Abstract
A set of mercury resistance plasmids was obtained from wheat rhizosphere soil amended or not amended with mercuric chloride via exogenous plasmid isolation by using Pseudomonas fluorescens R2f, Pseudomonas putida UWC1, and Enterobacter cloacae BE1 as recipient strains. The isolation frequencies were highest from soil amended with high levels of mercury, and the isolation frequencies from unamended soil were low. With P. putida UWC1 as the recipient, the isolation frequency was significantly enhanced in wheat rhizosphere compared to bulk soil. Twenty transconjugants were analyzed per recipient strain. All of the transconjugants contained plasmids which were between 40 and 50 kb long. Eight selected plasmids were distributed among five groups, as shown by restriction digestion coupled with a similarity matrix analysis. However, all of the plasmids formed a tight group, as judged by hybridization with two whole-plasmid probes and comparisons with other plasmids in dot blot hybridization analyses. The results of replicon typing and broad-host-range incompatibility (Inc) group-specific PCR suggested that the plasmid isolates were not related to any previously described Inc group. Although resistance to copper, resistance to streptomycin, and/or resistance to chloramphenicol was found in several plasmids, catabolic sequences were generally not identified. One plasmid, pEC10, transferred into a variety of bacteria belonging to the β and γ subdivisions of the class Proteobacteria and mobilized as well as retromobilized the IncQ plasmid pSUP104. A PCR method for detection of pEC10-like replicons was used, in conjunction with other methods, to monitor pEC10-homologous sequences in mercury-polluted and unpolluted soils. The presence of mercury enhanced the prevalence of pEC10-like replicons in soil and rhizosphere bacterial populations.
The potential use of genetically modified bacteria in agriculture has raised questions pertaining to the spread of introduced recombinant DNA through soil bacterial communities. Gene transfer in soil via conjugation has received much attention, and the focus of most studies has been the transfer and fate of introduced plasmids (6, 22, 27–29, 39). Under favorable conditions, in specific soil microhabitats, or under selection conditions, both self-transmissible and mobilizable plasmids present in introduced hosts can be transferred to introduced recipients, as well as to a variety of indigenous bacteria (15, 20, 27, 28, 33). In particular, rhizospheres of crop plants, such as wheat and sugar beet, provide conditions conducive to conjugal plasmid transfer between bacterial inhabitants (15, 36). When genetically modified bacteria are developed as inoculants for the rhizosphere, insertion of heterologous DNA into non-self-transmissible plasmids or the chromosome might restrict conjugal transfer of this DNA to members of the indigenous bacterial community. However, mobilizing or retromobilizing (33) plasmids present in indigenous soil bacteria could potentially still effect the transfer of the less mobile heterologous DNA via chromosome or plasmid mobilization, which may involve cointegration (9, 19, 31). Such plasmids might thus be responsible for the escape of heterologous DNA from genetically modified bacteria introduced into soil.
There is a paucity of knowledge concerning the incidence of plasmids with mobilizing capacity in soils and rhizospheres, as well as concerning the effects of soil factors, such as stresses resulting from pollution or from natural causes (e.g., rhizosphere acidity), on plasmid prevalence and transfer (e.g., reference 38). Whereas it has been suggested that chemical stress often does not enhance plasmid incidence in selected soil bacterial populations (40), pollution in river water or mines (in particular mercury pollution) has been found to exert a selective (enhancing) effect (4, 13).
Plasmids of environmental bacteria have classically been obtained by endogenous isolation procedures (20). Endogenous isolation implies that putative plasmid hosts with the phenotype of interest are isolated from soil, after which plasmids are extracted from pure cultures of these strains. On the other hand, pioneering studies performed with river stone epilithon (9) and later extended to soil and sediment (32) have shown that plasmids can be obtained directly from indigenous bacterial communities in new hosts by exogenous isolation. In this approach, plasmids are captured in selectable recipient strains by using mating between these strains and the total bacterial community obtained from an environmental sample. Following incubation, the mating mixture is plated with selection for the recipient and an additional marker gene presumedly located on a plasmid present in the indigenous bacteria (6). The advantage of the exogenous isolation procedure is that no culturing step is required in the mating, which thus allows isolation of plasmids from nonculturable hosts. Furthermore, plasmids are directly selected for their transfer capacity, in addition to the presence of a specific selectable marker.
In this study, exogenous plasmid isolation was employed to obtain transferable plasmids from soil bacteria by using mercury resistance as the selectable marker. The objective of this work was to gain insight into the potential present in soil bacterial populations to (retro)mobilize genes out of introduced bacteria into members of the soil bacterial community. Since the incidence of plasmids in soil bacteria is likely influenced by soil ecological factors and selection pressure, the presence of wheat roots and selection by mercury (25) were studied as experimental variables.
MATERIALS AND METHODS
Strains and plasmids used.
The strains and plasmids used in this study are listed in Table 1. The new plasmids exogenously isolated from soil, as summarized in Table 2, are listed in Table 3. All strains were routinely grown in Luria-Bertani (LB) broth (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl, 1 liter of H2O; pH 7.2) to which appropriate antibiotics (each at a concentration of 50 μg ml−1) were added (Table 1). Strains were maintained in 20% glycerol at −80°C.
TABLE 1.
Strains and plasmids used and host range of mercury resistance plasmid pEC10
| Organism | Marker(s)a | Phylogenetic group | Plasmid, reference, or sourceb | pEC10 transfer frequencyc |
|---|---|---|---|---|
| Agrobacterium tumefaciens GMi9023 | Rp | α Proteobacteria | 22 | 0d |
| Rhizobium leguminosarum bv. trifolii R62 | Rp | IPO-DLO | 0 | |
| Alcaligenes eutrophus AE815 | Rp | β Proteobacteria | VITO | 3.4 × 10−1 |
| Burkholderia cepacia P2 | Rp | IPO-DLO | 5.6 × 10−6 | |
| Acinetobacter calcoaceticus BD413 | Rp | γ Proteobacteria | University of Amsterdam | 0 |
| Acinetobacter calcoaceticus DSM418 | Rp | DSM | 0 | |
| Escherichia coli HB101 | Tce | pSUP104 | NAf | |
| Escherichia coli Sm10 (chr::Tn5::luxAB-tet) | Tc | IPO-DLO | 0 | |
| Escherichia coli CV601 | Rp | BGA | 0 | |
| Enterobacter cloacae BE1 | Rp | IPO-DLO | 1.4 × 10−2 | |
| Pseudomonas fluorescens R2f | Rp | IPO-DLO | 1 × 10−2 | |
| Pseudomonas fluorescens R2f (chr::Tn5) | Km Sm | IPO-DLO | NA | |
| Pseudomonas fluorescens R2f | Tce | pSUP104 | 10−3 | |
| Pseudomonas putida UWC1 | Rp | University of Wales, Cardiff | 2.0 × 10−4 | |
| Pseudomonas aeruginosa(pKT261) | Rp | Soil isolate | 9.1 × 10−5 | |
| Pseudomonas corrugata PD704 | Rp | PPS | 2.6 × 10−6 | |
| Pseudomonas stutzeri Rf3 | Rp | IPO-DLO | 5.5 × 10−7 | |
| Bacillus subtilis SEm-2 | Em | Gram-positive bacteria | IPO-DLO | 0 |
| Bacillus cereus FoTc30 | Tc | IPO-DLO | 0 | |
| Paenibacillus azotofixans P3L5 | Rp | IPO-DLO | 0 | |
| Arthrobacter sp. | IPO-DLO | 0 | ||
| Soil isolate F4 | Rp | Not α or γ Proteobacteria | IPO-DLO | 0 |
Resistance markers: Rp, rifampin; Tc, tetracycline; Km, kanamycin; Sm, streptomycin; Em, erythromycin.
VITO, Vlaams Instituut voor Technologische Onderzoek, Mol, Belgium; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany; BGA, Bundes Gesundheits Amt, Wernigerode, Germany; PPS, Plant Pathology Service, Wageningen, The Netherlands.
Plasmid pEC10 was obtained in this study. The transfer frequency was the number of transconjugants per recipient following standard filter mating with Pseudomonas fluorescens R2f chr::Tn5(pEC10) as the donor. The identities of transconjugants were confirmed by colony hybridization, plasmid extraction, or pEC10-specific colony PCR.
0, plasmid transfer was not detected (the transfer frequency was less than 10−8 to 10−9 transconjugant per recipient).
Resistance is plasmid encoded.
NA, not applicable.
TABLE 2.
