Abstract
Background
Myostatin, a cytokine produced by skeletal muscle, may influence Alzheimer’s disease (AD) pathogenesis, but sparse evidence exists in humans. We assessed the association between circulating levels of myostatin at Year 1 and plasma levels of β-amyloid 42/40 at Year 2, a marker of AD pathology, in a biracial cohort of older adults.
Methods
We studied 403 community-dwelling older adults enrolled in the Health, Aging and Body Composition Study from Memphis, Tennessee, and Pittsburgh, PA. Mean age was 73.8 ± 3 years; 54% were female; and 52% were Black. Serum myostatin levels were measured at Year 1, plasma β-amyloid 42/40 levels in Year 2 (higher ratio indicating lower amyloid load). Multivariable linear regression analyses tested the association of serum myostatin with plasma levels of β-amyloid 42/40 adjusted for computed-tomography-derived thigh muscle cross-sectional area, demographics, APOe4 allele, and risk factors for dementia. We tested for 2-way.interactions between myostatin and race or sex; results were stratified by race and sex.
Results
In multivariable models, myostatin was positively associated with plasma levels of β-amyloid 42/40 (standardized regression coefficient: 0.145, p = .004). Results were significant for white men and women (0.279, p = .009, and 0.221, p = .035, respectively) but not for Black men or women; interactions by race and gender were not statistically significant.
Conclusions
Higher serum myostatin was associated with lower amyloid burden, independently of APOe4 alleles, muscle area and other established risk factors for dementia. The role of myostatin in AD pathogenesis and the influence of race should be further investigated.
Keywords: Alzheimer’s, Biomarkers, Cognitive aging, Muscle
Currently, 6.5 million Americans are living with Alzheimer’s disease (AD) and this is projected to increase to 12.7 million in 2050 (1). AD contributes to loss of independence, decreased quality of life, increased mortality, and increased health care costs (2–4). Importantly, lifetime AD risk continues to have disparities with women and Black adults being disproportionally affected compared with their age-matched peers (5,6). Despite the wide impact of AD, the mechanisms underlying AD pathogenesis are largely unknown, and biomarkers for early identification of AD risk are limited.
An overlooked but well-recognized relationship is between physical and cognitive function (7–9). However, the mediators by which physical function alters AD pathogenesis are unclear. A possible explanation may be muscle-to-brain crosstalk, in which biologically active mediators from skeletal muscle, named myokines, have a role in influencing AD pathogenesis (10). Myostatin is a myokine from the TGF-B family and to date research has focused on its role in regulating skeletal muscle and frailty phenotypes (11). Although it is known as a marker of muscle damage in patient populations, studies in community-dwelling and nursing-home-dwelling older adults indicate a protective role of myostatin; higher levels of myostatin were associated with improved physical function (12–14). Recent literature in human studies has supported a beneficial role for myostatin in AD markers of pathology (15–17). Tanaka et al. in 2 separate cohorts (InCHIANTI, a prospective cohort in Tuscany, Italy, and the Baltimore Longitudinal Study of Aging [BLSA]) found that higher circulating levels of myostatin are associated with both slower hippocampal atrophy and lower probability of being positive on 11C Pittsburgh Compound B positron emission tomography in a subsample (16). Data from the BLSA and InCHIANTI cohorts suggest the possibility that higher levels of myostatin are associated with a neuroprotective phenotype, manifesting as lower AD burden cross-sectionally among healthy older adults (16). Conversely, the literature is mixed in the direction of the association of myostatin with cognition. Newman et al. did not find a cross-sectional association between myostatin with cognition or with incident dementia in a cohort from the Cardiovascular Health Study, but higher myostatin levels were associated with greater cognitive decline and incident dementia in the Health, Aging and Body Composition Study (Health ABC) cohort of older adults (15). If higher levels of myostatin were associated with protection against hallmarks of AD pathogenesis, this would help elucidate the role of myostatin as a neuroprotective myokine.
