Abstract
A method for quantifying bacterial populations introduced into an activated-sludge microbial community is described. The method involves extraction of DNA from activated sludge, appropriate dilution of the extracted DNA with DNA extracted from nonintroduced activated sludge, PCR amplification of a gyrB gene fragment from the introduced strain with a set of strain-specific primers, and quantification of the electrophoresed PCR product by densitometry. The adequacy of the method was examined by analyzing the population dynamics of two phenol-degrading bacteria, Pseudomonas putida BH and Comamonas sp. strain E6, that had been introduced into phenol-digesting activated sludge. The density of each of the two populations determined by the PCR method immediately after the introduction was consistent with the density estimated from a plate count of the inoculum. This quantitative PCR method revealed different population dynamics for the two strains in the activated sludge under different phenol-loading conditions. The behavior of both of these strains in the activated sludge reflected the growth kinetics of the strains determined in laboratory axenic cultures.
Bioaugmentation is a method for enhancing in situ pollutant biodegradation by introducing exogenous microorganisms with the desired catabolic traits. This method is considered useful when effective pollutant-degrading populations are not present at a polluted site. Successful bioaugmentation requires that strains suitable for each polluted site be selected; for example, at a highly contaminated site, a strain capable of rapidly degrading the pollutant present at a high concentration may be necessary, whereas for thorough degradation a strain with high affinity for the pollutant may be required. In this respect, it is desirable to establish laboratory evaluation methods for selecting the strains to be introduced. It has been found that phenol-degrading bacteria can be classified into several kinetically different groups (46), and competition between two phenol-degrading bacteria with different growth kinetics has been investigated in an axenic sequencing fed-batch reactor (10). Similarly, the growth kinetics of 2,4-dichlorophenoxyacetic acid-degrading bacteria have been compared in order to select strains suitable for bioaugmentation (15). However, it has not yet been clarified whether laboratory data can be extrapolated to predict and explain the growth and activity of strains introduced into the environment. Ka et al. (22) analyzed the competition among 2,4-dichlorophenoxyacetic acid-degrading bacteria in soil and suggested that the lag time for growth of these strains observed in laboratory batch culture experiments, rather than the specific growth rate, is the principal determinant for competitiveness in soil.
To further investigate the relationships between the growth properties of bacteria in test tubes and their behaviors in the natural environment, quantitative monitoring of bacteria introduced into the environment is a prerequisite. Selective plating has been used most widely for this purpose (12, 29, 30, 32); however, this method requires specific conditions under which only the introduced strain can grow, so that it cannot be used to detect anonymous natural isolates. In addition, it has recently been suggested that the plate count method fails to detect bacteria that become unculturable (but are still active) in response to environmental stress (20, 24, 34). Immunological methods have been used to detect nitrifying Nitrosomonas populations (36) and phenol-digesting populations (47, 48) in activated-sludge samples. Unfortunately, the sensitivity of these methods was relatively low; the detection limits were between 106 and 107 cells per ml. The use of gene probes in combination with hybridization and/or PCR is a more attractive method, because it provides higher specificity and higher sensitivity (33, 37, 41), although the success of this method when it is used for specific detection is highly dependent on the specificity of the nucleotide sequences used as the probes. The 16S rRNA sequence has been the most commonly used sequence (6, 33); this sequence has been used successfully to analyze overall bacterial community structures at the genus level (5, 25, 44, 51). The DNA sequences of genes encoding catabolic enzymes have also been used in many cases (4, 19, 33); however, horizontal transfer of these genes, either on the plasmid (9) or on the chromosome (27) of the introduced strains, to indigenous populations has been observed, especially when the environment contained substrates for the catabolic genes. DNA fragments amplified by repetitive sequence-based PCR have recently been used as strain-specific DNA probes (28), but this method is thought to be somewhat laborious. Use of the DNA sequence of the gyrB gene, in combination with PCR, has been proposed as a method which could be used for specific detection of bacterial strains in the natural environment (49). The gyrB gene encodes the subunit B protein of DNA gyrase (topoisomerase type II) (31). It has been claimed that an advantage of using the gyrB sequence as a strain-specific probe is the higher molecular evolution rate of the gyrB gene and thus the greater diversity in the sequence of this gene compared with the 16S rRNA sequence (16, 49). Thus, we decided to examine the use of the gyrB sequence for specific detection of strains in a complex microbial community, such as activated sludge.
An aim of this study was to develop a gyrB-targeted quantitative PCR method for analyzing the population dynamics of bacterial strains introduced into an activated-sludge microbial community. Quantitative PCR techniques have been shown to suffer from a number of practical difficulties, including the different efficiencies of PCR amplification in different samples and the narrow linear response range (8). Hence, several modified forms of PCR, such as a PCR coupled to limiting dilution (42) and a competitive PCR (11, 23, 24), have been developed for enumerating bacterial populations. However, these methods are laborious and have resulted in only limited applications to analyses of population dynamics (24). In this paper, we describe a less laborious quantitative PCR method that revealed the different population dynamics of two phenol-degrading strains introduced into activated sludge under different phenol-loading conditions.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A phenol-degrading strain, Pseudomonas putida BH, was isolated previously from activated sludge after enrichment in batch cultures (17), while Comamonas sp. strain E6 (previously identified as Pseudomonas sp.) was obtained from another activated sludge after enrichment in a continuous culture (45). These two strains were maintained on Luria-Bertani (LB) agar plates (35) and were stored at −80°C in the presence of 15% (wt/vol) glycerol. The cell numbers of these strains in pure cultures were determined by using LB plates. The synthetic media used were based on MP medium containing (per liter) 2.75 g of K2HPO4, 2.25 g of KH2PO4, 1.0 g of (NH4)2SO4, 0.2 g of MgCl2 · 6H2O, 0.1 g of NaCl, 0.02 g of FeCl3 · 6H2O, and 0.01 g of CaCl2; the pH of this medium was between 6.8 and 7.0.