Exogenous isolation of mercury resistance plasmids in three recipient strains from bulk soil and wheat rhizosphere soil portions 10 days after different amounts of HgCl2 had been added
| Recipient strain | Exogenous isolation frequency obtained with bacterial populations from soil treated witha:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No HgCl2
|
4 μg of HgCl2 g of dry soil−1
|
28 μg of HgCl2 g of dry soil−1
|
55 μg of HgCl2 g of dry soil−1
|
|||||
| Bulk soil | Rhizosphere soil | Bulk soil | Rhizosphere soil | Bulk soil | Rhizosphere soil | Bulk soil | Rhizosphere soil | |
| None | 0b | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudomonas putida UWC1 Rpr | 0 | 1 × 10−7 | 0 | 3 × 10−7 | 0 | 5 × 10−7 | 2 × 10−8 | 1 × 10−6 |
| Pseudomonas fluorescens R2f Rpr | 2 × 10−8 | 0 | 1 × 10−7 | 5 × 10−8 | 6 × 10−7 | 3 × 10−8 | 1 × 10−6 | 3 × 10−7 |
| Enterobacter cloacae BE1 Rpr | 0 | 7 × 10−9 | 2 × 10−7 | 2 × 10−7 | 4 × 10−7 | 4 × 10−7 | 5 × 10−7 | NDc |
CFU of recipients resistant to mercury (20 μg ml−1)/total CFU of recipients.
0, below the limit of detection (less than 10−9 transconjugant per recipient).
ND, not determined.
TABLE 3.
Grouping of eight mercury resistance plasmids based on replicon, broad-host-range Inc group PCR, and restriction-hybridization typing data
| Plasmid | Groupa | Size (kb)b | Detection of Inc group, marker gene or plasmidc
|
Positive resistance markersf | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IncP
|
IncN
|
IncW
|
IncQd
|
IncA/C
|
merRT
|
pEC10
|
|||||||||||
| Replicon typing | PCR-hybridization | Replicon typing | PCR-hybridization | Replicon typing | PCR-hybridization | Replicon typing | PCR-hybridization | Hybridization with probe | Replicon typing | PCR-hybridizatione | PCR-hybridization | Hybridization with probe | PCR-hybridization | ||||
| pEC10 | I | 45 | − | − | − | − | − | − | − | − | − | − | − | + | + | + | Hg Cu Sm Cm tcbCD |
| pEC8 | II | 46 | − | − | − | − | − | −g | − | − | − | − | − | w | + | + | Hg Cu Sm Cm |
| pF7 | II | 45 | − | −h | − | − | − | −i | − | − | − | − | − | − | + | − | Hg Cu Cmj |
| pP4 | II | 45 | − | − | − | − | − | −g | − | − | − | − | − | + | + | + | Hg Cu |
| pP2 | III | 42 | − | − | − | −k | − | −g | − | − | − | − | − | + | + | + | Hg Cu Sml |
| pF17 | III | 42 | − | − | − | − | − | −i | − | − | − | − | − | + | + | w | Hg Cu Sm Cmj |
| pP11 | IV | 49 | − | − | − | − | − | −g | − | − | − | − | − | + | w | + | Hg Cu Sml Cm As |
| pF10 | V | 40 | − | − | − | − | − | −i | − | − | − | − | − | + | + | + | Hg Cu Cmj |
Group based on PstI-generated restriction patterns and determined directly and after reaction with probes pEC10 and pP11.
Molecular size, as estimated from restriction digests.
Replicon typing was performed with Couturier probes (5). The PCR-hybridization analyses of Inc groups (7) were performed with the following systems: for IncP (α and β), trfA, oriT, korA, and traG; for IncN, repB, kikA, and oriT; for IncW, oriV, oriT, and trwA; for IncQ, repB, oriV, and oriT; and for IncA/C, rep (16). The PCR-hybridization analysis of merRT was performed with the merRTΔP system (consensus Tn501, Tn21, and pMJ100 mer genes), as described by Bruce et al. (3). The PCR-hybridization analysis of pEC10 was performed with the system developed in this study. The probes used for hybridization analysis were oriV (for IncA/C) and the whole-plasmid and PCR-generated pEC10-specific probe (for pEC10). +, positive signal; w, weak signal; −, no signal.
The IncQ oriV system generated nonspecific, nonhybridizing products of about 300 bp with all plasmids.
A weak, nonhybridizing band was detected with all plasmids.
In this study we tested resistance to Co, Ni, Cu, Zn, Ag, As, Cr, Cd, Hg, ampicillin, tetracycline, streptomycin (Sm), trimethoprim, chloramphenicol (Cm), gentamicin, kanamycin, nalidixic acid, erythromycin, lincomycin, and catabolic probes.
The IncW trwA system gave different nonspecific products with pEC8 and with pP11, pP4, and pP2.
The IncP traG system generated a nonspecific product with pF7.
The IncW oriV system generated a nonspecific, nonhybridizing product with pF7, pF10, and pF17.
Resistance to chloramphenicol was difficult to determine because of the high level of intrinsic resistance of the host.
The IncN oriT system generated a nonspecific product with pP2.
Resistance to streptomycin was difficult to determine because of the high level of intrinsic resistance of the host.
Soil-plant microcosms and sampling.
Flevo silt loam (FSL) freshly collected from a field microplot at the Instituut voor Plantenziektekundig Onderzoek, Directie Landbouwkundig Onderzoek (IPO-DLO; Wageningen, The Netherlands), was used in all microcosm experiments. The FSL soil had a low organic matter content (about 2%), and its pH was approximately neutral (22). In order to establish selective pressure for resistance to mercury, different concentrations of mercury (4, 28, and 55 μg of HgCl2 per g of soil) were added to separate soil portions. Control soil portions received equivalent amounts of water. To prepare soil microcosms, 60-g portions of soil with a moisture content of 35% (about 60% of the moisture-holding capacity, corresponding to pF 2) were, after treatment with the different concentrations of mercuric chloride, placed in polypropylene containers (diameter, 62 mm; height, 65 mm). One day after HgCl2 was added, a subset of microcosms was planted with pregerminated seeds of wheat (Triticum aestivum cv. Sicco) (three seeds per microcosm). The microcosms containing wheat were incubated for different periods of time with a cycle consisting of 16 h of light at 20°C and 8 h of darkness at 16°C per day. Soil microcosms not containing wheat seedlings were kept in the dark. The planted microcosms were weighed daily and watered until they were at their original weight when needed in order to keep the soil moisture content stable throughout the experiments.
Following incubation, samples were removed from duplicate or triplicate microcosms at regular times after the start of the experiment (at zero time and after 3 and 8 h and 1, 2, 3, 5, 14, and 28 days). Bulk soil samples were obtained from the unplanted soil microcosms. Rhizosphere samples were obtained by carefully removing plants from planted soil microcosms. Plants were shaken to remove loosely adhering soil particles, and roots were separated from shoots. Soil closely associated with the roots was considered rhizosphere soil.
For enumeration by selective plating, 5- to 10-g portions of bulk soil or rhizosphere soil (roots included) were shaken in 250-ml Erlenmeyer flasks containing 95 ml of sterile 0.1% sodium pyrophosphate and 10 g of gravel (diameter, 2 to 4 mm) for 10 min at 280 rpm. Appropriate dilutions of the resulting suspensions were plated onto 10% tryptic soy broth agar (0.1× TSA) (Oxoid) supplemented with 100 μg of cycloheximide per ml to estimate the total culturable bacterial populations and on 0.1× TSA supplemented with 20 μg of HgCl2 per ml and 100 μg of cycloheximide per ml to determine the mercury-resistant bacterial populations (12). The colonies appearing on plates were counted after 2 and 5 days of incubation at 27°C, and the average number of log CFU per gram of dry soil was inferred from the total counts obtained for (at least) duplicate experimental units.
For exogenous plasmid isolations, 5-g samples of bulk soil or rhizosphere soil were suspended in 95 ml of sterile 0.85% NaCl. The flasks were shaken on a gyratory shaker at 280 rpm for 30 min, and the bacterial suspensions were used as described below.
Exogenous isolation of mercury resistance plasmids from soil and rhizosphere.
On three occasions, samples of maize and wheat plants with adhering soil were obtained from agricultural fields around Wageningen, The Netherlands. These samples were immediately taken to the laboratory, where rhizospheres (root parts plus adhering soil) were separated, and rhizosphere soil suspensions were prepared as described above. In addition, suspensions were obtained in the same way from the rhizospheres of young (7-, 10-, or 14-day-old) wheat plants grown in microcosms.
