Abstract
Background
Based on studies from animal models, growth differentiation factor-11 (GDF-11) may have rejuvenating effects in humans. GDF-11 has high sequence homology with GDF-8 (also known as myostatin); follistatin and follistatin-like protein-3 (FSTL-3) are inhibitory proteins of both GDF-8 and GDF-11.
Methods
Using highly specific liquid chromatography with tandem mass spectrometry assays for GDF-11 and GDF-8 and immunoassays for follistatin and FSTL-3, we quantified the association of these factors with muscle size, strength, and physical performance in 2 prospective cohort studies of community-dwelling older adults (Health, Aging, and Body Composition study [Health ABC] and Cardiovascular Health Study [CHS]).
Results
GDF-8 levels were positively associated with thigh muscle cross-sectional area and density in Health ABC (data not available in CHS). GDF-8 levels were positively associated with lean mass (a surrogate of muscle mass) in Health ABC but not CHS, and grip strength in CHS but not Health ABC. FSTL-3 (and perhaps follistatin) was negatively associated with lean mass and had variable associations with other variables. In contrast, GDF-11 was not significantly associated with strength or performance.
Conclusions
GDF-8 and its binding proteins, follistatin and FSTL-3, may constitute a counterregulatory system (chalones) to restrain age-related loss of muscle mass and strength.
Keywords: Geriatric assessment, Physical function, Sarcopenia
The discovery of “geroproteins” that rejuvenate tissues of old mice (1) or accelerate aging in young mice has raised the possibility that treatments targeted to these proteins may reduce the risk of functional decline, mobility limitation, and other disabling aging-related conditions in older adults (2,3). Experiments in mice using heterochronic parabiosis have demonstrated that joining the circulation of young and aged mice appeared to reverse age-related changes in several systems in older mice, including muscle, heart, and brain (4–6). Proteomic analysis identified growth differentiation factor-11 (GDF-11) as a factor that may explain the effects of heterochronic parabiosis.
An initial report suggested that circulating concentrations of GDF-11 declined with advancing age, and supplementation of older mice with recombinant GDF-11 improved muscle strength, endurance, and muscle regeneration after injury (6). Ensuing studies in preclinical models, however, failed to observe either age-related changes in circulating GDF-11 concentrations or therapeutic benefits of GDF-11 administration on physical function. Some studies in mice reported that GDF-11 attenuates muscle regeneration and that very high concentrations induce muscle wasting (7–10). Differences in experimental conditions, dose regimens, and outcome assessments may have contributed to the heterogeneity of findings. A study in clinical populations found that GDF-11 levels did not vary by age but were associated with comorbidity and frailty (11). GDF-11 has not been widely studied in older adults.
The differences in findings among prior studies may also be related to the challenges in accurately measuring GDF-11. Concerns about the specificity and accuracy of the extant assays for the measurements of circulating GDF-11 have underscored the need for a novel GDF-11 assay that has a high degree of specificity and sensitivity. The GDF-11 protein shares high sequence homology with growth differentiation factor-8 (GDF-8, also known as myostatin), a negative regulator of muscle mass. Both GDF-8 and GDF-11 are generated in the precursor, inactive forms that are cleaved by a furin-like protease. The mature C-terminal dimer is maintained in an inactive latent complex with its respective propeptide (12–14). The circulating mature proteins are bound to their cognate binding proteins, including follistatin, follistatin-related protein-3 (FSTL-3), and GASP-1 and GASP-2, which regulate their biological activity (12,15–17). There are important differences in GDF-11 and GDF-8 concentrations: GDF-8 levels are higher than GDF-11 levels in both humans and mice, but GDF-8 levels are significantly lower in humans than in mice, potentially limiting the generalizability of mouse studies to humans (18). Many binding reagent assays (eg, immunoassays and aptamer-based methods, eg, SOMAlogic) used for the measurement of GDF-11 levels in earlier studies had varying degrees of cross-reactivity of GDF-8 (6,10). The methodological challenges of measuring GDF-11 and GDF-8 levels with a high level of specificity and sensitivity; uncertainty about mechanisms of their biological regulation; and the substantial differences in the concentration between mice and humans raise important questions about the role of these growth factors in human aging. A direct strategy to address this question is to study the relationship of GDF-11 and GDF-8, as well as their cognate binding proteins, with measures of muscle performance and physical function in humans.
In this report, we used a newly developed liquid chromatography with tandem mass spectrometry (LC-MS/MS) assay that can simultaneously measure GDF-8 and GDF-11 in the human serum with high degree of specificity and without detectable cross-reactivity of GDF-8 in the GDF-11 assay. We expected that higher GDF-8, follistatin, and FSTL-3 levels would be associated with worse physical function, decreased muscle size, and lower strength. Given previous divergent results of studies of GDF-11 with age-related outcomes, the directionality of the association of GDF-11 with outcomes was uncertain. These analyses leverage serum samples and data from community-dwelling older adults in the Health, Aging, and Body Composition study (Health ABC) and Cardiovascular Health Study (CHS) as part of the Comprehensive Evaluation of Aging-Related Geroproteins and Clinical Outcomes consortium.
Method
Study Cohorts
The CHS began in 1989 to evaluate determinants of cardiovascular disease (CVD) in adults aged ≥65 years and enrolled 5 201 men and women (19) at 4 U.S. field centers (Universities of Pittsburgh, California [Davis], Johns Hopkins, and Wake Forest). In 1992–93, 687 Black participants were enrolled (a total of 5 888 participants). Annual exams were conducted between 1992–93 and 1998–99, with follow-up every 6 months by phone alternating with annual visits to assess all hospitalizations and self- or proxy report of cardiovascular disease (CVD) events including myocardial infarction, stroke, and heart failure. We used data (and blood drawn) from the CHS visit conducted in 1994–95 (Year 7). A large repository of serum is maintained at −80°C at the CHS Core Lab, University of Vermont. Health ABC was established in 1998 to study how a change in body composition influences incident disability and mortality. In Health ABC, participants were recruited at 2 field centers: University of Pittsburgh and University of Memphis. Participants were aged 70–79 with no difficulty walking ¼ mile or up a flight of stairs. In total, 3 075 men and women (42% Black) were enrolled. A repository of serum and plasma, which has been stored at −70°C, is now located in the NIA Repository in Baltimore, MD. Baseline data from Health ABC including serum collected at the baseline examination (and follow-up incident mobility limitation) was used in the report. CHS and Health ABC participants provided written informed consent, and all clinical centers had protocols approved by local institutional review boards.
