inorganic mercury toxicity. Arch Environ Health. 1986 Jul-Aug;41(4):208–215. doi: 10.1080/00039896.1986.9938335. [DOI] [PubMed] [Google Scholar]
- SMITH J. C., WELLS A. R. A biochemical study of the urinary protein of men exposed to metallic mercury. Br J Ind Med. 1960 Jul;17:205–208. doi: 10.1136/oem.17.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicli A. G., Carretero O. A. Renal kallikrein-kinin system. Kidney Int. 1986 Jan;29(1):120–130. doi: 10.1038/ki.1986.14. [DOI] [PubMed] [Google Scholar]
- Stonard M. D., Chater B. V., Duffield D. P., Nevitt A. L., O'Sullivan J. J., Steel G. T. An evaluation of renal function in workers occupationally exposed to mercury vapour. Int Arch Occup Environ Health. 1983;52(2):177–189. doi: 10.1007/BF00405421. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Goto S., Hotta R. An epidemiological study on retinopathy due to carbon disulfide: CS2 exposure level and development of retinopathy. Int Arch Occup Environ Health. 1976 Apr 28;37(1):1–8. doi: 10.1007/BF00409360. [DOI] [PubMed] [Google Scholar]
- Tubbs R. R., Gephardt G. N., McMahon J. T., Pohl M. C., Vidt D. G., Barenberg S. A., Valenzuela R. Membranous glomerulonephritis associated with industrial mercury exposure. Study of pathogenetic mechanisms. Am J Clin Pathol. 1982 Apr;77(4):409–413. doi: 10.1093/ajcp/77.4.409. [DOI] [PubMed] [Google Scholar]
- Tucker S. M., Boyd P. J., Thompson A. E., Price R. G. Automated assay of N-acetyl-beta-glucosaminidase in normal and pathological human urine. Clin Chim Acta. 1975 Jul 23;62(2):333–339. doi: 10.1016/0009-8981(75)90245-4. [DOI] [PubMed] [Google Scholar]
- Verpooten G. F., Nouwen E. J., Hoylaerts M. F., Hendrix P. G., de Broe M. E. Segment-specific localization of intestinal-type alkaline phosphatase in human kidney. Kidney Int. 1989 Oct;36(4):617–625. doi: 10.1038/ki.1989.238. [DOI] [PubMed] [Google Scholar]
- Whiteman P. The quantitative determination of glycosaminoglycans in urine with Alcian Blue 8GX. Biochem J. 1973 Feb;131(2):351–357. doi: 10.1042/bj1310351. [DOI] [PMC free article] [PubMed] [Google Scholar]