Following shaking of the rhizosphere soil suspensions, large soil particles were allowed to settle by leaving the flasks untouched for 10 min. Thirty milliliters of each supernatant was filtered with filter paper (mittelschnell; Schleicher & Schuell, Dassel, Germany), and the filtrate was passed over a sterile membrane filter (pore size, 0.22 μm) by using a vacuum filtration unit (Millipore Corp., Bedford, Mass.). Washed overnight cultures (1 ml) of one of the recipient strains (Pseudomonas fluorescens R2f Rpr, Pseudomonas fluorescens R2f [chr::Tn5] [used only for exogenous isolation of added plasmid pEC10], Pseudomonas putida UWC1 Rpr, or Enterobacter cloacae BE1 Rpr) were then added to the filters. Control filters contained either only the soil bacterial fractions or one of the recipient strains. The filters with the mating mixtures, as well as the controls, were placed on LB agar plates containing 1.5% agar and incubated overnight at 27°C. Following incubation, the filters were each shaken in 5 ml of 0.85% NaCl to dislodge bacterial cells, and serial 10-fold dilutions were plated onto 0.1× TSA plates supplemented with rifampin (50 μg ml−1) and cycloheximide (100 μg ml−1) to enumerate the rifampin-resistant recipient CFU and onto 0.1× TSA plates supplemented with 20 μg of HgCl2 per ml, 50 μg of rifampin per ml, and 100 μg of cycloheximide per ml to select for transconjugants. For the Pseudomonas fluorescens R2f (chr::Tn5) recipient strain, King’s medium B (35) agar supplemented with kanamycin (50 μg ml−1), streptomycin (50 μg ml−1), HgCl2 (20 μg ml−1), and cycloheximide (100 μg ml−1) was used. The plates were incubated at 27°C for 2 to 5 days, after which they were used for CFU analyses, for colony hybridization experiments and PCR analysis, and for isolation procedures. Mutants resistant to mercury were not obtained with all recipient strains. Moreover, the indigenous bacterial populations sampled generally did not give rise to any colonies on the transconjugant-selective plates.
Plasmid extraction.
Plasmids were extracted by a modification of the method of Kado and Liu (11), as developed by Bailey and Lilley (1). Briefly, 1.5 ml of an overnight culture of each putative plasmid host was centrifuged (1 min, 14,000 rpm in an Eppendorf centrifuge), suspended in 200 μl of a solution containing 50 mM Tris, 3% sodium dodecyl sulfate, and 120 mM NaOH (pH 12.6), and kept at 57°C for 70 min. The DNA was extracted with 200 μl of phenol-chloroform (24). The aqueous phase was diluted with 200 μl of distilled H2O, and the DNA was precipitated by adding 50 μl of 3 M sodium acetate (pH 5.2) and 1 ml of absolute ethanol and incubating the preparation at −20°C. The pellet containing the plasmid DNA was taken up in 25 μl of distilled water, and 5 to 8 μl was analyzed by agarose gel electrophoresis by using 0.7% agarose gels electrophoresed in TBE buffer (24).
To obtain plasmid preparations that were free of chromosomal DNA and were sufficiently pure to permit digestion by restriction enzymes, plasmid DNA was isolated with a mini plasmid preparation kit (Qiagen Gmbh, Hilden, Germany). The protocol of the manufacturer was used, except that a 20-ml overnight culture was used and the elution buffer was heated to 50°C.
Determination of the host ranges of selected plasmids.
To determine the plasmid host ranges, filter matings were performed with selected rifampin-, tetracycline-, or erythromycin-resistant recipient strains in order to include a wide range of members of the α, β, and γ subdivisions of the class Proteobacteria, as well as gram-positive bacteria (Table 1). Pseudomonas fluorescens (R2f chr::Tn5) containing the plasmids was used as the donor. Millipore membrane filters onto which 100-μl aliquots of washed cultures of both donor and recipient strains had been pipetted were placed on LB agar plates and incubated overnight at 27°C. After incubation, the filters were each shaken in 5 ml of 0.85% NaCl, and appropriate dilutions were spread plated onto transconjugant-selective plates (LB agar supplemented with the appropriate antibiotic [rifampin, tetracycline, or erythromycin] at a concentration of 50 μg ml−1 and 20 μg of HgCl2 ml−1) and onto plates for enumeration of donor CFU (LB agar supplemented with 20 μg of HgCl2 per ml) and recipient CFU (LB agar supplemented with 50 μg of rifampin per ml, 50 μg of tetracycline per ml, or 50 μg of erythromycin per ml). Suspensions of donor and recipient bacteria were also incubated separately on filters and subsequently plated onto transconjugant-selective plates to check for the occurrence of spontaneous antibiotic- or mercury-resistant mutants.
After incubation of the plates for 2 to 5 days at 27°C, the numbers of putative transconjugants, donors, and recipients were recorded. The presence of plasmids in transconjugant colonies was confirmed by colony hybridization with the appropriate whole-plasmid or PCR-generated probes, as well as by plasmid extraction.
Determination of plasmid phenotypes.
The presence of plasmid-encoded antibiotic or heavy-metal resistance genes was assessed by streaking both plasmid-containing and corresponding plasmidless strains onto 0.1× TSA plates supplemented with various concentrations of different antibiotics and heavy metals and examining the plates for the development of single colonies. Presumptively resistant clones, which were identified by enhanced resistance compared to the corresponding plasmidless strains, were rechecked by using the same concentration of inhibitory agent. The following inhibitory agents and concentration ranges were used: Ag2SO4, 0.025 to 5.0 mM; Na2HAsO4 · 7H2O, 0.025 to 5.0 mM; CdNO3, 0.25 to 2.5 mM; CoCl2 · 2H2O, 0.25 to 5.0 mM; K2Cr2O7, 0.025 to 5.0 mM; CuSO4 · 5H2O, 0.15 to 1.6 mM; HgCl2, 0.015 to 1 mM; NiSO4 · 6H2O, 0.25 to 2.5 mM; ZnCl2, 2 to 12 mM; sodium ampicillin, 10 to 500 μg ml−1; chloramphenicol, 10 to 150 μg ml−1; erythromycin, 5 to 100 μg ml−1; gentamicin sulfate, 10 to 200 μg ml−1; kanamycin sulfate, 10 to 200 μg ml−1; lincomycin, 7.5 to 50 μg ml−1; nalidixic acid, 10 to 200 μg ml−1; streptomycin sulfate, 10 to 200 μg ml−1; tetracycline hydrochloride, 5 to 100 μg ml−1; and trimethoprim, 10 to 100 μg ml−1.
Hybridization studies.
To screen for homology between the exogenously isolated plasmids and selected DNA sequences, colony hybridization, dot blot hybridization, and Southern blot hybridization were used. To prepare probes for (reverse) hybridization, preparations of selected plasmids were digested with HindIII, and the DNA was labeled with digoxigenin-11-dUTP by using the megaprime labeling system (Boehringer, Mannheim, Germany). The filters used for hybridization (24) contained dot blots of a range of novel and reference plasmids, as well as colony material from a plasmid host (Escherichia coli) with a set of 26 plasmids belonging to various incompatibility (Inc) groups (kindly provided by Helmut Tschäpe, Bundes Gesundheits Amt, Wernigerode, Germany). Other filters contained restriction enzyme-digested DNAs of selected novel plasmid isolates, of replicon typing probes (based on genes involved in plasmid replication of about 20 Inc groups [5]), and of 17 genes selected for their involvement in xenobiotic compound-catabolic processes (kindly donated by Dirk Springael, Vlaams Instituut vor Technologische Onderzoek, Mol, Belgium). These genes were xylDLEGFJIHS, catA, benABC, catBCDE, pcaACBDFE, bphC, todC123BADE, alkST, alkBAC, tcbCD, bphABCD, dmpABCD, nahR/nahG, nahAa, Ab, tbuA1, tbmB, and tbmC.
Hybridizations were carried out under high-stringency conditions by using standard procedures (24). Blots were washed under high-stringency conditions before nonradioactive detection was performed with the chemiluminescent substrate disodium 3-(4-methoxyspiro{1,2-dioxetane-3,21-(51-chloro)tricyclo [3.3.1.13.7]decan}-4-yl)phenyl phosphate(CSPD) combined with the digoxigenin-11-dUTP labeling-enzymatic detection system (Boehringer).
Soil DNA extraction.
DNA was extracted directly from FSL bulk and rhizosphere soil samples by the method of Smalla et al. (26), as modified by van Elsas and Smalla (35). A good yield of pure DNA (25 μg g of soil−1; average size, >20 kb) was obtained in all cases in which a subsequent PCR analysis was performed.
PCR analysis.
PCR were performed with pure plasmid or chromosomal DNA, with colonies, and with soil DNA. A suite of primer systems specific for selected plasmid Inc groups (7, 16, 17), for mer genes (3), and for pEC10-like plasmids was used. Unless noted otherwise, the PCR mixtures and temperature cycling regimens used were the same as those described previously (7, 16, 37). PCR products were analyzed by electrophoresis in appropriate 1.4% agarose gels (24). The nature of the PCR products was determined by blotting gels onto nylon filters and hybridizing these filters with the appropriate amplicon-specific probes.
