Abstract
Telomere length (TL) may be a biomarker of aging processes as well as age-related diseases. However, most studies of TL and aging are conducted in high-income countries. Less is known in low- and middle-income countries (LMICs) such as South Africa, where life expectancy remains lower despite population aging. We conducted a descriptive analysis of TL in a cohort of older adults in rural South Africa. TL was assayed from venous blood draws using quantitative polymerase chain reaction (T/S ratio). We examined the correlation between TL and biomarkers, demographic characteristics, mental/cognitive health measures, and physical performance measures in a subsample of the Wave 1 2014–2015 “Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa” (HAALSI) cohort (n = 510). We used logistic regression to measure the association between TL and mortality through Wave 3 (2021–2022). In bivariate analyses, TL was significantly correlated with age (r = −0.29, p < .0001), self-reported female sex (r = 0.13, p = .002), mortality (r = −0.1297, p = .003), diastolic blood pressure (r = 0.09, p = .037), pulse pressure (r = −0.09, p = .045), and being a grandparent (r = −0.17, p = .0001). TL was significantly associated with age (β = −0.003; 95% confidence interval [CI] = −0.005, −0.003). TL was significantly associated in unadjusted multivariate analyses with mortality, but the relationship between TL and mortality was attenuated after adjusting for age (odds ratio [OR] = 0.19; 95% CI = 0.03, 1.27) and other covariates (OR = 0.17; 95% CI = 0.02, 1.19). Our study is the first analysis of TL in an older adult South African population. Our results corroborate existing relationships between TL and age, sex, cardiometabolic disease, and mortality found in higher-income countries.
Keywords: Biomarkers, Mortality, Population health, Telomeres
Telomere length (TL) has emerged as an important potential biomarker of biological aging processes. A telomere is a region of repetitive DNA sequences at the end of chromosomes that shorten with progressive cell division (1). Although originally thought to be genetically determined, both TL and its rate of shortening have been found to also be influenced by environmental factors and health behaviors (2,3). In prior research, TL has been associated with respiratory, musculoskeletal, and cardiovascular diseases, HIV, and diabetes, as well as many age-related conditions, including cancer and Alzheimer’s disease (2,4–10). TL has also been associated with increased mortality in older adults in the United States, Europe, and Asia (9–11). However, it is unclear if TL contributes to these conditions, and meta-analyses have yielded inconclusive results as to whether TL is a more effective biomarker for aging than chronological age (2,5). Chronological age reflects time since birth whereas biological “aging” may be better measured by time to death or functional decline (12). It is especially important to understand the relationship between chronological age and biological aging in low- and middle-income countries (LMICs), where life expectancy is shorter than in many high-income countries. Some studies have found no relationship between TL and cognitive or physical function or other age-related disease processes in older adults, potentially due to methodological differences (13,14). Recent research has also complicated the relationship between TL and morbidity, as studies have demonstrated that telomeres may lengthen through homeostatic mechanisms during cell division, especially when high concentrations of telomerase are present (15,16). Despite these mixed results, TL remains associated with some disorders and is strongly correlated with chronological age.
Of the research conducted on TL and aging, most have taken place in higher-income countries, especially European or North American cohorts (8–10,14,17–19). There remains a large gap in the knowledge of TL in LMIC settings, especially among older African populations. Many African countries have begun to experience demographic transitions with substantial population aging. The study of aging in this context is becoming increasingly critical, as the burden of age-related conditions is expected to increase (20). Yet, TL research on the African continent has primarily occurred in young- to middle-aged adult populations (21–24) or in urban settings (25,26). Our study is uniquely positioned to address this major gap in our understanding of TL characteristics, especially in rural older African adults. We aim to provide a descriptive analysis of TL in a community-based representative sample of older, rural South African adults in the Health and Demographic Surveillance System (HDSS) of Agincourt, South Africa. Our aims are to (a) describe cross-sectional patterns of association between TL and demographic, health, and social characteristics; (b) examine the association between age and TL; and (c) characterize associations between TL and mortality with and without adjustment for age.
Method
Study Sample
Data for this study were from “Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa” (HAALSI), an ongoing population-representative cohort study of Black adults aged 40+ years in the rural Agincourt subdistrict of Mpumalanga province, South Africa (27). Data are available publicly at haalsi.org/data. HAALSI is a harmonized international sister study to the U.S. Health and Retirement Survey (HRS), with international partner studies in at least 10 countries worldwide. Detailed information on recruitment and selection into the cohort is available elsewhere (27). The first wave of the HAALSI survey occurred in 2014–2015 with 5 059 individuals enrolled. Wave 2 occurred in 2018–2019 and Wave 3 in 2021–2022. Each survey wave included interviews, physical measurements, and venous and/or dried blood spot blood collection. Immediately following the Wave 1 survey, a subsample of 2 492 individuals aged 40–80 years participated in additional laboratory testing in 2015–2016 as part of the H3Africa AWI-Gen study (28,29), which included a venous blood draw with DNA extraction. Among those who provided blood for DNA extraction, we conducted a pilot study for TL of 510 individuals aged 40–80, randomly sampled stratified by age group, self-reported sex, and educational attainment.
