Abstract
Methylobacterium sp. strain DM4 and Methylophilus sp. strain DM11 can grow with dichloromethane (DCM) as the sole source of carbon and energy by virtue of homologous glutathione-dependent DCM dehalogenases with markedly different kinetic properties (the kcat values of the enzymes of these strains are 0.6 and 3.3 s−1, respectively, and the Km values are 9 and 59 μM, respectively). These strains, as well as transconjugant bacteria expressing the DCM dehalogenase gene (dcmA) from DM11 or DM4 on a broad-host-range plasmid in the background of dcmA mutant DM4-2cr, were investigated by growing them under growth-limiting conditions and in the presence of an excess of DCM. The maximal growth rates and maximal levels of dehalogenase for chemostat-adapted bacteria were higher than the maximal growth rates and maximal levels of dehalogenase for batch-grown bacteria. The substrate saturation constant of strain DM4 was much lower than the Km of its associated dehalogenase, suggesting that this strain is adapted to scavenge low concentrations of DCM. Strains and transconjugants expressing the DCM dehalogenase from strain DM11, on the other hand, had higher growth rates than bacteria expressing the homologous dehalogenase from strain DM4. Competition experiments performed with pairs of DCM-degrading strains revealed that a strain expressing the dehalogenase from DM4 had a selective advantage in continuous culture under substrate-limiting conditions, while strains expressing the DM11 dehalogenase were superior in batch culture when there was an excess of substrate. Only DCM-degrading bacteria with a dcmA gene similar to that from strain DM4, however, were obtained in batch enrichment cultures prepared with activated sludge from sewage treatment plants.
Dichloromethane (DCM) is an industrial solvent used mainly in the production of synthetic chemicals, as a paint remover, and as a degreasing agent (23). A total of 135,000 tons of this compound was produced in Western Europe in 1995 (15). DCM, with its very low boiling point (40°C), escapes into the environment mainly by evaporation into the atmosphere, and its efflux rate has been estimated to be similar to its production rate (34). A substantial reduction in DCM emissions has been achieved in recent years (19), but due to its high solubility in water (23), DCM has remained a significant component of industrial and communal wastewater streams (28, 49). Bacteria that mineralize DCM, such as aerobic methylotrophs (17, 27) and anaerobic acetogens (30), can be isolated readily from soil and groundwater that have been exposed to DCM. Methylotrophic DCM-degrading strains express a glutathione-dependent DCM dehalogenase that is encoded by the gene dcmA (2, 25) and is one of the few bacterial glutathione S-transferases whose function is known (46). The dcmA genes of several DCM-degrading methylotrophs have been isolated and sequenced (48), and all are closely related to the dcmA gene of Methylobacterium sp. strain DM4. The DM4 and DM11 dehalogenases display only 56% identity at the protein sequence level (47). The kinetic parameters kcat and Km of these enzymes differ significantly; the DCM dehalogenase of strain DM11 exhibits a sixfold-higher turnover rate and a sixfold-higher Km for DCM than the DCM dehalogenase of strain DM4 (48).
Several studies have explored the potential of using DCM-utilizing bacteria for biological treatment of industrial effluents, waste gases, and groundwater (10, 17, 42, 49). In these studies the efficiency of DCM removal depended not only on the technology of the process, but also on the degradation properties of the bacterial strains involved. Only a small amount of detailed information on the kinetics of pure cultures growing on halogenated aliphatic compounds is available (7, 44). The work reported here was undertaken to investigate how the kinetic properties of DCM dehalogenase affect the growth properties and the competitiveness of DCM-utilizing bacteria under substrate-limiting conditions and when there is an excess of growth substrate.
MATERIALS AND METHODS
Materials.
Restriction and DNA-modifying enzymes were purchased from Fermentas (Maechler, Basel, Switzerland) unless noted otherwise. Oligonucleotides were purchased from Microsynth (Balgach, Switzerland). All other chemicals were of the highest available purity and were purchased from Fluka (Buchs, Switzerland) unless noted otherwise.
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Transconjugants DM4-2cr(DM4) and DM4-2cr(DM11) of Methylobacterium sp. strain DM4-2cr (18) carrying plasmids pME1683 and pME1685, respectively (see below and Table 1), were constructed by biparental mating by using Escherichia coli S17-1 as the donor strain (41). The identities of the transconjugants were verified by selective plating, by PCR amplification of the dcmA gene, and by measuring DCM dehalogenase activity in cell extracts (see below).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 36 |
| S17-1 | thi pro hsdR hsdM+ recA, chromosomally integrated RP4-2 (Tc::Mu Km::Tn7) | 41 |
| Methylotroph strains | ||
| Methylobacterium sp. strain DM4(DSM 6343) | DCM+ | 17 |
| Methylobacterium sp. strain DM4-2cr | Smr DCM−, mutant of DM4 | 18 |
| Methylobacterium sp. strain DM4-2cr(DM4) | Smr Kmr, Methylobacterium sp. strain DM4-2cr carrying pME1683 | This study |
| Methylobacterium sp. strain DM4-2cr(DM11) | Smr Kmr, Methylobacterium sp. strain DM4-2cr carrying pME1685 | This study |
| Methylophilus sp. strain DM11(DSM 6813) | DCM+ | 39 |
| Plasmids | ||
| pBluescript II KS(+) | Cloning vector, Apr | Stratagene |
| pME1540 | 4.40 kb, 1.5-kb HindIII-PstI fragment with dcmA of Methylobacterium sp. strain DM4 in pBluescript II KS(+) (KpnI, Bsp120I, XhoI, SalI, and ClaI missing upstream of HindIII in the polylinker) | 37 |
| pME1919 | 4.0 kb, pET-derived expression vector with 0.9-kb DNA fragment containing the dcmA gene of strain DM11, Apr | 47 |
| pME1673 | 3.1 kb, BamHI-XbaI trpA terminator region from the pBAce derivative pGST3a in pBluescript II KS(+) | This study |
| pME1671 | pBluescript II KS(+) derivative containing a 1,060-bp fragment with the dcmA gene of Methylophilus sp. strain DM11, the promoter PA, and the trpA terminator as a HindIII-XbaI insert | This study |
| pME1681 | pBluescript II KS(+) derivative containing the 1,513-bp HindIII BamHI fragment of pME1540 with the dcmA gene of Methylobacterium sp. strain DM4 | This study |
| pJB3Km1 | 6.05 kb, broad-host-range cloning vector derived from RK2, Kmr Apr | 4 |
| pME1683 | 1,557-bp KpnI-XbaI fragment of pME1681 in KpnI-XbaI fragment of pJB3Km1 | This study |
| pME1685 | 1,115-bp KpnI-XbaI fragment of pME1671 in KpnI-XbaI fragment of pJB3Km1 | This study |
DNA manipulations.