Two primers were used to detect pEC10-like replicons. These primers were primers pEC10-f (5′-GCA CCC TGC CAT TTG CAGG-3′) and pEC10-r (5′-GGC TTT TGC CCT TCT GGTG-3′). A touchdown temperature cycling scheme (1 min at 94°C; 1.5 min at [sequentially, two cycles for each temperature] 65, 63, 61, 59, and 57°C; 2 min at 72°C) was used, followed by 40 similar cycles consisting of annealing at 55°C and, finally, extension for 10 min at 72°C. The PCR mixture was the mixture described previously for Taq polymerase (Stoffel fragment) (37). For PCR with colony DNA as well as soil DNA as the target, 0.1% skim milk was added since it was found to enhance specific target amplification. This system yielded a product that was about 250 bp long with pEC10 DNA, pEC10-containing colonies, and pEC10-supplemented soil DNA. The product generated on pEC10 was used as a probe for pEC10 sequences with blots of PCR products, of DNA, and of colonies.
The quantitative PCR for pEC10-like sequences in soil DNA, which included blotting and hybridization analysis, was based on a triplicate, threefold-dilution most-probable-number (MPN) scheme as described previously (23). The efficiency of PCR amplification in the soil DNA background was monitored by using a eubacterial 16S rRNA sequence-based PCR system, which yielded a product that was about 450 bp long.
Prevalence of pEC10 and homologous plasmids in soil bacterial populations subjected to mercury stress.
The effect of mercury in soil on the prevalence of pEC10 type plasmids was assessed in microcosms containing FSL not treated with HgCl2 (control) or treated with 28 μg of HgCl2 g−1. A subset of mercury-treated microcosms received about 105 CFU of Enterobacter cloacae BE1 Rpr(pEC10) per g of soil, which served as a control for the selective effect of mercury. Microcosms were incubated at 20°C by using a daily regimen consisting of 16 h of light and 8 h of darkness. Three days after incubation, replicate microcosms receiving each treatment were planted with young wheat seedlings.
The microcosms were sampled 3 h (zero time), 3 days, 7 days (for planted microcosms 10 days), and 15 days (for planted microcosms 18 days) after the start of the experiment.
Serial 10-fold dilutions of bulk and rhizosphere soil samples from microcosms with and without added mercury were plated onto 0.1× TSA and, for the 7- and 15-day samples, on Gould’s S1 Pseudomonas-specific agar (8) with and without HgCl2 (20 μg ml−1). In addition, dilutions of soil portions that had received Enterobacter cloacae BE1 Rpr(pEC10) were also plated onto 0.1× TSA supplemented with 50 μg of rifampin per ml. The colonies appearing on these media were counted after incubation at 27°C for 2 to 5 days. Selected plates, as well as randomly picked colonies, were used for colony lifts to obtain filters for colony hybridization and in PCR studies targeting pEC10-like plasmids. Hybridizations with total pEC10 DNA, as well as with the pEC10-specific probe generated by PCR, were performed to assess the occurrence of pEC10-homologous DNA in selected colonies from soil. PCR with pEC10-specific primers was then performed with a subset of the probe-positive colonies to confirm the presence of pEC10-like sequences. The numbers of colonies that reacted with the probe and the specific PCR system were determined, and the numbers of CFU that presumably contained pEC10-like plasmids were calculated. In addition, soil suspensions were used to assess the exogenous isolation frequencies of mercury resistance plasmids in matings between the bacterial suspensions and either Enterobacter cloacae BE1 Rpr, Pseudomonas fluorescens R2f Rpr, or Pseudomonas fluorescens R2f (chr::Tn5) [for the soil portions supplemented with Enterobacter cloacae BE1 Rpr(pEC10)]. The presence of pEC10-like sequences in transconjugant colonies was assessed by performing colony hybridization with the pEC10 probe, as well as by pEC10-specific PCR. Finally, the numbers of pEC10 targets were determined in soil DNA extracts as described above.
Statistics.
All experiments were performed in duplicate or triplicate. Means of bacterial counts and plasmid isolation frequencies, as well as standard errors, were calculated. Analysis of variance was used to assess the significance (at a P of <0.05) of the differences between means due to treatments or experimental factors. The pEC10 target numbers were estimated by MPN PCR in a triplicate, threefold-dilution set-up, with confidence levels of MPN/2.8 and MPN × 2.8 (23).
RESULTS
Effect of mercury on indigenous soil bacterial populations.
The addition of different concentrations of HgCl2 to FSL soil dramatically affected the initial total bacterial counts (Fig. 1A). Whereas without added mercury the total counts initially decreased slightly but then remained roughly constant at levels of 4 × 107 to 6.3 × 107 CFU g of dry soil−1, the counts after mercuric chloride was added decreased rapidly to 1 × 107 CFU g−1 (4 μg of HgCl2 g of dry soil−1) or even to 1 × 105 CFU g−1 (28 or 55 μg of HgCl2 g of dry soil−1). Following these initial decreases, the bacterial counts increased again. After a few days, they were in all cases back to approximately the initial levels. For the two highest mercury concentrations (28 and 55 μg of dry soil−1), the total counts even increased to significantly higher levels (around 1.6 × 108 CFU g of dry soil−1) than the counts in the control.
FIG. 1.
Effects of different concentrations of mercury in FSL soil on the total numbers of culturable CFU on 0.1× TSA (A) and the numbers of CFU resistant to mercuric chloride (20 μg ml−1) (B). Symbols: •, no HgCl2; ▴, 4 μg of HgCl2 g of soil−1; ▾, 28 μg of HgCl2 g of soil−1; ▪, 55 μg of HgCl2 g of soil−1. The variation at each data point was within the symbol dimensions. The data points marked with a or b (referring to both high-mercury-level treatments) were significantly higher than the corresponding data points for the low- and no-mercury treatments, with a > b (P < 0.05).
The counts of mercury-resistant bacteria were initially on the order of around 105 CFU g of dry soil−1, and they remained at this level in the control and low-mercury treatments throughout the experiment (Fig. 1B). However, in the soil portions that received the two highest mercury concentrations, the numbers of mercury-resistant CFU initially decreased, after which the values increased to levels significantly above those in the no-mercury and low-mercury treatments. The levels reached were up to 1 order of magnitude below those of the total counts (i.e., 1 × 107 to 1.6 × 107 CFU g of dry soil−1) after 14 days and remained stable thereafter.
Exogenous isolation of mercury resistance plasmids from bulk and rhizosphere soil.
When the exogenous plasmid isolation procedure was used with Pseudomonas fluorescens R2f Rpr, Pseudomonas putida UWC1 Rpr, or Enterobacter cloacae BE1 Rpr as the recipient strain, transconjugants were not obtained on three occasions with bacterial populations from the rhizospheres of mature maize or wheat plants growing in the field (data not shown). The total bacterial counts ranged from 1.5 × 108 to 4 × 108 CFU g of dry soil−1, whereas the counts for the mercury-resistant populations were around 2 × 104 CFU g of dry soil−1. The estimated limits of detection, expressed as exogenous isolation frequencies (number of transconjugants per recipient), were about 10−10 to 10−11 in both cases.
We subsequently analyzed populations from young wheat plants in mercury-amended FSL soil microcosms, as well as unamended FSL soil microcosms. Numerous colonies of putative transconjugants were produced after matings of all three recipient strains with the bacterial flora obtained from FSL bulk and wheat rhizosphere soil samples (Table 2). The exogenous isolation frequencies obtained with populations of both bulk and wheat rhizosphere soils increased as the concentrations of mercury in the soil increased. This effect was especially prominent for the Pseudomonas fluorescens and Enterobacter cloacae recipient strains, which captured exogenous plasmids at least about 100-fold more frequently when they were incubated with bacterial populations from bulk or rhizosphere soils containing 55 μg of added mercury per g than when they were incubated with bacterial populations from unamended soil (Table 2). Irrespective of the level of mercury, the wheat rhizosphere significantly enhanced the exogenous isolation frequencies obtained with the Pseudomonas putida recipient, but not the exogenous isolation frequencies obtained with the Pseudomonas fluorescens and Enterobacter cloacae recipients.
Twenty randomly picked putative transconjugants per recipient strain screened for the physical presence of plasmids contained plasmids whose estimated sizes ranged from about 40 to 50 kb (data not shown).
Molecular characterization of selected mercury resistance plasmids.
Eight plasmids of different sizes (two or three plasmids per recipient strain) were characterized further (Table 3). All of the plasmids transferred mercury resistance to a Pseudomonas fluorescens R2f (chr::Tn5) recipient strain, suggesting that their resistance determinant was readily expressed in Pseudomonas fluorescens.