Measures
Demographic characteristics
Participants were asked to self-report their age, sex, education, and country of origin during the Wave 1 individual interview (27). Education was categorized into 4 groups based on educational attainment (no formal education, some primary, some secondary, and secondary or more).
Telomere length
The Thermo Fisher QuantStudio 6 Flex Real-Time PCR System (Waltham, MA) was used to extract DNA from venous blood samples and measure TL relative to standard reference DNA (Supplementary Material—Methods). The standard curve assessed and compensated for interplate variations in polymerase chain reaction (PCR) efficiency. The slopes of the standard curve for both the telomere and 36B4 reactions were −3.33 ± 0.33 and the linear correlation coefficient (R2) values for both reactions were over 0.99. The T/S ratio (−dCt) for each sample was calculated by subtracting the average 36B4 Ct value from the average telomere Ct value. The relative T/S ratio (−ddCt) was determined by subtracting the T/S ratio value of the 5-ng standard curve point from the T/S ratio of each unknown sample (10). Quality control samples were interspersed throughout the test samples to assess interplate and intraplate variability of threshold cycle (Ct) values. A combined inter- and intra-assay coefficient of variation calculated from the relative T/S ratio (−ddCt) of quality control samples is 8.5%. The relative T/S ratio was exponentiated to adjust for skew, resulting in normally distributed data. In the resulting exponentiated T/S ratio measure, a 1-unit increase represents a 100% increase in TL as compared to the population standard sample.
Biomarkers
Biomarkers of cardiovascular function, inflammatory response, and metabolic function were measured through physical assessment and point-of-care testing. Cardiovascular function biomarkers include systolic blood pressure, diastolic blood pressure, heart rate, and pulse pressure. Systolic and diastolic blood pressure and heart rate were measured 3 times using OMRON© Automatic blood pressure monitor M6W. The final blood pressure and heart rate were recorded as the average of the second and third measurements. Pulse pressure was measured as the difference between the mean systolic and diastolic blood pressures. Detailed protocol on point-of-care testing is available through HAALSI (27). Lung function was measured as peak expiratory flow using a spirometer. Inflammatory response biomarkers were assessed through C-reactive protein (CRP) from dried blood spots. Metabolic function biomarkers used include high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, random blood glucose, and body mass index (BMI). Cholesterol and random blood glucose were measured using point-of*care tests, and BMI was calculated using participant height and weight.
Mental/cognitive health
Mental health was assessed using the abbreviated 8-item Center for Epidemiologic Studies—Depression Scale (CES-D; range 0–8) (30), as well as a short screening scale for post-traumatic stress disorder (PTSD; range 0–7), collected during the Wave 1 individual interview. Cognitive function was measured through a 26-point scale that included items on orientation, immediate and delayed recall, and numeracy, partially harmonized with HRS (27).
Physical performance
Physical performance was measured through a timed walk and grip strength. Walking speed was assessed using a timed walk of 2.5 m. Participants completed the walk twice, and then those times were added together and used to calculate a final walking speed measurement. Grip strength was measured in both hands using the Smedley Digital Hand Dynamometer (12–0286), alternating between each hand. The maximum grip strength measurement from either hand was used in this analysis.
Social characteristics
Household wealth was assessed using a quintile wealth index using household durable goods as a measure of socioeconomic status (31). Households were ranked according to the scores from principal components analysis of household ownership of items such as televisions, refrigerators, livestock, and vehicles, as well as housing characteristics and type of water and sanitation facilities (31). During the Wave 1 individual interview, participants were also asked if they had grandchildren and whether they were part of a religious denomination.
The individual interview also included 11 questions asking about adverse life experiences across the life course. These questions asked if participants had experienced any of the following: natural disaster, life-threatening illness or accident, serious physical assault, sexual assault, parental unemployment of at least 6+ months, parental argument or fighting, parental substance abuse, physical abuse from parents, spousal substance abuse, fired or fired on in combat, witnessed death, or injury in combat. These measures were aggregated to create an 11-point scale measuring adverse life experiences.
Mortality
HAALSI participants were tracked through biannual follow-up. Participant deaths and dates of death were documented either via cohort tracking phone calls, or during the regular annual census visit through the Agincourt Health and Demographic Surveillance System (HDSS) in which the HAALSI study is nested (32). All deaths were followed up by a verbal autopsy team as part of the HDSS protocol (33). The analysis presented here includes mortality records through the HAALSI Wave 3 survey, which occurred in 2021–2022.