Outcome Measures
In Health ABC, participants were queried every 6 months about their ability and difficulty in walking ¼ mile or climbing 10 steps without resting. Our major endpoint for mobility disability is persistent severe mobility limitation, in which participants indicated inability or severe difficulty in either walking ¼ mile or climbing 10 steps on at least 2 consecutive 6-monthly contacts. Our secondary endpoint for mobility disability is persistent mobility limitation, in which participants reported any difficulty walking ¼ mile or climbing 10 steps at the same 6-monthly contacts. These events were assessed over 16.8 years of follow-up. These measures of perceived function provide information about an individual’s self-assessed function that is distinct from objective measures of performance and strength (that are described in the following paragraph).
A usual walking pace over 6 m (m/s) was assessed in CHS and Health ABC studies. In Health ABC, isokinetic knee extensor strength at 60° was measured by KinCom devices. In both CHS and Health ABC, the maximum grip strength in both hands for 2 trials was measured by Jamar dynamometers (Sammons Preston Rolyan, Bolingbrook, IL). In Health ABC, endurance was measured by time to complete the 400-m walk, in which participants were instructed to walk “as quickly as possible.” In CHS, endurance was measured by the distance covered in 6 minutes in the 6-minute walk test (6MWT) (20) Although the 400-m and 6MWT tests are similar, walking speed over the 400-m test was faster than in the 6-minute test, suggesting that the 400-m test is more appropriate at assessing near-maximal capacity in older adults (21). In Health ABC, lean mass was measured by whole-body DXA on Hologic 4500 scanners. Appendicular lean mass/height2 (ALM/ht2) was used as an approximation of the skeletal muscle mass in the arms and legs. Thigh muscle cross-sectional area (CSA) and muscle density were assessed by thigh computed tomography scans (22).
Covariates
In CHS, height (cm) was measured 1 time using a stadiometer and weight (lb) was measured using a calibrated balance-beam scale. In Health ABC, body height was calculated as the average of 4 standing measurements (mm) and weight (kg) was measured using a calibrated balance-beam scale. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Blood pressure was calculated as the average of 2 seated measurements using a mercury sphygmomanometer. Current smoking and drinking were self-reported in both studies. In Health ABC, participants self-rated health status, and reported physician diagnoses of coronary heart disease, heart failure, cancer, atrial fibrillation, and chronic obstructive pulmonary disease (COPD). In CHS, adjudicated medical condition data at Year 7 (1994–95) was determined by combining review of hospital records, cardiovascular imaging, electrocardiogram, and blood test results to classify previous CVD (ie, adjudicated diagnosis of coronary heart disease, congestive heart failure, and angina prior to the Year 7 visit); other health conditions at Year 7 were determined by combination of self-report and discharge codes since baseline self-report. In both studies, race was by self-report.
The Simultaneous Measurement of Serum GDF-11 and GDF-8 Using Liquid Chromatography With Tandem Mass Spectrometry
The details of assay development and validation have been published (23). Briefly, aliquots of serum samples that had never thawed before were denatured and reduced in 6 M urea and 20 mM dithiothreitol at 60°C for 40 minutes and alkylated with 35 mM iodoacetamide at 37°C for 30 minutes. The acidified samples were loaded onto conditioned SCX-SPE 96/2 mL well plates (Strata—XL-C 100 µm, Phenomenex Inc., Torrance, CA) using 1 mL of methanol, followed by 1 mL of 0.1% formic acid (FA), and washed with 1 mL of 0.1% FA followed by 1 mL of 10 mM bis-Tris, pH 5.8 buffer. Bound proteins were eluted in a buffer solution containing 10% methanol in 50 mM Tris at pH 10.5. Eluents from SCX-SPE were digested with trypsin in 50 mM Tris buffer at pH 8. The digested solution was loaded into a Strata-X 33µm SPE system (PN:8E-S100-AGB, Phenomenex Inc.), and eluted with 1 mL methanol and evaporated to dryness. Residues were reconstituted with 50 µL of 5% methanol, 0.1% FA in deionized water for LC-MS/MS analysis. A Shimadzu UPLC system consisting of LC-20 ADXR Binary Pump and SIL-20 ACXR Autosampler (Framingham, MA) was used for liquid chromatographic separation using Aeris Peptide 3.6 µm XB-C18 column. Column and autosampler temperatures were set at 45°C and 10°C, respectively. The mobile phase consisted of 0.1% FA in deionized water (A) and 0.1% FA in methanol (B). An AB Sciex (Framingham, MA) QTRAP® 5 500 hybrid triple quadrupole/linear ion trap mass spectrometer equipped with a Turbo V ion source was used for detection. The mass spectrometer was operated in positive electrospray ionization mode using an ion-spray voltage of 5 500. Ion source optimization was performed by flow injection using 0.1% FA in deionized water/methanol at 70/30 (v/v) at 600 µL/min or 200 µL/min depending on the columns used. Calibration curves were prepared by assaying commercial pooled human plasma spiked with graded concentrations of GDF-11 or GDF-8 (0.5, 1, 5, 10, 25, and 50 ng/mL). Additionally, isotope-labeled IPGMVVD^R and NLGLDEHSSES^R peptides were added as internal standards to a final concentration of 5 ng/mL after pH-based fractionation by SCX-SPE and tryptic digestion.
The standard curve was linear from 0 to 50 ng/mL concentration range for GDF-11 and from 0 to 100 ng/mL range for GDF-8. The lower limit of quantitation was 0.5 ng/mL for GDF-8 as well as for GDF-11. The inter-assay coefficients of variation in quality control pools with GDF-11 concentrations of 3.4, 7.4, 12.5, and 52.0 ng/mL were 8.7%, 13.0%, 14.2%, and 12.8%, respectively; inter-assay coefficients of variation at 8.7, 14.1, 17.3, and 51.1 ng/mL of GDF-8 were 15.1%, 12.5%, 16.4%, and 12.0%, respectively. Neither GDF-8 nor IgG1 had detectable cross-reactivity in the GDF-11 assay; similarly, neither GDF-11 nor IgG1 had detectable cross-reactivity in the GDF-8 assay. The accuracy of the assay, determined as the percent recovery in pooled human plasma spiked with 1 of 3 concentration levels of GDF-11 and GDF-8 (5, 10, and 50 ng/mL), ranged from 80% to 116% for GDF-11, and 81% to 111% for GDF-8.
Follistatin and FSTL-3 were measured using ELISA kits (R&D systems) at the Laboratory for Clinical Biochemistry Research, University of Vermont. The detectable range of the follistatin assay is ~250–16 000 pg/mL, and the inter-assay CV on pooled serum controls was 6.1%. The detectable range of the FSTL-3 assay is ~313–24 622 pg/mL, and the inter-assay CV on pooled serum controls is 2.6%.