Recombinant DNA techniques were performed as described previously (1, 36). pME1683 and pME1685, two broad-host-range plasmids that allowed constitutive expression of the DCM dehalogenase of either strain DM4 or strain DM11 in strain DM4-2cr (18) with the dcmA gene of each of the strains, were constructed under the control of the PA promoter of the dcmA gene of strain DM4 (26). The HindIII-BamHI fragment of plasmid pME1540 (37) containing the dcmA gene and the PA promoter region from strain DM4 (starting 223 bases upstream from the GTG translation start codon) was subcloned into pBluescript-KS(+), which yielded plasmid pME1681. Plasmid pME1671, another pBluescript-KS(+) derivative containing the dcmA gene of strain DM11 behind the same promoter, was constructed as follows. A 280-bp fragment of the upstream region of dcmA from DM4 was amplified with universal primer T3 (5′-ATTAACCCTCACTAAAGG-3′) and reverse primer 5′-CGTTATCCTCCCCTTACTGTG-3′ (nucleotides 1 to −20 of the dcmA dehalogenase region of strain DM4; EMBL accession no. M32346) by using pME1540 (37) as the template. This amplicon was then treated with T4 DNA polymerase and cut with HindIII. The dcmA gene from strain DM11 (EMBL accession no. L26544) less the start codon was excised from plasmid pME1919 (47) by digestion with NdeI, digestion with mung bean nuclease (Boehringer, Mannheim, Germany), and digestion with BamHI. The 223-bp HindIII PCR fragment and the 837-bp BamHI dcmA fragment from pME1919 were then ligated in one step into BamHI- and HindIII-restricted pBluescript-KS(+) derivative pME1673, which carries the 26-bp trpA terminator of plasmid pBAce (9) as a HindIII-XbaI fragment in the multiple cloning site (Table 1).
Two mobilizable plasmids, pME1683 (containing dcmA from strain DM4) and pME1685 (containing dcmA from strain DM11), were then obtained by introducing the KpnI-XbaI fragments of pME1681 and pME1671, respectively, into broad-host-range vector pJB3Km1 (4) that had been cut with the same restriction enzymes.
PCR and hybridization techniques.
Synthetic oligonucleotides specific for either the DM4 dcmA gene or the DM11 dcmA gene were used for detection of these genes and for synthesis of digoxigenin (DIG)-labeled gene probes. The specific primers used for detection of the dcmA gene from strain DM4 were 5′-TACTTTATCATCCGGCG-3′ (positions 46 to 62 in the dcmA gene) and 5′-CTAAGCGACTGCCGCGCCCTCC-3′ (positions 866 to 845), while the dcmA gene from strain DM11 was detected with 5′-TCGTGCAGTTCATCAATTTATGC-3′ (positions 48 to 70 in the dcmA gene from DM11) and 5′-GAGTTTAACACCATCAT-3′ (positions 693 to 677). DNA amplifications were carried out in 50-μl (total volume) reaction mixtures containing 0.2 U of Taq polymerase, 1.75 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, 50 pmol of each primer, and 10 to 100 ng of total bacterial DNA or 1 μl of boiled cell suspension as the template, with 40 cycles consisting of annealing at 53°C, polymerization at 72°C, and denaturation at 94°C. A 5-μl PCR DIG labeling mixture (Boehringer) instead of deoxynucleoside triphosphates was used for PCR under otherwise identical conditions to prepare base-labile DIG-labeled gene probes. Primers 5′-GAATGACAACCGTGCGC-3′ (positions −185 to −169 relative to the dcmA gene) and 5′-TCCGGTCATCGAAGGAATGC-3′ (positions 155 to 136 downstream of the dcmA gene) were used to obtain the DM4 probe, and primers 5′-ATGAGTACTAAACTACGATAT-3′ (positions 1 to 21 in the dcmA gene) and 5′-GAGTTTAACACCATCAT-3′ (positions 693 to 677 in the dcmA gene) were used for synthesis of the DM11 probe.
Preparation of total DNA.
Total DNAs from methylotrophic bacteria and DCM-degrading enrichment cultures were prepared as described previously (8). Total DNAs from sludge samples were prepared as follows. A 100-ml portion of crude sewage sludge was centrifuged, and the pellet was resuspended in 50 ml of cell disruption buffer (100 mM Tris-HCl [pH 7.6], 10 mM EDTA, 5 mM thiourea, 10 mM dithiothreitol, 1.5% sodium dodecyl sulfate [SDS], 1% deoxycholic acid, 1% Nonidet P-40) made with high-performance liquid chromatography quality water (Fluka). The sludge suspension was mixed with 5 g of 0.1-mm-diameter glass beads (Sigma, Buchs, Switzerland), and the cells were broken by treating the suspension for 1 min at 4°C with a bead beater (Biospec Products, Bartlesville, Okla.). The resulting slurry was extracted with phenol and chloroform, and the DNA was precipitated with ethanol and purified by treatment with polyvinylpolypyrrolidone (Sigma) as described previously (22). The DNA concentration was estimated by using agarose gels or the DNA DipStick assay (Invitrogen, Leek, The Netherlands).
Gene hybridization analysis.
Slot blot hybridization was performed by applying serial dilutions containing 5 to 0.1 μg of total DNA onto a Porablot nitrocellulose membrane (Macherey-Nagel, Basel, Switzerland) with a slot blot manifold (Minifold II; Schleicher & Schuell, Basel, Switzerland). Hybridization with DIG-labeled gene probes, chemiluminescent detection, and stripping of the probes between repeated hybridizations of the same membrane were performed as recommended by the manufacturer (5).
Media and growth conditions.
E. coli strains were grown in Luria-Bertani medium (rich medium) at 37°C. Methylotrophic bacteria were grown at 30°C in gas-tight glass flasks in liquid minimal medium (MM) (47). DCM (10 mM) was added as the only source of carbon and energy after autoclaving. Solid media were obtained by adding 15 g of agar per liter of medium before autoclaving. Agar plates were incubated in gas-tight glass jars (volume, 3 liters) to which 100 μl of DCM, 200 μl of methanol, or 200 μl of ethanol was added. Kanamycin (25 mg liter−1) and ampicillin (100 mg liter−1) were used as required.
Continuous cultivation of bacteria was performed in a 2.5-liter chemostat (MBR, Zürich, Switzerland) filled with 1.6 liters of MM. Reservoir medium amended with 10 mM DCM was stored in 20-liter bottles and maintained at pH 1.5 to prevent bacterial contamination. All chemostat parts and media and the neutralization solution were autoclaved for 30 min at 121°C before use. Stirred medium was fed into the chemostat through Ismaprene tubing (Ismatec, Glattbrugg, Switzerland). The pH of the medium in the cultivation vessel was maintained at pH 7.15 by automatic addition of a sterile 1 N KOH-NaOH solution. The culture volume in the chemostat was kept constant with overflow metal tubing. The stirring rate was adjusted to 1,000 rpm, and the temperature was automatically maintained at 30°C. Oxygen was provided by pumping air into the culture at a rate of 2 to 4 ml min−1. Only negligible stripping of DCM from the medium was observed under these conditions.
Enrichment of DCM-degrading microorganisms from sewage sludge.