To address this question, we analyzed the association between myostatin and a circulating marker of AD pathogenesis, plasma β-amyloid, in a subsample of the Health ABC cohort. Recent research indicates that plasma levels of β-amyloid are associated with the development of β-amyloid plaques, a pathogenic mechanism of AD risk (18). Specifically, lower plasma levels of β-amyloid 42/40 are associated with higher cortical β-amyloid burden and are predictive of subsequent cognitive decline (18,19). To that end, assessing for an association between circulating levels of myostatin and serum β-amyloid 42/40 may advance the scientific understanding of the role of myostatin in AD pathogenesis. Therefore, the primary aim of this study was to evaluate the association of circulating myostatin levels at Year 1 with plasma levels of β-amyloid at Year 2 in a biracial sample of older adults without dementia at enrollment, participating in the Health ABC study. Given the known disparities of AD burden by race and gender, our secondary aim was to assess if the association between myostatin and amyloid burden differed between Black and White men and women.
Method
Study Population
The Health ABC study is a longitudinal cohort study that enrolled 3 075 community-dwelling adults aged 70–79 from Pittsburgh, PA and Memphis, TN between March 1997 and July 1998, previously described (20). At enrollment, all participants reported no difficulties completing activities of daily living, ability to walk a quarter of a mile, and climb 10 steps without needing to rest. This study was approved by the institutional review boards at the University of Pittsburgh, the University of Tennessee Memphis, and the Coordinating Center at the University of California San Francisco. All participants signed a written informed consent. Specifically, yearly examinations were conducted for the first 6 years of the study, and then in Years 8, 10, and 11. The main outcome of Health ABC was to assess the incidence of mobility disability. Participants were contacted every 6 months for 17 years for all hospitalizations, and medical records were collected. A repository of serum and plasma, stored at −70°C, is presently located in the National Institute on Aging (NIA) Repository in Baltimore. The analytical cohort was composed of a random sample of 403 sex- and race-stratified participants who had measurements for both myostatin and plasma β-amyloid 42/40.
Covariates
Sociodemographic variables were collected at the time of enrollment including age, postsecondary education, sex, and race. Chronic medical conditions relevant to the current study that were collected included diabetes, hypertension, and body mass index. Additionally, the amount of physical activity was self-reported by the participants at the baseline visit and then the modified Paffenbarger Scale was used to calculate total kilocalories expended per week (21). APOe4 alleles are associated with increased AD risk and were defined as positive if there were equal to or greater than one APOe4 allele. Note, APOe4 testing was available for 381 of the 403 participants enrolled.
Main Independent Variables
Myostatin
Blood samples from the participant’s baseline visit were used to assess plasma levels of myostatin and were measured by liquid chromatography–tandem mass spectrometry, to address cross-reactivity with GDF-11 (22).
Muscle measurements
Muscle imaging via CT scans was performed midway at the thigh during the baseline visit (Year 1) as previously described (23). Calculations were based on a single slice of the CT image and Hounsfield units and manual segmentation were used to demarcate muscle from other tissues. Measurements of muscle area were reported in centimeters squared (cm2) and have been previously validated (24). Knee extension muscle torque was measured using a isokinetic technique and a dynamometer with 60° of a knee extension (model 125 AP, Kin-Com, Chattanooga, TN) as previously described (20). Torque was reported in Newton-Meters. Lean body mass was assessed using fan beam DXA (Hologic QDR4500A, software version 8.21, Waltham, MA) as previously described (23).
Outcome variables
Plasma β-amyloid 42/40.
β-Amyloid levels of 42 and 40 were measured from stored blood samples from participants of the Health ABC study in Year 2. The frozen blood samples were shipped to Mayo Clinic in Jacksonville, FL and were analyzed using the Innogenetics (Ghent, Belgium) INNO-BIA assay that has previously been described (19).
Cognition.
Modified Mini-Mental Status Examination (3MS) and Digit Symbol Substitution test (DSST) were used to measure cognitive function at Year 1. The 3MS tests overall cognitive function and specific components using a battery that covers orientation, language, and immediate and delayed memory (19). The DSST is a test of psychomotor speed and attention.
Statistical analysis.
Of the 403 participants, full data were present for 381 participants with APOe4 data being unavailable for the remaining 22 participants. The analytic sample was 403 except in analysis with APOe4 as a variable in which the sample size was 381. To identify variables and adjust for linear regression models, Spearman Correlation Coefficients (ρ, continuous variables) and mean differences (categorical variables) of population characteristics were performed separately for myostatin and β-amyloid 42/40, Table 1. Associations were adjusted for muscle area to account for different muscle sizes between participants.
Table 1.