Other strains of P. putida, including strains A10L (39), FK715 (13), IFO14164 (40), IFO14671 (1), and JCM6156 (2), Comamonas terrigena IAM12052 (7), Comamonas testosteroni IAM12419 (7), and Comamonas acidovorans IFO13582 (14) were grown in LB medium at 30°C.
Sequencing of the gyrB gene.
Total DNA of each bacterium was extracted by the Marmur procedure (26). The gyrB gene was amplified from the extracted DNA by PCR with primers UP-1 and UP-2r (49). The amplified product was purified after electrophoresis on 1% low-melting-temperature agarose (NucSieve GTG; FMC Bioproducts, Vallensbaek Strand, Denmark) as described by Sambrook et al. (35). The sequence of each amplified fragment was determined with a DNA sequencing kit (dye terminator cycle sequencing kit; Perkin-Elmer, Foster City, Calif.) and a model 373A DNA sequencer (Applied Biosystems, Foster City, Calif.) by using the manufacturers’ instructions.
Activated-sludge samples.
Activated-sludge samples were obtained from two municipal sewage treatment plants, the Takinoshita plant (Kawagoe, Saitama, Japan) in September 1996 and the Ohdaira plant (Kamaishi, Iwate, Japan) in July 1997.
Laboratory activated-sludge experiments.
An activated-sludge process was simulated in a laboratory unit (Miyamoto Corp., Osaka, Japan) composed of an aeration tank (working volume, 3 liters) and a settling tank (working volume, 2 liters). The laboratory unit was inoculated with approximately 7 g (dry weight) of the activated sludge obtained from the Takinoshita plant, and MP medium supplemented with 200 ppm of phenol was continuously supplied at a flow rate of 6 liters per day. The phenol-loading rate was calculated to be 0.4 g per liter per day. The mixed-liquor suspended solid (MLSS) content was maintained between 1,800 and 2,000 ppm by discarding the excess sludge from the aeration tank. The mean sludge residence time was calculated to be approximately 10 days. Air was constantly supplied at a rate of 2 liters per min, and the temperature was maintained at approximately 25°C. The MLSS content was measured by weighing the dried sludge on a 0.22-μm-pore-size filter by using Japan Industrial Standards method K0102 (21). The total cell count in the activated sludge was determined by a fluorescent-microscopy method with acridine orange as described previously (48). The phenol concentration in the aeration tank was measured by a colorimetric assay with Phenol Test Wako (Wako Pure Chemicals, Osaka, Japan) as described previously (48).
Inocula added to the phenol-digesting activated sludge were prepared by cultivating strains BH and E6 at 30°C in 500 ml of LB medium. Cells in the late exponential growth phase were harvested by centrifugating at 10,000 × g for 5 min and were then washed with 500 ml of MP medium. The cells were resuspended in 50 ml of MP medium and used as inocula for the activated sludge in the laboratory unit.
To provide a shock load of phenol, MP medium supplemented with 5,000 ppm of phenol was supplied to the phenol-acclimated activated sludge at a flow rate of 6 liters per day (the phenol-loading rate was calculated to be 10 g per liter per day).
Extraction of DNA from activated sludge.
Five milliliters of an activated-sludge suspension was mixed with 0.5 ml of 50 mM sodium tripolyphosphate. In order to deflocculate the activated sludge, the mixture was treated in a blender (Wheaton Instruments, Millville, N.J.) for 2 min. The suspension was then centrifuged at 15,000 × g for 5 min, and the precipitate was resuspended in 0.5 ml of a cell-suspending buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 0.35 M sucrose, and 20 mg of lysozyme per ml). After incubation for 10 min at 37°C, 0.75 ml of a lysing solution (100 mM Tris-HCl [pH 8.0], 0.3 M NaCl, 20 mM EDTA, 2% [wt/vol] sodium dodecyl sulfate, and 2% [wt/vol] 2-mercaptoethanol) was added, and the suspension was incubated at 55°C for an additional 30 min. Next, the suspension was extracted four times with a phenol-chloroform solution (35), and 0.8 ml of the aqueous solution was recovered. Then, 0.8 ml of 2-propanol was added to the aqueous solution, and after gently mixing, the solution was incubated at −20°C for 30 min. Nucleic acids were precipitated by centrifugation at 20,000 × g for 10 min, and, after the preparation was washed with 1 ml of an 80% ethanol solution, the nucleic acids were dissolved in 0.5 ml of TE buffer (35) containing 100 μg of RNase A. This solution was gently shaken at 30°C for 12 h, and DNA was precipitated by adding 0.5 ml of 2-propanol before the preparation was washed with 1 ml of an 80% ethanol solution and dissolved in 0.2 ml of TE buffer. The extracted DNA was quantified by measuring its UV absorption spectrum (35) and was finally dissolved in TE buffer at a concentration of 100 μg per ml.
DNA was also extracted from cells incorporated into activated-sludge flocs. Five milliliters of an activated-sludge suspension was centrifuged at 500 × g for 5 min to precipitate the activated-sludge flocs. These flocs were resuspended in 5 ml of MP medium, and DNA was extracted from the suspension as described above.
Detection of strains BH and E6 by PCR.