Activated sludge samples were collected from two industrial sewage treatment plants (S1 and S2) in the Basel (Switzerland) region. DCM dehalogenation in sludge was determined after 4 h of incubation of a 5-g (wet weight) sludge sample in MM containing 10 mM DCM. Activated sludge samples were washed and incubated for 30 min in fresh MM containing 10 mM DCM in gas-tight vials, and the rate of chloride release was measured colorimetrically (3). Monooxygenase activity was inhibited by adding 2% acetylene to the headspace.
Microorganisms that mineralized DCM were enriched from sludge by incubating sludge samples (400 mg, wet weight) in gas-tight flasks containing 30 ml of MM supplemented with 10 mM DCM at 30°C. Degradation of DCM was monitored by measuring the formation of chloride (3) and the decrease in pH. The cultures were neutralized with 5 N NaOH and supplemented once with 10 mM DCM. Serial transfers were performed by inoculating 0.001 volume of the enrichment culture into fresh medium after all of the DCM had been consumed (4 to 6 days).
Competition experiments.
Sludge suspensions S1 and S2 (from sewage treatment plants S1 and S2, respectively) prepared as described above were spiked before enrichment with an amount of growing cells of strain DM11 or DM4-2cr(DM11) (Table 1) corresponding to 10% of the initial DCM-degrading activity of sludge suspension S2. The spiked samples were cultivated as described above.
In addition, pairwise competition experiments with pure cultures of Methylobacterium sp. strain DM4, DM4-2cr(DM4), or DM4-2cr(DM11) and Methylophilus sp. strain DM11 were performed by adding equal numbers of cells (∼108 cells) of two organisms to 30 ml of MM containing 10 mM DCM. The cocultures were grown to the exponential phase until the optical density was 0.3, and then 30-μl aliquots were used to inoculate fresh medium. This procedure was repeated up to seven times, and samples from each serial transfer were collected for subsequent analysis of the coculture composition by PCR. Alternatively, plating on MM containing ethanol as the sole carbon source was used to determine cell counts for DM4 wild-type and DM4-2cr transconjugant colonies in the cocultures with strain DM11; this method made use of the exclusive ability of strain DM4 to grow with ethanol as a carbon source (16).
Gas chromatography.
Liquid samples (4.5 ml) were withdrawn from the chemostat with sterile syringes that already contained 0.5 ml of 85% phosphoric acid to quench further metabolic activity. Portions (4.5 ml) of the resulting solutions were added to 5-ml gas-tight glass vials sealed with Teflon caps containing 0.5 ml of octane (purity, >99%; Fluka). The vials were shaken vigorously to extract the DCM into the octane phase.
Aliquots (2 μl) from the octane phase were injected into a Porapak P column (1,800 by 2 mm; 80/100 mesh; Supelco, Buchs, Switzerland) on a gas chromatograph (model PE8700; Perkin-Elmer, Rotkreuz, Switzerland) equipped with an electron capture detector. Nitrogen at a flow rate of 40 ml min−1 was used as the carrier and purge gas for the electron capture detector. The temperatures used were 160°C in the column, 220°C in the injector, and 300°C in the detector. Under these conditions, the retention time of DCM was 1.4 min, and the detection limit was 0.3 μM.
Preparation and analysis of cell extracts and measurement of DCM dehalogenase activity.
Cell extracts were obtained from methylotrophic bacteria by repeated passage through a French press as previously described (25). Proteins were separated by SDS-polyacrylamide gel electrophoresis on minigels (CBS Scientific Company, Axon Lab, Baden-Dättwil, Switzerland) by using standard protocols (1). The percentages of DCM dehalogenase protein in the cells were calculated from the enzyme activities in the cell extracts based on the specific activities of the purified enzymes (16.7 mkat kg−1 for the DM4 enzyme and 100 mkat kg−1 for the DM11 enzyme). Specific DCM degradation rates were determined by measuring formaldehyde formation from DCM turnover as previously described (43).
Growth kinetics.
Substrate saturation constants (Monod constants) (Ks) (32) were determined by a gas chromatography analysis of the residual substrate concentration S in the water phase of continuous cultures growing with different dilution rates at steady state (33). Five chemostat culture volumes was pumped through the system before measurements at a new dilution rate were obtained. From these determinations, Ks and maximal growth rates (μmax) were estimated by nonlinear least-squares fitting of the experimental data to the Monod equation (equation 1):
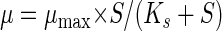 |
1 |
using Kaleidagraph (Synergy Software). Alternatively, the maximal growth rate was determined from washout curves as described previously (13).
A model of competition between two different DCM degraders in a serial batch culture was obtained with equation 2 (modified from the equation described by Duetz et al. [14]):
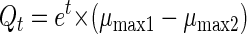 |
2 |
where μmax1 and μmax2 are the maximal growth rates of the two competing organisms and Qt is the time-dependent ratio of the cell numbers in the exponentially growing batch culture. Equation 2 is valid when equal numbers of the two bacterial strains are present at the beginning of the experiment.
The predicted growth rate (μp) at a given DCM concentration was calculated with equation 3, which was adapted from an equation published by van den Wijngaard et al. (44):
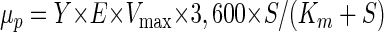 |
3 |
where Y is the growth yield (in liters per unit of optical density at 600 nm [OD600] per millimole of substrate), E is the yield of soluble protein (in kilograms of protein per liter per OD600 unit) estimated by determining the average value from 50 protein determinations in independent growth experiments performed with batch cultures, Vmax is the measured specific dehalogenase activity of the cell extracts (in millikatals per kilogram or millimoles per second per kilogram of protein), S is the DCM concentration (micromolar) in the medium, and Km is the Michaelis-Menten substrate saturation constant (micromolar).
RESULTS
Growth rates of DCM-utilizing strains in batch culture and specific expression of the dehalogenase.
We constructed plasmid derivatives that carry the dcmA gene from strain DM4 or from strain DM11 under the control of dcmA promoter PA of strain DM4 in broad-host-range plasmid pJB3Km1 (4). These plasmids were used to examine the relative importance of the properties of the bacterium and the characteristics of the DCM dehalogenase during growth with DCM. As shown in Table 2, plasmid pME1685 with the dcmA gene from Methylophilus sp. strain DM11 restored growth of DM4-2cr, a dcmA mutant of Methylobacterium sp. strain DM4 (18), when DCM was the sole carbon source. Control strain DM4-2cr(DM4) expressing the DCM dehalogenase from strain DM4 had kinetic properties similar to those of wild-type strain DM4. The maximal growth rates in batch culture with DCM as the sole carbon source were determined for Methylophilus sp. strain DM11, Methylobacterium sp. strain DM4, and two transconjugants, Methylobacterium sp. strains DM4-2cr(DM4) and DM4-2cr(DM11). The levels of expression of plasmid-encoded DCM dehalogenases in transconjugants were similar to the levels of expression in the wild-type strains, which ruled out the possibility that plasmid copy number effects were significant. In the DM4-2cr background, the dehalogenase from strain DM4 was expressed better than the dehalogenase from DM11. This may have resulted from nonoptimized codon usage of the DM11 dcmA gene in this context. The level of expression of the DM11 DCM dehalogenase, however, was comparatively low in its natural host as well (39).