Data Analysis
Pearson correlations were used to measure bivariate associations between TL and demographic characteristics, biomarkers, measures of physical and cognitive performance, and social characteristics. Point biserial correlation coefficients were calculated for the bivariate associations between TL and the above characteristics if they were binary variables. Linear regression was used to assess the predictive value of age on TL, and then the model was adjusted for social confounders significantly correlated with TL. We did not adjust for any health measures as covariates in order to avoid mistakenly conditioning on mediators. We then used logistic regression to measure the association between TL and mortality. Separate unadjusted and adjusted models were run for the linear regression between TL and age and the logistic regression between TL and mortality. Adjusted models included sex of respondent (self-defined), educational attainment, and being a grandparent to adjust for significant social confounders. For adjusted mortality models, age was included as a covariate to explore if TL is predictive of mortality independent of chronological age. Age2 was also included in the adjusted logistic regression models to account for any nonlinear relationships of age with TL or mortality. We also assessed the relationship between TL and mortality stratified by age and self-reported sex, but strata were too small to generate robust results. We ran further sensitivity analyses restricted to nonaccidental deaths. All analyses were conducted using Stata V. 17.0 (StataCorp LLC, College Station, TX).
Results
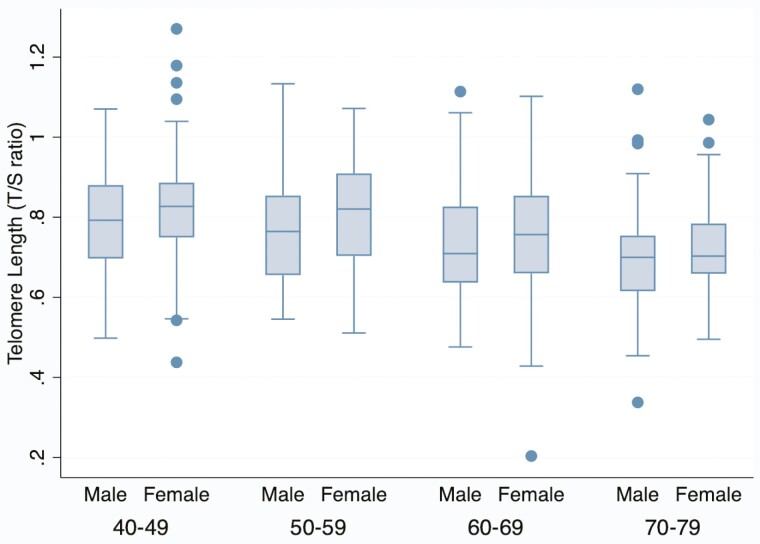
Descriptive statistics of the analytic sample are shown in Table 1. Mean age was 58 years, and approximately 53% of the sample was male. Nearly 15% of the sample died in the 7 years between Wave 1 and Wave 3 data collection in 2021–2022. Average blood pressure was in the hypertensive range (Table 1), indicating a high prevalence of cardiometabolic disease risk in the population. Average BMI was 25.83, slightly above healthy weight range. Generally, participants scored low on depression and PTSD scales (Table 1). Mean T/S ratio was 0.77; on average, TL in this sample was 77% the length of TL in the population standard sample (Table 1). TL generally decreased with older age and male sex (Figure 1).
Table 1.
Descriptive Statistics for Telomere Length, Demographic Characteristics, and Health Characteristics (HAALSI 2014–2015, N = 510)
| N | Descriptive Statistics | |
|---|---|---|
| Telomere length (T/S ratio), mean (SD) | 510 | 0.77 (0.1) |
| Demographic characteristics | ||
| Age (years), mean (SD) | 510 | 58.40 (11.2) |
| Sex of respondent, self-reported (% male) | 510 | 269 (52.7%) |
| Education (%) | 510 | |
| No formal education | 152 (29.8%) | |
| Some primary (1–7 years) | 148 (29.0%) | |
| Some secondary (8–11 years) | 122 (23.9%) | |
| Secondary of more (12+ years) | 88 (17.3%) | |
| Country of origin (% from South Africa) | 510 | 382 (74.9%) |
| Deceased as of Wave 3 (%) | 510 | 76 (14.9%) |
| Biomarkers | ||
| Systolic BP (mmHg), mean (SD) | 501 | 137.43 (21.0) |
| Diastolic BP (mmHg), mean (SD) | 501 | 82.65 (12.3) |
| Pulse pressure (mmHg), mean (SD) | 501 | 54.78 (14.7) |
| Heart rate (BPM), mean (SD) | 501 | 73.33 (12.1) |
| Lung function (L/s), mean (SD) | 398 | 4.20 (2.0) |
| HDL Cholesterol (mmol/L), mean (SD) | 427 | 1.57 (0.6) |
| LDL Cholesterol (mmol/L), mean (SD) | 387 | 2.00 (0.9) |
| Total Cholesterol (mmol/L), mean (SD) | 425 | 4.13 (1.2) |
| C-reactive protein, mean (SD) | 453 | 3.05 (3.0) |
| Blood glucose (mmol/L), mean (SD) | 477 | 6.78 (3.5) |
| BMI, mean (SD) | 510 | 25.83 (17.5) |
| Mental/cognitive health | ||
| CES-D score, mean (SD) | 504 | 1.32 (1.5) |
| PTSD symptoms, mean (SD) | 510 | 0.47 (1.3) |
| Cognitive function score, mean (SD) | 508 | 13.91 (4.6) |
| Physical performance | ||
| Timed walk speed (m/s), mean (SD) | 492 | 0.44 (0.1) |
| Grip strength (kg), mean (SD) | 498 | 29.63 (9.8) |
| Social characteristics | ||