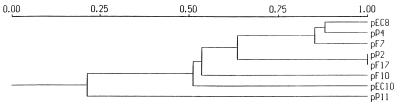
The restriction patterns of the eight plasmids generated with EcoRV, PstI, and XbaI revealed differences among the plasmids, as well as similarities. Digestion with XbaI resulted in up to 4 fragments, digestion with EcoRV resulted in 5 to 8 fragments, and digestion with PstI resulted in 9 to 13 fragments. A similarity matrix constructed with the Dice coefficient of similarity was based on the PstI digests, which were the most discriminating digests. The corresponding dendrogram (Fig. 2) showed that the plasmids fell into five groups that differed by a genetic distance of more than 20%. The largest group, designated group II, consisted of three plasmids (pEC8, pP4, and pF7), and another group (group III) consisted of two indistinguishable plasmids (pP2 and pF17) (Table 3). Several bands of all plasmids hybridized strongly with pEC10 and pP11 when they were used as probes, and the hybridization patterns of group II plasmids, as well as the hybridization patterns of group III plasmids, were internally consistent, whereas the hybridization patterns of the other groups were different. Hence, all of the plasmids were related to pEC10 at the sequence level, but their restriction and hybridization patterns clearly separated them.
FIG. 2.
Dendrogram (constructed by the neighbor-joining unweighted pair group method with mathematical averages) of genotypic relationships, showing the similarity among eight plasmids as determined by phenetic analysis with the Dice coefficient of similarity. The divergence between different classes is expressed as follows: 1 − coefficient.
A dot blot hybridization experiment performed in our laboratory and six other European laboratories showed that all eight plasmids produced hybridization signals with pEC10 when it was used as a probe, but not with a suite of eight divergent plasmid isolates. Furthermore, several plasmids (pEC10, pEC8, and pP4) showed homology with new plasmid pMOL96 (42), whereas the remaining plasmids reacted weakly or not at all. A hierarchical cluster analysis performed by A. K. Lilley at the Institute for Virology and Environmental Microbiology, Oxford, United Kingdom (with the average-between-group method), based on the positive and negative scores obtained, revealed that all eight plasmids clustered tightly together with each other and with no other plasmid (13a).
Replicon typing by hybridization with 17 replicon-specific probes (including those for the broad-host-range plasmids of the IncQ, IncP, IncN, IncW, and IncA/C groups) suggested that none of the eight plasmids had detectable homology with any of the 17 groups (Table 3). Furthermore, in a hybridization assay performed with 26 plasmids belonging to established Inc groups, weak signals were obtained with probes for the IncH2, IncFII, and IncFIX groups, but signals were not obtained with the remaining 23 plasmids. The lack of firm molecular assignment to any known Inc group (including the five broad-host-range groups) was confirmed by PCR broad-host-range Inc group typing (7), as no positive identification of an Inc group was obtained (Table 3). However, a weak band of the expected size, which did not hybridize with the appropriate probe, was obtained for all plasmids with a novel PCR system based on the rep gene of IncA/C plasmid RA1 (16). This suggested that all eight plasmids might share a rep gene distantly related to the IncA/C-related rep gene. On the other hand, none of the plasmids hybridized with an IncA/C oriV probe (Table 3).
Furthermore, all of the plasmids except one (pF7) produced a positive signal with consensus merRTΔP primers and probe (3). Hence, these plasmids contained sequences of prototypic mercury resistance transposon Tn501.
Screening for marker genes in selected plasmids.
All eight plasmids were subjected to a heavy-metal resistance and antibiotic resistance analysis, and one plasmid from each group (groups I through V) was also screened for the presence of catabolic genes (Table 3). All of the plasmids conferred resistance to 0.3 to 0.6 mM Cu to their hosts, and one plasmid, pP11, also showed As resistance (Table 3). Furthermore, five plasmids (pEC10, pEC8, pP2, pF17, and pP11) encoded resistance to 25 to 50 μg of streptomycin per ml, whereas resistance to chloramphenicol (100 to 200 μg ml−1) was found in six plasmids (pEC10, pEC8, pF7, pF17, pP11, and pF10). The latter resistance was difficult to detect in Pseudomonas putida UWC1 plasmids pP2 and pP4 due to the high intrinsic resistance of the host (Table 3). Plasmids pEC10 and pEC8 expressed resistance to chloramphenicol in both Pseudomonas fluorescens R2f and Enterobacter cloacae BE1. In the latter strain (but not in Pseudomonas fluorescens), they also expressed streptomycin resistance.
Hybridization studies performed with 17 probes based on xenobiotic compound-degrading genes or gene clusters revealed an absence of detectable homology with 16 gene sequences, whereas the only homology found for plasmid pEC10 was homology with the chlorocatechol dioxygenase gene, tcbCD (Table 3).
Host ranges of selected plasmids and (retro)mobilization by plasmid pEC10.
All eight plasmids were transferred between their original hosts and Pseudomonas fluorescens R2f (chr::Tn5). Several also were transferred to two members of the β subdivision of the Proteobacteria, Alcaligenes eutrophus AE815 and Burkholderia cepacia P2. None of the plasmids could be transferred to Agrobacterium tumefaciens Gmi9023 (a member of the α subdivision of the Proteobacteria), to Escherichia coli CV601 or Sm10 (chr::Tn5::luxAB-tet), or to the gram-positive bacteria Bacillus subtilis SEm-2 and Paenibacillus azotofixans P3L5. These limited data suggested that all of the plasmids had a preference for hosts belonging to the β and γ subdivisions of the Proteobacteria.
A more extensive host range study performed with plasmid pEC10 confirmed this preference for members of the β and γ subdivisions of the Proteobacteria (Table 1). Plasmid pEC10 was transferred to and maintained in all fluorescent pseudomonads tested, as well as Pseudomonas stutzeri, Enterobacter cloacae, Alcaligenes eutrophus, and Burkholderia cepacia. The Acinetobacter, Agrobacterium, Rhizobium, Bacillus, Paenibacillus, Escherichia coli, and soil isolate F4 strains tested did not support transfer or maintenance of pEC10.
In a mating between Enterobacter cloacae BE1(pEC10) and Pseudomonas fluorescens R2f(pSUP104), direct transconjugants (Pseudomonas fluorescens cells containing pEC10 in addition to pSUP104) were found at a frequency of 1.5 × 10−2 transconjugants per recipient, whereas retrotransconjugants (Enterobacter cloacae BE1 with pEC10 and pSUP104) appeared at a frequency of 7 × 10−7 transconjugants per recipient. In a subsequent mating between Pseudomonas fluorescens R2f(pEC10, pSUP104) and Pseudomonas fluorescens R2f (chr::Tn5), pSUP104 was mobilized by pEC10 at a frequency of 2.2 × 10−3 transconjugants per recipient. Both plasmids were found in several selected transconjugants, suggesting that transfer of pEC10 and mobilization of pSUP104 took place at similar rates. These data showed that pEC10 was capable of mobilizing as well as retromobilizing IncQ plasmid pSUP104. Plasmids belonging to group III (e.g., pF17) were also capable of mobilizing pSUP104 (data not shown).
Detection of pEC10-like plasmids.
A strategy for molecular detection of pEC10 was based on the observation that the IncQ-oriV-specific PCR system generated an amplification product of about 300 bp with pEC10 as the target (Table 3). This amplicon did not produce a hybridization signal with the IncQ-oriV-specific probe generated by PCR on IncQ plasmid RSF1010. The product was sequenced; alignment of the sequence with the sequences in the EMBL database (both total sequences and bacterial sequences) showed that the only similarities to the database sequences were in short (20-bp or smaller) regions of the amplicon. Primers for selected inner regions of the amplicon were designed, and the PCR system obtained was tested with 37 different plasmids (including the eight new plasmids and three IncQ plasmids, pSKTG, pSUP104, and pIE723), as well as with nine chromosomal DNAs obtained from seven different bacterial species (Escherichia coli, Enterobacter cloacae, Pseudomonas fluorescens, Pseudomonas putida, Acinetobacter calcoaceticus, Bacillus polymyxa, and Mycobacterium chlorophenolicum). The results revealed that neither the chromosomal DNAs of the nine strains nor 30 different plasmid DNAs produced a PCR product. On the other hand, all of the eight novel plasmids except pF7 were PCR positive (Table 3), and the products obtained hybridized with the probe generated by PCR with pEC10. Hence, we considered the PCR primers, as well as the probe generated on plasmid pEC10, specific for pEC10 and “like” replicons in the soil environment.
Effect of mercury addition to soil on the prevalence of mercury resistance and pEC10-like plasmids.