TABLE 2.
Kinetic parameters of DCM dehalogenases and of DCM-degrading methylotrophic bacteria
| Strain | DCM dehalogenase propertiesa
|
Strain properties
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Batch culture
|
Continuous culture
|
||||||||
| kcat for DCM (s−1) | Km for DCM (μM) | Maximal growth rate (h−1) | DCM dehalogenase sp act (mkat kg−1)b | % DCM dehalogenasec | Maximal growth rate (h−1)d | Ks for DCM (μM)d | DCM dehalogenase sp act (mkat kg−1)b,e | % DCM dehalogenasec | |
| Methylobacterium sp. strain DM4 | 0.6 | 9 | 0.072 | 3.8 ± 0.4 | 22 | 0.149 | 0.6 | 9.1 ± 1.4 | 54 |
| Methylophilus sp. strain DM11 | 3.3 | 59 | 0.160 | 9.4 ± 1.0 | 9 | 0.152 | 24.4 | 28.3 ± 1.9 | 28 |
| Methylobacterium sp. strain DM4-2cr(DM4) | 0.6 | 9 | 0.069 | 4.5 ± 0.3 | 27 | 0.151 (0.123)f | 0.6 | 9.6 ± 0.8 | 57 |
| Methylobacterium sp. strain DM4-2cr(DM11) | 3.3 | 59 | 0.086 | 9.2 ± 0.4 | 9 | 0.123 (0.131)f | 6.4 | 25.9 ± 2.4 | 26 |
Data from reference 47.
Mean ± standard deviation based on data from at least three independent measurements.
Amount of DCM dehalogenase expressed as a percentage of the total protein in cell extracts (see Materials and Methods).
Obtained by fitting the data to the Monod equation (see Materials and Methods).
Specific activities of DCM dehalogenases were determined with cell extracts from adapted chemostat cultures (>100 h in continuous culture).
The values in parentheses are the dilution rates at which washout occurred.
Methylophilus sp. strain DM11 had by far the highest specific growth rate on DCM. As expected from the turnover rates of the enzymes (47), the dehalogenase from strain DM11 enabled the DM4 transconjugant DM4-2cr(DM11) to grow with DCM at a higher maximal growth rate than the DM4 transconjugant DM4-2cr(DM4). On the other hand, strain DM4-2cr(DM4) had a growth rate similar to that of wild-type strain DM4 (Table 2). Since transconjugant DM4-2cr(DM11) was unable to achieve a maximal growth rate in the range of that observed in the original host, DM11, host-specific factors rather than the measured specific dehalogenase activity appeared to limit the growth of strain DM4 with DCM.
Competition in batch culture.
In all pairwise competition experiments performed in batch culture, the strain with the higher maximal growth rate with DCM outcompeted the other strain (Table 3). For reasons which are unknown at this time, the inferior strains were outcompeted significantly faster than expected based on differences in maximal growth rates (equation 2) except in the competition experiment performed with transconjugants DM4-2cr(DM11) and DM4-2cr(DM4). Transconjugant DM4-2cr(DM11), which expressed the DCM dehalogenase from DM11 in the strain DM4 background, was superior to strains containing the DCM dehalogenase from DM4. In contrast to wild-type strain DM11, however, it did not outcompete the indigenous DCM-degrading community in sewage sludge in a period corresponding to about 60 generation times (see below).
TABLE 3.
Competition between methylotrophic bacteria in serial batch cultures
| Competing strains
|
Δμmax (h−1)c | Ratio of cell no. after:
|
||||
|---|---|---|---|---|---|---|
| Higher maximal growth rate | Lower maximal growth rate | 48 ha
|
124 hb
|
|||
| Predictedd | Observed | Predictedd | Observed | |||
| DM11 (wild type) | DM4 (wild type) | 0.088 | 68 | 4,800 | 5.5 × 105 | >5.8 × 108 |
| DM4-2cr(DM4) | DM4 (wild type) | 0.003 | 1.15 | NDe | 1.45 | 44.4 |
| DM11 (wild type) | DM4-2cr(DM11) | 0.074 | 35 | 2,700 | 9,600 | >3.5 × 108 |
| DM4-2cr(DM11) | DM4-2cr(DM4) | 0.017 | 2.26 | ND | 8.2 | 8 |
After a single transfer at a 1,000-fold dilution into fresh medium.
After two transfers (three when wild-type strain DM11 was present) at a 1,000-fold dilution into fresh medium.
The difference in maximal growth rates (Δμmax) was computed from the data in Table 2.
Calculated with equation 2 (see Materials and Methods).
ND, not determined.
Enrichment and characterization of DCM-degrading microorganisms from sewage sludge.
Samples of activated sludge from two different sewage treatment plants (S1 and S2) were used as inocula for enrichment of DCM-degrading microorganisms. While S1 was fed in part with communal sewage which did not contain DCM, S2 was a wastewater treatment facility that was continuously exposed to large loads of DCM from the production of pharmaceuticals. The initial DCM-degrading capacity of the S2 sludge after induction with DCM was 5 mmol kg of sludge−1 h−1, but under the same conditions the initial DCM-degrading capacity of the S1 sludge was undetectable (<0.1 mmol kg of sludge−1 h−1). The extent to which DCM degradation in sewage treatment plants is performed by bacteria like DM4 or DM11 expressing glutathione-dependent dehalogenases is unknown. Since addition of acetylene did not inhibit dehalogenation (data not shown), it is unlikely that the initial DCM-degrading capacity of the sewage sludge samples arose from monooxygenase-mediated cometabolic degradation of DCM (45).
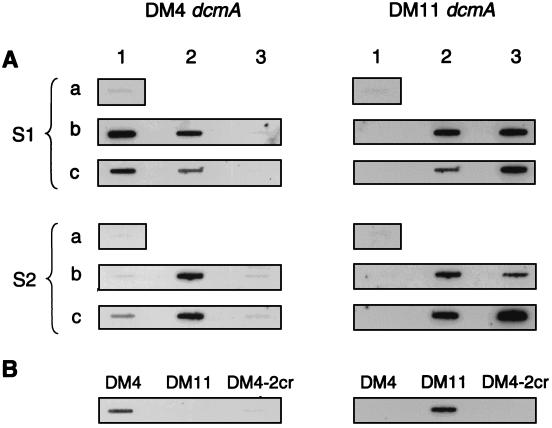
DCM-degrading microorganisms were readily enriched from sewage samples from both treatment plants. Total DNA was prepared directly from the sludge samples and from enrichment cultures obtained by using DCM as the sole carbon source. In all cases, total DNA directly isolated from the sludge failed to give a signal (Fig. 1A, positions a1). Genes similar to the dcmA gene from DM4, however, were detected in very low amounts in total DNA from sludge S2 after PCR amplification followed by detection of the amplified fragment by hybridization to the DM4 dcmA gene probe (data not shown). In contrast, PCR products similar to the dcmA gene of DM11 were never detected in total DNA from sludges or enrichment cultures. In enrichment cultures containing sewage sludge spiked with DM11 or DM4-2cr(DM11), Methylophilus sp. strain DM11 was the dominant organism after only three serial transfers and the DM4 type of dcmA could not be detected (Fig. 1A, positions b3). Strain DM4-2cr(DM11) remained in cocultures with native microorganisms containing a dcmA gene similar to the dcmA gene from DM4 for the entire duration of the experiment (six serial 1,000-fold dilutions over 28 days) (Fig. 1A, positions c2). Detection of only the DM4 type of dcmA in unspiked enrichment cultures (Fig. 1A, positions b1 and c1) therefore probably reflected the absence of DCM degraders with a DM11 type of DCM dehalogenase in sludge samples.