Analysis Approach
Each of the independent variables (GDF-8, GDF-11, follistatin, and FSTL-3) was log transformed as all had a skewed distribution. The relationship between these proteins and age was described with Spearman correlations and LOESS plots. In multivariate analyses, each protein as an independent variable was analyzed in separate models. Models were stratified by cohort and adjusted for age, race, and sex. For mobility limitation in Health ABC, Cox proportional hazards models were used to estimate hazard ratios (HRs) for persistent and severe persistent mobility limitation; follow-up time was time from enrollment in days to the first occurrence of limitation, death, or end of the follow-up period. Linear regression models were used to estimate beta-coefficients for continuous outcomes: grip strength, walking speed, 400-m walk time (Health ABC only), and 6-minute walk distance (CHS only). These outcomes were standardized to facilitate reporting of effect estimates across many different outcome measures. Multivariate models were further adjusted for self-rated health, BMI, cancer, COPD (Health ABC) or chronic bronchitis (CHS), angina, coronary heart disease, congestive heart failure, estimated glomerular filtration rate (eGFR) by cystatin C (24), cystatin C, C-reactive protein, smoking, and drinking status. Effect estimates (beta-coefficients and HRs) are reported per natural log(2) units of increase in the log-transformed predictor, which is equivalent to a doubling on the original scale. For the analyses above, we used a p value of .05 to determine statistical significance. Finally, to determine whether the association between each “geroprotein” and outcomes differed across levels of the other proteins (ie, effect modification), we tested pairwise statistical interaction terms between GDF-8, GDF-11, follistatin, and FSTL-3 for all outcomes. Each interaction model included only the main effect and interaction terms for the proteins tested, which were entered into the model as continuous transformed variables. For each outcome, this totaled 6 significance tests. Thus, to account for multiple comparisons, we used a Bonferroni-adjusted p value to interpret the significance of interaction within each outcome (p = .05/8 = .008).
Results
Distributions of GDF-8, GDF-11, Follistatin, and FSTL-3 Levels by Age and Sex
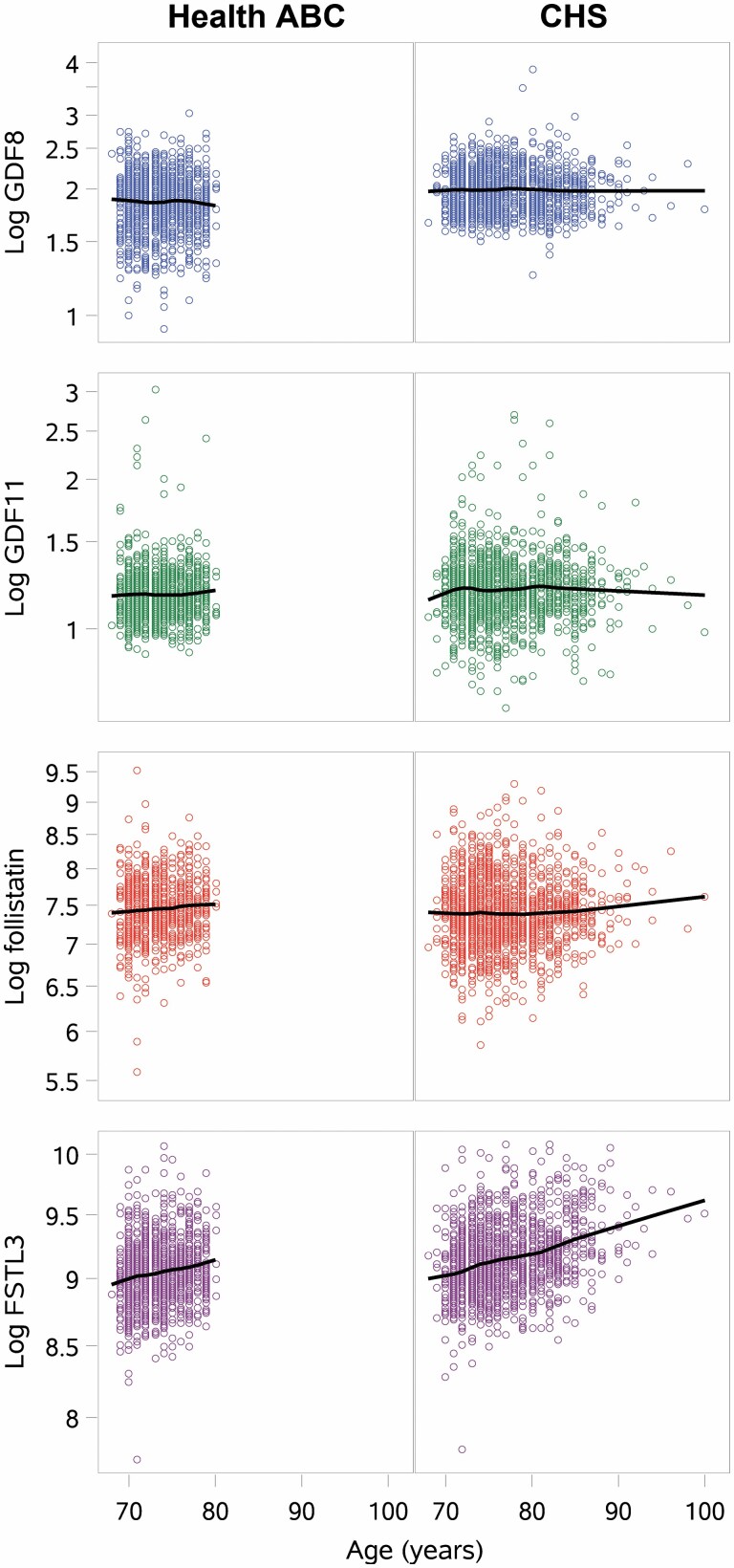
Circulating GDF-11 level was weakly but significantly and positively correlated with age overall and in white women. GDF-8 was weakly, but significantly and positively correlated with age in all participants, and in white women and men. Correlations between age and GDF-8 or GDF-11 did not reach statistical significance in other race/sex groups. FSTL-3 was modestly positively correlated with age overall and in all sex/race groups (r > 0.2, p < .001 for all; Figure 1). Follistatin level was not significantly correlated with age overall or in any race/sex subgroup, except for a weak association in Health ABC.
Figure 1.
Scatterplots of age and log GDF-8, log GDF-11, log follistain, and log FSTL-3 among participants in Health ABC and CHS. ALM/ht2 = appendicular lean mass/height2; CHS = Cardiovascular Health Study; FSTL-3 = follistatin-like protein-3; GDF = growth differentiation factor.
GDF-11 levels did not vary significantly by sex or race (p = .748); GDF-8, follistatin, and FSTL-3 varied by sex and race (Supplementary Table 1, p < .001 for all). GDF-11, GDF-8, FSTL-3, and follistatin were mostly weakly or not significantly correlated with one another (Supplementary Table 3); the strongest correlations observed were between GDF-8 and GDF-11 (Pearson product–moment correlation coefficient, ρ = 0.15 in both cohorts combined, p < .001; ρ = .07 in Health ABC, p = .018, ρ = 0.17 in CHS, p < .001); and between GDF-8 and follistatin (ρ = −0.09 in both cohorts combined, p < .001; ρ = −0.17 in Health ABC, p < .001; ρ = −0.03 in CHS, p = .198). All other correlations between these proteins were modest (ρ < 0.1) and often were not statistically significant.