Distribution of the Variables of Interest, and Their Associations With Myostatin and Plasma Β-Amyloid in N = 403 Study Participants
| Mean (SD) or N (%) | Myostatin† | Plasma β-Amyloid 42/40 | |
|---|---|---|---|
| Myostatin, ng/mL | 6.9 (1.90) | 0.128* | |
| Plasma β-amyloid 42/40, ng/mL | 0.19 (0.066) | 0.123* | |
| Age, y | 73.8 (2.87) | 0.064 | 0.020 |
| Female gender, (female–male) | 184 (54%) | −0.634 | −0.002 |
| White race, (White–Black) | (49%) | −0.697* | 0.004 |
| Education, postsecondary | 146 (36.32) | −0.151 | 0.009 |
| 3MS, mean points (interquartile range) | 91.3 (8.00) | −0.097 | 0.007 |
| DSST, points | 34.4 (13.34) | −0.046 | −0.019 |
| APOe4 allele ≥1‡ | 131 (34.38) | 0.400 | −0.015* |
| Diabetes, present | 162 (40.20) | 0.203 | −0.005 |
| Hypertension, present | 157 (38.96) | 0.577* | −0.009 |
| BMI, kg/m2 | 27.5 (4.72) | 0.022 | 0.050 |
| Thigh muscle torque, Nm | 106.8 (38.03) | −0.019 | −0.012 |
| Thigh muscle area, cm2 | 225.6 (57.16) | 0.227** | 0.038 |
| Physical activity, kcal/kg/wk | 85.8 (74.60) | 0.120* | 0.042 |
Notes: Spearman correlation (ρ) for continuous variables and mean differences are reported for categorical variables, respectively. 3MS = Modified Mini-Mental Status Examination; DSST = Digit Symbol Substitution test; SD = standard deviation.
†Adjusted for muscle area.
‡APOe4 measured in N = 381 participants.
*p < .05. **p < .01. All other p values of ≥.05.
To determine the association of myostatin with β-amyloid 42/40, we created multivariate linear regression models adjusting for APOe4 allele ≥1, demographics, and muscle area. Models were further adjusted for other known risk factors for AD, including education, physical activity, and hypertension.
To assess the association of myostatin with β-amyloid 42/40 by gender and race, we performed interaction analyses using the full sample (n = 403) to evaluate the interaction of myostatin with gender and race, respectively. Specifically, we included the interaction terms of myostatin with gender (myostatin × gender) and race (myostatin × race) in the full sample (n = 403). Due to the known disparity of AD risk in women and Black individuals and our a priori hypothesis that myostatin’s association with plasma β-amyloid 42/40 may be sex- and gender-dependent and these relationships may help to explain this disparity, we also established subgroups. Specifically, the subgroups were White men, Black men, White women, and Black women to further evaluate the association of myostatin with β-amyloid 42/40. Multivariate linear regression models adjusting for age and APOe4 allele ≥1 were created for each subgroup, with further adjustment for other dementia risk factors (education, physical activity, diabetes, and hypertension) in subsequent models.
Results
The baseline characteristics of the participants are presented in Table 1. Mean age at the start of the study was 73.0 years (standard deviation (SD): 2.87). Myostatin levels were higher in Black individuals compared to White individuals when adjusting for muscle area (Table 1) and remained significant after further adjustment for gender (p < .05). The presence of hypertension and higher physical activity was also associated with higher myostatin levels, independent of muscle area. Plasma levels of β-amyloid 42/40 were lower with the presence of the APOe4 allele, p < .05. Associations of myostatin or β-amyloid 42/40 with other population characteristics were not statistically significant (p > .05).