The extracted DNA was subjected to PCR amplification with a Trio-Thermoblock thermal cycler (Biometra, Göttingen, Germany). The primers used were primers BHS1 (5′-TGCTGAAGGATGAACGTAGT-3′) and BHR1 (5′-TACCCTTCAACGGCAGGATT) for strain BH and primers SSS (5′-TGCGTGAACGCGCTCAGCAA-3′) and SHR3 (5′-ACGCCGTTGTTCAGGAACGAG-3′) for strain E6. The PCR for strain BH was conducted in a reaction mixture (total volume, 100 μl) containing 2.5 U of Taq DNA polymerase (AmpliTaq Gold; Perkin-Elmer, Branchburg, N.J.), 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.001% (wt/vol) gelatin, each deoxynucleoside triphosphate at a concentration of 200 μM, 1 μg of DNA, and 100 pmol of each primer. The PCR for strain E6 was performed like the PCR for strain BH, except that 2.5 mM MgCl2 and 50 pmol of each primer were used. The thermal profile used for amplification of the gyrB fragment of strain BH consisted of 10 min of activation of the polymerase at 94°C, followed by 30 cycles consisting of 1 min at 94°C, 1 min at 60°C, and 2 min at 72°C, and finally extension for 10 min at 72°C. The thermal profile used for amplification of DNA from strain E6 consisted of activation of the polymerase for 10 min at 94°C, followed by 30 cycles consisting of 1 min at 94°C, 1 min at 64°C, and 2 min at 72°C, and finally extension for 10 min at 72°C.
Analysis of the PCR products.
Ten microliters of each PCR product was subjected to electrophoresis on an agarose gel (high-strength, analytical-grade agarose; Bio-Rad Laboratories, Hercules, Calif.) containing agarose at concentrations of 1.5% (wt/vol) for strain BH and 3.0% (wt/vol) for strain E6 in TAE buffer (35). To quantitatively analyze the PCR products, 5 μl of a DNA quantity standard (approximately 100 ng of DNA) was applied to the gel; this standard was prepared by 10-fold dilution of the PCR product amplified by the procedure described above from 1 μg of DNA extracted from a pure culture of strain BH or E6. After electrophoresis, the DNA in the gel was stained with SYBR Green I (FMC Bioproducts) for 3 h as recommended by the manufacturer and then photographed. The intensity of each PCR product was determined from a negatively printed photograph of the gel with a model TIAS 100 densitometer (TEF Corporation, Tokyo, Japan).
Statistics.
Data were statistically analyzed by the t test (P = 0.05).
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper have been deposited in the GSDB, DDBJ, EMBL, and NCBI nucleotide sequence databases under accession no. AB002234, AB002235, AB002236, and AB002237.
RESULTS AND DISCUSSION
Design of PCR primers for specific probing.
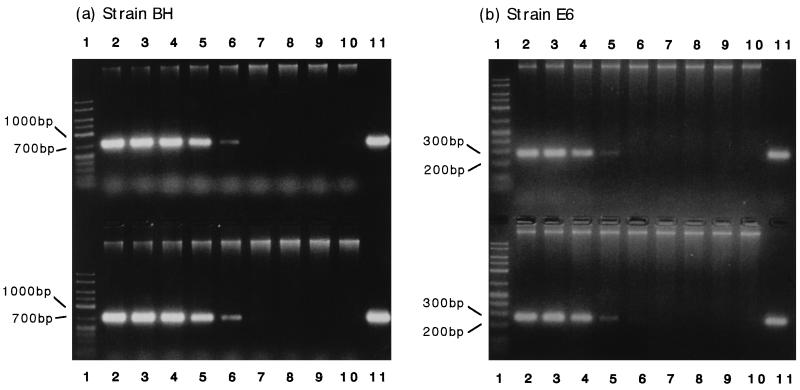
A phylogenetic analysis of the gyrB sequence of strain BH was conducted previously (16), and this strain was classified as a P. putida strain. PCR primers BHS1 and BHR1 for specific detection of strain BH were designed by comparing the gyrB sequence of strain BH with the gyrB sequences of other P. putida strains. The gyrB sequences of strains BH, A10L, FK715, IFO14671, and JCM6156 have appeared in nucleotide sequence databases under accession no. D86010, D86005, D86014, D86011, and D37926, respectively. The gyrB sequence of strain IFO14164 was identical to the sequence of P. putida PRS2000 (accession no. X54631). These primers allowed amplification of a 738-bp fragment from the total DNA of strain BH (Fig. 1a, lane 11), but not from the total DNAs of other P. putida strains (data not shown).
FIG. 1.
PCR amplification of the gyrB fragments of strains BH and E6. For the upper part of each gel 10-fold serial dilutions of each pure culture were mixed with the activated sludge, and DNA extraction from the mixed samples and PCR amplification of the extracted DNA were carried out. (a) Strain BH. Lane 1, 50-2500 DNA size marker (FMC Corporation); lane 2, 2.5 × 107 cells per ml; lane 3, 2.5 × 106 cells per ml; lane 4, 2.5 × 105 cells per ml; lane 5, 2.5 × 104 cells per ml; lane 6, 2.5 × 103 cells per ml; lane 7, 2.5 × 102 cells per ml; lane 8, 2.5 × 101 cells per ml; lane 9, 2.5 × 100 cells per ml; lane 10, uninoculated activated sludge; lane 11, pure BH culture. (b) Strain E6. Lane 1, 50-2500 DNA marker; lane 2, 1.9 × 107 cells per ml; lane 3, 1.9 × 106 cells per ml; lane 4, 1.9 × 105 cells per ml; lane 5, 1.9 × 104 cells per ml; lane 6, 1.9 × 103 cells per ml; lane 7, 1.9 × 102 cells per ml; lane 8, 1.9 × 101 cells per ml; lane 9, 1.9 × 100 cells per ml; lane 10, uninoculated activated sludge; lane 11, pure E6 culture. For the lower part of each gel DNA was extracted from activated sludge containing strains BH and E6 at densities of 2.5 × 107 and 1.9 × 107 cells per ml, respectively, before 10-fold serial dilution of the extracted DNA with DNA extracted from the uninoculated activated sludge. PCR amplification was carried out by using the diluted samples. (a) Strain BH. (b) Strain E6. Lanes 1, 50-2500 DNA size marker; lanes 2, undiluted DNA; lanes 3, 10-fold dilution; lanes 4, 102-fold dilution; lanes 5, 103-fold dilution; lanes 6, 104-fold dilution; lanes 7, 105-fold dilution; lanes 8, 106-fold dilution; lanes 9, 107-fold dilution; lanes 10, uninoculated activated sludge; lanes 11, pure culture.