| Household wealth quintile (%) | 510 | |
| Lowest quintile | 91 (17.8%) | |
| Quintile 2 | 92 (18.0%) | |
| Quintile 3 | 93 (18.2%) | |
| Quintile 4 | 96 (18.8%) | |
| Highest quintile | 138 (27.1%) | |
| Is a grandparent (%) | 510 | 400 (78.4%) |
| Part of a religious denomination, self-reported (%) | 510 | 422 (82.8%) |
| Cumulative adverse life experiences, mean (SD) | 510 | 2.67 (1.9) |
Notes: BMI = body mass index; BP = blood pressure; CES-D = Center for Epidemiologic Studies—Depression Scale; HAALSI = Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa; HDL = high-density lipoprotein; LDL = low-density lipoprotein; PTSD = post-traumatic stress disorder; SD = standard deviation.
Figure 1.
Telomere length by age and self-reported sex (HAALSI 2014–2015, N = 510). The center line indicates the median value (50th percentile). The box encompasses the 25th to 75th percentiles of the data in each category. The whiskers denote the 5th and 95th percentiles, and any dots beyond those boundaries represent outliers. HAALSI = Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa.
Table 2 shows the results of the Pearson’s correlations between TL and each demographic characteristic and measure of biological, physical, and social health. TL had a small but significant negative correlation with age, mortality, and pulse pressure. TL had a significant positive correlation with female sex, being a grandparent, and diastolic blood pressure. The correlation between TL and education was weakly positive but findings were not statistically significant at α = 0.05.
Table 2.
Correlations Between Telomere Length and Health/Social Characteristics (HAALSI 2014–2015, N = 510)
| Correlation Coefficient (r) | p Value | |
|---|---|---|
| Demographic characteristics | ||
| Age | −0.29 | <.0001*** |
| Female sex (self-reported) | 0.13 | .002** |
| Educational attainment (low to high) | 0.08 | .076+ |
| Country of origin (South Africa vs Mozambique) | 0.07 | .137 |
| Deceased (as of Wave 3) | −0.13 | .003** |
| Biomarkers | ||
| Systolic BP | −0.01 | .855 |
| Diastolic BP | 0.09 | .037* |
| Pulse pressure | −0.09 | .045* |
| Heart rate | −0.06 | .158 |
| Lung function (peak expiratory flow) | 0.02 | .728 |
| HDL cholesterol | −0.06 | .242 |
| LDL cholesterol | 0.00 | .969 |
| Total cholesterol | −0.05 | .308 |
| C-reactive protein | 0.05 | .292 |
| Blood glucose | −0.05 | .238 |
| BMI | −0.04 | .316 |
| Mental/cognitive health | ||
| CES-D score | −0.02 | .670 |
| PTSD symptoms | 0.02 | .734 |
| Cognitive function score | 0.03 | .479 |
| Physical performance | ||
| Timed walk | 0.04 | .429 |
| Grip strength | −0.03 | .503 |
| Social characteristics | ||
| Household wealth | −0.03 | .561 |
| Is a grandparent | 0.17 | .0001** |
| Part of a religious denomination (self-reported) | −0.03 | .530 |
| Cumulative adverse life experiences | −0.07 | .127 |
Notes: BMI = body mass index; BP = blood pressure; CES-D = Center for Epidemiologic Studies—Depression Scale; HAALSI = Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa; HDL = high-density lipoprotein; LDL = low-density lipoprotein; PTSD = post-traumatic stress disorder; SD = standard deviation.
+ p < .1. *p < .05. **p < .01. ***p < .001.
In the unadjusted linear model predicting TL from chronological age, a 1-year increase in age had a small but significant negative association with TL (Table 3). After adjusting for sex, education, household wealth, and being a grandparent, the association was still significant (β = −0.003, p < .0001). Increased TL was significantly associated with lower odds of mortality in both unadjusted (odds ratio [OR] = 0.06; 95% confidence interval [CI] = 0.01, 0.41) and adjusted (OR = 0.09, 95% CI = 0.01, 0.60) logistic regression models that did not include age (Table 4). When adjusted for all covariates including age, the association between increased TL and higher odds of mortality was no longer statistically significant (OR = 0.15, 95% CI = 0.02, 1.08). Our sensitivity analyses excluding mortality due to accidents did not yield any significantly different results.