FIG. 1.
Slot blot hybridization of total DNA from activated-sludge samples from two sewage treatment plants (S1 and S2) and from subsequently obtained enrichment cultures of DCM degraders with DIG-labeled dcmA gene probes specific for the DCM dehalogenase gene of strain DM4 (left) or strain DM11 (right). A 1-μg portion of genomic DNA was applied to each slot, and the same membrane was hybridized sequentially with both probes. (A) Total DNA from the original activated-sludge samples (rows a) and from enrichment cultures of DCM degraders after three serial transfers (rows b) or six serial transfers (rows c) into fresh medium, obtained without spiking with bacteria expressing the DM11 DCM dehalogenase (columns 1), spiked with DM4-2cr(DM11) (columns 2), or spiked with Methylophilus sp. strain DM11 (columns 3). (B) Total control DNA from Methylobacterium sp. strain DM4, Methylophilus sp. strain DM11 and dcmA mutant strain DM4-2cr.
Maximal growth rates and DCM Ks values in continuous culture.
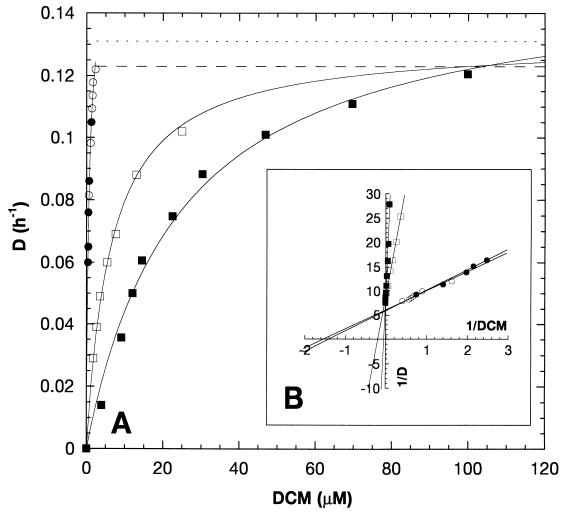
Kinetic parameters for growth of strains DM11, DM4, DM4-2cr(DM4), and DM4-2cr(DM11) on DCM were also determined in continuous cultures under substrate-limiting conditions (Table 2). The specific activities of DCM dehalogenase in cell extracts were up to threefold higher than the specific activities obtained with cells grown in batch mode. This correlated well with the increases in the amounts of the DCM dehalogenase observed in SDS-polyacrylamide gel electrophoresis gels (data not shown). The residual DCM concentrations were measured at different dilution rates in continuous cultures to determine the Ks of DCM-degrading strains (33). DM4 and DM4-2cr(DM4) had a very low Ks for DCM (Fig. 2), but DM4-2cr(DM4) was washed out at a dilution rate much lower than that predicted by fitting the experimental data to the Monod equation (equation 1) (Fig. 2). In contrast, the lowest dilution rate at which washout of transconjugant DM4-2cr(DM11) was observed was very similar to the maximal growth rate predicted by fitting the data to the Monod equation (Table 2).
FIG. 2.
(A) Plot of dilution rates (D) versus DCM residual concentrations in the water phase during steady-state growth with DCM in continuous cultures of wild-type strain DM4 (•), DM4-2cr(DM4) (○), DM4-2cr(DM11) (□), and wild-type strain DM11 (▪). The data points, representing the averages of three to five independent measurements, were fitted directly to the Monod equation as described in Materials and Methods. The horizontal lines indicate the dilution rates at which washout was observed for strain DM4-2cr(DM4) (dashed line) and for strain DM4-2cr(DM11) (dotted line). (B) Lineweaver-Burk plot of the data shown in panel A.
The Ks values for DCM were 10- to 15-fold lower than the Km values of the expressed DCM dehalogenases in the case of strain DM4 and the two DM4-2cr transconjugants (Table 2). The difference in the Ks values of strains DM4-2cr(DM4) and DM4-2cr(DM11) reflected the difference in the Km values of the dehalogenases of strains DM11 and DM4. In contrast, the Ks of strain DM11 was only 2.6-fold lower than the Km of the DCM dehalogenase of this organism. This suggested that strain DM4 was able to provide the DCM dehalogenase with a higher substrate concentration than that present in the medium.
Competition in continuous culture.
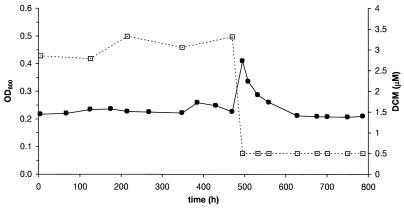
A competition experiment was performed with transconjugants DM4-2cr(DM4) and DM4-2cr(DM11) in continuous culture at a dilution rate of 0.039 (Fig. 3). This pair of strains allowed us to study the effect of the dehalogenase on strain competitiveness in the same background under substrate-limiting conditions. A pure culture of strain DM4-2cr(DM11) was first maintained at a steady state for about 20 generations (500 h). The residual concentration of DCM at this dilution rate was 2.5 ± 0.3 μM, which is slightly below the Ks determined for this strain (Table 2). When an equal amount of cells from a batch-grown culture of DM4-2cr(DM4) was added, the DCM concentration immediately fell to a value below the detection limit (0.3 μM), as expected from the Ks of the added transconjugant in pure culture (0.6 μM) (Table 2). Screening of single colonies isolated from the chemostat with specific primers used to determine the presence of either the DM4 dcmA gene or the DM11 dcmA gene revealed that only 120 h (five generations) after introduction of strain DM4-2cr(DM4) into the chemostat, all reisolated transconjugant clones contained the dcmA gene from strain DM4. This demonstrated the importance of the affinity of the DCM dehalogenase for the competitiveness of the host strain during growth when the concentrations of growth substrate are limiting, in sharp contrast to the observations made in batch culture when there was an excess of substrate (Table 3).
FIG. 3.
Competition between transconjugants DM4-2cr(DM11) and DM4-2cr(DM4) in a continuous culture containing DCM as the growth substrate. An equal amount of batch-grown DM4-2cr(DM4) was added at 500 h to a DCM-limited chemostat culture of strain DM4-2cr(DM11) grown with 10 mM DCM in the feed solution at a dilution rate of 0.039 h−1. Plots of OD600 versus time (•) and the residual DCM concentration versus time (□) are shown.