Characteristics of Participants by Levels of GDF-11 in Health ABC and CHS
In Health ABC, those in the higher quartiles of GDF-11 were older and less likely to be white than those in the lower quartiles (Table 1). Walking speed varied by GDF-11 quartile with the slowest speeds in the highest and the lowest quartiles. Persistent mobility limitation was also somewhat more common in the highest and lowest quartiles. No other characteristics varied significantly across quartiles of GDF-11. Characteristics of participants by GDF-11 in CHS, and by GDF-8, FSTL-3, and follistatin levels in CHS and Health ABC are reported in the Supplementary Results and Supplementary Tables 4–9. In CHS, only prior history of angina, GDF-8 levels and ALM/ht2 varied by quartile of GDF-11 (Supplementary Table 4).
Table 1.
Characteristics (mean ± SD or N [%]) by Quartile of GDF-11, Health ABC
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Value | |
|---|---|---|---|---|---|
| <2.97 ng/mL | ≥2.97–<3.17 ng/mL | ≥3.17–< 3.44ng/mL | ≥3.44 ng/mL | ||
| N = 297 | N = 311 | N = 314 | N = 315 | ||
| Age | 73.4 ± 2.8 | 73.5 ± 2.9 | 73.1 ± 2.8 | 73.7 ± 2.9 | .035 |
| Male | 150 (50.5) | 157 (50.5) | 164 (52.2) | 158 (50.2) | .954 |
| Race | |||||
| White | 201 (67.7) | 194 (62.4) | 188 (59.9) | 176 (55.9) | .024 |
| Black | 96 (32.3) | 117 (37.6) | 126 (40.1) | 139 (44.1) | |
| Other | |||||
| Weight, kg | 75.5 ± 14.1 | 75.0 ± 15.3 | 77.4 ± 16.0 | 75.0 ± 14.5 | .150 |
| Height, m | 1.66 ± 0.09 | 1.67 ± 0.09 | 1.68 ± 0.10 | 1.66 ± 0.09 | .139 |
| BMI category | .453 | ||||
| Normal weight | 90 (30.3) | 116 (37.3) | 101 (32.2) | 95 (30.2) | |
| Overweight | 136 (45.8) | 121 (38.9) | 134 (42.7) | 145 (46.0) | |
| Obese | 71 (23.9) | 74 (23.8) | 79 (25.2) | 75 (23.8) | |
| Total body fat, % | 35.3 ± 7.6 | 34.2 ± 7.7 | 34.6 ± 7.3 | 34.3 ± 7.7 | .334 |
| Persistent severe mobility limitation | 131 (44.1) | 121 (38.9) | 129 (41.1) | 129 (41.0) | .632 |
| Persistent mobility limitation | 234 (78.8) | 226 (72.7) | 240 (76.4) | 258 (81.9) | .043 |
| Current smoker | 33 (11.2) | 22 (7.1) | 39 (12.4) | 29 (9.2) | .128 |
| Current drinker | 155 (52.4) | 167 (54.1) | 158 (50.6) | 160 (50.8) | .814 |
| Good/excellent self-rated health | 258 (86.9) | 268 (86.5) | 267 (85.0) | 267 (84.8) | .846 |
| Congestive heart failure | 7 (2.4) | 6 (2.0) | 12 (3.9) | 6 (2.0) | .368 |
| Cancer | 63 (21.2) | 60 (19.3) | 68 (21.7) | 64 (20.3) | .891 |
| Chronic obstructive pulmonary diseasea | 2 (0.7) | 4 (1.3) | 1 (0.3) | 5 (1.6) | .357 |
| Coronary heart disease | 52 (17.5) | 66 (21.2) | 69 (22.0) | 57 (18.1) | .408 |
| Angina | 26 (8.9) | 35 (11.6) | 38 (12.5) | 24 (7.7) | .158 |
| Grip strength, kg | 32.8 ± 10.33 | 32.88 ± 10.45 | 34.1 ± 10.97 | 33.4 ± 11.04 | .407 |
| GDF-8, ng/mL | 6.60 ± 2.13 | 6.72 ± 1.74 | 6.63 ± 1.76 | 6.96 ± 2.18 | .097 |
| Follistatin, pg/mL | 1 915.8 ± 770.7 | 1 900.4 ± 858.2 | 1 936.5 ± 1 109.0 | 1 751.3 ± 699.5 | .094 |
| Follistatin-like protein-3, pg/mL | 8 813.8 ± 2 223.0 | 8 663.3 ± 2 465.9 | 8 623.5 ± 2 369.2 | 8 879.3 ± 2 268.4 | .493 |
| 6-m walk speed, m/s | 1.17 ± 0.22 | 1.21 ± 0.23 | 1.21 ± 0.24 | 1.18 ± 0.23 | .051 |
| Appendicular lean mass/height2 | 7.57 ± 1.34 | 7.65 ± 1.42 | 7.75 ± 1.39 | 7.66 ± 1.4 | .476 |
| Estimated glomerular filtration rate | 72.9 ± 18.3 | 74.2 ± 18.5 | 72.7 ± 19.1 | 73.6 ±- 17.8 | .742 |
Notes: BMI = body mass index; CHS = Cardiovascular Health Study; Health ABC = Health, Aging, and Body Composition study; GDF = growth and differentiation factor; SD = standard deviation.
aDefined as chronic obstructive pulmonary disease (COPD) in Health ABC and chronic bronchitis in CHS.
Incident Mobility Limitation
In Health ABC, GDF-11 levels were not significantly associated with incident mobility limitation (regardless of persistent or severe limitation subtypes, Table 2). Higher GDF-8 levels were associated with a decreased risk of persistent severe mobility limitation, but not persistent mobility limitation. Higher FSTL-3 levels were significantly associated with persistent severe mobility limitation and persistent mobility limitation in base models and after multivariate adjustment. Higher follistatin levels were also significantly associated with a modestly increased likelihood of persistent severe mobility limitation and persistent mobility limitation. Incident mobility limitation was not assessed in CHS.
Table 2.