Table 3.
Linear Regression Models of Age-Predicting Telomere Length (HAALSI 2014–2015, N = 510)
| Variable | Model 1 | Model 2 |
|---|---|---|
| β (95% CI) | β (95% CI) | |
| Age | −0.004 (−0.005, −0.003)*** | −0.003 (−0.004, −0.002)*** |
| Sex | ||
| Male | REF | |
| Female | 0.04 (0.01, 0.06)** | |
| Educational attainment | ||
| No formal education | REF | |
| Some primary (1–7 years) | 0.00 (−0.03, 0.03) | |
| Some secondary (8–11 years) | 0.00 (−0.03, 0.04) | |
| Secondary of more (12+ years) | 0.01 (−0.03, 0.04) | |
| Is a grandparent | 0.03 (−0.07, 0.00)* | |
Notes: Model 1 covariates: age. Model 2 covariates: age, sex (reference: male), educational attainment (reference: no formal education), being a grandparent. CI = confidence interval.
*p < .05. **p < .01. ***p < .001.
Table 4.
Logistic Regression Models of Telomere Length Predicting Mortality (HAALSI 2014–2015, N = 510)
| Variable | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Telomere length (T/S ratio) | 0.06 (0.01, 0.41)** | 0.09 (0.01, 0.60)* | 0.16 (0.02, 1.10)+ | 0.15 (0.02, 1.08)+ |
| Age | 1.05 (1.02, 1.07)*** | 1.29 (0.96, 1.73)+ | ||
| Age² | 0.998 (0.996, 1.000) | |||
| Sex | ||||
| Male | REF | REF | ||
| Female | 1.22 (0.73, 2.03) | 1.40 (0.83, 2.36) | ||
| Educational attainment | ||||
| No formal education | REF | REF | ||
| Some primary (1–7 years) | 0.91 (0.50, 1.66) | 0.94 (0.51, 1.73) | ||
| Some secondary (8–11 years) | 0.60 (0.30, 1.21) | 0.67 (0.33, 1.37) | ||
| Secondary of more (12+ years) | 0.52 (0.22, 1.20) | 0.70 (0.29, 1.70) | ||
| Is a grandparent | ||||
| Yes | REF | REF | ||
| No | 2.44 (1.06, 5.62)* | 1.42 (0.57, 3.51) | ||
Notes: Model 3 covariates: TL. Model 4 covariates: TL, sex (reference: male), educational attainment (reference: no formal education), being a grandparent. Model 5 covariates: TL, age. Model 6 covariates: TL, age, age2, sex (reference: male), educational attainment (reference: no formal education), being a grandparent. CI = confidence interval; OR = odds ratio; TL = telomere length.
+ p < .1. *p < .05. **p < .01. ***p < .001.
Discussion
This study describes the characteristics of TL in a cohort of older adults aged 40 and above in rural South Africa and presents the first analysis to assess the predictive value of TL on mortality in an older rural African population. Our findings are consistent with existing data from non-African settings demonstrating a strong relationship between TL and age; younger age was associated with longer TL. Of the few studies on TL done in South Africa, ours most closely reflects Ngwa et al.’s cross-sectional analysis of TL and cardiometabolic characteristics in an urban black South African population (n = 676; median age 42 years). Although about a year elapsed between the survey and the blood collection, we still consider our analysis to be cross-sectional, as TL does not change substantially over 1 year (1,34). Ngwa et al. found that TL was not associated with age, sex, or any metabolic conditions (25). Our results contrast with this study and suggest that TL trends in this rural South African community may have demographic patterns similar to European, Asian, or American populations. Most strikingly, people with longer telomeres had significantly lower odds of mortality between Wave 1 (2014–2015) and Wave 3 7 years later (2021–2022). When age was included in the model, TL was no longer statistically significantly predictive of mortality, but the direction of the relationship was still strongly negative, demonstrating that the trend of shorter TL and mortality may still be present even when chronological age is accounted for. Thus, even with a relatively small pilot sample of 510 participants, we demonstrate that TL is a good indicator of chronological age or age since birth and may have utility as a biomarker for aging in rural South African populations. Further work in this area with larger samples is needed to continue understanding these associations.