The 805-bp nucleotide sequence in the 5′ region of gyrB of strain E6 was determined. A phylogenetic analysis of this partial gyrB sequence revealed the close relationship between this strain and Comamonas strains. The E6 gyrB sequence was 87, 83, and 83% identical to the gyrB sequences of C. terrigena IAM12052, C. testosteroni IAM12419, and C. acidovorans IFO13582, respectively. PCR primers SSS and SHR3 for specific detection of strain E6 were designed by comparing the gyrB sequences of these strains. These primers allowed amplification of a 277-bp fragment from the total DNA of strain E6 (Fig. 1b, lane 11) but not from the total DNAs of other Comamonas strains (data not shown).
Development of quantitative PCR.
Use of the primers designed for specific amplification of the gyrB fragments of strains BH and E6 allowed a quantitative PCR method to be developed. First, experiments were conducted in which cultures of strains BH and E6 or serial dilutions of cultures were mixed with phenol-digesting activated sludge obtained from the laboratory unit (MLSS; 1,850 ppm; total cell count, 3.8 × 109 cells per ml); this was followed by DNA extraction and PCR amplification. The amount of DNA extracted from each of the activated-sludge samples was 51 ± 6.1 μg (n = 9). As shown in Fig. 1, only those fragments with the expected molecular sizes were amplified by PCR, not only from DNA extracted from each pure culture (lane 11), but also from DNA extracted from the activated sludge mixed with the BH and E6 cultures (lane 2). In addition, no fragments were amplified from DNA extracted from noninoculated activated sludge (lane 10). These results suggest that gyrB-targeted PCR can be used for specific detection of a BH or E6 population in activated sludge.
Figure 1 also shows that the quantitative ranges of the PCR method for the BH and E6 populations were 103 to 104 and 104 to 105 cells per ml, respectively. The lower detection limit for strain E6 was approximately 10 times higher than the lower detection limit for strain BH. This may have been due to a lower efficiency of PCR amplification for strain E6 than for strain BH. The relative efficiency of a PCR is influenced by a variety of factors, including the length and secondary structure of the target nucleic acid molecule (8). In the case of the E6 gyrB sequence, its high GC content may have reduced the efficiency of the PCR. Selvaratnam et al. (38) reported that the sensitivity of the reverse transcriptase-PCR method for detecting a dmpN-expressing pseudomonad population in activated sludge was 104 CFU per 10 mg of activated sludge, which is identical to the sensitivity of our method. The lower detection limit of a PCR for specific detection of Azoarcus tolulyticus in soil has been reported to be 102 cells per ml (51). The differences in sensitivity values may have been caused by differences in the amounts of inhibitory substances in the extracted DNA samples or in the PCR conditions. Activated sludge contains a large amount of extracellular polysaccharide which is coextracted with DNA and is known to be inhibitory to PCR (43). A number of modifications of the present method (for example, further purification of the extracted DNA, an increase in the number of PCR cycles, and improvement in the detection method [8]) may be possible and may increase the sensitivity of the PCR assay, although our present PCR method was sensitive enough to monitor the population dynamics of strains BH and E6 in the activated sludge.
One useful method for expanding the upper limit of detection of a quantitative PCR assay is to use a diluted sample (8), so we next tested the applicability of this method. DNA extracted from activated sludge containing BH and E6 cells was mixed with DNA extracted from uninoculated phenol-digesting activated sludge in different ratios so that the DNA concentration in each solution was 100 μg per ml, and 1 μg of each DNA sample was then subjected to PCR. The DNA extracted from uninoculated activated sludge was used to dilute DNA from the activated sludge containing the BH and E6 cells, because it was found that the efficiency of the PCR was strongly affected by the amount of sludge-extracted DNA subjected to the PCR (data not shown). As shown in Fig. 1, the band intensity of each PCR product amplified from the diluted DNA samples was similar to the band intensity of each PCR product amplified from DNA extracted from sludge containing the same number of cells of the strain. This result indicates that DNA dilution should allow quantitative PCR determinations for broad ranges of population densities of these strains.
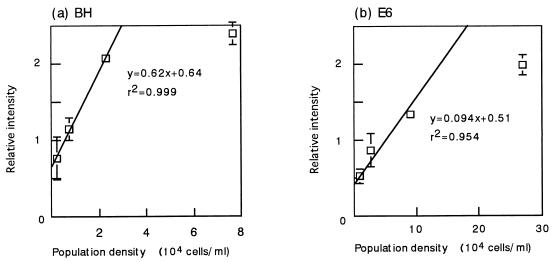
To determine more accurately the relationship between the amount of PCR products and the number of cells in the activated sludge, additional experiments were conducted, in which dilutions of BH and E6 cultures in LB medium were mixed with the phenol-digesting activated sludge; then the DNA was extracted, and a PCR was performed. The numbers of cells of these strains after they were mixed with the sludge were estimated from plate counts of the pure cultures. The PCR products were electrophoresed together with a DNA quantity standard prepared as described in Materials and Methods, and the relative intensity of the PCR product compared with the intensity of the DNA quantity standard was determined by densitometry. Figure 2 shows that there was a linear relationship between the relative intensity of each PCR product and the number of cells in the activated sludge, indicating that the PCR method was quantitative within the ranges examined. The population density could be interpolated from the relative intensity of the PCR product with the equations shown in Fig. 2.