DISCUSSION
Achieving low effluent concentrations of xenobiotic compounds is one of the aims of wastewater treatment and is heavily dependent on the metabolism of endogenous microorganisms that colonize the man-made treatment ecosystems (10, 12, 21). Chlorinated aliphatic chemicals are prominent problem compounds in such environments, but the factors that determine the efficiency of degradation of halogenated aliphatic compounds by microbial populations are not well understood. We therefore studied the parameters which determine the growth efficiency and competitiveness of two DCM-degrading bacteria that were previously characterized in some detail (27).
The level of expression of DCM dehalogenase increased two- to threefold when DCM-degrading organisms were cultivated in a chemostat under growth-limiting DCM supply conditions (Table 2) compared with that when they were cultivated in batch mode. Increased expression of key metabolic enzymes under substrate-limiting conditions is a well-known phenomenon (20). A high content of dehalogenase in bacteria growing in continuous culture correlated with a higher maximal growth rate compared to batch conditions, except for strain DM11, which had about the same maximal growth rate under batch conditions as under continuous culture conditions (Table 2). This suggested that strain DM11 had been under selection pressure to maximize its growth rate.
The experiments to determine maximal growth rates in continuous cultures by measuring washout rates at dilution rates higher than the maximal growth rates (13) were fraught with technical difficulties. Biomass washout was often impossible to observe, since at high growth rates the bacteria tended to form a thick biofilm on the walls of the fermentor glass vessel, a phenomenon previously observed by other workers (11). For strain DM4-2cr(DM4), in contrast, dilution rates higher than 0.123 h−1 resulted in washout of the culture, although fitting of the experimental data to the Monod equation suggested that the maximal growth rate was 0.15 h−1. The fact that the maximum growth rate is approached very slowly with increasing substrate concentrations is a possible weakness of the Monod kinetic model (35), and many alternative models have been developed to describe the kinetics of bacterial growth (see reference 24 for a review). For example, the specific affinity model (6, 13) often used to describe the relative ability of a bacterium to sequester growth substrates, in which specific affinity is defined as the ratio of maximal growth rate to Ks for the growth-limiting substrate, assumes that the lower end of the growth rate-versus-substrate concentration curve is linear. In the case studied here, however, all of the strains had very similar maximal growth rates, and the substrate affinity of the bacteria was most simply described by the Ks parameter alone. This also allowed us to directly compare the substrate affinity of the bacteria with the affinity constant (Km) of the key metabolic enzyme, DCM dehalogenase.
DCM-degrading bacteria were able to grow at substrate concentrations much lower than the Km of the expressed dehalogenase (Table 2), although this was true to a lesser degree in the case of strain DM11. Such a discrepancy between the observed Ks for the growth substrate of a bacterial strain and the Km of the key metabolic enzyme has been described well previously (13, 44) and has been explained by levels of expression of the key metabolic enzyme much greater than the levels of expression required for bacterial growth with the enzyme substrate. For example, in the Ancylobacter aquaticus mutant strain AD25 growing with 1,2-dichloroethane, haloalkane dehalogenase accounted for 30 to 40% of the total cell protein, a 10- to 15-fold-higher value than the value for the parent strain growing with a similar maximal growth rate (44). This overcapacity led to high conversion rates at substrate concentrations much lower than the Km of the dehalogenase, resulting in a low Ks for the strain. Also, Pseudomonas putida pWW0 was shown to have a 4- to 5-fold higher level of expression of enzymes of the TOL upper pathway, resulting in a 10-fold overcapacity for the oxidation of m-xylene, compared to the observed rate of m-xylene transformation in a chemostat (13).
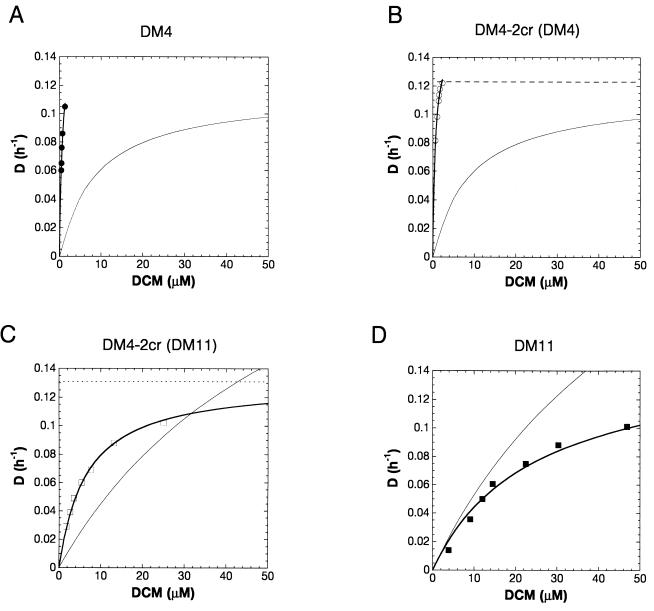
In the present study, the observed growth rates for chemostat-grown DCM-degrading bacteria under substrate-limiting conditions differed significantly from the growth rates predicted on the basis of the kinetics and level of expression of the dehalogenase (equation 3) (Fig. 4). On the one hand, the predicted growth rates of the two strains expressing the DM11 type of dehalogenase exceeded the experimental growth rates. This effect was observed at DCM concentrations greater than 30 μM for DM4-2cr(DM11) (Fig. 4C) and over the entire range of substrate concentrations for strain DM11 (Fig. 4D). Thus, factors other than the turnover number of the dehalogenase limited the growth of strains DM4-2cr(DM11) and DM11 with DCM at near-maximal growth rates. The predicted and experimental growth curves for strain DM11 (Fig. 4D) agreed well at low DCM concentrations, suggesting that the Ks of this organism was determined mainly by the kinetics and level of expression of the dehalogenase.
FIG. 4.
Plots of predicted (curves without symbols) and observed (curves with symbols) growth rates of wild-type strain DM4 (A), transconjugant DM4-2cr(DM4) (B), transconjugant DM4-2cr(DM11) (C), and wild-type strain DM11 (D) versus DCM concentrations in continuous cultures. The predicted growth rates are based on the kinetics of purified dehalogenase in vitro, the dehalogenase specific activities of cell extracts, and the growth yields of the strains with DCM (equation 3). The curves for the observed growth rates were obtained by directly fitting the experimental data to the Monod equation, as described in Materials and Methods. The horizontal lines indicate the dilution rates at which washout was observed for strain DM4-2cr(DM4) (B) and for strain DM4-2cr(DM11) (C). D, dilution rate.