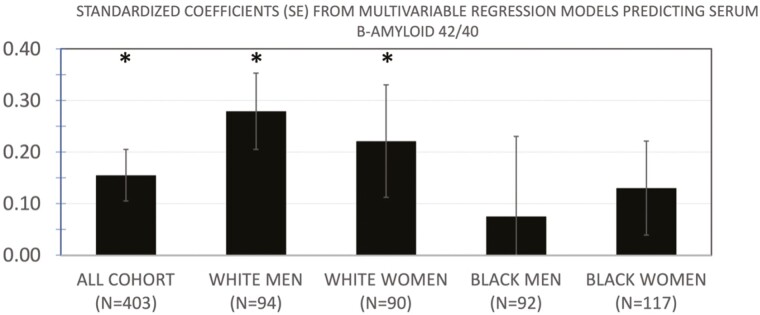
In multivariable models controlling for age, race, gender, muscle area and presence of the APOe4 allele, myostatin was positively associated with plasma levels of β-amyloid 42/40 for all participants (Table 2); for 1 SD increment of circulating levels of myostatin, there was a difference of 0.145 (95% confidence interval [CI]: 0.041, 0.250, p = .006) in β-amyloid 42/40. To help appreciate the meaning of the regression coefficient for myostatin, we note that for each APOe4 allele present, β-amyloid 42/40 was lowered by −0.112 (95% CI: −0.449, −0.023, p = .03). Further adjustment for education, hypertension, physical activity, and education in addition to age, race, gender, muscle area, and presence of the APOe4 allele yielded similar results for the full cohort with a regression coefficient for myostatin of 0.155 (0.05, 0.260), p = .004 and is shown in Figure 1. Two-way interactions of myostatin by gender or by race were not statistically significant (p > .3). The unadjusted plasma myostatin levels (ng/mL, SD) for the stratified groups were for White men (6.7, 1.8), White women (6.4, 1.8), Black men (7.8 2.1), and Black women (6.7, 1.7). The unadjusted plasma β-amyloid 42/40 levels (ng/mL, SD) for the stratified groups were for White men (0.18, 0.04), White women (0.19, 0.07), Black men (0.19, 0.09), and Black women (0.18, 0.07). In analyses stratified by gender and race (Figure 1), results were statistically significant for White men and women (standardized regression coefficient [95% CI]: 0.279 [0.052, 0.346], p = .009 and .221 [0.017, 0.452], p = .035, respectively), but not for Black men (0.075 [−0.212, 0.405], p = .5) and women (0.130 [−0.059, 0.301], p = .2); CIs for the point estimates overlapped for all race and gender groups. Race differences were similar when both sexes were combined, with associations significant in White but not men (data not shown).
Table 2.
Results of Multivariable Linear Regression Models of Myostatin as a Determinant of Plasma β-Amyloid 42/40
| Adjusted for Age and Muscle Area | Further Adjusted for Race | Further Adjusted for Gender | Further Adjusted for APOe4 Allele | |
|---|---|---|---|---|
| Myostatin | Standardized Regression Coefficients (95 % Confidence Intervals), p Value | |||
| 0.127 (0.023, 0.130) p = .016 |
0.123 (0.023, 0.129) p = .017 |
0.136 (0.032, 0.241) p = .010 |
0.145 (0.041, 0.250) p = .006 |
|
Figure 1.
Results of multivariable linear regression models of myostatin as a determinant of plasma β-amyloid 42/40, in the total cohort and stratified by race and gender. All models are adjusted for age, APOE4, muscle area, education, physical activity, and hypertension. Models in the full cohort are also adjusted for race and gender. *p < .05.
Discussion
In this study of older adults without dementia, circulating levels of myostatin were positively associated with plasma levels of β-amyloid 42/40. Using multivariable models, this finding was robust to adjustment of APOe4 allele, a well-recognized predictor of AD pathology accumulation, as well as other dementia risk factors. Collectively, these results suggest that higher levels of myostatin may be associated with lower amyloid burden in older adults.
Our finding of circulating myostatin levels being positively associated with plasma β-amyloid 42/40 levels is supported by 2 epidemiological cohorts of older adults, suggesting that myostatin may be neuroprotective in AD pathogenesis (16). Specifically, higher myostatin was associated with more favorable neuroimaging profile and with decreased odds of having mild cognitive impairment or dementia in the BLSA and the InCHIANTI cohorts. In a small subsample in the same study, associations with PET amyloid burden trended toward significance (p = .06) (16). Although there are differences between using PET and serum markers of β-amyloid, as used in the current study, they are both established methods to infer β-amyloid levels involved in the AD pathogenesis (19). Our study was able to leverage a large population (n = 403) to increase our power and discover an association between myostatin and β-amyloid. Importantly, the association between myostatin and β-amyloid remained significant after adjustment for APOe4 allele status, a known biomarker for amyloid pathology. For comparison, in this cohort APOe4 had a similar, but opposite, associations with β-amyloid, indicating the association of myostatin with β-amyloid is not negligible and deserves further study.