Hazard Ratios (HRs) for Mobility Limitation Per Doubling in Concentration for GDF-11, GDF-8, Follistatin, or Follistatin-Like Protein-3, Health ABC, adjusted for age, sex, and race; and the Multivariable Adjusted as Noted
| HR (95% CI) Per Doubling Concentration of Protein | ||
|---|---|---|
| Log GDF-11 | Model 1 | Model 2 |
| Persistent severe mobility limitation | 0.89 (0.58, 1.39) | 0.84 (0.43, 1.64) |
| Persistent mobility limitation | 1.01 (0.75, 1.37) | 1.04 (0.66, 1.62) |
| Log GDF-8 | ||
| Persistent severe mobility limitation | 0.79 (0.63, 0.98) | 0.67 (0.48, 0.94) |
| Persistent mobility limitation | 0.97 (0.82, 1.15) | 1.02 (0.79, 1.30) |
| Log Follistatin | ||
| Persistent severe mobility limitation | 1.20 (0.99, 1.47) | 1.13 (0.83, 1.55) |
| Persistent mobility limitation | 1.27 (1.10, 1.46) | 1.26 (1.01, 1.56) |
| Log FSTL-3 | ||
| Persistent severe mobility limitation | 2.47 (1.92, 3.17) | 2.41 (1.44, 4.02) |
| Persistent mobility limitation | 2.13 (1.76, 2.57) | 1.77 (1.11, 2.56) |
Notes: Model 1 is adjusted for age, sex, race; Model 2 is adjusted for covariates in Model 1 and self-rated health, body mass index, cancer, chronic obstructive pulmonary disease, angina, smoking status, drinking status, coronary heart disease, heart failure, cystatin C, C-reactive protein and estimated glomerular filtration rate. FSTL-3 = follistatin-like protein-3; GDF = growth differentiation factor; Health ABC = Health, Aging, and Body Composition study.
Measures of Muscle Strength, Walking Speed, and Body Composition
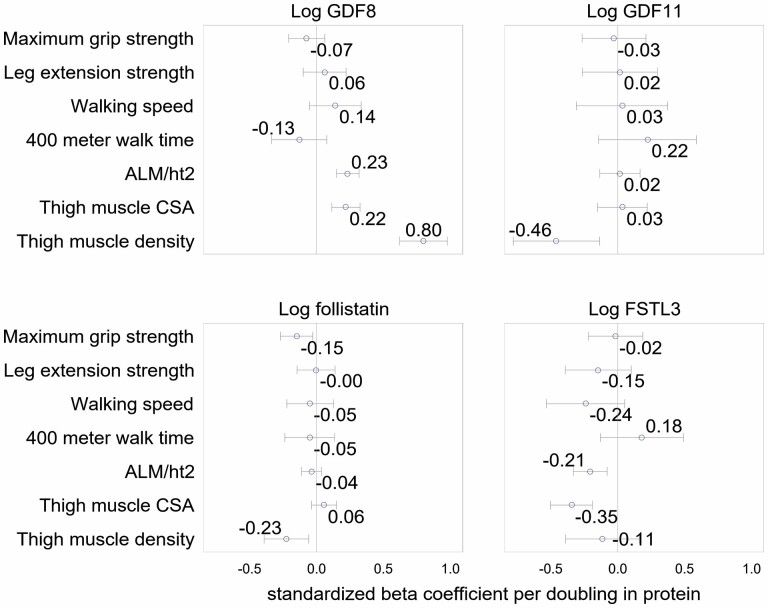
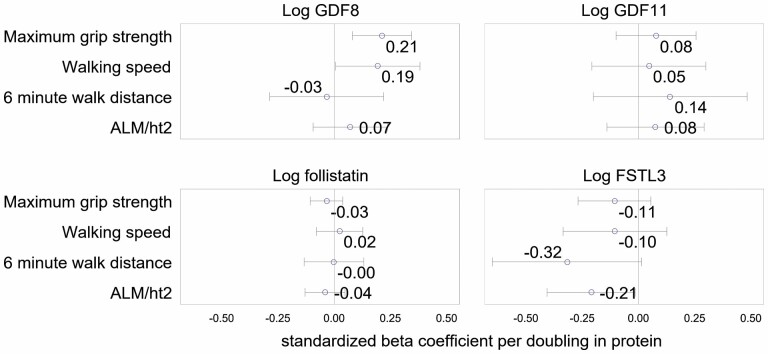
In Health ABC, fully adjusted models, each doubling of GDF-11 level was associated with a −0.46SD lower thigh muscle density (Figure 2). In both CHS and Health ABC, there were no significant associations between GDF-11 level with strength, thigh muscle CSA (assessed in Health ABC only), ALM/ht2, 400-m walk time (assessed in Health ABC only), 6-minute walk distance (assessed in CHS only) or 6-m walking speed (Figures 2 and 3). In models adjusted for age, sex, and race, the results were similar (Supplementary Figures 1 and 2).
Figure 2.
Multivariate adjusted beta-coefficients for log GDF-8, log GDF-11, log follistain, and log FSTL-3 with lean mass, strength, muscle size, muscle density, and walking performance in Health ABC. Notes: Model is adjusted for age, sex, race, self-rated health, body mass index, cancer, chronic obstructive pulmonary disease, angina, smoking status, drinking status, coronary heart disease, heart failure, cystatin C, C-reactive protein, and estimated glomerular filtration rate. ALM/ht2 = appendicular lean mass/height2; CSA = cross-sectional area; FSTL-3 = follistatin-like protein-3; GDF = growth differentiation factor.
Figure 3.
Multivariate adjusted beta-coefficients for log GDF-8, log GDF-11, log follistain, and log FSTL-3 with lean mass, strength, and walking performance in CHS. Notes: Model is adjusted for age, sex, race, self-rated health, body mass index, cancer, chronic obstructive pulmonary disease, angina, smoking status, drinking status, coronary heart disease, heart failure, cystatin C, C-reactive protein. and estimated glomerular filtration rate. CHS = Cardiovascular Health Study; FSTL-3 = follistatin-like protein-3; GDF = growth differentiation factor; Health ABC = Health, Aging, and Body Composition study.
In Health ABC, higher GDF-8 level was associated with greater thigh muscle CSA, thigh muscle density, and greater ALM/ht2 in minimally and fully adjusted models (Figure 2 and Supplementary Figure 1). Furthermore, in models adjusted for age, race, and sex, higher GDF-8 level was associated with faster walking speeds (Supplementary Figure 1); this association was attenuated to nonsignificance after full adjustment. In CHS, GDF-8 levels were positively associated with grip strength in both fully and minimally adjusted models. GDF-8 level was not significantly associated with 400-m walk time or knee extension strength in Health ABC, or 6-minute walk distance in CHS in fully or minimally adjusted models.
In fully adjusted models, FSTL-3 levels were significantly negatively associated with ALM/ht2 in both Health ABC and CHS. Higher FSTL-3 levels were also associated with lower thigh CSA, and this association persisted after multivariate adjustment. In both cohorts, after full adjustment, FSTL-3 levels were not associated with grip strength, leg extension strength, walking speed, 400-m walk time, or thigh muscle density.
Higher follistatin levels were associated with lower thigh muscle density in minimally and fully adjusted models in Health ABC (muscle density was not measured in CHS). Follistatin level was not associated with any other strength, performance, or body composition outcome in fully adjusted models in either Health ABC or CHS.