The correlations between TL and biomarkers, mental/cognitive health measures, and physical performance metrics were mostly inconclusive, which corresponds with existing literature. We did find significant correlations between TL and some sociodemographic characteristics: sex and being a grandparent. Our results agree with several studies showing that women have significantly higher TL than men (35–37). We found a significant correlation between being a grandparent and TL, and being a grandparent was significantly positively associated with TL in the linear regression models of age and TL. This may mean that being a grandparent or having meaningful familial relationships may be a protective factor against telomere shortening; previous research has shown that lack of social support has been associated with shorter TL, although results are far from conclusive (38,39). However, being a grandparent was not significantly associated with mortality in logistic regression models of TL and mortality after adjusting for age. Additionally, we found a statistically significant negative correlation between TL and pulse pressure, in that people with shorter telomeres had wider pulse pressure differences. This is consistent with other studies reporting the negative relationship between TL and pulse pressure (7,19). These results also correspond to findings from von Känel et al., who found that shorter TL was associated with elevated levels of circulating hemostatic factors in a South African cohort (40). However, the significantly positive correlation between TL and diastolic blood pressure contradicts the correlation between TL and pulse pressure. This is consistent with Ngwa et al.’s study of TL and cardiometabolic biomarkers in an adult South African cohort (25). Thus, we cannot say for certain if TL would be a sensitive biomarker for biological aging, as the relationship between TL and cardiometabolic factors is inconclusive. We did not find an association between TL and any of the other physical, metabolic, inflammatory, psychiatric, or cognitive measures.
Our study also contributes evidence to the established link between TL and mortality in older adults. Several studies and meta-analyses have found that shorter TL is associated with increased risk of all-cause mortality (9–11). This pilot study provides some supporting evidence that TL could offer predictive value of mortality in older South African populations. However, we cannot conclude that TL is more predictive of mortality than chronological age.
This study has several strengths. To our knowledge, our study is the first to examine the effects of TL in older South African adults. With the rapid demographic transition taking place on the African continent, life expectancy is increasing, and demographic composition is shifting to older populations. It is critical that research is conducted to document the health of middle-aged and older people. Understanding the possible measures and biomarkers for aging can help researchers and clinicians understand and quantify risk for aging-related conditions. Our analytic sample is also unique in that examines TL in adults living in rural South Africa. As TL has been shown to be influenced by social and environmental factors along the life course, people living in rural areas may have been exposed to different types of environmental exposures, infrastructure, and social contact than those living in cities (2,3). As Ngwa et al.’s paper on TL in South African adults was focused in Cape Town, our analysis opens the conversation about urban versus rural exposures and TL in South Africa. Furthermore, as the HAALSI study collects a vast quantity of data about cohort members, we were able to analyze the correlations between TL and many different biomarkers, mental/cognitive health measures, physical performance measures, and social characteristics in our sample.
Despite its strengths, our study is not without limitations. Because data on TL were only collected at Wave 1, we are unable to analyze the change in TL over time or determine how the rate of TL shortening may have an impact on aging-related health processes. We also cannot assume that TL only shortened over the life course; computational models developed by Bateson and Nettle have shown that in population-level TL data, a small portion of the sample could experience TL lengthening (15). Thus, there was no way to determine if TL was a cause, correlate, or consequence of biological aging and morbidity. More research using longitudinal data is necessary to establish causation between TL and poor health. Furthermore, the sample size was too small to operationalize TL in tertiles or quartiles. Therefore, we were unable to explore any potential impacts of extreme TL, long or short, on aging processes. We were also unable to yield any meaningful results from age- or sex-stratified models because strata were too small. Furthermore, although the results from this analysis can be generalized to the full HAALSI cohort because our sample was randomized, it cannot be used to make conclusions about all South African adults. Although qPCR is a useful method to collect population-level data on TL from large samples, the T/S ratio is a within-sample measure of TL and cannot be used for comparison with other samples. Overall, the Agincourt HDSS community is more rural and disadvantaged than other areas of South Africa, and thus may reflect different social and environmental factors that influence TL. In sum, more research must be done using large amounts of population-representative, longitudinal data to elucidate the complex relationship between TL and aging over the life course. This data could capture changes in TL over time and address many of the limitations of this—and other cross-sectional—studies of TL.
Our descriptive analysis examined the characteristics of TL in a cohort of older adults in rural South Africa and found significant associations with age, self-reported sex, pulse pressure, and mortality. Increased age was strongly associated with shorter TL. When TL and age were included jointly in models predicting mortality, remained significant, whereas TL was not. These results affirm the established trends in TL with age and sex in a previously unstudied population. Our analysis also demonstrates that although shorter TL is associated with higher risk mortality, it may not be a sensitive enough biomarker of aging to be clinically relevant in LMIC.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Steve Tollman and Kathleen Kahn for their leadership at Witwatersrand, as well as those involved in HAALSI and Agincourt health and demographic surveillance system (HDSS) fieldwork, including the field staff and data analysts. We are also grateful to the HAALSI participants for their invaluable role in this study.
Contributor Information
Sarah Gao, Harvard Center for Population and Development Studies, Harvard University, Cambridge, Massachusetts, USA.
Julia K Rohr, Harvard Center for Population and Development Studies, Harvard University, Cambridge, Massachusetts, USA.
Immaculata de Vivo, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Michele Ramsay, Sydney Brenner Institute for Molecular Science, University of the Witwatersrand, Johannesburg, South Africa.