However, there is other evidence that higher myostatin levels may have a neurodegenerative effect. A single animal study using a transgenic mouse model of APP1/PS1 of exclusively male mice indicated that myostatin may contribute to amyloid accumulation (25). The findings in male mice were from a small sample, have not been replicated, and emphasize the need for continued research for establishing the role of myostatin in amyloid deposition and AD pathogenesis. Of note, our study did not find a cross-sectional association between myostatin levels and cognitive test scores, consistent with findings in the CHS (15) and in the BLSA among cognitively normal adults (16). However, we note the direction of association was opposite to what was found for amyloid, with higher myostatin corresponding to a worse score on 3MS and DSST, consistent with what was found in the larger Health ABC cohort (15). There are also conflicting results pertaining to the longitudinal association of myostatin with cognitive decline in older adults. Among 4 cohort studies, myostatin was associated with a greater risk of dementia in the Health ABC, but slower cognitive decline in the BLSA, and InCHIANTI, and it did not show a significant association with cognitive decline or dementia in the CHS (15,16). One possible explanation for these conflicting results is the variability in the methods to assess myostatin levels across study cohorts. Another consideration is the significant amount of lead time that is required for amyloid aggregate into plaques and cause clinical cognitive impairment, it is not surprising that it may be difficult to discern the association of myostatin with cognition. While myostatin may be an early indicator of amyloid burden protection over 1 year, its association with cognitive changes over longer periods of time may not be as strong. Overall, a study replicating the association of myostatin to plasma β-amyloid 42/40 in an independent cohort is needed. Moreover, as trials involving the inhibition of the activin receptor II (to which myostatin binds) are already under way, understanding the contribution of myostatin to neurocognitive outcomes is critical and deserves more work (26).
To date, research on myostatin has focused on muscle health, hypertrophy, and frailty. Myostatin serum concentration is correlated to muscle size and is involved in inhibiting protein synthesis and promoting protein ubiquitination. Therefore, the science was aimed at establishing a negative relationship between myostatin and muscle health. Despite this, the literature associating physical frailty phenotypes with myostatin is conflicting as to whether high or low levels of myostatin are associated with frailty (12,14). In part, this literature has demonstrated the importance of adjusting for muscle size in statistical analysis involving myostatin. From a brain health standpoint, impaired autophagy and impaired protein breakdown are suspected to contribute to amyloid plaques and neurofibrillary tangles (11,27). Therefore, it is biologically plausible that, by decreasing protein synthesis and/or increasing cellular autophagy programs through protein ubiquitination, myostatin may be associated with a more favorable amyloid load.
We found that the associations between circulating levels of myostatin and β-amyloid 42/40 were statistically significant within White men and women, but not in Black participants. This finding should be interpreted with caution as the stratified groups may not have been adequately powered to find a difference and the tests for interaction were not statistically significant, and last the CIs for the beta-coefficients overlapped across all of the race and gender groups. However, results were significant for white but not black when both sexes were combined, suggesting that the effects of myostatin effects may be influenced by race (28). Since this is the first known study to investigate the association of myostatin with plasma levels β-amyloid 42/40 by race and gender, we lack studies to compare. The association of myostatin with frailty phenotypes also shows gender differences and generally demonstrated a stronger association in women compared with men (12,14). In the current study, we believe we are the first to report a significant difference in circulating myostatin levels in Black individuals compared with White individuals when adjusting for muscle area and gender. In regard to racial differences, studies reporting myostatin levels are lacking, but polymorphisms (R153) of the myostatin gene were seen in Black participants more than White participants in a study evaluating a strength training program (29). In summary, more research is needed to uncover gender and race-specific disparities in AD development.
Our study has several strengths including the statistical adjustment for muscle size in analyses concerning myostatin, relatively large sample size, gender, and race subgroup analysis, and the use of LC–MS for accurate measurement of myostatin. The LC–MS measurement is a notable strength as myostatin shares homology with GDF-11 and many other proteins from this family (22). Our study has limitations, including the study design in which all variables were collected at Year 1 except β-amyloid, which was collected at Year 2. Despite a year difference in collection time between myostatin and β-amyloid this is unlikely to significantly alter the results given that plasma β-amyloid levels have been demonstrated to change minimally over a 4-year period of time (30). Our study best resembles a cross-sectional study, and we would recommend longitudinal studies assessing the association between serum levels of myostatin and β-amyloid. Additionally, there is evidence that the role of myostatin may be further mediated by close family members including follistatin and GDF-11 that we did not include in this report. The sample size of our analyses may have limited the power to detect differences by race and gender, as well as other associations. For example, the association of β-amyloid with 3MS was not statistically significant; this was similar to what was shown in a larger Health ABC cohort, and other cross-sectional studies of not demented older adults thus this may reflect the limited nature of β-amyloid in uncovering cognitive impairment cross-sectional in a population of adults without dementia (15). The values of β-amyloid, myostatin, and other variables in our sample were similar to what has been previously published in larger subsamples of the Health ABC cohort, indicating selection bias, if present, was unlikely to have affected our results (15).