Evaluation of Effect Modification
In models where we tested for the presence of pairwise effect modification between the putative geroproteins, we found little evidence for the presence of statistical interaction. Although some of the p values for the interaction terms prior to adjustment for multiple comparisons were between p = .01 and p = .05, none was smaller than the Bonferroni-adjusted p value of .008. Thus, we found no evidence that the association of each protein with the outcomes studied varied by the level of any of the other proteins under examination.
Discussion
Previous data on circulating GDF-11 and GDF-8 levels in mice and in humans have found disparate results; studies in preclinical models have reported both beneficial (6,11) and detrimental (7–10) effects of GDF-11 onh muscle strength and endurance. These differing results may have been due to potential cross-reactivity between GDF-11 and GDF-8 in the binding assays (both immunoassays and aptamer assays) due to their high sequence homology (6,10). In this current report, we used an LC-MS/MS assay to measure circulating total GDF-11 and GDF-8 levels with excellent precision, no detectable cross-reactivity of GDF-11 in the GDF-8 assay, and sufficient sensitivity to be able to measure circulating GDF-8 concentrations in almost all men and women. Overall, we found that GDF-11 was only modestly related to age, and was not significantly associated with measures of muscle size, strength, and physical performance—the only significant association between GDF-11 and these outcomes was the negative association with thigh muscle density. In contrast, GDF-8 levels were positively associated with thigh muscle CSA and density, ALM/ht2 (in Health ABC but not CHS), and grip strength (in CHS but not Health ABC). In addition, in analyses of FSTL-3 and follistatin, we found that higher FSTL-3 was associated with a greater risk of incident mobility limitation, lower thigh CSA, and lower ALM/ht2, whereas higher follistatin was associated with decreased thigh muscle density, lower grip strength, and increased risk of mobility limitation. However, associations between follistatin and strength and other measures of physical performance were generally modest and often not statistically significant after multivariate adjustment.
The positive association of circulating GDF-8 levels with measures of skeletal muscle mass and strength may appear incongruent with its widely recognized role as a negative regulator of muscle mass that has been well documented by genetic studies in multiple species, including humans (25). Moreover, pharmacologic blockade of GDF-8 synthesis or action in mice, nonhuman primates, and humans by the administration of anti-GDF-8 antibodies, GDF-8 propeptide, inhibitors of the cleavage of its precursor proteins into its mature form, ActRIIB ligand traps or ActRIIB receptor blockers has been shown to increase muscle mass (26–29). If GDF-8 was the primary driver of age-related loss of skeletal muscle mass and strength, higher GDF-8 levels would be expected to be associated with lower levels of skeletal muscle mass and strength. Instead, the positive association of higher levels of GDF-8 with greater skeletal muscle mass and strength suggests that GDF-8 acts as a chalone—a counterregulatory hormone (30)—to minimize muscle loss in response to aging and to restrain unbridled skeletal muscle growth in response to anabolic stimuli (30), and are consistent with our prior report (31). Because skeletal muscle is the predominant source of circulating GDF-8 (32), we speculate that aging-associated loss of skeletal muscle mass leads to lower levels of GDF-8, which acts as a homeostatic feedback mechanism to brake further muscle loss. This speculation is supported by our findings that older adults with lower muscle mass and strength had lower GDF-8 levels (23). The role of GDF-8 as a chalone also is supported by our observations that testosterone administration increases skeletal muscle mass and GDF-8 levels (33).
Both GDF-8 and GDF-11 are regulated by several inhibitory binding proteins, including FSTL-3 and follistatin (12,16,25). Our analyses show that high levels of FSTL-3, and perhaps follistatin as well, are associated with lower muscle size and strength. In fact, circulating FSTL-3 level had the strongest and most consistent association with muscle strength, performance measures, and mobility limitation. However, these findings are the opposite of what one might expect if increased FSTL-3 and/or follistatin were the drivers of these effects, as high levels of these inhibitory proteins would be expected to suppress GDF-8 and activin-A activity and cause muscle growth. Indeed, overexpression of these inhibitory binding proteins in mice increases muscle mass, and conversely, targeted loss-of-function mutations of genes encoding some of these binding proteins (GASP-1, GASP-2, and follistatin) are associated with reduced muscle mass (34,35). This apparent discrepancy between the findings of our observational study in humans, and previously reported studies of the effects of gain-of-function or loss-of-function of FSTL-3 and follistatin in genetically modified mice could be due to 1or more of the following reasons. The TGF-ß family regulatory network is complex, and the effect of high levels of FSTL-3 and follistatin on GDF-8 and activin activity may be counteracted by other biological effects of these binding proteins. For example, follistatin is also known to bind and inhibit other TGF-ß family members, such as BMPs, and at least one study has suggested that BMP signaling can counteract GDF-8 signaling in muscle; specifically, this study reported that increased BMP signaling can drive muscle growth and, conversely, that inhibition of BMP signaling with BMP-specific inhibitor, namely noggin, can induce muscle atrophy (36). Nevertheless, we favor the chalone hypothesis that increased FSTL-3 and/or follistatin levels are a part of the counterregulatory homeostatic network and that the increased levels of these inhibitory binding proteins are a compensatory response to reduce muscle mass with aging. Consistent with this proposal, circulating FSTL-3 levels have been reported to increase with aging and frailty in a small clinical human study, and to be associated with circulating level of activins (37). Our data showing negative relation of FSTL-3 levels with muscle size, measures of muscle strength and physical performance, and positive relation with risk of mobility limitation also are indirectly supported by data from a subset of participants in the Baltimore Longitudinal Study of Aging (38); in this analysis, Semba et al. found that the antagonists of GDF-8 and GDF-11, but not GDF-8 and GDF-11 themselves, were independently associated with muscle strength; mobility outcomes were not studied.
GDF-11 levels were not consistently associated with either lean mass, or with measures of muscle strength, physical performance, or with mobility limitation. It has been proposed that the function of GDF-8 with respect to suppressing muscle growth is redundant with that of at least one other TGF-ß family member (39,40), that is, GDF-11. Several observations support this proposal. First, GDF-8 and GDF-11 share greater than 90% identity in the mature domains of these molecules, and the 2 proteins are virtually indistinguishable in vitro assays (15,35,41–43). Second, GDF-8 and GDF-11 share all of the known regulatory components, including extracellular binding proteins, and are capable of signaling through the same receptors (12,15–17,35,44). Third, genetic studies have shown that GDF-8 and GDF-11 are functionally redundant in another context, namely with respect to the control of anterior–posterior patterning of the axial skeleton (45). GDF-11 circulates in the blood and is produced by a number of tissues, including skeletal muscle (42,43,46). Hence, it is possible that GDF-11 protein has GDF-8-like activities in the muscle and offers redundancy with GDF-8. Indeed, systemic overexpression of GDF-11 has been shown to induce a cachexia-like syndrome in mice (8,47,48), similar to what has been described for overexpression of GDF-8. Moreover, germ-line replacement of the portion of the Gdf8 gene encoding the mature, C-terminal peptide with the corresponding region taken from Gdf11 had only a relatively modest effect on skeletal muscle mass and metabolic function, even though all the circulating GDF-8 was replaced with GDF-11 in these mice (49). Genetic disruption of Gdf11 in the skeletal muscle, however, revealed no effect of GDF-11 loss (45). Our findings that circulating GDF-8, but not GDF-11, levels are associated with measures of muscle mass and strength are consistent with a primary role of GDF-8 as a counterregulatory chalone in the skeletal muscle; in this chalone model, GDF-11 plays a secondary redundant role.