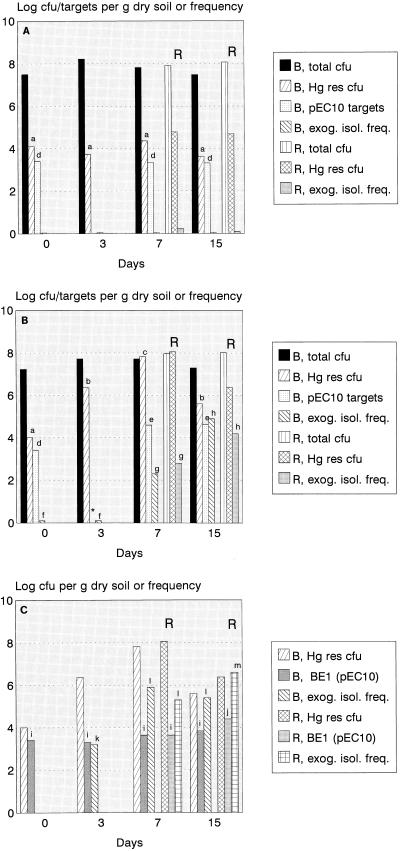
The effect of mercury as a selective agent on the occurrence of pEC10-like plasmids in soil and wheat rhizosphere bacterial populations was assessed in a microcosm study. At 3, 7, and 15 days after the start of the experiment, the mercury added had no effect on the total bacterial counts on 0.1× TSA (Fig. 3A and B). After 7 and 15 days, the counts on Gould’s S1 agar were equivalent to 0.1 to 0.5% of the total counts on 0.1× TSA, and effects of mercury were also not observed. In accordance with the experiment shown in Fig. 1, the numbers of CFU on 0.1× TSA amended with mercury increased progressively in mercury-amended soil, whereas the counts were roughly stable (at the initial level, around 104 CFU g of dry soil−1) in soil without added mercury. From day 3, the numbers of mercury-resistant CFU were significantly higher in the mercury-amended soil than in the unamended soil (Fig. 3A and B). The presence of wheat roots in all cases enhanced the total and mercury-resistant counts after 7 and 15 days, albeit not always significantly (Fig. 3A and B). On days 7 and 15 the proportions of mercury-resistant CFU in the total counts on Gould’s S1 agar obtained with unamended soil were 1 to 4%; however, the proportions in mercury-amended soil samples were 57% (bulk soil) and 80% (rhizosphere soil).
FIG. 3.
Effect of mercury added to FSL soil on the prevalence of pEC10-like mercury resistance plasmids, as shown by selective plating and colony probing-PCR, exogenous plasmid isolation, and pEC10-specific MPN PCR of soil DNA. (A) Untreated soil. (B) Mercury-treated soil. (C) Mercury-treated soil with added Enterobacter cloacae BE1 Rpr(pEC10). Abbreviations: B, bulk soil; R, rhizosphere soil; total cfu, total counts on 0.1× TSA; Hg res cfu, mercury-resistant CFU; exog. isol. freq., exogenous isolation frequency; BE1 (pEC10), Enterobacter cloacae BE1(pEC10) CFU. The log CFU per gram of dry soil, target numbers per gram of dry soil, or log exogenous plasmid isolation frequencies (10−12) are shown. For mercury-resistant counts (untreated soil and mercury-treated soil), a < b < c (P < 0.05). For pEC10 targets (untreated soil and mercury-treated soil), d < e. For exogenous isolation frequencies (mercury-treated soil), f < g < h (P < 0.05). For BE1 Rpr(pEC10) counts [mercury-treated soil with added BE1 Rpr(pEC10)], i < j (P < 0.05). For pEC10 isolation frequencies (mercury-treated soil with added BE1), k < l < m (P < 0.05). The asterisk indicates that values were not determined.
The concentration of Enterobacter cloacae BE1(pEC10), which was about 105 CFU g of dry soil−1 at the beginning of the experiment, decreased to 2.6 × 103 CFU g of dry soil−1 shortly after the organism was added to the mercury-amended soil. There was a small but significant increase in the number of organisms in the wheat rhizosphere after 15 days (Fig. 3C). Colony filter hybridization and a PCR analysis of randomly selected colonies indicated that pEC10 was present in all colonies grown on the selective plates.
The exogenous plasmid isolation frequencies obtained in matings performed with Enterobacter cloacae BE1 Rpr, Pseudomonas fluorescens R2f Rpr, and the bacterial flora from unamended soil were at or below the limit of detection throughout the experiment. With Pseudomonas fluorescens R2f Rpr, the levels of exogenous transconjugants increased to detectable values in the rhizosphere of wheat after 7 and 15 days. In matings performed with the bacterial flora from mercury-treated soil portions, the frequencies were initially (at zero time and on day 3) also below the limit of detection, whereas they had increased dramatically and significantly after 7 and 15 days (Fig. 3B). The presence of wheat roots did not affect these frequencies to a great extent after 7 and 15 days (Fig. 3B). A high proportion (52 to 62%) of the transconjugant colonies obtained contained pEC10-like sequences, as determined by colony PCR.
Plasmid pEC10 was exogenously isolated in Pseudomonas fluorescens R2f (chr::Tn5) from soil portions that had received Enterobacter cloacae BE1 Rpr(pEC10) at frequencies that differed significantly, from 1.3 × 10−9 transconjugants per recipient (day 3, bulk soil) to 4.8 × 10−6 transconjugants per recipient (day 15, rhizosphere soil). On day 15, the frequency of isolation was significantly greater with rhizosphere bacterial populations than with populations from corresponding bulk soil (Fig. 3C). The majority (82 to 85%) of the putative exogenous transconjugants from day-7 and -15 samples carried pEC10-like plasmids.
Furthermore, a significant (17-fold) increase in the number of colonies with homology to pEC10 in mercury-amended bulk soil samples was observed; the level increased from 2.3 × 103 CFU g of dry soil−1 initially to 4.16 × 104 CFU g of dry soil−1 after 15 days (day-3 and -7 data could not be assessed). Moreover, on days 7 and 15, with bulk and rhizosphere samples the number of colonies containing pEC10-homologous sequences on mercury-containing Gould’s S1 agar was approximately 104 CFU g of dry soil−1. In unamended soil portions, such increases were not noticeable, and the number of pEC10-homologous colonies on both 0.1× TSA and Gould’s S1 agar remained below the limit of detection (3 × 103 to 5 × 103 CFU g of dry soil−1).
The prevalence of pEC10-like sequences in bulk soil samples increased as a result of the addition of mercury (Fig. 3B). Whereas the initial pEC10 copy number was estimated to be 2.45 × 103 copies g of dry soil−1, the copy numbers increased to 3.98 × 104 and 4.2 × 104 copies g of dry soil−1 in mercury-amended soil on days 7 and 15, respectively. On the other hand, the levels of pEC10-like sequences in the unamended soil portions remained stable at about the initial level (Fig. 3A). The soil portions containing added Enterobacter cloacae BE1 Rpr(pEC10) at similar low levels also showed positive amplification and were used to monitor the PCR amplification efficiency.
DISCUSSION
In this study, we first assessed the responses of culturable bacterial populations in soil to different levels of mercury, and subsequently we assessed the possible involvement of plasmids in conferring mercury resistance, as well as gene-mobilizing capacity, to selected bacterial populations. We chose this combination since mercury resistance is a ubiquitous marker in bacterial populations in the environment which is often associated with mobile genetic elements (1a, 10, 12, 21, 30). Both the total culturable bacterial populations (Fig. 1) and the mercury-resistant culturable bacterial populations (Fig. 1 and 3) reacted quickly to high levels of added mercury, whereas a strong response was not noticeable at low mercury levels (Fig. 1). The highest mercury concentration used in this study (55 μg g of soil−1) was still about 10-fold below the concentration which has been found to inhibit soil microbial activity (34), but it was in the range which can elicit a response by bacteria in soil (12). The presence of mercury in soil imposes in situ stress on cells that are affected by sufficiently high local levels of mercury. In general, bacterial populations in soil can respond to mercury stress by a variety of mechanisms, including (i) inhibition of cellular metabolism, resulting in growth inhibition or death; (ii) induction of existing mercury resistance operons, possibly followed by outgrowth of resistant forms; and (iii) acquisition of plasmids with functional mercury resistance genes via conjugation. The population response can thus be observed as an increase in the frequency with which mercury-resistant bacteria are isolated (18). Cell death, induction of mer operons, gene transfer of mer determinants, and clonal selection of mer operon-containing organisms very likely all played a role in the response measured by CFU counting in our study.