FIG. 2.
Standard curves for determining the densities of BH and E6 populations in laboratory unit sludge. A quantitative PCR was carried out as described in the legend to Fig. 1, except that a DNA quantity standard was used to compare the results obtained in different gels. The ordinate indicates the intensity of the band of each PCR product relative to the intensity of the DNA quantity standard. The means of three determinations are shown, and the error bars indicate standard deviations.
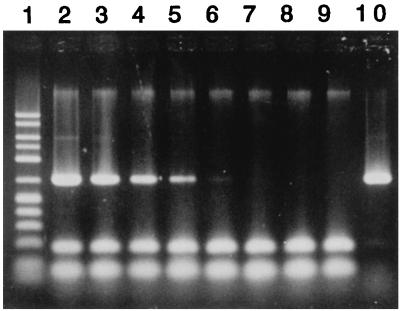
Finally, to examine the general applicability of the quantitative PCR method, strain BH was introduced into the Ohdaira activated sludge, and its population density was determined by the quantitative PCR method. Cells of strain BH were mixed with the Ohdaira activated sludge (MLSS; 1,730 ppm; total cell count, 2.4 × 109 cells per ml), and DNA was extracted from the inoculated sludge and uninoculated sludge and then subjected to PCR with the BH-specific primers. The PCR conditions were the same as those developed for detecting the BH population in the sludge of the laboratory unit except for the amount of DNA added to the PCR solution (0.2 μg) and the amplification cycle (40 cycles). The BH population in the Ohdaira sludge was specifically detected by this PCR method, as shown in Fig. 3, with a sensitivity similar to that observed with the phenol-digesting activated sludge. A standard curve for enumerating the BH population in the Ohdaira sludge was produced for a range of 1.3 × 103 to 7.8 × 103 cells per ml, a range similar to that observed for the BH population in the laboratory unit sludge. The standard curve could be expressed with the following equation: R = 0.25 × 10−3 D + 0.19 (r2 = 0.97), where R is the relative intensity and D is the population density (in cells per ml). In addition, when the DNA extracted from uninoculated sludge was used for dilution, a BH population density of 1.4 × 106 ± 0.3 × 106 cells per ml (n = 3) in the Ohdaira sludge was accurately determined by the PCR method (data not shown). These results suggest that although the PCR conditions need to be optimized depending on the sludge, the method for PCR quantitation developed in this study is widely applicable for quantifying the bacterial population introduced into an activated-sludge microbial community.
FIG. 3.
Specificity and sensitivity of the PCR method for detecting the BH population in Ohdaira activated sludge. Lane 1, 50-2500 DNA size marker; lane 2, 3.5 × 106 cells per ml; lane 3, 3.5 × 105 cells per ml; lane 4, 3.5 × 104 cells per ml; lane 5, 3.5 × 103 cells per ml; lane 6, 3.5 × 102 cells per ml; lane 7, 3.5 × 101 cells per ml; lane 8, 3.5 × 100 cells per ml; lane 9, uninoculated activated sludge; lane 10, pure BH culture.
Population dynamics of strains BH and E6 in phenol-digesting activated sludge.
BH and E6 cells grown in LB medium were introduced into the laboratory unit containing the phenol-digesting Takinoshita activated sludge, and the fate of each of these strains was investigated by the quantitative PCR method. Under stable operational conditions (a phenol-loading rate of 0.4 g per liter per day), the phenol concentration in the aeration tank never exceeded 0.5 ppm (the lower limit of detection of the phenol assay), and the total cell count ranged from 3 × 109 to 5 × 109 cells per ml throughout this experiment. Based on plate counts determined for the inocula, the numbers of BH and E6 cells in the aeration tank immediately after inoculation were estimated to be 5.2 × 107 ± 0.5 × 107 and 9.1 × 107 ± 0.4 × 107 cells per ml (n = 3), respectively. One hour after inoculation, the densities of the BH and E6 populations were determined by the quantitative PCR to be 6.3 × 107 ± 1.2 × 107 and 7.9 × 107 ± 2.8 × 107 cells per ml (n = 3), respectively; these values were not significantly different from the plate count values described above.
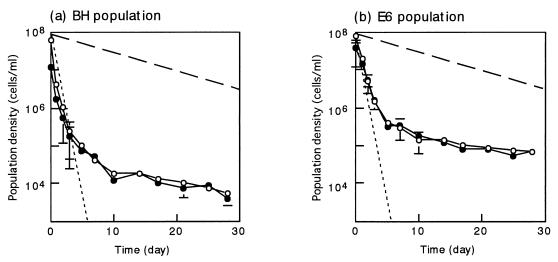
Figure 4 shows the fate of the BH and E6 populations in the activated sludge. It was observed that the decline in density of each of these two populations was multiphasic; there was an initial rapid decline, followed by a slower decline. However, the time at which the phase shift occurred was different in these two populations; the BH population entered the slow-decline phase on day 10, whereas the change occurred in the E6 population 5 days after inoculation. In both populations, most of the cells were incorporated into activated-sludge flocs in the slow-decline phase. In contrast, in the initial rapid-decline phase, the tendencies of the two populations to be incorporated into flocs were found to be different. For instance, approximately 75% of the cells in the E6 population were incorporated into flocs on day 1, whereas approximately 39% of the cells in the BH population were in flocs. These data indicate that the E6 population was more rapidly incorporated into the activated-sludge flocs than the BH population, which may have resulted in the earlier phase shift of the E6 population.
FIG. 4.