Our collective findings suggest that higher circulating levels of myostatin are associated with neuroprotective levels of plasma β-amyloid which may be in the opposite direction of the association of myostatin with dementia or cognitive decline. Moving forward, research should continue investigating how myokines, such as myostatin, contribute to AD pathogenesis and consider how the relationship may be modified by gender and race, due to the known disparities in AD risk. Given the established link between physical function and cognitive resilience in aging adults, it is possible that myostatin and other myokines have a role in mediating the protective effect of muscle on cognition and further research is encouraged.
Contributor Information
Brendan L McNeish, Department of Physical Medicine and Rehabilitation, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Iva Miljkovic, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Xiaonan Zhu, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Peggy M Cawthon, Research Institute, California Pacific Medical Center, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Anne B Newman, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Bret Goodpaster, AdventHealth, Orlando, Florida, USA.
Kristine Yaffe, Department of Psychiatry, Neurology, and Epidemiology and Biostatistics, University of California, San Francisco, California, USA; VA Medical Center, San Francisco, San Francisco, California, USA.
Caterina Rosano, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Funding
This work was supported by R01-AG052964 to A.B.N and P30-AG02482 to B.L.M. Health ABC was supported by National Institute on Aging Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. The research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
Conflict of Interest
A.N., B.L.M., X.Z., B.G., I.M., K.Y., and C.R. report no competing interests. P.C. receives consulting fees from BioAge.
Author Contributions
B.L.M. drafted the manuscript and revised analyses. C.R. developed analysis plan, completed analyses, and revised the manuscript. X.Z. developed analysis plan and completed analyses. B.L.M., I.M., P.C., A.B.N., K.Y., X.Z., B.G., and C.R. critically reviewed and edited the manuscript and A.B.N. secured funding for this project.
Data Availability
Data from Health ABC are available to investigators. For Health ABC, requests for data access may be made to https://healthabc.nia.nih.gov/.
References
- 1. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA.. Population estimate of people with clinical AD and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement J Alzheimers Assoc. 2021;17(12):1966–1975. doi: 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liang CS, Li DJ, Yang FC, et al. Mortality rates in Alzheimer’s disease and non-Alzheimer’s dementias: a systematic review and meta-analysis. Lancet Healthy Longev. 2021;2(8):e479–e488 doi: 10.1016/S2666-7568(21)00140-9 [DOI] [PubMed] [Google Scholar]
- 3. Walker MD, Salek SS, Bayer AJ.. A review of quality of life in Alzheimer’s disease. PharmacoEconomics. 1998;14(5):499–530 doi: 10.2165/00019053-199814050-00004 [DOI] [PubMed] [Google Scholar]
- 4. Zhao Y, Kuo TC, Weir S, Kramer MS, Ash AS.. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. BMC Health Serv Res. 2008;8(1):108. doi: 10.1186/1472-6963-8-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non-Hispanic black and white individuals in the United States, 2000–2016. JAMA Neurol. 2021;78(3):275–284 doi: 10.1001/jamaneurol.2020.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement J Alzheimers Assoc. 2015;11(3):310–320. doi: 10.1016/j.jalz.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol Ser A Biol Sci Med Sci. 2013;68(11):1379–1386. doi: 10.1093/gerona/glt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clouston SAP, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35(1):33–50 doi: 10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hörder H, Johansson L, Guo X, et al. Midlife cardiovascular fitness and dementia: A 44-year longitudinal population study in women. Neurology. 2018;90(15):e1298–e1305 doi: 10.1212/WNL.0000000000005290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pedersen BK. Physical activity and muscle–brain crosstalk. Nat Rev Endocrinol. 2019;15(7):383–392 doi: 10.1038/s41574-019-0174-x [DOI] [PubMed] [Google Scholar]