The exogenous plasmid isolations were performed primarily to assess the prevalence and nature of transferable plasmids with mercury resistance determinants in soil bacterial populations. Exogenous isolation in a preselected host is a good method to obtain and assess plasmids with self-transfer and possibly gene-mobilizing capacity. It abolishes the need to culture the plasmid hosts and therefore even permits isolation of plasmids from nonculturable bacteria (32). However, a lack of knowledge about the natural plasmid host obviously leaves doubts concerning the role of the isolated plasmid in its natural host.
The preferential isolation of mercury resistance plasmids from young wheat roots suggests that the dynamics of the plasmids and their hosts are related to temporal and spatial aspects of plant growth, inasmuch as these may reach a peak in abundance and/or activity at a specific time and in specific sites during root development. Although the original plasmid hosts are not known, they might be found in copiotrophic early-root-colonizing groups, such as the fluorescent pseudomonads or enteric bacteria. This hypothesis is also consistent with the host range data obtained with pEC10. Lilley et al. (14) also found that mercury resistance plasmids of similar types were prevalent only at specific times of plant development in three consecutive years in the phyllospheres and rhizospheres of sugar beet; this fluctuating prevalence very likely reflected the fluctuating abundance of the presumed plasmid hosts.
In the soil microcosms, the exogenous isolation frequencies of the mercury resistance plasmids increased as higher concentrations of mercury were added to soil. The wheat rhizosphere enhanced these frequencies only for one recipient strain. Top et al. (32) suggested that exogenous isolation frequencies could be used in a quantitative way to estimate the abundance of the plasmid types isolated. In our view, the enhanced isolation frequencies that resulted from mercury addition indicated that the mercury pressure increased either the prevalence of mercury resistance determinants located on mobile genetic elements or the activity of the determinants via induction of mercury resistance genes. Selection by mercury may also have resulted in clonal selection of cells containing plasmids with mercury resistance determinants, as well as plasmid transfer to new hosts at a stage when the densities of potential plasmid donor bacteria were increased.
Despite the proposed grouping of the eight selected plasmids in five groups based on restriction patterns and plasmid sizes (Fig. 2 and Table 3), all of these plasmids are probably related. This was evident from the clustering of the plasmids in the dot blot hybridization assay, from the cross-hybridization results, from the similar (albeit not identical) phenotypes of the plasmids, and from the reactions of most of the plasmids with the pEC10-specific detection systems, as well as the merRTΔP detection system. The (weak) reactions with the PCR system specific for the IncA/C rep gene and the absence of hybridization under high-stringency conditions suggested that these plasmids were related at lower levels to a rep gene of the broad-host-range IncA/C group, a synonym of the IncP3 group of Pseudomonas plasmids (2, 16, 17). Interestingly, the 88-kb prototype IncP3 plasmid, pBS73 (of Pseudomonas aeruginosa), also carries resistance to mercury, resistance to streptomycin, and resistance to chloramphenicol. It is possible that the bacterial populations from which the plasmids were obtained naturally contain a myriad of closely related plasmids, from which a closely knit yet diverse set of plasmids was obtained in the three gram-negative hosts. It is unclear whether each representative of this plasmid pool is restricted to a different specific host (which would result in mainly vertical inheritance) or whether ample gene transfer and recombination among the different (gram-negative) hosts can take place. The primary isolation of the group II plasmids pEC8, pP4, and pF7 in three different gram-negative hosts was interesting and may support the latter possibility. Moreover, as the group II plasmids, as well as pEC10 and pF10, reacted with a 0.7-kb tniA probe generated by PCR from novel plasmid pMOL96 (41), a transposon with the Tn5090 (Tn402) transposase of IncPβ plasmid R751 may be present. These findings support a myriad of different internal relationships among our plasmids, as well as the possible presence of a tniA gene.
The selected plasmids obtained from the rhizosphere of wheat could be transferred to a range of members of the β and γ subdivisions of the Proteobacteria at moderate to high frequencies. As exemplified by pEC10, they also mobilized and retromobilized IncQ plasmid pSUP104. Hence, these plasmids form a pool of elements which may play a role in conjugal gene transfer events in the initial stages of plant root development (i.e., at times when their hosts are prevalent). Given the presumably low levels of antibiotics (streptomycin, chloramphenicol) and heavy metals (Hg, Cu) in the FSL soil used, it is unclear from the plasmid phenotypes and genotypes what their actual role in the ecophysiology of their hosts is, other than conferring gene-mobilizing capacity. It would be interesting to assess whether these plasmids enhance their mobilizing activity and extend it to, for instance, the bacterial chromosome under stress conditions in soil and rhizospheres.
From the final experiment in mercury-treated soil it became clear that pEC10-like plasmids are indeed selected for or “activated” by mercury stress. The following three lines of evidence supported this contention: (i) the number of colonies reacting with the pEC10-specific probe, as well as the PCR system, increased; (ii) the exogenous plasmid isolation frequencies increased, and most of the plasmids isolated were pEC10-like; and (iii) direct PCR performed with soil-extracted DNA revealed that there was enhanced prevalence of pEC10-like plasmids under the influence of mercury. The simplest explanation for these three phenomena is that there was an increase in the abundance of bacteria carrying pEC10-like plasmids, although an increase in the plasmid copy number per cell, as well as progressive induction of repressed mercury resistance genes, may also have played a role, either by affecting the numbers of targets measured by direct soil DNA extraction and PCR or by affecting the numbers of probe-positive colonies and the exogenous isolation frequencies. The similar numbers of probe- and PCR-positive colonies (around 104 g of dry soil−1) found on 0.1× TSA and Gould’s S1 medium might indicate that fluorescent pseudomonads are prime carriers of these plasmids in bulk and wheat rhizosphere soils. If this is true, it might also explain the enhancement of the isolation frequency in the rhizosphere, as fluorescent pseudomonads are known to be favored by young rhizospheres. On the other hand, the introduced pEC10-carrying strain Enterobacter cloacae BE1 did not survive in high numbers in the mercury-treated soil, and its abundance was enhanced only to a small extent in the wheat rhizosphere. These observations suggested that Enterobacter cloacae BE1(pEC10) did not have a strong selective advantage over other competing soil microorganisms in the mercury-stressed soil system.
We suggest that the gene-mobilizing capacity of soil bacterial populations increases as mercury stress is applied, since the prevalence (dominance) of pEC10-like mercury resistance plasmids with gene-mobilizing capacity is enhanced. This observation has an important bearing on the potential for gene spread via conjugation in soil microbial communities under stress conditions. In contrast, Wickham and Atlas (40), in a limited survey, found that mercury selective pressure did not enhance the incidence of plasmids in selected culturable soil bacterial populations. However, the data of these authors were based solely on plasmid incidence as judged by plasmid extraction from soil isolates, which represents a very different level of resolution. In our view, this does not imply that the incidence of transfer-proficient mercury resistance plasmids is unaffected in total or specific soil bacterial fractions. As exemplified by the mercury resistance determinants present on the pEC10-like plasmids, the self-transmissible plasmids could well provide a means of genetic adaptation to changing environmental conditions to their hosts.
ACKNOWLEDGMENTS
This work was supported by grant BIO2-CT92-0491 from the EU-BIOTECH program.
We are grateful to all partners of the EU-BIOTECH consortium for their help, particularly for sharing dot blot hybridization results obtained with the pEC10-like plasmids. In particular, Helmut Tschäpe, Dirk Springael, and Annick Wilmotte are acknowledged for providing Inc determinants and catabolic genes for the hybridization studies and for sharing results obtained with the tniA probe. Andrew Lilley is thanked for his assistance with the dot blot analysis. Sander Worst, Alexandre Rosado, Gert-Jan ten Thij, and Ludwina Lankwarden are acknowledged for their help with some of the experiments.
REFERENCES
- 1.Bailey, M. J., and A. K. Lilley. 1996. Personal communication.