Dynamics of the BH (a) and E6 (b) populations introduced into phenol-digesting activated sludge under stable operational conditions at a phenol-loading rate of 0.4 g per liter per day. Symbols: ○, population density in total activated sludge; •, population density in activated-sludge flocs (per milliliter of liquid in the aeration tank); dashed line, theoretical washout curve at a Tr of 10 days; dotted line, theoretical washout curve at a Tr of 0.5 day. The theoretical curves were drawn by using the following equation: St = S0 · exp[−t/Tr], where St is the population density at time t and S0 is the initial population density. The means of three determinations are shown, and the error bars indicate standard deviations.
The E6 population survived in the activated sludge at a density that was more than 10 times higher than that of the BH population. Theoretical washout curves for dispersed cells (residence time [Tr], 0.5 day) and for cells incorporated into activated-sludge flocs (Tr, 10 days) are also shown in Fig. 4. The slope of the E6 population curve in the slow-decline phase was less steep than the slope of the theoretical curve (Tr, 10 days), clearly indicating that the E6 population grew in the activated-sludge flocs during this period. On the other hand, judging from the slope, the growth of the BH population in the slow-decline phase was not as noticeable as the growth of the E6 population. However, since predation must have occurred, it is likely that the BH population also grew at a rate that compensated for the decline in the population density due to predation. From these findings, we concluded that the E6 population grew more rapidly in the activated-sludge flocs than the BH population under stable operational conditions.
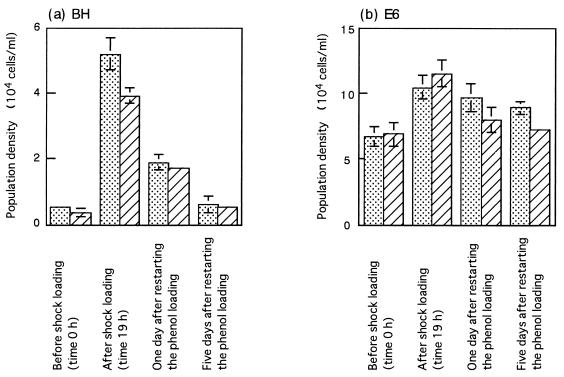
On day 28, the activated sludge was subjected to shock loading of phenol. One hour after the shock loading began, the phenol concentration in the aeration tank reached 271 ppm, and the shock loading was stopped at this moment. The phenol concentration gradually decreased after that, and it was approximately 1 ppm at 19 h. Phenol loading at a rate of 0.4 g per liter per day was restarted at 23 h. During and after shock loading, the MLSS concentration and the total cell counts ranged from 1,800 to 2,000 ppm and from 3 × 109 to 5 × 109 cells per ml, respectively. Before and after the phenol shock loading, the densities of the BH and E6 populations were determined by the PCR method (Fig. 5). It was found that the BH population significantly increased (approximately 10-fold) after shock loading. The increase in the BH population in the total activated sludge seemed to be greater than the increase in the activated-sludge flocs, although the difference was not statistically significant. The E6 population also increased after shock loading, although the increase was small and just exceeded the significant level. After shock loading was stopped, the BH population rapidly declined and reached a level similar to the level before shock loading. However, the decrease in the E6 population after shock loading was as slow as the decrease observed during the slow-decline phase under the stable operational conditions shown in Fig. 4. This observation indicates that the BH population grew more actively than the E6 population during the shock loading period.
FIG. 5.
Dynamics of BH (a) and E6 (b) populations in phenol-digesting activated sludge that was subjected to phenol shock loading as described in the text. Dotted bars, population densities in total activated sludge; cross-hatched bars, population densities in activated-sludge flocs (per milliliter of liquid in the aeration tank). The means of three determinations are shown, and the error bars indicate standard deviations.
It has been suggested that several factors affect the growth of bacteria in a microbial community (3, 34); some of these factors are nutrient availability, the presence of toxins, attachment of cells to matrices, and physical parameters. One of these, nutrient availability (i.e., the phenol concentration), was the only factor that apparently changed in the experiments described above, suggesting that the phenol concentration in the aeration tank influenced the changes in the growth tendencies of the BH and E6 populations. The growth kinetics of strains BH and E6 on phenol were determined in a laboratory pure-culture experiment by the method described by Watanabe et al. (47). The three kinetic constants in Haldane’s equation (18, 50), Ks, Ki, and μmax, determined were 22.2 ± 2.2 ppm, 107 ± 10 ppm, and 1.52 ± 0.07 h−1, respectively, for BH and 10.5 ± 1.8 ppm, 46.4 ± 5.4 ppm, and 0.91 ± 0.07 h−1, respectively, for E6 (estimated values ± standard errors). From the kinetics, it could be estimated by extrapolation that the specific growth rate of strain E6 was higher than that of strain BH in the presence of phenol concentrations below 5.7 ± 0.7 ppm. It is thus conceivable that the more rapid growth of the E6 population than of the BH population under stable operational conditions and the reverse growth trend after shock loading reflected the growth kinetics of the organisms. Additional experiments, such as experiments to determine the rates of incorporation into flocs and predation, are needed to predict the population dynamics in activated sludge.
ACKNOWLEDGMENTS
We thank Nobuhiro Takahashi for continuing support of this work. We also thank Ikuko Hiramatsu for technical assistance.