- 11. Baig MH, Ahmad K, Moon JS, et al. Myostatin and its regulation: a comprehensive review of myostatin inhibiting strategies. Front Physiol. 2022;13. Accessed November 10, 2022. https://www.frontiersin.org/articles/10.3389/fphys.2022.876078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergen HR, Farr JN, Vanderboom PM, et al. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet Muscle. 2015;5(1):21. doi: 10.1186/s13395-015-0047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arrieta H, Hervás G, Rezola-Pardo C, et al. Serum myostatin levels are higher in fitter, more active, and non-frail long-term nursing home residents and increase after a physical exercise intervention. Gerontology. 2019;65(3):229–239 doi: 10.1159/000494137 [DOI] [PubMed] [Google Scholar]
- 14. Choi SJ, Lee MS, Kang DH, et al. Myostatin/Appendicular Skeletal Muscle Mass (ASM) ratio, not myostatin, is associated with low handgrip strength in community-dwelling older women. Int J Environ Res Public Health. 2021;18(14):7344. doi: 10.3390/ijerph18147344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newman AB, Patel S, Kizer J, et al. Evaluation of associations of growth differentiation factor-11, growth differentiation factor-8 and their binding proteins follistatin and follistatin-like protein-3 with dementia and cognition. J Gerontol A Biol Sci Med Sci. 2023:glad019. doi: 10.1093/gerona/glad019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka T, Lavery R, Varma V, et al. Plasma proteomic signatures predict dementia and cognitive impairment. Alzheimers Dement Transl Res Clin Interv. 2020;6(1). doi: 10.1002/trc2.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fife E, Kostka J, Kroc Ł, et al. Relationship of muscle function to circulating myostatin, follistatin and GDF11 in older women and men. BMC Geriatr. 2018;18(1):200. doi: 10.1186/s12877-018-0888-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305(3):261–266 doi: 10.1001/jama.2010.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol Bethesda. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 21. Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K.. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708 doi: 10.1001/archinte.161.14.1703 [DOI] [PubMed] [Google Scholar]
- 22. Peng L, Gagliano-Jucá T, Pencina KM, et al. Age trends in growth and differentiation factor-11 and myostatin levels in healthy men, and differential response to testosterone, measured using liquid chromatography-tandem mass spectrometry. J Gerontol A Biol Sci Med Sci. 2022;77(4):763–769 doi: 10.1093/gerona/glab146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2002;50(5):897–904 doi: 10.1046/j.1532-5415.2002.50217.x [DOI] [PubMed] [Google Scholar]
- 24. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R.. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol Bethesda Md 1985. 2000;89(1):104–110. doi: 10.1152/jappl.2000.89.1.104 [DOI] [PubMed] [Google Scholar]
- 25. Lin YS, Lin FY, Hsiao YH.. Myostatin is associated with cognitive decline in an animal model of Alzheimer’s disease. Mol Neurobiol. 2019;56(3):1984–1991 doi: 10.1007/s12035-018-1201-y [DOI] [PubMed] [Google Scholar]
- 26. Rooks D, Swan T, Goswami B, et al. Bimagrumab vs optimized standard of care for treatment of sarcopenia in community-dwelling older adults: a randomized clinical trial. JAMA Netw Open. 2020;3(10):e2020836. doi: 10.1001/jamanetworkopen.2020.20836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Q, Liu Y, Sun M.. Autophagy and Alzheimer’s disease. Cell Mol Neurobiol. 2017;37(3):377–388 doi: 10.1007/s10571-016-0386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Velez LM, Van C, Moore T, et al. Genetic variation of putative myokine signaling is dominated by biological sex and sex hormones. eLife. 2022;11:e76887. doi: 10.7554/eLife.76887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrell RE, Conte V, Lawrence EC, Roth SM, Hagberg JM, Hurley BF.. Frequent sequence variation in the human myostatin (GDF8) gene as a marker for analysis of muscle-related phenotypes. Genomics. 1999;62(2):203–207 doi: 10.1006/geno.1999.5984 [DOI] [PubMed] [Google Scholar]
- 30. Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, Chang YF, Tracy R, DeKosky ST.. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70(19):1664–1671. doi: 10.1212/01.wnl.0000306696.82017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from Health ABC are available to investigators. For Health ABC, requests for data access may be made to https://healthabc.nia.nih.gov/.