Nancy Krieger, Department of Social and Behavioral Sciences, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Chodziwadziwa W Kabudula, MRC/Wits Rural Public Health and Health Transitions Research Unit, Agincourt, South Africa.
Meagan T Farrell, Harvard Center for Population and Development Studies, Harvard University, Cambridge, Massachusetts, USA.
Darina T Bassil, Harvard Center for Population and Development Studies, Harvard University, Cambridge, Massachusetts, USA.
Nigel W Harriman, Harvard Center for Population and Development Studies, Harvard University, Cambridge, Massachusetts, USA.
Diana Corona-Perez, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Katarina Pesic, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Lisa F Berkman, Harvard Center for Population and Development Studies, Harvard University, Cambridge, Massachusetts, USA.
Funding
This work was supported by the National Institute of Aging of the National Institutes of Health (P01 AG041710). HAALSI is nested within the Agincourt Health and Socio-Demographic Surveillance System, with funding from The Wellcome Trust, University of the Witwatersrand, and Medical Research Council, South Africa (058893/Z/99/A, 069683/Z/02/Z, 085477/ Z/08/Z, and 085477/B/08/Z).
Conflict of Interest
None.
References
- 1. Srinivas N, Rachakonda S, Kumar R.. Telomeres and telomere length: a general overview. Cancers. 2020;12(3):558. doi: 10.3390/cancers12030558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaiserman A, Krasnienkov D.. Telomere length as a marker of biological age: state-of-the-art, open issues, and future perspectives. Front Genet. 2021;11:630186. doi: 10.3389/fgene.2020.630186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robertson T, Batty GD, Der G, Fenton C, Shiels PG, Benzeval M.. Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiol Rev. 2013;35(1):98–111. doi: 10.1093/epirev/mxs001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P.. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith L, Luchini C, Demurtas J, et al. Telomere length and health outcomes: an umbrella review of systematic reviews and meta-analyses of observational studies. Ageing Res Rev. 2019;51:1–10. doi: 10.1016/j.arr.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 6. Araújo Carvalho AC, Tavares Mendes ML, da Silva Reis MC, Santos VS, Tanajura DM, Martins-Filho PRS.. Telomere length and frailty in older adults—a systematic review and meta-analysis. Ageing Res Rev. 2019;54:100914. doi: 10.1016/j.arr.2019.100914 [DOI] [PubMed] [Google Scholar]
- 7. Butt HZ, Atturu G, London NJ, Sayers RD, Bown MJ.. Telomere length dynamics in vascular disease: a review. Eur J Vasc Endovasc Surg. 2010;40(1):17–26. doi: 10.1016/j.ejvs.2010.04.012 [DOI] [PubMed] [Google Scholar]
- 8. Yaffe K, Lindquist K, Kluse M, et al. ; Health ABC Study. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011 Nov;32(11):2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider CV, Schneider KM, Teumer A, et al. Association of telomere length with risk of disease and mortality. JAMA Intern Med. 2022;182(3):291–300. doi: 10.1001/jamainternmed.2021.7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA.. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/s0140-6736(03)12384-7 [DOI] [PubMed] [Google Scholar]
- 11. Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S.. Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev. 2018;48:11–20. doi: 10.1016/j.arr.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 12. Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andrés V.. Biological versus chronological aging: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(8):919–930. doi: 10.1016/j.jacc.2019.11.062 [DOI] [PubMed] [Google Scholar]
- 13. Harris SE, Deary IJ, MacIntyre A, et al. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406(3):260–264. doi: 10.1016/j.neulet.2006.07.055 [DOI] [PubMed] [Google Scholar]
- 14. Brown LL, Zhang YS, Mitchell C, Ailshire J.. Does telomere length indicate biological, physical, and cognitive health among older adults? Evidence from the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2018;73(12):1626–1632. doi: 10.1093/gerona/gly001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bateson M, Nettle D.. The telomere lengthening conundrum—it could be biology. Aging Cell. 2017;16(2):312–319. doi: 10.1111/acel.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hug N, Lingner J.. Telomere length homeostasis. Chromosoma. 2006;115(6):413–425. doi: 10.1007/s00412-006-0067-3 [DOI] [PubMed] [Google Scholar]
- 17. Müezzinler A, Mons U, Dieffenbach AK, et al. Body mass index and leukocyte telomere length dynamics among older adults: results from the ESTHER cohort. Exp Gerontol. 2016;74:1–8. doi: 10.1016/j.exger.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 18. Scarabino D, Broggio E, Gambina G, Corbo RM.. Leukocyte telomere length in mild cognitive impairment and Alzheimer’s disease patients. Exp Gerontol. 2017;98:143–147. doi: 10.1016/j.exger.2017.08.025 [DOI] [PubMed] [Google Scholar]