REFERENCES
- 1.Arima K, Komagata K, Minoda S. Metabolism of aromatic compounds. Part 1. Bacterial oxidation of three isomers of monohydroxybenzoic acids. Agric Chem Soc Jpn. 1954;28:629–635. [Google Scholar]
- 2.Assinder S J, Williams P A. The TOL plasmids: determination of the catabolism of toluene and the xylenes. Adv Microb Physiol. 1990;31:1–69. doi: 10.1016/s0065-2911(08)60119-8. [DOI] [PubMed] [Google Scholar]
- 3.Atlas R M, Bartha R. Microbial ecology: fundamentals and applications. Redwood City, Calif: The Benjamin/Cummings Publishing Company Inc.; 1992. Microbial communities and ecosystems; pp. 130–162. [Google Scholar]
- 4.Blackburn J W, Jain R K, Sayler G S. Molecular microbial ecology of a naphthalene-degrading genotype in activated sludge. Environ Sci Technol. 1987;21:884–890. [Google Scholar]
- 5.Bond P L, Hugenholtz P, Keller J, Blackall L L. Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludge from sequencing batch reactors. Appl Environ Microbiol. 1995;61:1910–1916. doi: 10.1128/aem.61.5.1910-1916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLong E F, Wickham G S, Pace N R. Phylogenetic strains: ribosomal RNA-based probes for the identification of single cells. Science. 1989;243:1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- 7.de Vos P, Kersters K, Falsen E, Pot B, Gillis M, Segers P, de Ley J. Comamonas Davis and Park 1962 gen. nov., nom. rev. emend., and Comamonas terrigena Hugh 1962 sp. nov., nom. rev. Int J Syst Bacteriol. 1985;35:443–453. [Google Scholar]
- 8.Diaco R. Practical considerations for the design of quantitative PCR assays. In: Innis M A, Gelfand D H, Sninsky J J, editors. PCR strategies. San Diego, Calif: Academic Press; 1995. pp. 84–107. [Google Scholar]
- 9.Digiovanni G D, Neilson J W, Pepper I L, Sinclair N A. Gene transfer of Alcaligenes eutrophus JMP134 plasmid pJP4 to indigenous soil recipients. Appl Environ Microbiol. 1996;62:2521–2526. doi: 10.1128/aem.62.7.2521-2526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikshituru S, Baltzis B C, Lewandowski G A. Competition between two microbial populations in a sequencing fed-batch reactor: theory, experimental verification, and implications for waste treatment applications. Biotechnol Bioeng. 1993;42:643–656. doi: 10.1002/bit.260420513. [DOI] [PubMed] [Google Scholar]
- 11.Diviacco S, Norio P, Zentilin L, Menzo S, Clementi M, Biamonti G, Riva S, Falaschi A, Giacca M. A novel procedure for quantitative polymerase chain reaction by coamplification of competitive templates. Nucleic Acids Res. 1992;122:313–320. doi: 10.1016/0378-1119(92)90220-j. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Ike M, Uesugi K. Operation parameters affecting the survival of genetically engineered microorganisms in activated sludge processes. Water Res. 1994;28:1667–1672. [Google Scholar]
- 13.Furukawa K. Microbial degradation of polychlorinated biphenyls. In: Chakrabarty A M, editor. Biodegradation and detoxification of environmental pollutants. Boca Raton, Fla: CRC Press, Inc.; 1982. pp. 33–57. [Google Scholar]
- 14.Gray P H H. The formation of indigotin from indol by soil bacteria. Proc R Soc Lond B Biol Sci. 1928;102:263–279. [Google Scholar]
- 15.Greer L E, Joseph J A, Shelton D R. Kinetic comparison of seven strains of 2,4-dichlorophenoxyacetic acid-degrading bacteria. Appl Environ Microbiol. 1992;58:1027–1030. doi: 10.1128/aem.58.3.1027-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harayama S, Yamamoto S. Phylogenetic identification of Pseudomonas strains based on a comparison of gyrB and rpoD sequences. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C: American Society for Microbiology; 1996. pp. 250–258. [Google Scholar]
- 17.Hashimoto S, Fujita M. Identification of three phenol-degrading microorganisms isolated from activated sludges and their characteristics. J Jpn Sewage Works. 1987;9:655–660. [Google Scholar]
- 18.Hill G A, Robinson C W. Substrate inhibition kinetics: phenol degradation by Pseudomonas putida. Biotechnol Bioeng. 1975;17:1599–1615. [Google Scholar]
- 19.Holben W E, Schroeter B M, Calabrese V G M, Olsen R H, Kukor J K, Biederbeck V O, Smith A E, Tiedje J M. Gene probe analysis of soil microbial populations selected by amendment with 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1992;58:3941–3948. doi: 10.1128/aem.58.12.3941-3948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam M S, Hasan M K, Miah M A, Sur G C, Felsenstein A, Venkatesan M, Sack R B, Albert M J. Use of the polymerase chain reaction and fluorescent-antibody methods for detection of viable but nonculturable Shigella dysenteriae type 1 in laboratory microcosms. Appl Environ Microbiol. 1993;59:536–540. doi: 10.1128/aem.59.2.536-540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Japanese Industrial Standard Committee. Testing methods for industrial wastewater, JIS K0102. Tokyo, Japan: Japanese Standards Association; 1986. [Google Scholar]
- 22.Ka J O, Holben W E, Tiedje J M. Analysis of competition in soil among 2,4-dichlorophenoxyacetic acid-degrading bacteria. Appl Environ Microbiol. 1994;60:1121–1128. doi: 10.1128/aem.60.4.1121-1128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S-Y, Bollinger J, Bezdicek D, Ogram A. Estimation of the abundance of an uncultured soil bacterial strain by a competitive quantitative PCR method. Appl Environ Microbiol. 1996;62:3787–3793. doi: 10.1128/aem.62.10.3787-3793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leser T D, Boye M, Hendriksen N. Survival and activity of Pseudomonas sp. strain B13 (FR1) in a marine microcosm determined by quantitative PCR and an rRNA-targeting probe and its effect on indigenous bacterioplankton. Appl Environ Microbiol. 1995;61:1201–1207. doi: 10.1128/aem.61.4.1201-1207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manz W, Wagner M, Amann R, Schleifer K. In situ characterization of the microbial consortia active in two wastewater treatment plants. Water Res. 1994;28:1715–1723. [Google Scholar]