This study has many strengths. We reported results from 2 large, well-characterized cohorts of older adults, and used state-of-the art assays to measure the geroproteins of interest. These findings should be viewed in the context of the limitations of our work. First, while the LC-MS/MS assay for GDF-8 and GDF-11 measures GDF-8 and GDF-11 concentrations with a high level of specificity, we do not know whether the GDF-8 and GDF-11 levels accurately reflect the circulating levels or the local tissue levels of the biologically active forms of these ligands. Second, the concentrations were measured at a single time point; it is possible that changes in these circulating muscle hormones over time may be more robustly associated with age-related changes in function and mobility, particularly given our theory that GDF-8, follistatin, and FSTL-3 as chalones. Third, there were some inconsistencies in the results between CHS and Health ABC studies; these apparent differences could be due to factors that differed between the participants of the 2 studies (Health ABC had greater enrollment of Blacks, while CHS participants were lower functioning) or differences in the protocols for the measurement of outcomes or collection, or the storage of specimens between the studies. Fourth, we adjusted the results for several potential confounding factors, but we cannot rule out that unmeasured (or poorly measured) confounding factors may explain the associations observed. Fifth, we included DXA ALM/height2 as an approximation of muscle size, because the CT-derived measure of muscle CSA was only available in Health ABC. We acknowledge that DXA lean mass overestimates muscle mass (50), and that associations with CT-derived measure of muscle CSA and performance and mobility outcomes are more robust that the association of DXA ALM/height2 and these outcomes. Sixth, the participants in both cohorts were relatively old; it is possible that age-related changes in these geroproteins may occur at an earlier age. Seventh, we also note that several covariates, for example, comorbidities, may also be mediators of the association between GDF-8, GDF-11, follistatin, or FSTL-3, and the mobility, strength, and performance outcomes and may constitute overadjustment. Thus we have also presented minimally adjusted models for interpretation. Finally, there were differences in the associations reported across Health ABC and CHS. These differences could be due to measurement differences of our outcomes, or differences in how the serum was processed and stored between cohorts. The incongruent findings could also be due to differential associations of these putative geroproteins with strength, function, and muscle size by race or by the presence of comorbidity and health status since the CHS and Health ABC cohorts had different prevalence of disease and disability, and more Black participants were enrolled in Health ABC.
In conclusion, we theorize that GDF-8 and its binding proteins, FSTL-3 and follistatin, constitute a counterregulatory system to restrain age-related loss of skeletal muscle mass and muscle function (strength). This theory is supported by our results that demonstrated that GDF-8 levels are positively associated with ALM/height2 and grip strength; and FSTL-3 levels (and to some extent, follistatin as well) are negatively associated with ALM/height2 and grip strength and incident mobility limitation. In contrast, GDF-11 was not robustly associated with the strength or performance metrics under study, suggesting that GDF-11 is not an important regulator of muscle size or physical performance in older adults. It is possible that GDF-11 plays a secondary, redundant role in skeletal muscle homeostasis.
Supplementary Material
Contributor Information
Peggy M Cawthon, Research Institute, California Pacific Medical Center, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA.
Sheena Patel, Research Institute, California Pacific Medical Center, San Francisco, California, USA.
Anne B Newman, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Shalender Bhasin, Research Program in Men’s Health: Aging and Metabolism; Boston Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Liming Peng, Research Program in Men’s Health: Aging and Metabolism; Boston Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Russell P Tracy, Department of Pathology and Laboratory Medicine, University of Vermont Larner College of Medicine, Burlington, Vermont, USA.
Jorge R Kizer, Cardiology Section, San Francisco Veterans Affairs Health Care System, and Departments of Medicine, Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA.
Se-Jin Lee, The Jackson Laboratory and University of Connecticut School of Medicine, Farmington, Connecticut, USA.
Luigi Ferrucci, Office of the Scientific Director, National Institute on Aging, Bethesda, Maryland, USA.
Peter Ganz, Cardiology Division, Zuckerberg San Francisco General Hospital and Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Nathan K LeBrasseur, Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, Minnesota, USA.
Steven R Cummings, Research Institute, California Pacific Medical Center, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA.
Funding
The present research was supported by R01 AG052964 to J.R.K., P.G., A.B.N., R.P.T., and S.R.C. CHS was supported by R01 AG053325 from the National Institute on Aging (NIA); and by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the NIA. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Health ABC was supported by NIA Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. The research was funded in part by the Intramural Research Program of the NIH, NIA.
Conflict of Interest
None declared.
Author Contributions
P.M.C. developed analysis plan, completed analyses, and drafted the manuscript. S.P. completed analyses and critically reviewed the paper. A.B.N., P.G., S.R.C., R.P.T., and J.R.K. critically reviewed the manuscript and secured funding for this project. All other authors critically reviewed the manuscript.