On the other hand, the observed growth rates of strains DM4 and DM4-2cr(DM4) were higher than predicted (Fig. 4A and B), and the Ks values of DM4 and DM4-2cr transconjugants were far lower than those expected from the kinetic parameters and the observed expression of the DCM dehalogenases alone. In other words, the activities and substrate affinities of the dehalogenases measured in vitro were not high enough to account for the growth rates of strain DM4 and DM4-2cr transconjugants (Fig. 4A through C) at low dilution rates. The existence of a DCM accumulation system in Methylobacterium sp. strain DM4 would provide the dehalogenase with a higher substrate concentration than that present in the medium. In the case of common hydrophilic growth substrates, such as glucose, succinate, and acetate, transmembrane uptake systems which are often upregulated under substrate-limiting conditions (40) result in accumulation of the growth substrate in the bacterial cell (20) and contribute to an increase in substrate affinity (7, 40). The possibility that there is an accumulation mechanism involving enrichment of DCM in membrane compartments by passive diffusion (like the mechanisms observed for other lipophilic halogenated compounds) that would expose membrane-bound metabolic enzymes to increased substrate concentrations (12) can be eliminated in this case since the DCM dehalogenase of Methylobacterium sp. strain DM4 is located in the cytosol (27). Finally, although the possibility that in strain DM4 there is an effector that enhances DCM activity in vivo cannot be eliminated a priori, it is rather unlikely in our view since such an effector would have to result in increases in the kcat values of both DM4 and DM11 enzymes by factors of about 10 and 5, respectively, to yield a better fit with the observed Ks values in vivo (Fig. 4).
The properties of strains DM4 and DM11 and of the corresponding DCM dehalogenases may reflect differences in the natural environments of these bacteria. DM11 was enriched from a spill site that had been very heavily contaminated with DCM for decades (38), and this strain may have evolved under conditions that included a high concentration of DCM. DM4, on the other hand, was isolated from wastewater sludge. Sewage treatment plants have some typical characteristics of a continuous system, such as constant influx of nutrients, efflux of purified water, and continuous removal of biomass, in addition to low concentrations of DCM. In this environment, steady-state concentrations of growth-limiting substrates are as low as the metabolic activities of the endogenous microorganisms in the sludge permit, and Ks, rather than maximal growth rate, is the relevant parameter for bacterial competitiveness. Indeed, only DCM degraders with genes resembling the DM4 dcmA gene could be detected in or enriched from sewage sludge (Fig. 1), despite the fact that the batch method of cultivation used for enrichment strongly favored growth of organisms with properties similar to those of strain DM11.
Since strain DM4 is already highly optimized with respect to Ks, the efficiency of the dehalogenase appears to be the limiting step for growth with DCM in this bacterium. Therefore, cultivation of DM4-2cr(DM11) in a chemostat containing low DCM concentrations should favor the selection of faster-growing mutants in which the comparatively high Km of the wild-type DM11 DCM dehalogenase has been altered. Such mutants, if they can be obtained, may shed some light on the structural features of DCM dehalogenases which determine substrate affinity and catalytic efficiency (31, 47, 48).
ACKNOWLEDGMENTS
We are grateful to Svein Valla for his gift of plasmid pJB3Km1 and for providing details of its properties prior to publication, to Ming Tam for supplying plasmid pGST3, to Dietmar Stax for advice on the preparation of total DNA from sludge, and to Wouter Duetz for helpful suggestions on the manuscript.
This research was supported by grant 5002-037905 from the Biotechnology Priority Programme of the Swiss National Research Foundation.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1997. [Google Scholar]
- 2.Bader R, Leisinger T. Isolation and characterization of the Methylophilus sp. strain DM11 gene encoding dichloromethane dehalogenase/glutathione S-transferase. J Bacteriol. 1994;176:3466–3473. doi: 10.1128/jb.176.12.3466-3473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann J G, Sanik J. Determination of trace amounts of chlorine in naphtha. J Anal Chem. 1957;29:241–243. [Google Scholar]
- 4.Blatny J M, Brautaset T, Winther-Larsen H C, Haugan K, Valla S. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl Environ Microbiol. 1997;63:370–379. doi: 10.1128/aem.63.2.370-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehringer. The DIG system user’s guide for filter hybridization. Mannheim, Germany: Boehringer; 1995. [Google Scholar]
- 6.Button D K. Differences between the kinetics of nutrient uptake by microorganisms, growth and enzyme kinetics. Trends Biochem Sci. 1983;4:121–124. [Google Scholar]
- 7.Button D K. Kinetics of nutrient-limited transport and microbial growth. Microbiol Rev. 1985;49:270–297. doi: 10.1128/mr.49.3.270-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Kuo T. A simple and rapid method for the preparation of Gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993;21:2260. doi: 10.1093/nar/21.9.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig S P, Yuan L, Kuntz D A, McKerrow J H, Wang C C. High level expression in Escherichia coli of soluble, enzymatically active schistosomal hypoxanthine/guanine phosphoribosyltransferase and trypanosomal ornithine decarboxylase. Proc Natl Acad Sci USA. 1991;88:2500–2504. doi: 10.1073/pnas.88.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diks R M, Ottengraf S P. Verification studies of a simplified model for the removal of dichloromethane from waste gases using a biological trickling filter (part I) Bioprocess Eng. 1991;6:93–99. [Google Scholar]
- 11.Diks R M M. The removal of dichloromethane from waste gases in a biological trickling filter. Ph.D. thesis. Eindhoven, The Netherlands: Technical University; 1992. [Google Scholar]
- 12.Duetz W A. Physiology of toluene-degrading Pseudomonas strains under various conditions of nutrient limitation in chemostat culture. Ph.D. thesis. Groningen, The Netherlands: Rijksuniversiteit; 1996. [Google Scholar]
- 13.Duetz W A, Wind B, Kamp M, van Andel J G. Effect of growth rate, nutrient limitation and succinate on expression of TOL pathway enzymes in response to m-xylene in chemostat cultures of Pseudomonas putida (pWW0) Microbiology. 1997;143:2331–2338. doi: 10.1099/00221287-143-7-2331. [DOI] [PubMed] [Google Scholar]
- 14.Duetz W A, Winson M K, van Andel J G, Williams P A. Mathematical analysis of catabolic function loss in a population of Pseudomonas putida mt-2 during non-limited growth on benzoate. J Gen Microbiol. 1991;137:1363–1368. doi: 10.1099/00221287-137-6-1363. [DOI] [PubMed] [Google Scholar]