- 19. Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A.. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36(2):195–200. doi: 10.1161/01.hyp.36.2.195 [DOI] [PubMed] [Google Scholar]
- 20. Aboderin IAG, Beard JR.. Older people’s health in sub-Saharan Africa. Lancet. 2015;385(9968):e9–e11. doi: 10.1016/s0140-6736(14)61602-0 [DOI] [PubMed] [Google Scholar]
- 21. Nsereko E, Uwase A, Muvunyi CM, et al. Association between micronutrients and maternal leukocyte telomere length in early pregnancy in Rwanda. BMC Pregnancy Childbirth. 2020;20(1):692. doi: 10.1186/s12884-020-03330-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Auld E, Lin J, Chang E, et al. HIV infection is associated with shortened telomere length in Ugandans with suspected tuberculosis. PLoS One. 2016;11(9):e0163153. doi: 10.1371/journal.pone.0163153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malan-Müller S, Hemmings SMJ, Spies G, Kidd M, Fennema-Notestine C, Seedat S.. Shorter telomere length—a potential susceptibility factor for HIV-associated neurocognitive impairments in South African women. PLoS One. 2013;8(3):e58351. doi: 10.1371/journal.pone.0058351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Needham BL, Straight B, Hilton CE, Olungah CO, Lin J.. Family socioeconomic status and child telomere length among the Samburu of Kenya. Soc Sci Med. 2021;283:114182. doi: 10.1016/j.socscimed.2021.114182 [DOI] [PubMed] [Google Scholar]
- 25. Ngwa NE, Matsha TE, Lombard C, et al. Cardiometabolic profile and leukocyte telomere length in a Black South African population. Sci Rep. 2022;12(1):3323. doi: 10.1038/s41598-022-07328-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Womersley JS, Spies G, Tromp G, Seedat S, Hemmings SMJ.. Longitudinal telomere length profile does not reflect HIV and childhood trauma impacts on cognitive function in South African women. J Neurovirol. 2021;27(5):735–749. doi: 10.1007/s13365-021-01009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gómez-Olivé FX, Montana L, Wagner RG, et al. Cohort profile: health and ageing in Africa: a longitudinal study of an INDEPTH community in South Africa (HAALSI). Int J Epidemiol. 2018;47(3):689–690j. doi: 10.1093/ije/dyx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramsay M, Crowther N, Tambo E, et al. H3Africa AWI-Gen Collaborative Centre: a resource to study the interplay between genomic and environmental risk factors for cardiometabolic diseases in four sub-Saharan African countries. Glob Health Epidemiol Genomics. 20161:e20. doi: 10.1017/gheg.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ali SA, Soo C, Agongo G, et al. Genomic and environmental risk factors for cardiometabolic diseases in Africa: methods used for Phase 1 of the AWI-Gen population cross-sectional study. Glob Health Action. 2018;11(Suppl 2):1507133. doi: 10.1080/16549716.2018.1507133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 31. Riumallo-Herl C, Canning D, Kabudula C.. Health inequalities in the South African elderly: the importance of the measure of social-economic status. J Econ Ageing. 2019;14:100191. doi: 10.1016/j.jeoa.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kahn K, Collinson MA, Gómez-Olivé FX, et al. Profile: Agincourt health and socio-demographic surveillance system. Int J Epidemiol. 2012;41(4):988–1001. doi: 10.1093/ije/dys115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Byass P, Chandramohan D, Clark SJ, et al. Strengthening standardised interpretation of verbal autopsy data: the new InterVA-4 tool. Glob Health Action. 2012;5(1):19281. doi: 10.3402/gha.v5i0.19281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011;14(1):28–34. doi: 10.1097/MCO.0b013e32834121b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gardner M, Bann D, Wiley L, et al. ; Halcyon study team. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aviv A, Shay J, Christensen K, Wright W.. The longevity gender gap: are telomeres the explanation? Sci Aging Knowl Environ. 2005;2005(23):pe16. doi: 10.1126/sageke.2005.23.pe16 [DOI] [PubMed] [Google Scholar]
- 37. Slagboom PE, Droog S, Boomsma DI.. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55(5):876–882. [PMC free article] [PubMed] [Google Scholar]
- 38. Rentscher KE, Carroll JE, Mitchell C.. Psychosocial stressors and telomere length: a current review of the science. Annu Rev Public Health. 2020;41(1):223–245. doi: 10.1146/annurev-publhealth-040119-094239 [DOI] [PubMed] [Google Scholar]
- 39. Barbosa ARC, Nunes DP, Lima DB, et al. Association of social support network with telomere length: a cross-sectional study with community-dwelling older adults. Rejuvenation Res. 2022;25(6):253–259. doi: 10.1089/rej.2022.0037 [DOI] [PubMed] [Google Scholar]
- 40. von Känel R, Malan NT, Hamer M, van der Westhuizen FH, Malan L.. Leukocyte telomere length and hemostatic factors in a South African cohort: the SABPA Study. J Thromb Haemost. 2014;12(12):1975–1985. doi: 10.1111/jth.12733 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.