References
- 1. Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12(3):525–530. doi: 10.1111/acel.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bitto A, Kaeberlein MR. It’s in our blood. Cell Metab. 2014;20(1):2–4. doi: 10.1016/j.cmet.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brack AS. Ageing of the heart reversed by youthful systemic factors! EMBO J. 2013;32(16):2189–2190. doi: 10.1038/emboj.2013.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153(4):828–839. doi: 10.1016/j.cell.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344(6184):630–634. doi: 10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344(6184):649–652. doi: 10.1126/science.1251152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hinken AC, Powers JM, Luo G, Holt JA, Billin AN, Russell AJ. Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells. Aging Cell. 2016;15(3):582–584. doi: 10.1111/acel.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmers TA, Jiang Y, Wang M, et al. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res Cardiol. 2017;112(4):48. doi: 10.1007/s00395-017-0639-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammers DW, Merscham-Banda M, Hsiao JY, Engst S, Hartman JJ, Sweeney HL. Supraphysiological levels of GDF11 induce striated muscle atrophy. EMBO Mol Med. 2017;9(4):531–544. doi: 10.15252/emmm.201607231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22(1):164–174. doi: 10.1016/j.cmet.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schafer MJ, Atkinson EJ, Vanderboom PM, et al. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23(6):1207–1215. doi: 10.1016/j.cmet.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98(16):9306–9311. doi: 10.1073/pnas.151270098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ge G, Hopkins DR, Ho WB, Greenspan DS. GDF11 forms a bone morphogenetic protein 1-activated latent complex that can modulate nerve growth factor-induced differentiation of PC12 cells. Mol Cell Biol. 2005;25(14):5846–5858. doi: 10.1128/MCB.25.14.5846-5858.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thies RS, Chen T, Davies MV, et al. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors. 2001;18(4):251–259. doi: 10.3109/08977190109029114 [DOI] [PubMed] [Google Scholar]
- 15. Kondas K, Szlama G, Trexler M, Patthy L. Both WFIKKN1 and WFIKKN2 have high affinity for growth and differentiation factors 8 and 11. J Biol Chem. 2008;283(35):23677–23684. doi: 10.1074/jbc.M803025200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hill JJ, Davies MV, Pearson AA, et al. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277(43):40735–40741. doi: 10.1074/jbc.M206379200 [DOI] [PubMed] [Google Scholar]
- 17. Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol. 2003;17(6):1144–1154. doi: 10.1210/me.2002-0366 [DOI] [PubMed] [Google Scholar]
- 18. Latres E, Mastaitis J, Fury W, et al. Activin A more prominently regulates muscle mass in primates than does GDF8. Nat Commun. 2017;8:15153. doi: 10.1038/ncomms15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w [DOI] [PubMed] [Google Scholar]
- 20. Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387–398. doi: 10.1378/chest.123.2.387 [DOI] [PubMed] [Google Scholar]
- 21. Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x [DOI] [PubMed] [Google Scholar]
- 22. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 23. Peng L, Gagliano-Juca T, Pencina KM, et al. Age trends in growth and differentiation factor-11 and myostatin levels in healthy men, and differential response to testosterone, measured using liquid chromatography-tandem mass spectrometry. J Gerontol A Biol Sci Med Sci. 2022;77(4):763–769. doi: 10.1093/gerona/glab146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SJ. Sprinting without myostatin: a genetic determinant of athletic prowess. Trends Genet. 2007;23(10):475–477. doi: 10.1016/j.tig.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 26. Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531–543. doi: 10.1016/j.cell.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 27. Camporez JP, Petersen MC, Abudukadier A, et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci USA. 2016;113(8):2212–2217. doi: 10.1073/pnas.1525795113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rooks D, Praestgaard J, Hariry S, et al. Treatment of sarcopenia with bimagrumab: results from a phase II, randomized, controlled, proof-of-concept study. J Am Geriatr Soc. 2017;65(9):1988–1995. doi: 10.1111/jgs.14927 [DOI] [PubMed] [Google Scholar]
- 29. O’Connell KE, Guo W, Serra C, et al. The effects of an ActRIIb receptor Fc fusion protein ligand trap in juvenile simian immunodeficiency virus-infected rhesus macaques. FASEB J. 2015;29(4):1165–1175. doi: 10.1096/fj.14-257543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee SJ, McPherron AC. Myostatin and the control of skeletal muscle mass. Curr Opin Genet Dev. 1999;9(5):604–607. doi: 10.1016/s0959-437x(99)00004-0 [DOI] [PubMed] [Google Scholar]
- 31. Bergen HR, 3rd, Farr JN, Vanderboom PM, et al. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet Muscle. 2015;5:21. doi: 10.1186/s13395-015-0047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee SJ, Lehar A, Liu Y, et al. Functional redundancy of type I and type II receptors in the regulation of skeletal muscle growth by myostatin and activin A. Proc Natl Acad Sci U S A. 2020;117(49):30907–30917. doi: 10.1073/pnas.2019263117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lakshman KM, Bhasin S, Corcoran C, et al. Measurement of myostatin concentrations in human serum: circulating concentrations in young and older men and effects of testosterone administration. Mol Cell Endocrinol. 2009;302(1):26–32. doi: 10.1016/j.mce.2008.12.019 [DOI] [PubMed] [Google Scholar]
- 34. Lee SJ, Lee YS, Zimmers TA, et al. Regulation of muscle mass by follistatin and activins. Mol Endocrinol. 2010;24(10):1998–2008. doi: 10.1210/me.2010-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee YS, Lee SJ. Regulation of GDF-11 and myostatin activity by GASP-1 and GASP-2. Proc Natl Acad Sci USA. 2013;110(39):E3713–E3722. doi: 10.1073/pnas.1309907110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sartori R, Schirwis E, Blaauw B, et al. BMP signaling controls muscle mass. Nat Genet. 2013;45(11):1309–1318. doi: 10.1038/ng.2772 [DOI] [PubMed] [Google Scholar]
- 37. Roh JD, Hobson R, Chaudhari V, et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11(482):eaau8680. doi: 10.1126/scitranslmed.aau8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Semba RD, Zhang P, Zhu M, et al. Relationship of circulating growth and differentiation factors 8 and 11 and their antagonists as measured using liquid chromatography-tandem mass spectrometry with age and skeletal muscle strength in healthy adults. J Gerontol A Biol Sci Med Sci. 2019;74(1):129–136. doi: 10.1093/gerona/gly255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS One. 2007;2(8):e789. doi: 10.1371/journal.pone.0000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee SJ, Reed LA, Davies MV, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA. 2005;102(50):18117–18122. doi: 10.1073/pnas.0505996102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0 [DOI] [PubMed] [Google Scholar]
- 42. Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Dev Biol. 1999;208(1):222–232. doi: 10.1006/dbio.1998.9191 [DOI] [PubMed] [Google Scholar]
- 43. Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFbeta superfamily during mouse embryogenesis. Mech Dev. 1999;80(2):185–189. doi: 10.1016/s0925-4773(98)00205-6 [DOI] [PubMed] [Google Scholar]
- 44. Souza TA, Chen X, Guo Y, et al. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol Endocrinol. 2008;22(12):2689–2702. doi: 10.1210/me.2008-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev Biol. 2009;9:24. doi: 10.1186/1471-213X-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22(3):260–264. doi: 10.1038/10320 [DOI] [PubMed] [Google Scholar]
- 47. Zimmers TA, Davies MV, Koniaris LG, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296(5572):1486–1488. doi: 10.1126/science.1069525 [DOI] [PubMed] [Google Scholar]
- 48. Jones JE, Cadena SM, Gong C, et al. Supraphysiologic administration of GDF11 induces cachexia in part by upregulating GDF15. Cell Rep. 2018;22(6):1522–1530. doi: 10.1016/j.celrep.2018.01.044 [DOI] [PubMed] [Google Scholar]
- 49. Lee SJ, Lehar A, Rydzik R, et al. Functional replacement of myostatin with GDF-11 in the germline of mice. Skelet Muscle. 2022;12(1):7. doi: 10.1186/s13395-022-00290-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cawthon PM, Orwoll ES, Peters KE, et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74(6):844–852. doi: 10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.