- 15.European Chlorinated Solvents Association. Plain facts about chlorinated solvents. Brussels, Belgium: European Chlorinated Solvents Association; 1995. [Google Scholar]
- 16.Gälli R. Optimierung des mikrobiellen Abbaus von Dichlormethan in einem Wirbelschicht-Bioreaktor. Ph.D. thesis. Zürich, Switzerland: ETH Zürich; 1986. [Google Scholar]
- 17.Gälli R, Leisinger T. Specialized bacterial strains for the removal of dichloromethane from industrial waste. Conserv Recycling. 1985;8:91–100. [Google Scholar]
- 18.Gälli R, Leisinger T. Plasmid analysis and cloning of the dichloromethane-utilization genes of Methylobacterium sp. DM4. J Gen Microbiol. 1988;134:943–952. doi: 10.1099/00221287-134-4-943. [DOI] [PubMed] [Google Scholar]
- 19.Hanson D J. Toxics release inventory report shows chemical emissions continuing to fall. Chem Eng News. 1996;74:29–46. [Google Scholar]
- 20.Harder W, Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- 21.Hartmans S, Tramper J. Dichloromethane removal from waste gases with a trickle-bed bioreactor. Bioprocess Eng. 1991;6:83–92. [Google Scholar]
- 22.Holben W E, Jansson J K, Chelm B K, Tiedje J M. DNA probe method for the detection of specific microorganisms in the soil bacterial community. Appl Environ Microbiol. 1988;54:703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard P H. Handbook of environmental fate and exposure data for organic chemicals. Vol. 2. Chelsea, Mich: Lewis Publishers Inc.; 1990. pp. 176–183. [Google Scholar]
- 24.Jannasch H W, Egli T. Microbial growth kinetics: a historical perspective. Antonie Leeuwenhoek. 1993;63:213–224. doi: 10.1007/BF00871219. [DOI] [PubMed] [Google Scholar]
- 25.La Roche S D, Leisinger T. Sequence analysis and expression of the bacterial dichloromethane dehalogenase structural gene, a member of the glutathione S-transferase supergene family. J Bacteriol. 1990;172:164–171. doi: 10.1128/jb.172.1.164-171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Roche S D, Leisinger T. Identification of dcmR, the regulatory gene governing expression of dichloromethane dehalogenase in Methylobacterium sp. strain DM4. J Bacteriol. 1991;173:6714–6721. doi: 10.1128/jb.173.21.6714-6721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leisinger T, Bader R, Hermann R, Schmid-Appert M, Vuilleumier S. Microbes, enzymes and genes involved in dichloromethane utilization. Biodegradation. 1994;5:237–248. doi: 10.1007/BF00696462. [DOI] [PubMed] [Google Scholar]
- 28.Line D E, Wu J, Arnold J A, Jennings G D, Rubin A R. Water quality of first flush runoff from 20 industrial sites. Water Environ Res. 1997;69:305–310. [Google Scholar]
- 29.Liu L F, Hong J L, Tsai S P, Hsieh J C, Tam M F. Reversible modification of rat liver glutathione S-transferase 3-3 with 1-chloro-2,4-dinitrobenzene-specific labelling of Tyr-115. Biochem J. 1993;296:189–197. doi: 10.1042/bj2960189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mägli A, Wendt M, Leisinger T. Isolation and characterization of Dehalobacterium formicoaceticum gen. nov. sp. nov., a strictly anaerobic bacterium utilizing dichloromethane as source of carbon and energy. Arch Microbiol. 1996;166:101–108. [Google Scholar]
- 31.Marsh A, Ferguson D M. Knowledge based modeling of a bacterial dichloromethane dehalogenase. Proteins Struct Funct Genet. 1997;28:217–226. doi: 10.1002/(sici)1097-0134(199706)28:2<217::aid-prot10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Monod J. The growth of bacterial cultures. Annu Rev Microbiol. 1949;3:371–394. [Google Scholar]
- 33.Owens J D, Legan J D. Determination of the Monod substrate saturation constant for microbial growth. FEMS Microbiol Rev. 1987;46:419–432. [Google Scholar]
- 34.Pearson C R. C1 and C2 halocarbons. In: Hutzinger O, editor. The handbook of environmental chemistry. Vol. 3. Berlin, Germany: Springer-Verlag; 1982. pp. 69–88. [Google Scholar]
- 35.Powell E O, Evans C G T, Strange R E, Tempest D W. Microbial physiology and continuous culture. London, United Kingdom: Her Majesty’s Stationery Office; 1967. [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Schmid-Appert M, Zoller K, Traber H, Vuilleumier S, Leisinger T. Association of newly discovered IS elements with the dichloromethane utilization genes of methylotrophic bacteria. Microbiology. 1997;143:2557–2567. doi: 10.1099/00221287-143-8-2557. [DOI] [PubMed] [Google Scholar]
- 38.Scholtz R. Hydrolytische und reduktive Dehalogenierung von aliphatischen Chlorkohlenwasserstoffen durch Mikroorganismen. Ph. D. thesis. Zürich, Switzerland: ETH Zürich; 1989. [Google Scholar]
- 39.Scholtz R, Wackett L P, Egli C, Cook A M, Leisinger T. Dichloromethane dehalogenase with improved catalytic activity isolated from a fast-growing dichloromethane-utilizing bacterium. J Bacteriol. 1988;170:5698–5704. doi: 10.1128/jb.170.12.5698-5704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senn H, Lendenmann U, Snozzi M, Hamer G, Egli T. The growth of Escherichia coli in glucose-limited chemostat cultures: a re-examination of the kinetics. Biochim Biophys Acta. 1994;1201:424–436. doi: 10.1016/0304-4165(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 41.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–790. [Google Scholar]
- 42.Stucki G. Biological decomposition of dichloromethane from a chemical process effluent. Biodegradation. 1990;1:221–228. doi: 10.1007/BF00119759. [DOI] [PubMed] [Google Scholar]
- 43.Stucki G, Gälli R, Ebersold H R, Leisinger T. Dehalogenation of dichloromethane by cell extracts of Hyphomicrobium DM2. Arch Microbiol. 1981;130:366–371. [Google Scholar]
- 44.van den Wijngaard A J, Wind R D, Janssen D B. Kinetics of bacterial growth on chlorinated aliphatic compounds. Appl Environ Microbiol. 1993;59:2041–2048. doi: 10.1128/aem.59.7.2041-2048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Hylckama Vlieg J E T, de Koning W, Janssen D B. Transformation kinetics of chlorinated ethenes by Methylosinus trichosporium OB3b and detection of unstable epoxides by on-line gas chromatography. Appl Environ Microbiol. 1996;62:3304–3312. doi: 10.1128/aem.62.9.3304-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vuilleumier S. Bacterial glutathione S-transferases: what are they good for? J Bacteriol. 1997;179:1431–1441. doi: 10.1128/jb.179.5.1431-1441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vuilleumier S, Leisinger T. Protein engineering studies of dichloromethane dehalogenase/glutathione S-transferase from Methylophilus sp. strain DM11. Ser12 but not Tyr6 is required for enzyme activity. Eur J Biochem. 1996;239:410–417. doi: 10.1111/j.1432-1033.1996.0410u.x. [DOI] [PubMed] [Google Scholar]
- 48.Vuilleumier S, Sorribas H, Leisinger T. Identification of a novel determinant of glutathione affinity in dichloromethane dehalogenase/glutathione S-transferases. Biochem Biophys Res Commun. 1997;238:252–256. doi: 10.1006/bbrc.1997.7309. [DOI] [PubMed] [Google Scholar]
- 49.Zuber L. Trickling filter and three-phase airlift bioreactor for the removal of dichloromethane from air. Ph.D. thesis. Zürich, Switzerland: ETH Zürich; 1995. [Google Scholar]