- 26.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 27.Matheson V G, Forney L J, Suwa Y, Nakatsu C H, Sexstone A J, Holben W E. Evidence for acquisition in nature of a chromosomal 2,4-dichlorophenoxyacetic acid/α-ketoglutarate dioxygenase gene by different Burkholderia spp. Appl Environ Microbiol. 1996;62:2457–2463. doi: 10.1128/aem.62.7.2457-2463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matheson V G, Munakata-Marr J, Hopkins G D, McCarty P L, Tiedje J M, Forney L J. A novel means to develop strain-specific DNA probes for detecting bacteria in the environment. Appl Environ Microbiol. 1997;63:2863–2869. doi: 10.1128/aem.63.7.2863-2869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClure N C, Weightman A J, Fry J C. Survival of Pseudomonas putida UWC1 containing cloned catabolic genes in a model activated-sludge unit. Appl Environ Microbiol. 1989;55:2627–2634. doi: 10.1128/aem.55.10.2627-2634.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClure N C, Fry J C, Weightman A J. Survival and catabolic activity of natural and genetically engineered bacteria in a laboratory-scale activated-sludge unit. Appl Environ Microbiol. 1991;57:366–373. doi: 10.1128/aem.57.2.366-373.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMacken R, Silver L, Geogopoulos C. DNA replication. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 578–580. [Google Scholar]
- 32.Nüblein K, Maris D, Timmis K, Dwyer D F. Expression and transfer of engineered catabolic pathways harbored by Pseudomonas spp. introduced into activated sludge microcosms. Appl Environ Microbiol. 1992;58:3380–3386. doi: 10.1128/aem.58.10.3380-3386.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickup R W. Development of molecular methods for the detection of specific bacteria in the environment. J Gen Microbiol. 1991;137:1009–1019. [Google Scholar]
- 34.Roszak D B, Colwell R R. Survival strategy of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 36.Saraswat N, Alleman J E, Smith T J. Enzyme immunoassay detection of Nitrosomonas europaea. Appl Environ Microbiol. 1994;60:1969–1973. doi: 10.1128/aem.60.6.1969-1973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayler G S, Layton A C. Environmental application of nucleic acid hybridization. Annu Rev Microbiol. 1990;44:625–648. doi: 10.1146/annurev.mi.44.100190.003205. [DOI] [PubMed] [Google Scholar]
- 38.Selvaratnam S, Schoedel B A, Mcfarland B L, Kulpa C F. Application of reverse transcriptase PCR for monitoring expression of the catabolic dmpN gene in a phenol-degrading sequencing batch reactor. Appl Environ Microbiol. 1995;61:3981–3985. doi: 10.1128/aem.61.11.3981-3985.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimao M, Nakamura T, Okuda A, Abe M, Harayama S. Characterization of transposon insertion mutants of mandelic acid-utilizing Pseudomonas putida strain A10L. Biosci Biotechnol Biochem. 1996;60:1051–1055. doi: 10.1271/bbb.60.1051. [DOI] [PubMed] [Google Scholar]
- 40.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 41.Steffan R J, Atlas R M. Polymerase chain reaction: application in environmental microbiology. Annu Rev Microbiol. 1991;45:137–161. doi: 10.1146/annurev.mi.45.100191.001033. [DOI] [PubMed] [Google Scholar]
- 42.Sykes P J, Neoh S H, Brisco M J, Hughes E, Condon J, Morley A A. Quantitation of targets for PCR by use of limiting dilution. BioTechniques. 1992;13:444–449. [PubMed] [Google Scholar]
- 43.Taylor J L. A simple, sensitive, and rapid method for detecting seed contaminated with highly virulent Leptoshaeria maculans. Appl Environ Microbiol. 1993;59:3681–3685. doi: 10.1128/aem.59.11.3681-3685.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner M, Amann R, Lemmer H, Schleifer K H. Probing activated sludge with oligonucleotide specific for proteobacteria: inadequacy of culture-dependent methods for describing microbial community structure. Appl Environ Microbiol. 1993;59:1520–1525. doi: 10.1128/aem.59.5.1520-1525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe K, Hino S, Onodera K, Takahashi N. Abstracts of the 28th Annual Meeting of the Japan Society on Water Environment. Tokyo, Japan: The Japan Society on Water Environment; 1994. Studies on population dynamics of bacteria in a wastewater treatment process by using enzyme immunoassay, abstr. 3-F-9-35; pp. 720–721. [Google Scholar]
- 46.Watanabe K, Hino S, Onodera K, Kajie S, Takahashi N. Diversity in kinetics of bacterial phenol-oxygenating activity. J Ferment Bioeng. 1996;81:562–565. [Google Scholar]
- 47.Watanabe K, Hino S, Takahashi N. Effects of exogenous phenol-degrading bacteria on performance and ecosystem of activated sludge. J Ferment Bioeng. 1996;82:291–298. [Google Scholar]
- 48.Watanabe K, Hino S. Identification of a functionally important population in phenol-digesting activated sludge with antisera raised against isolated bacterial strains. Appl Environ Microbiol. 1996;62:3901–3904. doi: 10.1128/aem.62.10.3901-3904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto S, Harayama S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol. 1995;61:1104–1109. doi: 10.1128/aem.61.3.1104-1109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang R D, Humphrey A E. Dynamics and steady state studies of phenol degradation in pure and mixed cultures. Biotechnol Bioeng. 1975;17:1211–1235. doi: 10.1002/bit.260170809. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Palumbo A V, Tiedje J M. Sensitive detection of a novel class of toluene-degrading denitrifiers, Azoarcus tolulyticus, with small-subunit rRNA primers and probes. Appl Environ Microbiol. 1997;63:2384–2390. doi: 10.1128/aem.63.6.2384-2390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]