- 1a.Belliveau B, Trevors J T. Mercury resistance and detoxification in bacteria. Appl Organometall Chem. 1989;3:283–294. [Google Scholar]
- 2.Boronin A M. Diversity and relationships of Pseudomonas plasmids. In: Galli E, Silver S, Witholt B, editors. Pseudomonas: molecular biology and biotechnology. Washington, D.C: American Society for Microbiology; 1992. pp. 329–340. [Google Scholar]
- 3.Bruce K D, Hiorns W D, Hobman J L, Osborn A M, Strike P, Ritchie D A. Amplification of DNA from native populations of soil bacteria by using the polymerase chain reaction. Appl Environ Microbiol. 1992;58:3413–3416. doi: 10.1128/aem.58.10.3413-3416.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton N F, Day M J, Bull A T. Distribution of bacterial plasmids in clean and polluted sites in a South Wales river. Appl Environ Microbiol. 1982;44:1026–1029. doi: 10.1128/aem.44.5.1026-1029.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couturier M, Bex F, Bergquist P L, Maas W K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988;52:375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fry J C, Day M J. Plasmid transfer in the epilithon. In: Fry J C, Day M J, editors. Bacterial genetics in natural environments. London, United Kingdom: Chapman and Hall; 1990. pp. 55–80. [Google Scholar]
- 7.Götz A, Pukall R, Smit E, Tietze E, Prager R, Tschäpe H, van Elsas J D, Smalla K. Detection and characterization of broad-host-range plasmids in environmental bacteria by PCR. Appl Environ Microbiol. 1996;62:2621–2628. doi: 10.1128/aem.62.7.2621-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould W D, Hagedorn C, Bardinelli T R, Zablotowicz R M. New selective media for enumeration and recovery of fluorescent pseudomonads from various habitats. Appl Environ Microbiol. 1985;49:28–32. doi: 10.1128/aem.49.1.28-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill K E, Weightman A J, Fry J C. Isolation and screening of plasmids from the epilithon which mobilize recombinant plasmid pD10. Appl Environ Microbiol. 1992;58:1292–1300. doi: 10.1128/aem.58.4.1292-1300.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jobling M G, Peters S E, Ritchie D A. Plasmid-borne mercury resistance in aquatic bacteria. FEMS Microbiol Lett. 1988;49:31–37. [Google Scholar]
- 11.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly W J, Reanney D C. Mercury resistance among soil bacteria: ecology and transferability of gene encoding resistance. Soil Biol Biochem. 1984;16:1–8. [Google Scholar]
- 13.Khesin R, Karasyova E V. Mercury-resistance plasmids in bacteria from a mercury and antimony deposit area. Mol Gen Genet. 1984;197:280–285. doi: 10.1007/BF00330974. [DOI] [PubMed] [Google Scholar]
- 13a.Lilley, A. K. 1997. Unpublished results.
- 14.Lilley A K, Bailey M J, Day M J, Fry J C. Diversity of mercury resistance plasmids obtained by exogenous isolation from the bacteria of sugar beet in three successive years. FEMS Microbiol Ecol. 1996;20:211–227. [Google Scholar]
- 15.Lilley A K, Fry J C, Day M J, Bailey M J. In situ transfer of an exogenously isolated plasmid between Pseudomonas spp. in sugar beet rhizosphere. Microbiology. 1994;140:27–33. [Google Scholar]
- 16.Llanes C, Gabant P, Couturier M, Bayer L, Plesiat P. Molecular analysis of the replication elements of the broad-host-range repA/C replicon. Plasmid. 1996;36:26–35. doi: 10.1006/plas.1996.0028. [DOI] [PubMed] [Google Scholar]
- 17.Llanes C, Gabant P, Couturier M, Michel-Briand Y. Cloning and characterization of the IncA/C plasmid RA1 replicon. J Bacteriol. 1994;176:3403–3407. doi: 10.1128/jb.176.11.3403-3407.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborn A M, Bruce K D, Strike P, Ritchie D A. Polymerase chain reaction-restriction fragment length polymorphism analysis shows divergence among mer determinants from gram-negative soil bacteria indistinguishable by DNA-DNA hybridization. Appl Environ Microbiol. 1993;59:4024–4030. doi: 10.1128/aem.59.12.4024-4030.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell B J, Purdy K J, Thompson I P, Bailey M J. Demonstration of tra+ plasmid activity in bacteria indigenous to the phyllosphere of sugarbeet; gene transfer to a recombinant pseudomonad. FEMS Microbiol Ecol. 1993;12:195–206. [Google Scholar]
- 20.Pukall R, Tschäpe H, Smalla K. Monitoring the spread of broad host and narrow host range plasmids in soil microcosms. FEMS Microbiol Ecol. 1996;20:53–66. [Google Scholar]
- 21.Radford A J, Oliver J, Kelly W J, Reanney D C. Translocatable resistance to mercuric and phenylmercuric ions in soil bacteria. J Bacteriol. 1981;147:1110–1112. doi: 10.1128/jb.147.3.1110-1112.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richaume A, Smit E, Fauri G, van Elsas J D. Influence of soil type on the transfer of RP4p from Pseudomonas fluorescens to indigenous bacteria. FEMS Microbiol Ecol. 1992;101:263–292. [Google Scholar]
- 23.Rosado A, Seldin L, Wolters A C, van Elsas J D. Quantitative 16S rDNA-targeted polymerase chain reaction and oligonucleotide hybridization for the detection of Paenibacillus azotofixans in soil and the wheat rhizosphere. FEMS Microbiol Ecol. 1996;19:153–164. [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 25.Silver S, Misra T K. Plasmid-mediated heavy metal resistances. Annu Rev Microbiol. 1988;42:717–743. doi: 10.1146/annurev.mi.42.100188.003441. [DOI] [PubMed] [Google Scholar]
- 26.Smalla K, Cresswell N, Mendonca-Hagler L C, Wolters A, van Elsas J D. Rapid DNA extraction protocol from soil for polymerase chain reaction-mediated amplification. J Appl Bacteriol. 1993;74:78–85. [Google Scholar]
- 27.Smit E, Venne D, van Elsas J D. Mobilization of a recombinant IncQ plasmid between bacteria on agar and in soil via cotransfer or retrotransfer. Appl Environ Microbiol. 1993;59:2257–2263. doi: 10.1128/aem.59.7.2257-2263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smit E, van Elsas J D, van Veen J A, de Vos W M. Detection of plasmid transfer from Pseudomonas fluorescens to indigenous bacteria in soil using phage phiR2f for donor counterselection. Appl Environ Microbiol. 1991;57:3482–3488. doi: 10.1128/aem.57.12.3482-3488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stotzky G. Gene transfer among bacteria in soil. In: Levy S B, Miller R V, editors. Gene transfer in the environment. New York, N.Y: McGraw-Hill; 1989. pp. 165–222. [Google Scholar]
- 30.Tanaka M, Yamamoto T, Sawai T. Evolution of complex resistance transposons from an ancestral mercury transposon. J Bacteriol. 1983;153:1432–1438. doi: 10.1128/jb.153.3.1432-1438.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas C M. Promiscuous plasmids of gram-negative bacteria. London, United Kingdom: Academic Press; 1989. [Google Scholar]
- 32.Top E, de Smet I, Verstraete W, Dijkmans R, Mergeay M. Exogenous isolation of mobilizing plasmids from polluted soils and sludges. Appl Environ Microbiol. 1994;60:831–839. doi: 10.1128/aem.60.3.831-839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Top E M, de Rore H, Collard J-M, Gellens V, Slobodkina G, Verstraete W, Mergeay M. Retromobilization of heavy metal resistance genes in unpolluted and heavy metal polluted soil. FEMS Microbiol Ecol. 1995;18:191–203. [Google Scholar]
- 34.Trevors J T. Sterilization and inhibition of microbial activity in soil. J Microbiol Methods. 1996;26:53–59. [Google Scholar]
- 35.van Elsas J D, Smalla K. Extraction of DNA from soil. In: Akkermans A D L, van Elsas J D, de Bruijn F J, editors. Molecular microbial ecology manual, 1.3.3. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1–11. [Google Scholar]
- 36.van Elsas J D, Trevors J T, Starodub M-E. Bacterial conjugation in the rhizosphere of wheat. FEMS Microbiol Ecol. 1988;53:299–306. [Google Scholar]
- 37.van Elsas J D, Wolters A C. Polymerase chain reaction (PCR) analysis of soil microbial DNA. In: Akkermans A D L, van Elsas J D, de Bruijn F J, editors. Molecular microbial ecology manual, 2.7.2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1–10. [Google Scholar]
- 38.Verhagen C, Smit E, Janssen D B, van Elsas J D. Bacterial dichloropropene degradation in soil; screening of soils and involvement of plasmids carrying a dhlA-like gene. Soil Biol Biochem. 1995;27:1547–1557. [Google Scholar]
- 39.Wellington E M H, van Elsas J D. Genetic interaction among microorganisms in the natural environment. London, United Kingdom: Pergamon Press; 1992. [Google Scholar]
- 40.Wickham G S, Atlas R M. Plasmid frequency fluctuations in bacterial populations from chemically stressed soil communities. Appl Environ Microbiol. 1988;54:2192–2196. doi: 10.1128/aem.54.9.2192-2196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilmotte, A. 1997. Unpublished results.
- 42.Wilmotte A, Hennen A, van der Lelie D, Mergeay M. The broad host range plasmid pMOL96 contains a putative transposon similar to Tn402 (Tn5090) and Tn5053. In: Karagouni A, Koraki D, editors. Abstracts of the BAGECO-5 Conference, Nafplion, Greece. Athens, Greece: University of Athens Press; 1996. p. 30. [Google Scholar]